Ionic Liquid Modification of High-Pt-Loading Pt/C Electrocatalysts for Proton Exchange Membrane Fuel Cell Application
Abstract
:1. Introduction
2. Results and Discussion
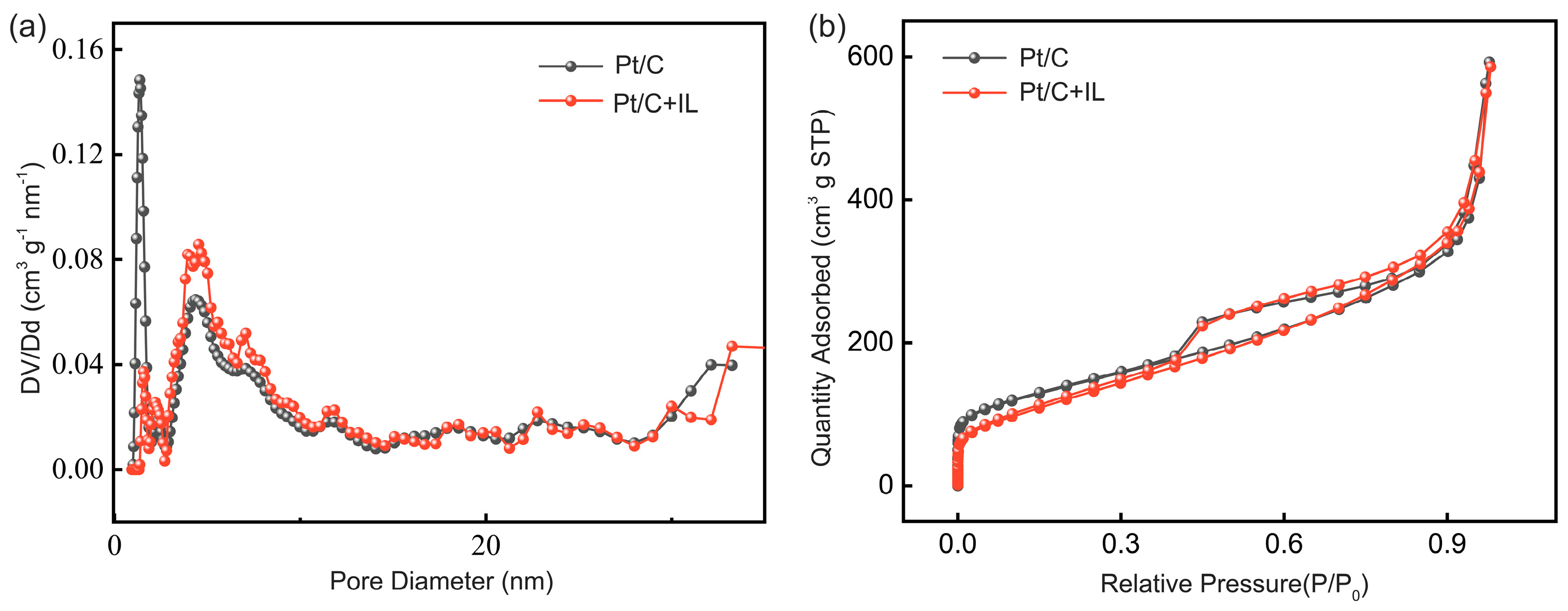
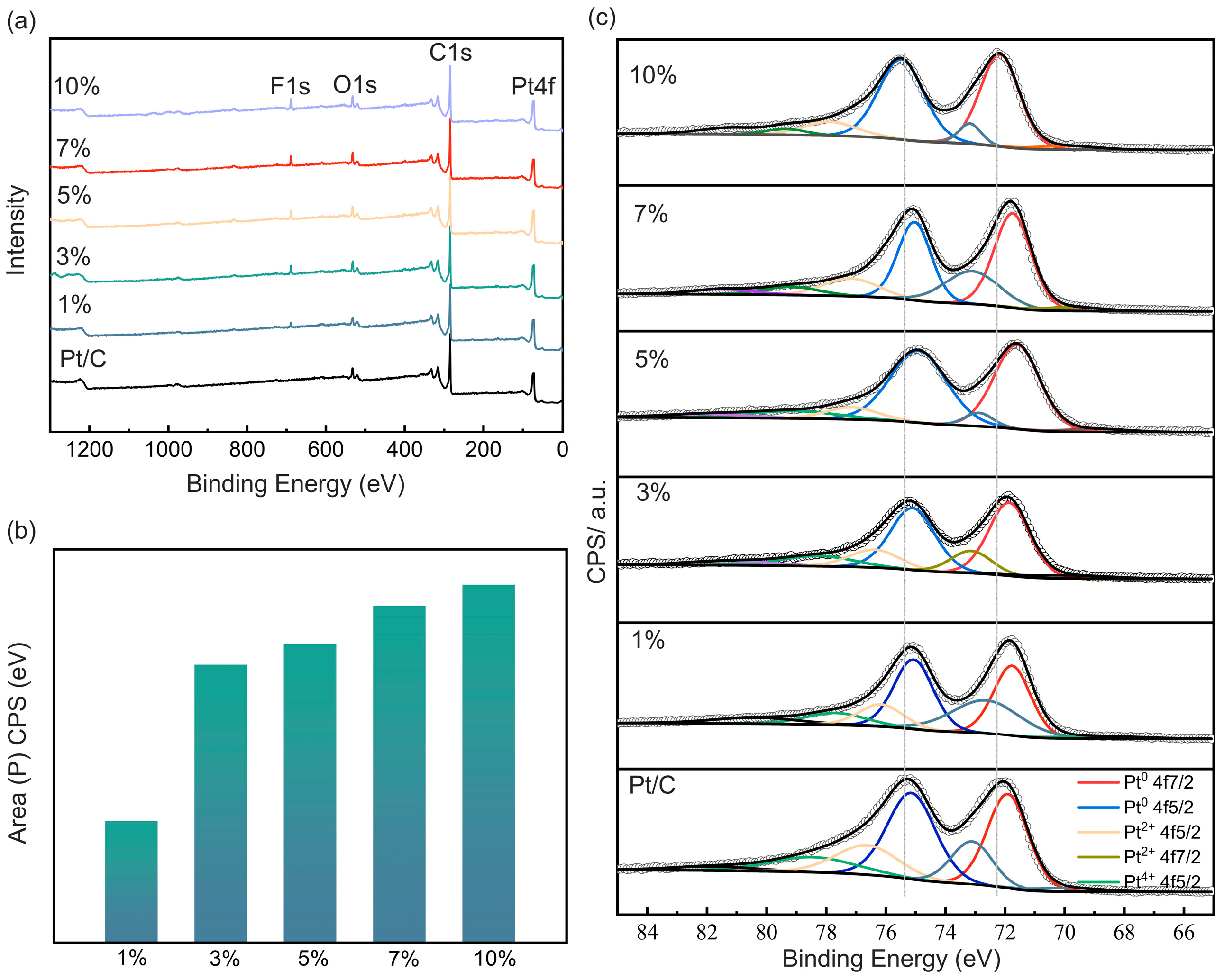
2.1. Characterization
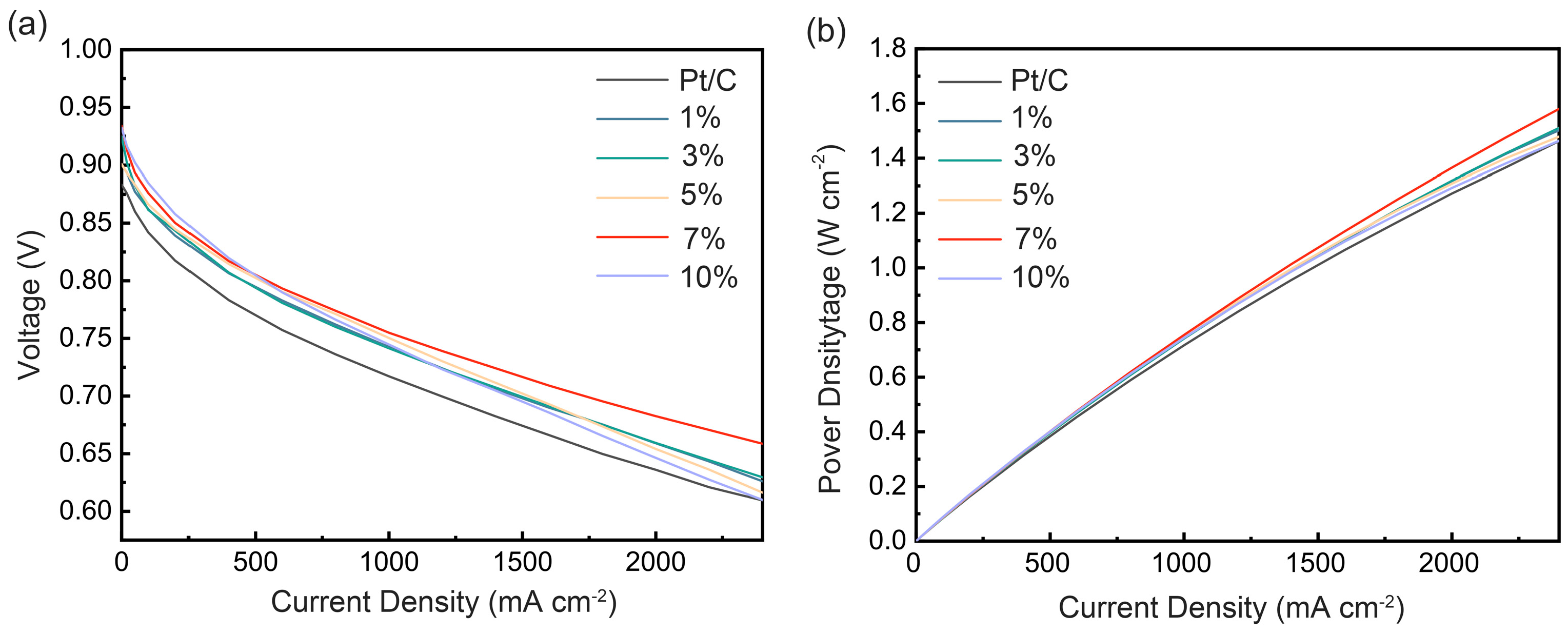
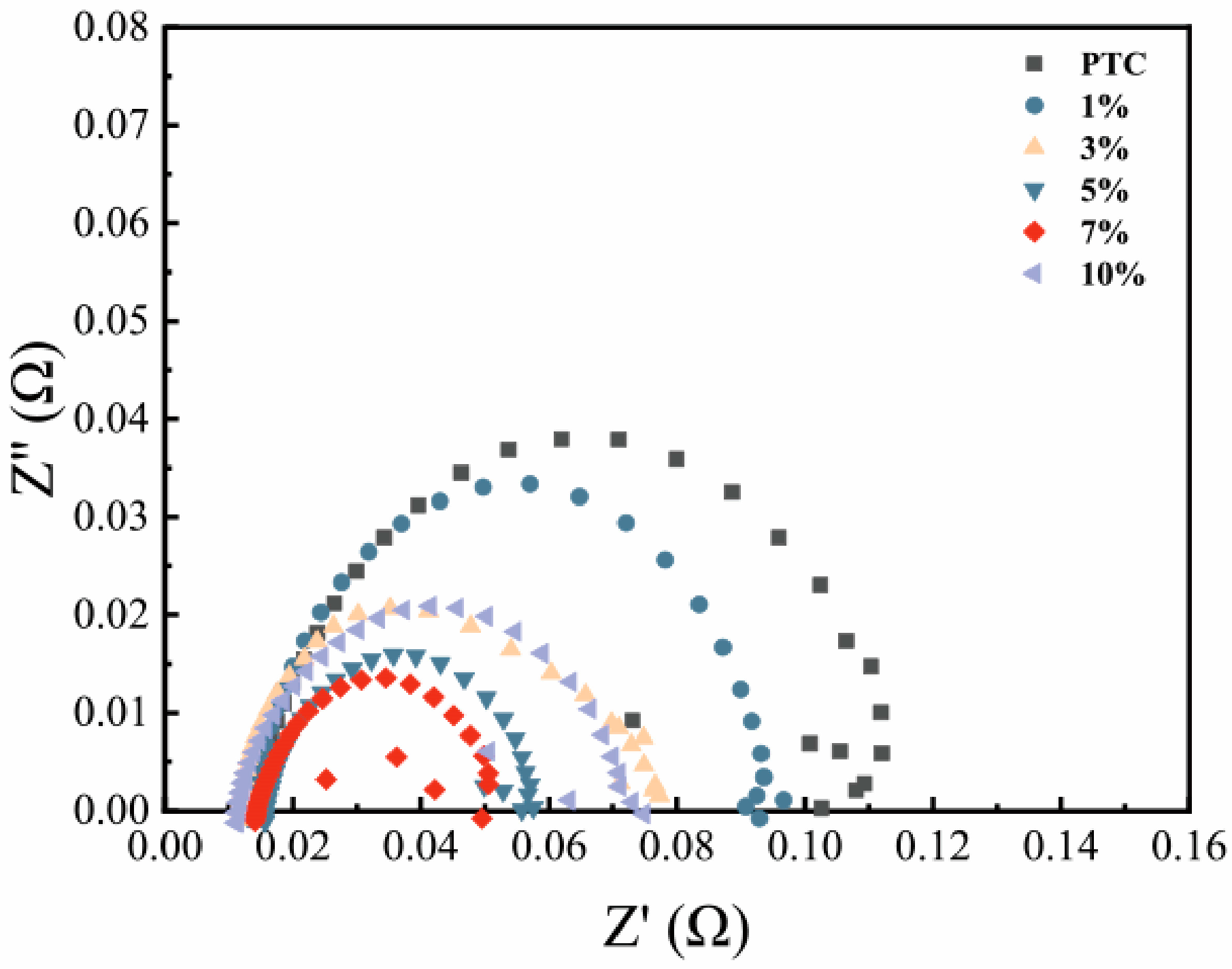
2.2. Electrochemical Measurements
3. Materials and Methods
3.1. Materials
3.2. Synthesis of IL [MTBD][beti]
3.3. [MTBD][beti] Modification of the Commercial Pt/C Electrocatalyst
3.4. Physical Characterization
3.5. Electrochemical Measurement
3.6. MEA Fabrication and Fuel Cell Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Z.; Liu, Z.; Zhang, A.; Yan, X.; Xue, W.; Peng, B.; Xin, H.L.; Pan, X.; Duan, X.; Huang, Y. Graphene-nanopocket-encaged PtCo nanocatalysts for highly durable fuel cell operation under demanding ultralow-Pt-loading conditions. Nat. Nanotechnol. 2022, 17, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, S.; Chen, S. Pt utilization in proton exchange membrane fuel cells: Structure impacting factors and mechanistic insights. Chem. Soc. Rev. 2022, 51, 1529–1546. [Google Scholar] [CrossRef]
- Zhao, J.; Tu, Z.; Chan, S.H. Carbon corrosion mechanism and mitigation strategies in a proton exchange membrane fuel cell (PEMFC): A review. J. Power Sources 2021, 488, 229434. [Google Scholar] [CrossRef]
