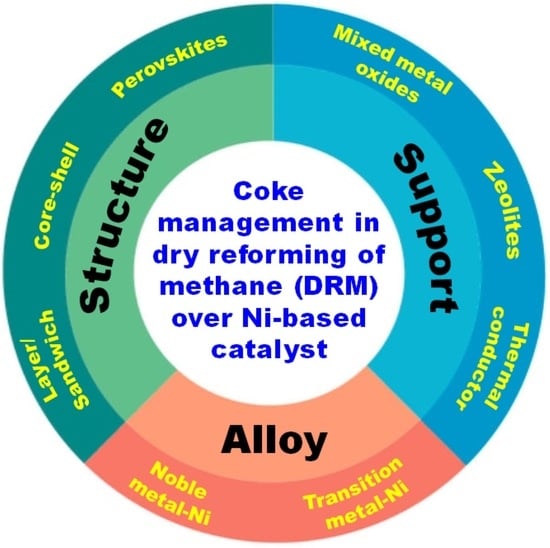
Recent Advances in Coke Management for Dry Reforming of Methane over Ni-Based Catalysts
Abstract
:1. Introduction
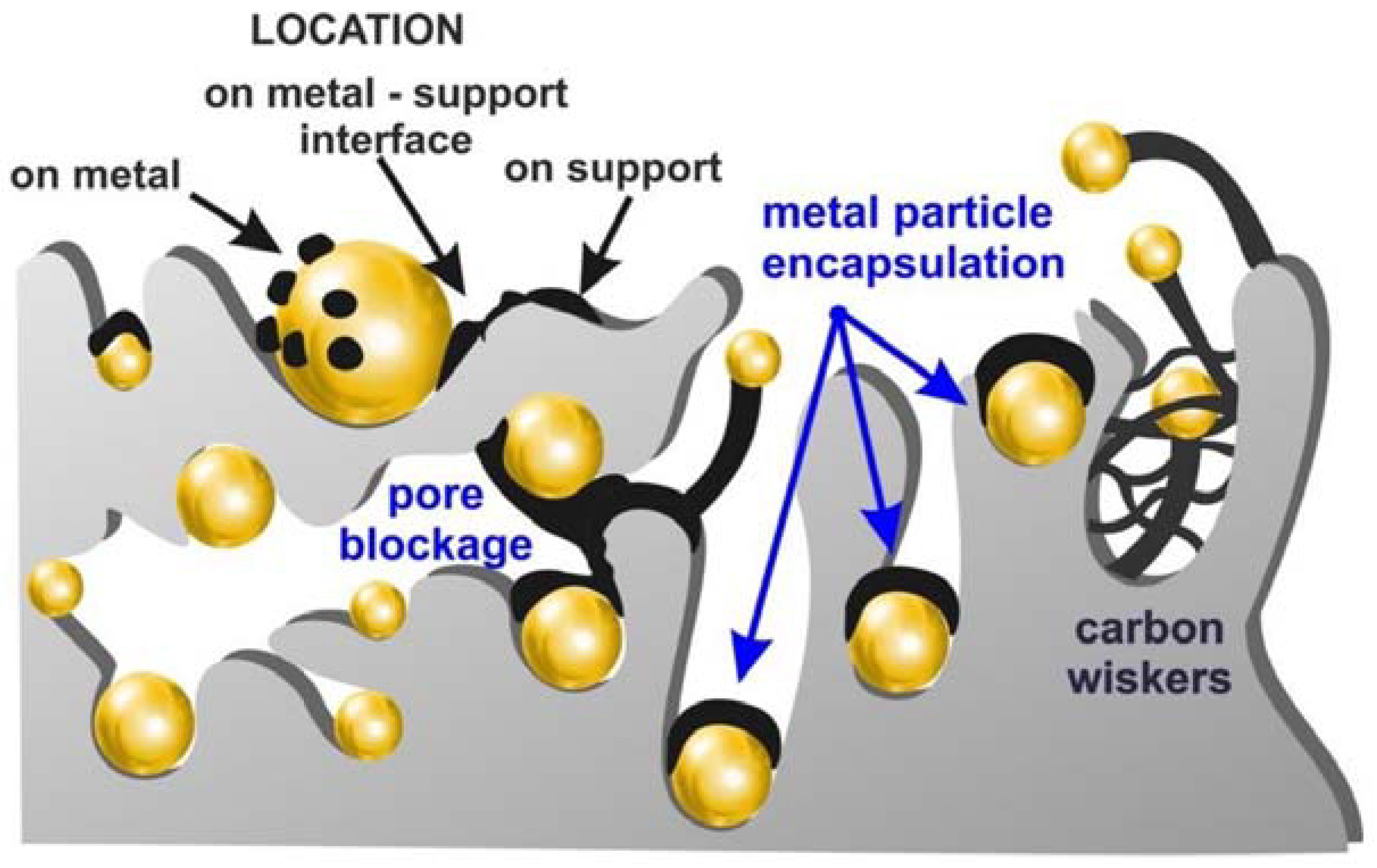
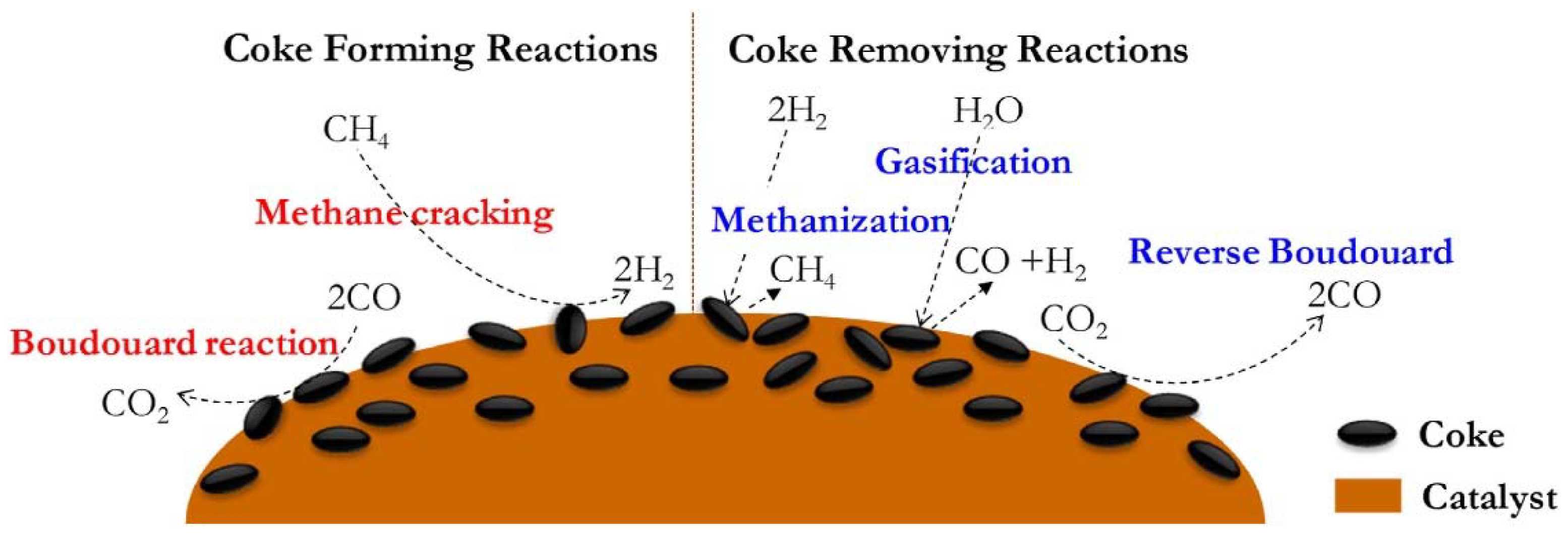
2. Mechanism of Coke Formation
2.1. Deep Methane Cracking
2.2. Carbon Monoxide Disproportionation
3. Design Strategies for Coke-Resistant Catalysts
3.1. Effect of Support
3.1.1. Oxide-Supported Catalysts
3.1.2. Zeolite-Supported Catalysts
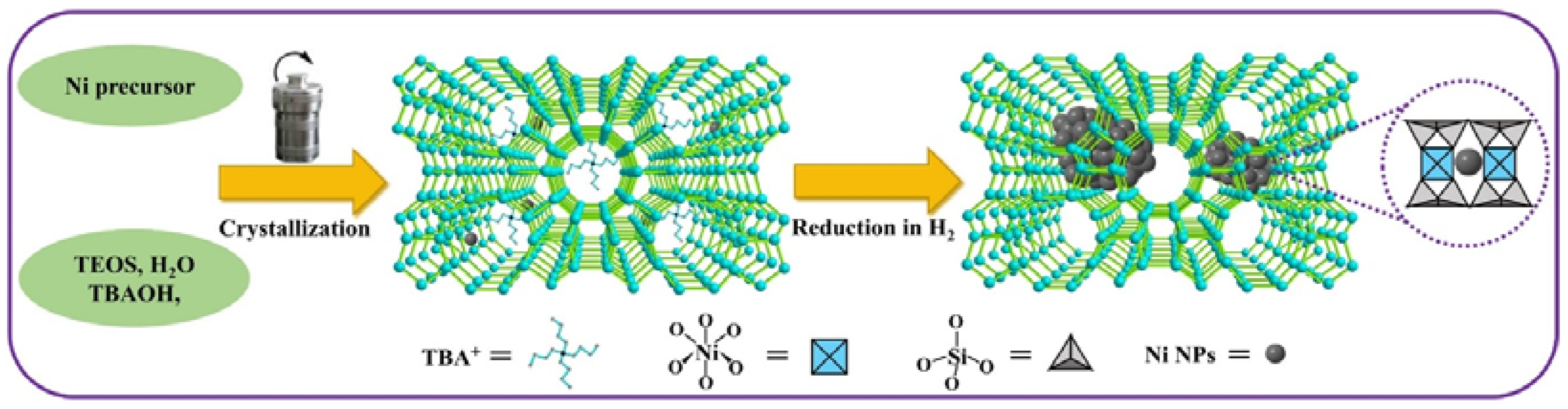
3.1.3. Other Supported Catalysts
3.2. Bimetallic and Alloying Ni-Based Catalysts
3.2.1. Noble Metal-Nickel Catalysts
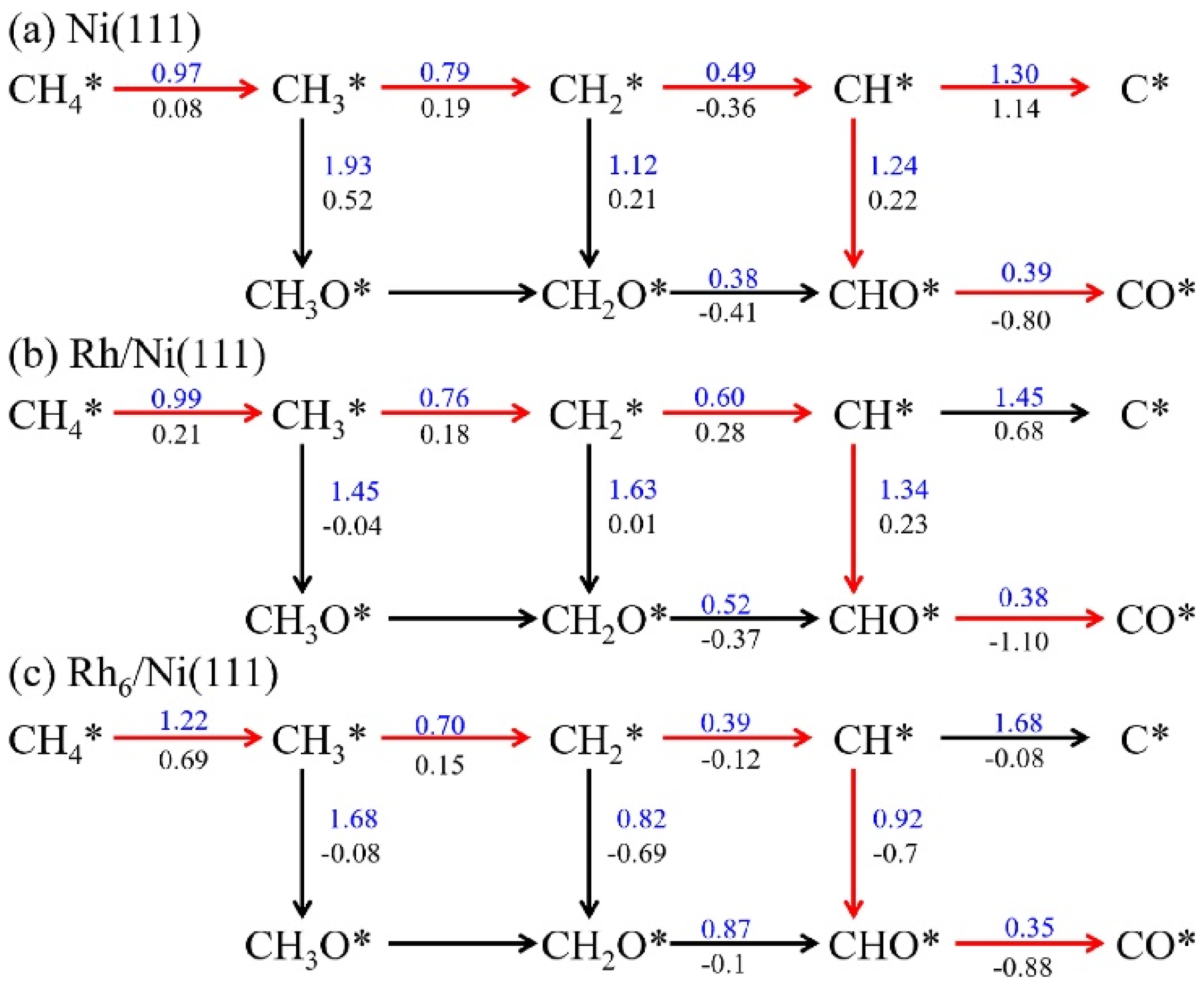
Rh-Ni Catalysts
Ru-Ni Catalysts
Pt-Ni Catalysts
3.2.2. Early Transition Metal-Nickel Catalysts
Co-Ni Catalysts
Fe-Ni Catalysts
Mo-Ni Catalysts
Cu-Ni Catalysts
3.3. Structured Approaches for Anti-Carbon Catalysts
3.3.1. Core–Shell Catalysts
3.3.2. Perovskite-Derived Catalysts
3.3.3. Other Structured Catalysts
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carley, S.; Konisky, D.M. The justice and equity implications of the clean energy transition. Nat. Energy 2020, 5, 569–577. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Chen, J.M. Carbon neutrality: Toward a sustainable future. Innovation 2021, 2, 100127. [Google Scholar] [CrossRef] [PubMed]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Koolen, C.D.; Luo, W.; Züttel, A. From Single Crystal to Single Atom Catalysts: Structural Factors Influencing the Performance of Metal Catalysts for CO2 Electroreduction. ACS Catal. 2023, 13, 948–973. [Google Scholar] [CrossRef]
- Zhang, J.; Pham, T.H.M.; Ko, Y.; Li, M.; Yang, S.; Koolen, C.D.; Zhong, L.; Luo, W.; Züttel, A. Tandem effect of Ag@C@Cu catalysts enhances ethanol selectivity for electrochemical CO2 reduction in flow reactors. Cell Rep. Phys. Sci. 2022, 3, 100949. [Google Scholar] [CrossRef]
- Wang, M.; Luo, L.; Wang, C.; Du, J.; Li, H.; Zeng, J. Heterogeneous Catalysts toward CO2 Hydrogenation for Sustainable Carbon Cycle. Acc. Mater. Res. 2022, 3, 565–571. [Google Scholar] [CrossRef]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K. The global methane budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Dummer, N.F.; Willock, D.J.; He, Q.; Howard, M.J.; Lewis, R.J.; Qi, G.; Taylor, S.H.; Xu, J.; Bethell, D.; Kiely, C.J.; et al. Methane Oxidation to Methanol. Chem. Rev. 2023, 123, 6359–6411. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Tang, J. Methane transformation by photocatalysis. Nat. Rev. Mater. 2022, 7, 617–632. [Google Scholar] [CrossRef]
- Meng, X.; Cui, X.; Rajan, N.P.; Yu, L.; Deng, D.; Bao, X. Direct Methane Conversion under Mild Condition by Thermo-, Electro-, or Photocatalysis. Chem 2019, 5, 2296–2325. [Google Scholar] [CrossRef]
- McConnachie, M.; Konarova, M.; Smart, S. Literature review of the catalytic pyrolysis of methane for hydrogen and carbon production. Int. J. Hydrogen Energy 2023, 48, 25660–25682. [Google Scholar] [CrossRef]
- Koo, C.W.; Rosenzweig, A.C. Biochemistry of aerobic biological methane oxidation. Chem. Soc. Rev. 2021, 50, 3424–3436. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Hu, Y.H. Steam reforming of methane: Current states of catalyst design and process upgrading. Renew. Sustain. Energy Rev. 2021, 149, 111330. [Google Scholar] [CrossRef]
- Parsapur, R.K.; Chatterjee, S.; Huang, K.-W. The Insignificant Role of Dry Reforming of Methane in CO2 Emission Relief. ACS Energy Lett. 2020, 5, 2881–2885. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Shi, C.; Wang, S.; Ge, X.; Deng, S.; Chen, B.; Shen, J. A review of different catalytic systems for dry reforming of methane: Conventional catalysis-alone and plasma-catalytic system. J. CO2 Util. 2021, 46, 101462. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the Development of Novel Cobalt Fischer−Tropsch Catalysts for Synthesis of Long-Chain Hydrocarbons and Clean Fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A review of recent developments in hydrogen production via biogas dry reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J.; Moon, D.H.; Kim, K.-H.; Kwon, E.E. Upgrading biogas into syngas through dry reforming. Renew. Sustain. Energy Rev. 2021, 143, 110949. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q.; Millar, G.J. Carbon Dioxide Reforming of Methane To Produce Synthesis Gas over Metal-Supported Catalysts: State of the Art. Energy Fuels 1996, 10, 896–904. [Google Scholar] [CrossRef]
- Pati, S.; Das, S.; Dewangan, N.; Jangam, A.; Kawi, S. Facile integration of core–shell catalyst and Pd-Ag membrane for hydrogen production from low-temperature dry reforming of methane. Fuel 2023, 333, 126433. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, T.; Song, S. Dry reforming of methane in a rotating gliding arc plasma: Improving efficiency and syngas cost by quenching product gas. J. CO2 Util. 2023, 70, 102448. [Google Scholar] [CrossRef]
- Song, Y.; Ozdemir, E.; Ramesh, S.; Adishev, A.; Subramanian, S.; Harale, A.; Albuali, M.; Fadhel, B.A.; Jamal, A.; Moon, D.; et al. Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science 2020, 367, 777–781. [Google Scholar] [CrossRef]
- Buelens, L.C.; Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. Super-dry reforming of methane intensifies CO2 utilization via Le Chatelier’s principle. Science 2016, 354, 449–452. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, H.; Wang, L.; Wang, L.; Wang, C.; Wang, H.; Fang, W.; He, M.; Wu, Q.; Xiao, F.-S. Enhanced CO2 utilization in dry reforming of methane achieved through nickel-mediated hydrogen spillover in zeolite crystals. Nat. Catal. 2022, 5, 1030–1037. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef]
- Baharudin, L.; Rahmat, N.; Othman, N.H.; Shah, N.; Syed-Hassan, S.S.A. Formation, control, and elimination of carbon on Ni-based catalyst during CO2 and CH4 conversion via dry reforming process: A review. J. CO2 Util. 2022, 61, 102050. [Google Scholar] [CrossRef]
- Grams, J.; Ruppert, A.M. Catalyst Stability—Bottleneck of Efficient Catalytic Pyrolysis. Catalysts 2021, 11, 265. [Google Scholar] [CrossRef]
- Hambali, H.U.; Jalil, A.A.; Abdulrasheed, A.A.; Siang, T.J.; Gambo, Y.; Umar, A.A. Zeolite and clay based catalysts for CO2 reforming of methane to syngas: A review. Int. J. Hydrogen Energy 2022, 47, 30759–30787. [Google Scholar] [CrossRef]
- Yousefi, M.; Donne, S. Technical challenges for developing thermal methane cracking in small or medium scales to produce pure hydrogen—A review. Int. J. Hydrogen Energy 2022, 47, 699–727. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Patel, R.; El Hassan, N.; Al-Zahrani, S.A.; Al-Awadi, A.S.; Frusteri, L.; Bayahia, H.; Alharth, A.I.; Al-Fatesh, A.S.; Kumar, R. Holmium promoted yttria-zirconia supported Ni catalyst for H2 production via dry reforming of methane. Int. J. Hydrogen Energy 2022, 47, 38242–38257. [Google Scholar] [CrossRef]
- Deng, J.; Bu, K.; Shen, Y.; Zhang, X.; Zhang, J.; Faungnawakij, K.; Zhang, D. Cooperatively enhanced coking resistance via boron nitride coating over Ni-based catalysts for dry reforming of methane. Appl. Catal. B Environ. 2022, 302, 120859. [Google Scholar] [CrossRef]
- Rosli, S.N.A.; Abidin, S.Z.; Osazuwa, O.U.; Fan, X.; Jiao, Y. The effect of oxygen mobility/vacancy on carbon gasification in nano catalytic dry reforming of methane: A review. J. CO2 Util. 2022, 63, 102109. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Carbon Deposition in Steam Reforming and Methanation. Catal. Rev. 1982, 24, 67–112. [Google Scholar] [CrossRef]
- Wang, J.; Fu, Y.; Kong, W.; Li, S.; Yuan, C.; Bai, J.; Chen, X.; Zhang, J.; Sun, Y. Investigation of Atom-Level Reaction Kinetics of Carbon-Resistant Bimetallic NiCo-Reforming Catalysts: Combining Microkinetic Modeling and Density Functional Theory. ACS Catal. 2022, 12, 4382–4393. [Google Scholar] [CrossRef]
- Kong, W.; Fu, Y.; Shi, L.; Li, S.; Vovk, E.; Zhou, X.; Si, R.; Pan, B.; Yuan, C.; Li, S.; et al. Nickel nanoparticles with interfacial confinement mimic noble metal catalyst in methane dry reforming. Appl. Catal. B Environ. 2021, 285, 119837. [Google Scholar] [CrossRef]
- Wang, J.; Fu, Y.; Kong, W.; Jin, F.; Bai, J.; Zhang, J.; Sun, Y. Design of a carbon-resistant Ni@S-2 reforming catalyst: Controllable Ni nanoparticles sandwiched in a peasecod-like structure. Appl. Catal. B Environ. 2021, 282, 119546. [Google Scholar] [CrossRef]
- Oh, K.H.; Lee, J.H.; Kim, K.; Lee, H.-K.; Kang, S.W.; Yang, J.-I.; Park, J.-H.; Hong, C.S.; Kim, B.-H.; Park, J.C. A new automated synthesis of a coke-resistant Cs-promoted Ni-supported nanocatalyst for sustainable dry reforming of methane. J. Mater. Chem. A 2023, 11, 1666–1675. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Al-Fatesh, A.S.; Srivastava, V.K.; Ibrahim, A.A.; Abahussain, A.A.M.; Abu-Dahrieh, J.K.; Alotibi, M.F.; Kumar, R. Hydrogen Production from Gadolinium-Promoted Yttrium-Zirconium-Supported Ni Catalysts through Dry Methane Reforming. ACS Omega 2023, 8, 22108–22120. [Google Scholar] [CrossRef] [PubMed]
