Construction of a Novel Ternary GQDs/g-C3N4/ZIF-67 Photocatalyst for Enhanced Photocatalytic Carbon Dioxide Reduction
Abstract
:1. Introduction
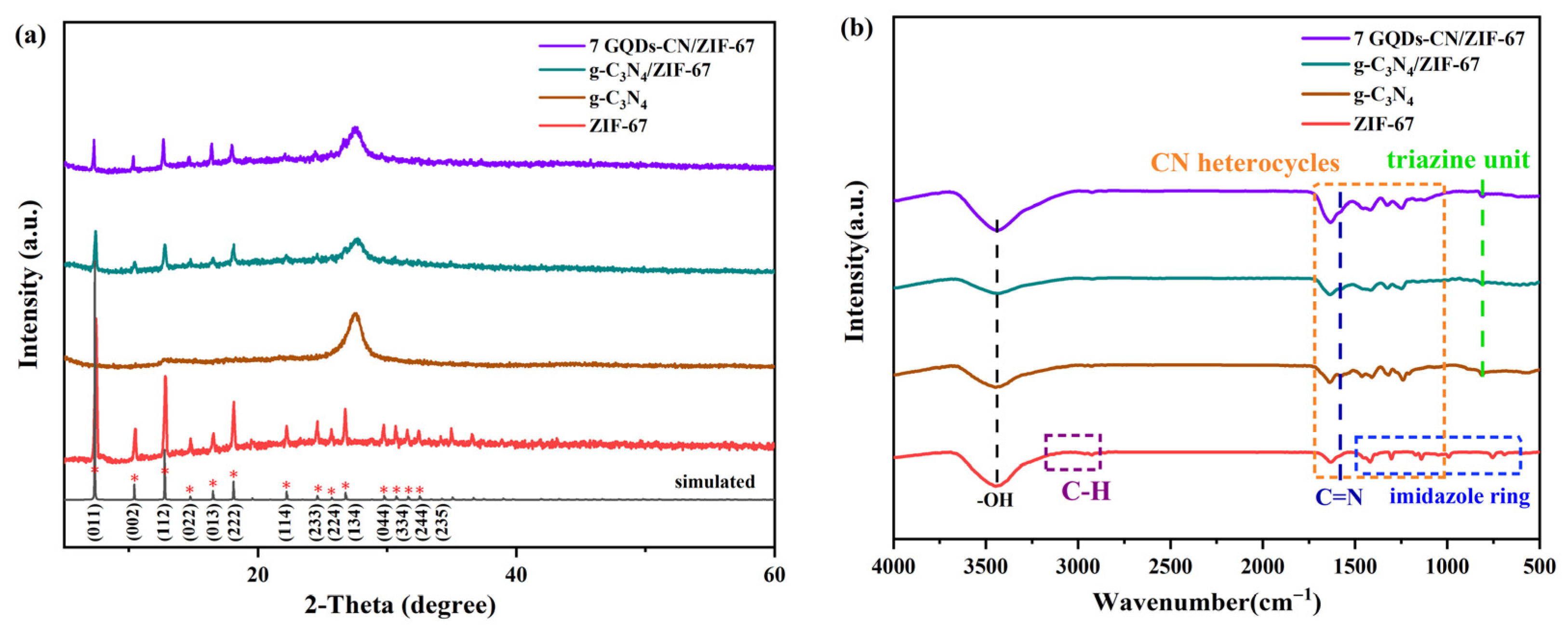
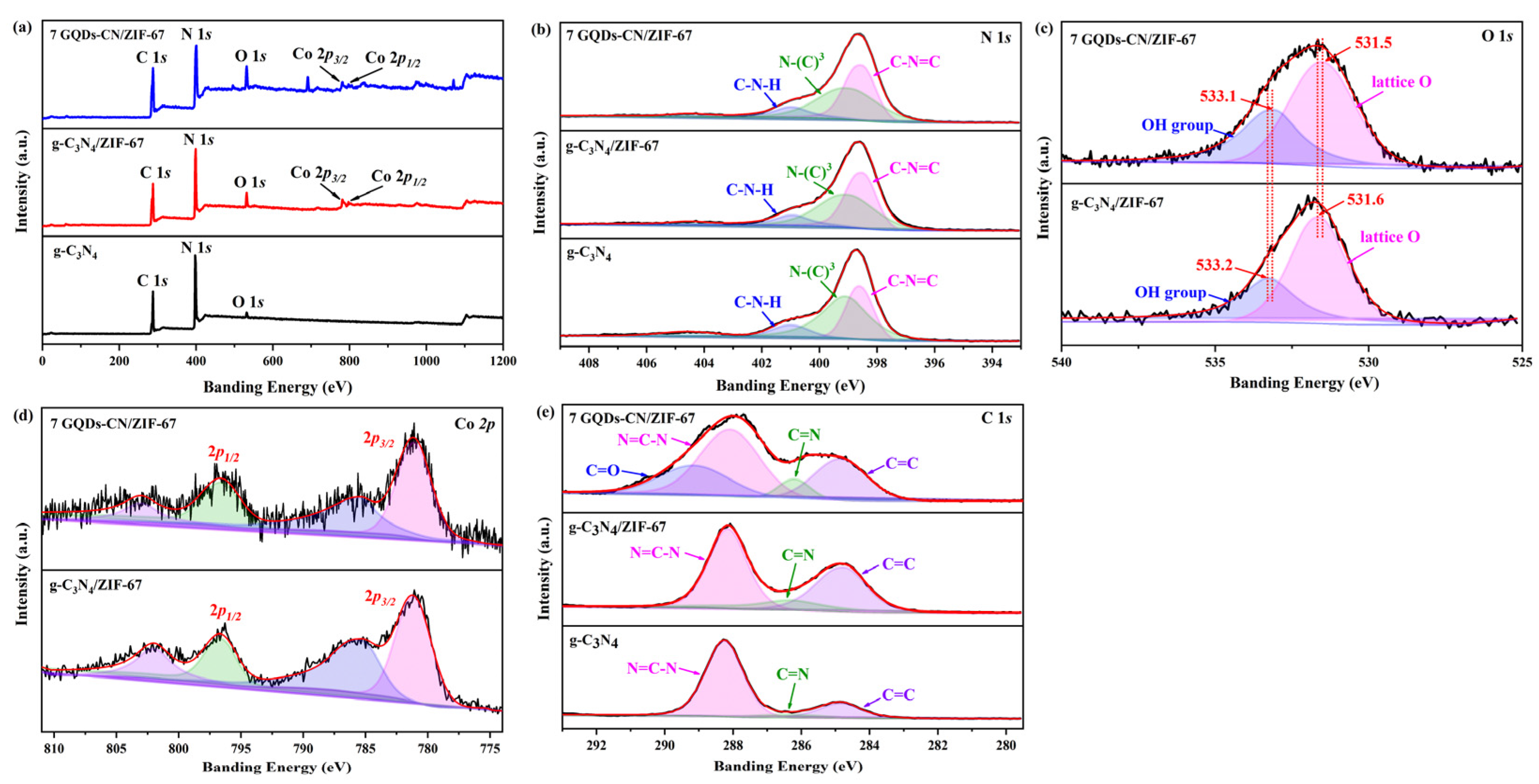
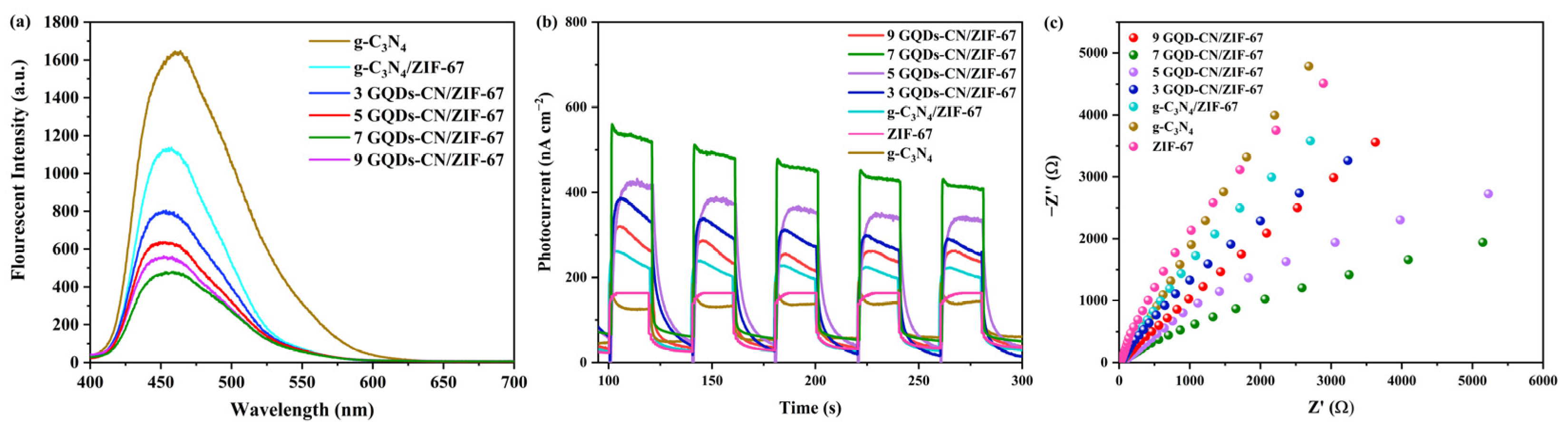
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Photocatalyst Construction
3.2.1. Synthesis of g-C3N4
3.2.2. Synthesis of GQDs
3.2.3. Synthesis of g-C3N4/ZIF-67 Sample
3.2.4. Synthesis of GQDs/g-C3N4/ZIF-67 Sample
3.3. Catalysts Characterization
3.4. Photocatalytic Reaction
3.5. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Habisreutinger, S.N.; Schmidt-Mende, P.L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, W.; Zhou, K. Metal-organic-framework-based catalysts for photoreduction of CO2. Adv. Mater. 2018, 30, 1705512. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, G.J.; Zhang, X.S.; Ba, X.; Shi, G.D.; Li, Y.; Wong, P.K.; Yu, J.; Yu, Y. Enhanced activity and stability of carbon-decorated cuprous oxide mesoporous nanorods for CO2 reduction in artificial photosynthesis. ACS Catal. 2016, 6, 6444–6454. [Google Scholar] [CrossRef]
- Lv, H.W.; Li, P.Y.; Li, X.J.; Chen, A.C.; Sa, R.J.; Zhu, H.; Wang, R.H. Boosting photocatalytic reduction of the diluted CO2 over covalent organic framework. Chem. Eng. J. 2023, 451, 138745. [Google Scholar] [CrossRef]
- Hong, D.C.; Tsukakoshi, Y.; Kotani, H.; Ishizuka, T.; Kojima, T. Visible-light-driven photocatalytic CO2 reduction by a Ni(II) complex bearing a bioinspired tetradentate ligand for selective CO production. J. Am. Chem. Soc. 2017, 139, 6538. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.L.; Zhang, H.B.; Wei, J.; Zhang, H.X.; Ye, J.H. Integrating the g-C3N4 nanosheet with B-H bonding decorated metal-organic framework for CO2 activation and photoreduction. ACS Nano 2018, 12, 5333–5340. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yang, Q.; Wang, Y.; Zhao, J.W.; Wang, D.B. Novel ternary heterojunction photcocatalyst of Ag nanoparticles and g-C3N4 nanosheets Co-modified BiVO4 for wider spectrum visible-light photocatalytic degradation of refractory pollutant. Appl. Catal. B-Environ. 2017, 205, 133–147. [Google Scholar] [CrossRef]