- Li, H.; You, J.; Feng, Y.; Yan, X.; Yin, J.; Luo, L.; He, M.; Cheng, X.; Shen, S.; Zhang, J. Comprehensive understanding of oxygen transport at Gas/ionomer/electrocatalyst triple phase boundary in PEMFCs. Chem. Eng. J. 2023, 478, 147454. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Carpenter, M.K.; Moylan, T.E.; Kukreja, R.S.; Koestner, R.; Gu, W.; Thompson, L.; Kongkanand, A. Boosting Fuel Cell Performance with Accessible Carbon Mesopores. ACS Energy Lett. 2018, 3, 618–621. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, D.; Li, B.; Yang, D.; Ming, P.; Zhang, C. Effect of Dispersion Solvents and Ionomers on the Rheology of Catalyst Inks and Catalyst Layer Structure for Proton Exchange Membrane Fuel Cells. ACS Appl. Mater. Interfaces 2021, 13, 27119–27128. [Google Scholar] [CrossRef]
- Han, B.; Yu, S.; Wang, Z.; Zhu, H. Imidazole polymerized ionic liquid as a precursor for an iron-nitrogen-doped carbon electrocatalyst used in the oxygen reduction reaction. Int. J. Hydrogen Energy 2020, 45, 29645–29654. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, J.; Wang, H.; Wang, K.; Tian, Y.; Xue, X.; Qiao, Y.; Ji, X.; Zhang, S. Ionic liquids as a new cornerstone to support hydrogen energy. Green Chem. 2023, 25, 4981–4994. [Google Scholar] [CrossRef]
- Zhang, G.-R.; Etzold, B.J. Ionic liquids in electrocatalysis. J. Energy Chem. 2016, 25, 199–207. [Google Scholar] [CrossRef]
- Snyder, J.; Fujita, T.; Chen, M.; Erlebacher, J. Oxygen reduction in nanoporous metal–ionic liquid composite electrocatalysts. Nat. Mater. 2010, 9, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.; Livi, K.; Erlebacher, J. Oxygen Reduction Reaction Performance of [MTBD][beti]-Encapsulated Nanoporous NiPt Alloy Nanoparticles. Adv. Funct. Mater. 2013, 23, 5494–5501. [Google Scholar] [CrossRef]
