Efficient Charge Transfer of p-n Heterojunction UiO-66-NH2/CuFe2O4 Composite for Photocatalytic Hydrogen Production
Abstract
:1. Introduction
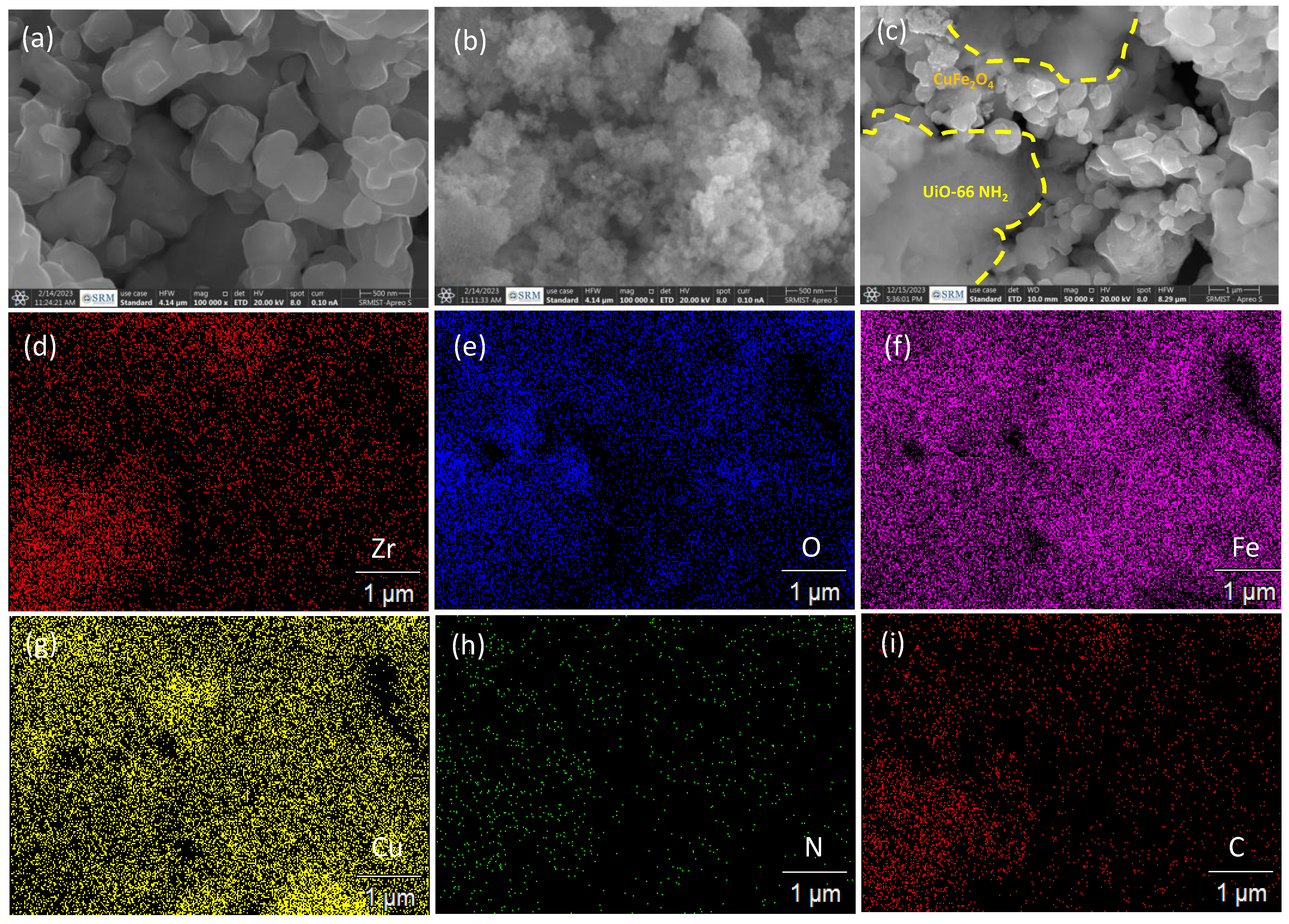
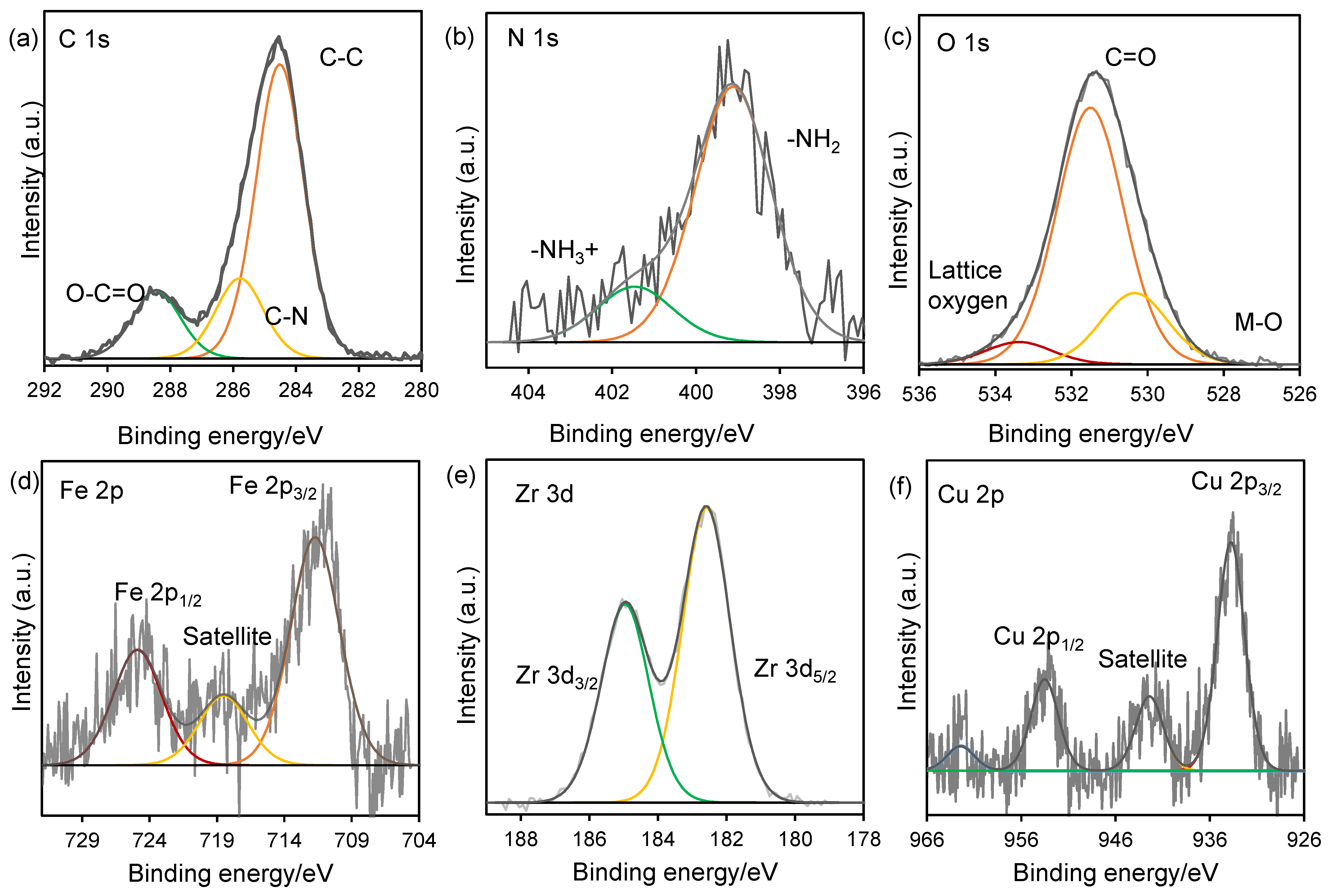
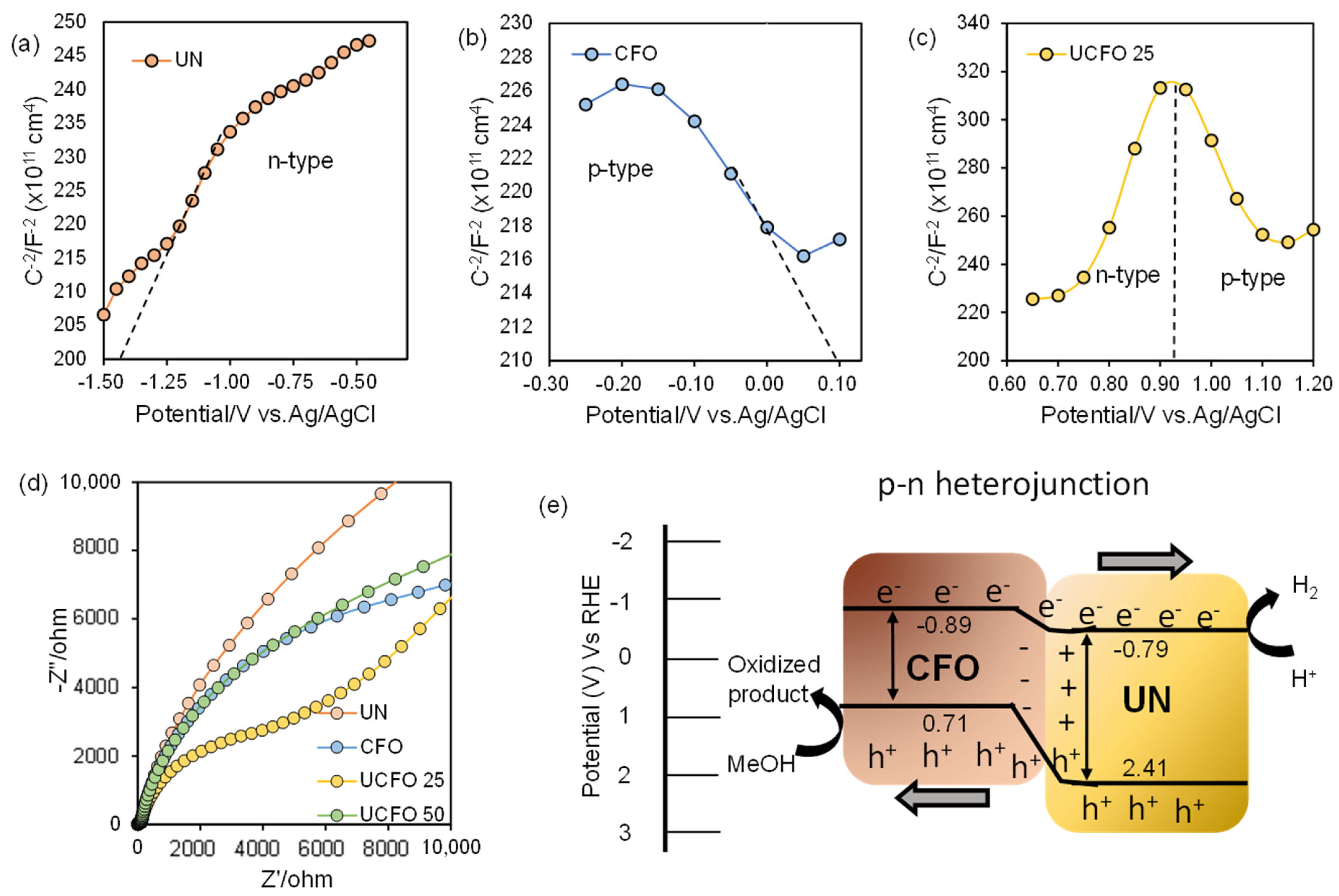
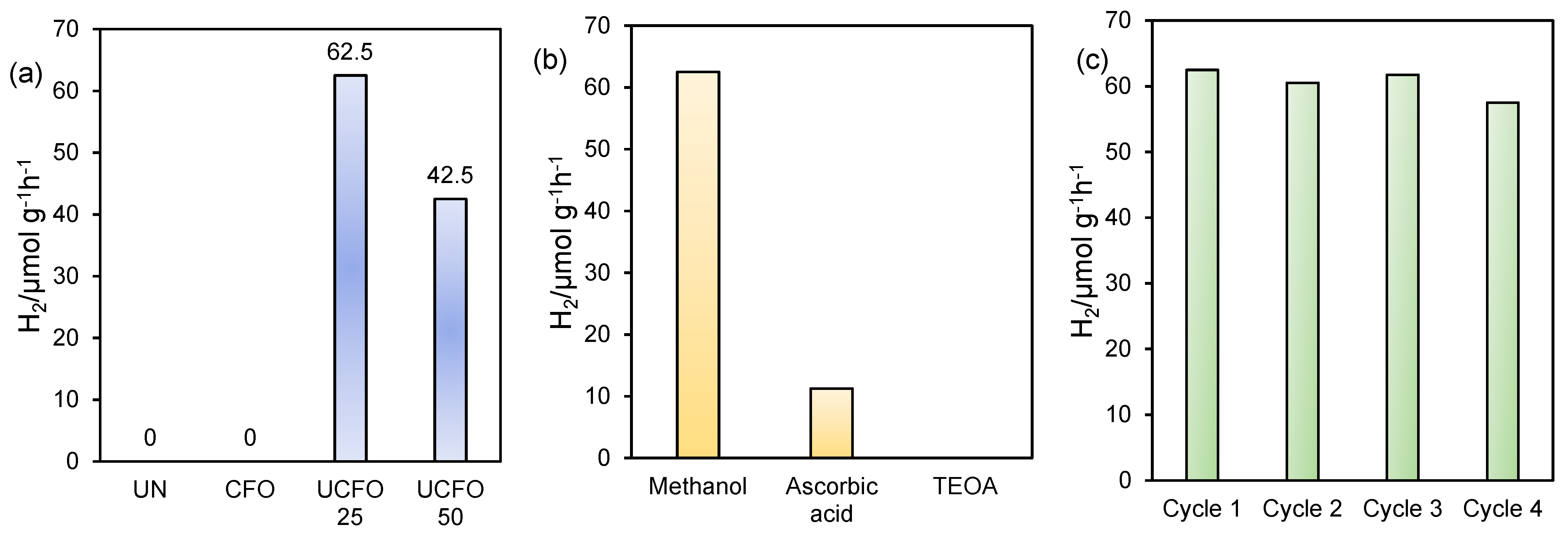
2. Result and Discussion
3. Experimental Details
3.1. Chemicals
3.2. Synthesis of UiO-66-NH2
3.3. Synthesis of CuFe2O4
3.4. Synthesis of UiO-66-NH2/CuFe2O4
3.5. Characterizations
3.6. Photocatalytic Experiment
3.7. Electrochemical and Photoelectrochemical Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yalew, S.G.; van Vliet, M.T.H.; Gernaat, D.E.H.J.; Ludwig, F.; Miara, A.; Park, C.; Byers, E.; De Cian, E.; Piontek, F.; Iyer, G.; et al. Impacts of climate change on energy systems in global and regional scenarios. Nat. Energy 2020, 5, 794–802. [Google Scholar] [CrossRef]
- Shindell, D.; Smith, C.J. Climate and air-quality benefits of a realistic phase-out of fossil fuels. Nature 2019, 573, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An Overview of Hydrogen Production: Current Status, Potential, and Challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Hu, Y.H. Steam reforming of methane: Current states of catalyst design and process upgrading. Renew. Sustain. Energy Rev. 2021, 149, 111330. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Shanmugam, M.; Augustin, A.; Mohan, S.; Honnappa, B.; Chuaicham, C.; Rajendran, S.; Hoang, T.K.A.; Sasaki, K.; Sekar, K. Conducting polymeric nanocomposites: A review in solar fuel applications. Fuel 2022, 325, 124899. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Wang, X.-L.; Sun, Y.-Y.; Xiao, Y.; Chen, X.-X.; Huang, X.-C.; Zhou, H.-L. Facile Solution-Refluxing Synthesis and Photocatalytic Dye Degradation of a Dynamic Covalent Organic Framework. Molecules 2022, 27, 8002. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Li, Z.; Chen, Z.; Liu, Q.; Song, M.; Huang, C. NiSe/Cd0.5Zn0.5S Composite Nanoparticles for Use in p–n Heterojunction-Based Photocatalysts for Solar Energy Harvesting. ACS Appl. Nano Mater. 2020, 3, 3665–3674. [Google Scholar] [CrossRef]
