Catalytic and Capacitive Properties of Hierarchical Carbon–Nickel Nanocomposites
Abstract
1. Introduction
2. Result and Discussion
2.1. Preparation of Hierarchical Graphitic Carbon–Nickel Nanocomposites
2.2. Characterization of Nanomaterials
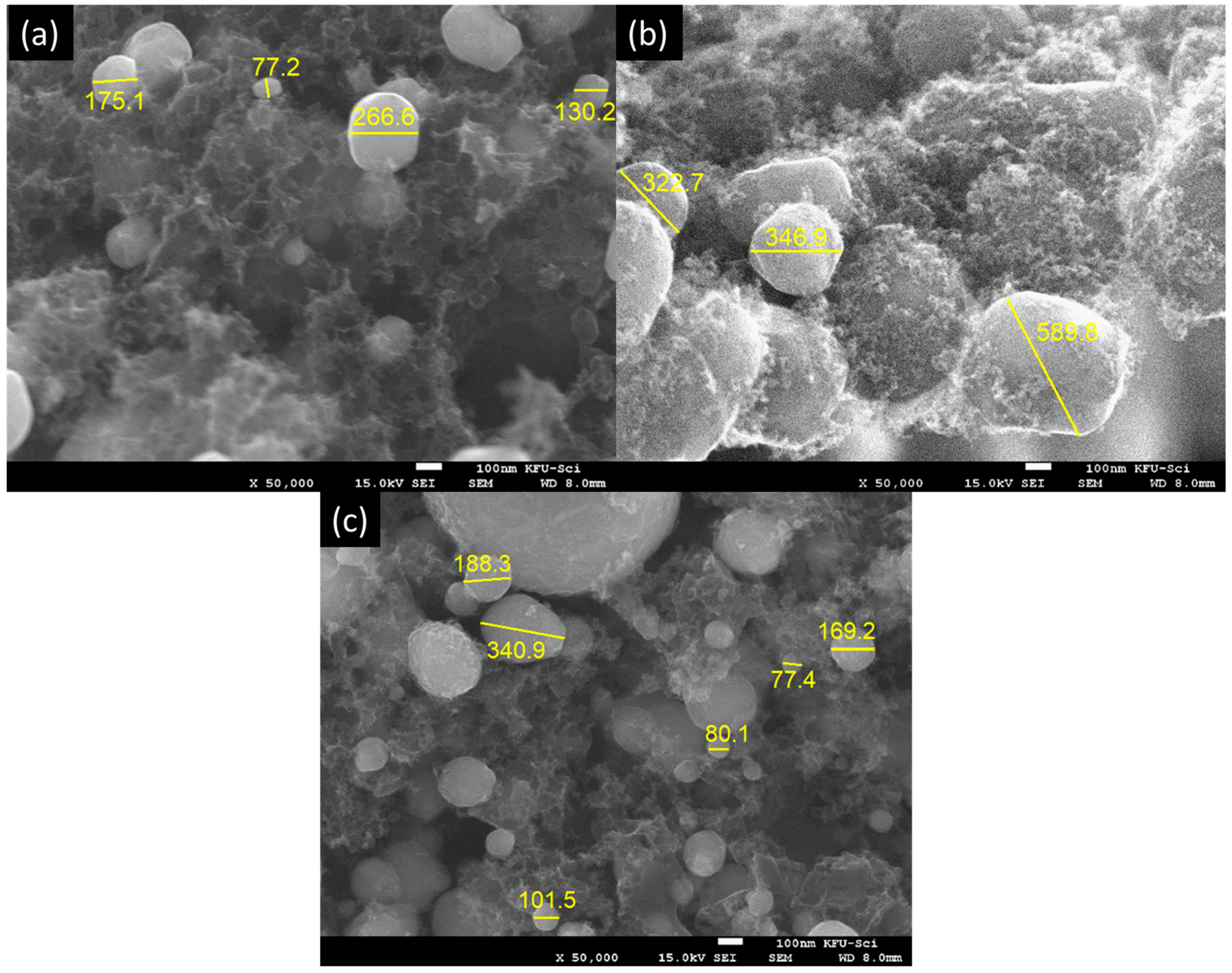
2.2.1. Scanning Electron Microscopy (SEM)
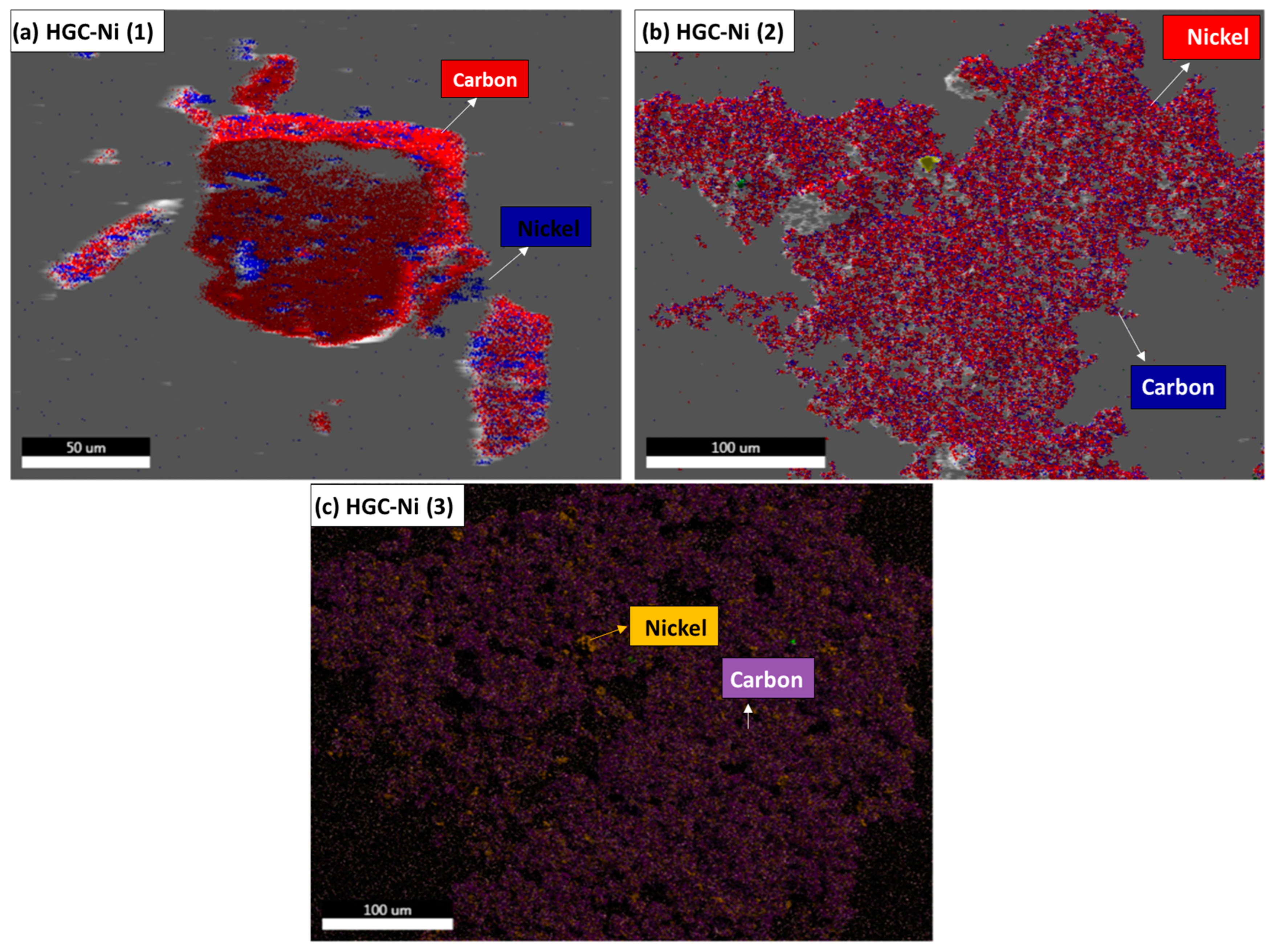
2.2.2. SEM-EDAX and Mapping
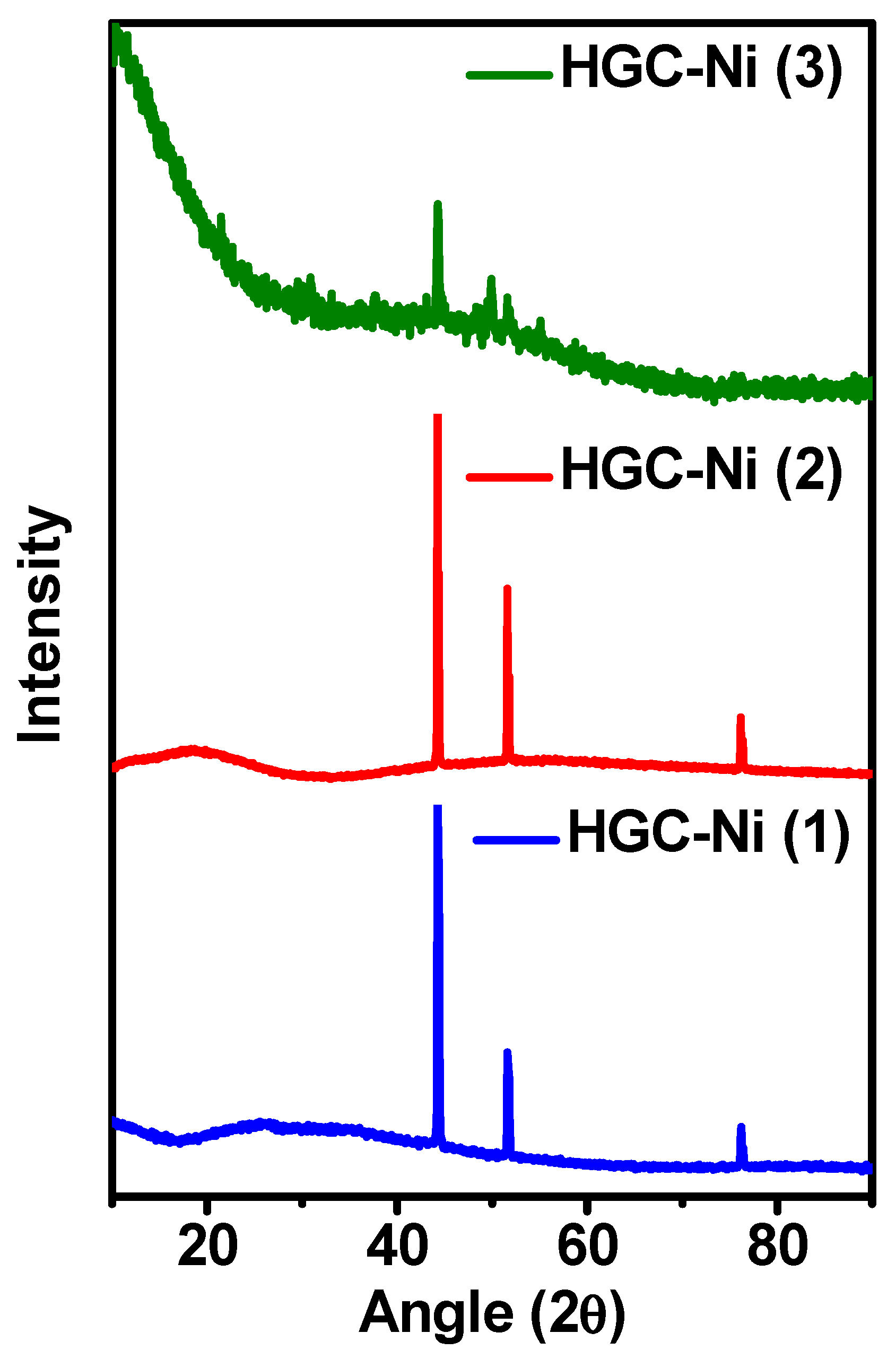
2.2.3. Powder X-ray Diffraction Analysis of the Nickel Nanocomposites
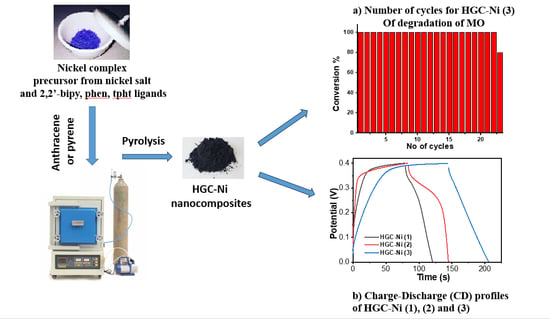
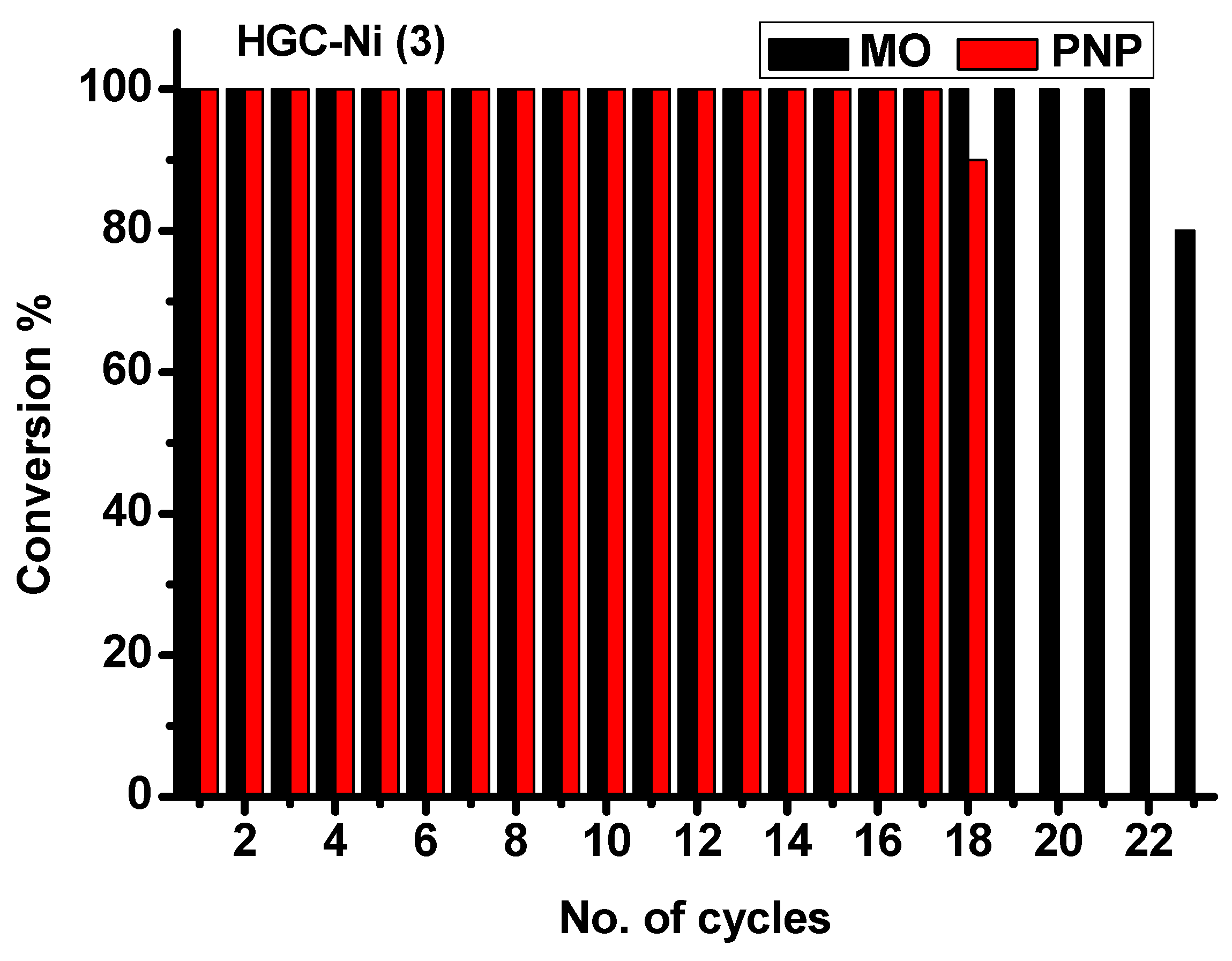
2.3. Catalytic Study
2.3.1. Determination of Catalytic Activity of HGC-Ni Nano-Catalyst for Hydrogenation of PNP and MO
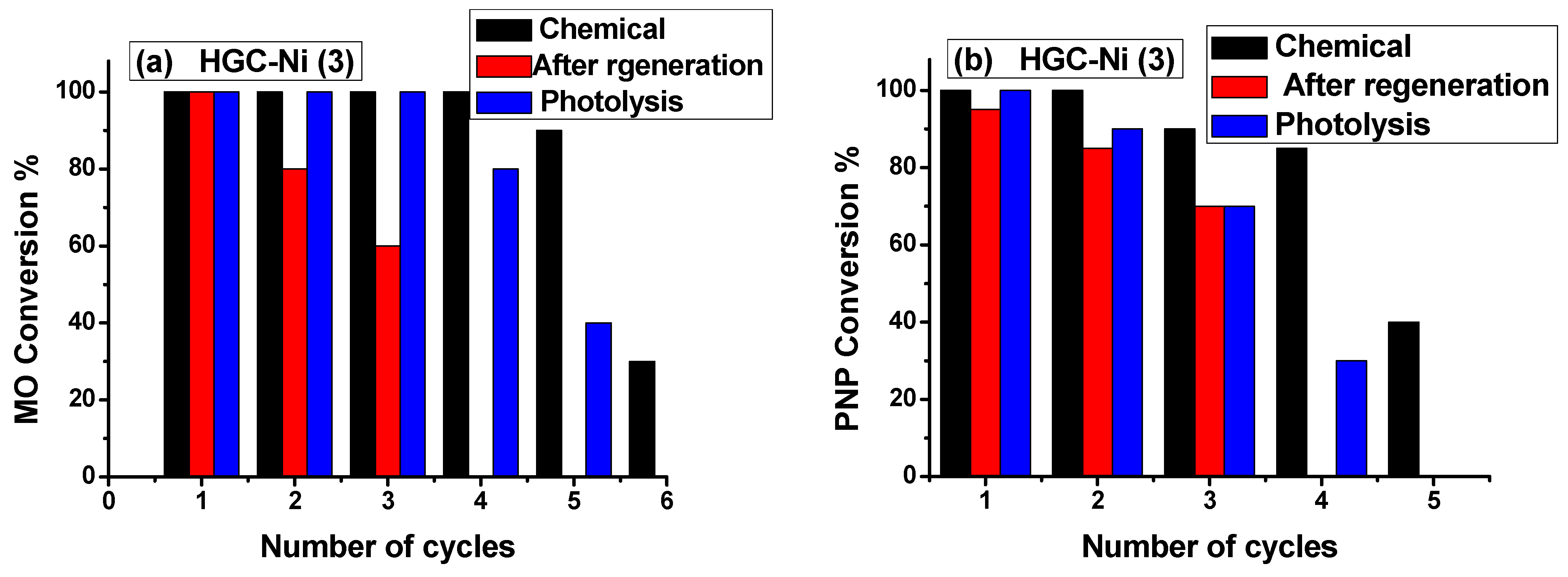
2.3.2. Batch Catalytic Experiment: Chemical, Photochemical, and Regeneration
2.3.3. Mechanism of Hydrogenation of PNP and MO by HGC-Ni Catalysts
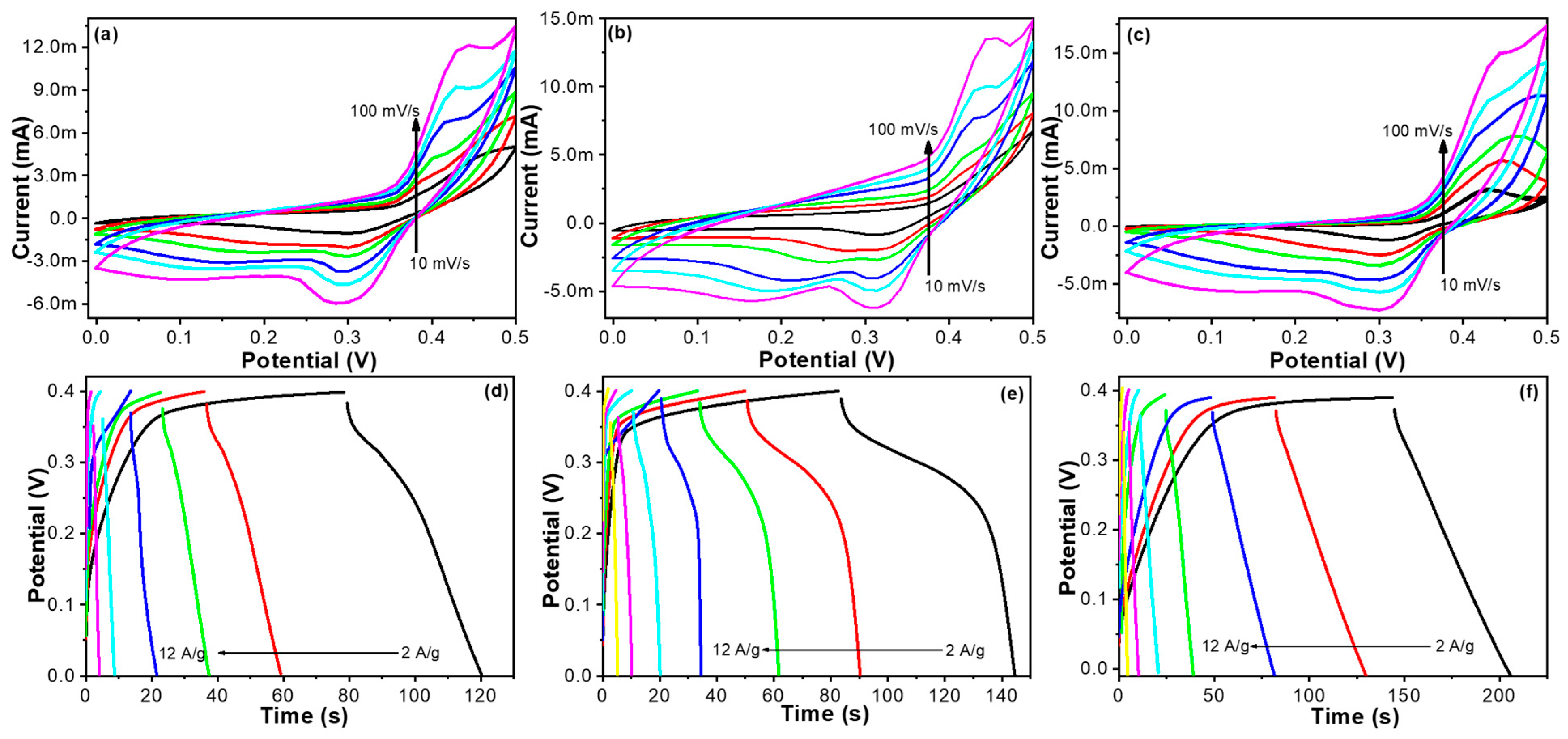
2.4. Electrochemical Properties of HGC-Ni Catalysts
3. Experimental Section
3.1. Equipment
3.2. Preparation of Metal Complexes
3.2.1. Ni(2,2′-bipyridine)Cl2.H2O
3.2.2. [Ni(tpht)(2,2′-bipy)].4H2O
3.2.3. [Ni(phen)2(H2O)2]SO4.5.6H2O
3.3. Preparation of Hierarchically Graphitic Carbon Containing Nickel Nanoparticles HGC-Ni
3.3.1. Preparation HGC-Ni (1)
3.3.2. Preparation of HGC-Ni (2)
3.3.3. Preparation of HGC-Ni (3)
3.4. Catalysis Experiment
3.4.1. Catalytic Reduction of p-Nitrophenol (P-NP)
3.4.2. Catalytic Reduction of Methyl Orange (MO)