- Zhang, G.-R.; Wolker, T.; Sandbeck, D.J.S.; Munoz, M.; Mayrhofer, K.J.J.; Cherevko, S.; Etzold, B.J.M. Tuning the Electrocatalytic Performance of Ionic Liquid Modified Pt Catalysts for the Oxygen Reduction Reaction via Cationic Chain Engineering. ACS Catal. 2018, 8, 8244–8254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-R.; Munoz, M.; Etzold, B.J. Boosting performance of low temperature fuel cell catalysts by subtle ionic liquid modification. ACS Appl. Mater. Interfaces 2015, 7, 3562–3570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-R.; Yong, C.; Shen, L.-L.; Yu, H.; Brunnengräber, K.; Imhof, T.; Mei, D.; Etzold, B.J. Increasing accessible active site density of non-precious metal oxygen reduction reaction catalysts through ionic liquid modification. ACS Appl. Mater. Interfaces 2023, 15, 18781–18789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, J.; Xia, B.; Li, Y.; Du, S. Ionic liquid modified Pt/C electrocatalysts for cathode application in proton exchange membrane fuel cells. Front. Chem. Sci. Eng. 2019, 13, 695–701. [Google Scholar] [CrossRef]
- Liu, W.; Di, S.; Wang, F.; Zhu, H. Ionic liquid modified fct-PtCo/C@ILs as high activity and durability electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2022, 47, 6312–6322. [Google Scholar] [CrossRef]