- Halmann, M. Photoelectrochemical reduction of aqueous carbon dioxide on p-type gallium phosphide in liquid junction solar cells. Nature 1978, 275, 115–116. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Tu, W.G.; Zhou, Y.; Zou, Z.G. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef]
- King, S.; Lin, R.B.; Wang, H.L.; Arman, H.; Chen, B.L. Two-dimensional metal–organic frameworks for selective separation of CO2/CH4 and CO2/N2. Mat. Chem. Front. 2017, 1, 1514–1519. [Google Scholar] [CrossRef]
- Zhang, N.; Long, R.; Gao, C.; Xiong, Y.J. Recent progress on advanced design for photoelectrochemical reduction of CO2 to fuels. Sci. China Mater. 2018, 61, 771–805. [Google Scholar] [CrossRef]
- Wu, H.L.; Li, X.B.; Tung, C.H.; Wu, L.Z. Semiconductor quantum dots: An emerging candidate for CO2 photoreduction. Adv. Mater. 2019, 31, 1900709. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, D.N.; Liao, Y.L.; Fan, J.J.; Xiang, Q.J. Structural engineering of 3D hierarchical Cd0.8Zn0.2S for selective photocatalytic CO2 reduction. Chin. J. Catal. 2021, 42, 131–140. [Google Scholar] [CrossRef]
- Kumar, S.; Karthikeyan, S.; Lee, A. g-C3N4-based nanomaterials for visible light-driven photocatalysis. Catalysts 2018, 8, 74. [Google Scholar] [CrossRef]
- Cao, S.W.; Low, J.X.; Yu, J.G.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lian, R.; Zhang, Y.; Ma, X.L.; Huang, J.W.; She, H.D.; Liu, C.; Wang, Q.Z. Rational preparation of cocoon-like g-C3N4/COF hybrids: Accelerated intramolecular charge delivery for photocatalytic hydrogen evolution. Appl. Catal. B-Environ. 2022, 315, 121568. [Google Scholar] [CrossRef]
- Ahilandeswari, G.; Arivuoli, D. Enhanced sunlight-driven photocatalytic activity in assembled ZrO2/g-C3N4 nanocomposite. J. Mater. Sci.-Mater. Electron. 2022, 33, 23986–24002. [Google Scholar] [CrossRef]
