Highly Tuning of Sunlight-Photocatalytic Properties of SnO2 Nanocatalysts: Function of Gd/Fe Dopants
Abstract
1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction (XRD)
2.2. FTIR Spectra
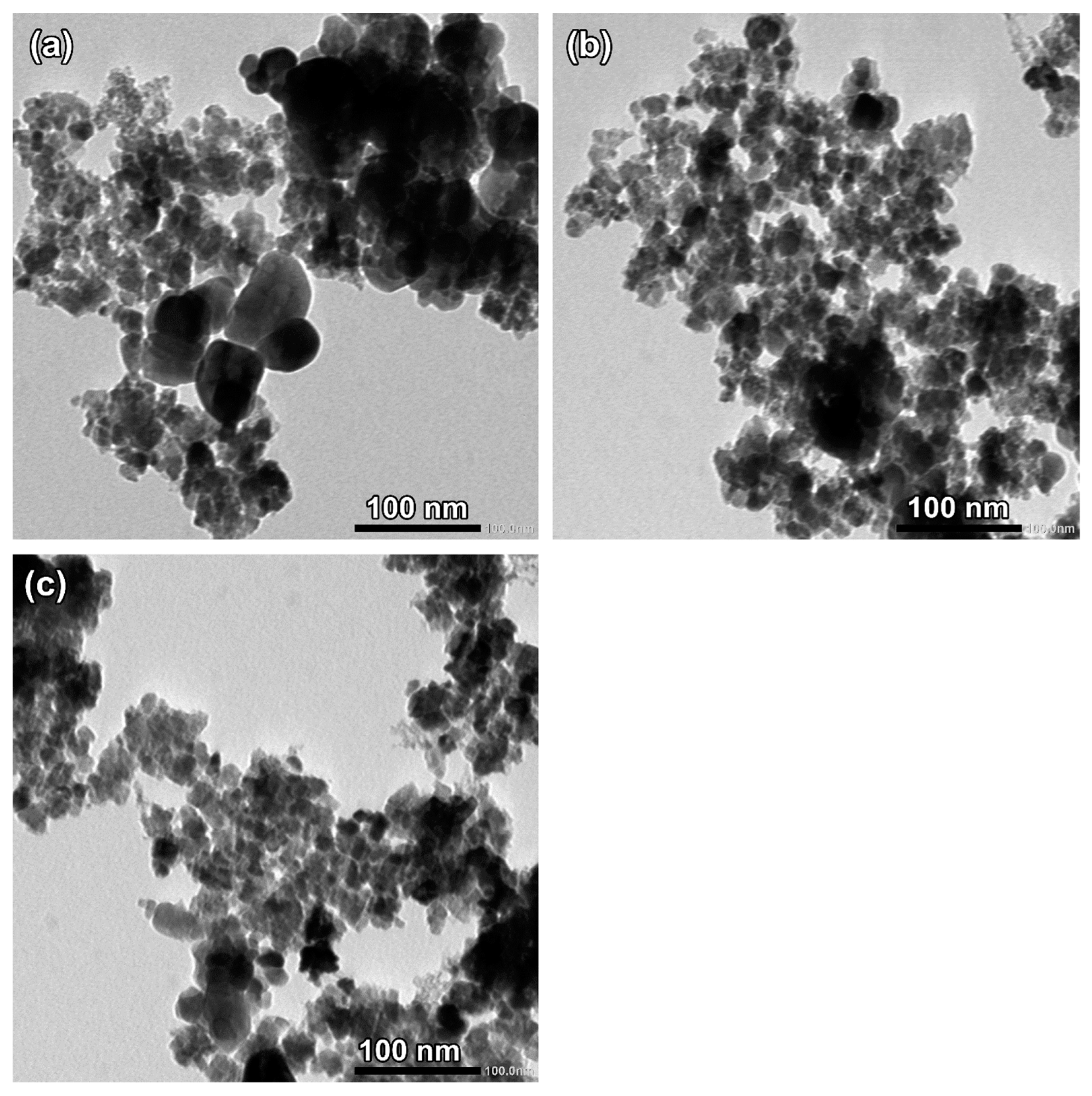
2.3. Transmission Electron Microscope (TEM)
2.4. Band Gap Energy and Optical Properties
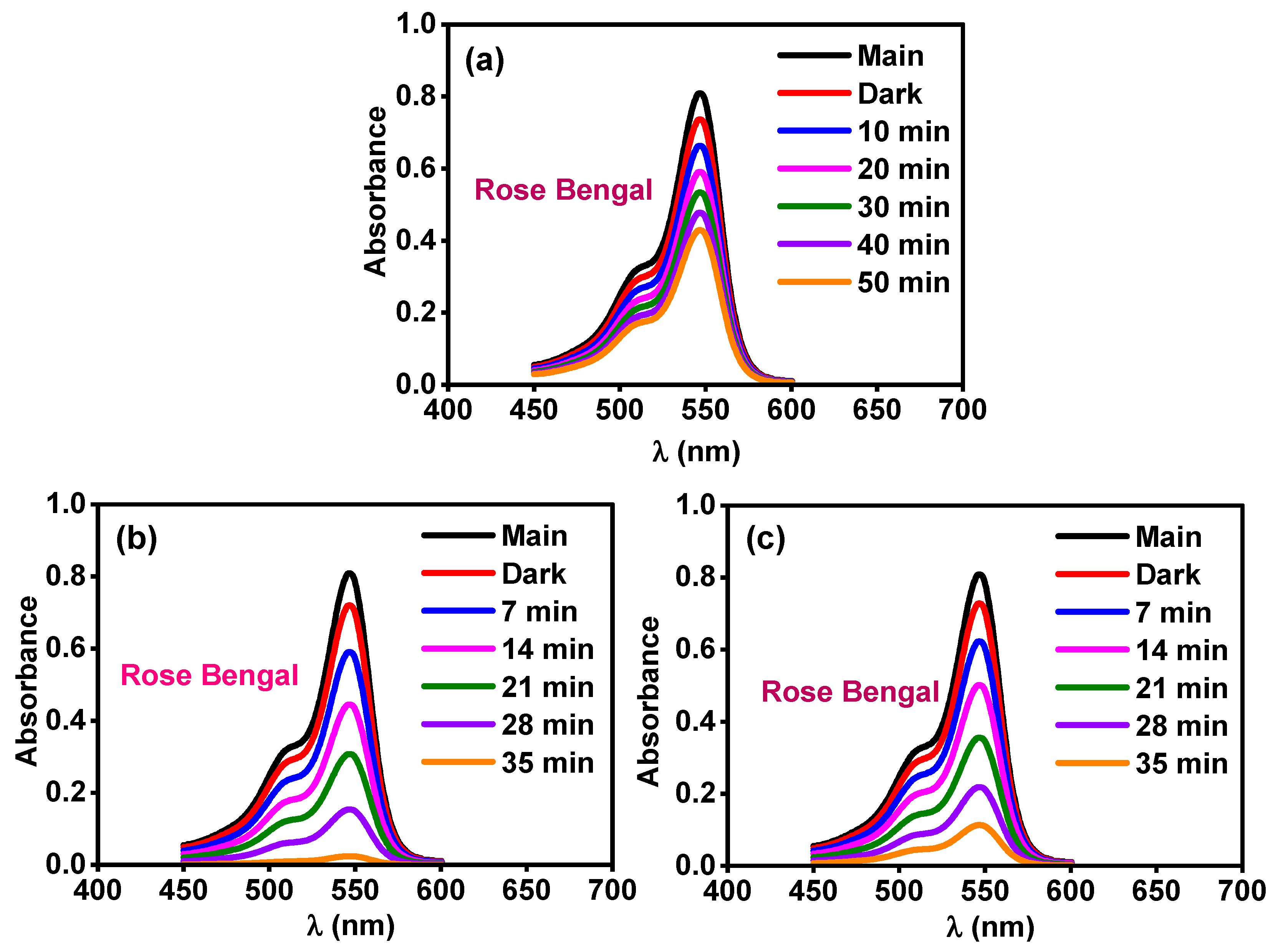
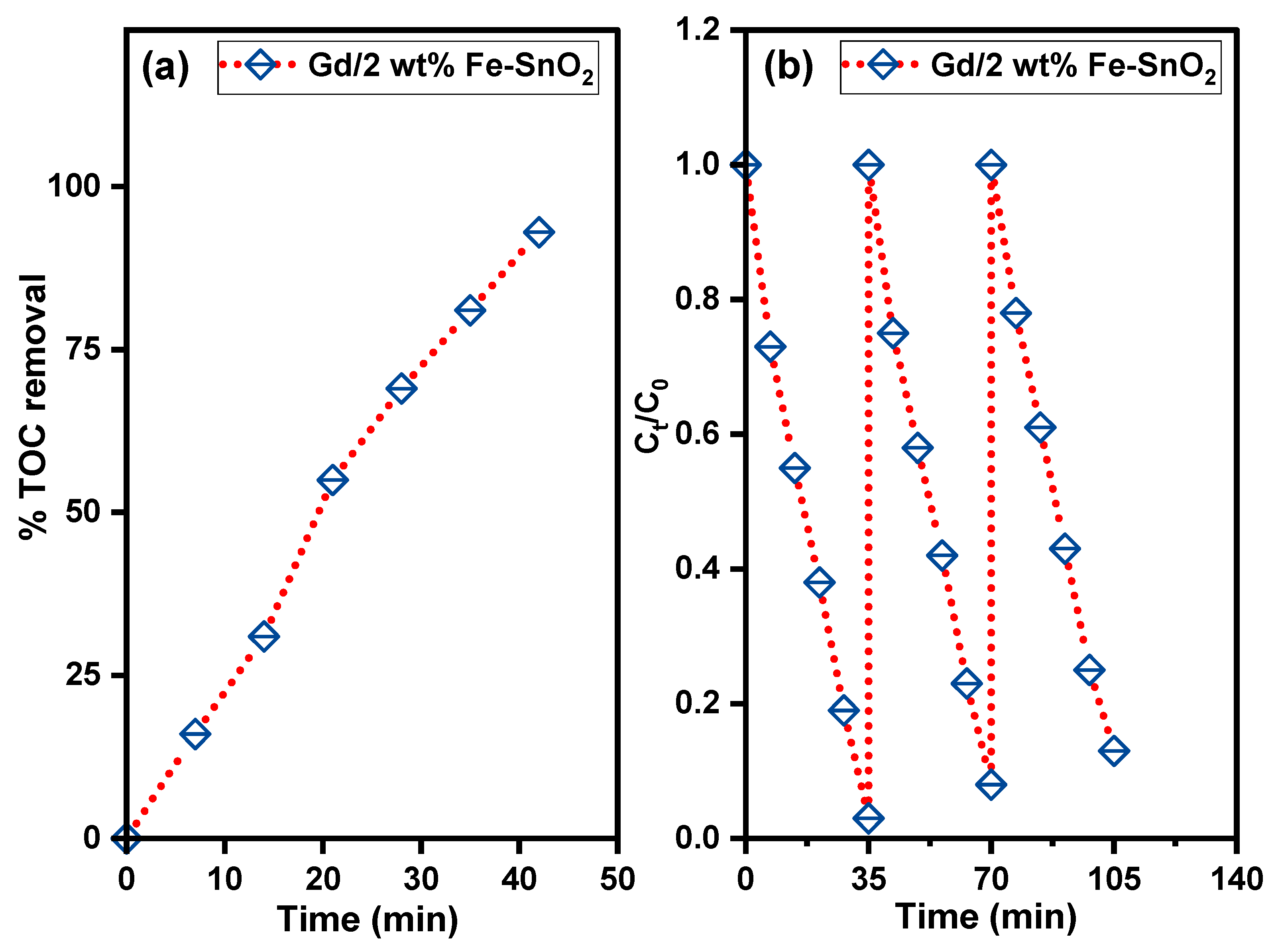
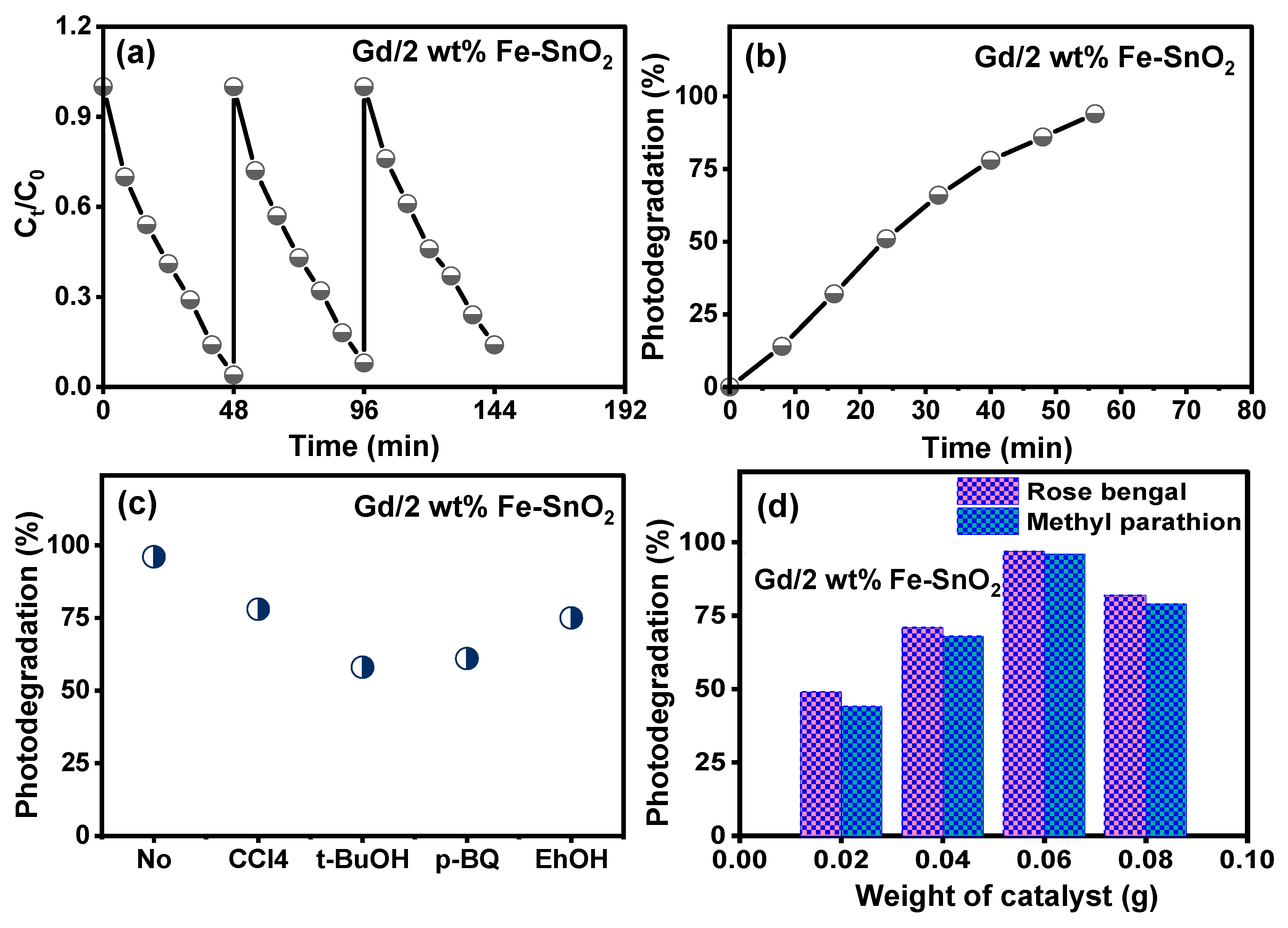
2.5. Activity for Removal of Organic Pollutants
3. Materials and Methods
3.1. Preparation of Nanosized Undoped and Gd/Fe-SnO2 Powders
3.2. Physical Analysis and Photocatalytic Procedure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basha, B.; Manzoor, A.; Alrowaili, Z.A.; Ihsan, A.; Shakir, I.; Al-Buriahig, M.S. Ba2−xHoxSr2−yNiyFe12O22 and its composite with MXene: Synthesis, characterization and enhanced visible light mediated photocatalytic activity for colored dye and pesticide. RSC Adv. 2023, 13, 29944–29958. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, M.; Taher, M.A.; Tamaddon, A.M. Facile synthesis and characterization of magnetic nanocomposite ZnO/CoFe2O4 hetero-structure for rapid photocatalytic degradation of imidacloprid. Heliyon 2019, 5, e02870. [Google Scholar] [CrossRef] [PubMed]
- Malini, B.; Gandhimathi, R. An overview of photocatalytic degradation of agricultural pollutants in water. Int. J. Green Energy 2024, 1–15. [Google Scholar] [CrossRef]
- Dihom, H.R.; Al-Shaibani, M.M.; Mohamed, R.M.S.R.; Al-Gheethi, A.A.; Sharma, A.; Khamidun, M.H.B. Photocatalytic degradation of disperse azo dyes in textile wastewater using green zinc oxide nanoparticles synthesized in plant extract: A critical review. J. Water Process Eng. 2022, 47, 102705. [Google Scholar] [CrossRef]