- Qiao, M.; Tang, C.; Tanase, L.C.; Teodorescu, C.M.; Chen, C.; Zhang, Q.; Titirici, M.-M. Oxygenophilic ionic liquids promote the oxygen reduction reaction in Pt-free carbon electrocatalysts. Mater. Horiz. 2017, 4, 895–899. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Thirunavukkarasu, G.; Salam, I.; Varcoe, J.R.; Mardle, P.; Li, X.; Mu, S.; Du, S. Ionic liquid-modified microporous ZnCoNC-based electrocatalysts for polymer electrolyte fuel cells. ACS Energy Lett. 2019, 4, 2104–2110. [Google Scholar] [CrossRef]
- Wang, C.; Kong, Y.; Soldemo, M.; Wu, Z.; Tissot, H.; Karagoz, B.; Marks, K.; Stenlid, J.H.; Shavorskiy, A.; Kokkonen, E. Stabilization of Cu2O through site-selective formation of a Co1Cu hybrid single-atom catalyst. Chem. Mater. 2022, 34, 2313–2320. [Google Scholar] [CrossRef]
- Addanki Tirumala, R.T.; Ramakrishnan, S.B.; Mohammadparast, F.; Khatri, N.; Arumugam, S.M.; Tan, S.; Kalkan, A.K.; Andiappan, M. Structure–Property–Performance Relationships of Dielectric Cu2O Nanoparticles for Mie Resonance-Enhanced Dye Sensitization. ACS Appl. Nano Mater. 2022, 5, 6699–6707. [Google Scholar] [CrossRef]
- Cozzi, D.; De Bonis, C.; D’Epifanio, A.; Mecheri, B.; Felice, V.; Tavares, A.; Licoccia, S. Functionalized metal oxides for PEMFC applications. ECS Trans. 2011, 41, 2297. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, S.-Y.; Popov, B.N. Mesoporous tin oxide as an oxidation-resistant catalyst support for proton exchange membrane fuel cells. J. Electrochem. Soc. 2010, 157, B1163. [Google Scholar] [CrossRef]
