Co–HOAT Complexes Change Their Antibacterial and Physicochemical Properties with Morphological Evolution
Abstract
:1. Introduction
2. Experimental Sections
2.1. Materials and Characterization
2.2. Synthesis of Various Co–HOAT Nanoparticles
2.3. The Test of Cobalt Ions in Co–HOAT Nanoparticles
2.4. Culture and Treatment of Microorganisms
2.5. The Videos of Optical Microscope for Anchoring Bacteria
2.6. Molecular Geometries of Calculated Structures
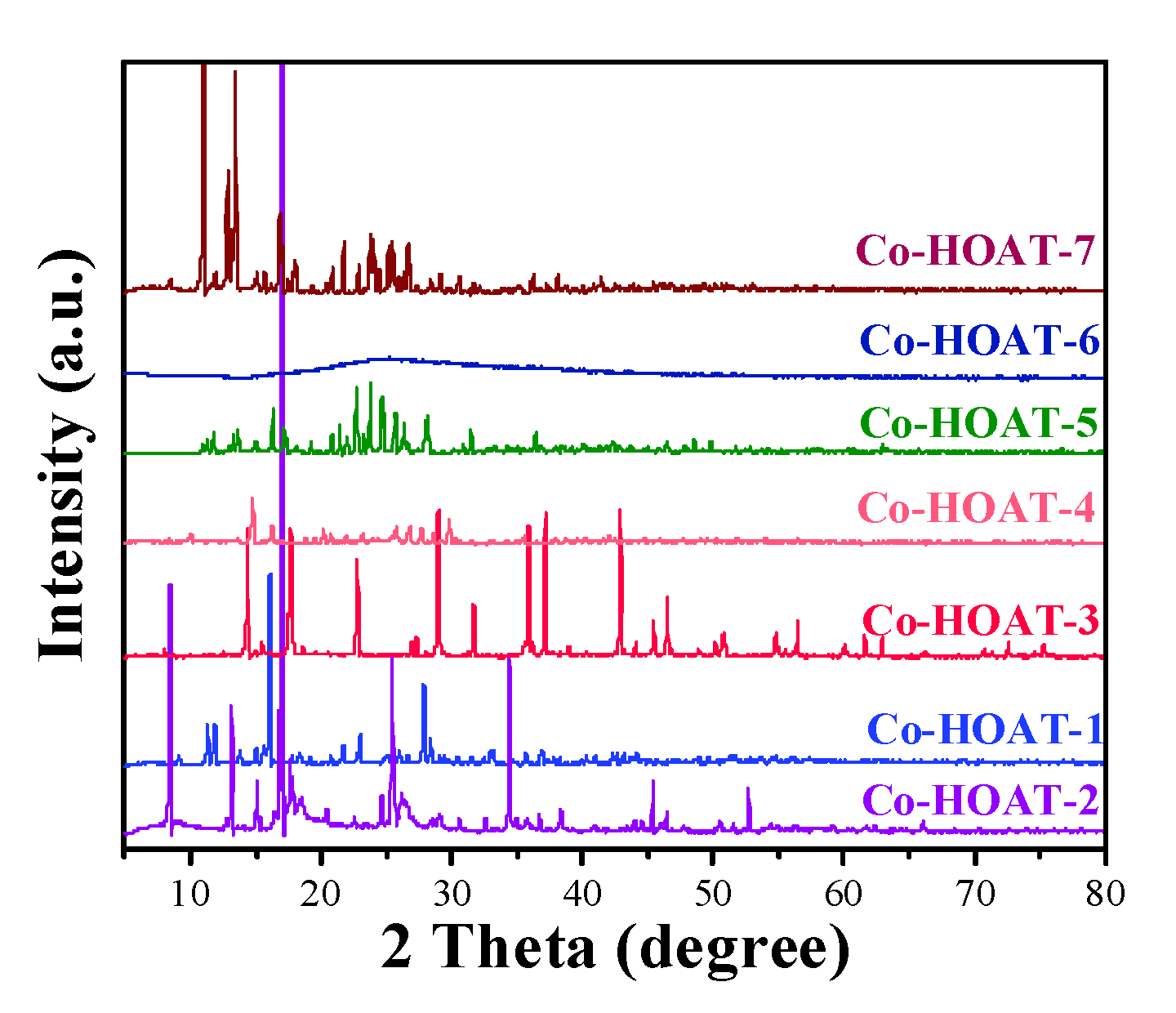
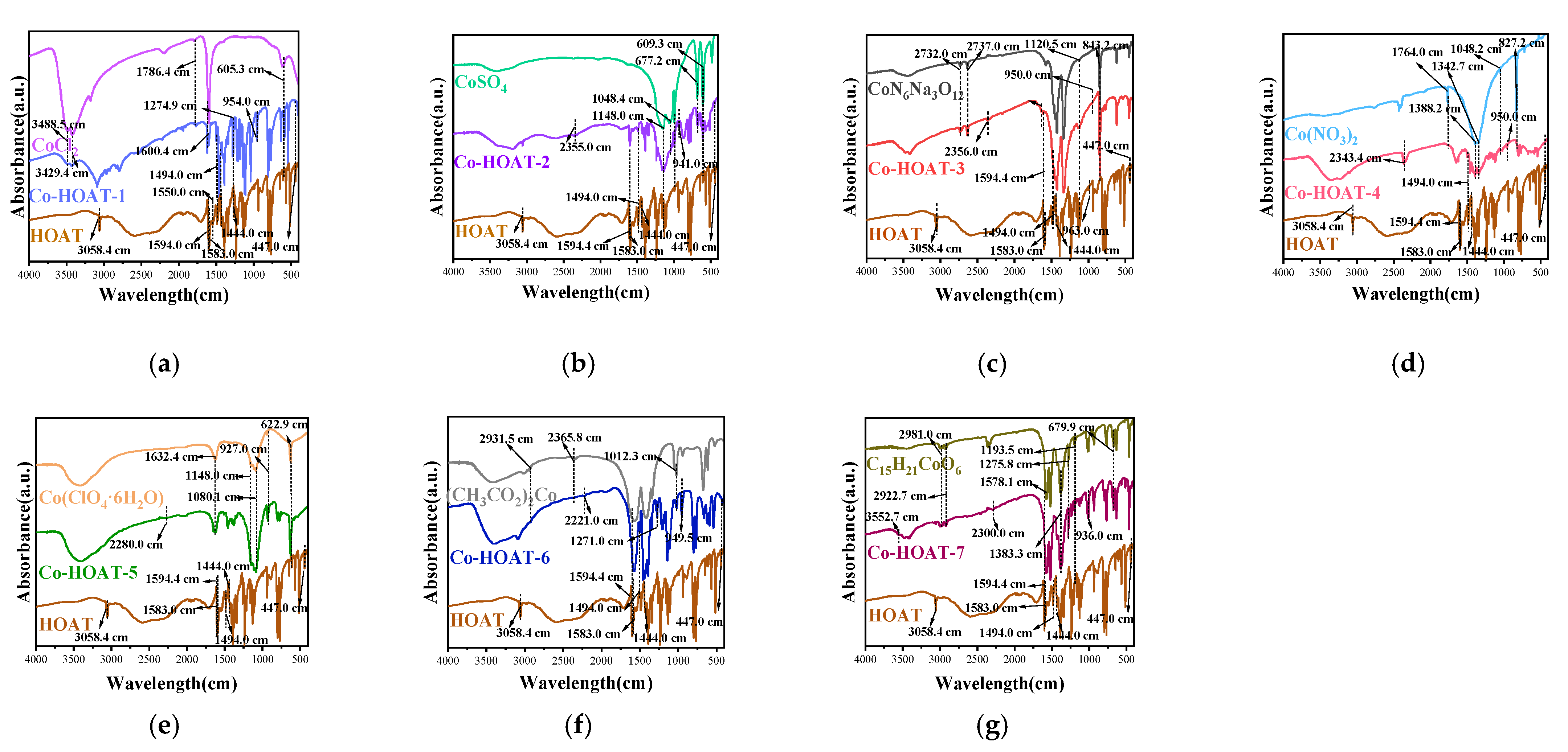
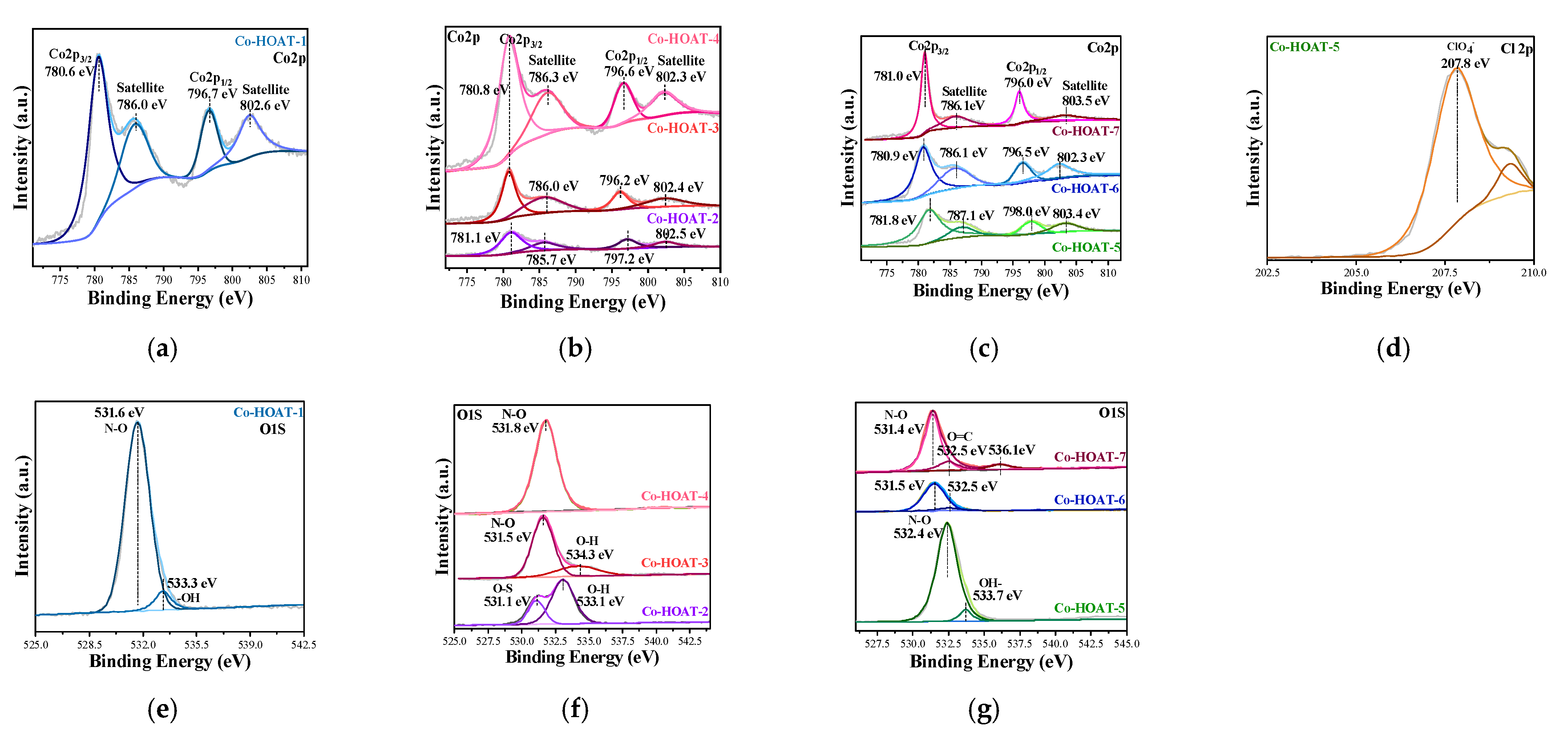
3. Results and Analysis
Characterization of Samples
4. Antibacterial Assay
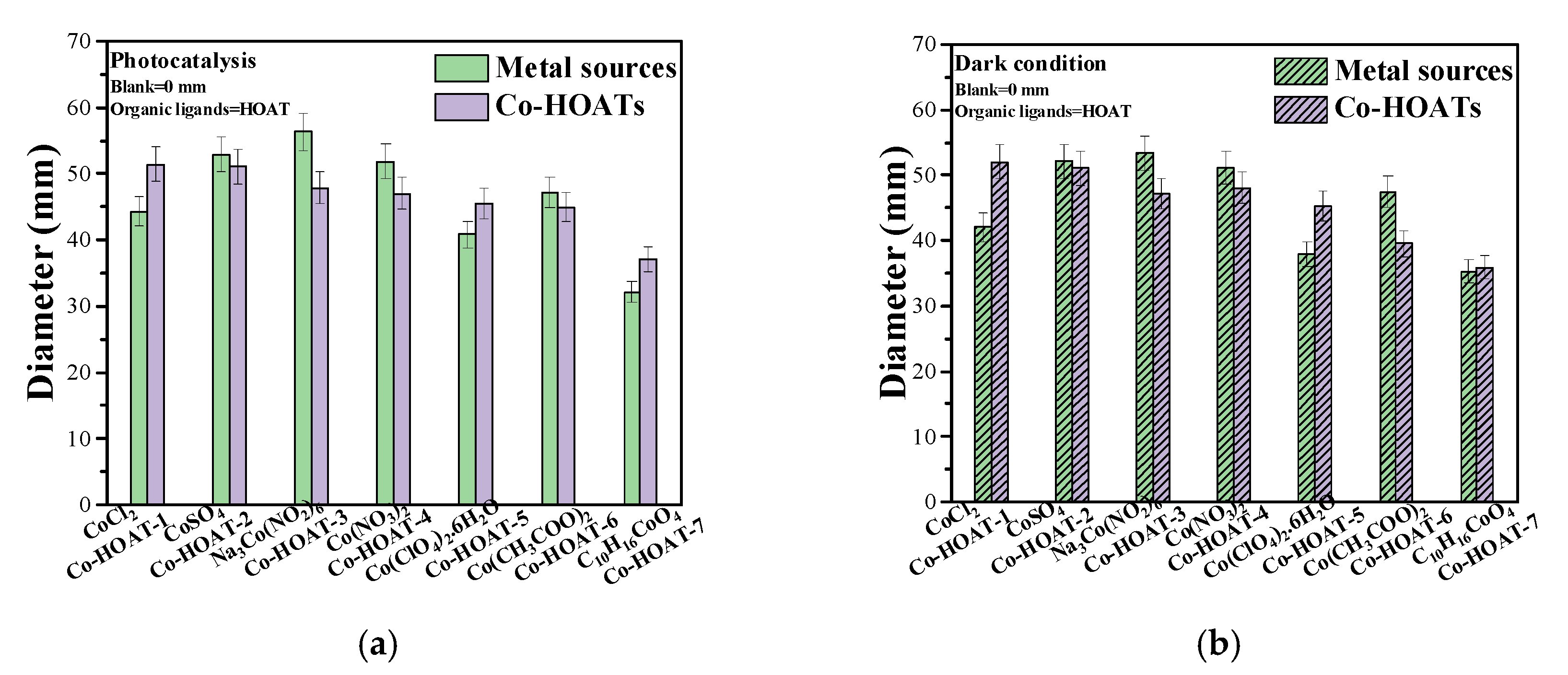
The Zone of Inhibition Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, N.; Verma, D.; Saini, N.; Arbi, R.; Munir, M.; Jovic, M.; Turak, A. Antiviral nanoparticles for sanitizing surfaces: A roadmap to self-sterilizing against COVID-19. Nano Today 2021, 40, 101267. [Google Scholar] [CrossRef] [PubMed]
- Wegener, M.; Hansen, M.J.; Driessen, A.A.-O.; Szymanski, W.A.-O.; Feringa, B.A.-O. Photocontrol of Antibacterial Activity: Shifting from UV to Red Light Activation. J. Am. Chem. Soc. 2017, 139, 17979–17986. [Google Scholar] [CrossRef] [PubMed]
- Dodd, M.C.; Kohler, H.P.E.; Von Gunten, U. Oxidation of antibacterial compounds by ozone and hydroxyl radical: Elimination of biological activity during aqueous ozonation processes. Environ. Sci. Technol. 2009, 43, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Xie, R.; Imlay, K.; Shang, J.K. Visible-light-induced bactericidal activity of titanium dioxide codoped with nitrogen and silver. Environ. Sci. Technol. 2010, 44, 6992–6997. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, Z.; Li, Z.; Zou, Z. Photocatalytic and Thermocatalytic Conversion of Methane. Solar RRL 2021, 5, 2000596. [Google Scholar] [CrossRef]
- Valenzuela, L.; Faraldos, M.; Bahamonde, A.; Rosal, R. Critical review on the use of photocatalysis and photoelectrocatalysis to create antimicrobial surfaces. Curr. Opin. Chem. Eng. 2021, 34, 100762. [Google Scholar] [CrossRef]
- Kim, W.; Zhu, W.; Hendricks, G.L.; Van Tyne, D.; Steele, A.D.; Keohane, C.E.; Fricke, N.; Conery, A.L.; Shen, S.; Pan, W.; et al. A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature 2018, 556, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Shi, F.; Zhu, H.; Yang, Y.; Zhong, J.; Luo, L.; Huo, Y.; Li, H. Photoelectrocatalytic bacterial inactivation of Acinetobacter baumannii on Cu2O/TiO2@Cu mesh photoanodes. Catal. Sci. Technol. 2020, 10, 7378–7385. [Google Scholar] [CrossRef]