- Ekeoma, B.C.; Yusuf, M.; Johari, K.; Abdullah, B. Mesoporous silica supported Ni-based catalysts for methane dry reforming: A review of recent studies. Int. J. Hydrogen Energy 2022, 47, 41596–41620. [Google Scholar] [CrossRef]
- Vogt, C.; Weckhuysen, B.M. The concept of active site in heterogeneous catalysis. Nat. Rev. Chem. 2022, 6, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cao, X.-M.; Zhu, J.; Hu, P. Activity and coke formation of nickel and nickel carbide in dry reforming: A deactivation scheme from density functional theory. J. Catal. 2014, 311, 469–480. [Google Scholar] [CrossRef]
- Wang, D.; Littlewood, P.; Marks, T.J.; Stair, P.C.; Weitz, E. Coking Can Enhance Product Yields in the Dry Reforming of Methane. ACS Catal. 2022, 12, 8352–8362. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, S.; Yang, B. Promoted coke resistance of Ni by surface carbon for the dry reforming of methane. iScience 2023, 26, 106237. [Google Scholar] [CrossRef]
- Shen, D.; Wang, J.; Bai, Y.; Lyu, S.; Zhang, Y.; Li, J.; Li, L.; Wang, G. Carbon-confined Ni based catalyst by auto-reduction for low-temperature dry reforming of methane. Fuel 2023, 339, 127409. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Qian, K.; Huang, W. Metal–Support Interactions in Metal/Oxide Catalysts and Oxide–Metal Interactions in Oxide/Metal Inverse Catalysts. ACS Catal. 2022, 12, 1268–1287. [Google Scholar] [CrossRef]
- Pu, T.; Zhang, W.; Zhu, M. Engineering Heterogeneous Catalysis with Strong Metal–Support Interactions: Characterization, Theory and Manipulation. Angew. Chem. Int. Ed. 2023, 62, e202212278. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, W.-X. Sabatier principle of metal-support interaction for design of ultrastable metal nanocatalysts. Science 2021, 374, 1360–1365. [Google Scholar] [CrossRef]
- Frey, H.; Beck, A.; Huang, X.; van Bokhoven, J.A.; Willinger, M.G. Dynamic interplay between metal nanoparticles and oxide support under redox conditions. Science 2022, 376, 982–987. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Patel, N.; Fakeeha, A.H.; Alotibi, M.F.; Alreshaidan, S.B.; Kumar, R. Reforming of methane: Effects of active metals, supports, and promoters. Catal. Rev. Sci. Eng. 2023, 1–99. [Google Scholar] [CrossRef]
- Ahmad, Y.H.; Mohamed, A.T.; El-Sayed, H.A.; Kumar, A.; Al-Qaradawi, S.Y. Design of Ni/La2O3 catalysts for dry reforming of methane: Understanding the impact of synthesis methods. Int. J. Hydrogen Energy 2022, 47, 41294–41309. [Google Scholar] [CrossRef]
- Ahn, S.-Y.; Jang, W.-J.; Shim, J.-O.; Jeon, B.-H.; Roh, H.-S. CeO2-based oxygen storage capacity materials in environmental and energy catalysis for carbon neutrality: Extended application and key catalytic properties. Catal. Rev. Sci. Eng. 2023, 1–84. [Google Scholar] [CrossRef]
- Bahari, M.B.; Setiabudi, H.D.; Duy Nguyen, T.; Phuong, P.T.T.; Duc Truong, Q.; Abdul Jalil, A.; Ainirazali, N.; Vo, D.-V.N. Insight into the influence of rare-earth promoter (CeO2, La2O3, Y2O3, and Sm2O3) addition toward methane dry reforming over Co/mesoporous alumina catalysts. Chem. Eng. Sci. 2020, 228, 115967. [Google Scholar] [CrossRef]
- Scheiber, P.; Fidler, M.; Dulub, O.; Schmid, M.; Diebold, U.; Hou, W.; Aschauer, U.; Selloni, A. (Sub)Surface Mobility of Oxygen Vacancies at the TiO2 Anatase (101) Surface. Phys. Rev. Lett. 2012, 109, 136103. [Google Scholar] [CrossRef] [PubMed]
- Onn, T.M.; Zhang, S.; Arroyo-Ramirez, L.; Chung, Y.-C.; Graham, G.W.; Pan, X.; Gorte, R.J. Improved Thermal Stability and Methane-Oxidation Activity of Pd/Al2O3 Catalysts by Atomic Layer Deposition of ZrO2. ACS Catal. 2015, 5, 5696–5701. [Google Scholar] [CrossRef]
- Bu, K.; Kuboon, S.; Deng, J.; Li, H.; Yan, T.; Chen, G.; Shi, L.; Zhang, D. Methane dry reforming over boron nitride interface-confined and LDHs-derived Ni catalysts. Appl. Catal. B Environ. 2019, 252, 86–97. [Google Scholar] [CrossRef]
- Tuci, G.; Liu, Y.; Rossin, A.; Guo, X.; Pham, C.; Giambastiani, G.; Pham-Huu, C. Porous Silicon Carbide (SiC): A Chance for Improving Catalysts or Just Another Active-Phase Carrier? Chem. Rev. 2021, 121, 10559–10665. [Google Scholar] [CrossRef]
- Shen, D.; Huo, M.; Li, L.; Lyu, S.; Wang, J.; Wang, X.; Zhang, Y.; Li, J. Effects of alumina morphology on dry reforming of methane over Ni/Al2O3 catalysts. Catal. Sci. Technol. 2020, 10, 510–516. [Google Scholar] [CrossRef]
- Gholizadeh, F.; Izadbakhsh, A.; Huang, J.; Zi-Feng, Y. Catalytic performance of cubic ordered mesoporous alumina supported nickel catalysts in dry reforming of methane. Microporous Mesoporous Mater. 2021, 310, 110616. [Google Scholar] [CrossRef]
- Ali, S.; Khader, M.M.; Almarri, M.J.; Abdelmoneim, A.G. Ni-based nano-catalysts for the dry reforming of methane. Catal. Today 2020, 343, 26–37. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, X.; Deng, J.; Kuboon, S.; Faungnawakij, K.; Xiao, S.; Zhang, D. Coking-resistant dry reforming of methane over BN–nanoceria interface-confined Ni catalysts. Catal. Sci. Technol. 2020, 10, 4237–4244. [Google Scholar] [CrossRef]
- Ma, Q.; Han, Y.; Wei, Q.; Makpal, S.; Gao, X.; Zhang, J.; Zhao, T.-S. Stabilizing Ni on bimodal mesoporous-macroporous alumina with enhanced coke tolerance in dry reforming of methane to syngas. J. CO2 Util. 2020, 35, 288–297. [Google Scholar] [CrossRef]
- Al-Swai, B.M.; Osman, N.; Alnarabiji, M.S.; Adesina, A.A.; Abdullah, B. Syngas Production via Methane Dry Reforming over Ceria–Magnesia Mixed Oxide-Supported Nickel Catalysts. Ind. Eng. Chem. Res. 2019, 58, 539–552. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Djinović, P.; Davlyatova, L.F.; Pintar, A.; Efstathiou, A.M. Origin and reactivity of active and inactive carbon formed during DRM over Ni/Ce0.38Zr0.62O2-δ studied by transient isotopic techniques. Catal. Today 2018, 299, 201–211. [Google Scholar] [CrossRef]
- Chong, C.C.; Cheng, Y.W.; Bukhari, S.N.; Setiabudi, H.D.; Jalil, A.A. Methane dry reforming over Ni/fibrous SBA-15 catalysts: Effects of support morphology (rod-liked F-SBA-15 and dendritic DFSBA-15). Catal. Today 2021, 375, 245–257. [Google Scholar] [CrossRef]
- Jin, F.; Fu, Y.; Kong, W.; Wang, J.; Cai, F.; Yuan, C.; Pan, B.; Zhang, J.; Sun, Y. Stable Trimetallic NiFeCu Catalysts with High Carbon Resistance for Dry Reforming of Methane. ChemPlusChem 2020, 85, 1120–1128. [Google Scholar] [CrossRef]
- Bawah, A.-R.; Malaibari, Z.O.; Muraza, O. Syngas production from CO2 reforming of methane over Ni supported on hierarchical silicalite-1 fabricated by microwave-assisted hydrothermal synthesis. Int. J. Hydrogen Energy 2018, 43, 13177–13189. [Google Scholar] [CrossRef]
- Danghyan, V.; Calderon Novoa, S.; Mukasyan, A.; Wolf, E.E. Pressure dilution, a new method to prepare a stable Ni/fumed silica catalyst for the dry reforming of methane. Appl. Catal. B Environ. 2018, 234, 178–186. [Google Scholar] [CrossRef]
- Hambali, H.U.; Jalil, A.A.; Abdulrasheed, A.A.; Siang, T.J.; Abdullah, T.A.T.; Ahmad, A.; Vo, D.-V.N. Fibrous spherical Ni-M/ZSM-5 (M: Mg, Ca, Ta, Ga) catalysts for methane dry reforming: The interplay between surface acidity-basicity and coking resistance. Int. J. Energy Res. 2020, 44, 5696–5712. [Google Scholar] [CrossRef]
- Chang, K.; Zhang, H.; Cheng, M.-j.; Lu, Q. Application of Ceria in CO2 Conversion Catalysis. ACS Catal. 2020, 10, 613–631. [Google Scholar] [CrossRef]
- Muravev, V.; Parastaev, A.; van den Bosch, Y.; Ligt, B.; Claes, N.; Bals, S.; Kosinov, N.; Hensen, E.J.M. Size of cerium dioxide support nanocrystals dictates reactivity of highly dispersed palladium catalysts. Science 2023, 380, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Al-Fatesh, A.S.; Arafat, Y.; Kasim, S.O.; Ibrahim, A.A.; Abasaeed, A.E.; Fakeeha, A.H. In situ auto-gasification of coke deposits over a novel Ni-Ce/W-Zr catalyst by sequential generation of oxygen vacancies for remarkably stable syngas production via CO2-reforming of methane. Appl. Catal. B Environ. 2021, 280, 119445. [Google Scholar] [CrossRef]
- Damaskinos, C.M.; Zavašnik, J.; Djinović, P.; Efstathiou, A.M. Dry reforming of methane over Ni/Ce0.8Ti0.2O2-δ: The effect of Ni particle size on the carbon pathways studied by transient and isotopic techniques. Appl. Catal. B Environ. 2021, 296, 120321. [Google Scholar] [CrossRef]
- da Fonseca, R.O.; Ponseggi, A.R.; Rabelo-Neto, R.C.; Simões, R.C.C.; Mattos, L.V.; Noronha, F.B. Controlling carbon formation over Ni/CeO2 catalyst for dry reforming of CH4 by tuning Ni crystallite size and oxygen vacancies of the support. J. CO2 Util. 2022, 57, 101880. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Yan, B. Ni/Ce0.9Eu0.1O1.95 with enhanced coke resistance for dry reforming of methane. J. Catal. 2022, 407, 77–89. [Google Scholar] [CrossRef]
- Ding, X.; Yang, Y.; Li, Z.; Huang, P.; Liu, X.; Guo, Y.; Wang, Y. Engineering a Nickel–Oxygen Vacancy Interface for Enhanced Dry Reforming of Methane: A Promoted Effect of CeO2 Introduction into Ni/MgO. ACS Catal. 2023, 13, 15535–15545. [Google Scholar] [CrossRef]