- Kawsar, M.; Hossain, M.S.; Tabassum, S.; Bahadur, N.M.; Ahmed, S. Synthesis of different types of nanohydroxyapatites for efficient photocatalytic degradation of textile dye (Congo red): A crystallographic characterization. RSC Adv. 2024, 14, 11570–11583. [Google Scholar] [CrossRef] [PubMed]
- Madkhali, N.; Prasad, C.; Malkappa, K.; Choi, H.Y.; Govinda, V.; Bahadur, I.; Abumousa, R.A. Recent update on photocatalytic degradation of pollutants in waste water using TiO2-based heterostructured materials. Results Eng. 2023, 17, 100920. [Google Scholar] [CrossRef]
- Natsathaporn, P.; Jenjob, R.; Pattanasattayavong, P.; Yiamsawas, D.; Crespy, D. Photocatalytic degradation of pesticides by nanofibrous membranes fabricated by colloid-electrospinning. Nanotechnology 2020, 31, 215603. [Google Scholar] [CrossRef]
- Khan, K.A.; Shah, A.; Nisar, J. Electrochemical detection and removal of brilliant blue dye via photocatalytic degradation and adsorption using phyto-synthesized nanoparticles. RSC Adv. 2024, 14, 2504–2517. [Google Scholar] [CrossRef]
- Yeganeh, M.; Charkhloo, E.; Sobhi, H.R.; Esrafili, A.; Gholami, M. Photocatalytic processes associated with degradation of pesticides in aqueous solutions: Systematic review and meta-analysis. Chem. Eng. J. 2022, 428, 130081. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Rafiee, E.; Eavani, S. Photocatalytic removal of toxic dyes, liquorice and tetracycline wastewaters by a mesoporous photocatalyst under irradiation of different lamps and sunlight. J. Environ. Manag. 2022, 313, 115023. [Google Scholar] [CrossRef]
- Germani, R.; Mancinelli, M.; Roselli, A.; Tiecco, M.; Fantacci, S.; di Bona, S.; Giacco, T.D. Kinetic effects of cationic surfactants on the photocatalytic degradation of anionic dyes in aqueous TiO2 dispersions. New J. Chem. 2023, 47, 392–401. [Google Scholar] [CrossRef]