- Helali, S.; Polo-López, M.I.; Fernández-Ibáñez, P.; Ohtani, B.; Amano, F.; Malato, S.; Guillard, C. Solar photocatalysis: A green technology for E. coli contaminated water disinfection. Effect of concentration and different types of suspended catalyst. J. Photochem. Photobiol. A Chem. 2014, 276, 31–40. [Google Scholar] [CrossRef]
- Gong, J.; Li, C.; Wasielewski, M.R. Advances in solar energy conversion. Chem. Soc. Rev. 2019, 48, 1862–1864. [Google Scholar] [CrossRef]
- Ran, B.; Ran, L.; Wang, Z.; Liao, J.; Li, D.; Chen, K.; Cai, W.; Hou, J.; Peng, X. Photocatalytic Antimicrobials: Principles, Design Strategies, and Applications. Chem. Rev. 2023, 123, 12371–12430. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Ding, M.; Tong, H.; Yang, Y.; Zhang, J.; Wang, L.; Li, H.; Huo, Y. Photoelectrocatalytic sterilization on thorn-like ZIF-67/ZnO hybrid photoanodes. J. Environ. Chem. Eng. 2022, 10, 107385. [Google Scholar] [CrossRef]
- Liu, J.; Wu, D.; Zhu, N.; Wu, Y.; Li, G. Antibacterial mechanisms and applications of metal-organic frameworks and their derived nanomaterials. Trends Food Sci. Technol. 2021, 109, 413–434. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, L.-N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.-H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Gupta, V.K.; Fakhri, A.; Tahami, S.; Agarwal, S. Zn doped CdO nanoparticles: Structural, morphological, optical, photocatalytic and anti-bacterial properties. J. Colloid Interface Sci. 2017, 504, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Polo, C.; Larumbe, S.; Gil, A.; Muñoz, D.; Fernández, L.R.; Barquín, L.F.; García-Prieto, A.; Fdez-Gubieda, M.L.; Muela, A. Improved photocatalytic and antibacterial performance of Cr doped TiO2 nanoparticles. Surf. Interfaces 2021, 22, 100867. [Google Scholar] [CrossRef]
- Chen, C.; Wang, D.; Li, Y.; Huang, H.; Ke, Y. ‘Flower-like AgBr/CeO2 Z-scheme heterojunction photocatalyst with enhanced visible light photocatalytic and antibacterial activities. Appl. Surf. Sci. 2021, 565, 150534. [Google Scholar] [CrossRef]
- Yuzer, B.; Aydın, M.I.; Con, A.H.; Inan, H.; Can, S.; Selcuk, H.; Kadmi, Y. Photocatalytic, self-cleaning and antibacterial properties of Cu(II) doped TiO2. J. Environ. Manag. 2022, 302, 114023. [Google Scholar] [CrossRef]
- Wang, K.; Lv, M.; Si, T.; Tang, X.; Wang, H.; Chen, Y.; Zhou, T. Mechanism analysis of surface structure-regulated Cu2O in photocatalytic antibacterial process. J. Hazard. Mater. 2024, 461, 132479. [Google Scholar] [CrossRef]