- Tsoukalou, A.; Imtiaz, Q.; Kim, S.M.; Abdala, P.M.; Yoon, S.; Müller, C.R. Dry-reforming of methane over bimetallic Ni–M/La2O3 (M=Co, Fe): The effect of the rate of La2O2CO3 formation and phase stability on the catalytic activity and stability. J. Catal. 2016, 343, 208–214. [Google Scholar] [CrossRef]
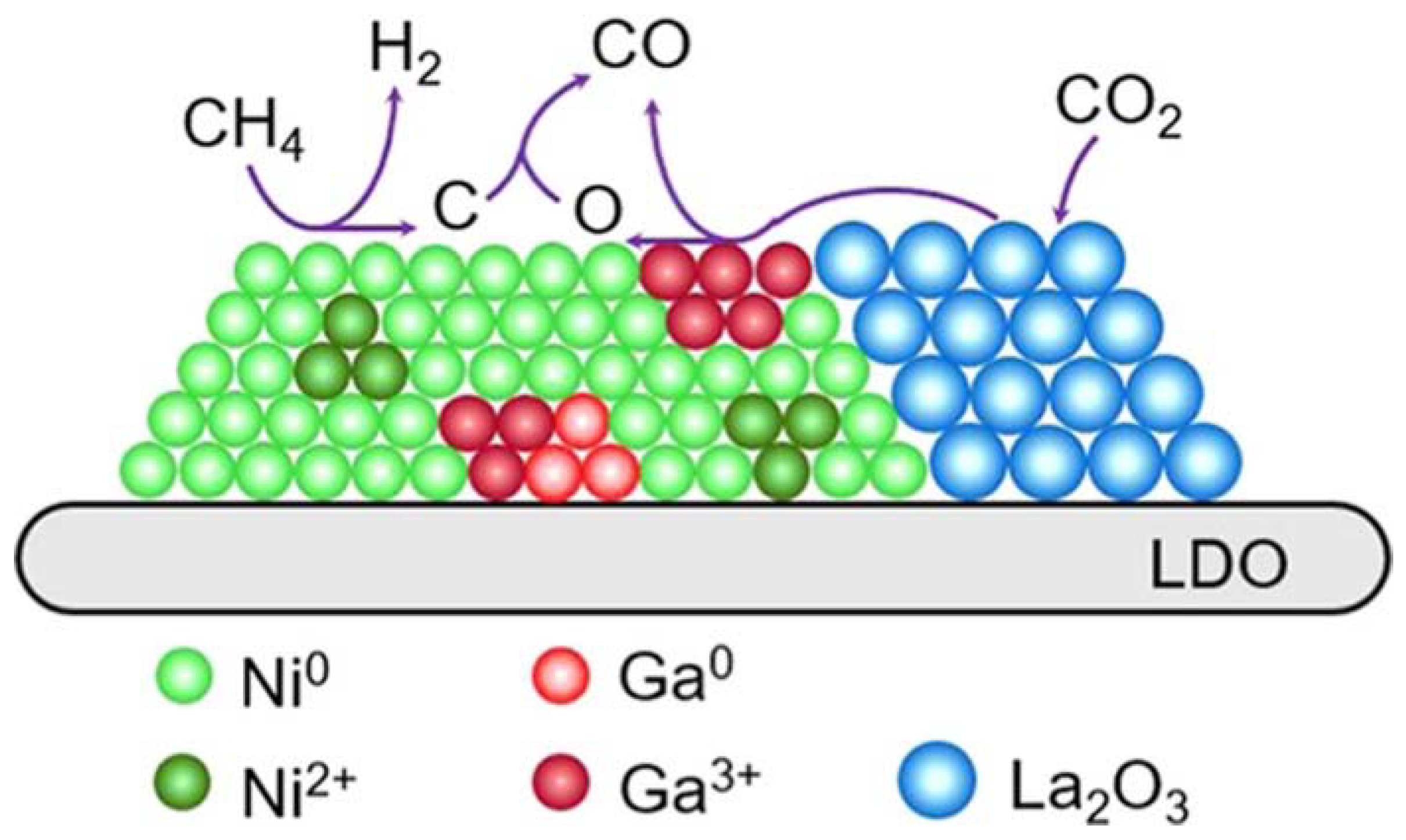
- Zeng, F.; Wei, B.; Lan, D.; Ge, J. Highly Dispersed NixGay Catalyst and La2O3 Promoter Supported by LDO Nanosheets for Dry Reforming of Methane: Synergetic Catalysis by Ni, Ga, and La2O3. Langmuir 2021, 37, 9744–9754. [Google Scholar] [CrossRef]
- Song, J.; Duan, X.; Zhang, W. Methane dry reforming over mesoporous La2O3 supported Ni catalyst for syngas production. Microporous Mesoporous Mater. 2021, 310, 110587. [Google Scholar] [CrossRef]
- Haug, L.; Thurner, C.; Bekheet, M.F.; Bischoff, B.; Gurlo, A.; Kunz, M.; Sartory, B.; Penner, S.; Klötzer, B. Zirconium Carbide Mediates Coke-Resistant Methane Dry Reforming on Nickel-Zirconium Catalysts. Angew. Chem. Int. Ed. 2022, 61, e202213249. [Google Scholar] [CrossRef]
- Haug, L.; Thurner, C.; Bekheet, M.F.; Ploner, K.; Bischoff, B.; Gurlo, A.; Kunz, M.; Sartory, B.; Penner, S.; Klötzer, B. Pivotal Role of Ni/ZrO2 Phase Boundaries for Coke-Resistant Methane Dry Reforming Catalysts. Catalysts 2023, 13, 804. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, J.; Yan, T.; Deng, J.; Fang, J.; Zhang, D. Emergent Ni catalysts induced by nitride-to-oxide transformation for coking and sintering resistant dry reforming of methane. New J. Chem. 2023, 47, 10604–10612. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Li, G.; Zhao, Q.; Wu, X.S.; Wang, Y.; Sun, Y.; Hu, C. Synergy of Oxygen Vacancies and Ni0 Species to Promote the Stability of a Ni/ZrO2 Catalyst for Dry Reforming of Methane at Low Temperatures. ACS Catal. 2023, 13, 6486–6496. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Hu, Y.H. Carbon dioxide reforming of methane over nickel/alkaline earth metal oxide catalysts. Appl. Catal. A Gen. 1995, 133, 149–161. [Google Scholar] [CrossRef]
- Zuo, Z.; Liu, S.; Wang, Z.; Liu, C.; Huang, W.; Huang, J.; Liu, P. Dry Reforming of Methane on Single-Site Ni/MgO Catalysts: Importance of Site Confinement. ACS Catal. 2018, 8, 9821–9835. [Google Scholar] [CrossRef]
- Rosdin, R.D.B.; Yusuf, M.; Abdullah, B. Dry reforming of methane over Ni-based catalysts: Effect of ZrO2 and MgO addition as support. Mater. Lett. X 2021, 12, 100095. [Google Scholar] [CrossRef]
- Bagabas, A.; Al-Fatesh, A.S.; Kasim, S.O.; Arasheed, R.; Ibrahim, A.A.; Ashamari, R.; Anojaidi, K.; Fakeeha, A.H.; Abu-Dahrieh, J.K.; Abasaeed, A.E. Optimizing MgO Content for Boosting γ-Al2O3-Supported Ni Catalyst in Dry Reforming of Methane. Catalysts 2021, 11, 1233. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, J.; Xu, R.; Zhang, R.; Ge, J. Highly dispersed Ni/MgO-mSiO2 catalysts with excellent activity and stability for dry reforming of methane. Nano Res. 2022, 15, 5004–5013. [Google Scholar] [CrossRef]
- Han, R.; Xing, S.; Wang, Y.; Wei, L.; Li, Z.; Yang, C.; Song, C.; Liu, Q. Two birds with one stone: MgO promoted Ni-CaO as stable and coke-resistant bifunctional materials for integrated CO2 capture and conversion. Sep. Purif. Technol. 2023, 307, 122808. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Yan, B. Enhancing Coke Resistance of Mg1–xNixAl2O4 Catalysts for Dry Reforming of Methane via a Doping-Segregation Strategy. ChemCatChem 2023, 15, e202300220. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Liu, J.; Li, T.; Wang, Y.; Zhao, Y.; Li, G.; Zhang, Y. Dry reforming of methane over Ni/SiO2 catalysts: Role of support structure properties. Fuel 2023, 340, 127490. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Liu, J.; Li, T.; Zhang, X.; Wang, Y.; Zhao, Y.; Li, G.; Zhang, Y. Insight into the role of preparation method on the structure and size effect of Ni/MSS catalysts for dry reforming of methane. Fuel Process. Technol. 2023, 250, 107891. [Google Scholar] [CrossRef]
- Song, Q.; Ran, R.; Wu, X.; Si, Z.; Weng, D. Dry reforming of methane over Ni catalysts supported on micro- and mesoporous silica. J. CO2 Util. 2023, 68, 102387. [Google Scholar] [CrossRef]
- Chen, S.; Niu, J.; Zheng, X.; Liu, H.; Jin, Y.; Ran, J. Unraveling the effect of particle size of active metals in Ni/MgO on methane activation and carbon growth mechanism. Phys. Chem. Chem. Phys. 2024, 26, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cheng, F.; Xie, K. Stable Ni nanocrystals on porous single-crystalline MgO particles for enhanced dry reforming activity and durability of CH4/CO2. Catal. Sci. Technol. 2024, 14, 681–688. [Google Scholar] [CrossRef]
- Li, G.; Hao, H.; Jin, P.; Wang, M.; Yu, Y.; Zhang, C. Dry reforming of methane over Ni/Al2O3 catalysts: Support morphological effect on the coke resistance. Fuel 2024, 362, 130855. [Google Scholar] [CrossRef]
- Yang, G.S.; Kang, J.; Park, E.D. Aqueous-Phase Partial Oxidation of Methane over Pd−Fe/ZSM-5 with O2 in the Presence of H2. ChemCatChem 2023, 15, e202201630. [Google Scholar] [CrossRef]
- Xu, Z.; Kang, J.; Park, E.D. Continuous gas-phase oxidation of methane into methanol over Cu-mordenite. Microporous Mesoporous Mater. 2023, 360, 112727. [Google Scholar] [CrossRef]
- Huang, T.; Huang, W.; Huang, J.; Ji, P. Methane reforming reaction with carbon dioxide over SBA-15 supported Ni–Mo bimetallic catalysts. Fuel Process. Technol. 2011, 92, 1868–1875. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Wongsakulphasatch, S.; Vo, D.-V.N. Understanding the role of surface basic sites of catalysts in CO2 activation in dry reforming of methane: A short review. Catal. Sci. Technol. 2020, 10, 35–45. [Google Scholar] [CrossRef]
- Sumi, T.; Murata, D.; Kitamura, H.; Kubota, S.; Miyake, K.; Uchida, Y.; Miyamoto, M.; Nishiyama, N. Formation of Ni Species Anchored on a Silicalite-1 Zeolite Framework as a Catalyst with High Coke Deposition Resistance on Dry Reforming of Methane. Cryst. Growth Des. 2023, 23, 3308–3313. [Google Scholar] [CrossRef]
- Cheng, Q.; Yao, X.; Ou, L.; Hu, Z.; Zheng, L.; Li, G.; Morlanes, N.; Cerrillo, J.L.; Castaño, P.; Li, X.; et al. Highly Efficient and Stable Methane Dry Reforming Enabled by a Single-Site Cationic Ni Catalyst. J. Am. Chem. Soc. 2023, 145, 25109–25119. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, Y.; Qin, X.; Liu, L.; Ren, Z.; Tao, X.; Wang, C.; Wang, H.; Li, L.; Liu, X.; et al. Zeolite fixed cobalt–nickel nanoparticles for coking and sintering resistance in dry reforming of methane. Chem. Eng. Sci. 2023, 280, 119030. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Xiao, F.-S. Metal@Zeolite Hybrid Materials for Catalysis. ACS Cent. Sci. 2020, 6, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Slater, T.J.A.; Huang, H.; Zhou, Y.; Jiao, Y.; Parlett, C.M.A.; Guan, S.; Chansai, S.; Xu, S.; Wang, X.; et al. Developing silicalite-1 encapsulated Ni nanoparticles as sintering-/coking-resistant catalysts for dry reforming of methane. Chem. Eng. J. 2022, 446, 137439. [Google Scholar] [CrossRef]
- Fujitsuka, H.; Kobayashi, T.; Tago, T. Development of Silicalite-1-encapsulated Ni nanoparticle catalyst from amorphous silica-coated Ni for dry reforming of methane: Achieving coke formation suppression and high thermal stability. J. CO2 Util. 2021, 53, 101707. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Gao, Z.; Zhang, X.; Zhang, L.; Wang, M.; Chen, B.; Diao, Y.; Li, Y.; Xiao, D.; et al. Embedding high loading and uniform Ni nanoparticles into silicalite-1 zeolite for dry reforming of methane. Appl. Catal. B Environ. 2022, 307, 121202. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Han, D.; He, B.; Sun, X.; Liu, M.; Mei, Y.; Zu, Y. Small-sized Ni nanoparticles embedded nickel phyllosilicate as a metal-acid bifunctional zeolite catalyst for cooperatively boosting CO2-CH4 reforming. Fuel 2023, 331, 125957. [Google Scholar] [CrossRef]
- Kulkarni, S.R.; Velisoju, V.K.; Tavares, F.; Dikhtiarenko, A.; Gascon, J.; Castaño, P. Silicon carbide in catalysis: From inert bed filler to catalytic support and multifunctional material. Catal. Rev. 2023, 65, 174–237. [Google Scholar] [CrossRef]
- Gunduz Meric, G.; Arbag, H.; Degirmenci, L. Coke minimization via SiC formation in dry reforming of methane conducted in the presence of Ni-based core–shell microsphere catalysts. Int. J. Hydrogen Energy 2017, 42, 16579–16588. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, G.; Bi, G.; Guo, Y.; Xie, J. Monolithic SiC-foam supported Ni-La2O3 composites for dry reforming of methane with enhanced carbon resistance. Fuel Process. Technol. 2021, 212, 106627. [Google Scholar] [CrossRef]
- Feng, K.; Zhang, J.; Li, Z.; Liu, X.; Pan, Y.; Wu, Z.; Tian, J.; Chen, Y.; Zhang, C.; Xue, Q.; et al. Spontaneous regeneration of active sites against catalyst deactivation. Appl. Catal. B Environ. 2024, 344, 123647. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Liu, Y.; Zheng, J.; Deng, J.; Yan, T.; Cheng, D.; Zhang, D. Unraveling the Unique Promotion Effects of a Triple Interface in Ni Catalysts for Methane Dry Reforming. Ind. Eng. Chem. Res. 2023, 62, 4965–4975. [Google Scholar] [CrossRef]