3.4.3. Electrochemical Measurements and Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karakas, K.; Celebioglu, A.; Celebi, M.; Uyar, T.; Zahmakiran, M. Nickel nanoparticles decorated on electrospun polycaprolactone/chitosan nanofibers as flexible, highly active and reusable nanocatalyst in the reduction of nitrophenols under mild conditions. Appl. Catal. B Environ. 2017, 203, 549–562. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Mariam, E.; Krishnamurthy, S.; Sundaram, S.; Mallick, T.K. Effectual visible light photocatalytic reduction of para-nitro phenol using reduced graphene oxide and ZnO composite. Sci. Rep. 2023, 13, 9521. [Google Scholar] [CrossRef]
- Emmanuel, S.S.; Adesibikan, A.A.; Opatola, E.A.; Olawoyin, C.O. A pragmatic review on photocatalytic degradation of methyl orange dye pollutant using greenly biofunctionalized nanometallic materials: A focus on aquatic body. Appl. Organomet. Chem. 2023, 37, e7108. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Dattatraya, G.; Ganesh, R.; Fernando, L.; Ferreira, R.; Bilal, M.; Chandra, R.; Naresh, R. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Ali, N.; Khan, I.; Zhang, B.; Sadiq, M. Heterogeneous photodegradation of industrial dyes: An insight to different mechanisms and rate affecting parameters. J. Environ. Chem. Eng. 2020, 8, 104364. [Google Scholar] [CrossRef]
- Sam, S.P.; Tan, H.T.; Sudesh, K.; Adnan, R.; Ting, A.S.Y.; Ng, S.L. Phenol and p-nitrophenol biodegradations by acclimated activated sludge: Influence of operational conditions on biodegradation kinetics and responding microbial communities. J. Environ. Chem. Eng. 2021, 9, 105420. [Google Scholar] [CrossRef]
- Nekoeinia, M.; Yousefinejad, S.; Hasanpour, F.; Dezaki, M.Y. Highly efficient catalytic degradation of p-nitrophenol by Mn3O4.CuO nanocomposite as a heterogeneous fenton-like catalyst. J. Exp. Nanosci. 2020, 15, 322–336. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Song, C.; Shi, X.; Du, H. An Eco-Friendly Iron Cathode Electro-Fenton System Coupled with a pH-Regulation Electrolysis Cell for p-Nitrophenol Degradation. Front. Chem. 2022, 9, 837761. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhou, J.; Zhang, A.; Zhong, Y. Electrochemical degradation of p-nitrophenol with different processes. J. Environ. Sci. 2010, 22, 500–506. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, X.; Zhong, H.; Wang, H.; Zeng, G.; Chen, X.; Wang, H.; Zhang, L.; Shao, J. Enhanced adsorptive removal of p-nitrophenol from water by aluminum metal-organic framework/reduced graphene oxide composite. Sci. Rep. 2016, 6, 25638. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S.; Srivastava, V.C. Electro-oxidation of nitrophenol by ruthenium oxide coated titanium electrode: Parametric, kinetic and mechanistic study. Chem. Eng. J. 2015, 263, 135. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114. [Google Scholar] [CrossRef]
- Khan, A.U.; Zahoor, M.; Rehman, M.U.; Shah, A.B.; Zekker, I.; Khan, F.A.; Ullah, R.; Albdrani, G.M.; Bayram, R.; Mohamed, H.R.H. Biological Mineralization of Methyl Orange by Pseudomonas aeruginosa. J. Water 2022, 14, 1551. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Fawzy, M.E.; Nassar, H.F. Effective Chemical Coagulation Treatment Process for Cationic and Anionic Dyes Degradation, Egypt. J. Chem. 2022, 65, 301–310. [Google Scholar] [CrossRef]
- He, W.; Liu, Y.; Ye, J.; Wang, G. Electrochemical degradation of azo dye methyl orange by anodic oxidation on Ti4O7 electrodes. J. Mater. Sci. Mater. Electron. 2018, 29, 14065–14072. [Google Scholar] [CrossRef]
