Impact of Metal Impregnation of Commercial Zeolites in the Catalytic Pyrolysis of Real Mixture of Post-Consumer Plastic Waste
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalytic Materials
2.2. Catalytic Performance of the Metal-Impregnated Zeolites
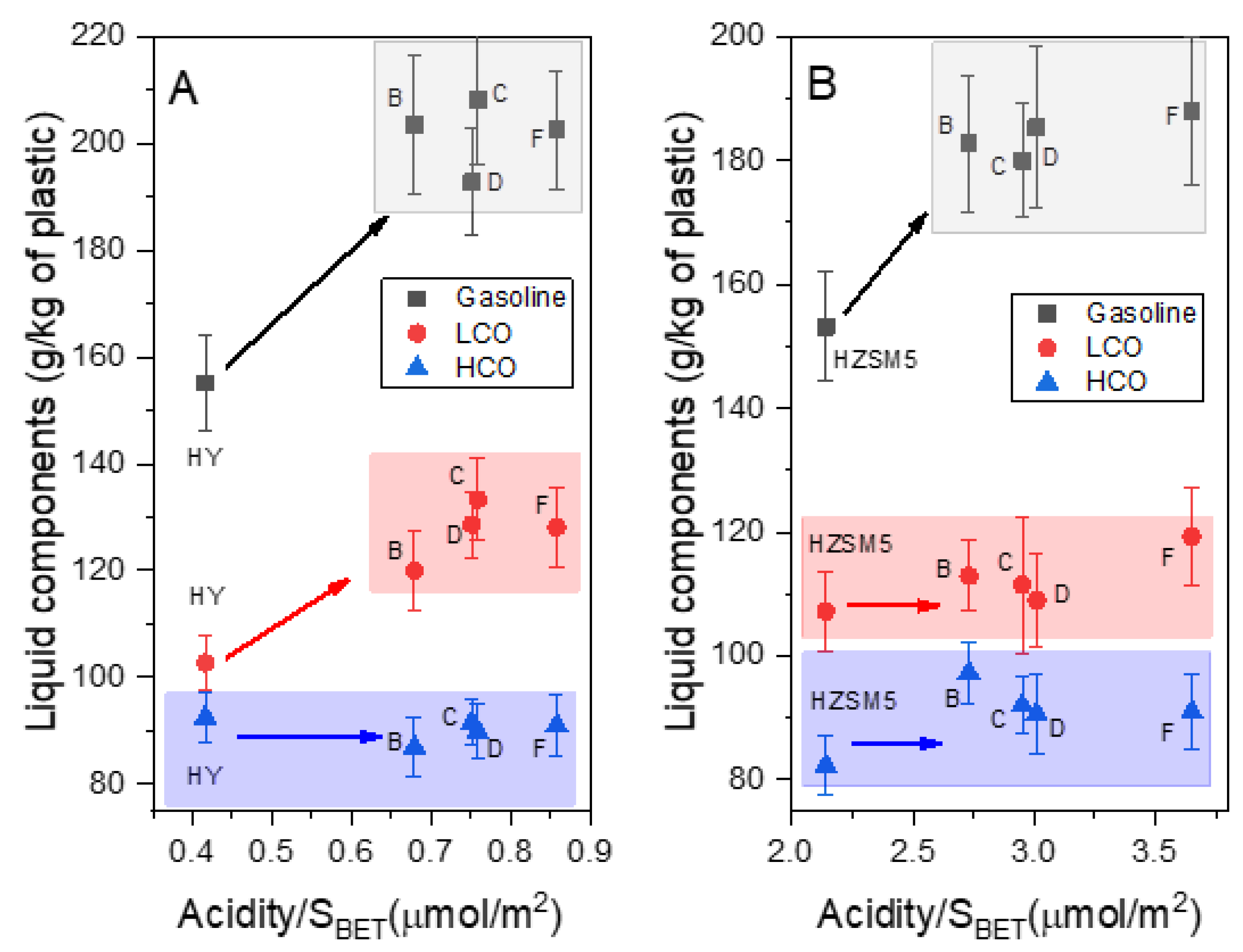
2.2.1. Effect on Product Yields and the Functional Groups of the Liquid Products
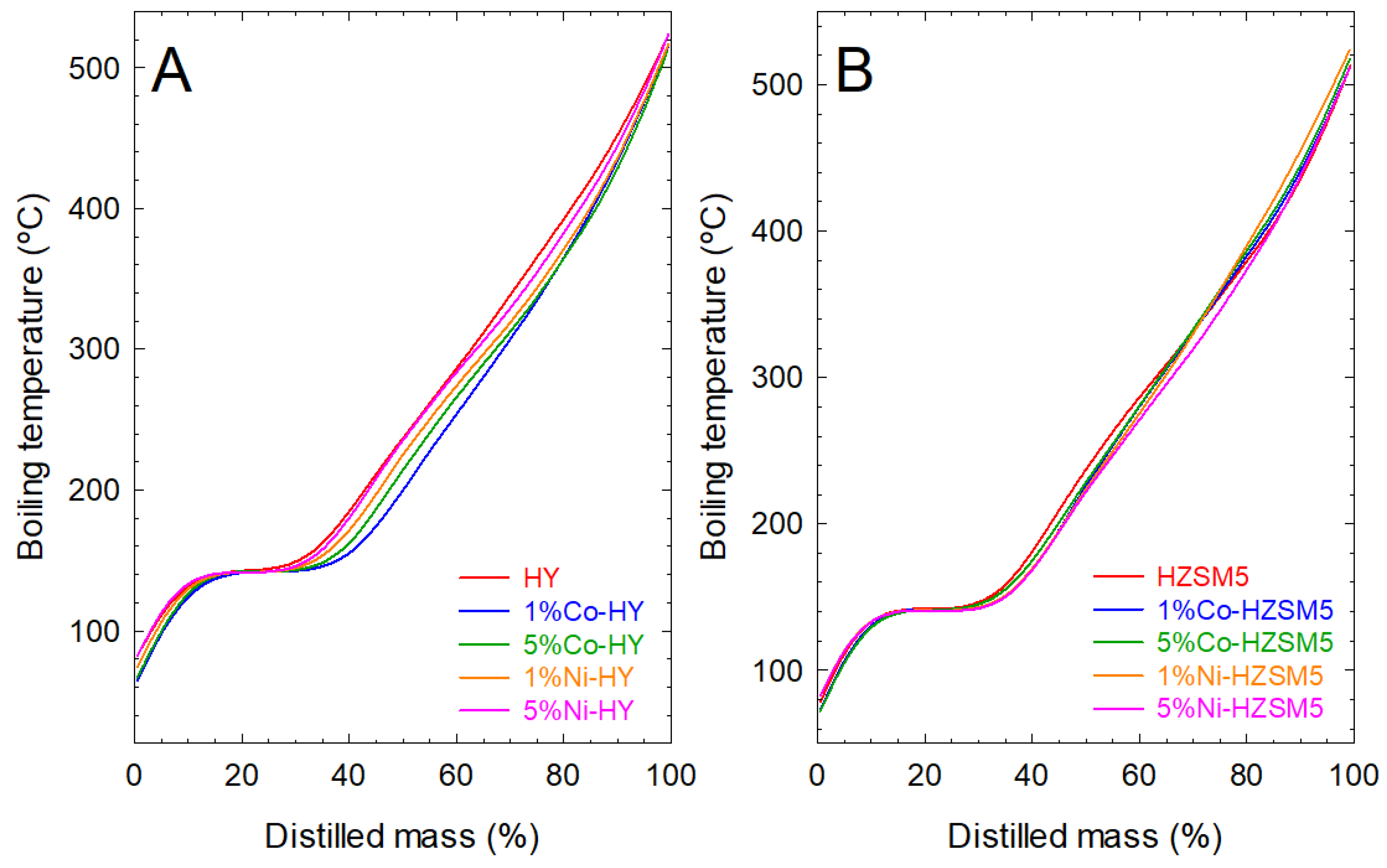
2.2.2. Effect on Simulated Distillation Boiling Points of the Liquid Products
3. Materials and Methods
3.1. Raw Material
3.2. Preparation and Characterization of the Catalysts
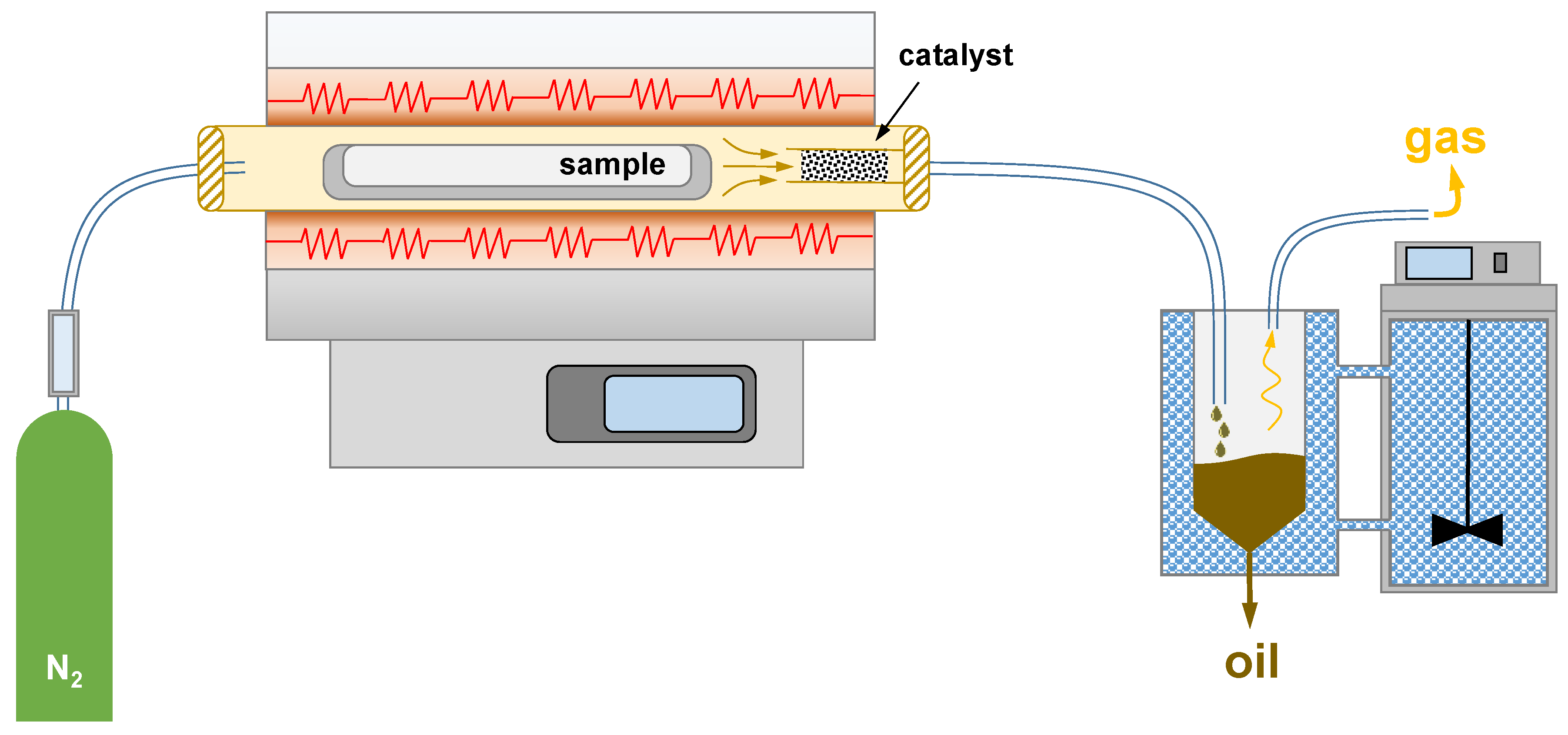
3.3. Pyrolysis Reactor and Pyrolysis Conditions
3.4. Liquid Product Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nation Environment Programme. Single-Use Plastics: A Roadmap for Sustainability; Canno, T., Ed.; United Nations Environment Programe: Nairobi, Kenya, 2018. [Google Scholar]
- Plastics Europe. Plastics Europe Association of Plastics Manufacturers Plastics—The Facts 2021 An analysis of European Plastics Production, Demand and Waste Data. In Plastics-Facts 2021; Plastics Europe: Bruxelles, Belgium, 2021; p. 34. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (accessed on 1 January 2024).
- Wan, Y.; Chen, X.; Liu, Q.; Hu, H.; Wu, C.; Xue, Q. Informal landfill contributes to the pollution of microplastics in the surrounding environment. Environ. Pollut. 2022, 293, 118586. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, Z.; Wu, D.; Zhan, L.; Shi, H.; Xie, B. Occurrence of microplastics in landfill systems and their fate with landfill age. Water Res. 2019, 164, 114968. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhu, Z.-R.; Li, W.-H.; Yan, X.; Wang, L.-K.; Zhang, L.; Jin, J.; Dai, X.; Ni, B.-J. Revisiting Microplastics in Landfill Leachate: Unnoticed Tiny Microplastics and Their Fate in Treatment Works. Water Res. 2020, 190, 116784. [Google Scholar] [CrossRef] [PubMed]
- European Commission. European Strategy for Plastics in a Circular Economy. EUR-Lex. 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A52018DC0028 (accessed on 23 November 2022).
- Scheirs, J.; Kaminsky, W. Feedstock Recycling and Pyrolysis of Waste Plastics: Converting Waste Plastics into Diesel and Other Fuels; John Wiley & Sons, Ltd.: West Sussex, UK, 2006. [Google Scholar]
- Kusenberg, M.; Eschenbacher, A.; Delva, L.; De Meester, S.; Delikonstantis, E.; Stefanidis, G.D.; Ragaert, K.; Van Geem, K.M. Towards high-quality petrochemical feedstocks from mixed plastic packaging waste via advanced recycling: The past, present and future. Fuel Process. Technol. 2022, 238, 107474. [Google Scholar] [CrossRef]
- Huysveld, S.; Ragaert, K.; Demets, R.; Nhu, T.; Civancik-Uslu, D.; Kusenberg, M.; Van Geem, K.; De Meester, S.; Dewulf, J. Technical and market substitutability of recycled materials: Calculating the environmental benefits of mechanical and chemical recycling of plastic packaging waste. Waste Manag. 2022, 152, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Harscoet, E.; Chouvenc, S.; Faugeras, A.-C.; Ammenti-Deloitte, F. Chemical and Physico-Chemical Recycling of Plastic Waste; Final Report; Record: Villeurbanne, France, 2022; Available online: www.record-net.org (accessed on 1 January 2024).