- Zheng, J.; Impeng, S.; Liu, J.; Deng, J.; Zhang, D. Mo promoting Ni-based catalysts confined by halloysite nanotubes for dry reforming of methane: Insight of coking and H2S poisoning resistance. Appl. Catal. B Environ. 2024, 342, 123369. [Google Scholar] [CrossRef]
- Alli, R.D.; Mahinpey, N. Influence of organic ligand and nickel loading on the performance of MOF-derived catalysts for dry reforming of methane. Fuel 2024, 361, 130756. [Google Scholar] [CrossRef]
- De, S.; Zhang, J.; Luque, R.; Yan, N. Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef]
- Zou, Z.; Zhang, T.; Lv, L.; Tang, W.; Zhang, G.; Kumar Gupta, R.; Wang, Y.; Tang, S. Preparation adjacent Ni-Co bimetallic nano catalyst for dry reforming of methane. Fuel 2023, 343, 128013. [Google Scholar] [CrossRef]
- Palanichamy, K.; Umasankar, S.; Ganesh, S.; Sasirekha, N. Highly coke resistant Ni–Co/KCC-1 catalysts for dry reforming of methane. Int. J. Hydrogen Energy 2023, 48, 11727–11745. [Google Scholar] [CrossRef]
- Duan, X.; Pan, J.; Yang, X.; Wan, C.; Lin, X.; Li, D.; Jiang, L. Nickel–cobalt bimetallic catalysts prepared from hydrotalcite-like compounds for dry reforming of methane. Int. J. Hydrogen Energy 2022, 47, 24358–24373. [Google Scholar] [CrossRef]
- Nataj, S.M.M.; Alavi, S.M.; Mazloom, G. Modeling and optimization of methane dry reforming over Ni–Cu/Al2O3 catalyst using Box–Behnken design. J. Energy Chem. 2018, 27, 1475–1488. [Google Scholar] [CrossRef]
- Danghyan, V.; Kumar, A.; Mukasyan, A.; Wolf, E.E. An active and stable NiOMgO solid solution based catalysts prepared by paper assisted combustion synthesis for the dry reforming of methane. Appl. Catal. B Environ. 2020, 273, 119056. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Li, X.; Bai, Y.; Zheng, L.; Wu, L.; Han, X. Effect of Gd Promoter on the Structure and Catalytic Performance of Mesoporous Ni/Al2O3–CeO2 in Dry Reforming of Methane. Ind. Eng. Chem. Res. 2018, 57, 17076–17085. [Google Scholar] [CrossRef]
- Sheng, K.; Luan, D.; Jiang, H.; Zeng, F.; Wei, B.; Pang, F.; Ge, J. NixCoy Nanocatalyst Supported by ZrO2 Hollow Sphere for Dry Reforming of Methane: Synergetic Catalysis by Ni and Co in Alloy. ACS Appl. Mater. Interfaces 2019, 11, 24078–24087. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Kumar, R.; Kasim, S.O.; Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Atia, H.; Armbruster, U.; Kreyenschulte, C.; Lund, H.; et al. Effect of Cerium Promoters on an MCM-41-Supported Nickel Catalyst in Dry Reforming of Methane. Ind. Eng. Chem. Res. 2022, 61, 164–174. [Google Scholar] [CrossRef]
- Wang, H.; Dong, X.; Zhao, T.; Yu, H.; Li, M. Dry reforming of methane over bimetallic Ni-Co catalyst prepared from La(CoxNi1−x)0.5Fe0.5O3 perovskite precursor: Catalytic activity and coking resistance. Appl. Catal. B Environ. 2019, 245, 302–313. [Google Scholar] [CrossRef]
- Liang, T.-Y.; Senthil Raja, D.; Chin, K.C.; Huang, C.-L.; Sethupathi, S.A.P.; Leong, L.K.; Tsai, D.-H.; Lu, S.-Y. Bimetallic Metal–Organic Framework-Derived Hybrid Nanostructures as High-Performance Catalysts for Methane Dry Reforming. ACS Appl. Mater. Interfaces 2020, 12, 15183–15193. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Chen, Y.; Zhang, B.; Li, Z.; Cui, X.; Guo, C.; Zhao, Y.; Zhang, J. Tailoring the stability of Ni-Fe/mayenite in methane–Carbon dioxide reforming. Fuel 2021, 284, 118909. [Google Scholar] [CrossRef]
- Wan, C.; Song, K.; Pan, J.; Huang, M.; Luo, R.; Li, D.; Jiang, L. Ni–Fe/Mg(Al)O alloy catalyst for carbon dioxide reforming of methane: Influence of reduction temperature and Ni–Fe alloying on coking. Int. J. Hydrogen Energy 2020, 45, 33574–33585. [Google Scholar] [CrossRef]
- Xiao, Z.; Hou, F.; Zhang, J.; Zheng, Q.; Xu, J.; Pan, L.; Wang, L.; Zou, J.; Zhang, X.; Li, G. Methane Dry Reforming by Ni–Cu Nanoalloys Anchored on Periclase-Phase MgAlOx Nanosheets for Enhanced Syngas Production. ACS Appl. Mater. Interfaces 2021, 13, 48838–48854. [Google Scholar] [CrossRef] [PubMed]
- Sagar, T.V.; Padmakar, D.; Lingaiah, N.; Sai Prasad, P.S. Influence of Solid Solution Formation on the Activity of CeO2 Supported Ni–Cu Mixed Oxide Catalysts in Dry Reforming of Methane. Catal. Lett. 2019, 149, 2597–2606. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, J.; Pupucevski, M.; Impeng, S.; Yang, B.; Chen, G.; Kuboon, S.; Zhong, Q.; Faungnawakij, K.; Zheng, L.; et al. High-Performance Binary Mo–Ni Catalysts for Efficient Carbon Removal during Carbon Dioxide Reforming of Methane. ACS Catal. 2021, 11, 12087–12095. [Google Scholar] [CrossRef]
- Károlyi, J.; Németh, M.; Evangelisti, C.; Sáfrán, G.; Schay, Z.; Horváth, A.; Somodi, F. Carbon dioxide reforming of methane over Ni–In/SiO2 catalyst without coke formation. J. Ind. Eng. Chem. 2018, 58, 189–201. [Google Scholar] [CrossRef]
- Araiza, D.G.; Arcos, D.G.; Gómez-Cortés, A.; Díaz, G. Dry reforming of methane over Pt-Ni/CeO2 catalysts: Effect of the metal composition on the stability. Catal. Today 2021, 360, 46–54. [Google Scholar] [CrossRef]
- Andraos, S.; Abbas-Ghaleb, R.; Chlala, D.; Vita, A.; Italiano, C.; Laganà, M.; Pino, L.; Nakhl, M.; Specchia, S. Production of hydrogen by methane dry reforming over ruthenium-nickel based catalysts deposited on Al2O3, MgAl2O4, and YSZ. Int. J. Hydrogen Energy 2019, 44, 25706–25716. [Google Scholar] [CrossRef]
- García-Diéguez, M.; Pieta, I.S.; Herrera, M.C.; Larrubia, M.A.; Alemany, L.J. RhNi nanocatalysts for the CO2 and CO2 + H2O reforming of methane. Catal. Today 2011, 172, 136–142. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, L.; Zheng, X.; Liu, W.; Cao, Z.; Peng, H. Coke-resistance over Rh–Ni bimetallic catalyst for low temperature dry reforming of methane. Int. J. Hydrogen Energy 2023, 48, 13890–13901. [Google Scholar] [CrossRef]
- Tang, L.; Huang, X.; Ran, J.; Guo, F.; Niu, J.; Qiu, H.; Ou, Z.; Yan, Y.; Yang, Z.; Qin, C. Density functional theory studies on direct and oxygen assisted activation of C–H bond for dry reforming of methane over Rh–Ni catalyst. Int. J. Hydrogen Energy 2022, 47, 30391–30403. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, T.; Sui, Z.; Zhu, Y.-A.; Han, C.; Zhu, K.; Zhou, X. A single source method to generate Ru-Ni-MgO catalysts for methane dry reforming and the kinetic effect of Ru on carbon deposition and gasification. Appl. Catal. B: Environ. 2018, 233, 143–159. [Google Scholar] [CrossRef]
- Álvarez, M.A.; Bobadilla, L.F.; Garcilaso, V.; Centeno, M.A.; Odriozola, J.A. CO2 reforming of methane over Ni-Ru supported catalysts: On the nature of active sites by operando DRIFTS study. J. CO2 Util. 2018, 24, 509–515. [Google Scholar] [CrossRef]
- Miao, C.; Chen, S.; Shang, K.; Liang, L.; Ouyang, J. Highly Active Ni–Ru Bimetallic Catalyst Integrated with MFI Zeolite-Loaded Cerium Zirconium Oxide for Dry Reforming of Methane. ACS Appl. Mater. Interfaces 2022, 14, 47616–47632. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Liland, E.S.; Regli, K.S.; Yang, J.; Rout, K.R.; Luo, J.; Rønning, M.; Ran, J.; Chen, D. Unraveling Enhanced Activity, Selectivity, and Coke Resistance of Pt–Ni Bimetallic Clusters in Dry Reforming. ACS Catal. 2021, 11, 2398–2411. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, B.; Miao, S.; Liu, W.; Xie, J.; Lee, S.; Pellin, M.J.; Xiao, D.; Su, D.; Ma, D. Lattice Strained Ni-Co alloy as a High-Performance Catalyst for Catalytic Dry Reforming of Methane. ACS Catal. 2019, 9, 2693–2700. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.; Tian, D.; Jiang, L.; Li, Z.; Wang, H.; Li, K. Optimization of Ni-Based Catalysts for Dry Reforming of Methane via Alloy Design: A Review. Energy Fuels 2022, 36, 5102–5151. [Google Scholar] [CrossRef]
- Horlyck, J.; Lawrey, C.; Lovell, E.C.; Amal, R.; Scott, J. Elucidating the impact of Ni and Co loading on the selectivity of bimetallic NiCo catalysts for dry reforming of methane. Chem. Eng. J. 2018, 352, 572–580. [Google Scholar] [CrossRef]
- Tu, W.; Ghoussoub, M.; Singh, C.V.; Chin, Y.-H.C. Consequences of Surface Oxophilicity of Ni, Ni-Co, and Co Clusters on Methane Activation. J. Am. Chem. Soc. 2017, 139, 6928–6945. [Google Scholar] [CrossRef] [PubMed]
- Turap, Y.; Wang, I.; Fu, T.; Wu, Y.; Wang, Y.; Wang, W. Co–Ni alloy supported on CeO2 as a bimetallic catalyst for dry reforming of methane. Int. J. Hydrogen Energy 2020, 45, 6538–6548. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Liang, Z.; Zhang, G.; Wang, Y.; Zhao, Y.; Li, G.; Lv, Y. Enhancing the dry reforming of methane over Ni-Co-Y/WC-AC catalyst: Influence of the different Ni/Co ratio on the catalytic performance. Fuel 2023, 335, 127082. [Google Scholar] [CrossRef]
- Kumari, R.; Sengupta, S. Catalytic CO2 reforming of CH4 over MgAl2O4 supported Ni-Co catalysts for the syngas production. Int. J. Hydrogen Energy 2020, 45, 22775–22787. [Google Scholar] [CrossRef]
- Shakir, M.; Prasad, M.; Ray, K.; Sengupta, S.; Sinhamahapatra, A.; Liu, S.; Vuthaluru, H.B. NaBH4-Assisted Synthesis of B–(Ni–Co)/MgAl2O4 Nanostructures for the Catalytic Dry Reforming of Methane. ACS Appl. Nano Mater. 2022, 5, 10951–10961. [Google Scholar] [CrossRef]