- George, M.; Zhang, G.-R.; Schmitt, N.; Brunnengräber, K.; Sandbeck, D.J.; Mayrhofer, K.J.; Cherevko, S.; Etzold, B.J. Effect of ionic liquid modification on the ORR performance and degradation mechanism of trimetallic PtNiMo/C catalysts. ACS Catal. 2019, 9, 8682–8692. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, O.; Kultin, D.; Zakharov, A.; Kustov, L. Advances in application of ionic liquids: Fabrication of surface nanoscale oxide structures by anodization of metals and alloys. Surf. Interfaces 2022, 34, 102345. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Wolker, T.; Brunnengraeber, K.; Martinaiou, I.; Lorenz, N.; Zhang, G.-R.; Kramm, U.I.; Etzold, B.J. The effect of temperature on ionic liquid modified Fe-NC catalysts for alkaline oxygen reduction reaction. J. Energy Chem. 2022, 68, 324–329. [Google Scholar] [CrossRef]
- Farooq, A.; Reinert, L.; Levêque, J.-M.; Papaiconomou, N.; Irfan, N.; Duclaux, L. Adsorption of ionic liquids onto activated carbons: Effect of pH and temperature. Microporous Mesoporous Mater. 2012, 158, 55–63. [Google Scholar] [CrossRef]
- Oh, H.; Lee, Y.i.; Lee, G.; Min, K.; Yi, J.S. Experimental dissection of oxygen transport resistance in the components of a polymer electrolyte membrane fuel cell. J. Power Sources 2017, 345, 67–77. [Google Scholar] [CrossRef]
- Kernchen, U.; Etzold, B.; Korth, W.; Jess, A. Solid catalyst with ionic liquid layer (SCILL)–A new concept to improve selectivity illustrated by hydrogenation of cyclooctadiene. Chem. Eng. Technol. Ind. Chem. Plant Equip. Process Eng. Biotechnol. 2007, 30, 985–994. [Google Scholar] [CrossRef]
