Abstract
In this study, we report the synthesis of eight Schiff bases (3–10) type N-heterocycle (N-het) using conventional refluxing conditions as well as different eco-friendly techniques such as grinding, thermal fusion, microwave irradiation (MWI) and ultrasound, all of them in the presence of a catalytic amount of acetic acid. These procedures had the additional advantage of being environmentally friendly and high-yield, making these protocols an alternative for Schiff-base syntheses. The obtained Schiff bases were coordinated to palladium, generating new complexes of type [Pd2Cl4(N-het)2]. Complexes [Pd2Cl4(5)2] and [Pd2Cl4(9)2] showed high activity and selectivity for a model Mizoroki–Heck C-C coupling reaction of styrene with iodobenzene and bromobenzaldehydes. All compounds and complexes were evaluated for antifungal activity against clinically important fungi such as Candida albicans and Cryptococcus neoformans. Although the Schiff bases (3–10) showed low antifungal activity against both fungi, some of their palladium complexes such as [Pd2Cl4(3)2], [Pd2Cl4(5)2], [Pd2Cl4(8)2] and [Pd2Cl4(10)2] showed comparatively higher antifungal effects mainly against C. neoformans. The product of the Mizoroki–Heck-type C-C coupling reactions, 4-styrylbenzaldehyde, was isolated and purified to be later used in the synthesis of four new nitrophenylpyrazole derivatives of styrylimine, which also displayed antifungal activity, especially against C. neoformans.
1. Introduction
The pyrazole scaffold is present in a plethora of biologically relevant molecules that show a wide variety of pharmaceutical and biological properties, including antifungal, anticancer, antiviral, anti-inflammatory, antioxidant and other activities [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19]. This scaffold is also useful as a synthetic building block and in coordination chemistry [20,21,22,23,24,25,26,27,28,29,30,31,32]. Hence, considerable efforts have been made in the synthesis of new pyrazole-based complex structures and in the development of new methods for their construction [33,34,35,36]. The palladium–pyrazole complexes are active in Mizoroki–Heck reactions, which is the most efficient route for the vinylation of aryl/vinyl halides or triflates involving the formation of new C-C bonds [37,38,39]. Among various commonly used reactions for C-C coupling (viz. Mizoroki–Heck, Suzuki, Stille, and Sonogashira reactions), the Mizoroki–Heck reaction may be the most attractive organic reaction catalyzed by palladium complexes to produce chemicals for the elaborate synthesis of pharmaceuticals and natural products [40,41,42,43]. In recent years, fungi have emerged as a major cause of human infections, especially among immunocompromised hosts, with a tremendous impact on morbidity and mortality [44]. Although there are diverse drugs available for the treatment of systemic and superficial mycoses, many of them are not completely effective for their eradication [45,46]. In addition, the currently available compounds possess varied degrees of toxicity, and fungi quickly develop resistance due to their large-scale use [47,48].
Herein, the synthesis of the nitrophenylpyrazole derivatives of hydroxyphenyl imines was carried out because the pyrazole ring is pπ–excedent, so it can transfer negative charge density to substituents such as the nitrophenyl groups. This can therefore allow the charge-density transfer from the dπ orbitals of palladium to the pπ* of the pyrazole ring, generating a strong metal-N-pyrazole bond. Thus, these stable complexes can be used in Mizoroki–Heck C-C coupling reactions, even at high temperatures and in an aqueous medium. We also report the synthesis of new nitrophenylpyrazole-derived Schiff bases and their palladium complexes, which have potential biological activities [49,50,51]. These new compounds were tested for their antifungal properties against clinically important fungal species such as Candida albicans and Cryptococcus neoformans.
2. Results and Discussion
2.1. Synthesis of Ligands (3–10)
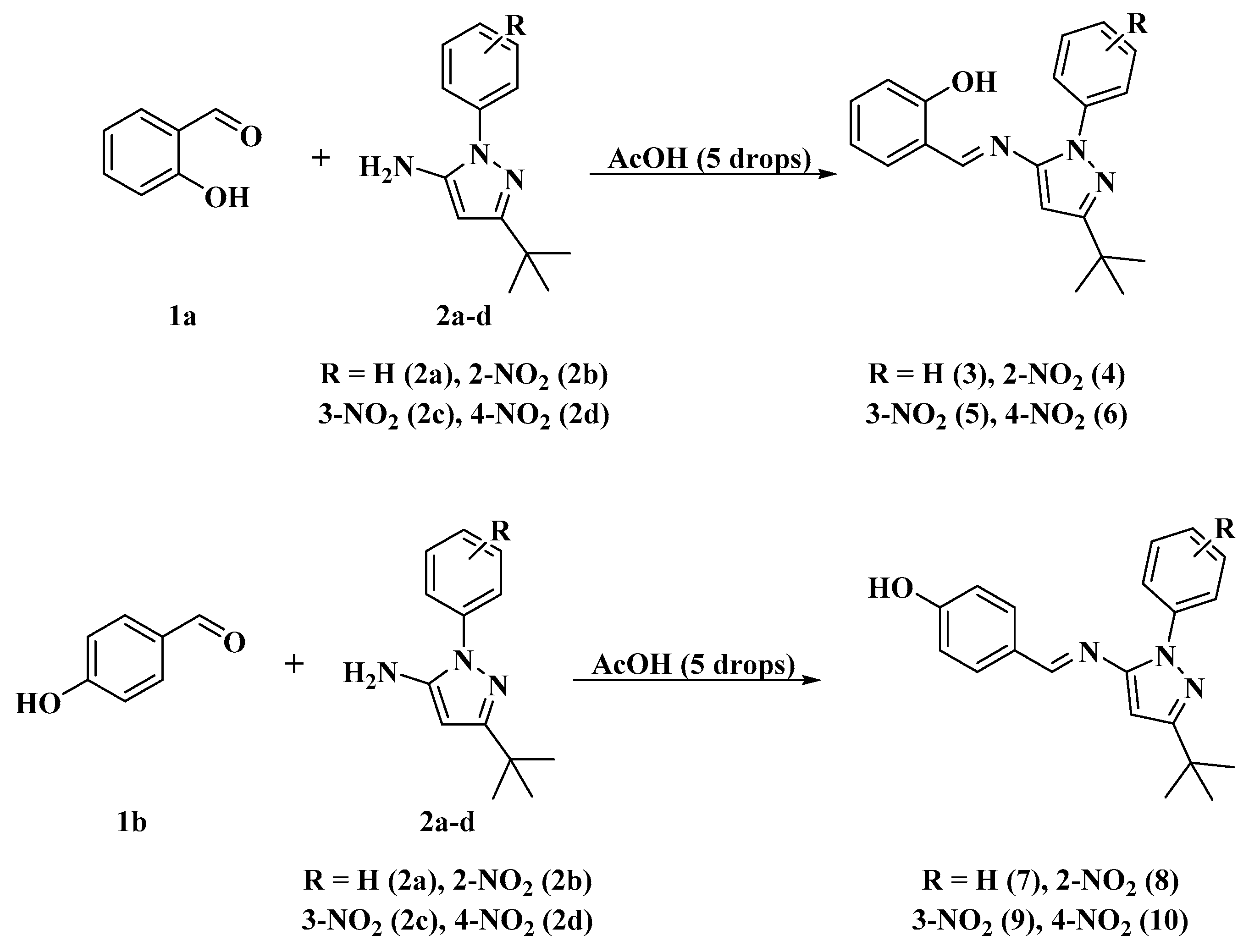
The synthesis of the Schiff bases (Scheme 1) was performed through a condensation reaction between the hydroxybenzaldehydes 1a (R: 2-OH) [52,53] or 1b (R: 4-OH) [30] and the aminopyrazoles 2a–d using either conventional heating (reflux in ethanol) or environmentally friendly techniques such as mechanical milling, thermal fusion, microwave irradiation (MWI) and ultrasound, using acetic acid as a catalyst. These approaches yielded the compounds 3–10 in very good yields (75–90%) by reflux in ethanol, (82–97%) by mechanical milling, (90–99%) by thermal fusion, (84–95%) by MWI and (86–96%) by ultrasound. We note that high yields, short reaction times and solvent-free conditions can be obtained with these four techniques (mechanical grinding, thermal fusion, microwave irradiation and ultrasound), making them good alternatives to conventional heating methods (Scheme 1 and Figure 1). Thus, compounds (3–7) have already been reported by our group [52,53,54].
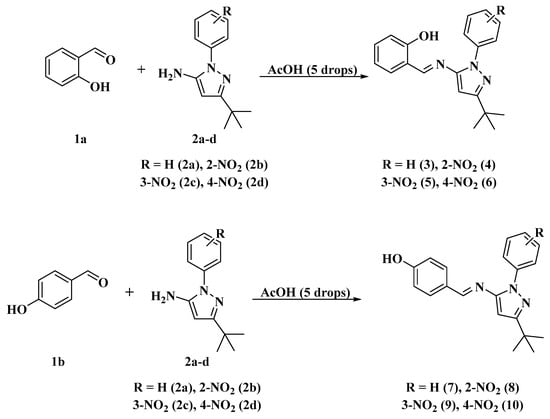
Scheme 1.
Synthesis of Schiff base-ligands (3–10).
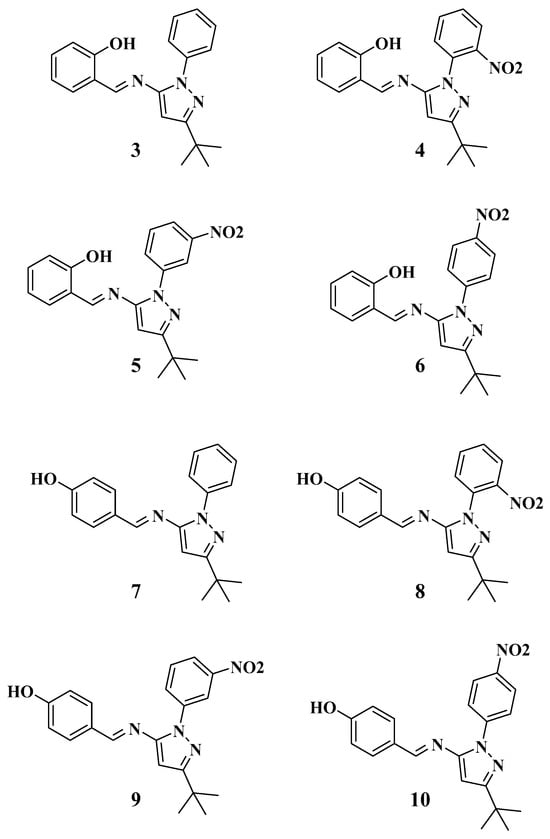
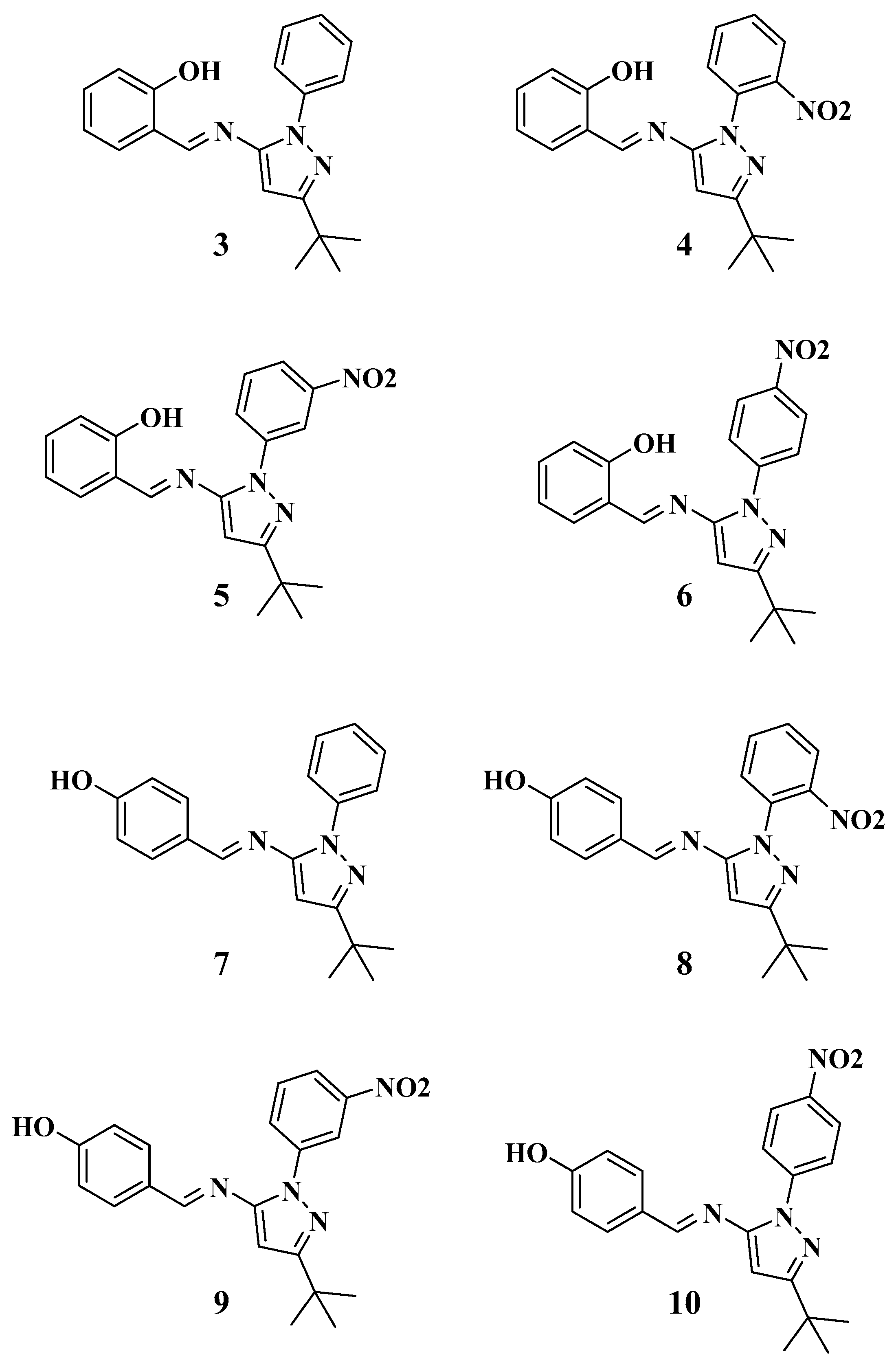
Figure 1.
Structures of the Schiff base-ligands 3–10.