- Aihemaiti, X.; Wang, X.; Li, Y.; Wang, Y.; Xiao, L.; Ma, Y.; Qi, K.; Zhang, Y.; Liu, J.; Li, J. Enhanced photocatalytic and antibacterial activities of S-scheme SnO2/Red phosphorus photocatalyst under visible light. Chemosphere 2022, 296, 134013. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Ma, H.; Wang, K.; Zeng, Q.; Li, P.; Lyu, S.; Li, B.; Luo, X.; Jiang, L.; Cao, M.; Liao, B.; et al. Photocatalytic properties and antibacterial mechanisms of microbial-derived ZnS/CuS nanocomposites. J. Environ. Chem. Eng. 2023, 11, 111425. [Google Scholar] [CrossRef]
- Agboola, P.O.; Haider, S.; Shakir, I. Copper sulfide and their hybrids with carbon nanotubes for photocatalysis and antibacterial studies. Ceram. Int. 2022, 48, 10136–10143. [Google Scholar] [CrossRef]
- Huo, P.; Liu, C.; Wu, D.; Guan, J.; Li, J.; Wang, H.; Tang, Q.; Li, X.; Yan, Y.; Yuan, S. Fabricated Ag/Ag2S/reduced graphene oxide composite photocatalysts for enhancing visible light photocatalytic and antibacterial activity. J. Ind. Eng. Chem. 2018, 57, 125–133. [Google Scholar] [CrossRef]
- Nivetha, P.; Kavitha, B.; Kalanithi, M. Investigation of photocatalytic and antimicrobial activities of BaWO4–MoS2 nanoflowers. J. Sci. Adv. Mater. Dev. 2021, 6, 65–74. [Google Scholar] [CrossRef]
- Dou, L.; Yuan, M.; Li, D.; Zou, S.; Tan, S.; Zhao, Z.; Cai, G. Friction spun spandex/rGO/Ag/polyester core-sheath yarn with antibacterial activity for wearable sensors. Surf. Interfaces 2024, 44, 103746. [Google Scholar] [CrossRef]
- Chilivery, R.; Zhang, R.; Chen, G.; Yao, D.; Fan, D.; Lu, F.; Song, Y. Facile in situ construction of novel hybrid 3D-BiOCl@PDA heterostructures with vacancy induced charge transfer for efficient visible light driven photocatalysis and antibacterial activity. Colloid. Surface A 2023, 656, 130415. [Google Scholar] [CrossRef]
- Sun, J.; Mo, S.; Zhang, Z.; Wen, J.; Guo, D.; Li, Y.; Liu, L. Optimizing the electronic structure of BiOBr via constructing oxygen-rich vacancies for highly efficient NIR light-driven antibacterial activity. Chem. Eng. J. 2022, 450, 137980. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Li, Y.-J.; Jian, H.-J.; Harroun, S.G.; Wei, S.-C.; Ravindranath, R.; Lai, J.-Y.; Huang, C.-C.; Chang, H.-T. Green synthesis of catalytic gold/bismuth oxyiodide nanocomposites with oxygen vacancies for treatment of bacterial infections. Nanoscale 2018, 10, 11808–11819. [Google Scholar] [CrossRef]