- Peng, Y.; Wang, Y.; Ke, L.; Dai, L.; Wu, Q.; Cobb, K.; Zeng, Y.; Zou, R.; Liu, Y.; Ruan, R. A review on catalytic pyrolysis of plastic wastes to high-value products. Energy Convers. Manag. 2022, 254, 115243. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, S.; Pramanik, H. Pyrolysis of waste expanded polystyrene and reduction of styrene via in-situ multiphase pyrolysis of product oil for the production of fuel range hydrocarbons. Waste Manag. 2021, 120, 330–339. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Wang, X.; Wang, R.; Wang, K.; Pang, S.; Zhong, Z.; Sun, Y.; Ruan, R.; Ragauskas, A.J. Converting polycarbonate and polystyrene plastic wastes into aromatic hydrocarbons via catalytic fast co-pyrolysis. J. Hazard. Mater. 2020, 386, 121970. [Google Scholar] [CrossRef]
- Xue, Y.; Johnston, P.; Bai, X. Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics. Energy Convers. Manag. 2017, 142, 441–451. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Muhammad, C.; Williams, P.T. Influence of catalyst bed temperature and properties of zeolite catalysts on pyrolysis-catalysis of a simulated mixed plastics sample for the production of upgraded fuels and chemicals. J. Energy Inst. 2019, 92, 1337–1347. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.; Laresgoiti, M.; Adrados, A.; Torres, A. Pyrolysis of municipal plastic wastes II: Influence of raw material composition under catalytic conditions. Waste Manag. 2011, 31, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, B.; Valizadeh, S.; Kim, H.; Choi, Y.J.; Seo, M.W.; Yoo, K.S.; Lin, K.-Y.A.; Hussain, M.; Park, Y.-K. Production of light olefins and monocyclic aromatic hydrocarbons from the pyrolysis of waste plastic straws over high-silica zeolite-based catalysts. Environ. Res. 2023, 245, 118076. [Google Scholar] [CrossRef] [PubMed]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Abdelnaby, M.A. Synthesis of value-added aromatic chemicals from catalytic pyrolysis of waste wind turbine blades and their kinetic analysis using artificial neural network. J. Anal. Appl. Pyrolysis 2024, 177, 106330. [Google Scholar] [CrossRef]
- Muneer, B.; Zeeshan, M.; Qaisar, S.; Razzaq, M.; Iftikhar, H. Influence of in-situ and ex-situ HZSM-5 catalyst on co-pyrolysis of corn stalk and polystyrene with a focus on liquid yield and quality. J. Clean. Prod. 2019, 237, 117762. [Google Scholar] [CrossRef]
- Kartik, S.; Balsora, H.K.; Sharma, M.; Saptoro, A.; Jain, R.K.; Joshi, J.B.; Sharma, A. Valorization of plastic wastes for production of fuels and value-added chemicals through pyrolysis—A review. Therm. Sci. Eng. Prog. 2022, 32, 101316. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Y.; Kong, G.; Liu, Q.; Zhang, G.; Wang, K.; Cao, T.; Cheng, Q.; Zhang, Z.; Ji, G.; et al. CO2-mediated catalytic upcycling of plastic waste for H2-rich syngas and carbon nanomaterials. J. Hazard. Mater. 2023, 460, 132500. [Google Scholar] [CrossRef] [PubMed]
- Uemichi, Y.; Makino, Y.; Kanazuka, T. Degradation of polyethylene to aromatic hydrocarbons over metal-supported activated carbon catalysts. J. Anal. Appl. Pyrolysis 1989, 14, 331–344. [Google Scholar] [CrossRef]
- Nishino, J.; Itoh, M.; Fujiyoshi, H.; Uemichi, Y. Catalytic degradation of plastic waste into petrochemicals using Ga-ZSM-5. Fuel 2008, 87, 3681–3686. [Google Scholar] [CrossRef]
- Pinto, F.; Costa, P.; Gulyurtlu, I.; Cabrita, I. Pyrolysis of plastic wastes: 2. Effect of catalyst on product yield. J. Anal. Appl. Pyrolysis 1999, 51, 57–71. [Google Scholar] [CrossRef]
- Kang, J.; Kim, J.Y.; Sung, S.; Lee, Y.; Gu, S.; Choi, J.-W.; Yoo, C.-J.; Suh, D.J.; Choi, J.; Ha, J.-M. Chemical upcycling of PVC-containing plastic wastes by thermal degradation and catalysis in a chlorine-rich environment. Environ. Pollut. 2024, 342, 123074. [Google Scholar] [CrossRef]
- Upare, D.P.; Park, S.; Kim, M.; Kim, J.; Lee, D.; Lee, J.; Chang, H.; Choi, W.; Choi, S.; Jeon, Y.-P.; et al. Cobalt promoted Mo/beta zeolite for selective hydrocracking of tetralin and pyrolysis fuel oil into monocyclic aromatic hydrocarbons. J. Ind. Eng. Chem. 2016, 35, 99–107. [Google Scholar] [CrossRef]
- Guo, H.; He, H.; Miao, C.; Hua, W.; Yue, Y.; Gao, Z. Ethane conversion in the presence of CO2 over Co-based ZSM-5 zeolite: Co species controlling the reaction pathway. Mol. Catal. 2022, 519, 112155. [Google Scholar] [CrossRef]
- Ewuzie, R.N.; Genza, J.R.; Abdullah, A.Z. Catalytic hydrogenolysis of lignin in isopropanol as hydrogen donor over nickel-cobalt supported on zeolite to produce aromatic and phenolic monomers. Appl. Catal. A Gen. 2023, 667, 119443. [Google Scholar] [CrossRef]
- Kurniawan, R.G.; Karanwal, N.; Park, J.; Verma, D.; Kwak, S.K.; Kim, S.K.; Kim, J. Direct conversion of furfural to 1,5-pentanediol over a nickel–cobalt oxide–alumina trimetallic catalyst. Appl. Catal. B Environ. 2023, 320, 121971. [Google Scholar] [CrossRef]
- Cai, Y.; Fan, Y.; Li, X.; Chen, L.; Wang, J. Preparation of refined bio-oil by catalytic transformation of vapors derived from vacuum pyrolysis of rape straw over modified HZSM-5. Energy 2016, 102, 95–105. [Google Scholar] [CrossRef]
- Pedrosa, A.M.G.; Souza, M.J.; Melo, D.M.; Araujo, A.S. Cobalt and nickel supported on HY zeolite: Synthesis, characterization and catalytic properties. Mater. Res. Bull. 2006, 41, 1105–1111. [Google Scholar] [CrossRef]
- Lai, S.; Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Zhang, Z.; Lu, G. The promotional role of Ce in Cu/ZSM-5 and in situ surface reaction for selective catalytic reduction of NOx with NH3. RSC Adv. 2015, 5, 90235–90244. [Google Scholar] [CrossRef]