2.2. Synthesis of Complexes [Pd2Cl4(3)2]-[Pd2Cl4(10)2]
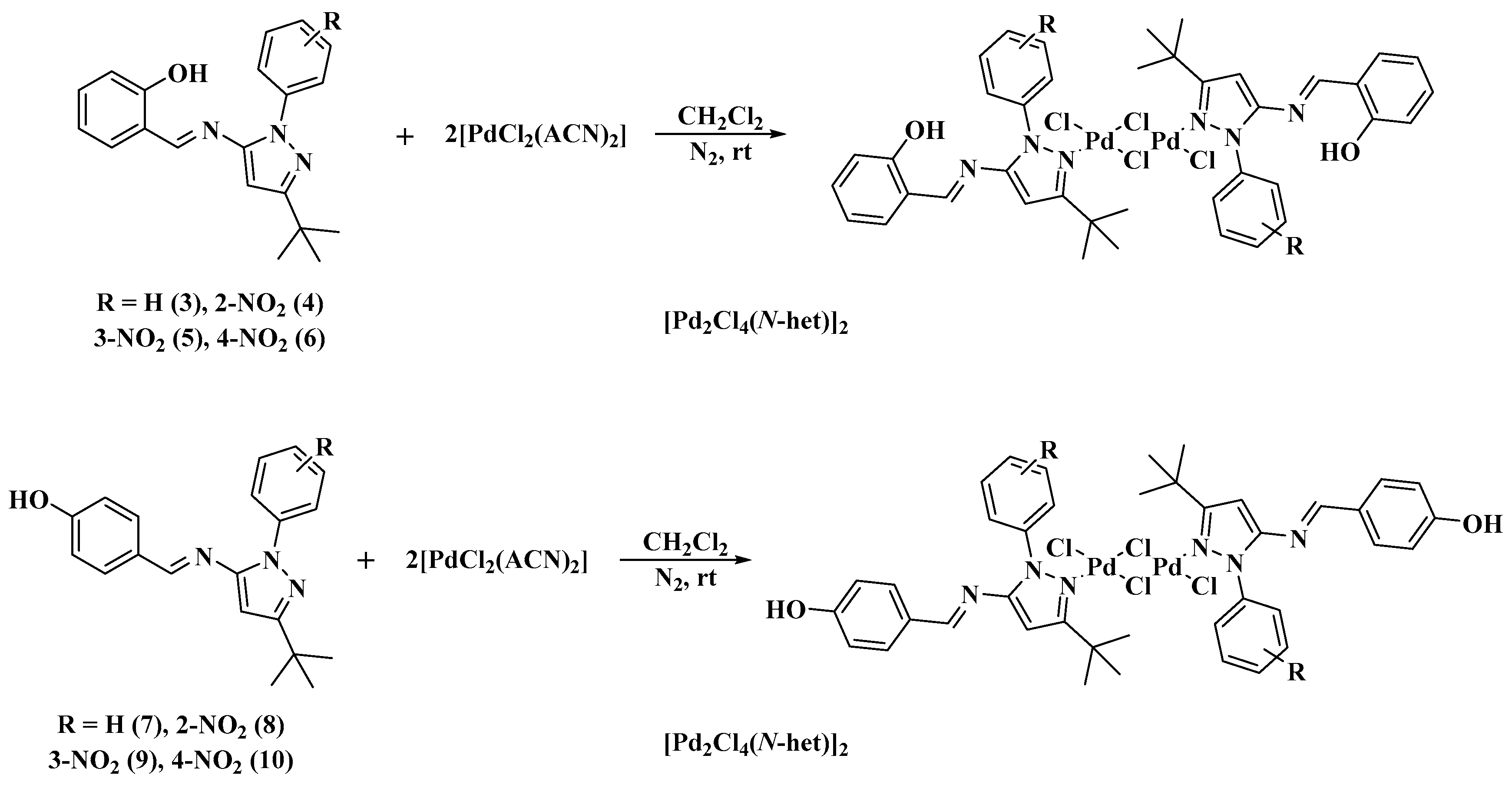
The coordination of the Schiff bases 3–10 to Pd(II) was achieved by treating compounds 3–10 with PdCl2(ACN)2 (ACN=acetonitrile) in CH2Cl2 at room temperature under a nitrogen atmosphere (Scheme 2). Dimeric Pd complexes of the form [Pd2Cl4(N-het)2] were obtained, where each Pd(II) is linked to a Schiff-base ligand, to a terminal chlorine and to two bridging chlorines, forming a square planar configuration on each Pd(II) center (Figure 2). The elemental analyses were in agreement with the formulation of the complexes [Pd2Cl4(N-het)2], showing a 1:1 ratio of Pd to Schiff bases.
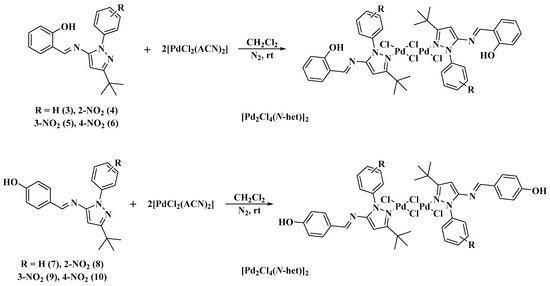
Scheme 2.
Synthesis of palladium complexes.
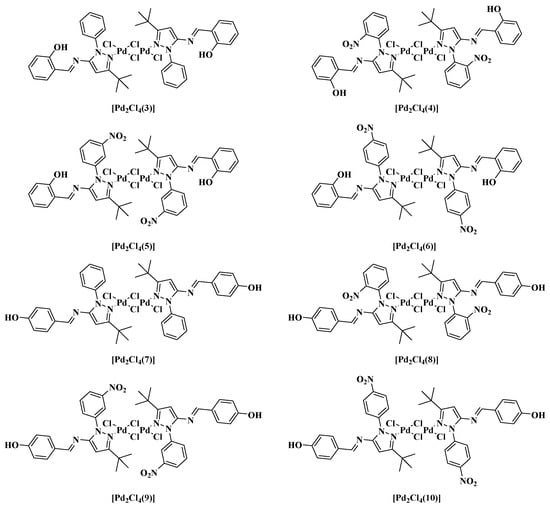
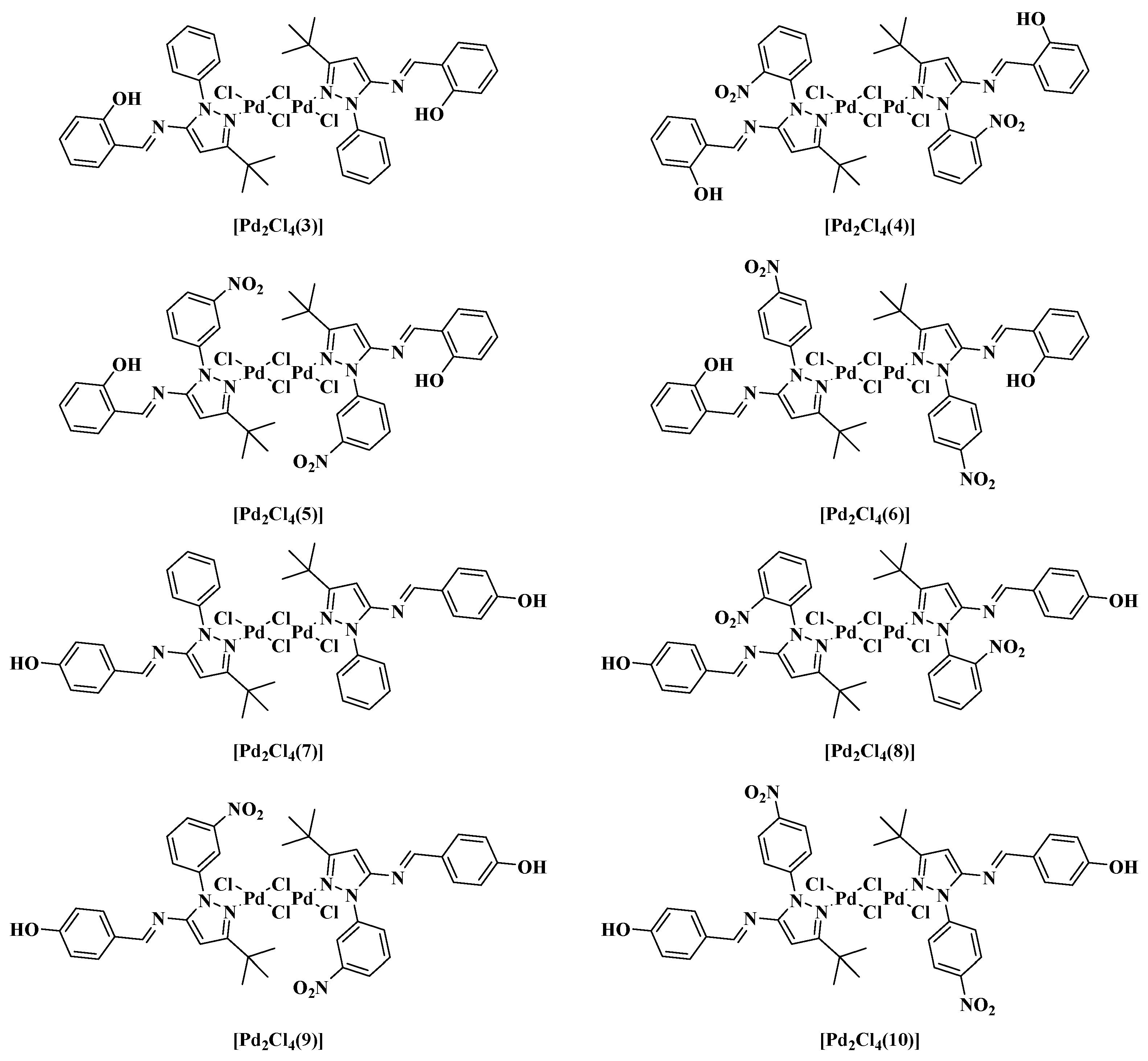
Figure 2.
Structures of the new Pd-Schiff base complexes.
2.3. Mass Spectrometry (EI-MS)
The mass spectra of all the Schiff bases prepared here, namely 3–10, show peaks corresponding to their respective parent molecular ions [M+]. For the nitro-containing Schiff bases, 4–6 and 8–10, the [M+] peaks correspond to their base peaks. For compounds 4–6 and 8–10, which have the nitro group on the aromatic ring, in the MS, the fragmentation pattern is characteristic of the six compounds, with a base peak corresponding to the molecular ion [M]+ (364 m/z). The MS of compounds 3 and 7 show base peaks corresponding to their [M-CH3]+ ions (304 m/z, 100) as previously shown for this class of compounds [52,53,54], as well as a peak for the molecular ion [M]+ (319 m/z, 83.43). The MS spectra of the Schiff bases are shown in Figures S1–S3 (Supplementary Material).
2.4. Infrared Spectroscopy (IR)
The IR spectra of compounds 7–10 each showed an intense band in the 3455–3326 cm−1 range assigned to the OH group, and for compounds 3–6, these bands were in the 3450–3419 cm−1 range. These observations for compounds 3–6 are consistent with the presence of an intramolecular hydrogen bond between the phenolic H atom and the imine N atom [52,53,54,55]. Additional bands in the IR spectra of Schiff bases 3–10 were assigned on the basis of spectra of analogous compounds [52,53,56]: (i) the band between 3141–3120 cm−1 was assigned to the H-C= stretching of the pyrazole; (ii) the band between 3095–3022 cm−1 was assigned to the C-H stretching of the aromatic rings; (iii) the band between 2968–2954 cm−1 was assigned to the asymmetric stretching of the C-H aliphatic tert-butyl groups (symmetric stretching at 2871–2861 cm−1); (iv) the band at 1623–1596 cm−1 was assigned to the -C=N vibration of the imine groups; (v) the band at 1535–1511 cm−1 was assigned to the asymmetric stretching of the –NO2 group (1357–1334 cm−1 for the symmetric stretching; see Figures S4–S6, Supplementary Materials).
In the IR spectra of the metal derivatives [Pd2Cl4(N-het)2] (Figures S7–S14 Supplementary Materials), the characteristic bands of the ligands were observed, but with slight shifts to higher frequencies for the hydroxyl group, C-H of the pyrazole, νas and νs of the tert-butyl group and νC=N of the imino group, consistent with the coordination to the metal [55,56,57]. As noted above, the hydroxyl groups of compounds 3–6 showed bands of lower intensity, but upon coordination to Pd(II), these bands increased in intensity, suggesting that the intramolecular hydrogen bonds had been broken due to coordination to the metal.