- Kong, J.Z.; Zhai, H.F.; Zhang, W.; Wang, S.S.; Zhao, X.R.; Li, M.; Li, H.; Li, A.D.; Wu, D. Visible light-driven photocatalytic performance of N-doped ZnO/g-C3N4 nanocomposites. Nanoscale Res. Lett. 2017, 12, 526. [Google Scholar] [CrossRef]
- Bi, L.L.; Xu, D.D.; Zhang, L.J.; Lin, Y.H.; Wang, D.J.; Xie, T.F. Metal Ni-loaded g-C3N4 for enhanced photocatalytic H2 evolution activity: The change in surface band bending. Phys. Chem. Chem. Phys. 2015, 17, 29899–29905. [Google Scholar] [CrossRef]
- Wen, J.Q.; Xie, J.; Chen, X.B.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, H.Y.; Zhang, Z.T.; Zhang, Y.; Huang, J.W.; She, H.D.; Liu, C.L.; Wang, Q.Z. Rational design of honeycomb-like APTES-TiO2/COF heterostructures: Promoted intramolecular charge transfer for visible-light-driven catalytic CO2 reduction. Chem. Eng. J. 2023, 456, 140990. [Google Scholar] [CrossRef]
- Zhao, C.; Liao, Z.Z.; Liu, W.; Liu, F.Y.; Ye, J.Y.; Liang, J.L.; Li, Y.Y. Carbon quantum dots modified tubular g-C3N4 with enhanced photocatalytic activity for carbamazepine elimination: Mechanisms, degradation pathway and DFT calculation. J. Hazard. Mater. 2019, 381, 120957. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, G.F.; Zhang, L.; Lian, R.; Huang, J.W.; She, H.D.; Liu, C.L.; Wang, Q.Z. Construction of TiO2-covalent organic framework Z-Scheme hybrid through coordination bond for photocatalytic CO2 conversion. J. Energy Chem. 2022, 64, 85–92. [Google Scholar] [CrossRef]
- Domingo Tafalla, B.; Eugenia Martínez, F.; Franco, F.; Palomares Gil, E. Applications of carbon dots for the photocatalytic and electrocatalytic reduction of CO. Molecules 2022, 27, 1081. [Google Scholar] [CrossRef]
- Mandal, S.; Adhikari, S.; Choi, S.; Lee, Y.; Kim, D. Fabrication of a novel Z-scheme Bi2MoO6/GQDs/MoS2 hierarchical nanocomposite for the photo-oxidation of ofloxacin and photoreduction of Cr(VI) as aqueous pollutants. Chem. Eng. J. 2022, 444, 136609. [Google Scholar] [CrossRef]
