Abstract
In this work, a coupling system consisting of bismuth vanadate (BiVO4) and cobalt-based polyoxometalates (Co-POMs) was developed to enhance the oxygen evolution reaction. Crystallization-driven self-assembly and the wet chemical synthesis method were deployed in synthesizing Co-POMs and monoclinic–tetragonal mixed–phase BiVO4, respectively. The introduction of Co-POMs into a BiVO4-containing mixture significantly enhanced the water oxidation reaction, with a more than twofold increment in the total amount of oxygen evolved. For instance, 461.2 µmol of oxygen was evolved from the system containing 20 mg of Co-POMs compared to 195 µmol of oxygen produced from a pristine BiVO4 system. This extraordinary improvement in the oxygen evolution reaction indicates the existence of a positive synergic effect between BiVO4 and Co-POMs, in which Co-POMs could act as effective cocatalysts to extract photogenerated charge carriers generated by BiVO4 and improve the charge transfer process. However, the amount of oxygen produced was slightly reduced to 440.7 µmol with an increase in AgNO3 loading from 30 mg to 60 mg. This unforeseen phenomenon could be elucidated by the shielding effect of silver particles, in which a higher AgNO3 loading led to a more prominent shielding effect. The presence of silver nanoparticles on post-reaction BiVO4 was confirmed by TEM and XPS analysis. This newly established process scheme provides an insight into the development of an efficient photocatalytic oxygen evolution system in realizing future commercial applications toward green energy production.
1. Introduction
Solar hydrogen generation is widely considered as one of the most auspicious approaches to overcome the energy crisis. Catalysis is a common method that is deployed in water splitting reactions; the coupled water oxidation reaction is often regarded as a kinetic bottleneck due to its sluggish kinetic and complex multistep electron transfer process [1]. To date, a variety of semiconductors have been explored, which have the ability to oxidize water under light illumination, such as TiO2 [2,3], BiVO4 [4,5], Fe2O3 [6,7,8], ZrO2 [9], ZnO [10] and WO3 [11]. Among these, the inexpensive and non-toxic mixed-metal oxide of BiVO4 has gained popularity in the field of light-induced catalytic reaction and has been intensively researched because of its superior material properties. In contrast to most semiconductors which are only active within the UV light range, BiVO4 is active towards visible–light irradiation. In addition, it has with high stability and its band gap is appropriate for the catalyzation of water oxidation reactions [4]. However, BiVO4 often suffers from the rapid recombination of photogenerated electron–hole pairs and sluggish water oxidation kinetics, which negatively impact its catalytic performance and lower its overall efficiency [12]. The introduction of a cocatalyst is one of the effective strategies that has been deployed to improve the overall catalytic activity of BiVO4. For instance, Liu et al. [13] and Lee et al. [14] reported the effectiveness of rhodium and gold-based cocatalysts in boosting the photocatalytic water oxidation performance of BiVO4, but the exorbitant cost of noble metals limits their potential in large-scale development and application.
POMs, a class of molecular metal-oxo cluster compounds, with structural diversity and excellent redox properties are emerging materials in the field of catalysis. POMs are made up of set of transferable building blocks, and an assortment of POMs can be produced with the addition of appropriate precursors in a suitable reaction condition. The structural diversity and flexibility of POMs enable them to perform well in diverse applications. Among these, POMs are most prevalently applied in the field of catalysis due to their ability to catalyze fast and invertible stepwise multielectron–-transfer reactions. In this regard, POMs have been proven to be able to act as robust catalysts in the simulation of various reactions, including water oxidation [15,16,17], water reduction [18,19], arsenic oxidation [20], alcohol oxidation [21], gasoline oxidative desulphurization [22] and pollutant remediation [23,24]. In addition, POMs have been widely applied in dye-sensitized solar cells [25], fuel cell [26]., rechargeable batteries [27], electrochromism [28], gas sensing [29], membrane technology [30] and the biological sector [23]. In addition to their widespread application, POMs are generally stable, easily accessible and non-toxic, which are favorable characteristics for long term application, and POMs are one step closer towards future large-scale commercialization.
In terms of photocatalytic water oxidation application, POMs are often coupled with suitable materials, particularly semiconductors, to boost their catalytic performance. In photocatalytic reactions, semiconductors and POMs commonly act photocatalysts and cocatalysts, respectively. The introduction of POMs as cocatalysts contributes to an increase in the reaction rate owing to the role of POMs in preventing the rapid recombination of photogenerated electron–hole pairs. For example, Lauinger et al. [31] illustrated the effectiveness of POMs in the enhancement of photoelectrocatalytic activity, in which the introduction of ruthenium-based POMs on TiO2 film quadrupled its photocurrent density compared to that of pristine TiO2 under UV light illumination. Although ruthenium-based POMs were among the pioneer types of POMs to be explored, their extortionate cost is not economically viable. The limitations of precious metals in long-term product development result in the need to develop alternative materials. Thus, the recent discovery of low-cost transitional metals, such as cobalt, nickel, iron and copper, as POMs building blocks are indeed important, as they are low-priced and abundant in the Earth’s crust. Ma et al. [18] divulged that the addition of copper-based POMs into a TiO2-based photocatalytic system significantly increased the quantity of generated hydrogen and improved the water reduction reaction owing to the positive synergic effect between TiO2 and POMs.
In this work, an inexpensive transition metal of cobalt was applied as a base in POMs synthesis. Both the cobalt-based POMs, Na10[Co4(H2O)2(PW9O34)2]·28H2O and BiVO4 were fabricated under mild conditions without the involvement of a strong acid and base. This synthesis technique was chosen owing to its simplicity and the involvement of minimal hazard [32]. Nascent materials were utilized to catalyze the water oxidation reaction under a visible–light illumination with the presence of sacrificial agent. This study presents an overview of the potential of the POMs/BiVO4 coupling system in the field of solar fuel production.
2. Materials and Methods
2.1. Materials Synthesis
All chemicals were of reagent grade obtained from Sigma-Aldrich, St. Louis, MO, USA and used directly without further purification unless otherwise stated. Deionized water (DI water) with resistivity of 18.2 Ω·cm was used for reagent preparation. A mild synthesis technique was deployed for the fabrication of BiVO4 nanoparticles [5]. 8.0 g polyethylene glycol 6000 (PEG 6000) was added into a beaker filled with 700 mL DI water. The solution was stirred for 5 min, followed by the addition of 0.48 g bismuth (III) nitrate pentahydrate (Bi(NO3)3·5H2O) and 0.12 g ammonium metavanadate (NH4VO3). The beaker was then sealed with aluminum foil and the resulting solution was stirred constantly with a magnetic stirrer at room temperature for 4 h. The yellowish suspension was then placed in an electronic oven at the temperature of 60 °C overnight. BiVO4 nanoparticles formed were then obtained by centrifugation and dried at 70 °C. It was followed by annealing at the temperature of 400 °C for 12 h to improve the crystallinity of BiVO4 nanoparticles.
The detailed synthesis procedure of cobalt–based POMs, Na10[Co4(H2O)2(PW9O34)2]·28H2O was reported in our previous study [33]. Typically, 6.98 g cobalt (II) nitrate hexahydrate (Co(NO3)2·6H2O), 35.62 g sodium tungstate dihydrate (Na2WO4·2H2O) and 4.30 g sodium phosphate dibasic dodecahydrate (Na2HPO4·12H2O) were dissolved in 100 mL DI water. The solution pH was adjusted to pH 7 by dripping hydrochloric acid 37% (HCl, Merck, Rahway, NJ, USA) into the mixture, subsequently it was heated at 100 °C for 2 h. The resulting heated solution was then filtered to eliminate impurities. The filtrate was left aside uninterrupted for few days until the emergence of dark purple crystals. Chemical formula of the purple crystal formed is Na10[Co4(H2O)2(PW9O34)2]·28H2O and denoted as Co-POMs.
2.2. Materials Characterization
Fourier–transform infrared spectroscopy (FTIR) spectrum was gathered using Perkin Elmer Frontier spectrometer (PerkinElmer, Waltham, MA, USA) with the sample was cold-pressed into a pallet by potassium bromide (KBr) mixing. Brunauer–Emmett-Teller (BET) specific surface area test was conducted using ASAP Tristar II 3020 (Micromeritics, Norcross, GA, USA) and analyzed by nitrogen adsorption-desorption isotherm. The sample was degassed at 90 °C overnight under vacuum condition prior BET experiment. Bruker D8 advanced diffractometer (Bruker, Billerica, MA, USA, 40 kV, 40 mA) with Cu Kα radiation and FESEM JEOL 7600F (JEOL, Tokyo, Japan) were used to conduct X-ray diffraction (XRD) and field emission scanning electron microscope (FESEM) experiment respectively. Transmission electron microscopy (TEM, JEOL, Tokyo, Japan), scanning transmission electron microscopy (STEM) and energy dispersive X-ray (EDX) analysis were performed by TEM JOEL-2100 (JEOL, Tokyo, Japan) equipped with EDX Oxford Instruments detector with an accelerating voltage of 200 kV. AXIS SUPRA with Al Kα radiation was utilized to gather the X-ray photoelectron spectroscopy (XPS, Shimadzu, Kyoto, Japan) spectrum. The obtained spectral position was corrected by using carbon as reference with the shifting of C 1s core level position to 284.4 eV and was fitted according to Shirley background subtraction.