- Ramadevi, P.; Shanmugavadivu, R.; Venkatesan, R.; Mayandi, J.; Sagadevan, S. Photocatalytic dye degradation efficiency and reusability of aluminium substituted nickel ferrite nanostructures for wastewater remediation. Inorg. Chem. Commun. 2023, 150, 110532. [Google Scholar] [CrossRef]
- Geldasa, F.T.; Kebede, M.A.; Shura, M.W.; Hone, F.G. Experimental and computational study of metal oxide nanoparticles for the photocatalytic degradation of organic pollutants: A review. RSC Adv. 2023, 13, 18404–18442. [Google Scholar] [CrossRef] [PubMed]
- Roy, H.; Ur Rahman, T.; Md. Khan, A.J.R.; Al-Mamun, M.R.; Islam, S.Z.; Khaleque, M.A.; Hossain, M.I.; Khan, M.Z.H.; Islam, M.S.; Marwani, H.M.; et al. Toxic dye removal, remediation, and mechanism with doped SnO2-based nanocomposite photocatalysts: A critical review. J. Water Process Eng. 2023, 54, 104069. [Google Scholar] [CrossRef]
- Zieliński, B.; Miądlicki, P.; Przepiórski, J. Development of activated carbon for removal of pesticides from water: Case study. Sci. Rep. 2022, 12, 20869. [Google Scholar] [CrossRef]
- Nyankson, E.; Kumar, R.V. Removal of water-soluble dyes and pharmaceutical wastes by combining the photocatalytic properties of Ag3PO4 with the adsorption properties of halloysite nanotubes. Mater. Today Adv. 2019, 4, 100025. [Google Scholar] [CrossRef]
- Moghaddam, S.S.; Moghaddam, M.R.A.; Arami, M. Coagulation/flocculation process for dye removal using sludge from water treatment plant: Optimization through response surface methodology. J. Hazard. Mater. 2010, 175, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Fradj, A.B.; Boubakri, A.; Hafiane, A.; Hamouda, S.B. Removal of azoic dyes from aqueous solutions by chitosan enhanced ultrafiltration. Results Chem. 2020, 2, 100017. [Google Scholar] [CrossRef]