- Chen, X.; Yin, L.; Long, K.; Sun, H.; Sun, M.; Wang, H.; Zhang, Q.; Ning, P. The reconstruction of Ni particles on SBA-15 by thermal activation for dry reforming of methane with excellent resistant to carbon deposition. J. Energy Inst. 2020, 93, 2255–2263. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A.K. Effects of metal content on activity and stability of Ni-Co bimetallic catalysts for CO2 reforming of CH4. Appl. Catal. A Gen. 2008, 339, 121–129. [Google Scholar] [CrossRef]
- Chen, S.; Yang, B. Activity and stability of alloyed NiCo catalyst for the dry reforming of methane: A combined DFT and microkinetic modeling study. Catal. Today 2022, 400–401, 59–65. [Google Scholar] [CrossRef]
- Ou, Z.; Ran, J.; Qiu, H.; Huang, X.; Qin, C. Understanding the role of Co segregation on the carbon deposition over Ni–Co bimetal catalyst in dry reforming of methane. Sep. Purif. Technol. 2024, 333, 125868. [Google Scholar] [CrossRef]
- Liu, K.; Xing, F.; Xiao, Y.; Yan, N.; Shimizu, K.-I.; Furukawa, S. Development of a Highly Stable Ternary Alloy Catalyst for Dry Reforming of Methane. ACS Catal. 2023, 13, 3541–3548. [Google Scholar] [CrossRef]
- Li, B.; Yuan, X.; Li, L.; Wang, X.; Li, B. Stabilizing Ni-Co Alloy on Bimodal Mesoporous Alumina to Enhance Carbon Resistance for Dry Reforming of Methane. Ind. Eng. Chem. Res. 2021, 60, 16874–16886. [Google Scholar] [CrossRef]
- Gai, X.; Yang, D.; Tang, R.; Luo, M.; Lu, P.; Xing, C.; Yang, R.; Ma, Q.; Li, Y. Preparation of Ni-Co/SiO2 catalyst by ammonia reflux impregnation and its CH4-CO2 reforming reaction performance. Fuel 2022, 316, 123337. [Google Scholar] [CrossRef]
- Kuboon, S.; Deng, J.; Gao, M.; Faungnawakij, K.; Hasegawa, J.-Y.; Zhang, X.; Shi, L.; Zhang, D. Unraveling the promotional effects of NiCo catalysts over defective boron nitride nanosheets in dry reforming of methane. Catal. Today 2022, 402, 283–291. [Google Scholar] [CrossRef]
- Panda, S.; Joshi, V.; Shrivastaw, V.K.; Das, S.; Poddar, M.; Bal, R.; Bordoloi, A. Enhanced coke-resistant Co-modified Ni/modified alumina catalyst for the bireforming of methane. Catal. Sci. Technol. 2023, 13, 4506–4516. [Google Scholar] [CrossRef]
- Huang, J.; Jones, A.; Waite, T.D.; Chen, Y.; Huang, X.; Rosso, K.M.; Kappler, A.; Mansor, M.; Tratnyek, P.G.; Zhang, H. Fe(II) Redox Chemistry in the Environment. Chem. Rev. 2021, 121, 8161–8233. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Marin, G.B. Enhanced Carbon-Resistant Dry Reforming Fe-Ni Catalyst: Role of Fe. ACS Catal. 2015, 5, 3028–3039. [Google Scholar] [CrossRef]
- Kim, S.M.; Abdala, P.M.; Margossian, T.; Hosseini, D.; Foppa, L.; Armutlulu, A.; van Beek, W.; Comas-Vives, A.; Copéret, C.; Müller, C. Cooperativity and Dynamics Increase the Performance of NiFe Dry Reforming Catalysts. J. Am. Chem. Soc. 2017, 139, 1937–1949. [Google Scholar] [CrossRef]
- Song, Z.; Wang, Q.; Guo, C.; Li, S.; Yan, W.; Jiao, W.; Qiu, L.; Yan, X.; Li, R. Improved Effect of Fe on the Stable NiFe/Al2O3 Catalyst in Low-Temperature Dry Reforming of Methane. Ind. Eng. Chem. Res. 2020, 59, 17250–17258. [Google Scholar] [CrossRef]
- Margossian, T.; Larmier, K.; Kim, S.M.; Krumeich, F.; Müller, C.; Copéret, C. Supported Bimetallic NiFe Nanoparticles through Colloid Synthesis for Improved Dry Reforming Performance. ACS Catal. 2017, 7, 6942–6948. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Cao, M.; Li, S.; Song, Z.; Qiu, L.; Yu, F.; Li, R.; Yan, X. Structural evolution of robust Ni3Fe1 alloy on Al2O3 in dry reforming of methane: Effect of iron-surplus strategy from Ni1Fe1 to Ni3Fe1. Appl. Catal. B: Environ. 2023, 331, 122669. [Google Scholar] [CrossRef]
- Huang, L.; Ma, Y.; Niu, M.; Ren, S.; Guo, Q.; Xu, C.; Shen, B. Formation of H2O in the CH4-CO2 dry reforming process and its activation to this reaction over Ni-Fe/MC12A7 catalysts. Appl. Catal. B Environ. 2023, 334, 122822. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Z.; Zhu, Y.-A.; Liu, Z.; Sui, Z.; Zhu, K.; Zhou, X. Dry reforming of methane on Ni-Fe-MgO catalysts: Influence of Fe on carbon-resistant property and kinetics. Appl. Catal. B Environ. 2020, 264, 118497. [Google Scholar] [CrossRef]
- Gaillard, M.; Virginie, M.; Khodakov, A.Y. New molybdenum-based catalysts for dry reforming of methane in presence of sulfur: A promising way for biogas valorization. Catal. Today 2017, 289, 143–150. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Q. Fewer defects, better catalysis? Science 2020, 367, 737. [Google Scholar] [CrossRef]
- Yao, L.; Galvez, M.E.; Hu, C.; Da Costa, P. Mo-promoted Ni/Al2O3 catalyst for dry reforming of methane. Int. J. Hydrogen Energy 2017, 42, 23500–23507. [Google Scholar] [CrossRef]
- Abdel Karim Aramouni, N.; Zeaiter, J.; Kwapinski, W.; Leahy, J.J.; Ahmad, M.N. Molybdenum and nickel-molybdenum nitride catalysts supported on MgO-Al2O3 for the dry reforming of methane. J. CO2 Util. 2021, 44, 101411. [Google Scholar] [CrossRef]
- Tang, J.; Meng, J.; Pan, W.; Gu, T.; Zhang, Q.; Zhang, J.; Wang, X.; Bu, C.; Piao, G. Effect of hydroxyl and Mo doping on activity and carbon deposition resistance of hydroxyapatite supported NixMoy catalyst for syngas production via DRM reaction. Int. J. Hydrogen Energy 2023, 48, 19033–19045. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Liu, J.-H.; Lv, C.-Q.; Ren, R.-R.; Wang, G.-C. Dry reforming of methane on Ni(111) surface with different Mo doping ratio: DFT-assisted microkinetic study. Appl. Surf. Sci. 2022, 581, 152310. [Google Scholar] [CrossRef]
- Song, K.; Lu, M.; Xu, S.; Chen, C.; Zhan, Y.; Li, D.; Au, C.; Jiang, L.; Tomishige, K. Effect of alloy composition on catalytic performance and coke-resistance property of Ni-Cu/Mg(Al)O catalysts for dry reforming of methane. Appl. Catal. B: Environ. 2018, 239, 324–333. [Google Scholar] [CrossRef]
- Han, K.; Wang, S.; Liu, Q.; Wang, F. Optimizing the Ni/Cu Ratio in Ni–Cu Nanoparticle Catalysts for Methane Dry Reforming. ACS Appl. Nano Mater. 2021, 4, 5340–5348. [Google Scholar] [CrossRef]
- Chatla, A.; Ghouri, M.M.; El Hassan, O.W.; Mohamed, N.; Prakash, A.V.; Elbashir, N.O. An experimental and first principles DFT investigation on the effect of Cu addition to Ni/Al2O3 catalyst for the dry reforming of methane. Appl. Catal. A: Gen. 2020, 602, 117699. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Y.-A.; Yan, X.; Chen, F.; Shen, Q.; Zhang, L.; Yan, N. Cooperative Atom Motion in Ni–Cu Nanoparticles during the Structural Evolution and the Implication in the High-Temperature Catalyst Design. ACS Appl. Energy Mater. 2019, 2, 8894–8902. [Google Scholar] [CrossRef]
- Bekheet, M.F.; Delir Kheyrollahi Nezhad, P.; Bonmassar, N.; Schlicker, L.; Gili, A.; Praetz, S.; Gurlo, A.; Doran, A.; Gao, Y.; Heggen, M.; et al. Steering the Methane Dry Reforming Reactivity of Ni/La2O3 Catalysts by Controlled In Situ Decomposition of Doped La2NiO4 Precursor Structures. ACS Catal. 2021, 11, 43–59. [Google Scholar] [CrossRef]
- Wang, F.; Han, B.; Zhang, L.; Xu, L.; Yu, H.; Shi, W. CO2 reforming with methane over small-sized Ni@SiO2 catalysts with unique features of sintering-free and low carbon. Appl. Catal. B Environ. 2018, 235, 26–35. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Li, H. NiCo@SiO2 core-shell catalyst with high activity and long lifetime for CO2 conversion through DRM reaction. Nano Energy 2018, 45, 101–108. [Google Scholar] [CrossRef]
- Pang, Y.; Dou, Y.; Zhong, A.; Jiang, W.; Gu, L.; Feng, X.; Ji, W.; Au, C.-T. Nanostructured Ru-Co@SiO2: Highly efficient yet durable for CO2 reforming of methane with a desirable H2/CO ratio. Appl. Catal. A Gen. 2018, 555, 27–35. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Zhang, X.; Wang, Z.; Wang, X.; Peng, H. Design of Ni-ZrO2@SiO2 catalyst with ultra-high sintering and coking resistance for dry reforming of methane to prepare syngas. J. CO2 Util. 2018, 27, 297–307. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, D.; Ruan, Y.; Zhao, Y.; Wang, S.; Ma, X. Facile one-pot synthesis of Ni@HSS as a novel yolk-shell structure catalyst for dry reforming of methane. J. CO2 Util. 2018, 24, 190–199. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, B.; Wang, Z.; Kawi, S. High carbon resistant Ni@Ni phyllosilicate@SiO2 core shell hollow sphere catalysts for low temperature CH4 dry reforming. J. CO2 Util. 2018, 27, 238–246. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Q.; Shen, W.; Zhu, Z.; Fang, Y. (Ni/MgAl2O4)@SiO2 core–shell catalyst with high coke-resistance for the dry reforming of methane. React. Kinet. Mech. Catal. 2018, 125, 127–139. [Google Scholar] [CrossRef]
- Dou, J.; Zhang, R.; Hao, X.; Bao, Z.; Wu, T.; Wang, B.; Yu, F. Sandwiched SiO2@Ni@ZrO2 as a coke resistant nanocatalyst for dry reforming of methane. Appl. Catal. B: Environ. 2019, 254, 612–623. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, Y.; Li, H.; Li, H. CO2 conversion to synthesis gas via DRM on the durable Al2O3/Ni/Al2O3 sandwich catalyst with high activity and stability. Green Chem. 2018, 20, 2781–2787. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.H.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Silica–Ceria sandwiched Ni core–shell catalyst for low temperature dry reforming of biogas: Coke resistance and mechanistic insights. Appl. Catal. B Environ. 2018, 230, 220–236. [Google Scholar] [CrossRef]