- Cairns, R.W.; Ott, E. X-Ray Studies of the System Nickel-Oxygen-Water. J. Am. Chem. Soc. 1933, 55, 527–533. [Google Scholar] [CrossRef]
- Chupin, C.; Vanveen, A.; Konduru, M.; Després, J.; Mirodatos, C. Identity and location of active species for NO reduction by CH4 over Co-ZSM-5. J. Catal. 2006, 241, 103–114. [Google Scholar] [CrossRef]
- Seo, S.M.; Lim, W.T.; Seff, K. Single-crystal structures of fully and partially dehydrated zeolite Y (FAU, Si/Al = 1.56) largely Co2+ exchanged at pH 5.1. Microporous Mesoporous Mater. 2013, 170, 67–74. [Google Scholar] [CrossRef]
- Núñez, F.; Chen, L.; Wang, J.A.; Flores, S.O.; Salmones, J.; Arellano, U.; Noreña, L.E.; Tzompantzi, F. Bifunctional Co3O4/ZSM-5 Mesoporous Catalysts for Biodiesel Production via Esterification of Unsaturated Omega-9 Oleic Acid. Catalysts 2022, 12, 900. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. The role of the Auger parameter in XPS studies of nickel metal, halides and oxides. Phys. Chem. Chem. Phys. 2012, 14, 2434–2442. [Google Scholar] [CrossRef]
- Peck, M.A.; Langell, M.A. Comparison of Nanoscaled and Bulk NiO Structural and Environmental Characteristics by XRD, XAFS, and XPS. Chem. Mater. 2012, 24, 4483–4490. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Valero-Romero, M.J.; Sartipi, S.; Sun, X.; Rodríguez-Mirasol, J.; Cordero, T.; Kapteijn, F.; Gascon, J. Carbon/H-ZSM-5 composites as supports for bi-functional Fischer–Tropsch synthesis catalysts. Catal. Sci. Technol. 2016, 6, 2633–2646. [Google Scholar] [CrossRef]
- Vovchok, D.; Tata, J.; Orozco, I.; Zhang, F.; Palomino, R.M.; Xu, W.; Harper, L.; Khatib, S.J.; Rodriguez, J.A.; Senanayake, S.D. Location and chemical speciation of Cu in ZSM-5 during the water-gas shift reaction. Catal. Today 2019, 323, 216–224. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Scherzer, J. Correlation between Catalyst Formulation and Catalytic Properties. In Fluid Catalytic Cracking: Science and Technology; Magee, J.S., Mitchell, M.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; Volume 76, pp. 145–182. [Google Scholar]
- Reschetilowski, W.; Unger, B.; Wendlandt, K.-P. Study of the ammonia–zeolite interaction in modified ZSM-5 by temperature-programmed desorption of ammonia. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 2941–2944. [Google Scholar] [CrossRef]
- Karge, H.G.; Dondur, V. Investigation of the distribution of acidity in zeolites by temperature-programmed desorption of probe molecules. 2. Dealuminated Y-Type Zeolites. J. Phys. Chem. 1991, 95, 282–288. [Google Scholar] [CrossRef]
- Razzaq, M.; Zeeshan, M.; Qaisar, S.; Iftikhar, H.; Muneer, B. Investigating use of metal-modified HZSM-5 catalyst to upgrade liquid yield in co-pyrolysis of wheat straw and polystyrene. Fuel 2019, 257, 116119. [Google Scholar] [CrossRef]
- Veses, A.; Puértolas, B.; Callén, M.; García, T. Catalytic upgrading of biomass derived pyrolysis vapors over metal-loaded ZSM-5 zeolites: Effect of different metal cations on the bio-oil final properties. Microporous Mesoporous Mater. 2015, 209, 189–196. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Li, J.; Su, L.; Zuo, J.; Komarneni, S.; Wang, Y. Improving the aromatic production in catalytic fast pyrolysis of cellulose by co-feeding low-density polyethylene. Appl. Catal. A Gen. 2013, 455, 114–121. [Google Scholar] [CrossRef]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Aromatic fuel oils produced from the pyrolysis-catalysis of polyethylene plastic with metal-impregnated zeolite catalysts. J. Energy Inst. 2019, 92, 195–202. [Google Scholar] [CrossRef]
- Calero, M.; Solís, R.R.; Muñoz-Batista, M.J.; Pérez, A.; Blázquez, G.; Martín-Lara, M. Oil and gas production from the pyrolytic transformation of recycled plastic waste: An integral study by polymer families. Chem. Eng. Sci. 2023, 271, 118569. [Google Scholar] [CrossRef]
- Paucar-Sánchez, M.F.; Calero, M.; Blázquez, G.; Solís, R.R.; Muñoz-Batista, M.J.; Martín-Lara, M.Á. Thermal and catalytic pyrolysis of a real mixture of post-consumer plastic waste: An analysis of the gasoline-range product. Process Saf. Environ. Prot. 2022, 168, 1201–1211. [Google Scholar] [CrossRef]
- Miskolczi, N.; Bartha, L.; Deák, G. Thermal degradation of polyethylene and polystyrene from the packaging industry over different catalysts into fuel-like feed stocks. Polym. Degrad. Stab. 2006, 91, 517–526. [Google Scholar] [CrossRef]
- Iliopoulou, E.; Stefanidis, S.; Kalogiannis, K.; Delimitis, A.; Lappas, A.; Triantafyllidis, K. Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Appl. Catal. B Environ. 2012, 127, 281–290. [Google Scholar] [CrossRef]
- Richardson, J.T. Principles of Catalyst Development, 2nd ed.; Springer Science: New York, NY, USA, 1992. [Google Scholar]
- Altomare, A.; Corriero, N.; Cuocci, C.; Falcicchio, A.; Moliterni, A.; Rizzi, R. QUALX2.0: A qualitative phase analysis software using the freely available database POW_COD. J. Appl. Crystallogr. 2015, 48, 598–603. [Google Scholar] [CrossRef]
- Paucar-Sánchez, M.F.; Calero, M.; Blázquez, G.; Muñoz-Batista, M.J.; Martín-Lara, M.A. Characterization of liquid fraction obtained from pyrolysis of post-consumer mixed plastic waste: A comparing between measured and calculated parameters. Process Saf. Environ. Prot. 2022, 159, 1053–1063. [Google Scholar] [CrossRef]
- ASTM D2789-95; Standard Test Method for Hydrocarbon Types in Low Olefinic Gasoline by Mass Spectrometry. American Society for Testing and Materials, ASTM International: West Conshohocken, PA, USA, 2016; pp. 1–7.