- Kong, X.; Liu, X.; Zheng, Y.; Chu, P.K.; Zhang, Y.; Wu, S. Graphitic carbon nitride-based materials for photocatalytic antibacterial application. Mater. Sci. Eng. R Rep. 2021, 145, 100610. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, J.; Sun, Z.; Wang, M.; Zou, X.; Mao, H.; Yan, F. Enhanced photocatalytic and antibacterial activity of acridinium-grafted g-C3N4 with broad-spectrum light absorption for antimicrobial photocatalytic therapy. Acta Biomater. 2022, 146, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Qiao, Q.; Pei, Z.; Wang, Z.; Guo, J.; Li, Y.; Zhai, G.; Fei, P.; Lu, J.; Jia, H. Polyurethane-Based T-ZIF-8 Nanofibers as Photocatalytic Antibacterial Materials. ACS Appl. Nano Mater. 2023, 6, 22173–22184. [Google Scholar] [CrossRef]
- Zhang, J.; Li, P.; Zhang, X.; Ma, X.; Wang, B. Aluminum Metal–Organic Frameworks with Photocatalytic Antibacterial Activity for Autonomous Indoor Humidity Control. ACS Appl. Mater. Interfaces 2020, 12, 46057–46064. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, R.M.; Darwesh, O.M.; El-Shahat, M. Titanium-based metal-organic framework capsulated with magnetic nanoparticles: Antimicrobial and photocatalytic degradation of pesticides. Microporous Mesoporous Mater. 2023, 354, 112543. [Google Scholar] [CrossRef]
- Zhou, S.; Gao, J.; Zhu, J.; Peng, D.; Zhang, Y.; Zhang, Y. Self-cleaning, antibacterial mixed matrix membranes enabled by photocatalyst Ti-MOFs for efficient dye removal. J. Membr. Sci. 2020, 610, 118219. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, A.; Kaur, R.; Singh, S.; Bhatti, M.S.; Umar, A.; Baskoutas, S.; Kansal, S.K. Cu-BTC metal organic framework (MOF) derived Cu-doped TiO2 nanoparticles and their use as visible light active photocatalyst for the decomposition of ofloxacin (OFX) antibiotic and antibacterial activity. Adv. Powder Technol. 2021, 32, 1350–1361. [Google Scholar] [CrossRef]
- Savić-Gajić, I.M.; Savić, I.M. Drug design strategies with metal-hydroxyquinoline complexes. Expert Opin. Drug Dis. 2020, 15, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Crespi, A.F.; Zomero, P.N.; Pérez, A.L.; Brondino, C.D.; Molina, A.I.; Linck, Y.G.; Monti, G.A.; Fernández, M.A.; Rodríguez-Castellón, E.; Lázaro-Martínez, J.M. Montmorillonite materials with paramagnetic metal complexes: Structural studies and catalytic degradation of emerging pollutants. J. Environ. Chem. Eng. 2023, 11, 111420. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, C.; Peng, L.; Li, Z.; Wang, J.; Liao, X.; Guo, W. Discovery of metal complexes with antibacterial properties in aqueous extracts of Radix scutellariae and a study of the antibacterial properties of the baicalin–manganese complex. Inorg. Chem. Front. 