- Dhananjay, P.; Abhilash, M.R.; Shilpa, N.; Hemanth, K.N.K.; Gowtham, H.G.; Aiyaz, M.; Brijesh, S.S.; Abdul, M.; Suhail, A.; Murali, M. Solar irradiation driven catalytic dye degradation by novel biosynthesized zinc oxide nanoparticles (ZnO–NPs) from Barleria mysorensis: Kinetics, reusability and mineralization studies. J. Mol. Struct. 2024, 1303, 137549. [Google Scholar]
- Fatima, S.; Munawar, T.; Nadeem, M.S.; Mukhtar, F.; Khan, S.A.; Koc, M.; Iqbal, F. Boosted natural sunlight driven photodegradation of organic dyes using rGO anchored Pr/Cu dual-doped ZnO nanocomposite: Characterization and mechanistic insight. Opt. Mater. 2023, 136, 113397. [Google Scholar] [CrossRef]
- Xu, J.; Shen, J.; Jiang, H.; Yu, X.; Qureshi, W.A.; Maouche, C.; Gao, J.; Yang, J.; Liu, Q. Progress and challenges in full spectrum photocatalysts: Mechanism and photocatalytic applications. J. Ind. Eng. Chem. 2023, 119, 112–129. [Google Scholar] [CrossRef]
- Ullah, S.; Ferreira-Neto, E.P.; Hazra, C.; Parveen, R.; Rojas-Mantilla, H.D.; Calegaro, M.L.; Serge-Correales, Y.E.; Rodrigues-Filho, U.P.; Ribeiro, S.J.L. Broad spectrum photocatalytic system based on BiVO4 and NaYbF4:Tm3+ upconversion particles for environmental remediation under UV-vis-NIR illumination. Appl. Catal. B Environ. 2019, 243, 121–135. [Google Scholar] [CrossRef]
- Chong, C.Y.; Sum, J.Y.; Lai, L.S.; Toh, P.Y.; Chang, Z.H. Visible light-driven dye degradation by magnetic cobalt-doped zinc oxide/iron oxide photocatalyst. Next Mater. 2024, 2, 100074. [Google Scholar] [CrossRef]
- Sultana, M.; Mondal, A.; Islam, S.; Khatun, M.A.; Rahaman, M.H.; Chakraborty, A.K.; Rahman, M.S.; Rahman, M.M.; Nur, A.S.M. Strategic development of metal doped TiO2 photocatalysts for enhanced dye degradation activity under UV–Vis irradiation: A review. Curr. Res. Green Sustain. Chem. 2023, 7, 100383. [Google Scholar] [CrossRef]