- MNL10902M; Standard Test Method for Boiling Range Distribution of Petroleum Fractions by Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2008; pp. 444–454. [CrossRef]
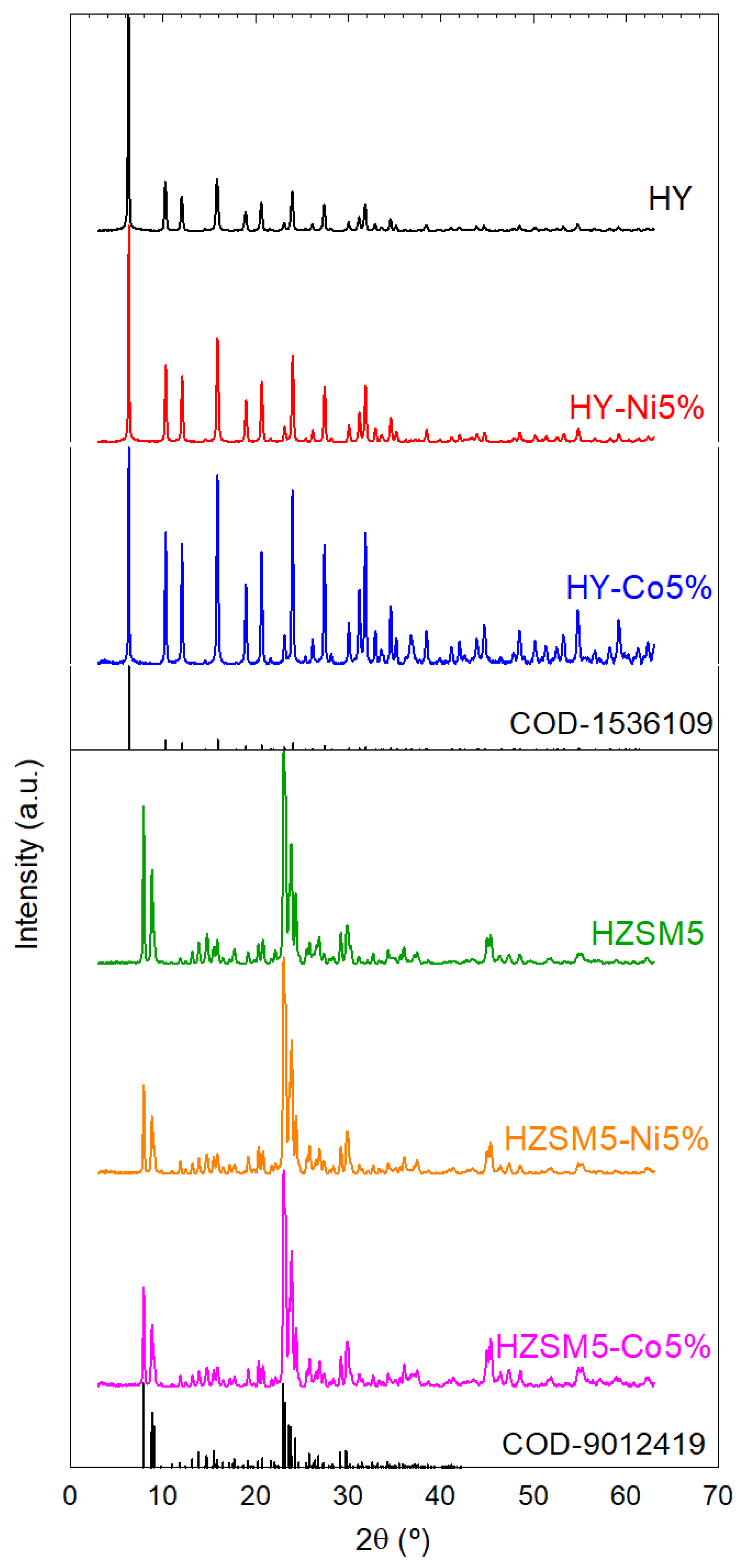
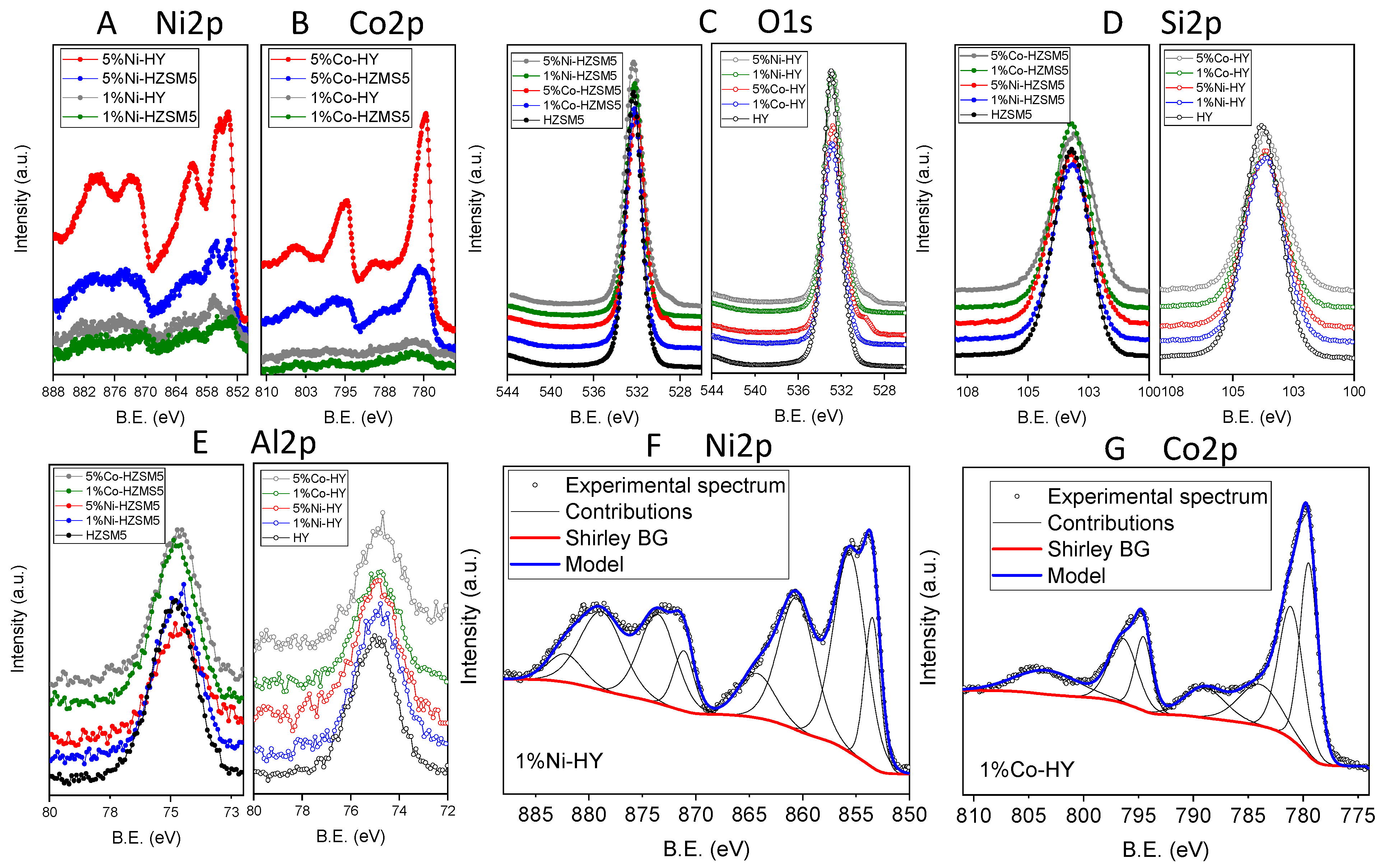

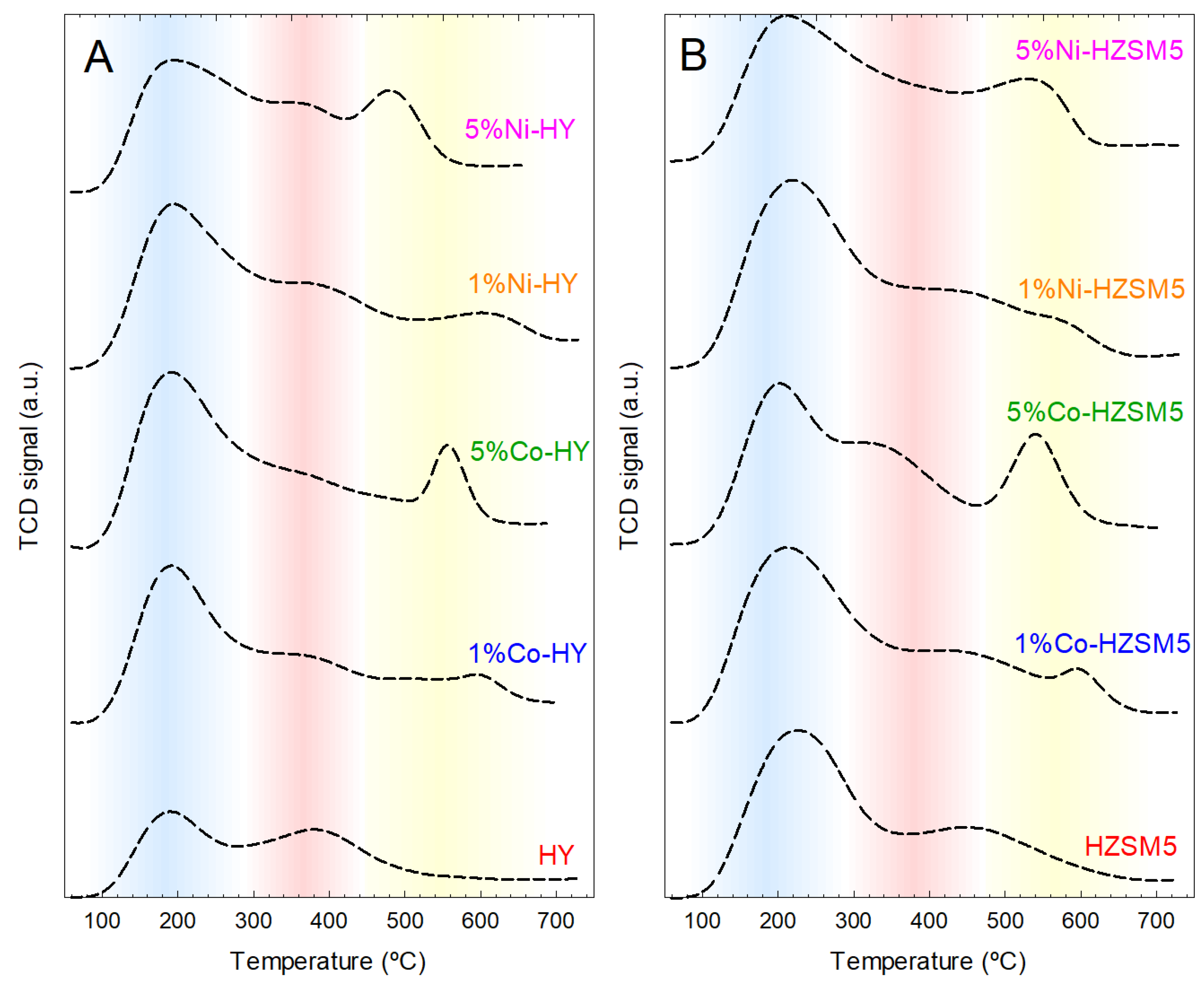
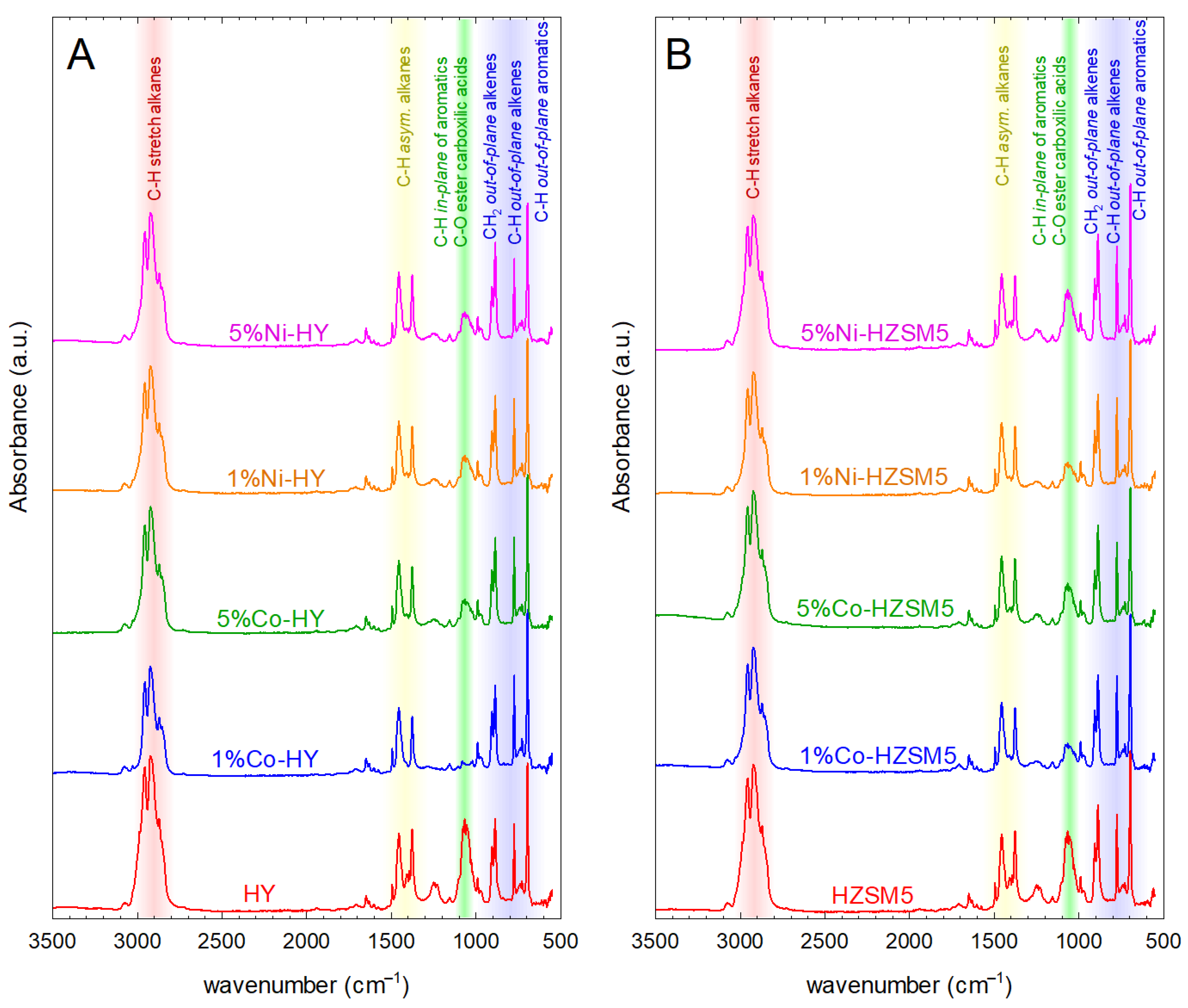
| Catalyst | L (nm) | SBET (m2/g) | SMP (m2/g) | VT (cm3/g) | VMP (cm3/g) | VMP/VT (%) | Dave (Å) | Total Acidity (μmol/g) |
|---|---|---|---|---|---|---|---|---|
| HY | 51.91 | 1384 | 1274 | 0.705 | 0.432 | 61.3 | 50.5 | 576 |
| 1%Co-HY | - | 1044 | 961 | 0.521 | 0.326 | 62.6 | 55.5 | 709 |
| 5%Co-HY | 59.38 | 959 | 884 | 0.476 | 0.300 | 63.0 | 54.9 | 728 |
| 1%Ni-HY | - | 1022 | 943 | 0.511 | 0.320 | 62.6 | 56.6 | 768 |
| 5%Ni-HY | 51.91 | 974 | 893 | 0.494 | 0.304 | 61.5 | 54.0 | 836 |
| HZSM5 | 47.03 | 488 | 440 | 0.241 | 0.149 | 61.8 | 31.4 | 1045 |
| 1%Co-HZSM5 | - | 472 | 423 | 0.230 | 0.143 | 62.2 | 40.2 | 1300 |
| 5%Co-HZSM5 | 52.90 | 432 | 388 | 0.230 | 0.131 | 56.9 | 41.0 | 1302 |
| 1%Ni-HZSM5 | - | 469 | 422 | 0.230 | 0.142 | 61.7 | 32.7 | 1383 |
| 5%Ni-HZSM5 | 47.02 | 413 | 368 | 0.215 | 0.124 | 57.7 | 36.4 | 1509 |
| Catalyst | Coke/Catalyst (%wt.) | Gas (%wt.) | Liquid (%wt.) | Char (%wt.) |
|---|---|---|---|---|
| HY | 4.4 ± 0.4 | 58.1 ± 1.8 | 35.0 ± 2.2 | 6.9 ± 0.6 |
| 1%Co-HY | 6.2 ± 0.6 | 50.9 ± 2.6 | 41.0 ± 2.6 | 6.6 ± 0.5 |
| 5%Co-HY | 4.1 ± 0.5 | 50.2 ± 2.5 | 43.1 ± 2.5 | 6.6 ± 0.6 |
| 1%Ni-HY | 4.2 ± 0.4 | 51.9 ± 2.0 | 41.3 ± 2.0 | 6.8 ± 0.5 |
| 5%Ni-HY | 4.1 ± 0.4 | 49.6 ± 2.4 | 43.2 ± 2.4 | 7.2 ± 0.7 |
| HZSM5 | 1.3 ± 0.3 | 58.3 ± 2.7 | 34.3 ± 2.0 | 7.5 ± 0.6 |
| 1%Co-HZSM5 | 2.0 ± 0.4 | 53.6 ± 2.0 | 39.3 ± 2.0 | 7.2 ± 0.7 |
| 5%Co-HZSM5 | 2.0 ± 0.4 | 57.1 ± 2.6 | 38.5 ± 2.6 | 6.9 ± 0.5 |
| 1%Ni-HZSM5 | 2.2 ± 0.5 | 57.2 ± 1.8 | 38.3 ± 1.8 | 6.7 ± 0.5 |
| 5%Ni-HZSM5 | 1.5 ± 0.2 | 53.2 ± 2.6 | 39.8 ± 2.6 | 7.0 ± 0.7 |