2023, 10, 6506–6518. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Y.; Liu, G. Green and cost-effective carboxylic acid Fe complex functionalized cotton fabrics: Sunlight-driven catalytic and antibacterial activities, mechanical and thermal properties. Cellulose 2018, 25, 3663–3678. [Google Scholar] [CrossRef]
- Liang, J.; Sun, D.; Yang, Y.; Li, M.; Li, H.; Chen, L. Discovery of metal-based complexes as promising antimicrobial agents. Eur. J. Med. Chem. 2021, 224, 113696. [Google Scholar] [CrossRef] [PubMed]
- El-Sawaf, A.K.; El-Essawy, F.; Nassar, A.A.; El-Samanody, E.-S.A. Synthesis, spectral, thermal and antimicrobial studies on cobalt(II), nickel(II), copper(II), zinc(II) and palladium(II) complexes containing thiosemicarbazone ligand. J. Mol. Struct. 2018, 1157, 381–394. [Google Scholar] [CrossRef]
- Pettinari, C.; Pettinari, R.; Di Nicola, C.; Tombesi, A.; Scuri, S.; Marchetti, F. Antimicrobial MOFs. Coord. Chem. Rev. 2021, 446, 214121. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Peng, Y.; Yang, X. Exploring the Antibacteria Performance of Multicolor Ag, Au, and Cu Nanoclusters. ACS Appl. Mater. Interfaces 2019, 11, 8461–8469. [Google Scholar] [CrossRef]
- DelRe, C.; Jiang, Y.; Kang, P.; Kwon, J.; Hall, A.; Jayapurna, I.; Ruan, Z.; Ma, L.; Zolkin, K.; Li, T.; et al. Near-complete depolymerization of polyesters with nano-dispersed enzymes. Nature 2021, 592, 558–563. [Google Scholar] [CrossRef]
- Han, C.; Lee, J.P.; Lobkovsky, E.; Porco, J.A. Catalytic Ester−Amide Exchange Using Group (IV) Metal Alkoxide−Activator Complexes. J. Am. Chem. Soc. 2005, 127, 10039–10044. [Google Scholar] [CrossRef]
- Ferrer, F.J.; Carpino, L.A. Synthesis of the Two Isomeric Benzo Derivatives of 1-Hydroxy-7-azabenzotriazole and Preliminary Studies of Their Effectiveness as Coupling Reagents. In Peptides: The Wave of the Future, Proceedings of the Second International and the Seventeenth American Peptide Symposium, San Diego, CA, USA, 9–14 June 2001; Lebl, M., Houghten, R.A., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 95–96. [Google Scholar]
- Chen, Y.; Liu, Y.W.; Xie, Y.; Zhang, H.H.; Zhang, Z. Preparation and anti-corrosion performance of superhydrophobic silane/graphene oxide composite coating on copper. Surf. Coat. Technol. 2021, 423, 127622. [Google Scholar] [CrossRef]
- Stierstorfer, J.; Klapötke, T.M. High Energy Materials. Propellants, Explosives and Pyrotechnics. By Jai Prakash Agrawal. Angew. Chem. Int. Ed. 2010, 49, 6253. [Google Scholar] [CrossRef]