- Tran, V.A.; Kadam, A.N.; Lee, S.W. Adsorption-assisted photocatalytic degradation of methyl orange dye by zeolite-imidazole-framework-derived nanoparticles. J. Alloys Compd. 2020, 835, 155414. [Google Scholar] [CrossRef]
- Rajput, N.L.; Mughal, M.A.; Balouch, A.; Khan, K.M.; Tunio, S.A.; Kanwal; Sohu, S. Effective photocatalytic methylene orange dye degradation ability in coloured textile contaminated water by highly efficient catalyst Schiff-based resin-encapsulated supported on TiO2@SiO2 metal oxide nanoparticles. Int. J. Environ. Anal. Chem. 2022, 102, 3561–3575. [Google Scholar] [CrossRef]
- Xingu-Contreras, E.; García-Rosales, G.; García-Sosa, I.; Cabral-Prieto, A. Degradation of methyl orange using iron nanoparticles with/without support at different conditions. Microporous Mesoporous Mater. 2020, 292, 109782. [Google Scholar] [CrossRef]
- Khan, S.A.; Bakhsh, E.M.; Akhtar, K.; Khan, S.B. A template of cellulose acetate polymer-ZnAl/C layered double hydroxide composite fabricated with Ni NPs: Applications in the hydrogenation of nitrophenols and dyes degradation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 241, 118671. [Google Scholar] [CrossRef]
- Xia, J.; He, G.; Zhang, L.; Sun, X.; Wang, X. Hydrogenation of nitrophenols catalyzed by carbon black-supported nickel nanoparticles under mild conditions. Appl. Catal. B 2016, 180, 408–415. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, M.O.; Ansari, S.A.; Kumar, P. Nanostructured titanium nitride and its composites as high-performance supercapacitor electrode material. J. Nanomater. 2023, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Alsulaim, G.M.; Alsharif, S.A.; Almutairi, H.H.; Alali, H.A.; Ansari, S.A.; Ahmad, M.M. Renewable biopolymer-derived carbon–nickel oxide nanocomposite as an emerging electrode material for energy storage applications. J. Sci. Adv. Mater. Dev. 2023, 8, 100591. [Google Scholar] [CrossRef]