2.3. Photocatalytic Test
The photocatalytic oxygen evolution experiment was performed in a sealed quartz flask reactor with silver nitrate (AgNO3) as a sacrificial agent. Typically, 10 mg BiVO4 and 30 mg AgNO3 were dispersed in 10 mL DI water, followed by sonication to agitate the particles homogeneously. For the BiVO4/Co-POMs coupling system, a variable quantity of Co-POMs was introduced into the reactor prior sonication. The as-prepared reaction mixture was deaerated by purging with Argon (Ar) gas for 20 min to drive away the residual oxygen. The oxygen evolution reaction was stimulated by irradiating the reaction mixture with a 300 W compact xenon lamp source (MAX-303, Asahi Spectra, Tokyo, Japan) equipped with a 420 nm cutoff filter. In gas analysis, gas chromatograph (Agilent 7890 A, Santa Clara, CA, USA) with a thermal conductivity detector and Ar carrier gas was utilized to compute the amount of oxygen produced from the photocatalytic reaction. The gas sample was taken at certain time interval and injected manually into gas chromatograph by using a gastight syringe (500 µL, Hamilton SampleLock Syringe, Reno, NV, USA). All the analysis were conducted at least in triplicates and displayed as mean ± standard deviation for data precision. The potency of the designed system towards water oxidation reaction is denoted by the amount of oxygen produced from the system.
3. Discussion
3.1. Materials Characterization
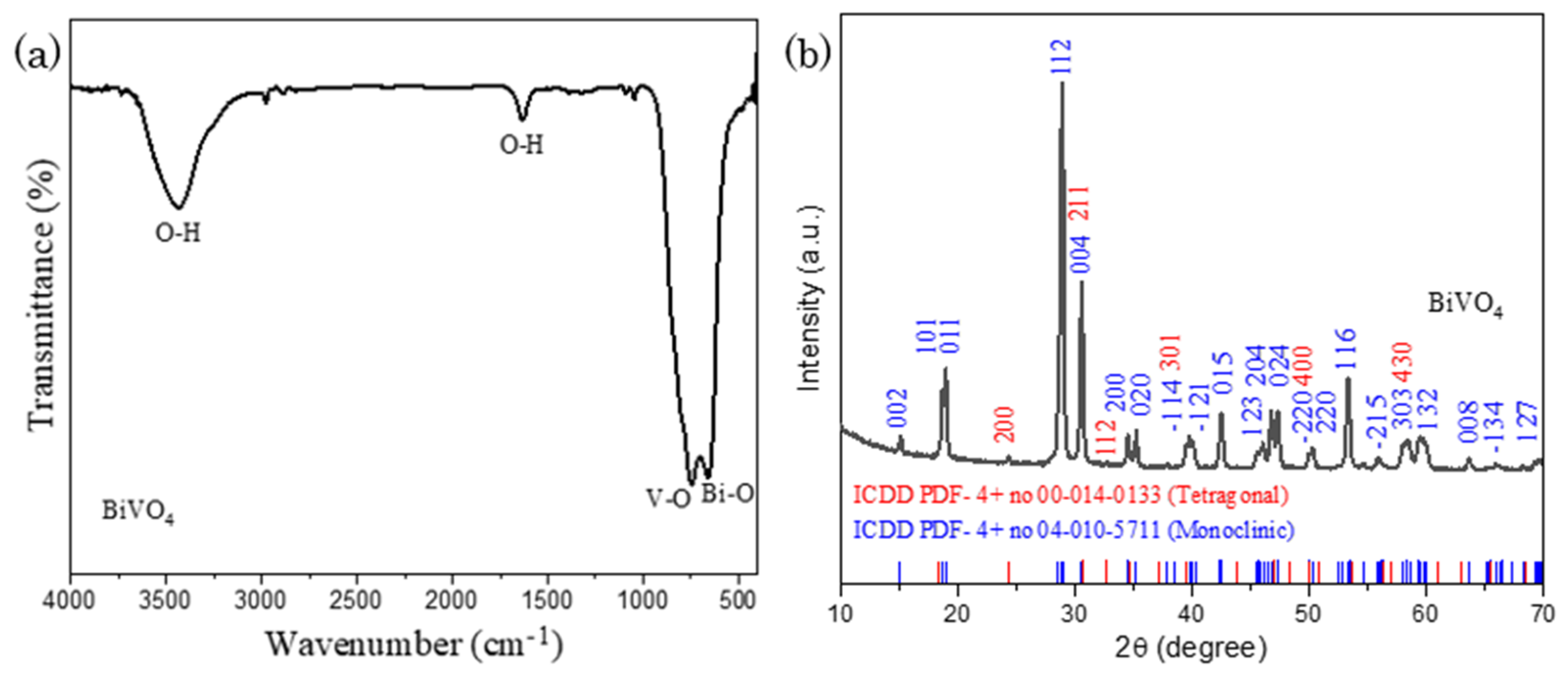
The structure of Co-POMs has been defined and well-presented in our previous study [33]. Thus, this section will solely focus on the characterization of BiVO4. Figure 1a presents FTIR spectrum of BiVO4, with the occurrence of sharp peaks at 746 cm−1 and 658 cm−1, corresponding to the V-O and Bi-O stretching vibration respectively. A minor peak at 410 cm−1 exhibits the bending mode of VO4 units. The broad peaks at 3435 cm−1 and 1632 cm−1 illustrates O-H stretching vibration of water that was adsorbed from the atmosphere during sample analysis [34,35,36]. In BET surface area study, it is worth to mention that as-fabricated BiVO4 has a relatively high BET surface area of 9.36 m2g−1 in comparison to the BiVO4 synthesized by other reported techniques such as hydrothermal and sonication that was with an average BET surface area of 4.5 m2g−1. Indeed, a high BET value is beneficial towards the improvement in catalytic activity due to the abundance of surface–active adsorption and reaction sites for photocatalysis. A larger quantity of reactant can be adsorbed on the photocatalyst and being converted into desired product via the catalytic reaction [37,38].
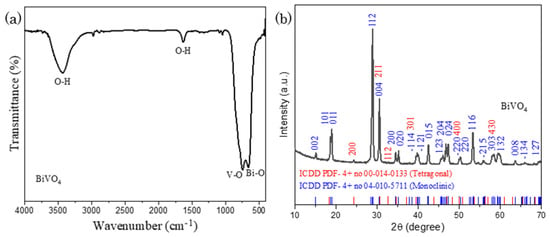
Figure 1.
(a) FTIR spectrum and (b) XRD pattern of BiVO4, showing the formation of monoclinic-tetragonal mixed phase BiVO4.
Besides, XRD experiment was carried out with a scan rate of 1°/min to examine the molecular structure of BiVO4 and gather its crystallographic data. In Figure 1b, XRD pattern with 2θ range of 10° and 70° manifests typical BiVO4 diffraction peaks which are well accordance to International Centre of Diffraction Data, ICDD (PDF-4+ no 04-010-5711; Space group = I2/b, a = 5.194 Å, b = 11.697 Å, c = 5.090 Å, α = 90.00°, β = 90.39°, γ = 90.00°), illustrating the monoclinic phase of BiVO4. Apart from that, several low signal peaks that are not well-indexed to monoclinic BiVO4 is observed in Figure 1b. A further analysis was conducted with the findings of BiVO4 second phases, in which the additional diffraction peaks are well-matched with ICDD (PDF-4+ no 00-014-0133; Space group = I41/amd, a = 7.300 Å, c = 6.457 Å), validating existence of tetragonal BiVO4. Thus, the nascent BiVO4 was proven to be a mixed phase compound, consisting of both monoclinic and tetragonal phases within its pristine structure.