| Products | Fraction | HY | 1%Co-HY | 5%Co-HY | 1%Ni-HY | 5%Ni-HY |
|---|---|---|---|---|---|---|
| Gasoline | Light Naphtha | 1.7 ± 0.4 | 3.4 ± 0.2 | 3.1 ± 0.3 | 2.5 ± 0.2 | 1.7 ± 0 4 |
| Mid Naphtha | 30.8 ± 2.5 | 36.4 ± 2.0 | 34.5 ± 2.3 | 33.1 ± 2.5 | 32.3 ± 2.0 | |
| Heavy Naphtha | 11.8 ± 0.9 | 10.6 ± 1.0 | 10.6 ± 0.8 | 11.1 ± 1.1 | 11.2 ± 1.0 | |
| Total | 44.3 ± 2.5 | 50.3 ± 2.0 | 48.3 ± 2.3 | 46.7 ± 2.5 | 45.2 ± 2.0 | |
| LCO | Light Gas Oil | 9.8 ± 0.7 | 9.6 ± 0.6 | 9.8 ± 0.5 | 9.9 ± 0.9 | 9.8 ± 0.8 |
| Mid Gas Oil | 9.2 ± 0.4 | 9.2 ± 0.7 | 9.9 ± 0.5 | 9.9 ± 0.7 | 9.6 ± 0.7 | |
| Heavy Gas Oil | 10.3 ± 0.8 | 9.5 ± 0.6 | 11.2 ± 1.1 | 11.3 ± 1.4 | 11.2 ± 0.9 | |
| Total | 29.3 ± 0.8 | 28.2 ± 0.7 | 30.9 ± 1.1 | 31.1 ± 1.4 | 30.6 ± 0.9 | |
| HCO | Light Vacuum Gas Oil | 12.0 ± 0.9 | 9.3 ± 0.5 | 10.1 ± 0.8 | 10.8 ± 0.8 | 11.9 ± 1.0 |
| Mid Vacuum Gas Oil | 7.2 ± 0.5 | 5.7 ± 0.6 | 5.2 ± 0.3 | 5.5 ± 0.5 | 6.1 ± 0.5 | |
| Heavy Vacuum Gas Oil | 7.2 ± 0.3 | 6.5 ± 0.4 | 5.6 ± 0.4 | 5.9 ± 0.5 | 6.2 ± 0.3 | |
| Total | 26.4 ± 0.9 | 21.5 ± 0.6 | 20.8 ± 0.8 | 22.2 ± 0.8 | 24.2 ± 1.0 |
| Products | Fraction | HZSM5 | 1%Co-HZSM5 | 5%Co-HZSM5 | 1%Ni-HZSM5 | 5%Ni-HZSM5 |
|---|---|---|---|---|---|---|
| Gasoline | Light Naphtha | 2.0 ± 0.4 | 2.5 ± 0.3 | 2.9 ± 0.3 | 1.7 ± 0.2 | 1.7 ± 0.3 |
| Mid Naphtha | 31.9 ± 2.3 | 34.0 ± 2.7 | 34.7 ± 2.6 | 34.8 ± 2.4 | 34.9 ± 2.2 | |
| Heavy Naphtha | 10.8 ± 0.9 | 10.0 ± 1.0 | 10.6 ± 1.1 | 10.6 ± 0.8 | 10.6 ± 0.7 | |
| Total | 44.7 ± 2.3 | 46.5 ± 2.7 | 48.2 ± 2.6 | 47.1 ± 2.4 | 47.2 ± 2.2 | |
| LCO | Light Gas Oil | 9.5 ± 0.7 | 9.0 ± 0.7 | 9.0 ± 0.9 | 9.3 ± 0.7 | 9.6 ± 0.9 |
| Mid Gas Oil | 9.6 ± 0.8 | 8.9 ± 0.6 | 9.0 ± 0.8 | 8.7 ± 0.6 | 9.5 ± 0.7 | |
| Heavy Gas Oil | 12.2 ± 1.1 | 10.8 ± 0.9 | 10.3 ± 0.8 | 9.4 ± 0.9 | 10.8 ± 0.8 | |
| Total | 31.3 ± 1.1 | 28.7 ± 0.9 | 28.3 ± 0.9 | 27.4 ± 0.9 | 29.9 ± 0.9 | |
| HCO | Light Vacuum Gas Oil | 12.8 ± 1.2 | 12.2 ± 1.1 | 11.7 ± 0.9 | 10.9 ± 1.1 | 10.9 ± 0.6 |