- Al-Abawi, B.T.; Parveen, N.; Ansari, S.A. Controllable synthesis of sphere-shaped interconnected interlinked binder-free nickel sulfide@nickel foam for high-performance supercapacitor applications. Sci. Rep. 2022, 12, 14413. [Google Scholar] [CrossRef] [PubMed]
- Hammud, H.H.; Karnati, R.K.; Alotaibi, N.; Hussain, S.G.; Prakasam, T. Cobalt–carbon silica nanoparticles for uptake of cationic and anionic dye from polluted water. Molecules 2021, 26, 7489. [Google Scholar] [CrossRef]
- Alramadhan, S.A.; Hammud, H.H. Graphene Nickel Silica Supported Nanocomposites, Application in Water Treatment. J. Appl. Nanosci. 2020, 11, 273–291. [Google Scholar] [CrossRef]
- Ng, S.C.; Brockhouse, B.N.; Hallman, E.D. Characterization of large alloy single crystals by neutron diffraction. Mater. Res. Bull. 1967, 2, 69–73. [Google Scholar] [CrossRef]
- Hammud, H.H.; Traboulsi, H.; Karnati, R.K.; Hussain, S.G.; Bakir, E. Hierarchical Graphitic Carbon-Encapsulating Cobalt Nanoparticles for Catalytic Hydrogenation of 2,4-Dinitrophenol. Catalysts 2022, 12, 39. [Google Scholar] [CrossRef]
- Bukhamsin, H.A.; Hammud, H.H.; Awada, C.; Prakasam, T. Catalytic Reductive Degradation of 4-Nitrophenol and Methyl orange by Novel Cobalt Oxide Nanocomposites. Catalysts 2024, 14, 89. [Google Scholar] [CrossRef]
- Hammud, H.H.; Traboulsi, H.; Karnati, R.K.; Bakir, E. Photodegradation of Congo Red by Modified P25-Titanium Dioxide with Cobalt-Carbon Supported on SiO2 Matrix, DFT Studies of Chemical Reactivity. Catalysts 2022, 12, 248. [Google Scholar] [CrossRef]