- Matalkeh, M.; Nasrallah, G.K.; Shurrab, F.M.; Al-Absi, E.S.; Mohammed, W.; Elzatahry, A.; Saoud, K.M. Visible light photocatalytic activity of Ag/WO3 nanoparticles and its antibacterial activity under ambient light and in the dark. Results Eng. 2022, 13, 100313. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, N.; Diery, W.A.; Moujaes, E.A.; Mittal, A.; Singh, P.; Sharma, S. Co+2, Ni+2, Cu+2 doped Indium oxide as visible active nano-photocatalyst: A facile solution combustion synthesis, electronic band structure analysis by DFT approach and photocatalytic decontamination of RhB and Triclopyr. J. Mol. Liq. 2023, 392, 123508. [Google Scholar] [CrossRef]
- Mezyen, M.; El Fidha, G.; Bitri, N.; Harrathi, F.; Ly, I.; Llobet, E. Visible light activated SnO2:Dy thin films for the photocatalytic degradation of methylene blue. RSC Adv. 2023, 13, 31151–31166. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar]
- Kong, Y.; Li, Y.; Cui, X.; Su, L.; Ma, D.; Lai, T.; Yao, L.; Xiao, X.; Wang, Y. SnO2 nanostructured materials used as gas sensors for the detection of hazardous and flammable gases: A review. Nano Mater. Sci. 2022, 4, 339–350. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, Q.; Zhang, L.; Han, C.; Gao, H.; Zhang, Y.; Yan, H. SnO2-based electron transporting layer materials for perovskite solar cells: A review of recent progress. J. Energy Chem. 2019, 35, 144–167. [Google Scholar] [CrossRef]
- Shabna, S.; Dhas, S.S.J.; Biju, C.S. Potential progress in SnO2 nanostructures for enhancing photocatalytic degradation of organic pollutants. Catal. Commun. 2023, 177, 106642. [Google Scholar] [CrossRef]
- Arif, M.; Shah, M.Z.U.; Ahmad, S.A.; Shah, M.S.; Ali, Z.; Ullah, A.; Idrees, M.; Zeb, J.; Song, P.; Huang, T.; et al. High photocatalytic performance of copper-doped SnO2 nanoparticles in degradation of Rhodamine B dye. Opt. Mater. 2022, 134, 113135. [Google Scholar] [CrossRef]