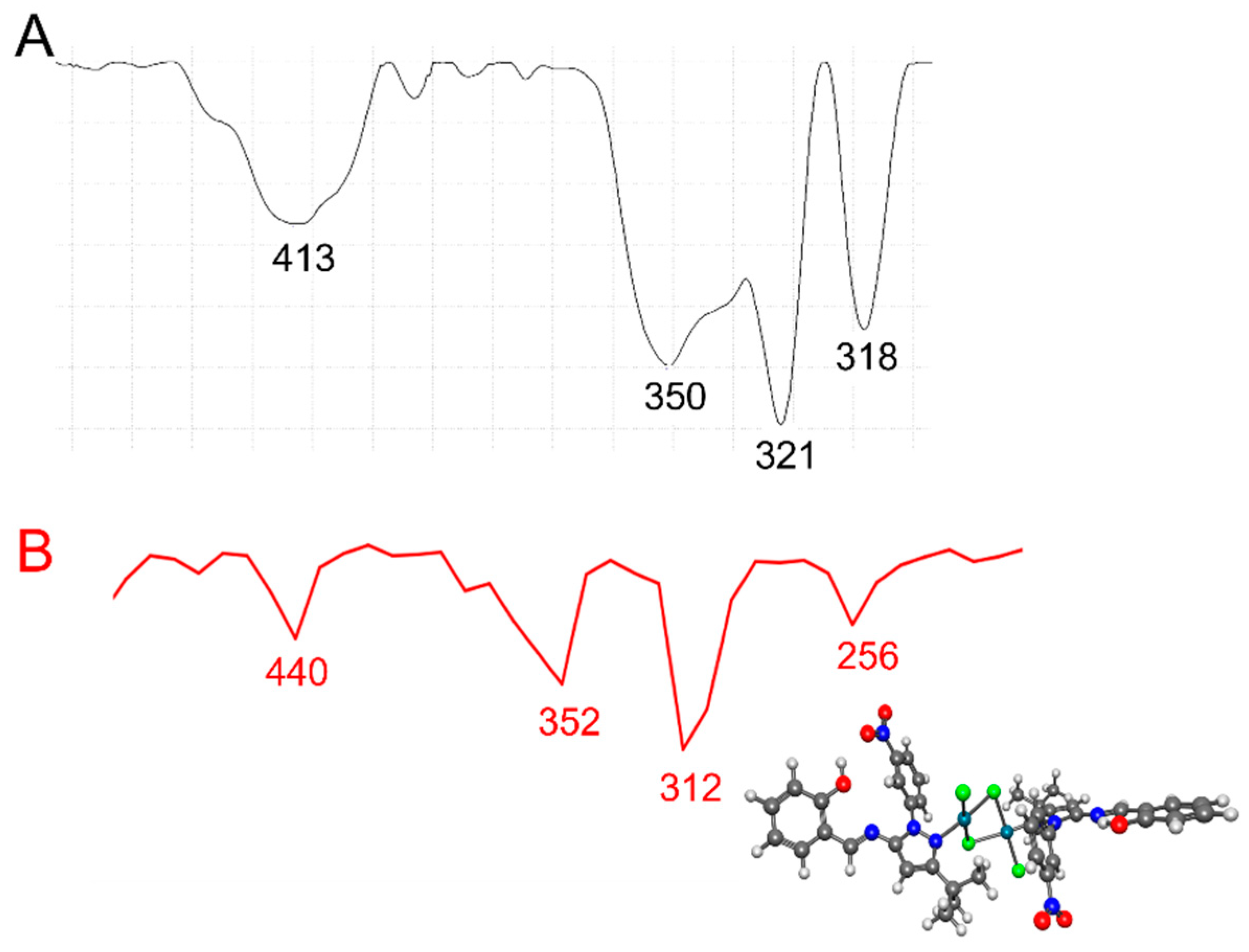
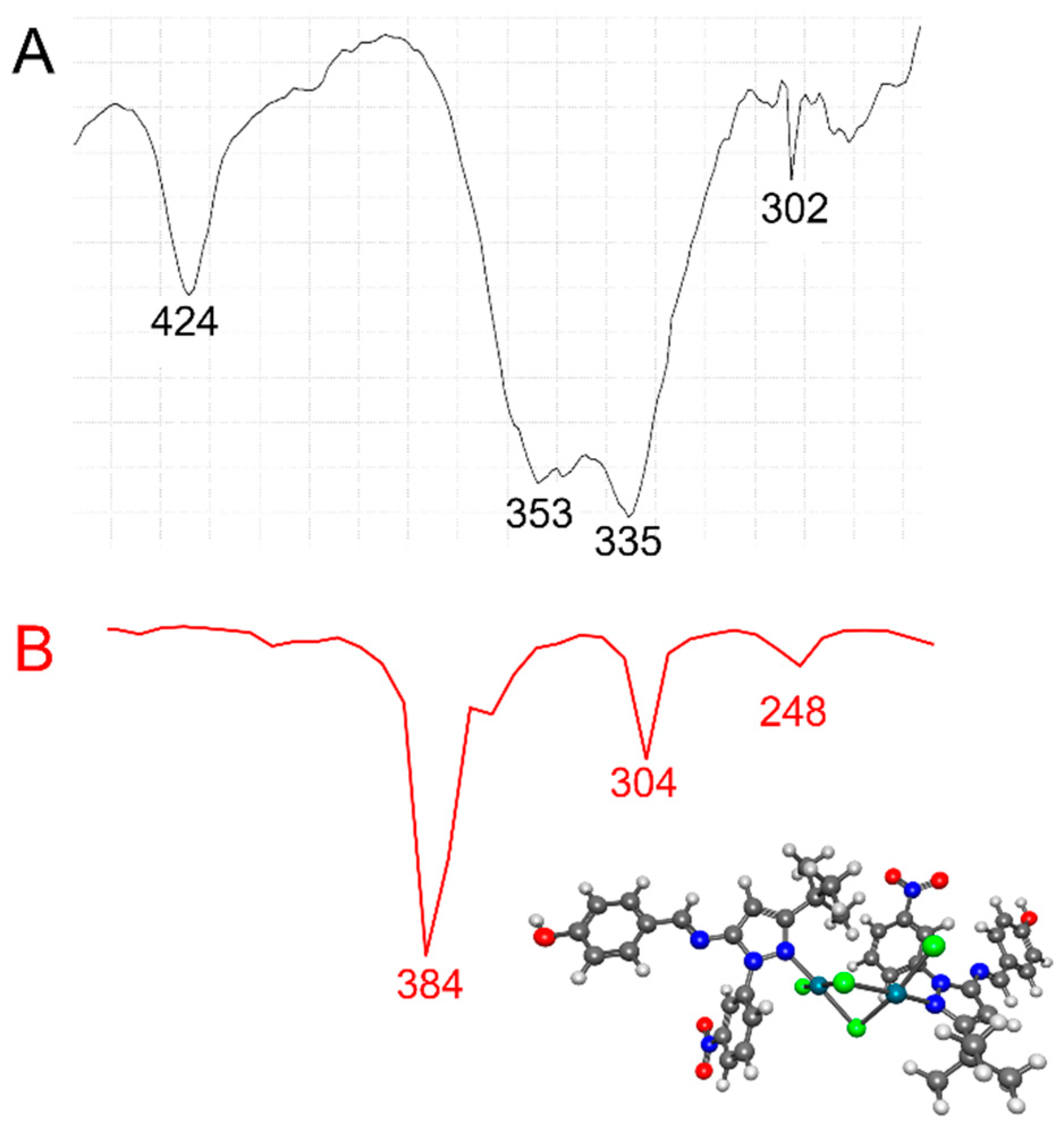
In general, bridging Pd-Cl stretching frequencies [νb(Pd-Cl)] appeared at lower frequencies than did terminal Pd-Cl stretching frequencies [νt(Pd-Cl)] [58]. In addition, trans-planar [Pd2Cl4(N-het)2]-type complexes with C2h symmetry exhibited three IR-active ν (Pd-Cl) absorption bands [i.e., one νt(Pd-Cl) and two νb(Pd-Cl)] in the 360–294 cm−1 region [59]. In the low-frequency region of the IR spectra of [Pd2Cl4(5)2] and [Pd2Cl4(9)2] (Figure 3 and Figure 4, Table 1), an absorption at 350–353 cm−1 was observed, which we assigned to the νt(Pd-Cl) mode, and two additional absorption bands at 321–335 cm−1 and 318–302 cm−1 were observed, which we assigned to the two νb(Pd-Cl) modes. The bands at 336–338 cm−1 involved a νb(M-X) cis to Cl mode, while the band at 318–302 cm−1 involved a νb(Pd-Cl) trans to (N-het) mode, as it was sensitive to the nature of the ligand [58]. The IR spectra thus suggest a planar geometry around each Pd(II) atom and its two ligands in [Pd2Cl4(5)2] and [Pd2Cl4(9)2], as well as the existence of one terminal Pd-Cl and two bridging Pd-Cl bonds in its structure, in agreement with reports in the literature regarding palladium complexes [58]. Finally, the absorption band at 424–440 cm−1 was assigned to a Pd-N stretching frequency. The experimentally recorded frequencies for [Pd2Cl4(5)2] and [Pd2Cl4(9)2] were compared with those calculated obtained by DFT) using ωB97X-D as the functional and LANL2DZ as the basis set. Scaling factors are usually entered to change the calculated frequency values. In our calculations, the scaling factor for ωB97X-D/LANL2DZ was 1.000. In the experimental low-frequency region of the IR spectra of [Pd2Cl4(5)2] and [Pd2Cl4(9)2], absorptions were observed at 350 and 353 cm−1 respectively, which we assigned to the νt(Pd-Cl) mode, and two additional absorption bands at 321–335 cm−1 and 318–302 cm−1 were observed, which we assigned to the two νb(Pd-Cl) modes. The calculated values showed an absorption at 352 and 384 cm−1 for the νt(Pd-Cl) terminal mode and two bridging Pd-Cl bonds at 312–304 cm−1 and 256 and 248 cm−1, respectively. The calculated frequencies were in good agreement with the experimental data. The discrepancies between the calculated and experimental vibrational frequencies arose for two fundamental reasons: first, the experimental frequencies corresponded to the solid phase of KBr, while the calculated frequencies belonged to the gas phase. Second, the calculation was performed for an isolated molecule in the gas phase, while the experimental values include intermolecular interactions.
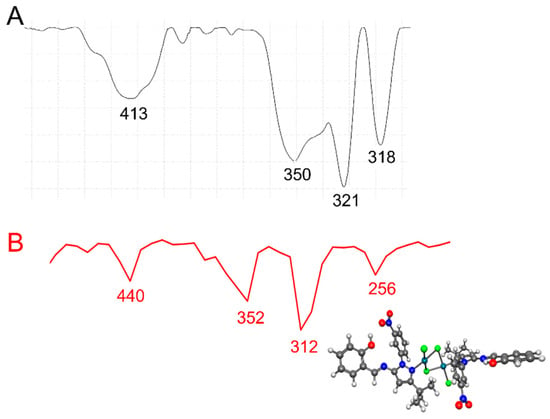
Figure 3.
(A) Experimental (black line) and (B) calculated (red line) IR at lower frequencies for the [Pd2Cl4(5)2] complex using ωB97X-D/LANL2DZ.
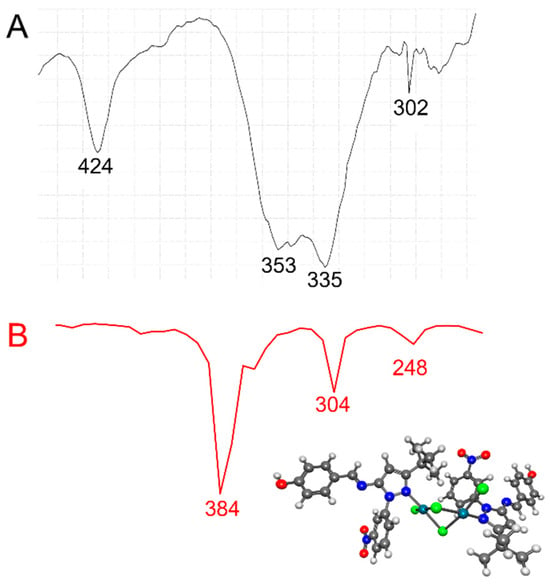
Figure 4.
(A) Experimental (black line) and (B) calculated (red line) IR at lower frequencies for the [Pd2Cl4(9)2] complex using ωB97X-D/LANL2DZ.

Table 1.
Experimental low-frequency IR absorption bands for the Pd(II) complexes [Pd2Cl4(3)2]-[Pd2Cl4(10)2].
These observations are consistent with the results reported by Nelana and coworkers for (pyrazol-1-yl)phenylmethanone Pd(II) complexes [60]. The far-IR spectra of the Pd(II) complexes can be found in the Supplementary Materials, Figures S15–S20.
2.5. Nuclear Magnetic Resonance (NMR)
The 1H NMR spectra of compounds 3–10 show four singlets at approximately 1, 6, 9 and 11 ppm, which are assigned to the tert-butyl group (H-7), pyrazole hydrogen (H-4), imino group (H-15) and OH group protons, respectively. It can be observed that the imino and OH protons of the compound 3 appear downfield relative to those of compound 7. The presence of an electron-withdrawing group such as –NO2 shifts all the Schiff-base protons downfield. The 13C NMR and DEPT spectra allowed us to assign the quaternary, tertiary, and primary carbon atoms of compounds 3–10. The unambiguous assignment of the signals in the 1H and 13C NMR spectra of compounds 3–10 was deduced from the direct and broad heteronuclear chemical shifts in these spectra. The chemical-shift correlations of the proton-carbon bond and the higher-rank correlations over two or three bonds (2J or 3J couplings) were determined using HSQC and HMBC techniques, respectively (see Figures S21–S35 in the Supplementary Materials for compounds 8–10).
Coordination of the Schiff bases to Pd(II) results, not unexpectedly in small chemical shift difference of the free and bound ligands consistent with this coordination (Figures S36–S51 Supplementary Materials for complexes [Pd2Cl4(3)2]-[Pd2Cl4(10)2]).
2.6. Molecular Orbitals
Frontier molecular orbitals (FMOs), which include the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), can provide an insight into electronic and optical properties, as well as the chemistry of complex reactions because they help explain the way in which the molecule interacts with other species [61]. In addition, they are also used to predict the identity of the most reactive moieties in π-electronic systems. We performed DFT calculations to explore the FMOs and their respective energies for compounds 3–10 in order to predict reactivity in Mizoroki–Heck reactions. The FMOs of all the compounds show similar characteristics. The HOMO is mainly located on the pyrazole ring, the imino group and the phenol ring, which is consistent with the electron-donor capacity of these moieties. The LUMO is located on the phenol and pyrazole rings in the compounds 3 and 7, which do not have the nitro group. In compounds 4–6 and 8–10, which contain the NO2 group, the LUMO is located only on the phenyl rings that possess the nitro group, this localization being in concordance with its electron-acceptor capacity.
The energy difference between the HOMO and LUMO (GAP or ΔE), as well as the hardness (η) and the softness (σ), help to characterize the chemical reactivity and kinetic stability of the molecule. A molecule with a small gap is more polarizable and is generally associated with a high chemical reactivity and low kinetic stability because it is energetically favorable to add electrons in a LUMO and receive electrons from a HOMO. In addition, such molecules are also known as soft molecules [52,62].
Using the HOMO and LUMO energy values for each molecule, hardness (η) and softness (σ) can be calculated by using equations 1 and 2 [63,64]. The values obtained for compounds 3–10 and the energy differences of the FMOs are shown in Table 2. The values in the Table 2 for compounds 3 and 6 were taken from references [52,53,54].

Table 2.
HOMO-LUMO energies, GAP, hardness and softness of compounds 3–10 calculated using B3LYP/6-311**G(d,p). Values are given in electron volts (eV).
- Chemical hardness can be calculated as follows:
- Global softness is the inverse of global hardness, as follows:
In Table 2, it can be observed that compounds 3 and 7 have the greatest energy difference (3.912 and 3.911 eV respectively) and the greatest chemical hardness (1.956 and 1.955 eV respectively). This indicates that they are less polarizable and less reactive than compounds 4–6 and 8–10. Compounds 3 and 7 also have a lower capacity to generate a metal-ligand charge transfer; therefore, their Pd(II) complexes are expected to be less stable and less active in catalytic Mizoroki–Heck reactions.
Compounds 5 and 9 exhibited the lowest energy difference (3.188 and 3.129 eV, respectively) and the lowest chemical hardness (1.594 and 1.565 eV respectively), making them the softest compounds. This indicates that they are more polarizable. This observation predicts that charge-transfer transition from the dπ orbitals of the palladium to the pπ* of the orbitals of the pyrazole ligands will be facilitated, strengthening palladium–pyrazole bonding. It is known that polarizable chemical species facilitate electron donation to metals and that they form stable complexes with soft and polarizable metal ions. Therefore, it can be assumed that the Pd(II) complexes with ligands 5 and 9 will be the most stable and will be more active in catalytic Mizoroki–Heck reactions.
2.7. Reactivity toward the Mizoroki–Heck C-C Coupling Reaction
2.7.1. Catalysis between Iodobenzene and Styrene
All complexes were tested as catalysts for the Mizoroki–Heck C-C coupling reaction between styrene and iodobenzene (Scheme 3, Table 3). All the complexes were active and selective for the formation of trans-stilbene or trans-styrylbenzaldehyde. Interestingly, the [Pd2Cl4(5)2] and [Pd2Cl4(9)2] complexes exhibited high catalytic activity and considerable selectivity towards trans-stilbene, with a complete conversion (100%, TOF 500 and 89–90% selectivity respectively) within two hours. We also observed that the [Pd2Cl4(3)2] and [Pd2Cl4(7)2] complexes exhibited lower catalytic activity than did the other Pd(II) complexes in which the Schiff bases bear a nitro group on the phenyl ring. Therefore, electron-withdrawing groups in the aromatic ring reduce the donor capacity of the pyrazole, thus favoring the backdonation of electron density by the metal. This strengthens the Pd–pyrazole bond and weakens the Pd-Cl bond, thus promoting a rapid reductive elimination of the H and Cl ligands. This in turn generates the intermediate Pd(0)-Schiff base, increasing the activity and selectivity towards trans-stilbene.