- Delmas, J.; Laversenne, L.; Rougeaux, I.; Capron, P.; Garron, A.; Bennici, S.; Swierczynski, D.; Auroux, A. Improved hydrogen storage capacity through hydrolysis of solid NaBH4 catalysed with cobalt boride. Int. J. Hydrog. Energy 2011, 36, 2145–2153. [Google Scholar] [CrossRef]
- Dorraj, M.; Sadjadi, S.; Heravi, M.M. Pd on poly(1-vinylimidazole) decorated magnetic S-doped grafitic carbon nitride: An efficient catalyst for catalytic reduction of organic dyes. Sci. Rep. 2020, 10, 13440. [Google Scholar] [CrossRef]
- Tchieno, F.M.M.; Tonle, I.K. p-Nitrophenol determination and remediation: An overview. Rev. Anal. Chem. 2018, 37, 20170019. [Google Scholar] [CrossRef]
- Elder, R.L. Final report on the safety assessment of p-aminophenol, m-aminophenol, and o-aminophenol. J. Am. Coll. Toxicol. 1988, 7, 279–333. [Google Scholar]
- Sakir, M.; Onses, M.S. Solid substrates decorated with Ag nanostructures for the catalytic degradation of methyl orange. Results Phys. 2019, 12, 1133–1141. [Google Scholar] [CrossRef]
- Khan, S.B.; Ismail, M.; Bakhsh, E.M.; Asiri, A.M. Design of simple and efficient metal nanoparticles templated on ZnO-chitosan coated textile cotton towards the catalytic reduction of organic pollutants. J. Ind. Text. 2022, 51 (Suppl. S1), 1703S–1728S. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Seo, K.M.; Kim, S.H.; Ansari, S.A. Critical aspects of various techniques for synthesizing metal oxides and fabricating their composite-based supercapacitor electrodes: A review. J. Nanomater. 2022, 12, 1873. [Google Scholar] [CrossRef] [PubMed]