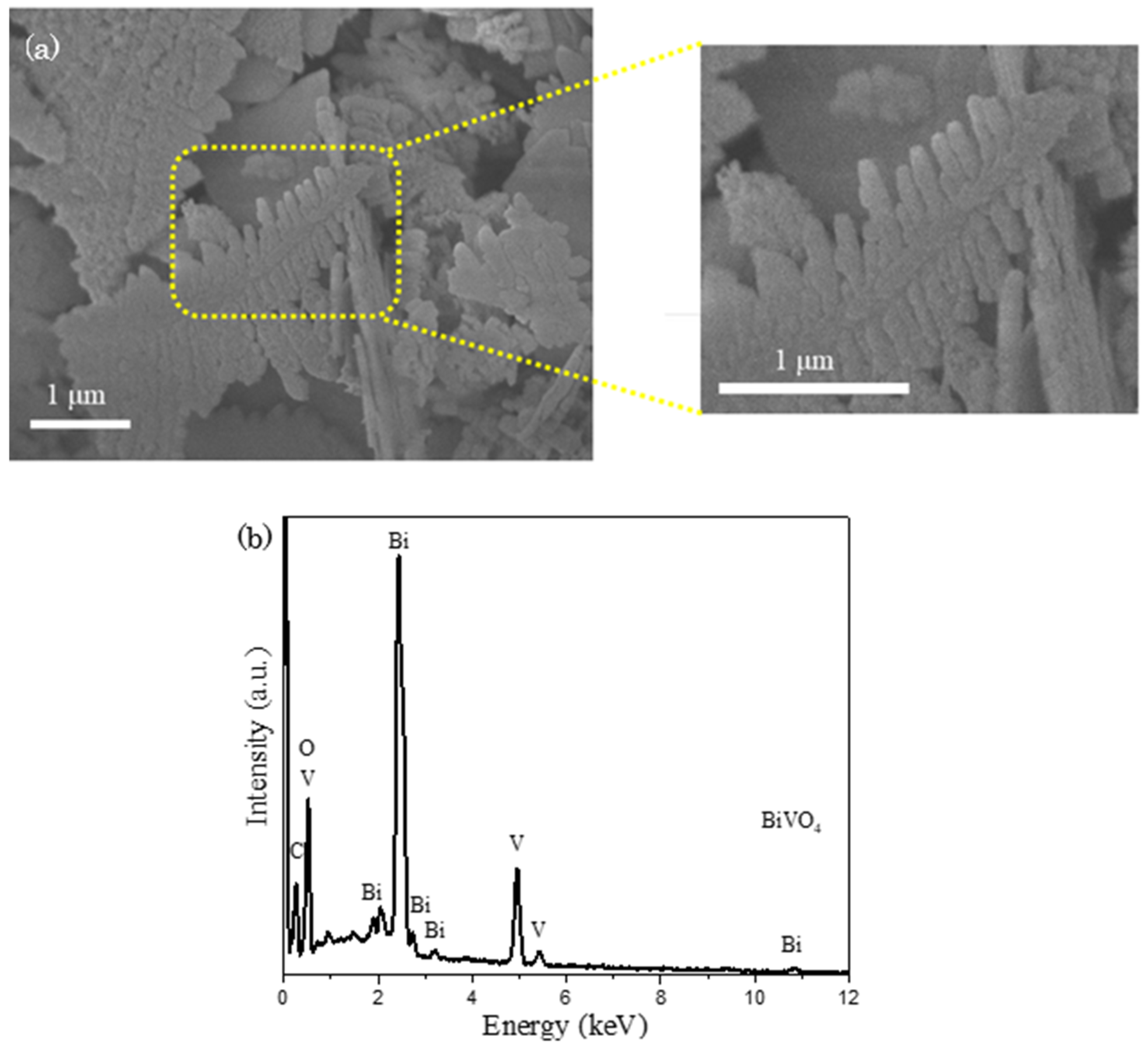
FESEM study was carried out to discern the surface morphology of as–synthesized BiVO4. In Figure 2a, a unique fishbone–like structure is noticed with an approximate dimension of 2.0 µm in length and 0.8 µm in width. The corresponded EDX spectrum in Figure 2b signifies the appearance of signals that are indexed to the elements of Bi, V and O, where the atomic percentages of Bi, V and O were quantified as 16%, 16% and 68% respectively. The gathered values were approximated to the actual BiVO4 atomic ratio of 1:1:4, suggesting the formation of BiVO4. The occurrence of additional peaks that are well-indexed to the element of C is speculated as the signals coming from carbon tape that is used to hold the sample during the experimental analysis.
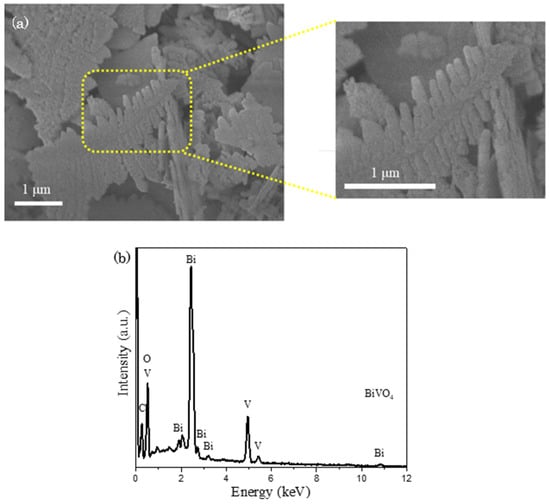
Figure 2.
(a) FESEM image with zoomed parts presents an enlarged image at the designated spot and (b) EDX spectrum of BiVO4, revealing the emergence of fishbone-like BiVO4 particle.
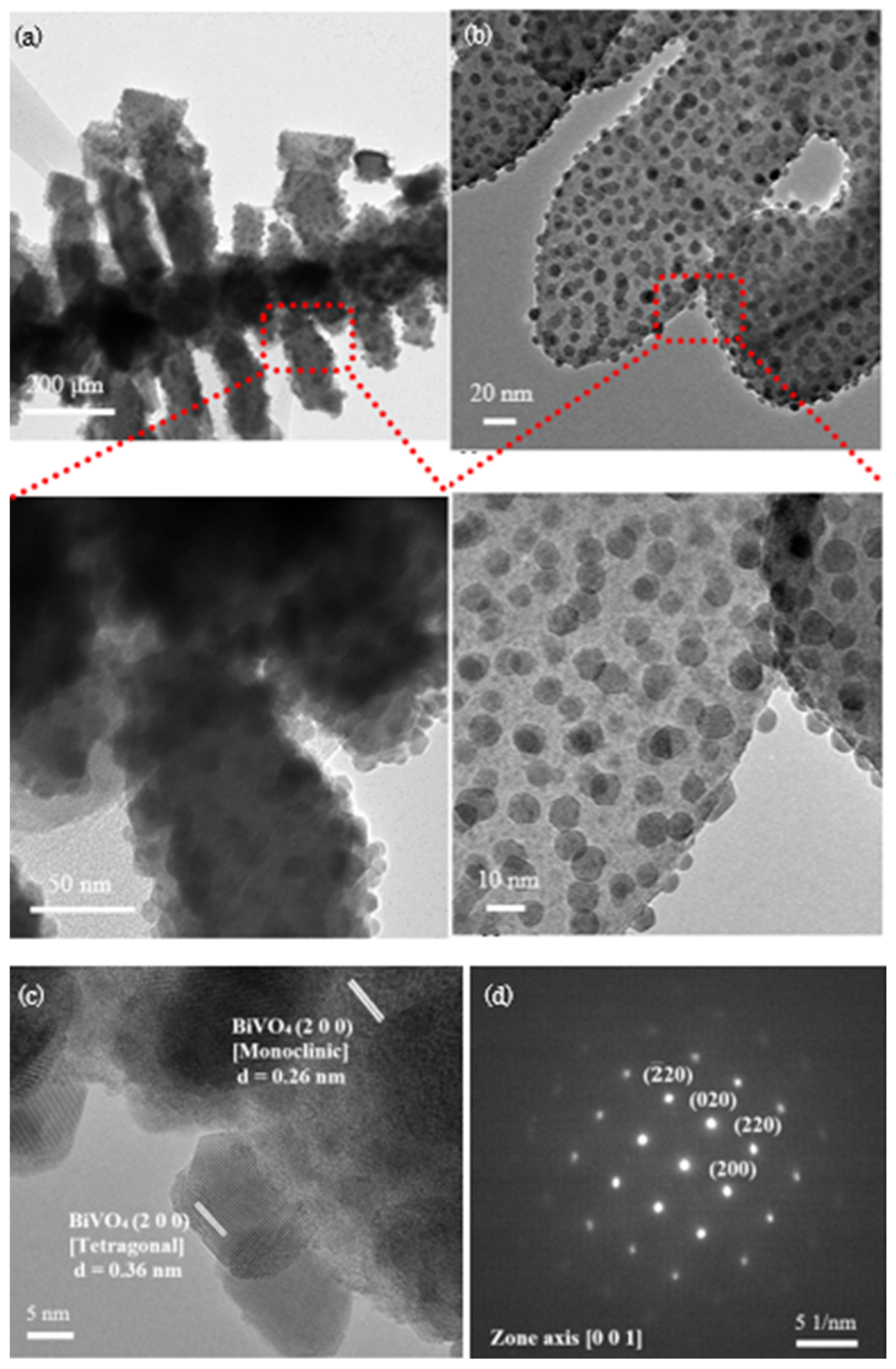
Following the observation of this distinctive structural morphology, TEM analysis was conducted to delve into the architecture of material in more detail. TEM images in Figure 3a,b reveal a fishbone–like structure BiVO4 that is well-matched with the micrograph attained by FESEM. Remarkably, the high magnification TEM images disclose the presence of small nanoparticles structure that are scattered around the main fishbone–like structure. Based on a thorough analysis as displayed in Figure 3c, the dissimilarity in BiVO4 lattice fringe values suggest the existence of different phases with the main body structure and scattered nanoparticles are scrutinized as monoclinic and tetragonal phases respectively. This finding is consistent with XRD spectrum shown in Figure 1b with the presence of both monoclinic and tetragonal phases in as–synthesized BiVO4. Selected area electron diffraction (SAED) pattern in Figure 3d that is taken along the zone axis of [0 0 1] confirms the crystallization of tetragonal BiVO4.
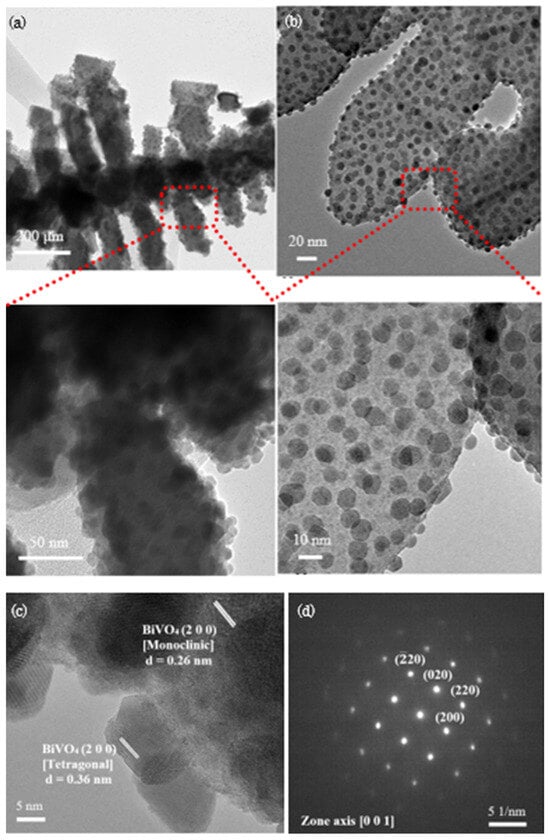
Figure 3.