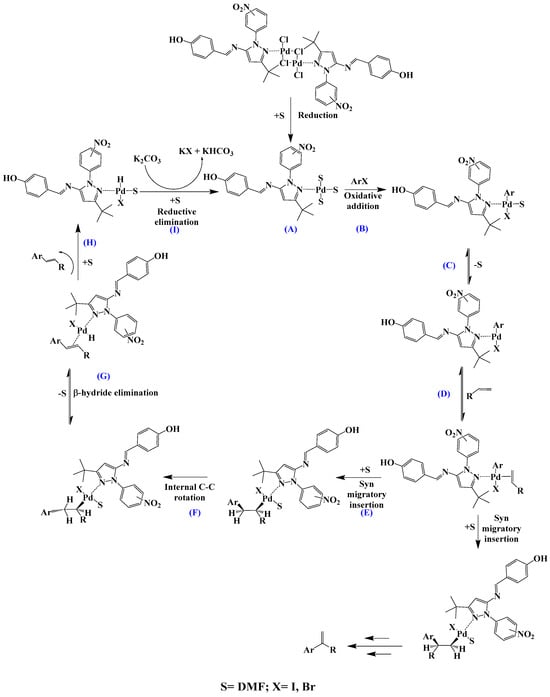
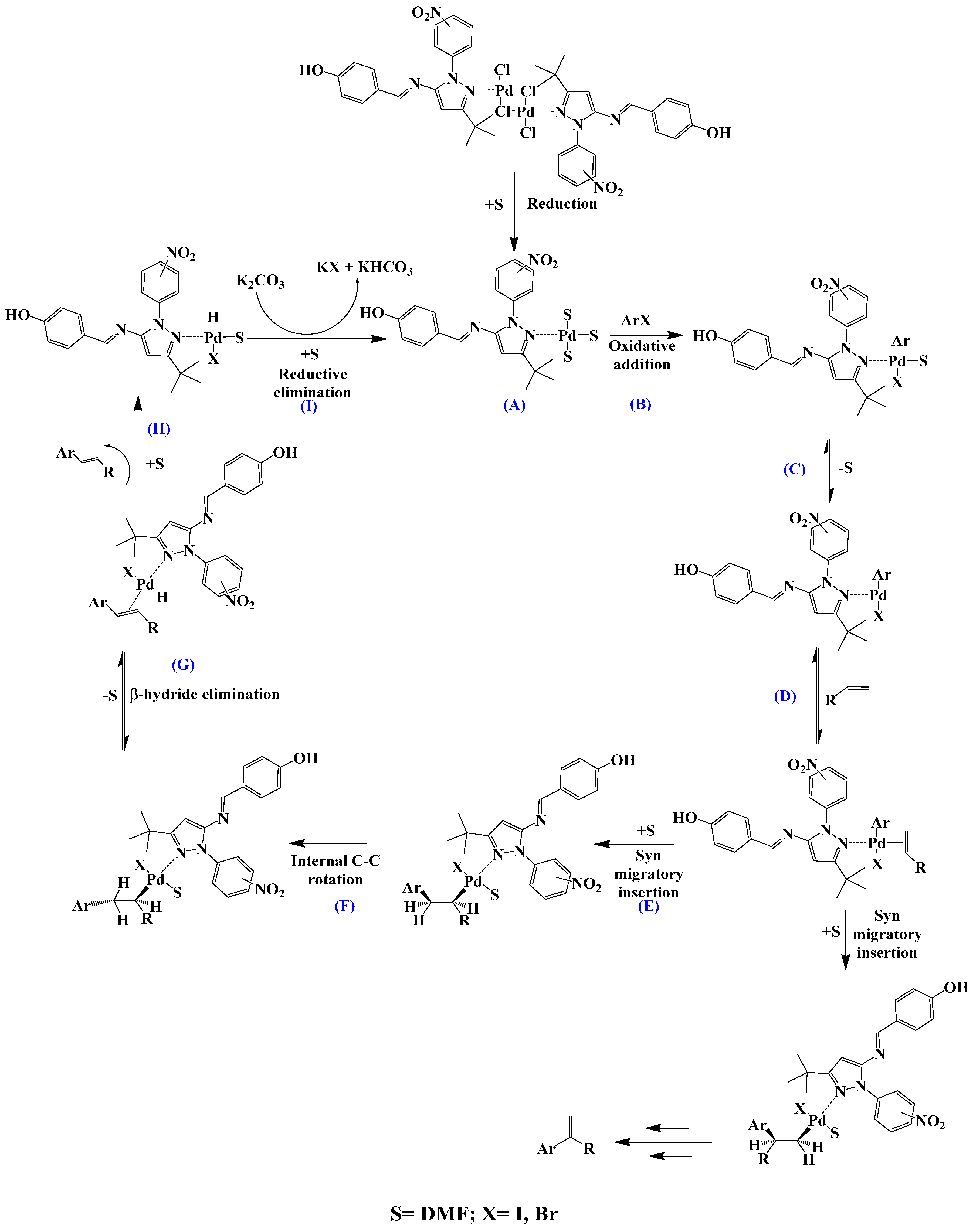
Scheme 3.
Proposed mechanism for a model Mizoroki–Heck C-C coupling reaction (A – I: proposed intermediates and reaction steps).

Table 3.
Mizoroki–Heck C-C coupling reaction of styrene with iodobenzene catalyzed by [Pd2Cl4(N-het)2].
According to the results shown in Table 3, it can be observed that the electronic effects rather than the steric ones are determinant in the catalytic activity, since the selectivity towards trans-stilbene was very similar in all the experiments. Such results are in concordance with the theoretical data detailed in Table 2, where [Pd2Cl4(3)2]-[Pd2Cl4(7)2] are the hardest and least reactive complexes and [Pd2Cl4(5)2]-[Pd2Cl4(9)2] are the softest and most reactive complexes.
Comparing the obtained results with those reported by Taira and co-workers [65], who achieved a conversion to trans-stilbene of 60%, our catalysts [Pd2Cl4(5)2] and [Pd2Cl4(9)2] achieved a conversion of 100% in two hours, showing excellent activity towards this olefin.
2.7.2. Mercury Drop Test Using [Pd2Cl4(5)2] and [Pd2Cl4(9)2] Complexes
To determine the homogeneity of the [Pd2Cl4(5)2] and [Pd2Cl4(9)2] complexes, the mercury drop test was performed [66,67]. The reactions were carried out in the presence of an excess of mercury, and similar behavior was observed when the reaction was carried out without mercury (see Table 4). Moreover, after three cycles of catalyst recycling, the selectivity remained at 96–93% for [Pd2Cl4(9)2] and 89–90% for [Pd2Cl4(5)2], confirming that the complexes are stable at high temperatures. In addition, it was possible to verify that theoretical and experimental data correlate with each other, since both complexes showed the smallest energy differences (see Table 2), with [Pd2Cl4(9)2] being softer than [Pd2Cl4(5)2]. This indicates that a softer compound is more reactive and stable in the catalytic Mizoroki–Heck reactions, which yielded conversion percentages of 99% for [Pd2Cl4(9)2] and 74% for [Pd2Cl4(5)2]. This experiment also showed that the amalgamation of Pd particles or, for example, the poisoning of [Pd2Cl4(5)2]-[Pd2Cl4(9)2] complexes, does not occur during the reaction, indicating the homogeneity of the reaction during the catalytic cycle.

Table 4.
Mercury drop test and catalyst recycling by [Pd2Cl4(5)2] and [Pd2Cl4(9)2] in DMF.
Table 5 shows the results of the Hg drop test for the [Pd2Cl4(5)2] and [Pd2Cl4(9)2] complexes using DMF/water in a 1:1 ratio as solvent. It can be observed that there is a decrease in their activity but an increase in selectivity towards trans-stilbene, indicating that [Pd2Cl4(5)2] and [Pd2Cl4(9)2] complexes remain active throughout the three catalytic cycles. This demonstrates that these Pd(II) complexes are stable in aqueous medium and that Pd clusters are not formed.

Table 5.
Mercury drop test and catalyst recycling by [Pd2Cl4(5)2] and [Pd2Cl4(9)2] in DMF/H2O.
2.7.3. Effect of Catalyst Recycling on the Catalytic Activity and Selectivity of the [Pd2Cl4(9)2] Complex
Since Table 3 shows that the [Pd2Cl4(9)2] complex achieves 100% conversion in 2 h, the catalytic recycling of the complex was carried out for ten hours. During that time, equal amounts of initial substrates (iodobenzene, styrene and K2CO3) were added every two hours. The catalytic activity showed that over five cycles, the selectivity towards trans-stilbene remains constant, showing a high regioselectivity, but that after the fifth addition of substrates, the percentage conversion decreases to 68% (see Table 6). This result is important from the industrial and environmental points of view, since the [Pd2Cl4(9)2] complex shows high activity and excellent recyclability, making it a very interesting Pd(II) catalyst for the synthesis of new compounds.

Table 6.
Catalyst recycling by [Pd2Cl4(9)2].
2.7.4. Effect of the Concentration on the Reactivity and Selectivity of the [Pd2Cl4(9)2] Complex
The effect of the concentration was tested using different concentrations of [Pd2Cl4(9)2] (Table 7). The results for catalytic activity showed that up to a catalyst-to-substrate ratio of 1:3000, the percentage of conversion remains unchanged and without a decrease in the selectivity towards trans-stilbene, but at a ratio of 1:10,000, the percentage of conversion decreases to 74% and the selectivity towards trans-stilbene decreases to 88%.

Table 7.
Effect of the concentration by [Pd2Cl4(9)2].
2.7.5. Effect of the Base on the Reactivity and Selectivity of the [Pd2Cl4(9)2] Complex
In Table 8, the study of the effect of the base is presented, finding the best bases under our reaction conditions were: potassium carbonate (K2CO3) and trimethylamine (Et3N), respectively. Potassium carbonate presented the higher percentage of conversion (99%, TOF 1485 and 89% selectivity), this is due to the basic strength of the oxygen increases with the size of the alkaline cation (K > Na > Li), accelerating the reduction of Pd(II) to Pd(0). This is consistent with the results obtained, since the highest percentage of conversion was K2CO3 (99%), followed by Na2CO3 (53%) and finally Li2CO3 (37%), showing that the catalyst activity is influenced by the base used. It should be noted that the bases Et3N and NaOAc also generate a high catalytic activity, due to their good solubility in DMF, which facilitates their function in the catalytic cycle.

Table 8.
Effect of the base by [Pd2Cl4(9)2].
2.7.6. Effect of the Temperature on the Reactivity and Selectivity of the [Pd2Cl4(9)2] Complex
Keeping the same reaction conditions, the temperature was set to the following values: 100, 120, 140 and 160 °C. Potassium carbonate was used as the base because of its higher conversion percentage in the previous experiment. It can be observed that increasing the temperature from 100 °C to 120, 140 and 160 °C (Table 9) improved the reactivity of [Pd2Cl4(9)2], with TOFs of 1020, 1155, 1380 and 1485, respectively. Interestingly, the selectivity towards trans-stilbene remained the same in all four experiments (89%), indicating that [Pd2Cl4(9)2] is stable, active and regioselective. In contrast, it has been reported that the efficiency of Pd(II) complexes based on monodentate PPh3 used as catalysts for Mizoroki–Heck C-C coupling reactions often decreases when the temperature is above 140 °C. This drawback is often related to the oxidation of phosphorus in such ligands [68,69,70].

Table 9.
Effect of temperature on [Pd2Cl4(9)2].
2.7.7. Effect of Excess Ligand on the Reactivity and Selectivity of the [Pd2Cl4(9)2] Complex
Upon addition of the free ligand (9), the [Pd2Cl4(9)2] complex loses catalytic activity, exhibiting a conversion percentage of 88% after four hours for a relatively high catalyst-to-ligand ratio (i.e., 1:5), and a percentage of conversion of 23% for a relatively low catalyst-to-ligand ratio (i.e., 1:10) (Table 10). This is consistent with the blocking of central metal coordination sites by Schiff-base ligands.

Table 10.
Effect of excess ligand on [Pd2Cl4(9)2].
2.7.8. Catalysis between Bromobenzaldehydes and Styrene in DMF
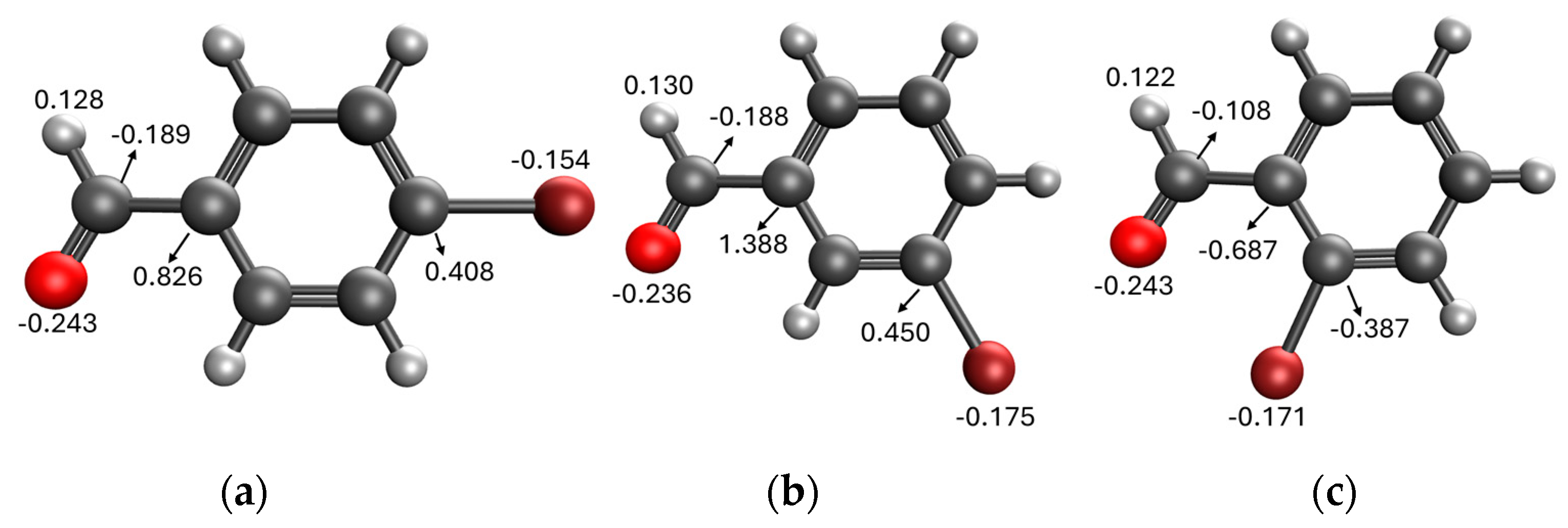
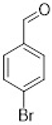
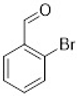
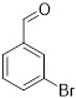
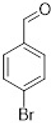
Comparing the results obtained in the Mizoroki–Heck reaction with those achieved with bromobenzaldehydes in Table 11, it can be seen that aryl bromides substituted with aldehyde in the meta and para positions are more reactive than those substituted with aldehyde in the ortho position. Figure 5 shows the Mulliken charges obtained using the DFT method (B3LYP) and basis function 6-311**G(d,p) for each atom of the o-bromobenzaldehyde, m-bromobenzaldehyde and p-bromobenzaldehyde molecules. It can be seen that the energies for the carbon atom bonded to bromine are: −0.387 for o-bromobenzaldehyde, 0.45 for m-bromobenzaldehyde and 0.408 for p-bromobenzaldehyde. Accordingly, the ortho-substituted aryl bromide has a negative charge on this carbon atom, which causes the nucleophilic attack of palladium on that carbon to be disadvantaged, making the oxidative addition slower. This is why reactions with this substrate are slower compared to reactions with the other substrates. Conversely, the meta-substituted aryl bromide exhibits the most positive charge on the carbon atom, favoring the nucleophilic attack of palladium and making the oxidative addition faster. The para-substituted aryl bromide should then undergo the second-fastest reaction. This theoretical explanation is in agreement with the results obtained experimentally.