- Kaviani, M.; Rezaei, M.; Mehdi Alavi, S.; Akbari, E. High coke resistance Ni-SiO2@SiO2 core-shell catalyst for biogas dry reforming: Effects of Ni loading and calcination temperature. Fuel 2022, 330, 125609. [Google Scholar] [CrossRef]
- Wang, C.; Wu, H.; Jie, X.; Zhang, X.; Zhao, Y.; Yao, B.; Xiao, T. Yolk–Shell Nanocapsule Catalysts as Nanoreactors with Various Shell Structures and Their Diffusion Effect on the CO2 Reforming of Methane. ACS Appl. Mater. Interfaces 2021, 13, 31699–31709. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lauterbach, J.; Sasmaz, E. Yolk–Shell Pt-NiCe@SiO2 Single-Atom-Alloy Catalysts for Low-Temperature Dry Reforming of Methane. ACS Catal. 2021, 11, 8247–8260. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Lin, S.; Luo, Y.; Bao, Z.; Mao, Y.; Li, K.; Wu, D.; Peng, H. Confined Ni-In intermetallic alloy nanocatalyst with excellent coking resistance for methane dry reforming. J. Energy Chem. 2022, 65, 34–47. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, D.; Zhao, Y.; Zhao, Y.; Wang, S.; Ma, X. Enhanced performance of xNi@yMo-HSS catalysts for DRM reaction via the formation of a novel SiMoOx species. Appl. Catal. B: Environ. 2021, 291, 120075. [Google Scholar] [CrossRef]
- Shi, Y.; Han, K.; Wang, F. Ni–Cu Alloy Nanoparticles Confined by Physical Encapsulation with SiO2 and Chemical Metal–Support Interaction with CeO2 for Methane Dry Reforming. Inorg. Chem. 2022, 61, 15619–15628. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, D.; Zhao, Y.; Moyo, P.S.; Zhao, Y.; Wang, S.; Ma, X. Confined high dispersion of Ni nanoparticles derived from nickel phyllosilicate structure in silicalite-2 shell for dry reforming of methane with enhanced performance. Microporous Mesoporous Mater. 2021, 313, 110842. [Google Scholar] [CrossRef]
- Qu, H.; Yang, H.; Han, L.; He, S.; Liu, J.; Hu, R.; Su, H.; Su, Y. Sandwich-structured nickel/kaolinite catalyst with boosted stability for dry reforming of methane with carbon dioxide. Chem. Eng. J. 2023, 453, 139694. [Google Scholar] [CrossRef]
- Zhang, Q.; Lee, I.; Joo, J.B.; Zaera, F.; Yin, Y. Core–Shell Nanostructured Catalysts. Acc. Chem. Res. 2013, 46, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Goswami, A.; Asefa, T.; Guo, H.; Biradar, A.V.; Peng, D.-L.; Zboril, R.; Varma, R.S. Core–shell nanoparticles: Synthesis and applications in catalysis and electrocatalysis. Chem. Soc. Rev. 2015, 44, 7540–7590. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Pérez-Ramírez, J.; Gong, J.; Dewangan, N.; Hidajat, K.; Gates, B.C.; Kawi, S. Core–shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2. Chem. Soc. Rev. 2020, 49, 2937–3004. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, F. Coke-resistant Ni@SiO2 catalyst for dry reforming of methane. Appl. Catal. B Environ. 2015, 176-177, 513–521. [Google Scholar] [CrossRef]
- Park, K.S.; Kwon, J.H.; Yu, J.S.; Jeong, S.Y.; Jo, D.H.; Chung, C.-H.; Bae, J.W. Catalytically stable monodispersed multi-core Ni-Co nanoparticles encapsulated with SiO2 shells for dry reforming of CH4 with CO2. J. CO2 Util. 2022, 60, 101984. [Google Scholar] [CrossRef]
- Lin, S.; Wang, J.; Mi, Y.; Yang, S.; Wang, Z.; Liu, W.; Wu, D.; Peng, H. Trifunctional strategy for the design and synthesis of a Ni-CeO2@SiO2 catalyst with remarkable low-temperature sintering and coking resistance for methane dry reforming. Chin. J. Catal. 2021, 42, 1808–1820. [Google Scholar] [CrossRef]
- Das, S.; Lim, K.H.; Gani, T.Z.H.; Aksari, S.; Kawi, S. Bi-functional CeO2 coated NiCo-MgAl core-shell catalyst with high activity and resistance to coke and H2S poisoning in methane dry reforming. Appl. Catal. B Environ. 2023, 323, 122141. [Google Scholar] [CrossRef]
- Han, K.; Wang, S.; Hu, N.; Shi, W.; Wang, F. Alloying Ni–Cu Nanoparticles Encapsulated in SiO2 Nanospheres for Synergistic Catalysts in CO2 Reforming with Methane Reaction. ACS Appl. Mater. Interfaces 2022, 14, 23487–23495. [Google Scholar] [CrossRef]
- Kosari, M.; Askari, S.; Seayad, A.M.; Xi, S.; Kawi, S.; Borgna, A.; Zeng, H.C. Strong coke-resistivity of spherical hollow Ni/SiO2 catalysts with shell-confined high-content Ni nanoparticles for methane dry reforming with CO2. Appl. Catal. B: Environ. 2022, 310, 121360. [Google Scholar] [CrossRef]
- Singh, S.; Zubenko, D.; Rosen, B.A. Influence of LaNiO3 Shape on Its Solid-Phase Crystallization into Coke-Free Reforming Catalysts. ACS Catal. 2016, 6, 4199–4205. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, W.; Song, H.; Zhao, J.; Yang, J.; Yan, L.; Qiao, B.; Chou, L. Highly coke-resistant Ni-La2O2CO3 catalyst with low Ni loading for dry reforming of methane with carbon dioxide. Catal. Today 2022, 402, 189–201. [Google Scholar] [CrossRef]
- Moogi, S.; Hyun Ko, C.; Hoon Rhee, G.; Jeon, B.-H.; Ali Khan, M.; Park, Y.-K. Influence of catalyst synthesis methods on anti-coking strength of perovskites derived catalysts in biogas dry reforming for syngas production. Chem. Eng. J. 2022, 437, 135348. [Google Scholar] [CrossRef]
- Rabelo-Neto, R.C.; Sales, H.B.E.; Inocêncio, C.V.M.; Varga, E.; Oszko, A.; Erdohelyi, A.; Noronha, F.B.; Mattos, L.V. CO2 reforming of methane over supported LaNiO3 perovskite-type oxides. Appl. Catal. B Environ. 2018, 221, 349–361. [Google Scholar] [CrossRef]
- Bonmassar, N.; Bekheet, M.F.; Schlicker, L.; Gili, A.; Gurlo, A.; Doran, A.; Gao, Y.; Heggen, M.; Bernardi, J.; Klötzer, B.; et al. In Situ-Determined Catalytically Active State of LaNiO3 in Methane Dry Reforming. ACS Catal. 2020, 10, 1102–1112. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jang, J.S.; Ra, E.C.; Kim, K.Y.; Kim, E.H.; Lee, J.S. Reduced perovskite LaNiO3 catalysts modified with Co and Mn for low coke formation in dry reforming of methane. Appl. Catal. A Gen. 2019, 575, 198–203. [Google Scholar] [CrossRef]
- Shahnazi, A.; Firoozi, S. Improving the catalytic performance of LaNiO3 perovskite by manganese substitution via ultrasonic spray pyrolysis for dry reforming of methane. J. CO2 Util. 2021, 45, 101455. [Google Scholar] [CrossRef]
- Shahnazi, A.; Firoozi, S. Mesoporous LaNi1-xMnxO3 perovskite with enhanced catalytic performance and coke resistance synthesized via glycine-assisted spray pyrolysis for methane dry reforming. Mol. Catal. 2023, 547, 113320. [Google Scholar] [CrossRef]
- Yadav, P.K.; Das, T. Production of syngas from carbon dioxide reforming of methane by using LaNixFe1−xO3 perovskite type catalysts. Int. J. Hydrogen Energy 2019, 44, 1659–1670. [Google Scholar] [CrossRef]
- Iftikhar, S.; Martin, W.; Gao, Y.; Yu, X.; Wang, I.; Wu, Z.; Li, F. LaNixFe1−xO3 as flexible oxygen or carbon carriers for tunable syngas production and CO2 utilization. Catal. Today 2023, 416, 113854. [Google Scholar] [CrossRef]
- Yao, X.; Cheng, Q.; Attada, Y.; Ould-Chikh, S.; Ramírez, A.; Bai, X.; Mohamed, H.O.; Li, G.; Shterk, G.; Zheng, L.; et al. Atypical stability of exsolved Ni-Fe alloy nanoparticles on double layered perovskite for CO2 dry reforming of methane. Appl. Catal. B Environ. 2023, 328, 122479. [Google Scholar] [CrossRef]
- Das, S.; Bhattar, S.; Liu, L.; Wang, Z.; Xi, S.; Spivey, J.J.; Kawi, S. Effect of Partial Fe Substitution in La0.9Sr0.1NiO3 Perovskite-Derived Catalysts on the Reaction Mechanism of Methane Dry Reforming. ACS Catal. 2020, 10, 12466–12486. [Google Scholar] [CrossRef]
- Bai, J.; Fu, Y.; Kong, W.; Pan, B.; Yuan, C.; Li, S.; Wang, J.; Zhang, J.; Sun, Y. Design of Ni-substituted La2(CeZrNi)2O7 Pyrochlore Catalysts for Methane Dry Reforming. ChemNanoMat 2022, 8, e202100422. [Google Scholar] [CrossRef]
- Chai, Y.; Fu, Y.; Feng, H.; Kong, W.; Yuan, C.; Pan, B.; Zhang, J.; Sun, Y. A Nickel-Based Perovskite Catalyst with a Bimodal Size Distribution of Nickel Particles for Dry Reforming of Methane. ChemCatChem 2018, 10, 2078–2086. [Google Scholar] [CrossRef]
- Wei, T.; Qiu, P.; Jia, L.; Tan, Y.; Yang, X.; Sun, S.; Chen, F.; Li, J. Power and carbon monoxide co-production by a proton-conducting solid oxide fuel cell with La0.6Sr0.2Cr0.85Ni0.15O3−δ for on-cell dry reforming of CH4 by CO2. J. Mater. Chem. A 2020, 8, 9806–9812. [Google Scholar] [CrossRef]
- Dama, S.; Ghodke, S.R.; Bobade, R.; Gurav, H.R.; Chilukuri, S. Active and durable alkaline earth metal substituted perovskite catalysts for dry reforming of methane. Appl. Catal. B Environ. 2018, 224, 146–158. [Google Scholar] [CrossRef]
- Peng, W.; Li, Z.; Liu, B.; Qiu, P.; Yan, D.; Jia, L.; Li, J. Enhanced activity and stability of Ce-doped PrCrO3-supported nickel catalyst for dry reforming of methane. Sep. Purif. Technol. 2022, 303, 122245. [Google Scholar] [CrossRef]
- Chen, C.; Wei, J.; Lu, Y.; Duyar, M.S.; Huang, Y.; Lin, L.; Ye, R. Confinement effects over Ni-based catalysts for methane dry reforming. Catal. Sci. Technol. 2023, 13, 6089–6101. [Google Scholar] [CrossRef]