(a,b) TEM images with the zoomed parts shows the enlarged image at the designated spot; (c) TEM image with the fringe spacing that matched with XRD data, illustrating the crystallization of monoclinic-tetragonal mixed phase BiVO4 and (d) SAED pattern of BiVO4.
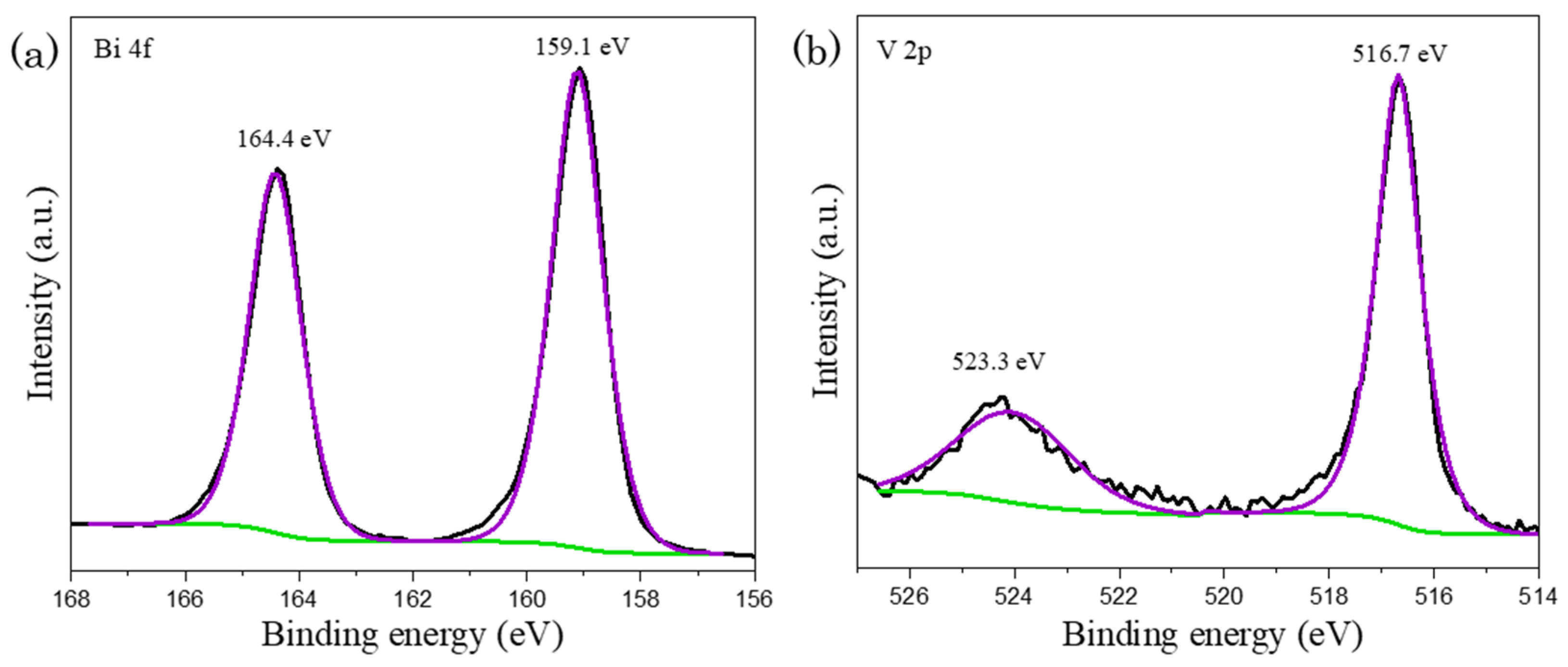
The chemical states of fabricated materials were ascertained by XPS study. In Figure 4, the occurrence of peaks at 164.4 eV and 159.1 eV in Bi 4f spectrum, which were corresponded to Bi 4f5/2 and Bi 4f7/2 indicates Bi3+ species. Besides, the signals in V 2p spectrum at the binding energy of 523.3 eV and 516.7 eV could be ascribed to V 2p1/2 and V 2p3/2 signals respectively that corresponds to V5+ state [39,40]. The presence significant peaks in both Bi 4f and V 2p XPS spectra as presented in Figure 4 illustrate the formation of BiVO4.
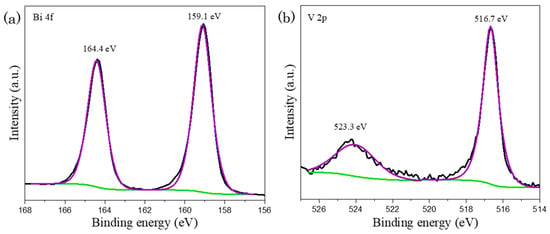
Figure 4.
XPS mapping of as-synthesized sample, proposing the formation of BiVO4 with the occurrence of peaks in (a) Bi 4f and (b) V 2p spectra, where black, purple and green lines represent raw experimental data, fitted peaks and background lines respectively.
3.2. Photocatalytic Oxygen Evolution
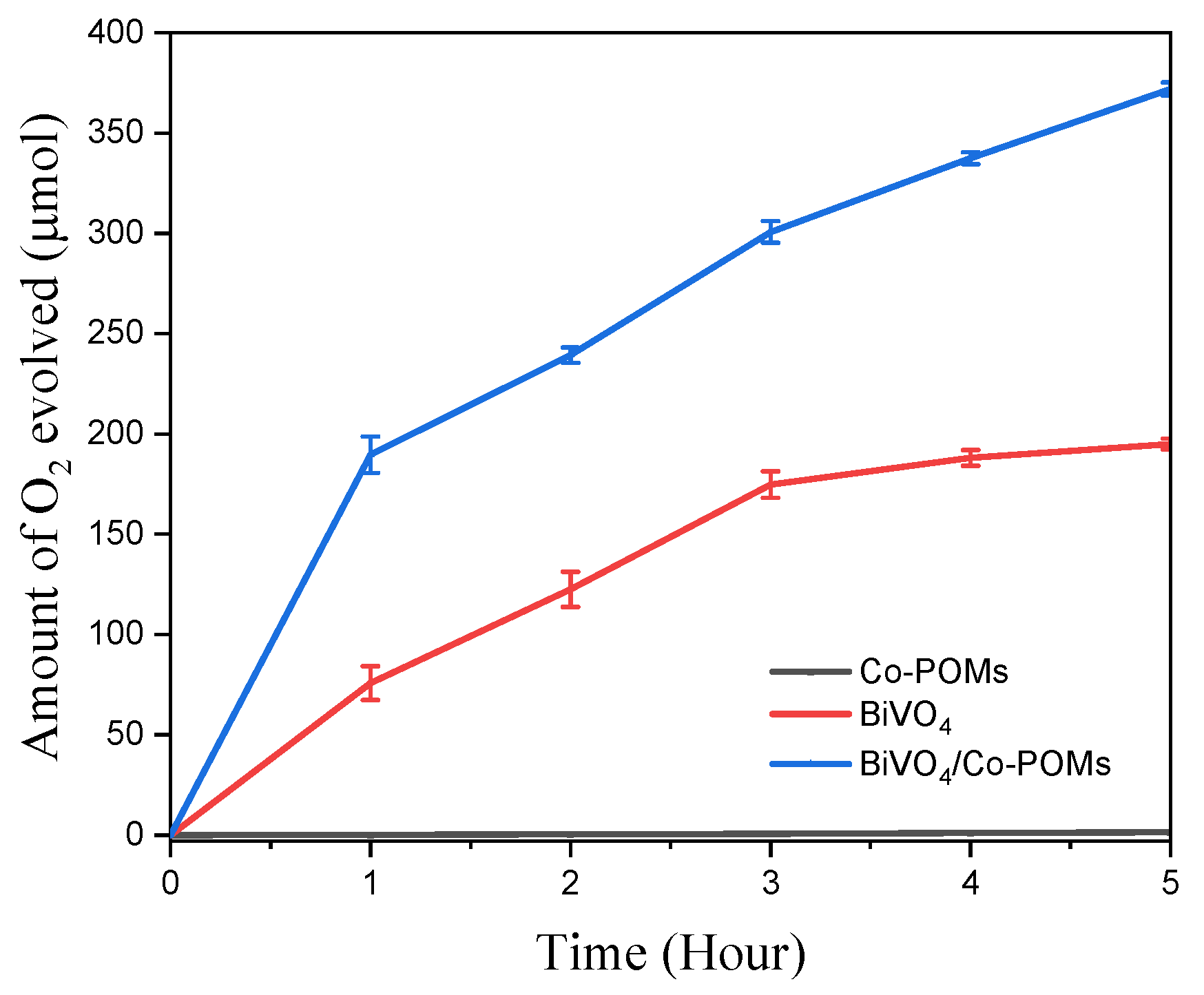
The photocatalytic activity of nascent material in the water oxidation reaction was evaluated by measuring the amount of oxygen evolved from the reaction under visible–light illumination. Oxygen gas sample was taken hourly with the analysis performed by gas chromatograph. Figure 5 depicts that Co-POMs alone was ineffective towards water oxidation reaction with no noticeable oxygen is detected after prolong reaction time. On the other hand, BiVO4 exhibited significant photocatalytic activity with 75.8 µmol oxygen was evolved in 1 h. Moreover, a remarkable amount of 195.0 µmol oxygen was generated at the end of 5 h reaction. The capability of nascent BiVO4 as an effective photocatalyst to oxidize water under visible–light illumination is on-par with the findings discerned by worldwide researchers. For instance, Thalluri et al. [41] revealed that approximately 70.0 µmol oxygen was evolved in 1 h, signifying the findings from this study was closed to the reported one. However, the catalyst was fabricated under harsh condition with the involvement of strong acid at pH 0 in contrast to the mild synthesis condition presented in this study. Indeed, harsh reaction condition is not favourable due to the experimental hazard [42,43]. The utilization of weak base instead of strong base in BiVO4 synthesis was illustrated in a work conducted by Usai et al. [44] with as-synthesized material showed prominent water oxidation reaction with the evolution of 53.0 µmol oxygen within the first hour of reaction. This finding demonstrated the potential of materials fabrication under mild conditions with the retaining of excellent catalytic activity.