Table 11.
Mizoroki–Heck C-C coupling reaction of styrene with bromobenzaldehydes catalyzed by [Pd2Cl4(N-het)2] in DMF.
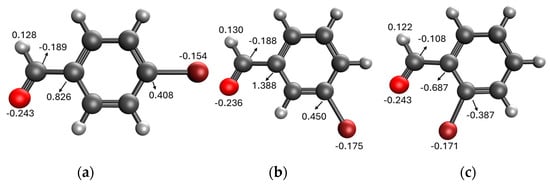
Figure 5.
Mulliken charges for (a) p-bromobenzaldehyde, (b) m-bromobenzaldehyde and (c) o-bromobenzaldehyde.
The Mizoroki–Heck reaction products (trans-stilbene, 2-styrylbenzaldehyde, 3-styrylbenzaldehyde and 4-styrylbenzaldehyde) were separated and characterized by different spectroscopic techniques (See Figures S52–S69, Supplementary Materials).
Catalysis between Bromobenzaldehydes and Styrene in DMF/H2O
Table 12 shows the catalysis results with different bromobenzaldehydes and styrene using DMF/water in a 1:1 ratio as the solvent. It can be observed that there is a decrease in their activity but an increase in the selectivity towards styrylaldehyde (see Table 10), showing that the Pd(II) complexes are stable in aqueous medium and do not undergo decomposition; however, the [Pd2Cl4(5)2] complex was the only one inactive with the substrate 2-bromobenzaldehyde, showing no conversion after 6 h.

Table 12.
Mizoroki–Heck C-C coupling reaction of styrene with bromobenzaldehydes catalyzed by the [Pd2Cl4(5)2] and [Pd2Cl4(9)2] in DMF/H2O.
2.7.9. Proposed Mizoroki–Heck Cycle
The proposed Mizoroki–Heck cycle is shown in Scheme 3. (A) This consists of the formation of the catalytically active species Pd(0), which involves breaking the chloride bridge by heating action; this step is followed by the oxidative addition (B) with aryl halide, which generates a Pd(II) C-aryl complex; the next step (C) consists of a balance between solvent coordination and non-coordination that generates a coordination vacancy and leads to the olefin (styrene) addition (D). Once styrene is coordinated to palladium, a syn migratory insertion occurs. This step is fundamental in determining the regioselectivity of the catalyst, arene (Ar) is inserted into one of the two carbons of unsaturation; the regioselectivity is governed by steric factors, so Ar will generally bind to the least-hindered carbon. An internal C-C rotation (F) then occurs to orient the β-hydride in a syn conformation to the metal. This geometry allows the β-hydride to be eliminated (G), yielding the product (H) and the corresponding Pd(II) complex, which, after the base-assisted reductive elimination (I) of HX, regenerates the active species of Pd(0) to restart the catalytic cycle.
2.8. Synthesis of New Styrylimines
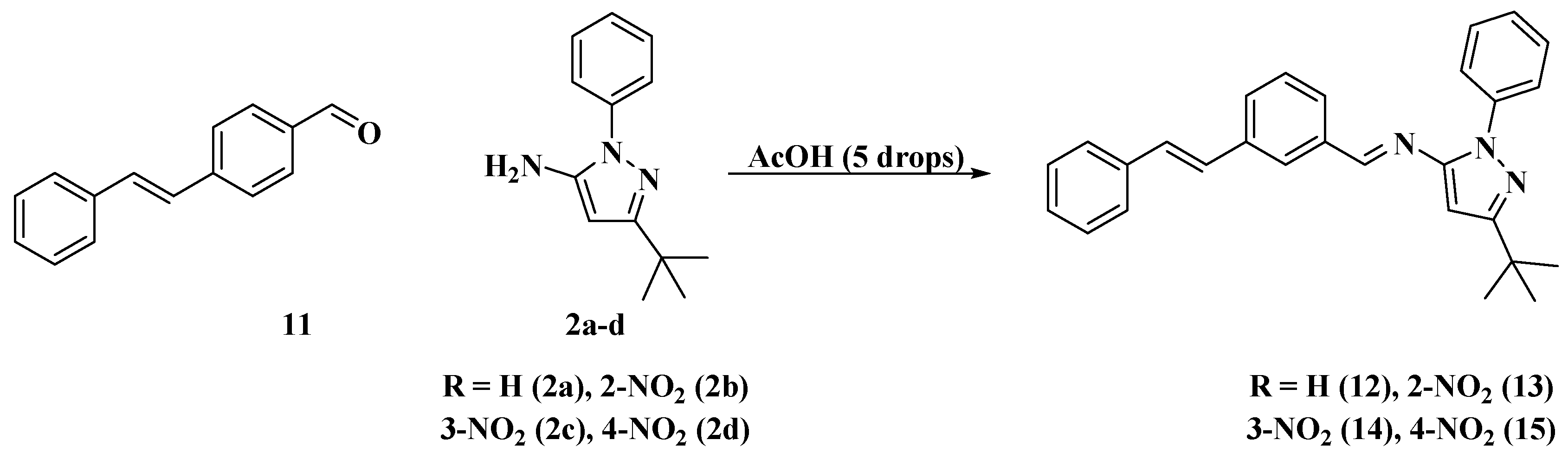
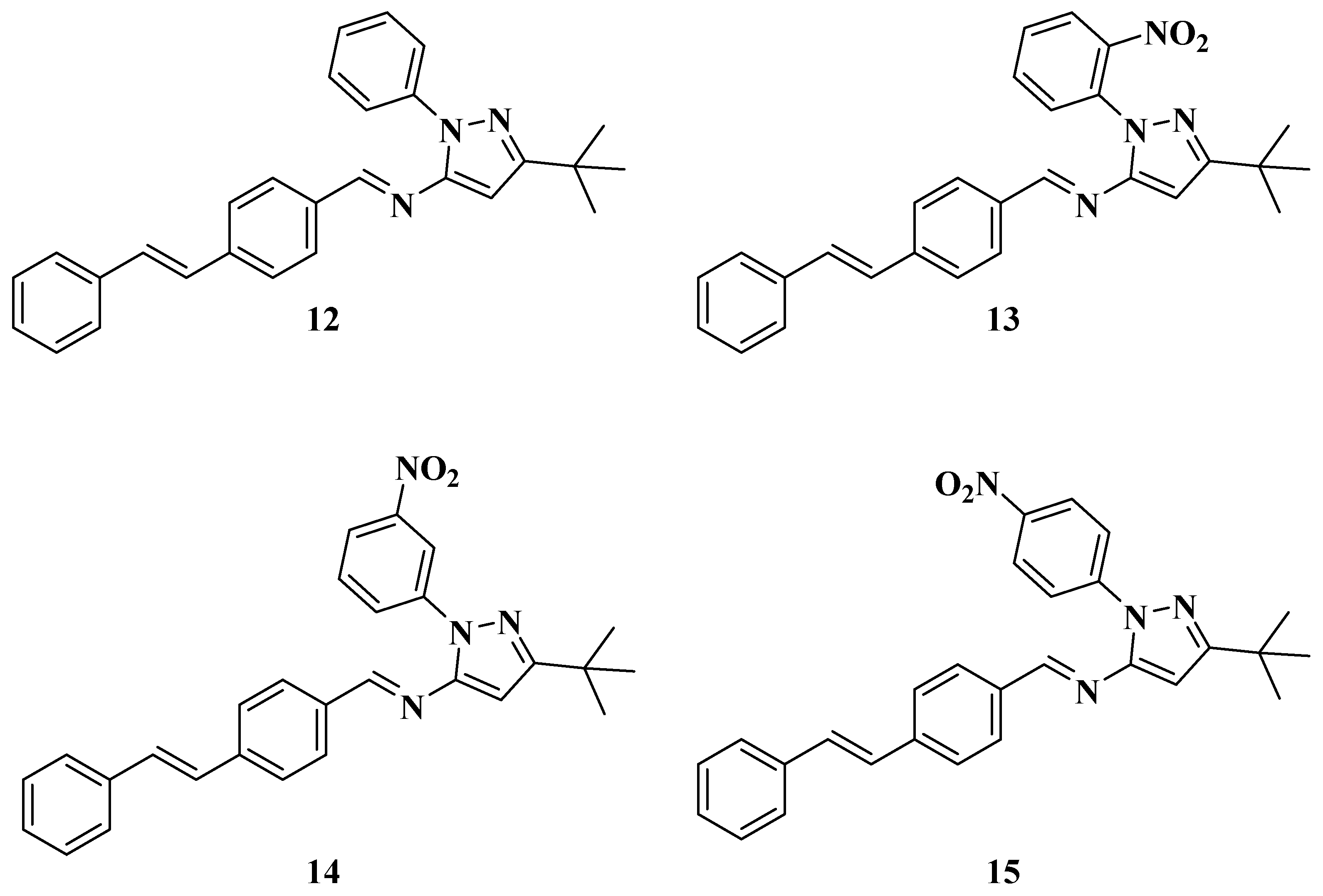
One of the products of the catalysis, (E)-4-styrylbenzaldehyde (11), was isolated for the synthesis of the new styrylimines (see Scheme 4 and Figure 6). This was achieved by a condensation reaction between this reagent (11) [52,53,54] and aminopyrazoles 2a–d using acetic acid (five drops in all cases) as a catalyst. These approaches yielded (E)-N-(3-(tert-butyl)-1-phenyl-1H-pyrazol-5-yl)-1-(4-((E)-styryl)phenyl)methanimine (12), (E)-N-(3-(tert-butyl)-1-(2-nitrophenyl)-1H-pyrazol-5-yl)-1-(4-((E)-styryl)phenyl)methanimine (13), (E)-N-(3-(tert-butyl)-1-(3-nitrophenyl)-1H-pyrazol-5-yl)-1-(4-((E)-styryl)phenyl)methanimine (14), and (E)-N-(3-(tert-butyl)-1-(4-nitrophenyl)-1H-pyrazol-5-yl)-1-(4-((E)-styryl)phenyl)methanimine (15).
Scheme 4.
Synthesis of new styryl-imines.
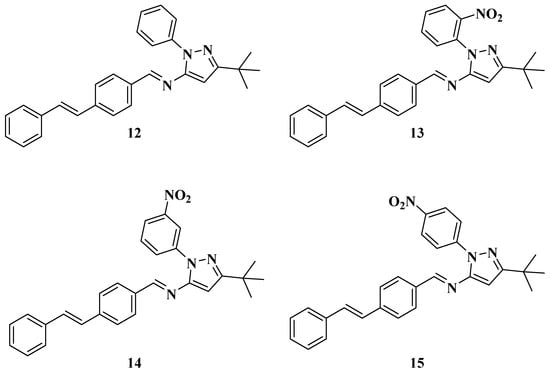
Figure 6.
Structures of the new Styrylimines 12–15.
2.8.1. Mass Spectrometry (EI-MS)
In the Supplementary Material (Figures S70–S73), the mass spectrometry of compounds 12–15 is shown. The molecular ion corresponds to the base peak M+′, with a mass of 405 m/z (100%) for the phenyl compound (12) and 450 m/z for the nitrated compounds (13–15). Other masses with similar relative intensities can be observed, such as 57m/z (23.1%), assigned to tert-butyl. The M-57 ion can also be observed at 393 m/z (0.79%). Phenyl can be observed at 77 m/z (5.45%) and its counterpart at 373 m/z (0.4%). The ion at 435 m/z (59.58%), corresponds to the loss of a methyl group (M-15). The ion 389 m/z (5.08%) corresponds to the loss of methyl and nitro groups (M-61).
2.8.2. Infrared Spectroscopy (IR)
The IR spectra of compounds (12–15) are shown in the Supplementary Material (Figures S74–S77). The spectra show the vibrational frequencies due to the asymmetric and symmetric stretching of the CH bonds, which are in the ranges of 2960 to 2950 cm−1 and 2870 to 2850 cm−1, respectively, corresponding to the tert-butyl group; the H-C= stretching of the pyrazole ring ranges from 3180 to 3120 cm−1; the vibration of the -C=N bond of the azomethine can be seen from 1612 to 1595 cm−1, and the asymmetric and symmetric stretching vibrations of the -NO2 group can be seen from 1530 to 1510 cm−1 and from 1352 to 1328 cm−1, respectively.
2.9. Antifungal Activity
Schiff bases 3–10, their Pd complexes (i.e., [Pd2Cl4(3)2]-[Pd2Cl4(10)2]) and the styrylimines 12–15 were tested for antifungal properties against C. albicans ATCC 10231 and C. neoformans ATCC 32264 using the standardized microbroth-dilution method M-27A3 of CLSI [71], which ensures reliable and reproducible results. Amphotericin B was used as a positive control.
Supplementary Materials Table S87 summarizes the antifungal results expressed as percentage of inhibition of each fungal growth in the range 250–3.9 µg/mL, along with the minimum concentrations of the Schiff bases and their complexes that inhibited the growth of each fungus by either 80% or 50% (IC80 and IC50). These values are accepted as representative of the in vitro activity of the target compounds [72,73,74,75,76]. Supplementary Materials Table S87 shows that all Schiff bases 3–10 and their complexes [Pd2Cl4(3)2]-[Pd2Cl4(10)2] inhibited both C. albicans and C. neoformans to different degrees at the different concentrations tested. The values given in Table S87 and Figure S88A for compounds 3–7 were taken from references [48,49,50,51,52,53,54].