- Abdelbasir, S.M.; Attia, S.Y.; Mohamed, S.G.; El-Hout, S.I. Waste-derived Ni/C composite material for supercapacitor applications. J. Energy Storage 2023, 65, 107332. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Deng, S.; Liu, J.; Qi, N.; Chen, Z. Metal–organic framework derived NiO/Ni@C composites as electrode materials with excellent electrochemical performance for supercapacitors. J. Energy Storage 2021, 37, 102426. [Google Scholar] [CrossRef]
- Brewer, B.; Brooks, N.R.; Abdul-Halim, S.; Sykes, A.G. Differential metathesis reactions of 2,2’-bipyridine and 1,10-phenanthroline complexes of cobalt(II) and nickel(II): Cocrystallization of ionization isomers {[cis-Ni(phen)2(H2O)2][cis-Ni(phen)2(H2O)Cl]}(PF6)3·4.5H2O, and a synthetic route to asymmetric t. J. Chem. Crystallogr. 2003, 33, 651–662. [Google Scholar] [CrossRef]
- Rogan, J.; Poleti, D.; Karanović, L.; Bogdanović, G.; Biré, A.S.-D.; Petrović, D.M.; Co, M.L. Ni(II) and Cu(II) complexes containing terephthalato ligands. Crystal structures of diaqua(2,2′-dipyridylamine)(terephthalato)metal(II) trihydrates (metal = cobalt or nickel). J. Polyhedron. 2000, 19, 1415–1421. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, L.H.; Liu, B.Y.; An, Z. Crystal structure of diaquabis(1,10-phenanthroline)nickel(II) sulfate hydrate (1:5.6), [Ni(H2O)2(C12H8N2)2][SO4]·5.6H2O. Z. Kristallogr. NCS 2006, 221, 451–452. [Google Scholar] [CrossRef]

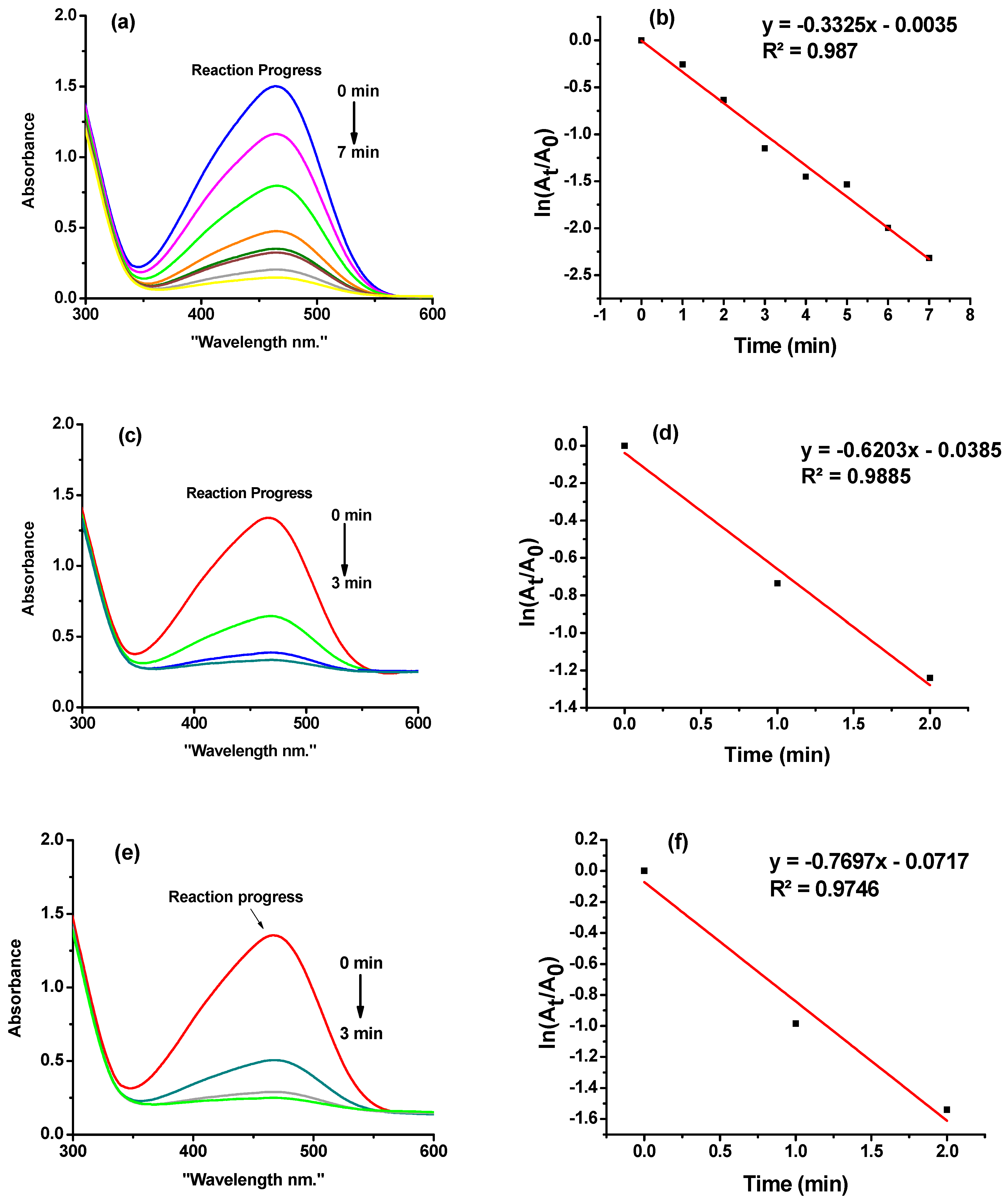




| Sample | PNP mL (mg) | No. of Cycles | Duration of All Cycles (min) | First-Order Rate Constant k min−1 (R2) | TON mg PNP/mg Nano (mmol PNP/mg Nano) | TOF mg PNP/mg Nano)/min (mmol PNP/mg Nano)/min) |
|---|---|---|---|---|---|---|
| HGC-Ni (1) | 0.8 (0.224) | 7.63 cycles | 109 min | 0.141 (0.97) | 1.0682 (0.0077) | 0.0098 (7.045 × 10−5) |
| HGC-Ni (2) | 0.5 (0.14) | 4.58 cycles | 68 min | 0.104 (0.871) | 0.6412 (0.0046) | 0.0094 (6.778 × 10−5) |