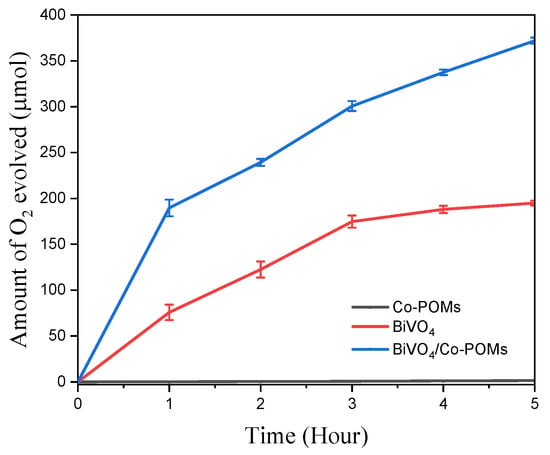
Figure 5.
Photocatalytic oxygen evolution activity per grams of catalyst under visible–light illumination with the presence of AgNO3 as sacrificial agent. The BiVO4/Co-POMs coupling system exhibits the best performance in terms of the amount of oxygen production, indicating the effectiveness of the coupling system in the water oxidation reaction.
For BiVO4/Co-POMs coupling system, 5 mg Co-POMs were introduced as cocatalyst into the BiVO4–containing system. Extraordinarily, the addition of Co-POMs showed drastic improvement towards the overall photocatalytic performance with the increases in the quantity of generated oxygen. In the first hour of reaction, 189.7 µmol oxygen has been collected from the BiVO4/Co-POMs coupling system. Furthermore, when the reaction was continued for additional hour, the amount of produced oxygen continued to rise over time, with a total of 372.0 µmol oxygen was detected at the end of 5 h. Thus, it was divulged that the oxygen produced from the novel BiVO4/Co-POMs coupling system was approximately twofold that of the pristine BiVO4 system. These promising results illustrated the existence of positive synergic effect between BiVO4 and Co-POMs. It is speculated that the role of Co-POMs as effective cocatalyst by extracting the photogenerated holes from BiVO4 before the occurrence of charge recombination.
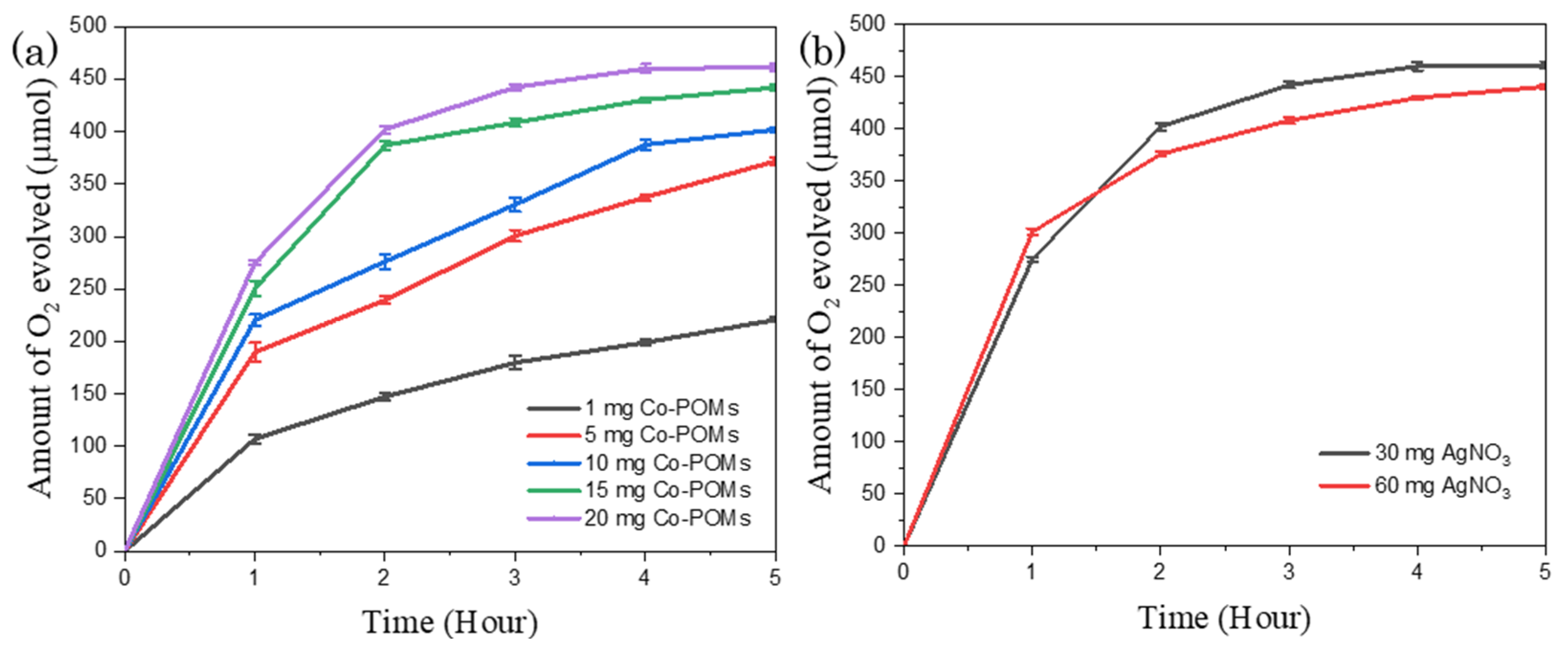
Moreover, a further study was carried out to examine the relationship between the reaction reactivity with the Co-POMs concentration. The analysed data are plotted as displayed in Figure 6a. The decrease of Co-POMs dosage from 5 mg to 1 mg for BiVO4-containing system resulted in the reduction of the amount of oxygen generation by 82.8 µmol within the first hour of reaction. A total of 220.6 µmol oxygen was gathered from 1 mg Co-POMs system towards the end of 5 h reaction, with a slight increment of 25.6 µmol oxygen compared to the pristine system without Co-POMs. This implies that 1 mg Co-POMs was insufficient to significantly increase the water oxidation activity. The photogenerated holes generated from BiVO4 were not utterly extracted by Co-POMs. On the other hand, it was discovered that the amount of produced oxygen escalated with the increase in Co-POMs concentration. For instance, 441.9 µmol oxygen was generated from 15 mg Co-POMs system. The amount of oxygen production was further increased to 461.2 µmol with 20 mg Co-POMs.
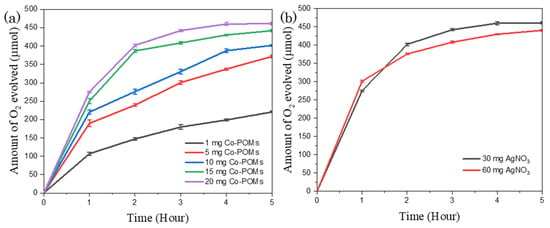
Figure 6.
Influence of (a) Co-POMs concentration and (b) AgNO3 loading in the Co-POMs-BiVO4 system towards the amount of oxygen produced, illustrating that higher Co-POMs concentration ameliorated the water oxidation reaction and high AgNO3 loading impeded the water oxidation activity of Co-POMs-BiVO4 system.
Apart from Co-POMs concentration, AgNO3 loading was varied with the results as depicted in Figure 6b. The total quantity of oxygen evolved from the 20 mg Co-POMs-BiVO4 system was slightly reduced from 461.2 µmol to 440.7 µmol oxygen with the increment of AgNO3 dosage from 30 mg to 60 mg. In the first hour of reaction, a larger amount of oxygen was generated from the system with higher AgNO3 loading owing to the presence of more electron acceptors in completing the overall water splitting reaction. However, the water oxidation reaction rate was declined beyond 2 h reaction time. This unforeseen phenomenon could be elucidated by shielding effects arise from the particles formed via the sacrificial reduction reaction, the photoreduction of AgNO3, with a higher AgNO3 loading lead to a more prominent shielding effect [45].