Schiff ligands 3–10 were not good inhibitors of either C. albicans (light gray rows) or C. neoformans (dark grey rows), since their IC80 and IC50 values were between 125 and >250 µg/mL. It should be noted that the IC80 and IC50 should ideally be lower than 20 µg/mL for a compound to be considered a good inhibitor. Nevertheless, styrylimines 12–15 are somewhat more active against these two strains, especially against C. neoformans (see Supplementary Material Figure S88).
Importantly, the complexes [Pd2Cl4(3)2]-[Pd2Cl4(10)2] showed better antifungal activity against C. albicans and especially against C. neoformans. Figure S88A,B show the higher percentages of inhibition achieved with the complexes compared to the ligands (see Figure S88A) against C. albicans at concentrations lower than 50 µg/mL. However, the complexes do not achieve 50% inhibition of C. albicans growth; thus, although they are more active than the ligands, their activities are not sufficient.
In contrast, the activity of complexes against C. neoformans is different from that of ligands. Some complexes [Pd2Cl4(3)2], [Pd2Cl4(5)2], [Pd2Cl4(8)2] and [Pd2Cl4(10)2] showed much stronger antifungal activity (Figure S88B) than the ligands (Figure S88A) did at concentrations lower than 50 µg/mL; these results can be clearly seen to the left of the dotted line.
In addition, [Pd2Cl4(3)2], [Pd2Cl4(5)2] and [Pd2Cl4(10)2] showed high anti-C. neoformans activity, with the following IC50 values: <3.9, 15.6 and 50 µg/mL, respectively, which are values of great interest for the future development of these compounds as antifungal agents. They also show the greatest differences in activities compared to their own ligands, as can be seen in Supplementary Materials Figures S89A–C.
Supplementary Materials Figure S90 shows the percent inhibition of C. neoformans vs. concentration of Schiff bases 3, 5 and 8, each in comparison with its complex [Pd2Cl4(3)2], [Pd2Cl4(5)2] and [Pd2Cl4(8)2] (A, B and C, respectively). The values for compounds 3 and 5 were taken from references [52,53,54].
It should be noted that although [Pd2Cl4(10)2] also showed a significantly lower IC50 (62.5 µg/mL) than did its own ligand 10 (>250 µg/mL) its IC50 was not low enough for it to be considered for further research. With these few examples, some relationships between structure and anti-cryptococcal activity can be inferred: the two most active complexes [Pd2Cl4(3)2] and [Pd2Cl4(5)2] (IC50 < 3.9 and 15.6 µg/mL, respectively) possess an o-OH-substituted benzyl ring, while the N-phenyl ring is either unsubstituted or m-NO2 substituted. By contrast, among the complexes with a p-OH substituted benzyl ring, the only active complex ([Pd2Cl4(8)2]; IC50 = 50 µg/mL) has an o-NO2 substituent on the N-phenyl ring.
3. Materials and Methods
3.1. General Information
Reagents and solvents used were purchased from commercial sources. Product characterization was performed using thin-layer chromatography (TLC) plates on silica-gel plates (Merck 60 F254). A variety of techniques including elemental analysis, IR, NMR (1H, 13C, DEPT-135, HSQC and HMBC) spectroscopy and mass spectrometry were used to characterize the new Schiff bases and their palladium complexes. The uncorrected melting and decomposition points were obtained on a Büchi M-565 melting-point apparatus. Elemental analyses were performed on a Thermal Scientific Flash 2000 (Thermo Fisher Scientific, Waltham, MA, USA). Mass spectra were obtained on a SHIMADZU-GCMS 2010-DI-2010 spectrometer equipped with a direct input probe operating at 70 eV. Microwave experiments were performed with a focused microwave reactor (CEM Discover TM) using the dynamic method. Infrared spectra were recorded on a Perkin Elmer FT 2000 series spectrophotometer using KBr disks. NMR spectra were recorded on a 400 MHz Bruker advance II spectrophotometer in DMSO-d6, chloroform-d and acetone-d6. Catalytic reactions were carried out in a 50 mL two-necked round-bottom flask fitted with a condenser, immersed into an oil bath and maintained at the desired temperature. Analysis of the reaction mixtures was performed using an Agilent 6890A Chromatograph with a 30 m x 0.25 mm x 0.1 μm AT5 capillary column (5% phenylmethylsiloxane). The GC-MS FIDs were obtained using a Jeol-GC-mate and Shimadzu GCMS-QP 2010 mass selective detector. Identification of the reaction products was carried out by GC-MS analysis (comparing them with standard samples). Pure product samples were subsequently obtained after column chromatography on silica gel with DCM-AcOEt mixtures as eluents and characterized by NMR spectroscopy. DFT calculations were performed in the gas phase to optimize the geometries and energies of the Schiff bases using B3LYP as functional with the basis set 6-311**G(d,p) and the Gaussian packet 09 [77] without any obstacle to geometry.
3.2. Synthesis of Schiff Bases
Schiff bases were synthesized by mixing different hydroxybenzaldehydes 1a (R: 2-OH) or 1b (R:4-OH) and aminopyrazoles (2a–2d) using various approaches such as conventional heating, mechanical milling, thermal fusion, microwave irradiation (MWI) and ultrasound, all of them in the presence of a catalytic amount of acetic acid (see Scheme 1).
Approach 1. Conventional heating: A mixture of 2-hydroxybenzaldehyde 1a or 4-hydroxybenzaldehyde 1b (200.00 mg, 1.64 mmol), aminopyrazole 2a–2d (1.64 mmol), ethanol (5 mL) and glacial acetic acid (5 drops) was refluxed for 4 h (monitored by TLC). The solvent was then evaporated to dryness using a rotary evaporator. The crude yellow solid product was washed with cold water (5 × 20 mL) and filtered under vacuum.
Approach 2. Mechanical milling: The above reaction mixture was placed in a pestle and ground with a mortar for 10–17 min at room temperature. At the end of the reaction (monitored by TLC), the crude yellow solid product was washed with cold water (5 × 20 mL) and filtered to dryness under vacuum.
Approach 3. Thermal fusion: The above reaction mixture was added to a test tube. The mixture was heated at 120 °C for 10–15 min (monitored by TLC). The crude yellow solid product was washed with cold water (5 × 20 mL) and filtered under vacuum.
Approach 4. Under MWI: The above reaction mixture was subjected to MWI for 8–10 min at 125–135 °C and 250 W of power. After completion of the reaction (monitored by TLC), the reaction mixture was allowed to cool to room temperature and the precipitate was washed with cold water (5 × 20 mL) and filtered under vacuum.
Approach 5. Under ultrasound: The above reaction mixture was subjected to ultrasound for 30–40 min (monitored by TLC). At the end of the reaction, the precipitate was washed with cold water (5 × 20 mL) and filtered under vacuum.
3.2.1. Characterization of Schiff Bases
(E)-2-(((3-(tert-butyl)-1-phenyl-1H-pyrazol-5-yl)imino)methyl)phenol (3)
Beige solid, m.p. 128–129 °C. MS [(70 eV) m/z (%)] 319 (M+, 100), 302 (M+-17) (14.58), 304 (M+-15) (77.76), 57 (6.61). IR (KBr, cm−1): νOH 3450, νC-H 3120 (pyrazole), νC-H 3060 (aromatic), νas–CH3 2958, νs–CH3 2861, ν–C=N 1619. 1H NMR (400 MHz, DMSO-d6, ppm): δ = 1.33 (s, 9H, H–7), 6.69 (s, 1H, H-4), 6.93 (d, 1H, 3J = 8.4 Hz, H-18), 6.99 (t, 1H, 3J = 7.4 Hz, H-11), 7.41 (m, 1H, 3J = 7.6 Hz, H-19), 7.42 (m, 1H, H-20), 7.54 (t, 2H, 3J = 7.4 Hz, H-10), 7.61 (d, 2H, 3J = 7.8 Hz, H-9), 7.67 (d, 1H, 3J = 7.6 Hz, H-21), 9.12 (s, 1H, H-15), 11.76 (s, 1H, H-17). 13C NMR (400 MHz, DMSO-d6, ppm): δ 30.7 (C-7), 32.7 (C-6), 91.5 (C-4), 117.1 (C-18), 119.9 (C-11), 120.1 (C-16), 124.5 (C-9), 127.4 (C-19), 129.3 (C-10), 132.4 (C-21), 134.3 (C-20), 139.3 (C-8), 148.7 (C-5), 160.11 (C-17), 162.0 (C-3), 163.1 (C-15). Anal. Calc. for C20H21N3O: C, 75.21; H, 6.63; N, 13.16%. Found: C, 75.31; H, 6.59; N, 13.12%. The atoms were numbered according to Figure S91.
(E)-2-(((3-(tert-butyl)-1-(2-nitrophenyl)-1H-pyrazol-5-yl)imino)methyl)phenol (4)
Beige solid, m.p. 102–104°C. MS [(70 eV) m/z (%)] 364 (M+,100), 349 (M+-15, 51.95), 318 (M+-46, 16.11), 307 (M+-57, 0.37) 122 (1.66), 57 (5.30). IR (KBr, cm−1): νOH 3450, νC-H 3135 (pyrazole), νC-H 3089 (aromatic), νas–CH3 2954, νs–CH3 2863, ν–C=N 1623, νas–NO2 1519, νs–NO2 1342. 1H NMR (400 MHz, CDCl3, ppm): δ= 1.40 (s, 9H, H-7), 6.41 (s, 1H, H-4), 6.96 (m, 2H, H-18 and H-20), 7.40 (m, 2H, H-21 and H-19), 7.61 (t, 1H, 3J = 7.8 Hz, H-11), 7.72 (d, 1H, H-13), 7.79 (t, 1H, H-12), 8.04 (d, 1H, 3J = 7.9 Hz, H-10), 8.75 (s, 1H, H-15), 11.68 (s, 1H, H-17). 13C NMR (100 MHz, CDCl3, ppm): δ= 30.2 (C-7), 32.7 (C-6), 90.3 (C-4), 117.3 (C-18), 118.7 (C-20), 119.6 (C-16), 125.2 (C-10), 129.2 (C-11), 129.2 (C-13), 132.1 (C-21), 132.9 (C-8), 133.2 (C-12), 134.1 (C-19), 146.1 (C-9), 148.8 (C-5), 160.6 (C-17), 163.2 (C-15), 164.3 (C-3). Anal. Cal. for C20H20N4O3: C, 65.92; H, 5.53; N, 15.38%. Found: C, 65.86; H, 5.47; N, 15.17%. The atoms were numbered according to Figure S91.
(E)-2-(((3-(tert-butyl)-1-(3-nitrophenyl)-1H-pyrazol-5-yl)imino)methyl)phenol (5)
Yellow solid, m.p. 147–148 °C. MS [(70 eV) m/z (%)] 364 (M+, 100), 349 (M+-15, 51.95), 318 (M+-46, 16), 307 (M+-57, 1.37) 122 (1.64), 57 (5.29). IR (KBr, cm−1): νOH 3427, νC-H 3128 (pyrazole), νC-H 3095 (aromatic), νas–CH3 2968, νs–CH3 2863, ν–C=N 1623, νas–NO2 1527, νs–NO2 1348. 1H NMR (400 MHz, CDCl3, ppm): δ= 1.44 (s, 9H, H-7), 6.41 (s, 1H, H-4), 7.00 (m, 2H, H-18 and H-20), 7.44 (m, 2H, H-19 and H-21), 7.69 (t, 1H, 3J = 8.1 Hz, H-12), 8.01 (d, 1H, H-13), 8.24 (d. 1H, 3J = 8.1 Hz, H-11), 8.58 (s, 1H, H-9), 8.80 (s, 1H, H-15), 11.85 (s, 1H, H-17). 13C NMR (100 MHz, CDCl3, ppm): δ= 30.3 (C-7), 32.7 (C-6), 91.3 (C-4), 117.5 (C-18), 118.7 (C-20), 119.5 (C-16), 119.7 (C-9), 121.7 (C-11), 129.8 (C-12), 129.8 (C-13), 132.9 (C-21), 134.4 (C-19), 140.0 (C-8), 148.1 (C-5), 148.7 (C-10), 160.8 (C-17), 163.4 (C-15), 163.7 (C-3). Anal. Cal. for C20H20N4O3: C, 65.92; H, 5.53; N, 15.38%. Found: C, 65.98; H, 5.64; N, 15.42%. The atoms were numbered according to Figure S91.
(E)-2-(((3-(tert-butyl)-1-(4-nitrophenyl)-1H-pyrazol-5-yl)imino)methyl)phenol (6)
Yellow solid, m.p. 204–205 °C. MS [(70 eV) m/z (%)] 364 (M+, 100), 349 (M+-15, 71.70), 318 (M+-46, 1.40), 307 (M+-57, 2.13), 122 (1.84), 57 (12.97). IR (KBr, cm−1): νOH 3419, νC-H 3135 (pyrazole), νC-H 3087 (aromatic), νas–CH3 2954, νs–CH3 2861, ν–C=N 1623, νas–NO2 1519, νs–NO2 1342. 1H NMR (400 MHz, CDCl3, ppm): δ= 1.43 (s, 9H, H-7), 6.41 (s, 1H, H-4), 7.03 (m, 2H, H-18 and H-20), 7.46 (m, 2H, H-21 and H-19), 7.90 (d, 2H, 3J = 9.1 Hz, H-9), 8.39 (d, 2H, 3J = 9.1 Hz, H-10), 8.80 (s, 1H, H-15), 11.92 (s, 1H, H-17). 13C NMR (100 MHz, CDCl3, ppm): δ= 30.2 (C-7), 32.7 (C-6), 92.1 (C-4), 117.5 (C-18), 118.7 (C-20), 119.8 (C-16), 123.9 (C-9), 124.7 (C-10), 133.0 (C-21), 134.5 (C-19), 144.1 (C-8), 145.8 (C-11), 148.5 (C-5), 160.7 (C-17), 163.7 (C-15), 164.1 (C-3). Anal. Cal. for C20H20N4O3: C, 65.92; H, 5.53; N, 15.38%. Found: C, 66.03; H, 5.57; N, 15.27%. The atoms were numbered according to Figure S91.
(E)-4-(((3-(tert-butyl)-1-phenyl-1H-pyrazol-5-yl)imino)methyl)phenol (7)
Schiff base 7 was synthesized and characterized by the following methodology, as previously reported [53].