| HGC-Ni (3) | 1.8 (0.504) | 17.9 cycles | 325 min | 0.173 (0.98) | 2.4100 (0.017) | 0.0074 (5.33 × 10−5) |
| Sample | MO mL (mg) | No. of Cycles | Duration of All Cycles (min) | Rate Constant k min−1 (R2) | TON mg MO/mg Nano (mmol MO/mg nano) | TOF mg MO/mg Nano)/min (mmol MO/mg Nano)/min) |
|---|---|---|---|---|---|---|
| HGC-Ni (1) | 0.7 (0.896) | 13.9 cycles | 128 min | 0.404 (0.956) | 4.448 (0.0134) | 0.0347 (1.0616 × 10−4) |
| HGC-Ni (2) | 0.95 (1.23) | 18.8 cycles | 148 min | 0.365 (0.945) | 5.6928 (0.0174) | 0.0385 (1.175 × 10−4) |
| HGC-Ni (3) | 1.15 (1.47) | 22.8 cycles | 220 min | 0.363 (0.934) | 7.3024 (0.0223) | 0.0332 (1.014 × 10−4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammud, H.H.; Aljamhi, W.A.; Al-Hudairi, D.E.; Parveen, N.; Ansari, S.A.; Prakasam, T. Catalytic and Capacitive Properties of Hierarchical Carbon–Nickel Nanocomposites. Catalysts 2024, 14, 181. https://doi.org/10.3390/catal14030181
Hammud HH, Aljamhi WA, Al-Hudairi DE, Parveen N, Ansari SA, Prakasam T. Catalytic and Capacitive Properties of Hierarchical Carbon–Nickel Nanocomposites. Catalysts. 2024; 14(3):181. https://doi.org/10.3390/catal14030181
Chicago/Turabian StyleHammud, Hassan H., Waleed A. Aljamhi, Dolayl E. Al-Hudairi, Nazish Parveen, Sajid Ali Ansari, and Thirumurugan Prakasam. 2024. "Catalytic and Capacitive Properties of Hierarchical Carbon–Nickel Nanocomposites" Catalysts 14, no. 3: 181. https://doi.org/10.3390/catal14030181
APA StyleHammud, H. H., Aljamhi, W. A., Al-Hudairi, D. E., Parveen, N., Ansari, S. A., & Prakasam, T. (2024). Catalytic and Capacitive Properties of Hierarchical Carbon–Nickel Nanocomposites. Catalysts, 14(3), 181. https://doi.org/10.3390/catal14030181