| Mid Vacuum Gas Oil | 5.6 ± 0.4 | 6.4 ± 0.5 | 5.8 ± 0.6 | 7.1 ± 0.4 | 5.8 ± 0.6 | |
| Heavy Vacuum Gas Oil | 5.6 ± 0.3 | 6.2 ± 0.4 | 6.0 ± 0.5 | 7.5 ± 0.8 | 6.1 ± 0.7 | |
| Total | 24.0 ± 1.2 | 24.8 ± 1.1 | 23.5 ± 0.9 | 25.5 ± 1.1 | 22.9 ± 0.7 |
| Catalyst | Gasoline | LCO | HCO |
|---|---|---|---|
| HY | 155.1 ± 8.8 | 102.7 ± 5.3 | 92.5 ± 4.8 |
| 1%Co-HY | 203.4 ± 13.0 | 120.0 ± 7.6 | 86.9 ± 5.5 |
| 5%Co-HY | 208.2 ± 12.0 | 133.3 ± 7.7 | 89.8 ± 5.2 |
| 1%Ni-HY | 192.8 ± 10.0 | 128.6 ± 6.2 | 91.6 ± 4.4 |
| 5%Ni-HY | 202.5 ± 11.0 | 132.1 ± 7.3 | 97.8 ± 5.4 |
| HZSM5 | 153.2 ± 8.9 | 107.2 ± 6.3 | 82.3 ± 4.8 |
| 1%Co-HZSM5 | 182.7 ± 11.0 | 112.9 ± 5.7 | 97.3 ± 5.0 |
| 5%Co-HZSM5 | 185.4 ± 13.0 | 109.0 ± 7.4 | 90.6 ± 6.1 |
| 1%Ni-HZSM5 | 180.0 ± 9.2 | 111.5 ± 5.2 | 92.0 ± 4.3 |
| 5%Ni-HZSM5 | 188.0 ± 12.0 | 119.3 ± 7.8 | 91.0 ± 6.0 |
| Catalyst | Paraffins, % | Naphthenics, % | Aromatics, % | |||||
|---|---|---|---|---|---|---|---|---|
| Monocycloparaffins | Dicycloparaffins | Total | Alkylbenzenes | Indan or Tetralins | Naphthalenes | Total | ||
| HY | 19.2 ± 1.2 | 19.9 ± 1.2 | 29.1 ± 2.4 | 49.3 ± 2.4 | 22.1 ± 1.6 | 7.8 ± 1.4 | 1.6 ± 0.5 | 31.5 ± 1.6 |
| 1%Co-HY | 18.1 ± 2.0 | 19.7 ± 1.7 | 30.0 ± 1.9 | 49.7 ± 1.9 | 22.3 ± 2.2 | 8.1 ± 1.0 | 1.8 ± 1.0 | 32.2 ± 2.2 |
| 5%Co-HY | 19.8 ± 1.9 | 18.8 ± 1.4 | 25.8 ± 1.9 | 44.6 ± 1.9 | 24.0 ± 2.0 | 10.2 ± 0.8 | 1.4 ± 0.4 | 35.6 ± 2.0 |
| 1%Ni-HY | 19.2 ± 1.2 | 20.1 ± 0.9 | 30.0 ± 2.1 | 50.1 ± 2.1 | 20.7 ± 1.5 | 8.2 ± 1.2 | 1.8 ± 0.6 | 30.7 ± 1.5 |
| 5%Ni-HY | 19.4 ± 2.4 | 20.5 ± 2.0 | 27.5 ± 1.4 | 48.0 ± 2.0 | 23.2 ± 1.6 | 8.01 ± 0.6 | 1.4 ± 0.5 | 32.6 ± 1.6 |
| HZSM5 | 19.4 ± 2.1 | 20.4 ± 2.2 | 25.3 ± 2.3 | 45.6 ± 2.3 | 26.3 ± 1.0 | 8.2 ± 1.1 | 0.5 ± 0.2 | 35.0 ± 1.1 |
| 1%Co-HZSM5 | 20.5 ± 1.5 | 19.8 ± 3.1 | 27.0 ± 1.9 | 46.8 ± 3.1 | 22.5 ± 3.4 | 8.8 ± 0.6 | 1.3 ± 0.5 | 32.7 ± 3.4 |
| 5%Co-HZSM5 | 20.3 ± 2.5 | 21.1 ± 1.8 | 30.0 ± 3.4 | 51.2 ± 3.4 | 20.3 ± 1.9 | 7.2 ± 0.7 | 1.1 ± 0.4 | 28.6 ± 1.9 |