Beige solid. m.p. 155–156 °C. MS [(70 eV) m/z (%)] 319 (M+, 83.43), 304 (M+-15, 100), 262 (M+-57, 3.49), 226 (M+-93, 5.39), 77 (23.29), 57 (13.54). IR (KBr, cm−1): νOH 3455, νC-H 3124 (pyrazole), νC-H 3058 (aromatic), νas–CH3 2962, νs–CH3 2865, ν–C=N 1618. 1H NMR (400 MHz, DMSO-d6, ppm): δ = 1.34 (s, 9H, H-7), 6.48 (s, 1H, H-4), 6.90 (d, 2H, 3J = 8.4 Hz, H-18), 7.33 (t, 1H, 3J = 7.4 Hz, H-11), 7.50 (t, 2H, 3J = 7.6 Hz, H-10), 7.75 (m, 4H, 3J = 8.4 Hz, H-9 and H-17), 8.76 (s, 1H, H-15), 10.28 (s, 1H, H-19). 13C NMR (100 MHz, DMSO-d6, ppm): δ = 30.7 (C-7), 32.7 (C-6), 90.9 (C-4), 116.4 (C-18), 123.9 (C-9), 126.5 (C-11), 127.6 (C-16), 129.1 (C-10), 131.5 (C-17), 140.0 (C-8), 151.0 (C-5), 161.5 (C-15), 161.7 (C-3), 161.8 (C-19). Anal. Calc. for C20H21N3O: C, 75.17; H, 6.52; N, 13.28%. Anal. Calc. for C20H21N3O: C, 75.17; H, 6.52; N, 13.28%. Found: C, 75.21; H, 6.63; N, 13.16%. The atoms were numbered according to Figure S91.
(E)-4-(((3-(tert-butyl)-1-(2-nitrophenyl)-1H-pyrazol-5-yl)imino)methyl)phenol (8)
Yellow solid. m.p. 173–175 °C. MS [(70 eV) m/z (%)] 364 (M+, 100), 349 (M+-15) (78.33), 318 (M+-46) (21.14), 77 (14.44), 57 (4.54). IR (KBr, cm−1) νOH 3436, νC-H pyrazole 3131, νPh-H 3066, νas–CH3 2962, νs–CH3 2871, ν–C=N 1606. 1H NMR (400 MHz, acetone-d6, ppm): δ = 1.37 (s, 9H, H-7), 6.50 (s, 1H, H-4), 6.96 (d, 2H, 3J= 8.8 Hz, H-18), 7.67 (t, 1H, 3J = 7.3 Hz, 4J = 1.7 Hz, H-11), 7.75 (d, 2H, 3J = 8.6 Hz, H-17), 7.83 (d, 1H, 3J = 8 Hz, 4J = 1.7 Hz, H-13), 7.87 (t, 1H, 3J = 8.0 Hz, 4J = 1.3 Hz, H-12), 8.07 (d, 1H, 3J = 8.1 Hz, 4J = 1.3 Hz, H-10), 8.74 (s, 1H, H-15), 9.22 (s, 1H, H-19). 13C NMR (400 MHz, acetone-d6, ppm): δ = 29.7 (C-7), 32.4 (C-6), 89.5 (C-4), 115.8 (C-18), 124.6 (C-10), 127.8 (C-16), 127.9 (C-11), 128.8 (C-13), 131.3 (C-17), 132.4 (C-8), 133.0 (C-12), 145.5 (C-9), 151.2 (C-5), 161.0 (C-15), 161.4 (C-19), 163.3 (C-3). Anal. Cald. For C20H20N4O3: C, 65.92; H, 5.53; N, 15.38%. Found: C, 66.24; H, 5.32; N, 15.41%. The atoms were numbered according to Figure S91.
(E)-4-(((3-(tert-butyl)-1-(3-nitrophenyl)-1H-pyrazol-5-yl)imino)methyl)phenol (9)
Yellow solid. m.p. 176–177 °C. MS [(70 eV) m/z (%)] 364 (M+, 100), 349 (M+-15) (65.32), 318 (M+-46) (17.91), 77 (32.15), 57 (31.89). IR (KBr, cm−1) νOH 3423, νC-H pyrazole 3124, νPh-H 3095, νas–CH3 2958, νs–CH3 2863, ν–C=N 1596. 1H NMR (400 MHz, DMSO-d6, ppm): δ = 1.35 (s, 9H, H-7), 6.61 (s, 1H, H-4), 6.95 (d, 2H, 3J = 8.6 Hz, H-18), 7.79 (t, 1H, 3J = 8.2 Hz, H-12), 7.83 (d, 2H, 3J = 8.6 Hz, H-17), 8.15 (d, 1H, 3J = 8.2 Hz, H-11), 8.33 (d, 1H, 3J = 8.0 Hz, H-13), 8.82 (s, 1H, H-15), 8.84 (s, 1H, H-9), 10.36 (s, 1H, H-19). 13C NMR (400 MHz, DMSO-d6, ppm): δ = 30.5 (C-7), 32.8 (C-6), 92.0 (C-4), 116.5 (C-18), 117.3 (C-9), 120.6 (C-11), 127.4 (C-16), 128.8 (C-13), 130.8 (C-12), 131.8 (C-17), 140.7 (C-8), 148.4 (C-10), 151.7 (C-5), 162.2 (C-19), 162.5 (C-15), 162.8 (C-3). Anal. Cald. For C20H20N4O3: C, 65.92; H, 5.53; N, 15.38%. Found: C, 65.84; H, 5.67; N, 15.27%. The atoms were numbered according to Figure S91.
(E)-4-(((3-(tert-butyl)-1-(4-nitrophenyl)-1H-pyrazol-5-yl)imino)methyl)phenol (10)
Yellow solid. m.p. 177–178 °C. MS [(70 eV) m/z (%)] 364 (M+, 100), 349 (M+-15) (64.23), 318 (M+-46) (21.03), 77 (34.90), 57 (33.95). IR (KBr, cm−1) νOH 3326, νC-H pyrazole 3141, νPh-H 3022, νas–CH3 2962, νs–CH3 2863, ν–C=N 1596, 1H NMR (400 MHz, DMSO-d6, ppm): δ = 1.35 (s, 9H, H-7), 6.61 (s, 1H, H-4), 6.95 (d, 2H, 3J = 8.6 Hz, H-18), 7.83 (d, 2H, 3J =8.6 Hz, H-17), 8.20 (d, 2H, 3J = 9.4 Hz, H-9), 8.37 (d, 2H, 3J = 9.4 Hz, H-10), 8.81 (s, 1H, H-15), 10.37 (s, 1H, H-19). 13C NMR (400 MHz, DMSO-d6, ppm): δ = 30.4 (C-7), 32.8 (C-6), 92.6 (C-4), 116.5 (C-18), 123.0 (C-9), 125.1 (C-10), 127.3 (C-16), 132.0 (C-17), 144.8 (C-8), 145.1 (C-11), 152.3 (C-5), 162.2 (C-19), 162.8 (C-15), 163.4 (C-3). Anal. Cald. For C20H20N4O3: C, 65.92; H, 5.53; N, 15.38%. Found: C, 66.33; H, 5.46; N, 15.31%. The atoms were numbered according to Figure S91.
3.3. Synthesis of Palladium(II) Complexes [Pd2Cl4(N-het)2]
The new Pd-coordination complexes were synthesized by following the methodology previously described in the literature [55] (Scheme 2). Figure 2 shows the synthesized coordination compounds: [Pd2Cl4(3)2], [Pd2Cl4(4)2], [Pd2Cl4(5)2], [Pd2Cl4(6)2], [Pd2Cl4(7)2], [Pd2Cl4(8)2], [Pd2Cl4(9)2] and [Pd2Cl4(10)2]. In general, dichloro-bis(acetonitrile)palladium(II) (80.00 mg, 0.31 mM) was dissolved in dry CH2Cl2 (2 mL). The corresponding Schiff base 7–10 (0.32 mM) was also dissolved in dry CH2Cl2 (2 mL) and was added to the above mixture. The reaction was stirred for 8 h under N2 atmosphere at room temperature. After the reaction finished (monitored by TLC), the solvent was removed using a rotary evaporator at room temperature and the scarlet red solid formed was washed with ethyl ether (3 × 5 mL).
3.3.1. Characterization of Palladium(II) Complexes [Pd2Cl4(N-het)2]
[Pd2Cl4(3)2]
Scarlet red solid. Decomposition point 195–197 °C. IR (KBr, cm−1) νOH 3444, νC-H 3128 (pyrazole), νPh-H 3058 (aromatic), νas–CH3 2960, νs–CH3 2863, ν–C=N 1662. 1H NMR (400 MHz, DMSO-d6, ppm): δ = 1.34 (s, 9H, H–7), 6.69 (s, 1H, H-4), 6.91 (d, 1H, 3J = 8.2 Hz, H-18), 6.99 (t, 1H, 3J = 7.1 Hz, H-11), 7.41 (t, 1H, 3J = 7.2 Hz, H-19), 7.43 (t, 1H, H-20), 7.53 (t, 2H, 3J = 7.4 Hz, H-10), 7.60 (d, 2H, 3J = 7.2 Hz, H-9), 7.66 (d, 1H, 3J = 7.2 Hz, H-21), 9.10 (s, 1H, H-15), 11.78 (s, 1H, H-17). 13C NMR (400 MHz, DMSO-d6, ppm): δ 30.6 (C-7), 32.7 (C-6), 91.6 (C-4), 117.1 (C-18), 120.0 (C-11), 124.9 (C-16), 127.7 (C-9), 129.5 (C-19), 132.5 (C-10), 134.4 (C-21), 138.4 (C-20), 139.2 (C-8), 148.4 (C-5), 160.1 (C-17), 162.2 (C-3), 163.2 (C-15). The atoms were numbered according to Figure S92.
[Pd2Cl4(4)2]
Scarlet red solid. Decomposition point 193–194 °C. IR (KBr, cm−1): νOH 3440, νC-H 3135 (pyrazole), νC-H 3068 (aromatic), νas–CH3 2960, νs–CH3 2865, ν–C=N 1662, νas–NO2 1535, νs–NO2 1344. 1H NMR (400 MHz, DMSO-d6, ppm): δ= 1.30 (s, 9H, H-7), 6.71 (s, 1H, H-4), 6.92 (m, 2H, H-18), 6.97 (m, 2H, H-20), 7.41 (t, 2H, H-21), 7.43 (t, 2H, H-19), 7.62 (m, 1H, 3J = 7.8 Hz, H-11), 7.81 (m, 1H, H-13), 7.89 (t, 1H, H-12), 8.10 (d, 1H, 3J = 8.0 Hz, H-10), 9.10 (s, 1H, H-15), 11.29 (s, 1H, H-17). 13C NMR (100 MHz, DMSO-d6, ppm): δ= 30.5 (C-7), 32.8 (C-6), 91.3 (C-4), 117.1 (C-18), 120.1 (C-20), 120.3 (C-16), 125.4 (C-10), 129.3 (C-11), 129.7 (C-13), 131.6 (C-21), 134.1 (C-8), 134.6 (C-12), 138.4 (C-19), 145.9 (C-9), 149.7 (C-5), 159.9 (C-17), 162.6 (C-15), 163.6 (C-3). The atoms were numbered according to Figure S92.
[Pd2Cl4(5)2]
Scarlet red solid. Decomposition point 198–200 °C. IR (KBr, cm−1): νOH 3469, νC-H 3120 (pyrazole), νC-H 3087 (aromatic), νas–CH3 2966, νs–CH3 2869, ν–C=N 1606, νas–NO2 1535, νs–NO2 1351. 1H NMR (400 MHz, DMSO-d6, ppm): δ= 1.35 (s, 9H, H-7), 6.74 (s, 1H, H-4), 6.95 (m, 2H, H-18), 7.01 (m, 2H, H-20), 7.44 (m, 2H, H-19), 7.45 (m, 2H, H-21), 7,59 (t, 1H, 3J = 8.5 Hz, H-12), 7.78 (m, 1H, H-13), 7.84 (m. 1H, H-11), 8.10 (s, 1H, 3J = 8.5 Hz, H-9), 9.15 (s, 1H, H-15), 11.29 (s, 1H, H-17). 13C NMR (100 MHz, DMSO-d6, ppm): δ= 30.5 (C-7), 32.8 (C-6), 92.6 (C-4), 117.2 (C-18), 118.3 (C-20), 120.2 (C-16), 120.7 (C-9), 121.6 (C-11), 129.9 (C-12), 131.0 (C-13), 134.7 (C-21), 138.4 (C-19), 140.1 (C-8), 148.5 (C-5), 150.0 (C-10), 159.9 (C-15), 162.4 (C-17), 163.2 (C-3). The atoms were numbered according to Figure S92.
[Pd2Cl4(6)2]
Scarlet red solid. Decomposition point 201–203 °C. IR (KBr, cm−1) νOH 3438, νC-H 3120 (pyrazole), νC-H 3074 (aromatic), νas–CH3 2962, νs–CH3 2863, ν–C=N 1623, νas–NO2 1525, νs–NO2 1346. 1H NMR (400 MHz, DMSO-d6, ppm): δ= 1.36 (s, 9H, H-7), 6.75 (s, 1H, H-4), 6.99 (m, 2H, H-18), 7.00 (m, 2H, H-20), 7.46 (t, 1H, 4J = 6.9 Hz, H-19), 7.80 (d, 2H, 4J = 6.4 Hz, H-21), 8.04 (d, 2H, 3J = 9.1 Hz, H-9), 8.39 (d, 2H, 3J = 9.1 Hz, H-10), 9.15 (s, 1H, H-15), 11.27 (s, 1H, H-17). 13C NMR (100 MHz, DMSO-d6, ppm): δ= 30.4 (C-7), 32.9 (C-6), 93.3 (C-4), 117.2 (C-18), 120.2 (C-20), 120.8 (C-16), 124.0 (C-9), 124.1 (C-10), 131.1 (C-21), 134.7 (C-19), 144.5 (C-8), 145.5 (C-11), 150.6 (C-5), 160.0 (C-15), 162.5 (C-17), 163.3 (C-3). The atoms were numbered according to Figure S92.
[Pd2Cl4(7)2]
Scarlet red solid. Decomposition point 196–198 °C. IR (KBr, cm−1) νOH 3465, νC-H pyrazole 3131, νPh-H 3072, νas–CH3 2962, νs–CH3 2865, ν–C=N 1629. 1H NMR (400 MHz, DMSO-d6, ppm): δ = 1.34 (s, 9H, H-7), 6.69 (s, 1H, H-4), 6.91 (d, 2H, 3J = 8.6 Hz, H-18), 7.33 (t, 1H, 3J = 7.2 Hz, H-11), 7.50 (t, 2H, 3J = 7.6 Hz, H-10), 7.74 (bd, 4H, 3J = 8.6 Hz, H-9 and H-17), 8.76 (s, 1H, H-15), 10.28 (s, 1H, H-19). 13C NMR (100 MHz, DMSO-d6, ppm): δ = 30.7 (C-7), 32.7 (C-6), 90.9 (C-4), 116.4 (C-18), 123.9 (C-9), 126.5 (C-11), 127.6 (C-16), 129.1 (C-10), 131.5 (C-17), 140.0 (C-8), 151.0 (C-5), 161.5 (C-15), 161.7 (C-3), 161.8 (C-19). Anal. Cald. For Pd2C40H42Cl4N6O2: C, 48.36; H, 4.26; N, 8.46%. Found: C, 48.73; H, 4.17; N, 8.58%. The atoms were numbered according to Figure S92.