- Shenoy, S.; Chuaicham, C.; Okumura, T.; Sekar, K.; Sasaki, K. Simple tactic polycondensation synthesis of Z-scheme quasi-polymeric g-C3N4/CaFe2O4 composite for enhanced photocatalytic water depollution via p-n heterojunction. Chem. Eng. J. 2023, 453, 139758. [Google Scholar] [CrossRef]
- Tang, J.-y.; Guo, R.-t.; Zhou, W.-g.; Huang, C.-y.; Pan, W.-g. Ball-flower like NiO/g-C3N4 heterojunction for efficient visible light photocatalytic CO2 reduction. Appl. Catal. B Environ. 2018, 237, 802–810. [Google Scholar] [CrossRef]
- Shanmugam, M.; Agamendran, N.; Sekar, K.; Natarajan, T.S. Metal–organic frameworks (MOFs) for energy production and gaseous fuel and electrochemical energy storage applications. Phys. Chem. Chem. Phys. 2023, 25, 30116–30144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Navalón, S.; Dhakshinamoorthy, A.; Álvaro, M.; Ferrer, B.; García, H. Metal–Organic Frameworks as Photocatalysts for Solar-Driven Overall Water Splitting. Chem. Rev. 2023, 123, 445–490. [Google Scholar] [CrossRef] [PubMed]
- Melillo, A.; Cabrero-Antonino, M.; Navalón, S.; Álvaro, M.; Ferrer, B.; García, H. Enhancing visible-light photocatalytic activity for overall water splitting in UiO-66 by controlling metal node composition. Appl. Catal. B Environ. 2020, 278, 119345. [Google Scholar] [CrossRef]
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dincă, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The Current Status of MOF and COF Applications. Angew. Chem. Int. Ed. 2021, 60, 23975–24001. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-X.; Xiong, Y.-Y.; Zhong, X.; Lan, P.C.; Wei, Z.-W.; Pan, H.; Su, P.-Y.; Song, Y.; Chen, Y.-F.; Nafady, A.; et al. Enhancing Photocatalytic Hydrogen Production via the Construction of Robust Multivariate Ti-MOF/COF Composites. Angew. Chem. Int. Ed. 2022, 61, e202114071. [Google Scholar] [CrossRef] [PubMed]