- Batvandi, M.; Haghighatzadeh, A.; Mazinani, B.; Dutta, J. Visible-light-driven photocatalysis with Z-scheme Ag3PO4@N-GQDs@g-C3N4 nano/hetero-junctions. Appl. Phys. A-Mater. Sci. Process. 2022, 128, 853. [Google Scholar] [CrossRef]
- You, F.H.; Zhu, M.Y.; Ding, L.J.; Xu, Y.H.; Wang, K. Design and construction of Z-scheme Bi2S3/nitrogen-doped graphene quantum dots: Boosted photoelectric conversion efficiency for high-performance photoelectrochemical aptasensing of sulfadimethoxine. Biosens. Bioelectron. 2019, 130, 230–235. [Google Scholar] [CrossRef]
- Goswami, L.; Aggarwal, N.; Verma, R.; Bishnoi, S.; Husale, S.; Pandey, R.; Gupta, G. Graphene quantum dot-sensitized ZnO-nanorod/GaN-nanotower heterostructure-based high-performance UV photodetectors. ACS Appl. Mater. Interfaces 2020, 12, 47038–47047. [Google Scholar] [CrossRef]
- Wei, D.; Tang, W.; Gan, Y.D.; Xu, X.Q. Graphene quantum dot-sensitized Zn-MOFs for efficient visible-light-driven carbon dioxide reduction. Catal. Sci. Technol. 2020, 10, 5666–5676. [Google Scholar] [CrossRef]
- Park, J.; Wang, Z.Y.; Sun, L.B.; Chen, Y.P.; Zhou, H.C. Introduction of functionalized mesopores to metal-organic frameworks via metal-ligand-fragment coassembly. J. Am. Chem. Soc. 2012, 134, 20110–20116. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.T.; Han, Q.; Liu, Y.; Zhong, J.B.; Chen, J.F.; Huang, J.W.; She, H.D.; Wang, Q.Z. Preparation of CdS-P25/ZIF-67 composite material and its photocatalytic CO2 reduction performance. Appl. Surf. Sci. 2022, 584, 584. [Google Scholar] [CrossRef]
- Ge, J.H.; Liu, Y.J.; Jiang, D.C.; Zhang, L.; Du, P.W. Integrating non-precious-metal cocatalyst Ni3N with g-C3N4 for enhanced photocatalytic H2 production in water under visible-light irradiation. Chin. J. Catal. 2019, 40, 160–167. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, F.J.; Lu, H.Y.; Hong, X.; Jiang, H.L.; Wu, Y.; Li, Y.D. Hollow Zn/Co ZIF particles derived from core–shell ZIF-67@ZIF-8 as selective catalyst for the semi-hydrogenation of acetylene. Angew. Chem. Int. Edit. 2015, 54, 10889–10893. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.N.; Wang, S.B.; Wang, X.C. Visible-light reduction CO2 with dodecahedral zeolitic imidazolate framework ZIF-67 as an efficient co-catalyst. Appl. Catal. B-Environ. 2017, 209, 476–482. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.B.; Qian, L.; Zhong, T.; Ran, Q.; Huang, J.; Hou, Y.P.; Li, F.Y.; Li, M.J.; Sun, Q.Q.; Zhang, H.Q. Enhanced visible light photocatalytic activity of CdS through controllable self-assembly compositing with ZIF-67. Mol. Catal. 2020, 485, 110797. [Google Scholar] [CrossRef]
- Guan, W.H.; Gao, X.C.; Ji, G.F.; Xing, Y.X.; Du, C.F.; Liu, Z.L. Fabrication of a magnetic nanocomposite photocatalysts Fe3O4@ZIF-67 for degradation of dyes in water under visible light irradiation. J. Solid State Chem. 2017, 255, 150–156. [Google Scholar] [CrossRef]
- Qian, J.F.; Sun, F.; Qin, L.Z. Hydrothermal synthesis of zeolitic imidazolate framework-67 (ZIF-67) nanocrystals. Mater. Lett. 2012, 82, 220–223. [Google Scholar] [CrossRef]
- Yu, M.Q.; Qu, Y.; Pan, K.; Wang, G.F.; Li, Y.D. Enhanced photoelectric conversion efficiency of dye-sensitized solar cells by the synergetic effect of NaYF4:Er3+/Yb3+ and g-C3N4. Sci. China Mater. 2017, 60, 228–238. [Google Scholar] [CrossRef]
- Shi, G.D.; Yang, L.; Liu, Z.W.; Chen, X.; Zhou, J.Q.; Yu, Y. Photocatalytic reduction of CO2 to CO over copper decorated g-C3N4 nanosheets with enhanced yield and selectivity. Appl. Surf. Sci. 2017, 427, 1165–1173. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.X.; Guo, C.M.; Gao, X.S.; Gong, C.H.; Wang, Y.; Liu, B.; Sun, L.C. Metal-organic frameworks (ZIF-67) as efficient cocatalysts for photocatalytic reduction of CO2: The role of the morphology effect. J. Mater. Chem. A 2018, 6, 4768–4775. [Google Scholar] [CrossRef]