- Nandanwar, S.K.; Kim, H.J. Anticancer and Antibacterial Activity of Transition Metal Complexes. ChemistrySelect 2019, 4, 1706–1721. [Google Scholar] [CrossRef]
- Utthra, P.P.; Pravin, N.; Raman, N. Scrutinizing the DNA damaging and antimicrobial abilities of triazole appended metal complexes. J. Photochem. Photobiol. B Biol. 2016, 158, 136–144. [Google Scholar] [CrossRef]
- Andiappan, K.; Sanmugam, A.; Deivanayagam, E.; Karuppasamy, K.; Kim, H.-S.; Vikraman, D. Detailed investigations of rare earth (Yb, Er and Pr) based inorganic metal-ion complexes for antibacterial and anticancer applications. Inorg. Chem. Commun. 2023, 150, 110510. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Al-Saif, F.A.; Al-Humaidi, J.Y.; Binjawhar, D.N.; Refat, M.S. Six new palladium(II) mixed ligand complexes of 2-, 3-, 4-monosubstituted derivative of pyridine ring with caffeine moiety: Synthesis, spectroscopic, morphological structures, thermal, antimicrobial and anticancer properties. J. Mol. Struct. 2020, 1218, 128547. [Google Scholar] [CrossRef]
- Nikolić, A.; Jović, B.; Krstić, V.; Tričković, J. N–H...O hydrogen bonding. FT-IR, NIR and 1H NMR study of N-methylpropionamide—Dialkyl ether systems. J. Mol. Struct. 2008, 889, 328–331. [Google Scholar] [CrossRef]
- Alt, H.C.; Gomeniuk, Y.V.; Bittersberger, F.; Kempf, A.; Zemke, D. Analysis of electrically active N–O complexes in nitrogen-doped CZ silicon crystals by FTIR spectroscopy. Mater. Sci. Semicond. Process. 2006, 9, 114–116. [Google Scholar] [CrossRef]
- Wu, L.; Wang, X. A Concise Handbook of Spectral Interpretation; People’s Medical Publishing House: Beijing, China, 2013; pp. 55–86. [Google Scholar]
- Zhang, H.; Peng, Q.; Zhang, Y.; Zhang, R. Organic Spectroscopy; Chemical Industry Press: Beijing, China, 2005; pp. 254–300. [Google Scholar]
- Wang, L.; Morita, A.; North, N.M.; Baumler, S.M.; Springfield, E.W.; Allen, H.C. Identification of Ion Pairs in Aqueous NaCl and KCl Solutions in Combination with Raman Spectroscopy, Molecular Dynamics, and Quantum Chemical Calculations. J. Phys. Chem. B 2023, 127, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yu, D.; Wu, X.; Xie, D.; Sun, Y.; Vishniakov, P.; Hu, F.; Li, L.; Li, C.; Maximov, M.Y.; et al. Interfacial coupling porous cobalt nitride nanosheets array with N-doped carbon as robust trifunctional electrocatalysts for water splitting and Zn-air battery. Chem. Eng. J. 2022, 437, 135281. [Google Scholar] [CrossRef]