- Baig, A.A.B.; Rathinam, V.; Ramya, V. Synthesis and investigation of Fe doped SnO2 nanoparticles for improved photocatalytic activity under visible light and antibacterial performances. Mater. Technol. 2020, 36, 623–635. [Google Scholar] [CrossRef]
- Alam, U.; Khan, A.; Raza, W.; Khan, A.; Bahnemann, D.; Muneer, M. Highly efficient Y and V co-doped ZnO photocatalyst with enhanced dye sensitized visible light photocatalytic activity. Catal. Today 2017, 284, 169–178. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, S.; Liu, J.; Zhang, Y.; Wang, Y.; Yu, J.; Yuan, M.; Zhang, P.; Liu, W.; Zhang, J. C, F co-doping Ag/TiO2 with visible light photocatalytic performance toward degrading Rhodamine B. Environ. Res. 2023, 232, 116311. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, C.; Chen, W. Fabrication of SnO-SnO2 nanocomposites with p-n heterojunctions for low-temperature sensing of NO2 gas. Nanoscale 2015, 7, 12133–12142. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, N.M.; Hamad, D.; Abdel-Latief, A.Y.; Abdel-Rahim, M.A. Preparation of quantum size of tin oxide: Structural and physical characterization. Prog. Nat. Sci. Mater. Int. 2016, 26, 145–151. [Google Scholar] [CrossRef]
- Chen, H.; Ding, L.; Sun, W.; Jiang, Q.; Hu, J.; Li, J. Synthesis and characterization of Ni doped SnO2 microspheres with enhanced visible-light photocatalytic activity. RSC Adv. 2015, 5, 56401–56409. [Google Scholar] [CrossRef]
- Raji, R.; Jyothi, G.; Sasidharan, S.; Gopchandran, K.G. White light-emitting ZnO:Dy3+ nanophosphors: Delving into the spectroscopic parameters via Judd–Ofelt analysis. Dalton Trans. 2024, 53, 6234–6244. [Google Scholar] [CrossRef]