- Du, Z.; Cai, H.Y.; Zhao, Z.Y.; Guo, Z.L.; Lin, J.; Huang, Y.; Tang, C.C.; Chen, G.F.; Fang, Y. Facile synthesis of graphene quantum dots and C-doping porous BN nanoribbon heterojunctions for boosting CO2 photoreduction. Sep. Purif. Technol. 2023, 311, 123321. [Google Scholar] [CrossRef]
- Ullah, M.; Bai, X.; Chen, J.K.; Lv, H.; Liu, Z.; Zhang, Y.; Wang, J.; Sun, B.H.; Li, L.; Shi, K.Y. Metal-organic framework material derived Co3O4 coupled with graphitic carbon nitride as highly sensitive NO2 gas sensor at room temperature. Colloid Surf. A-Physicochem. Eng. Asp. 2021, 612, 125972. [Google Scholar] [CrossRef]
- Han, C.Q.; Li, J.; Ma, Z.Y.; Xie, H.Q.; Waterhouse, G.; Ye, L.Q.; Zhang, T.R. Black phosphorus quantum dot/g-C3N4 composites for enhanced CO2 photoreduction to CO. Sci. China Mater. 2018, 61, 1159–1166. [Google Scholar] [CrossRef]
- Wang, J.Y.; Xu, Z.L.; Zhuang, C.S.; Wang, H.; Peng, T.Y. Dramatically enhanced visible-light driven H2 evolution by anchoring TiO2 nanoparticles on the molecularly grafted carbon nitride nanosheets via a multiple modification strategy. Dalton Trans. 2018, 47, 14556–14565. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yin, F.X.; Chen, B.H.; He, X.B.; Lv, P.L.; Ye, C.Y.; Liu, D.J. ZIF-67 incorporated with carbon derived from pomelo peels: A highly efficient bifunctional catalyst for oxygen reduction/evolution reactions. Appl. Catal. B-Environ. 2017, 205, 55–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhang, D.; Li, S.D.; Jiang, C.; Su, Y. Confinement preparation of Au nanoparticles embedded in ZIF-67-derived N-doped porous carbon for high-performance detection of hydrazine in liquid/gas phase. Sens. Actuator B-Chem. 2019, 285, 607–616. [Google Scholar] [CrossRef]
- Cui, Y.; Huang, X.X.; Wang, T.; Jia, L.H.; Nie, Q.Q.; Tan, Z.C.; Yu, H.S. Graphene quantum dots/carbon nitride heterojunction with enhanced visible-light driven photocatalysis of nitric oxide: An experimental and DFT study. Carbon 2022, 191, 502–514. [Google Scholar] [CrossRef]