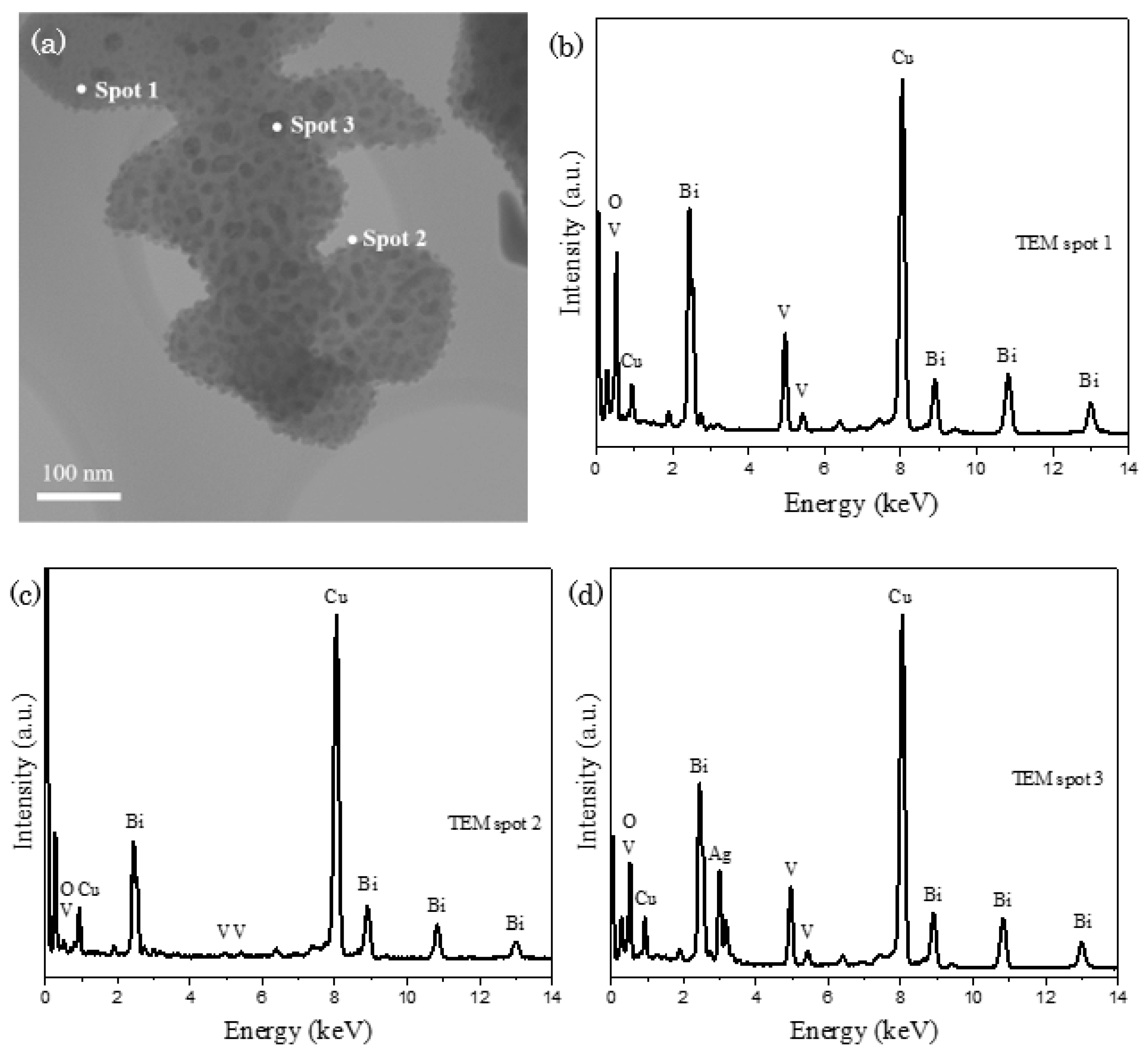
Post–reaction BiVO4 was collected and characterized to scrutinize the effect of photocatalytic reaction towards the alteration to its catalytic property. In Figure 7a, inhomogeneous distribution of unknown black spots is discerned on the surface of BiVO4. EDX analysis was conducted on several points to confirm the nature of black spots, in which spots 1, 2 and 3 in Figure 7b–d refer to the probing of EDX detector on BiVO4 main body structure, BiVO4 scattered nanoparticle and black spot respectively. The appearance of distinctive silver signals in spot 3 reveals that the black spot is corresponded to the attachment of silver-based particles or compounds on BiVO4. Besides, it is worth to mention that the signals correspond to phosphorus, cobalt and tungsten are absent in the EDX spectra, indicating that there is no bond-forming reaction between Co-POMs and BiVO4 without the deposition of Co-POMs on BiVO4.
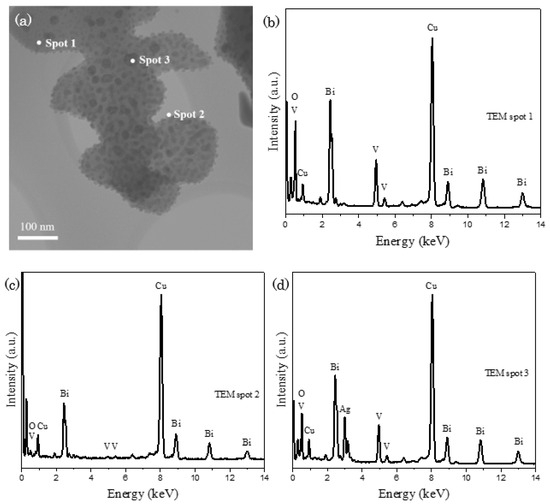
Figure 7.
(a) TEM image of post-reaction BiVO4 and (b–d) EDX spectrum, demonstrating the scattering of silver on the main body of BiVO4.
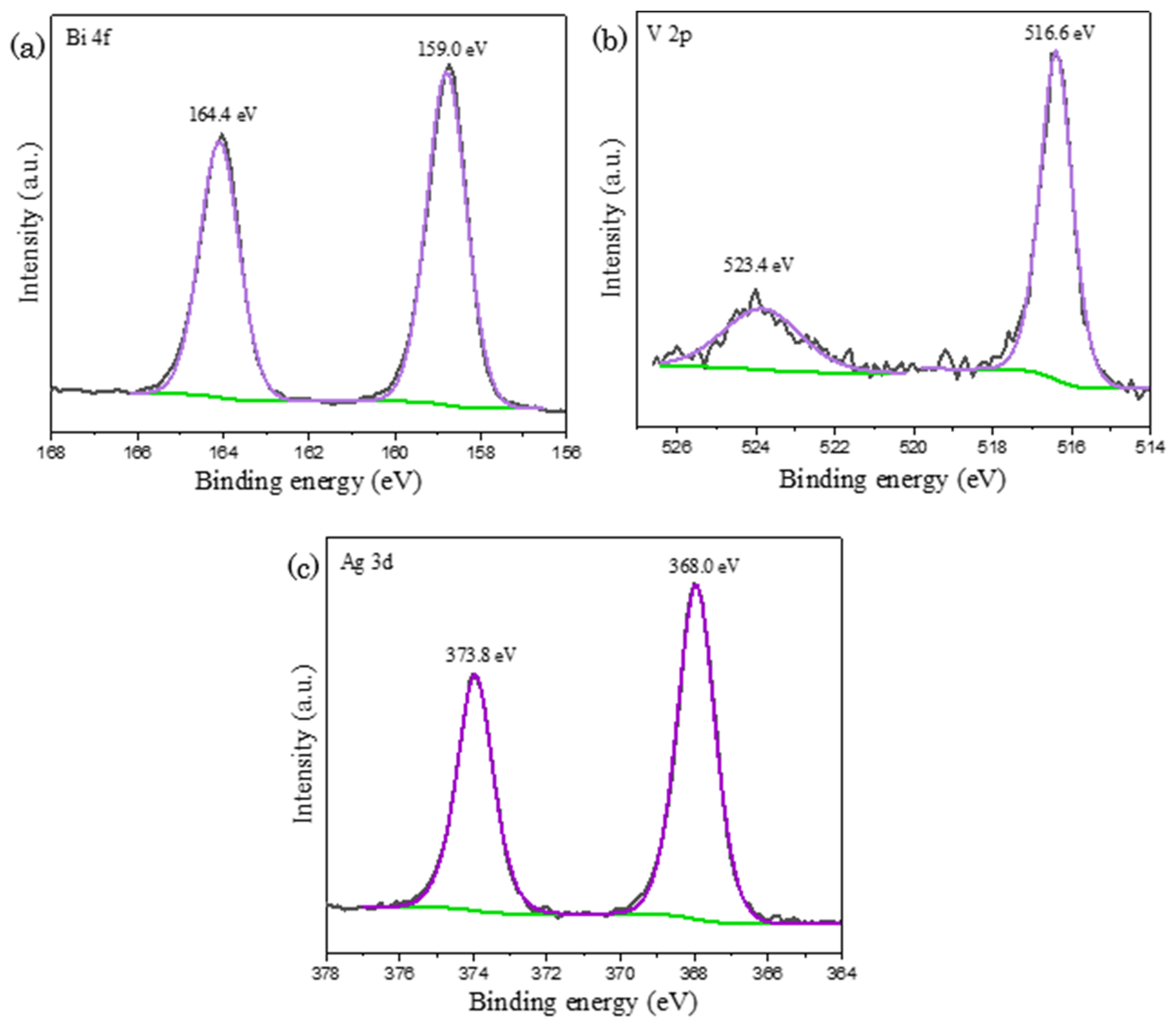
In the post–reaction XPS spectra as depicted in Figure 8, it is noticed that the catalyst’s chemical states are identical as compared to pre-reaction BiVO4 with the appearance of signals in both Bi 4f and V 2p spectra. This observation elucidates that the catalyst remains intact and does not undergo transformation upon the water oxidation reaction. In accordance with TEM EDX study, the signal corresponding to silver was detected on post-reaction BiVO4, in which it could be existed either as nanoparticles or ionic compounds. In Figure 8c, the existence of metallic Ag is confirmed by the appearance of peaks in Ag 3d spectrum at the binding energy of 373.8 eV and 368.0 eV, assigning to Ag 3d3/2 orbital and Ag 3d5/2 orbital respectively. Ag+ ions dissociated from AgNO3 was reduced into silver nanoparticles upon the acceptance of electron generated from the water oxidation reaction [46]. The deposition of silver nanoparticles on BiVO4 could conceivably explain the reduction in the overall reaction rate after prolong period. Light penetration was hindered by the suspended silver nanoparticles, causing a reduction in photocatalyst light exposure. It was accompanied with the decline in the amount of photogenerated electron–hole pairs and hence loss in the overall photocatalytic activity [45,47]. Although electron acceptors such as iron chloride, FeCl3 and cerium sulfate, Ce(SO4)2 are available in solving the issue of silver deposition, AgNO3 is still the most used sacrificial agent owing to its superior capacity to scavenge electrons than any other alternative option [48].