- Peng, X.; Zhao, S.; Omasta, T.J.; Roller, J.M.; Mustain, W.E. Activity and durability of Pt-Ni nanocage electocatalysts in proton exchange membrane fuel cells. Appl. Catal. B Environ. 2017, 203, 927–935. [Google Scholar] [CrossRef]
- Chen, Y.; Rodenbücher, C.; Wippermann, K.; Korte, C. Revealing Interfacial Reactions on Pt Electrodes in Ionic Liquids by In Situ Fourier-Transform Infrared Spectroscopy. Anal. Chem. 2023, 95, 16618–16624. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Morales-Collazo, O.; Chen, Z.; Song, T.; Wang, L.; Lin, H.; Brennecke, J.F.; Jia, H. The Activity Enhancement Effect of Ionic Liquids on Oxygen Reduction Reaction Catalysts: From Rotating Disk Electrode to Membrane Electrode Assembly. Catalysts 2021, 11, 989. [Google Scholar] [CrossRef]
- Sobota, M.; Happel, M.; Amende, M.; Paape, N.; Wasserscheid, P.; Laurin, M.; Libuda, J. Ligand effects in SCILL model systems: Site-specific interactions with Pt and Pd nanoparticles. Adv. Mater. 2011, 22, 2617–2621. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Du, S.; Steinberger-Wilckens, R. Temperature-controlled growth of single-crystal Pt nanowire arrays for high performance catalyst electrodes in polymer electrolyte fuel cells. Appl. Catal. B Environ. 2015, 164, 389–395. [Google Scholar] [CrossRef]
- Avid, A.; Ochoa, J.L.; Huang, Y.; Liu, Y.; Atanassov, P.; Zenyuk, I.V. Revealing the role of ionic liquids in promoting fuel cell catalysts reactivity and durability. Nat. Commun. 2022, 13, 6349. [Google Scholar] [CrossRef] [PubMed]
- Lexow, M.; Maier, F.; Steinrück, H.-P. Ultrathin ionic liquid films on metal surfaces: Adsorption, growth, stability and exchange phenomena. Adv. Phys. X 2020, 5, 1761266. [Google Scholar] [CrossRef]