- Bian, Z.; Kawi, S. Sandwich-like silica@ Ni@ silica multicore–shell catalyst for the low-temperature dry reforming of methane: Confinement effect against carbon formation. ChemCatChem 2018, 10, 320–328. [Google Scholar] [CrossRef]
- Kim, D.H.; Seo, J.-C.; Kim, Y.J.; Kim, J.; Yoon, S.; Ra, H.; Kim, M.-J.; Lee, K. Ni-Co alloy catalyst derived from NixCoy/MgAl2O4 via exsolution method for high coke resistance toward dry reforming of methane. Catal. Today 2024, 425, 114337. [Google Scholar] [CrossRef]
- Roh, J.; Park, H.; Kwon, H.; Joo, C.; Moon, I.; Cho, H.; Ro, I.; Kim, J. Interpretable machine learning framework for catalyst performance prediction and validation with dry reforming of methane. Appl. Catal. B Environ. 2024, 343, 123454. [Google Scholar] [CrossRef]
- Alotaibi, F.N.; Berrouk, A.S.; Saeed, M. Optimization of Yield and Conversion Rates in Methane Dry Reforming Using Artificial Neural Networks and the Multiobjective Genetic Algorithm. Ind. Eng. Chem. Res. 2023, 62, 17084–17099. [Google Scholar] [CrossRef]
- Yoon, Y.; You, H.M.; Kim, H.J.; Curnan, M.T.; Kim, K.; Han, J.W. Computational Catalyst Design for Dry Reforming of Methane: A Review. Energy Fuels 2022, 36, 9844–9865. [Google Scholar] [CrossRef]
- Lei, Y.; Ye, J.; García-Antón, J.; Liu, H. Recent advances in the built-in electric-field-assisted photocatalytic dry reforming of methane. Chin. J. Catal. 2023, 53, 72–101. [Google Scholar] [CrossRef]
- Malik, M.I.; Achouri, I.E.; Abatzoglou, N.; Gitzhofer, F. Intensified performance of methane dry reforming based on non-thermal plasma technology: Recent progress and key challenges. Fuel Process. Technol. 2023, 245, 107748. [Google Scholar] [CrossRef]
- Zheng, L.; Ambrosetti, M.; Tronconi, E. Joule-Heated Catalytic Reactors toward Decarbonization and Process Intensification: A Review. ACS Eng. Au 2024, 4, 4–21. [Google Scholar] [CrossRef]








| Catalyst | Preparation Method | Reaction Conditions | Performance | Carbon Deposits (wt%) | Coke Formation Rate (mgC/gcat./h) | Main Type of Coke | Comments on Coke Formation | Ref. |
|---|---|---|---|---|---|---|---|---|
| 6% Ni/Al2O3 nanofiber (F) | Incipient wetness impregnation | 750 °C, 10 h, GHSV = 36 L·g−1·h−1 | XCO2 ≈ 81% XCH4 ≈ 83% | 12 | 12 | Graphitic carbon | Catalytic performance depended on alumina morphology. | [64] |
| 6% Ni/Al2O3 nanosheet (S) | Incipient wetness impregnation | 750 °C, 10 h, GHSV = 36 L·g−1·h−1 | XCO2 ≈ 83% XCH4 ≈ 85% | 6 | 6 | Graphitic carbon | Catalytic performance depended on alumina morphology. | [64] |
| 10–20% Ni/Al2O3 (COMA) | One-pot | 750 °C, 210 h, GHSV = 18 L·g−1·h−1 | XCO2 = 96% XCH4 = 99% H2/CO = 0.89 | 5 | 0.24 | Graphitic carbon | Cage-like pore structure of COMA resisted coke growth by limiting available space around Ni. | [65] |
| Ni-SCS | Solution combustion synthesis | 800 °C, 50 h, GHSV = 14.4 L·g−1·h−1 | XCO2 = 98% XCH4 = 95% H2/CO = 0.90 | 20.03 | 4 | Filamentous carbon | Higher oxygen content and oxygen defect of the Ni-SCS catalyst led to surface carbon gasification. | [66] |
| Ni/BN-NC | Impregnation | 750 °C, 100 h | XCO2 = 82% XCH4 = 72% H2/CO = 1.00 | Negligible | / | Graphitic carbon | Introducing BN support generated more oxygen vacancies. CeO2 having oxygen vacancies increased carbon gasification rate. | [67] |
| Ni/MM-A | Incipient wetness impregnation | 700 °C, 100 h, GHSV= 30 L·g−1·h−1 | XCO2 = 85% XCH4 = 75% H2/CO = 0.76 | 2.5 | 0.25 | Carbon nanotubes | The fabricated macropores were beneficial to mass transfer and then to strong coke resistance. | [68] |
| Ni/15%CeO2-MgO | Co-precipitation | 800 °C, 30 h, GHSV = 36 L·g−1·h−1 | XCO2 = 95.2% XCH4 = 93.7% H2/CO = 1.03 | Negligible | / | MWCNTs | The occurrence of Ce3+ created charge imbalance, which led to the formation of oxygen vacancies through the transition from Ce3+ to Ce4+, thereby increasing the amount of available oxygen. | [69] |
| Ni/CeO0.38Zr0.62O2-δ | Homogeneous deposition-precipitation | 750 °C, 20 h | XCO2 = 60% XCH4 = 50% H2/CO = 0.5~0.75 | 0.3 | 0.15 | Graphitic and whisker carbon | Carbon formed from the CH4 dissociation reacted with surface lattice oxygen of the catalyst support to form CO. | [70] |
| 5%Ni/F-SBA-15 | Ultrasonic impregnation | 800 °C, 50 h, GHSV = 15 L·g−1·h−1 | XCO2 = 85% XCH4 = 86% H2/CO = 1.27 | 16.97 | 3.4 | Amorphous carbon | The nickel fibrous SBA-15 catalysts showed moderate basicity, which boosted coke removal by reverse Boudouard reaction. | [71] |
| 5%Ni/DFSBA-15 | Ultrasonic impregnation | 800 °C, 50 h, GHSV = 15 L·g−1·h−1 | XCO2 = 87% XCH4 = 88% H2/CO = 0.84 | 8.08 | 1.6 | Crystalline graphite | The nickel fibrous SBA-15 catalysts showed moderate basicity, which boosted coke removal by reverse Boudouard reaction. | [71] |
| Ni3Fe1Cu1-MA | Co-precipitation | 650 °C, 20 h, GHSV = 432 L·g−1·h−1 | XCH4≈15% H2/CO≈0.5 | 5 | 2.5 | Graphitic carbon | Cu enhanced the interaction between Ni and Fe, inhibiting Fe segregation and reducing carbon deposition. | [72] |
| 20Ni/ZnS-1 | Incipient wet impregnation | 750 °C, 12 h, GHSV = 51.4 L·g−1·h−1 | XCO2 = 75.4% XCH4 = 84.5% H2/CO = 1.87 | 38.3 | 31.9 | Crystalline carbon | The stability of these catalysts was highly improved by reducing mass transfer constraints during DRM process. | [73] |
| Ni/fumed SiO2 | Pressure dilation | 700 °C, 19 h, GHSV = 1440 L·gNi−1·h−1 | XCH4 = 92% | 5.7 | 3 | Carbon nanotubes | A significant increase in Ni dispersion resulted in a higher catalyst activity and increased stability. | [74] |
| Ni/ZSM-5 | Microemulsion | 750 °C, 80 h, GHSV = 30 L·g−1·h−1 | XCO2 = 62% XCH4 = 53% H2/CO = 0.80 | 12 | 1.5 | Filamentous carbon | Catalytic activity was improved by homogenous distribution of surface acid–basic sites, thereby reducing the propensity of coke deposition. | [75] |
| Catalyst | Preparation Method | Reaction Conditions | Performance | Carbon Deposits (wt%) | Coke Formation Rate (mgC/gcat/h) | Main Type of Carbon | Comments on Coke Formation | Ref. |
|---|---|---|---|---|---|---|---|---|
| 5Ni-Ta/FZSM-5 | Microemulsion | 750 °C, 80 h, GHSV = 30 L·g−1·h−1 | XCO2 = 97% XCH4 = 91% H2/CO = 0.97 | 5 | 0.65 | Filamentous carbon | Catalytic activity was improved by homogenous distribution of surface acid–basic sites, thereby reducing the propensity of coke deposition. | [75] |
| 5Ni5Co/SiO2 | Microemulsion anti-solvent extraction | 750 °C, 25 h, GHSV = 24 L·g−1·h−1 | XCO2 = 88% XCH4 = 91% H2/CO = 0.9 | Negligible | / | / | Ni/Co ratio determined carbon–oxygen balance eliminating coking. | [123] |
| 10Ni0Co/SiO2 | Microemulsion anti-solvent extraction | 750 °C, 25 h, GHSV = 24 L·g−1·h−1 | / | 7.2 | 2.88 | Graphitic carbon | / | [123] |
| 5Ni/MSS-EG | Ethylene glycol-assisted Impregnation | 800 °C, 24 h, GHSV = 78 L·g−1·h−1 | XCO2 = 87.5% XCH4 = 90.5% H2/CO = 0.9 | 0.31 | 0.13 | Graphitic carbon | Ethylene glycol-assisted impregnation minimized Ni particle size. | [98] |
| 2.5% Ni-7.5% Co/KCC-1 | Wet impregnation | 700 °C, 8 h, GHSV = 36 L·g−1·h−1 | XCO2 = 92% XCH4 = 85% H2/CO = 0.9 | 14.5 | 18.1 | graphitic carbon | The fibrous support restricted the deposition of coke. | [124] |
| 3% Ni–9% Co/Mg(Al)O | Co-precipitation | 600 °C, 25 h, GHSV = 120L·g−1·h−1 | XCO2 = 33% XCH4 = 33% H2/CO = 0.9 | 15.5 | 6.2 | Graphitic carbon, carbon filaments | Alloying Ni with Co promoted the elimination of carbon species. | [125] |
| 7.5Ni1Cu/Al2O3 | Impregnation | 700 °C, 7 h, GHSV = 14 L·g−1·h−1 | XCO2 = 70% XCH4 = 64.5% H2/CO = 0.85 | 3.5 | 5 | Carbon nanofiber | A small amount of Cu promoted resistance to carbon deposits. | [126] |
| 10NiO−MgO | Paper assisted combustion synthesis | 700 °C, 26 h, GHSV = 14 L·g−1·h−1 | XCO2 = 95% XCH4 = 82% H2/CO = 0.85 | 3.9 | 1.5 | Carbon nanotubes | Combustion synthesis prevented sintering and lead to the formation of a smaller crystallite size. | [127] |
| Ni/Al2O3-CeO2-1.2% Gd2O3 | One pot | 800 °C, 6 h, GHSV = 27 L·g−1·h−1 | XCO2 = 94% XCH4 = 86% H2/CO =0.9 | 1.35 | 9.5 | Filamentous carbon | Gd loading influenced the reducibility of Ni by weakening the formation of NiAl2O4. | [128] |
| Ni0.8-Co0.2/ZrO2 | Impregnation | 700 °C, 80 h, GHSV = 275 L·g−1·h−1 | XCO2 = 93% XCH4 = 92.8% H2/CO = 0.78 | 5.00 | / | / | The adsorbed oxygen remaining on Co oxidized the carbon deposits on Ni, restoring the metal surface. | [129] |
| 5% Ni-0.5% Ce/MCM-41 | Capillary impregnation | 800 °C, 100 h, GHSV = 21.6 L·g−1·h−1 | XCO2 = 78% XCH4 = 70% H2/CO = 0.93 | 3 | 0.3 | Carbon nanotubes | The small amount of surface ceria provided oxygen species for the quick oxidation of carbon deposits. | [130] |
| La(Co0.1Ni0.9)0.5 Fe0.5O3 | Sol-gel self-combustion | 750 °C, 30 h, GHSV = 12 L·g−1·h−1 | XCO2 = 80% XCH4 = 70% H2/CO < 0.9 | 0.08 | 0.026 | Filamentous carbon | The synergistic effect between Ni and Co improved the anti-coking properties of the catalysts | [131] |
| La(Co0.3Ni0.7)0.5 Fe0.5O3 | Sol-gel self-combustion | 750°C, 30 h, GHSV = 12 L·g−1·h−1 | / | 0.15 | 0.05 | Filamentous carbon | / | [131] |
| Ni-Co-MOF/Al2O3 | MOF synthesis | 700 °C, 8 h, GHSV = 60 L·g−1·h−1 | XCO2 = 57% XCH4 = 43% H2/CO = 0.87 | 3.1 | 3.9 | Amorphous carbon | The metal–organic framework showed low coke deposition at low temperatures. | [132] |