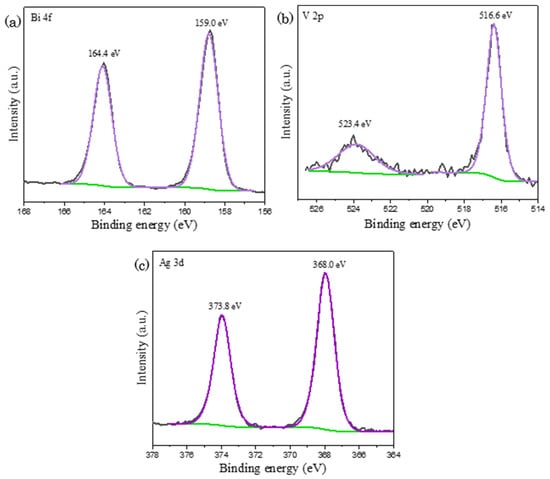
Figure 8.
XPS mapping of post-reaction BiVO4 with the occurrence of peaks in (a) Bi 4f; (b) V 2p and (c) Ag 3d spectra, indicating the deposition of silver nanoparticles on BiVO4, where black, purple and green lines represent raw experimental data, fitted peaks and background lines respectively.
4. Conclusions
This study develops an effective water oxidation system with the coexistence of Co-POMs and BiVO4 as photocatalyst and cocatalyst respectively. As-synthesized fishbone-like monoclinic-tetragonal mixed phase BiVO4 displayed significant performance in the oxygen evolution reaction with the production of 195 µmol oxygen within 5 h reaction under visible–light irradiation. However, its photocatalytic activity was limited by the fast recombination rate of photogenerated electron–hole pairs. Thus, an innovative system with the coupling of BiVO4 with Co-POMs was developed with notable enhancement in the oxygen evolution reaction, approximately twofold that of the pristine system with the absence of Co-POMs. The highest reaction activity was achieved by the system with 20 mg Co-POMs towards the generation of 461.2 µmol oxygen at the end of 5 h reaction. The presence of Co-POMs as cocatalyst has retarded the charge recombination rate with the extraction of holes by Co-POMs prior charge recombination. These promising results prove the effectiveness of BiVO4/Co-POMs coupling system in catalyzing the water oxidation rection. In conclusion, this propitious finding divulges the potential of coupling system to improve the oxygen evolution reaction and provides an insight towards innovative system design apart from as developed BiVO4/Co-POMs system.
Author Contributions
B.C.O.: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Visualization, Writing—Original draft; T.-T.L.: Supervision; C.X.: Resources, Funding Acquisition; Z.D.: Supervision, Resources, Funding Acquisition, Writing—Original Review & Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Singapore (Grant No. 2018-T1-001-077-02; Project ID 2018-T1-001-077) for the completion of this work. C. Xue thanks the support from the Ministry of Education, Singapore, under AcRF-Tier 1 (2021-T1-002-012; RG 65/21).
Data Availability Statement
The data presented in this study are available upon request from corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farooq, U.; Ahmad, T.; Naaz, F.; Islam, S.u. Review on metals and metal oxides in sustainable energy production: Progress and perspectives. Energy Fuels 2023, 37, 1577–1632. [Google Scholar] [CrossRef]
- Chen, C.; Wei, Y.; Yuan, G.; Liu, Q.; Lu, R.; Huang, X.; Cao, Y.; Zhu, P. Synergistic effect of Si doping and heat treatments enhances the photoelectrochemical water oxidation performance of TiO2 nanorod arrays. Adv. Funct. Mater. 2017, 27, 1701575. [Google Scholar] [CrossRef]
- Ghanem, M.A.; Arunachalam, P.; Amer, M.S.; Al-Mayouf, A.M. Mesoporous titanium dioxide photoanodes decorated with gold nanoparticles for boosting the photoelectrochemical alkali water oxidation. Mater. Chem. Phys. 2018, 213, 56–66. [Google Scholar] [CrossRef]
- Xi, L.; Jin, Z.; Sun, Z.; Liu, R.; Xu, L. Enhanced photoelectrocatalytic performance for water oxidation by polyoxometalate molecular doping in BiVO4 photoanodes. Appl. Catal. A Gen. 2017, 536, 67–74. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, W.; Yan, S.; Feng, J.; Zhao, Z.; Zhu, Y.; Li, Z.; Zou, Z. BiVO4 nano–leaves: Mild synthesis and improved photocatalytic activity for O2 production under visible light irradiation. CrystEngComm 2011, 13, 2500–2504. [Google Scholar] [CrossRef]
- Devi, H.R.; Ong, B.C.; Zhao, X.; Dong, Z.; Nanda, K.K.; Chen, Z. Insights into Improving Photoelectrochemical Water-Splitting Performance Using Hematite Anode. Energy Technol. 2021, 10, 2100457. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Y.; Luo, H.; Wang, W.; Liang, Q.; Chen, Z. Synthesis and photoelectrochemical properties of CoOOH/phosphorus-doped hematite photoanodes for solar water oxidation. Chem. Eng. J. 2019, 363, 23–32. [Google Scholar] [CrossRef]
- Farooq, U.; Chaudhary, P.; Ingole, P.P.; Kalam, A.; Ahmad, T. Development of cuboidal KNbO3@α-Fe2O3 hybrid nanostructures for improved photocatalytic and photoelectrocatalytic applications. Acs Omega 2020, 5, 20491–20505. [Google Scholar] [CrossRef]
- Hernández, S.; Gionco, C.; Husak, T.; Castellino, M.; Muñoz-Tabares, J.A.; Tolod, K.R.; Giamello, E.; Paganini, M.C.; Russo, N. Insights into the sunlight-driven water oxidation by Ce and Er-doped ZrO2. Front. Chem. 2018, 6, 368. [Google Scholar] [CrossRef]
- Lin, Y.G.; Hsu, Y.K.; Chen, Y.C.; Lee, B.W.; Hwang, J.S.; Chen, L.C.; Chen, K.H. Cobalt-Phosphate-Assisted Photoelectrochemical Water Oxidation by Arrays of Molybdenum-Doped Zinc Oxide Nanorods. ChemSusChem 2014, 7, 2748–2754. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, S.; Liu, C.; Li, J.; Lu, X.; Tong, Y. Facile synthesis of tungsten oxide nanostructures for efficient photoelectrochemical water oxidation. J. Power Sources 2014, 269, 98–103. [Google Scholar] [CrossRef]
- Cao, X.; Xu, C.; Liang, X.; Ma, J.; Yue, M.; Ding, Y. Rationally designed/assembled hybrid BiVO4-based photoanode for enhanced photoelectrochemical performance. Appl. Catal. B: Environ. 2020, 260, 118136. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.J.; Zhang, H.; Yuan, H.Y.; Zhang, Q.; Gu, L.; Wang, H.F.; Hu, P.; Liu, P.F.; Jiang, Z. Boosting photocatalytic water oxidation over bifunctional Rh0-Rh3+ sites. Angew. Chem. 2021, 133, 22943–22950. [Google Scholar] [CrossRef]
- Lee, M.G.; Moon, C.W.; Park, H.; Sohn, W.; Kang, S.B.; Lee, S.; Choi, K.J.; Jang, H.W. Dominance of Plasmonic Resonant Energy Transfer over Direct Electron Transfer in Substantially Enhanced Water Oxidation Activity of BiVO4 by Shape-Controlled Au Nanoparticles. Small 2017, 13, 1701644. [Google Scholar] [CrossRef]
- Han, X.-B.; Zhang, Z.-M.; Zhang, T.; Li, Y.-G.; Lin, W.; You, W.; Su, Z.-M.; Wang, E.-B. Polyoxometalate-based cobalt–phosphate molecular catalysts for visible light-driven water oxidation. J. Am. Chem. Soc. 2014, 136, 5359–5366. [Google Scholar] [CrossRef]
- Ong, B.C.; Lim, T.-T.; Dong, Z. Mixed-addenda polyoxometalates for enhanced electrochemical water oxidation. MRS Adv. 2021, 6, 588–593. [Google Scholar] [CrossRef]
- Zhu, G.; Glass, E.N.; Zhao, C.; Lv, H.; Vickers, J.W.; Geletii, Y.V.; Musaev, D.G.; Song, J.; Hill, C.L. A nickel containing polyoxometalate water oxidation catalyst. Dalton Trans. 2012, 41, 13043–13049. [Google Scholar] [CrossRef]
- Ma, K.; Dong, Y.; Zhang, M.; Xu, C.; Ding, Y. A homogeneous Cu-based polyoxometalate coupled with mesoporous TiO2 for efficient photocatalytic H2 production. J. Colloid Interface Sci. 2021, 587, 613–621. [Google Scholar] [CrossRef]
- Lv, H.; Gao, Y.; Guo, W.; Lauinger, S.M.; Chi, Y.; Bacsa, J.; Sullivan, K.P.; Wieliczko, M.; Musaev, D.G.; Hill, C.L. Cu-based Polyoxometalate Catalyst for Efficient Catalytic Hydrogen Evolution. Inorg. Chem. 2016, 55, 6750–6758. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Fang, W.; Liu, C.; Sun, Z.; Li, F.; Xu, L. Effect of mixed Mo/W polyoxometalate modification on photoelectrocatalytic activity of CdS nanocrystals for arsenic (III) oxidation. J. Phys. Chem. Solids 2020, 141, 109395. [Google Scholar] [CrossRef]