- Gomes Silva, C.; Luz, I.; Llabrés i Xamena, F.X.; Corma, A.; García, H. Water Stable Zr–Benzenedicarboxylate Metal–Organic Frameworks as Photocatalysts for Hydrogen Generation. Chem. A Eur. J. 2010, 16, 11133–11138. [Google Scholar] [CrossRef] [PubMed]
- Winarta, J.; Shan, B.; McIntyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A Decade of UiO-66 Research: A Historic Review of Dynamic Structure, Synthesis Mechanisms, and Characterization Techniques of an Archetypal Metal–Organic Framework. Cryst. Growth Des. 2020, 20, 1347–1362. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Song, F.; Huang, T.; Ji, J.; Zhong, Q.; Chu, W.; Xu, Q. UiO-66-NH2/GO Composite: Synthesis, Characterization and CO2 Adsorption Performance. Materials 2018, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Melillo, A.; Cabrero-Antonino, M.; Ferrer, B.; Dhakshinamoorthy, A.; Baldoví, H.G.; Navalón, S. MOF-on-MOF Composites with UiO-66-Based Materials as Photocatalysts for the Overall Water Splitting under Sunlight Irradiation. Energy Fuels 2023, 37, 5457–5468. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- He, S.; Li, L.-X.; Zhang, L.-T.; Zeng, S.; Feng, C.; Chen, X.-X.; Zhou, H.-L.; Huang, X.-C. Elucidating influences of defects and thermal treatments on CO2 capture of a Zr-based metal–organic framework. Chem. Eng. J. 2024, 479, 147605. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, M.; Liu, Y.; Zhang, G.; Li, L.; Du, L.; Li, B.; Xiao, S.; Wang, G.; Yang, X. Modified UiO-66 as photocatalysts for boosting the carbon-neutral energy cycle and solving environmental remediation issues. Coord. Chem. Rev. 2022, 458, 214428. [Google Scholar] [CrossRef]
- Yang, H.; Yan, J.; Lu, Z.; Cheng, X.; Tang, Y. Photocatalytic activity evaluation of tetragonal CuFe2O4 nanoparticles for the H2 evolution under visible light irradiation. J. Alloys Compd. 2009, 476, 715–719. [Google Scholar] [CrossRef]