- Zangmeister, C.D.; Pemberton, J.E. Raman Spectroscopy and Atomic Force Microscopy of the Reaction of Sulfuric Acid with Sodium Chloride. J. Am. Chem. Soc. 2000, 122, 12289–12296. [Google Scholar] [CrossRef]
- Chlebda, D.K.; Jodłowski, P.J.; Jędrzejczyk, R.J.; Łojewska, J. 2D-COS of in situ μ-Raman and in situ IR spectra for structure evolution characterisation of NEP-deposited cobalt oxide catalyst during n-nonane combustion. Spectrochim. Acta A 2017, 186, 44–51. [Google Scholar] [CrossRef]
- Liu, J.J.; Siegler, M.A.; Karlin, K.D.; Moënne-Loccoz, P. Direct Resonance Raman Characterization of a Peroxynitrito Copper Complex Generated from O2 and NO and Mechanistic Insights into Metal-Mediated Peroxynitrite Decomposition. Angew. Chem. Int. Ed. 2019, 58, 10936–10940. [Google Scholar] [CrossRef] [PubMed]
- Waterland, M.R.; Myers Kelley, A. Far-ultraviolet resonance Raman spectroscopy of nitrate ion in solution. J. Chem. Phys. 2000, 113, 6760–6773. [Google Scholar] [CrossRef]
- Zapata, F.; Ortega-Ojeda, F.; García-Ruiz, C.; González-Herráez, M. Selective Monitoring of Oxyanion Mixtures by a Flow System with Raman Detection. Sensors 2018, 18, 2196. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Lv, M.; Bai, H.; Hou, Q.; Li, M.; Wang, Z. Effects of Metamorphism and Deformation on the Coal Macromolecular Structure by Laser Raman Spectroscopy. Energy Fuels 2017, 31, 1136–1146. [Google Scholar] [CrossRef]
- Yuan, X.; Ge, H.; Wang, X.; Dong, C.; Dong, W.; Riaz, M.S.; Xu, Z.; Zhang, J.; Huang, F. Controlled Phase Evolution from Co Nanochains to CoO Nanocubes and Their Application as OER Catalysts. ACS Energy Lett. 2017, 2, 1208–1213. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, B.; Fu, Y.; Zhang, Z.; Yan, P.; Liu, T. MOF-derived Co/CoO particles prepared by low temperature reduction for microwave absorption. Chem. Eng. J. 2021, 410, 128378. [Google Scholar] [CrossRef]
- Ahmad, Z.; Li, Y.; Yang, J.; Geng, N.; Fan, Y.; Gou, X.; Sun, Q.; Chen, J. A Membrane-Supported Bifunctional Poly(amidoxime-ethyleneimine) Network for Enhanced Uranium Extraction from Seawater and Wastewater. J. Hazard. Mater. 2022, 425, 127995. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Bae, J.; Kim, K.S. Metal organic framework derived NaCoxOy for room temperature hydrogen sulfide removal. Sci. Rep. 2021, 11, 14740. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, X.; Gao, B.; Li, Y.; Kong, J.; Shang, Y.; Song, W.; Zhang, Q. Capture of perchlorate by a surface-modified bio-sorbent and its bio-regeneration properties: Adsorption, computations and biofouling. Chemosphere 2017, 185, 152–161. [Google Scholar] [CrossRef]