- Bannur, M.S.; Antony, A.; Maddani, K.I.; Poornesh, P.; Manjunatha, K.B.; Kulkarni, S.D.; Choudhari, K.S. Role of Ba in engineering band gap, photoluminescence and nonlinear optical properties of SnO2 nanostructures for photovoltaic and photocatalytic applications. Superlattices Microstruct. 2018, 122, 156–164. [Google Scholar] [CrossRef]
- Baig, A.B.A.; Rathinam, V.; Palaninathan, J. Facile synthesis of Ce-doped SnO2 nanoparticles with enhanced performance for photocatalytic degradation of organic dye. J. Iran. Chem. Soc. 2021, 18, 13–27. [Google Scholar] [CrossRef]
- Khatun, M.H.; Amin, R.; Sarker, M.S.I.; Shikder, M.R.; Islam, S.; Shahjahan, M. Structural and magnetic properties of Fe and Ni co-doped SnO2 nanoparticles prepared by co-precipitation method. Mater. Res. Express 2024, 11, 016102. [Google Scholar] [CrossRef]
- Zhao, X.; Wen, T.; Zhang, J.; Ye, J.; Ma, Z.; Yuan, H.; Ye, X.; Wang, Y. Fe-Doped SnO2 catalysts with both BA and LA sites: Facile preparation and biomass carbohydrates conversion to methyl lactate MLA. RSC Adv. 2017, 7, 21678–21685. [Google Scholar] [CrossRef]
- Singh, G.; Singh, R.C. Synthesis and characterization of Gd-doped SnO2 nanostructures and their enhanced gas sensing properties. Ceram. Int. 2017, 43, 2350–2360. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, N.; Feng, L.; Wen, S.; An, Y.; Liu, J. Local structure and magnetic properties of Fe-doped SnO2 films. J. Alloys Compd. 2017, 698, 863–867. [Google Scholar] [CrossRef]
- Lin, X.; Chen, Y.; Zhou, D.; Chen, M.; Liang, W.; Guo, H. Aminated graphene quantum dots/CdS nanobelts for enhanced photocatalytic degradation of RhB dye under visible light. RSC Adv. 2024, 14, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Lua, X.-X.; Luo, Y.-H.; Liu, Y.-S.; Ma, W.-W.; Xu, Y.; Zhang, H. Assembly of three stable POM-based pillar-layer CuI coordination polymers with visible light driven photocatalytic properties. CrystEngComm 2016, 18, 3650–3654. [Google Scholar] [CrossRef]