- Park, S.; Baek, J.H.; Zhang, L.; Lee, J.M.; Stone, K.H.; Cho, I.S.; Guo, J.; Jung, H.S.; Zheng, X. Rapid Flame-Annealed CuFe2O4 as Efficient Photocathode for Photoelectrochemical Hydrogen Production. ACS Sustain. Chem. Eng. 2019, 7, 5867–5874. [Google Scholar] [CrossRef]
- Lai, Y.-J.; Chang, J.-S.; Lee, D.-J. Synthesis of a novel solid mediator Z-scheme heterojunction photocatalysis CuFe2O4/Cu/UiO-66-NH2 for oxidation of dye in water. Chemosphere 2022, 296, 134080. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Ghosh, S.; Maiyalagan, T.; Basu, R.N. Band Edge Engineering of BiOX/CuFe2O4 Heterostructures for Efficient Water Splitting. ACS Appl. Energy Mater. 2022, 5, 3821–3833. [Google Scholar] [CrossRef]
- Shenoy, S.; Chuaicham, C.; Shanmugam, M.; Okumura, T.; Balijapalli, U.; Li, W.; Balakumar, V.; Sasaki, K.; Sekar, K. Tailoring Interfacial Physicochemical Properties in Cu2O-TiO2@rGO Heterojunction: Insights from EXAFS and Electron Trap Distribution Analysis. ACS Appl. Mater. Interfaces 2023, 15, 54105–54118. [Google Scholar] [CrossRef]
- Treger, M.; Hannebauer, A.; Schaate, A.; Budde, J.L.; Behrens, P.; Schneider, A.M. Tuning the optical properties of the metal–organic framework UiO-66 via ligand functionalization. Phys. Chem. Chem. Phys. 2023, 25, 6333–6341. [Google Scholar] [CrossRef] [PubMed]
- Mehtab, A.; Banerjee, S.; Mao, Y.; Ahmad, T. Type-II CuFe2O4/Graphitic Carbon Nitride Heterojunctions for High-Efficiency Photocatalytic and Electrocatalytic Hydrogen Generation. ACS Appl. Mater. Interfaces 2022, 14, 44317–44329. [Google Scholar] [CrossRef] [PubMed]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, Y.; Jiang, H.; Chen, J.; Liu, Y.; Liang, Q.; Zhou, M.; Li, Z.; Zhou, Y. Combined Effects of Octahedron NH2-UiO-66 and Flowerlike ZnIn2S4 Microspheres for Photocatalytic Dye Degradation and Hydrogen Evolution under Visible Light. J. Phys. Chem. C 2019, 123, 18037–18049. [Google Scholar] [CrossRef]
- Peñas-Garzón, M.; Sampaio, M.J.; Wang, Y.L.; Bedia, J.; Rodriguez, J.J.; Belver, C.; Silva, C.G.; Faria, J.L. Solar photocatalytic degradation of parabens using UiO-66-NH2. Sep. Purif. Technol. 2022, 286, 120467. [Google Scholar] [CrossRef]