- Cremer, T.; Wibmer, L.; Calderón, S.K.; Deyko, A.; Maier, F.; Steinrück, H.-P. Interfaces of ionic liquids and transition metal surfaces—Adsorption, growth, and thermal reactions of ultrathin [C1C1Im][Tf2N] films on metallic and oxidised Ni(111) surfaces. Phys. Chem. Chem. Phys. 2012, 14, 5153–5163. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, F.; Guo, Y.; Liang, X.; Yue, F.; Yan, Y.; Li, Y.; Zhu, Y.; He, Y.; Du, S. Ionic Liquid Modification of High-Pt-Loading Pt/C Electrocatalysts for Proton Exchange Membrane Fuel Cell Application. Catalysts 2024, 14, 344. https://doi.org/10.3390/catal14060344
Cheng F, Guo Y, Liang X, Yue F, Yan Y, Li Y, Zhu Y, He Y, Du S. Ionic Liquid Modification of High-Pt-Loading Pt/C Electrocatalysts for Proton Exchange Membrane Fuel Cell Application. Catalysts. 2024; 14(6):344. https://doi.org/10.3390/catal14060344
Chicago/Turabian StyleCheng, Fengshun, Yuchen Guo, Xinhong Liang, Fanqiushi Yue, Yichang Yan, Yang Li, Yuanzhi Zhu, Yanping He, and Shangfeng Du. 2024. "Ionic Liquid Modification of High-Pt-Loading Pt/C Electrocatalysts for Proton Exchange Membrane Fuel Cell Application" Catalysts 14, no. 6: 344. https://doi.org/10.3390/catal14060344
APA StyleCheng, F., Guo, Y., Liang, X., Yue, F., Yan, Y., Li, Y., Zhu, Y., He, Y., & Du, S. (2024). Ionic Liquid Modification of High-Pt-Loading Pt/C Electrocatalysts for Proton Exchange Membrane Fuel Cell Application. Catalysts, 14(6), 344. https://doi.org/10.3390/catal14060344