- Xiao, L.; Liao, R.; Yang, S.; Qiu, Y.; Wang, M.; Zhang, Z.; Du, J.; Xie, Z. Facile fabrication of F-doped SnO2 nanomaterials for improved photocatalytic activity. Coatings 2022, 12, 795. [Google Scholar] [CrossRef]
- Ahmad, S.; Almehmadi, M.; Janjuhah, H.T.; Kontakiotis, G.; Abdulaziz, O.; Saeed, K.; Ahmad, H.; Allahyani, M.; Aljuaid, A.; Alsaiari, A.A.; et al. The Effect of Mineral Ions Present in Tap Water on Photodegradation of Organic Pollutants: Future Perspectives. Water 2023, 15, 175. [Google Scholar] [CrossRef]








| Composition | SnCl4·5H2O (g) | Gd(NO3)3·6H2O (g) | Fe(NO3)3·9H2O (g) |
|---|---|---|---|
| SnO2 | 10.518 | - | - |
| Sn0.96Gd0.02Fe0.02O2 | 10.097 | 0.27 | 0.24 |
| Sn0.94Gd0.02Fe0.04O2 | 9.88 | 0.27 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsulaim, G.M.; Alsharif, S.A. Highly Tuning of Sunlight-Photocatalytic Properties of SnO2 Nanocatalysts: Function of Gd/Fe Dopants. Catalysts 2024, 14, 347. https://doi.org/10.3390/catal14060347
Alsulaim GM, Alsharif SA. Highly Tuning of Sunlight-Photocatalytic Properties of SnO2 Nanocatalysts: Function of Gd/Fe Dopants. Catalysts. 2024; 14(6):347. https://doi.org/10.3390/catal14060347
Chicago/Turabian StyleAlsulaim, Ghayah M., and Shada A. Alsharif. 2024. "Highly Tuning of Sunlight-Photocatalytic Properties of SnO2 Nanocatalysts: Function of Gd/Fe Dopants" Catalysts 14, no. 6: 347. https://doi.org/10.3390/catal14060347
APA StyleAlsulaim, G. M., & Alsharif, S. A. (2024). Highly Tuning of Sunlight-Photocatalytic Properties of SnO2 Nanocatalysts: Function of Gd/Fe Dopants. Catalysts, 14(6), 347. https://doi.org/10.3390/catal14060347