- Cao, M.; Yang, F.; Zhang, Q.; Zhang, J.; Zhang, L.; Li, L.; Wang, X.; Dai, W.-L. Facile construction of highly efficient MOF-based Pd@UiO-66-NH2@ZnIn2S4 flower-like nanocomposites for visible-light-driven photocatalytic hydrogen production. J. Mater. Sci. Technol. 2021, 76, 189–199. [Google Scholar] [CrossRef]
- Faungnawakij, K.; Shimoda, N.; Fukunaga, T.; Kikuchi, R.; Eguchi, K. Crystal structure and surface species of CuFe2O4 spinel catalysts in steam reforming of dimethyl ether. Appl. Catal. B Environ. 2009, 92, 341–350. [Google Scholar] [CrossRef]
- Shenoy, S.; Farahat, M.M.; Chuaicham, C.; Sekar, K.; Ramasamy, B.; Sasaki, K. Mixed-Phase Fe2O3 Derived from Natural Hematite Ores/C3N4 Z-Scheme Photocatalyst for Ofloxacin Removal. Catalysts 2023, 13, 792. [Google Scholar] [CrossRef]
- Lyu, J.; Ge, M.; Hu, Z.; Guo, C. One-pot synthesis of magnetic CuO/Fe2O3/CuFe2O4 nanocomposite to activate persulfate for levofloxacin removal: Investigation of efficiency, mechanism and degradation route. Chem. Eng. J. 2020, 389, 124456. [Google Scholar] [CrossRef]
- Gelderman, K.; Lee, L.; Donne, S.W. Flat-Band Potential of a Semiconductor: Using the Mott–Schottky Equation. J. Chem. Educ. 2007, 84, 685. [Google Scholar] [CrossRef]
- Ge, W.; Song, J.; Deng, S.; Liu, K.; Yang, P. Construction of Z-scheme CoFe2O4@ZnIn2S4 p-n heterojunction for enhanced photocatalytic hydrogen production. Sep. Purif. Technol. 2024, 328, 125059. [Google Scholar] [CrossRef]
- Yin, W.; Bai, L.; Zhu, Y.; Zhong, S.; Zhao, L.; Li, Z.; Bai, S. Embedding Metal in the Interface of a p-n Heterojunction with a Stack Design for Superior Z-Scheme Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 23133–23142. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, S.; Paramanik, L.; Sultana, S.; Mansingh, S.; Mohapatra, P.; Parida, K. A type-II interband alignment heterojunction architecture of cobalt titanate integrated UiO-66-NH2: A visible light mediated photocatalytic approach directed towards Norfloxacin degradation and green energy (Hydrogen) evolution. J. Colloid Interface Sci. 2020, 568, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ding, G.; Liu, X.; Huo, P.; Yan, Y.; Yan, Y.; Liao, G. Photocatalysis over NH2-UiO-66/CoFe2O4/CdIn2S4 double p-n junction: Significantly promoting photocatalytic performance by double internal electric fields. Chem. Eng. J. 2022, 435, 134740. [Google Scholar] [CrossRef]
- Rajan, A.; Neppolian, B. Coordinative integration of amorphous nickel-imidazole framework with graphitic carbon nitride for enhanced photocatalytic hydrogen production. Appl. Mater. Today 2022, 28, 101524. [Google Scholar] [CrossRef]
- Domínguez-Arvizu, J.L.; Jiménez-Miramontes, J.A.; Hernández-Majalca, B.C.; Valenzuela-Castro, G.E.; Gaxiola-Cebreros, F.A.; Salinas-Gutiérrez, J.M.; Collins-Martínez, V.; López-Ortiz, A. Study of NiFe2O4/Cu2O p-n heterojunctions for hydrogen production by photocatalytic water splitting with visible light. J. Mater. Res. Technol. 2022, 21, 4184–4199. [Google Scholar] [CrossRef]
- Hao, X.; Jin, Z.; Yang, H.; Lu, G.; Bi, Y. Peculiar synergetic effect of MoS2 quantum dots and graphene on Metal-Organic Frameworks for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2017, 210, 45–56. [Google Scholar] [CrossRef]
- Preeyanghaa, M.; Chuaicham, C.; Shenoy, S.; Vellaichamy, B.; Li, W.; Manokaran, K.; Varathan, E.; Neppolian, B.; Ohtani, B.; Sasaki, K.; et al. Unveiling the influence of Fe2O3 nanoparticles on CuxO–TiO2(B) nanofibers for dual Z-scheme electron transfer visible light photocatalysts: Investigation on local atomic structures and electronic properties. Environ. Sci. Nano 2023, 10, 1268–1283. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Q.; Li, L.; Zong, X.; Sun, H.; Tao, R.; Fan, X. Fe2O3 nanorods/CuO nanoparticles p-n heterojunction photoanode: Effective charge separation and enhanced photoelectrochemical properties. J. Colloid Interface Sci. 2021, 602, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jin, Z.; Bi, Y. Charge transmission channel construction between a MOF and rGO by means of Co–Mo–S modification. Catal. Sci. Technol. 2017, 7, 4478–4488. [Google Scholar] [CrossRef]