- Lafta, M.; Ammar, S. Synthesis and photocatalytic activity of polyoxometalates immobilized onto g-C3N4/ZIF-67 heterostructures. Mater. Sci. Semicond. Process. 2023, 153, 107131. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, Y.L.; Rao, Y.F.; Zhu, D.D.; Cao, J.J.; Shen, Z.X.; Ho, W.K.; Lee, S.C. Environment-friendly carbon quantum dots/ZnFe2O4 photocatalysts: Characterization, biocompatibility, and mechanisms for NO removal. Environ. Sci. Technol. 2017, 51, 2924–2933. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Li, S.J.; Xu, H.Y.; Wang, G.F.; Wang, D.S. Co-MOF as an electron donor for promoting visible-light photoactivities of g-C3N4 nanosheets for CO2 reduction. Chin. J. Catal. 2020, 41, 514–523. [Google Scholar] [CrossRef]
- Wei, Y.A.; Li, X.; Zhang, Y.L.; Yan, Y.S.; Huo, P.W.; Wang, H.Q. G-C3N4 quantum dots and Au nano particles Co-modified CeO2/Fe3O4 micro-flowers photocatalyst for enhanced CO2 photoreduction. Renew. Energy 2021, 179, 756–765. [Google Scholar] [CrossRef]
- Tao, F.F.; Dong, Y.L.; Yang, L.A. NiO/g-C3N4 quantum dots for photocatalytic CO2 reduction. Appl. Surf. Sci. 2023, 638, 158044. [Google Scholar] [CrossRef]
- Bika, P.; Papailias, I.; Giannakopoulou, T.; Tampaxis, C.; Steriotis, T.; Trapalis, C.; Dallas, P. Prominent COF, g-C3N4, and teir heterojunction materials for selective photocatalytic CO2 reduction. Catalysts 2023, 13, 1331. [Google Scholar] [CrossRef]
- Tian, F.Y.; Wu, X.Y.; Chen, J.H.; Sun, X.B.; Yan, X.M.; Liao, G.F. One-step photodeposition of spatially separated CuOx and MnOx dual cocatalysts on g-C3N4 for enhanced CO2 photoreduction. Dalton Trans. 2023, 52, 11934–11940. [Google Scholar] [CrossRef]
- Zhu, L.; Qiao, Z.P. SrTiO3@NiFe LDH core-shell composites for photocatalytic CO2 conversion. RSC Adv. 2022, 12, 10592–10597. [Google Scholar] [CrossRef]



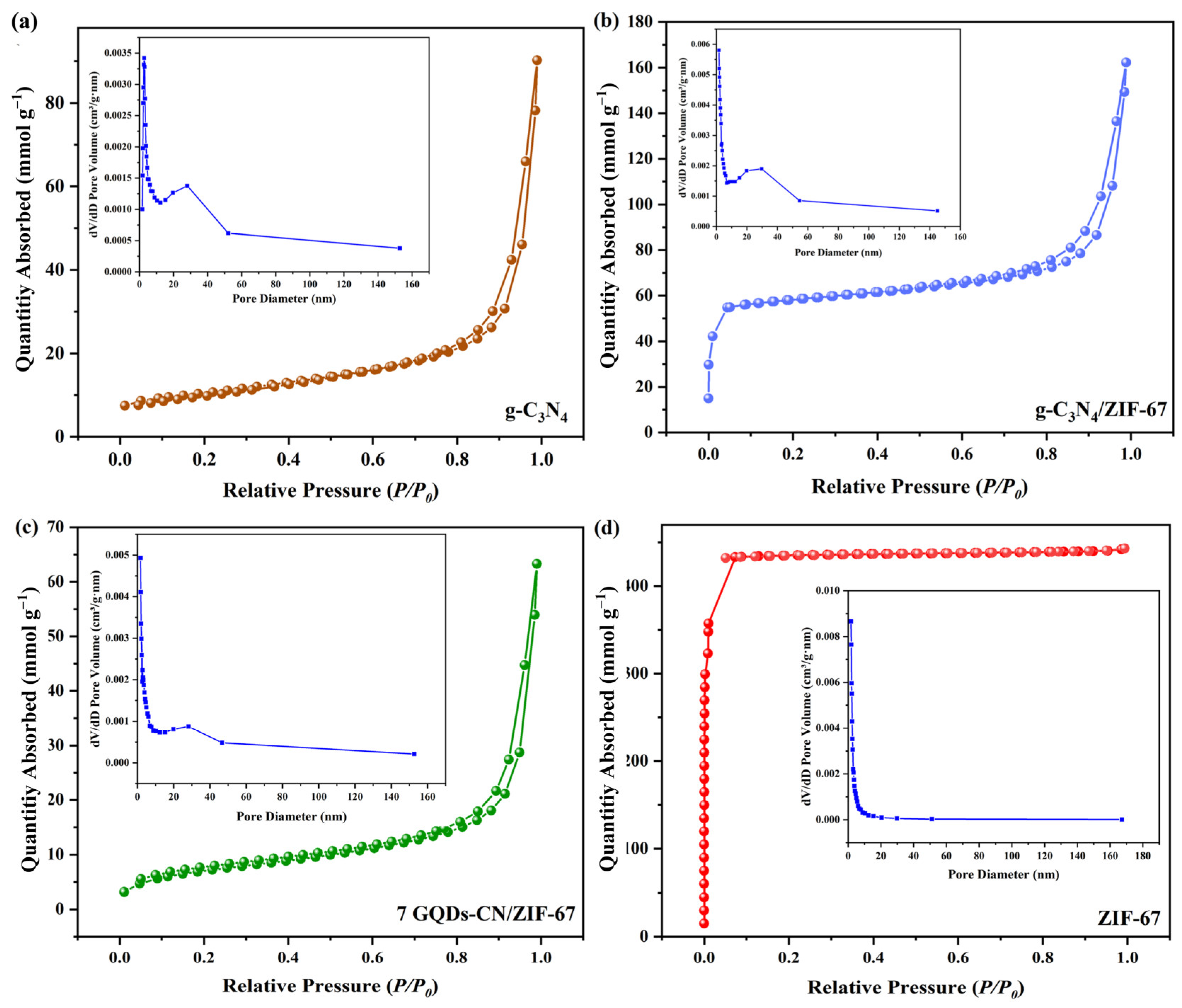