- Fernandez, V.; Morgan, D.; Bargiela, P.; Fairley, N.; Baltrusaitis, J. Combining PCA and nonlinear fitting of peak models to re-evaluate C 1s XPS spectrum of cellulose. Appl. Surf. Sci. 2023, 614, 156182. [Google Scholar] [CrossRef]
- Pang, L.; Dai, C.; Bi, L.; Guo, Z.; Fan, J. Biosafety and Antibacterial Ability of Graphene and Graphene Oxide In Vitro and In Vivo. Nanoscale Res. Lett. 2017, 12, 564. [Google Scholar] [CrossRef]
- Xu, X.; Ding, M.; Liu, K.; Lv, F.; Miao, Y.; Liu, Y.; Gong, Y.; Huo, Y.; Li, H. The synthesis and highly effective antibacterial properties of Cu-3, 5-dimethy l-1, 2, 4-triazole metal organic frameworks. Front. Chem. 2023, 11, 1124303. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-Structure-Dependent Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef]
- Miao, Y.; Xu, X.; Liu, K.; Wang, N. Preparation of novel Cu/TiO2 mischcrystal composites and antibacterial activities for Escherichia coli under visible light. Ceram. Int. 2017, 43, 9658–9663. [Google Scholar] [CrossRef]



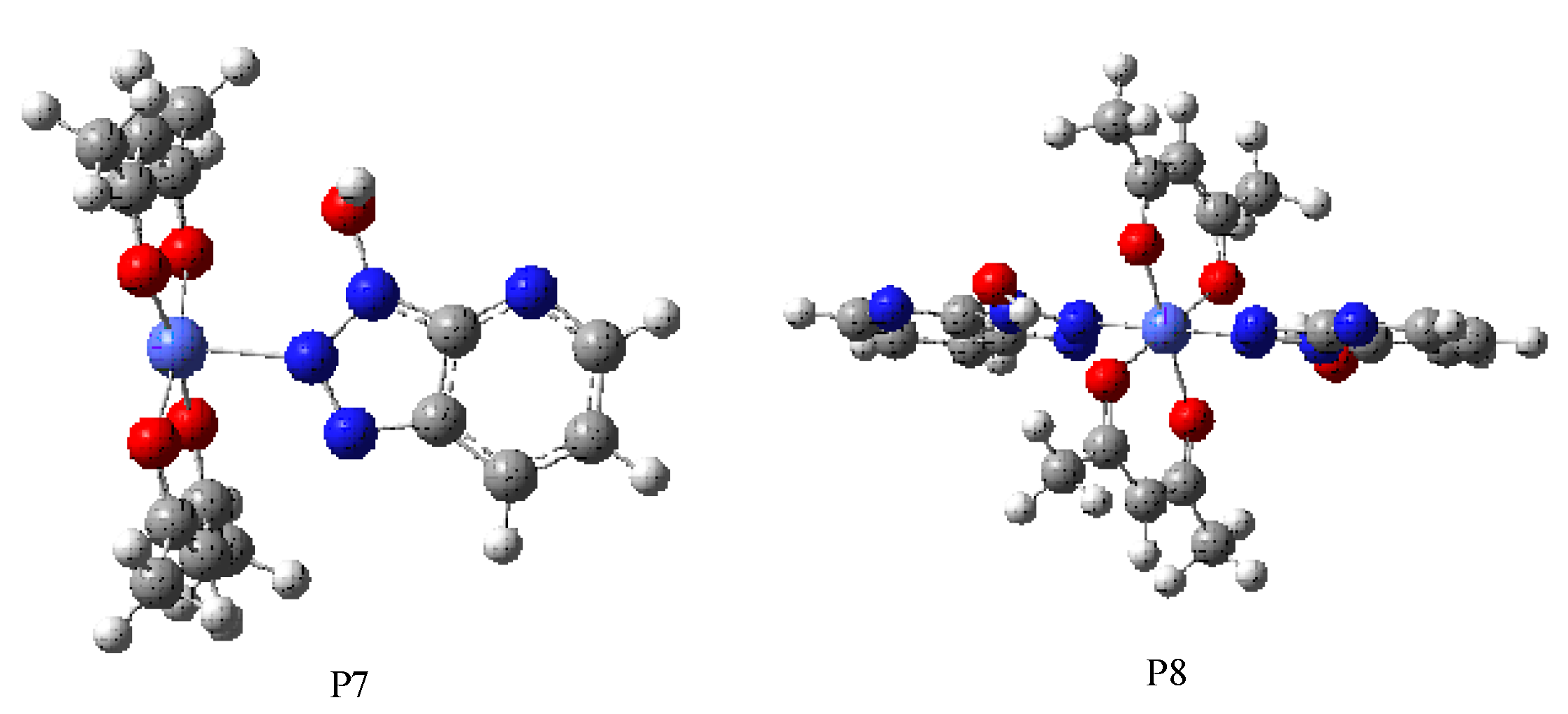
| Species | ECo2+ (Hatree) | EHOAT (Hatree) | EComplex (Hatree) | ∆E (Hatree) | ∆E (eV) |
|---|---|---|---|---|---|
| P21 | −142.6476629 | −974.439454 | −1117.5374599 | −0.2535470 | −6.90 |
| P22 | −142.6476629 | −974.439454 | −1117.517432 | −0.2335195 | −6.35 |
| P23 | −142.6476629 | −974.439454 | −1117.540615 | −0.2566421 | −6.98 |
| P3 | −142.6476629 | −1461.659181 | −1604.735925 | −0.1936112 | −5.27 |
| P4 | −142.6476629 | −1948.878908 | −2092.063041 | −0.1430971 | −3.90 |
| P5 | −142.6476629 | −2436.098635 | −2579.282433 | −0.041063 | −1.12 |
| P6 | −142.6476629 | −2923.318362 | −3066.657249 | −0.095178 | −2.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Ding, M.; Yu, S.; Lv, F.; Zhang, Y.; Miao, Y.; Bian, Z.; Li, H. Co–HOAT Complexes Change Their Antibacterial and Physicochemical Properties with Morphological Evolution. Catalysts 2024, 14, 173. https://doi.org/10.3390/catal14030173
Xu X, Ding M, Yu S, Lv F, Zhang Y, Miao Y, Bian Z, Li H. Co–HOAT Complexes Change Their Antibacterial and Physicochemical Properties with Morphological Evolution. Catalysts. 2024; 14(3):173. https://doi.org/10.3390/catal14030173
Chicago/Turabian StyleXu, Xiaolin, Mengna Ding, Shiwen Yu, Fujian Lv, Yun Zhang, Yingchun Miao, Zhenfeng Bian, and Hexing Li. 2024. "Co–HOAT Complexes Change Their Antibacterial and Physicochemical Properties with Morphological Evolution" Catalysts 14, no. 3: 173. https://doi.org/10.3390/catal14030173