| 7.5 wt% Ni-0.1Fe/Ca12Al14O33 | Impregnation | 700 °C, 60 h | XCO2 = 86% XCH4 = 90.1% H2/CO = 0.94 | 14.9 | 2.49 | Filamentous carbon | Oxygen transferred by Fe/FeO redox enhanced the removal of carbon on the Ni surface. | [133] |
| Ni4Fe1/Mg (Al)O | Coprecipitation | 700 °C, 25 h, GHSV = 60 L·g−1·h−1 | XCO2 = 86.8% XCH4 = 79.5% H2/CO = 0.85 | 0.7 | 0.28 | Graphitic carbon | NiFe alloying inhibited CH4 dissociation and promoted CO2 activation, thus contributing to the suppression of coke deposition. | [134] |
| 6Ni6Cu/MgAlO | Hydrothermal crystallization | 700 °C, 70 h, GHSV = 40 L·g−1·h−1 | XCO2 = 87.2% XCH4 = 85.2% H2/CO = 0.96 | 2.7 | 0.386 | Carbon nanotubes | The periclase phase provided abundant basic sites for the DRM and enhanced the metal–support interactions to promote the dispersion of nickel. | [135] |
| 5 wt% Ni-15 wt% Cu/CeO2 | Co-impregnation | 800 °C, 20 h, GHSV = 28.8 L·g−1·h−1 | XCO2 = 81% XCH4 = 80% H2/CO = 0.96 | 0.27 | 0.135 | Filamentous carbon | The abundance of reactive oxygen species in Ni-O-Ce solid solution resulted in great coke resistance. | [136] |
| Ni-Mo0.2/ZSM-5 | Sequential impregnation | 750 °C, 100 h, GHSV = 25 L·g−1·h−1 | XCO2 = 90% XCH4 = 90% H2/CO = 0.9 | 0.17 | 0.017 | Graphitic carbon | Mo species anchored on zeolite contributed to the variation between MoO species and oxycarbide/carbide species, enabling dynamic carbon removal. | [137] |
| Ni3.49-Mo/MgO | Autothermal combustion | 800 °C, GHSV = 60 L·g−1·h−1 | XCO2 = 100% XCH4 = 100% H2/CO = 1 | Negligible | / | / | The locking mechanism of nickel nanoparticle growth was a crucial element in resisting coking and sintering. | [27] |
| 3 wt% Ni-2 wt% In/SiO2 | Deposition-precipitation | 675 °C, 24 h, GHSV = 40 L·g−1·h−1 | XCO2 = 70% XCH4 = 30% H2/CO = 0.77 | Negligible | / | / | Ni-Ir dilution effect increased the dispersion of Ni on the catalyst surface and inhibited the formation of multi-bonded carbon species. | [138] |
| Pt25Ni75/CeO2 | Incipient wetness impregnation | 650 °C, 24 h, GHSV = 72 L·g−1·h−1 | XCO2 = 52% XCH4 = 50% H2/CO = 0.7 | 0.013 | 0.54 | Amorphous carbon | Metal–metal interactions and synergistic interactions improved the dispersion and reduction of metals and the reduction of cerium. | [139] |
| 5 wt% Ru-1 wt% Ni/MgAl2O4 | Wetness impregnation | 750 °C, 6 h, GHSV = 60 L·g−1·h−1 | XCO2 = 96% XCH4 = 93% H2/CO = 0.96 | 0.7 | 1.17 | Filamentous carbon | The addition of Ru reduced the metal Ni-support interaction dependent on the support. | [140] |
| Catalyst | Preparation Method | Reaction Conditions | Performance | Carbon Deposits | Coke Formation Rate (mgC/gcat/h) | Main Type of CARBON | Reason for Coke Resistance | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ni@SiO2 (core–shell) | Microemulsion | 650–800 °C, 50 h, GHSV = 18 L·g−1·h−1 | XCO2 = 71–90% XCH4 = 63–85% H2/CO = 1 | 0.7 | 0.14 | Amorphous carbon | High coke resistance attributed to small Ni particle size and confinement effect of shell. | [184] |
| NiCo@SiO2 (core–shell) | Microemulsion | 800 °C, 1000 h, GHSV = 300 L·g−1·h−1 | XCO2 = 88.9 XCH4 = 87.2 H2/CO = 1 | Negligible | / | / | NiCo alloy core–shell showed higher activity and selectivity than monometallic catalysts. | [185] |
| RuCo@SiO2 (core–shell) | Hydrothermal and modified Stöber | 700 °C, 10 h, GHSV = 54 L·g−1·h−1 | XCO2 = 84.7 XCH4 = 74.4 H2/CO = 0.98 | 0.5 | 0.5 | Amorphous carbon | Surface distribution of Ru and shell porosity were important factors in the DRM performance. | [186] |
| Ni-ZrO2@SiO2 (core–shell) | One-pot and microemulsion | 800 °C, 240 h, GHSV = 18 L·g−1·h−1 | XCO2 = 93.2 XCH4 = 90.5 H2/CO = 0.95 | Negligible | / | / | ZrO2 promoted reducibility of NiO, available oxygen species and increased Ni dispersion. | [187] |
| Ni@HSS (yolk–shell) | One-pot microemulsion | 800 °C, 55 h, GHSV = 144 L·g−1·h−1 | XCO2 = 95 XCH4 = 94.5 H2/CO = 0.93 | Negligible | / | / | Facile synthesis method for multiple small Ni nanoparticles anchored strongly inside silica hollow sphere. | [188] |
| Ni@NiPhy @SiO2 (core–shell) | Hydrothermal and Stöber | 700 °C, 600 h, GHSV = 36 L·g−1·h−1 | XCO2 = 95 XCH4 = 94.5 H2/CO = 0.8 | 5.5 | 0.09 | Carbon nanotubes | Confinement effect improved metal–support interaction and prevented carbon nanotube growth. | [189] |
| Ni/MgAl2O4 @SiO2 (core–shell) | Sol-gel coating | 750 °C, 10 h, GHSV = 12 L·g−1·h−1 | XCO2 = 80 XCH4 = 70 H2/CO = 1 | Negligible | / | / | Ni/MgAl2O4 prepared using solution-combustion were coated with silica, showing good coating and coke resistance. | [190] |
| @SiO2@Ni @ZrO2 (sandwich) | Sol-gel coating | 700 °C, 20 h, GHSV = 24 L·g−1·h−1 | XCO2 = 60 XCH4 = 60 H2/CO = 0.75 | Negligible | / | / | Higher binding energy of CO2 caused CO2 enrichment on surface and lowered coke formation. | [191] |
| Al2O3@Ni @Al2O3 (sandwich) | Atomic layer deposition | 800 °C, 70 h, GHSV = 300 L·g−1·h−1 | XCO2 = 95 XCH4 = 92 H2/CO = 0.75 | Negligible | / | / | Double interaction between Ni and γ-alumina support and alumina coating increased resistance to sintering and deactivation. | [192] |
| Ni-SiO2@CeO2 (core–shell) | Ammonia evaporation | 600 °C, 72 h, GHSV = 200 L·g−1·h−1 | XCO2 = 94 XCH4 = 92 H2/CO = 0.5 | Negligible | / | / | The confinement effect of the ceria shell on the Ni nanoparticles and strong redox ability of ceria contributed to the coke inhibition properties of Ni-SiO2@CeO2. | [193] |
| Ni-SiO2@SiO2 (core–shell) | Ammonia evaporation | 700 °C, 12 h, GHSV = 18 L·g−1·h−1 | XCO2 = 93 XCH4 = 72.5 H2/CO = 0.95 | Negligible | / | / | The encapsulation structure of the Ni-SiO2@CeO2 catalyst provided strong metal–support interaction that led to anti-sintering and low coke formation. | [194] |
| Ni@SiO2 (yolk–shell) | Reverse microemulsion | 700 °C, 30 h, GHSV = 48 L·g−1·h−1 | XCO2 = 80 XCH4 = 72.5 H2/CO = 0.9 | Negligible | / | / | The yolk–shell nanoreactors had excellent resistance to metal sintering and coke formation. | [195] |
| Pt0.25-NiCe @SiO2 (yolk–shell) | Reverse microemulsion + wetness impregnation | 500 °C, 20 h, GHSV = 60 L·g−1·h−1 | XCO2 = 6% XCH4 = 10% H2/CO = 0.49 | 3.5 | 1.75 | Filamentous and graphitic carbon | A synergetic combination of the confined yolk–shell morphology and Pt-Ni SAA structures prevented carbon formation and provided excellent catalyst stability. | [196] |
| In0.5Ni@SiO2 (core–shell) | One-pot micelle | 800 °C, 430 h, GHSV = 18 L·g−1·h−1 | XCO2 = 96% XCH4 = 95% H2/CO = 1.0 | 5% | 0.12 | Amorphous and graphitic carbon | In0.5Ni@SiO2 bimetallic catalysts exhibited excellent carbon resistance owing to the electronic, structural, and confinement effects of indium. | [197] |
| 6Ni6Cu/M @gAlO (sandwich) | Hydrothermal crystallization | 700 °C, 70 h, GHSV = 40 L·g−1·h−1 | XCO2 = 87.2% XCH4 = 85.2% H2/CO = 0.96 | 0.27 | 0.039 | Carbon nanotubes | The periclase phase provided abundant basic sites and enhanced the metal–support interactions to promote the dispersion of Ni. | [135] |
| 2Ni@1Mo-HSS (yolk–shell) | One-pot microemulsion | 800 °C, 72 h, GHSV = 240 L·g−1·h−1 | XCO2 = 92% XCH4 = 92% H2/CO = 0.91 | Negligible | / | / | The formation of SiMoO species promoted an increase in the number of acidic sites on the support, which helped activate methane. | [198] |
| Ni-CeO2@SiO2 (core–shell) | One-pot | 750 °C, 50 h, GHSV = 60 L·g−1·h−1 | XCO2 = 82 XCH4 = 80 H2/CO = 0.88 | 0.1 | 0.02 | Graphitic carbon | The SMSI promoted the ceria surface lattice oxygen mobility and generated more oxygen vacancies, reducing carbon deposition. | [199] |
| Ni@S-1 (core–shell) | Microemulsion and crystallization | 700 °C, 70 h, GHSV = 240 L·g−1·h−1 | XCO2 = 75 XCH4 = 75 H2/CO = 0.95 | 0.9 | 0.13 | Graphitic carbon | The core–shell structure with active metal nanoparticles encapsulated by the support shell prevented sintering and coke formation. | [200] |
| Ni@SiO2-S1 (core–shell) | Seed-directed synthesis | 700 °C, 28 h, GHSV = 750 L·g−1·h−1 | XCO2 = 80 XCH4 = 73 H2/CO = 0.9 | 0.5 | 0.18 | Graphitic carbon | The full encapsulation of Ni in the support with small Ni particle sizes and strong metal–support interactions protected Ni aggregation and inhibited coke formation. | [111] |
| Ni/Kaol (sandwich) | Wet impregnation | 750 °C, 100 h, GHSV = 6 L·g−1·h−1 | XCO2 = 60 XCH4 = 58 H2/CO = 0.86 | 5.9 | 0.59 | Graphitic carbon | Owing to the confinement and isolation effects of the kaolinite nanosheets, the Ni-Kaol catalyst maintained a high catalytic stability. | [201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Park, E.D. Recent Advances in Coke Management for Dry Reforming of Methane over Ni-Based Catalysts. Catalysts 2024, 14, 176. https://doi.org/10.3390/catal14030176
Xu Z, Park ED. Recent Advances in Coke Management for Dry Reforming of Methane over Ni-Based Catalysts. Catalysts. 2024; 14(3):176. https://doi.org/10.3390/catal14030176
Chicago/Turabian StyleXu, Zhenchao, and Eun Duck Park. 2024. "Recent Advances in Coke Management for Dry Reforming of Methane over Ni-Based Catalysts" Catalysts 14, no. 3: 176. https://doi.org/10.3390/catal14030176