| Photocatalyst | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| g-C3N4 | 37.10 | 0.068 | 19.965 |
| ZIF-67 | 1742.47 | 0.681 | 4.430 |
| g-C3N4/ZIF-67 | 221.29 | 0.160 | 18.535 |
| 7 GQDs-CN/ZIF-67 | 57.36 | 0.078 | 16.791 |
| Sample | Light (nm) | Time (h) | CO Product (umol/g) | Yields (μmol/g/h) | Selectivity(%) | Reference |
|---|---|---|---|---|---|---|
| 7 GQDs-CN/ZIF-67 | λ > 420 | 6 | 51.71 | 8.62 | 81.06 | This Work |
| g-C3N4/Au/CeO2/Fe3O4 | λ > 420 | 4 | 28.00 | 7.00 | 42.42 | [53] |
| NiO/g-C3N4 QDs | 200 < λ < 900 | 5 | 18.90 | 3.78 | 64.80 | [54] |
| COF/g-C3N4 (Pt) | λ < 400 | 18 | 117.23 | 6.51 | 72.98 | [55] |
| CuOx/g-C3N4/MnOx | λ > 420 | 4 | 21.96 | 5.49 | none | [56] |
| SrTiO3@NiFe LDH | λ > 420 | 6 | 47.40 | 7.90 | 56.83 | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Wang, J.; Xu, C.; Du, Z.; Yu, R.; Zhao, Y.; Han, J.; Zuo, J.; Guo, Z.; Tang, C.; et al. Construction of a Novel Ternary GQDs/g-C3N4/ZIF-67 Photocatalyst for Enhanced Photocatalytic Carbon Dioxide Reduction. Catalysts 2024, 14, 334. https://doi.org/10.3390/catal14060334
Zhao Z, Wang J, Xu C, Du Z, Yu R, Zhao Y, Han J, Zuo J, Guo Z, Tang C, et al. Construction of a Novel Ternary GQDs/g-C3N4/ZIF-67 Photocatalyst for Enhanced Photocatalytic Carbon Dioxide Reduction. Catalysts. 2024; 14(6):334. https://doi.org/10.3390/catal14060334
Chicago/Turabian StyleZhao, Zhiyuan, Jingjing Wang, Congnian Xu, Zhao Du, Rongrong Yu, Yongqi Zhao, Jiayi Han, Jingtao Zuo, Zhonglu Guo, Chengchun Tang, and et al. 2024. "Construction of a Novel Ternary GQDs/g-C3N4/ZIF-67 Photocatalyst for Enhanced Photocatalytic Carbon Dioxide Reduction" Catalysts 14, no. 6: 334. https://doi.org/10.3390/catal14060334
APA StyleZhao, Z., Wang, J., Xu, C., Du, Z., Yu, R., Zhao, Y., Han, J., Zuo, J., Guo, Z., Tang, C., & Fang, Y. (2024). Construction of a Novel Ternary GQDs/g-C3N4/ZIF-67 Photocatalyst for Enhanced Photocatalytic Carbon Dioxide Reduction. Catalysts, 14(6), 334. https://doi.org/10.3390/catal14060334