- Sekar, K.; Chuaicham, C.; Vellaichamy, B.; Li, W.; Zhuang, W.; Lu, X.; Ohtani, B.; Sasaki, K. Cubic Cu2O nanoparticles decorated on TiO2 nanofiber heterostructure as an excellent synergistic photocatalyst for H2 production and sulfamethoxazole degradation. Appl. Catal. B Environ. 2021, 294, 120221. [Google Scholar] [CrossRef]
- Hou, X.; Wu, L.; Gu, L.; Xu, G.; Du, H.; Yuan, Y. Maximizing the photocatalytic hydrogen evolution of Z-scheme UiO-66-NH2@Au@CdS by aminated-functionalized linkers. J. Mater. Sci. Mater. Electron. 2019, 30, 5203–5211. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Li, R.; Liu, H.; Zhang, W.; Ling, L.; Duan, W.; Liu, B. Hydrogen production with ultrahigh efficiency under visible light by graphene well-wrapped UiO-66-NH2 octahedrons. J. Mater. Chem. A 2017, 5, 20136–20140. [Google Scholar] [CrossRef]
- Fortage, J.; Collomb, M.-N.; Costentin, C. Turnover Number in Photoinduced Molecular Catalysis of Hydrogen Evolution: A Benchmarking for Catalysts? ChemSusChem 2024, e202400205. [Google Scholar] [CrossRef] [PubMed]



| S. No | Materials | Experimental Conditions | Hydrogen Efficiency | Ref. |
|---|---|---|---|---|
| 1. | Cu2O/TiO2 | 200 W Hg-Xe arc lamp (λ > 420 nm), 20 mg catalyst, 50 mL distilled water with 0.5 M Na2SO3 as a sacrificial agent | 60.96 μmol g−1 h−1 | [30] |
| 2. | NH2-UiO-66/CoFe2O4/CdIn2S4 | 20 mg of catalyst, TEOA as a sacrificial agent with Pt cocatalyst in presence of visible light λ > 420 nm | 55 μmol h−1 | [45] |
| 3. | NiFe2O4/Cu2O | 250 W metal halides lamp, model Philips. Methanol at 2% v/v was employed as a scavenger | 2.8 μmol g−1 h−1 | [47] |
| 4. | MoS2 QDs/UiO-66-NH2/G | 100 mL of 10% TEOA solution with Eosin Y dye kept in 300-W Xe lamp | 62 μmol h−1 | [48] |