- Saini, M.K.; Gupta, R.; Parbhakar, S.; Singh, S.; Hussain, F. Lanthano-phosphotungstate: A water soluble and reusable catalyst for oxidation of alcohols using H2O2 as an oxidant. RSC Adv. 2014, 4, 38446–38449. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Oveisi, M.; Asli, M.A.N. Phosphotungestovanadate immobilized on PVA as an efficient and reusable nano catalyst for oxidative desulphurization of gasoline. J. Mol. Catal. A Chem. 2015, 410, 121–132. [Google Scholar] [CrossRef]
- Ong, B.C.; Lim, H.K.; Tay, C.Y.; Lim, T.-T.; Dong, Z. Polyoxometalates for bifunctional applications: Catalytic dye degradation and anticancer activity. Chemosphere 2022, 286, 131869. [Google Scholar] [CrossRef]
- Xu, L.-J.; Zhou, W.-Z.; Zhang, L.-Y.; Li, B.; Zang, H.-Y.; Wang, Y.-H.; Li, Y.-G. Organic–inorganic hybrid assemblies based on Ti-substituted polyoxometalates for photocatalytic dye degradation. CrystEngComm 2015, 17, 3708–3714. [Google Scholar] [CrossRef]
- Wang, S.-M.; Liu, L.; Chen, W.-L.; Su, Z.-M.; Wang, E.-B.; Li, C. Polyoxometalate/TiO2 interfacial layer with the function of accelerating electron transfer and retarding recombination for dye-sensitized solar cells. Ind. Eng. Chem. Res. 2014, 53, 150–156. [Google Scholar] [CrossRef]
- Kim, Y.; Ketpang, K.; Jaritphun, S.; Park, J.S.; Shanmugam, S. A polyoxometalate coupled graphene oxide–Nafion composite membrane for fuel cells operating at low relative humidity. J. Mater. Chem. A 2015, 3, 8148–8155. [Google Scholar] [CrossRef]
- Wang, H.; Hamanaka, S.; Nishimoto, Y.; Irle, S.; Yokoyama, T.; Yoshikawa, H.; Awaga, K. In operando X-ray absorption fine structure studies of polyoxometalate molecular cluster batteries: Polyoxometalates as electron sponges. J. Am. Chem. Soc. 2012, 134, 4918–4924. [Google Scholar] [CrossRef]
- Wang, S.-M.; Liu, L.; Chen, W.-L.; Zhang, Z.-M.; Su, Z.-M.; Wang, E.-B. A new electrodeposition approach for preparing polyoxometalates-based electrochromic smart windows. J. Mater. Chem. A 2013, 1, 216–220. [Google Scholar] [CrossRef]
- Shi, H.; Li, N.; Sun, Z.; Wang, T.; Xu, L. Interface modification of titanium dioxide nanoparticles by titanium-substituted polyoxometalate doping for improvement of photoconductivity and gas sensing applications. J. Phys. Chem. Solids 2018, 120, 57–63. [Google Scholar] [CrossRef]
- Abouzari-Lotf, E.; Jacob, M.V.; Ghassemi, H.; Ahmad, A.; Nasef, M.M.; Zakeri, M.; Mehdipour-Ataei, S. Enhancement of fuel cell performance with less-water dependent composite membranes having polyoxometalate anchored nanofibrous interlayer. J. Power Sources 2016, 326, 482–489. [Google Scholar] [CrossRef]
- Lauinger, S.M.; Sumliner, J.M.; Yin, Q.; Xu, Z.; Liang, G.; Glass, E.N.; Lian, T.; Hill, C.L. High stability of immobilized polyoxometalates on TiO2 nanoparticles and nanoporous films for robust, light-induced water oxidation. Chem. Mater. 2015, 27, 5886–5891. [Google Scholar] [CrossRef]
- Nakayama, J.; Sakamoto, J.; Kasai, N.; Shibutani, T.; Miyake, A. Preliminary hazard identification for qualitative risk assessment on a hybrid gasoline-hydrogen fueling station with an on-site hydrogen production system using organic chemical hydride. Int. J. Hydrogen Energy 2016, 41, 7518–7525. [Google Scholar] [CrossRef]
- Ong, B.C.; Chen, Z.; Lim, T.-T.; Dong, Z. Immobilization of cobalt-based polyoxometalates on titanium dioxide nanorod for enhancement in photoelectrochemical water oxidation. Mater. Chem. Phys. 2023, 301, 127690. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, J. Hydrothermal processing for obtaining of BiVO4 nanoparticles. Mater. Lett. 2009, 63, 1939–1942. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, J. Characterization of visible-light-driven BiVO4 photocatalysts synthesized via a surfactant-assisted hydrothermal method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ali, S.; Mansha, M.; Qurashi, A. Sonochemical assisted hydrothermal synthesis of pseudo-flower shaped Bismuth vanadate (BiVO4) and their solar-driven water splitting application. Ultrason. Sonochemistry 2017, 36, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, W.; Liu, S.; Zhang, L.; Xu, H.; Zhu, W. A sonochemical route to visible-light-driven high-activity BiVO4 photocatalyst. J. Mol. Catal. A Chem. 2006, 252, 120–124. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Q.; Che, Y.; Zhang, L.; Zhang, D. Characterization and photocatalytic properties of N-doped BiVO4 synthesized via a sol–gel method. J. Alloys Compd. 2013, 548, 70–76. [Google Scholar] [CrossRef]
- Han, W.; Lin, H.; Fang, F.; Zhang, Y.; Zhang, K.; Yu, X.; Chang, K. The effect of Fe(iii) ions on oxygen-vacancy-rich BiVO4 on the photocatalytic oxygen evolution reaction. Catal. Sci. Technol. 2021, 11, 7598–7607. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Zhao, W.; Wang, B. Magnetic composite photocatalyst ZnFe2O4/BiVO4: Synthesis, characterization, and visible-light photocatalytic activity. Dalton Trans. 2013, 42, 15464–15474. [Google Scholar] [CrossRef]
- Thalluri, S.M.; Suarez, C.M.; Hernández, S.; Bensaid, S.; Saracco, G.; Russo, N. Elucidation of important parameters of BiVO4 responsible for photo-catalytic O2 evolution and insights about the rate of the catalytic process. Chem. Eng. J. 2014, 245, 124–132. [Google Scholar] [CrossRef]
- Han, N.; Park, S.; Kaang, B.K.; Jang, W.; Koo, H.Y.; Choi, W.S. An Active Absorbent for Cleanup of High-Concentration Strong Acid and Base Solutions. Materials 2019, 12, 3389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Richter, S.M.; Rozema, M.J.; Schellinger, A.; Smith, K.; Napolitano, J.G. Potential safety hazards associated with using acetonitrile and a strong aqueous base. Org. Process Res. Dev. 2017, 21, 1501–1508. [Google Scholar] [CrossRef]
- Usai, S.; Obregón, S.; Becerro, A.I.; Colón, G. Monoclinic–tetragonal heterostructured BiVO4 by yttrium doping with improved photocatalytic activity. J. Phys. Chem. C 2013, 117, 24479–24484. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Toyao, T.; Miyahara, K.; Zakary, L.; Van, D.D.; Kamata, Y.; Kim, T.-H.; Lee, S.W.; Matsuoka, M. Visible-light-driven photocatalytic water oxidation catalysed by iron-based metal–organic frameworks. Chem. Commun. 2016, 52, 5190–5193. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, R.; Ma, Y.-J.; Luo, S.-L.; Au, C.-T.; Yin, S.-F. Controllable synthesis of hollow and porous Ag/BiVO4 composites with enhanced visible-light photocatalytic performance. RSC Adv. 2013, 3, 24354–24361. [Google Scholar] [CrossRef]
- Thalluri, S.M.; Hussain, M.; Saracco, G.; Barber, J.; Russo, N. Green-Synthesized BiVO4 Oriented along {040} Facets for Visible-Light-Driven Ethylene Degradation. Ind. Eng. Chem. Res. 2014, 53, 2640–2646. [Google Scholar] [CrossRef]
- Martin, D.J. Investigation into High Efficiency Visible Light Photocatalysts for Water Reduction and Oxidation; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).