| 1%Ni-HZSM5 | 19.2 ± 3.0 | 20.9 ± 2.9 | 29.6 ± 4.4 | 50.5 ± 4.4 | 20.9 ± 2.3 | 7.8 ± 0.8 | 1.7 ± 0.6 | 30.3 ± 2.3 |
| 5%Ni-HZSM5 | 18.7 ± 1.9 | 20.8 ± 1.0 | 29.1 ± 1.6 | 49.9 ± 1.6 | 22.1 ± 3.1 | 8.1 ± 1.2 | 1.3 ± 0.3 | 31.4 ± 3.1 |
| Products | Fraction | Boiling Point (°C) |
|---|---|---|
| Gasoline | Light Naphtha | 36–90 |
| Mid Naphtha | 90–160 | |
| Heavy Naphtha | 160–205 | |
| LCO | Light Gas Oil | 205–260 |
| Mid Gas Oil | 260–315 | |
| Heavy Gas Oil | 315–370 | |
| HCO | Light Vacuum Gas Oil | 370–430 |
| Mid Vacuum Gas Oil | 430–480 | |
| Heavy Vacuum Gas Oil | 480–565 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paucar-Sánchez, M.F.; Calero, M.; Blázquez, G.; Solís, R.R.; Muñoz-Batista, M.J.; Martín-Lara, M.Á. Impact of Metal Impregnation of Commercial Zeolites in the Catalytic Pyrolysis of Real Mixture of Post-Consumer Plastic Waste. Catalysts 2024, 14, 168. https://doi.org/10.3390/catal14030168
Paucar-Sánchez MF, Calero M, Blázquez G, Solís RR, Muñoz-Batista MJ, Martín-Lara MÁ. Impact of Metal Impregnation of Commercial Zeolites in the Catalytic Pyrolysis of Real Mixture of Post-Consumer Plastic Waste. Catalysts. 2024; 14(3):168. https://doi.org/10.3390/catal14030168
Chicago/Turabian StylePaucar-Sánchez, Marco F., Mónica Calero, Gabriel Blázquez, Rafael R. Solís, Mario J. Muñoz-Batista, and María Ángeles Martín-Lara. 2024. "Impact of Metal Impregnation of Commercial Zeolites in the Catalytic Pyrolysis of Real Mixture of Post-Consumer Plastic Waste" Catalysts 14, no. 3: 168. https://doi.org/10.3390/catal14030168
APA StylePaucar-Sánchez, M. F., Calero, M., Blázquez, G., Solís, R. R., Muñoz-Batista, M. J., & Martín-Lara, M. Á. (2024). Impact of Metal Impregnation of Commercial Zeolites in the Catalytic Pyrolysis of Real Mixture of Post-Consumer Plastic Waste. Catalysts, 14(3), 168. https://doi.org/10.3390/catal14030168