| 5. | Fe2O3/CuxO/TiO2 | 20 mg of catalyst was used in 50 mL of 5 wt.% of MeOH solution in the presence of a 500 W Xenon lamp | 65.82 μmol g−1 h−1 | [49] |
| 6. | Cu2O/Fe2O3/rGO | 50 mg catalyst was dispersed into 140 mL of distilled water in the presence of 300-W Xe lamp | 4.86 μmol g−1 h−1 | [50] |
| 7. | rGO/ UiO-66-NH2/Co-Mo-S | 10 mg catalyst was added to 30 mL TEOA (15%) solution. After 10 min of ultrasonic dispersion, 20 mg of Eosin Y was added and kept in 300-W Xe lamp | 67.8 μmol h−1 | [51] |
| 8. | Cubic Cu2O/nano fiberTiO2 | 20 mg of catalyst was used in 50 mL of 5 wt.% of MeOH solution in the presence of a 500 W Xenon lamp | 48 μmol g−1 h−1 | [52] |
| 9. | UiO-66-NH2@Au@CdS | A 300 W xenon lamp, 10 mg photocatalysts was dispersed in 20 mL aqueous solution of L-ascorbic acid (pH = 4.0; 0.1 M). In particular, Pt (0.25 wt.%) was the cocatalyst for hydrogen production | 39.5 μmol h−1 | [53] |
| 10. | UiO-66 (NH2)/CuFe2O4 | 5 mg catalyst dispersed in 10 wt.% of MeOH solution kept in direct sunlight | 62.5 μmol g−1 h−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanmugam, M.; Agamendran, N.; Sekar, K. Efficient Charge Transfer of p-n Heterojunction UiO-66-NH2/CuFe2O4 Composite for Photocatalytic Hydrogen Production. Catalysts 2024, 14, 341. https://doi.org/10.3390/catal14060341
Shanmugam M, Agamendran N, Sekar K. Efficient Charge Transfer of p-n Heterojunction UiO-66-NH2/CuFe2O4 Composite for Photocatalytic Hydrogen Production. Catalysts. 2024; 14(6):341. https://doi.org/10.3390/catal14060341
Chicago/Turabian StyleShanmugam, Mariyappan, Nithish Agamendran, and Karthikeyan Sekar. 2024. "Efficient Charge Transfer of p-n Heterojunction UiO-66-NH2/CuFe2O4 Composite for Photocatalytic Hydrogen Production" Catalysts 14, no. 6: 341. https://doi.org/10.3390/catal14060341
APA StyleShanmugam, M., Agamendran, N., & Sekar, K. (2024). Efficient Charge Transfer of p-n Heterojunction UiO-66-NH2/CuFe2O4 Composite for Photocatalytic Hydrogen Production. Catalysts, 14(6), 341. https://doi.org/10.3390/catal14060341