[Pd2Cl4(8)2]
Scarlet red solid. Decomposition point 200–202 °C. IR (KBr, cm−1) νOH 3463, νC-H pyrazole 3143, νPh-H 3060, νas–CH3 2966, νs–CH3 2865, ν–C=N 1635. 1H NMR (400 MHz, DMSO-d6, ppm): δ = 1.32 (s, 9H, H-7), 6.55 (s, 1H, H-4), 6.88 (d, 2H, 3J = 8.8 Hz, H-18), 7.65 (t, 1H, 3J = 6.2 Hz, H-11), 7.76 (d, 2H, 3J = 8.4 Hz, H-17), 7.86 (t, 1H, 3J = 6.8 Hz, H-12), 7.80 (d, 1H, 3J = 7.2 Hz, H-13), 8.07 (d, 1H, 3J = 6.8 Hz, H-10), 8.72 (s, 1H, H-15), 10.29 (s, 1H, H-19). 13C NMR (400 MHz, DMSO-d6, ppm): δ = 30.4 (C-7), 32.4 (C-6), 90.5 (C-4), 116.3 (C-18), 125.2 (C-10), 127.2 (C-16), 128.8 (C-12), 129.1 (C-13), 131.7 (C-17), 132.0 (C-8), 134.0 (C-11), 145.3 (C-9), 151.2 (C-5), 162.0 (C-19), 162.1 (C-15), 163.3 (C-3). Anal. Cald. For Pd2C40H40Cl4N8O6: C, 44.34; H, 3.72; N, 10.34%. Found: C, 44.63; H, 3.81; N, 10.19%. The atoms were numbered according to Figure S92.
[Pd2Cl4(9)2]
Scarlet red solid. Decomposition point 201–203 °C. IR (KBr, cm−1) νOH 3428, νC-H pyrazole 3128, νPh-H 3091, νas–CH3 2964, νs–CH3 2865, ν–C=N 1631, 1H NMR (400 MHz, DMSO-d6, ppm): δ = 1.35 (s, 9H, H-7), 6.61 (s, 1H, H-4), 6.94 (d, 2H, 3J = 8.6 Hz, H-18), 7.83 (m, 3H, H-12 and H-17), 8.15 (d, 1H, 3J = 8.0 Hz, H-11), 8.33 (d, 1H, 3J = 8.2 Hz, H-13), 8.82 (s, 1H, H-15), 8.84 (s, 1H, H-9), 10.36 (s, 1H, H-19). 13C NMR (400 MHz, DMSO-d6, ppm): δ = 30.5 (C-7), 32.8 (C-6), 92.0 (C-4), 116.5 (C-18), 117.3 (C-9), 120.6 (C-11), 127.4 (C-16), 128.8 (C-13), 130.8 (C-12), 131.8 (C-17), 140.7 (C-8), 148.4 (C-10), 151.7 (C-5), 162.1 (C-19), 162.5 (C-15), 162.8 (C-3). Anal. Cald. For Pd2C40H40Cl4N8O6: C, 44.34; H, 3.72; N, 10.34%. Found: C, 44.22; H, 3.62; N, 10.45%. The atoms were numbered according to Figure S92.
[Pd2Cl4(10)2]
Scarlet red solid. Decomposition 203–204 °C. IR KBr, cm−1) νOH 3450, νC-H pyrazole 3178, νPh-H 3070, νas–CH3 2964, νs–CH3 2865, ν–C=N 1631. 1H NMR (400 MHz, DMSO-d6, ppm): δ = 1.35 (s, 9H, H-7), 6.62 (s, 1H, H-4), 6.95 (d, 2H, 3J = 8.6 Hz, H-18), 7.83 (d, 2H, 3J = 8.6 Hz, H-17), 8.20 (d, 2H, 3J = 9.2 Hz, H-9), 8.38 (d, 2H, 3J = 9.2 Hz, H-10), 8.81 (s, 1H, H-15), 10.37 (s, 1H, H-19). 13C NMR (400 MHz, DMSO-d6, ppm): δ = 30.4 (C-7), 32.8 (C-6), 92.6 (C-4), 116.5 (C-18), 123.1 (C-9), 125.1 (C-10), 127.3 (C-16), 132.0 (C-17), 144.8 (C-8), 145.1 (C-11), 152.3 (C-5), 162.2 (C-19), 162.8 (C-15), 163.5 (C-3). Anal. Cald. For Pd2C40H40Cl4N8O6: C, 44.34; H, 3.72; N, 10.34%. Found: C, 44.58; H, 3.77; N, 10.41%. The atoms were numbered according to Figure S92.
3.4. Synthesis of Styrylimines
Styrylimines were synthesized by mixing 4-stirylbenzaldehyde and aminopyrazoles (2a–2d) using the following methodology: a mixture of 4-stirylbenzaldehyde (200.00 mg, 0.96 mmol), aminopyrazole 2a–2d (0.96 mmol) and glacial acetic acid (5 drops) was placed in a pestle and ground with a mortar for 10–17 min at room temperature. After the reaction was complete (monitored by TLC), the crude yellow solid product was washed with cold water (5 × 20 mL) and filtered under vacuum to dryness (See Scheme 4).
3.4.1. Characterization of Styrylimines
(E)-N-(3-(tert-butyl)-1-phenyl-1H-pyrazol-5-yl)-1-(4-((E)-styryl)phenyl)methanimine (12)
Light yellow solid, m.p. 143.5–144 °C. m/z (%) 405 (M+, 94.93), 390 (M+-15, 100), 363 (53.43). IR (KBr, cm−1) νH–C= 3077 (pyrazol), ν Ph-H 3023, νas–CH3 2956, νs–CH3 2861, ν–C=N 1594. NMR 1H (400 MHz, DMSO-d6 (δ en ppm) 1.35 (s, 9H) H-7; 6.60 (s, 1H) H-4; 7.30 (m, 6H) H-22; 7.35 (m, 6H) H-17; 7.39 (m, 6H) H-18, 7.41 (m, 6H) H-21, 7.44 (m, 6H) H-11; 7.53 (t, 2H) H-10; 7.66 (d, 2H) H-20; 7.77 (dd, 4H) H-14 y H-15; 7.90 (d, 2H) H-9; 8.90 (s, 1H) H-12. NMR 13C (100 MHz, DMSO-d6 (δ en ppm): 30.69 C-7; 32.70 C-6; 91.46 C-4; 124.08 C-14; 126.78 C-20; 127.29 C-15; 127.48 C-17; 128.15 C-22; 128.65 C-10; 129.15 C-11; 129.28 C-9; 129.76 C-21; 131.12 C-18; 135.36 C-13; 137.20 C-19; 139.84 C-16; 141.18 C-8; 150.49 C-5; 161.28 C-12; 161.86 C-3. The atoms were numbered according to Figure S93.
(E)-N-(3-(tert-butyl)-1-(2-nitrophenyl)-1H-pyrazol-5-yl)-1-(4-((E)-styryl)phenyl)methanimine (13)
Light yellow solid, m.p. 144.5–145.7 °C. m/z (%) 450 (M+, 100), 435 (M+-15, 52.97), 408 (23.36). IR (KBr, cm−1) νH–C= 3079, ν Ph-H 3023, νas–CH3 2956, νs–CH3 2865, ν–C=N 1606 (pyrazole) y 1594 (imine), νas-NO2 1529, νs-NO2 1351. NMR H1 (300 MHz, DMSO-d6 (δ en ppm)) 1.32 (s, 9H) H-7; 6.66 (s, 1H) H-4; 7.35 (m, 5H) H-19 (J=15.09); 7.35 (m, 5H) H-20 (J=15.94); 7.35 (m, 5H) H-23; 7.35 (m, 5H) H-24; 7.64 (d, 2H) H-22; 7.71 (m, 6H) H-10; 7.75 (m, 6H) H-12; 7.75 (m, 6H) H-16; 7.75 (m, 6H) H-17; 7.88 (t, 1H) H-11; 8.10 (d, 1H) H-13; 8.85 (s, 1H) H-14. NMR 13C (75 MHz, DMSO-d6 (δ en ppm)): 30.53 C-7; 32.81 C-6; 125.2 C-13; 127.37 C-20; 127.53 C-12; 128.03 C-19; 128.67 C-24, 129.07 C-22; 129.20 C-23; 129.27 C-17; 129.85 C-16; 131.85 C-9; 134.10 C-11; 134.87 C-15; 137.13 C-21; 140.57 C-10; 141.40 C-18; 148.4 C-8; 150.69 C-5; 161.9 C-4; 161.9 C-14; 163.5 C-3. The atoms were numbered according to Figure S93.
(E)-N-(3-(tert-butyl)-1-(3-nitrophenyl)-1H-pyrazol-5-yl)-1-(4-((E)-styryl)phenyl)methanimine (14)
Light yellow solid, m.p. 158–160 °C. m/z (%) 450 (M+, 100), 435 (M+-15, 59.58), 408 (45.52). IR (KBr, cm−1) νH–C= 3124, ν Ph-H 3079, νas–CH3 2958, νs–CH3 2863, ν–C=N 1612 (pyrazol), 1594 (imine), νas-NO2 1527, νs-NO2 1351. NMR H1 (300 MHz, DMSO-d6 (δ en ppm)) 1.32 (s, 9H) H-7; 6.66 (s, 1H) H-4; 7.35 (m, 5H) H-19 (J=15.09); 7.35 (m, 5H) H-20 (J=15.94); 7.35 (m, 5H) H-23; 7.35 (m, 5H) H-24; 7.64 (d, 2H) H-22; 7.71 (m, 6H) H-10; 7.75 (m, 6H) H-12; 7.75 (m, 6H) H-16; 7.75 (m, 6H) H-17; 7.88 (t, 1H) H-11; 8.10 (d, 1H) H-13; 8.85 (s, 1H) H-14. NMR 13C (75 MHz, DMSO-d6 (δ en ppm)): 30.53 C-7; 32.81 C-6; 125.23ppm C-13; 127.37 C-20; 127.53 C-12; 128.03 C-19; 128.67 C-24, 129.07 C-22; 129.20 C-23; 129.27 C-17; 129.85 C-16; 131.85 C-9; 134.10 C-11; 134.87 C-15; 137.13 C-21; 140.57 C-10; 141.40 C-18; 148.4 C-8; 150.69 C-5; 161.9 C-4; 161.9 C-14; 163.5 C-3. The atoms were numbered according to Figure S93.
(E)-N-(3-(tert-butyl)-1-(4-nitrophenyl)-1H-pyrazol-5-yl)-1-(4-((E)-styryl)phenyl)methanimine (15)
Light yellow solid, m.p. 205.5–207 °C. m/z (%) 450 (M+, 100); 435(M+-15, 58.28); 218 (23.2). IR (KBr, cm−1) νH–C= 3180 pyrazole, νas–CH3 2958, νs–CH3 2861, ν Ph-H 3022, ν–C=N 1645 (pyrazole), 1591 (imine), νas-NO2 1510, νs-NO2 1328. NMR H1 (400 MHz, DMSO-d6 (δ en ppm)) 1.35 (s, 9H) H-7; 6.72 (s, 1H) H-4; 7.32 (m, 1H) H-22; 7.39 (m, 4H) H-18 (J=15.089 Hz), 7.42 (m, 4H) H-21 (3J=7.275 Hz), 7.44 ppm (m, 4H) H-17 (J=15.266 Hz); 7.66 (d, 2H) H-20; 7.79 (d, 2H) H-15 (3J=8.223 Hz); 7.97 (d, 2H) H-14 (3J=8.317 Hz); 8.17 (d, 2H) H-9 (3J=9.196 Hz); 8.40 (d, 2H) H-10 (3J=9.194 Hz); 8.95 (s, 1H) H-12. NMR 13C (100 MHz, DMSO-d6 (δ en ppm)): 30.41 C-7; 32.81 C-6; 93.19 C-4; 123.34 C-9; 125.08 C-10; 127.31 C-20; 127.52 C-15; 128.11 C-22; 128.69 C-18; 129.27 C-21; 130.16 C-14; 131.35 C-17; 135.01 C-13; 137.15 C-19; 141.58 C-16; 144.84 C-8; 144.98 C-11; 151.75 C-5; 162.72 C-12; 163.52 C-3. The atoms were numbered according to Figure S93.
3.5. Catalytic Activity toward a Model Mizoroki–Heck C-C Coupling Reactions
In a typical experiment (Scheme 3), a mixture of iodobenzene (0.897 mmol) or bromobenzaldehyde (0.541 mmol), styrene (1.167 mmol), palladium complexes (0.889 mmol) and K2CO3 (1.167 mmol) at a complex-to-substrate ratio of 1:3000 was prepared in DMF (4 mL) or a DMF–H2O mixture (1:1, 4 mL) in a 20 mL two-necked bottle was immersed in a pre-heated oil bath at 160 °C with magnetic stirring. Aliquots were removed from the reaction mixture at regular intervals and analyzed by GC-MS. In addition, to determine the reactivity and selectivity of the palladium complexes, various experiments were performed to test, e.g., the effect of the concentration, the effect of the base, the effect of the temperature and the effect of excess ligand. Additionally, the Hg drop test was carried out to ensure the homogeneity of the catalysts.
3.6. Antifungal Assays
3.6.1. Fungal Species
The antifungal activities of ligands 3–10, 12–15 and the Pd-complexes [Pd2Cl4(3)2]-[Pd2Cl4(10)2] were determined against the strains of the American Type Culture Collection, (ATCC, Rockville, MD, USA) C. albicans ATCC 10231 and C. neoformans ATCC 32264. The inocula were adjusted to 1–5 × 103 colony-forming units per mL (CFU/mL) according to the procedures of the Clinical and Laboratory Standards Institute (CLSI) [71].
3.6.2. Fungal Growth Inhibition Percentage Determination
Broth microdilutions were performed in 96-well microplates following the guidelines of the Clinical and Laboratory Standards Institute for yeasts (M27-A3) [71]. For the assay, compound test wells (CTWs) were prepared with stock solutions of each compound in DMSO (maximum concentration ≤ 1%) diluted with RPMI-1640 to final concentrations of 250–3.9 μg/mL. An inoculum suspension (100 μL) was added to each well (final volume in the well = 200 μL). A growth-control well (GCW) containing medium, inoculum and the same amount of DMSO used in a CTW but no compound and a sterility-control well (SCW) containing sample, medium and sterile water instead of inoculum were included for each fungus tested. Microtiter trays were incubated in a moist, dark chamber at 30 °C for 48 h for both yeasts. Microplates were read in a VERSA Max microplate reader (Molecular Devices, Sunnyvale, CA, USA). Tests were performed in triplicate. Reduction of growth for each compound concentration was calculated as follows: % inhibition = 100 − (OD 405 CTW − OD 405 SCW)/(OD 405 GCW − OD 405 SCW). The means ± SEM were used to construct the dose-response curves representing % inhibition vs. concentration of each compound. Dose-response curves were constructed with SigmaPlot 11.0 software. The antifungal drug amphotericin B (Sigma Chemical Co., St Louis, MO, USA) was included as a positive control. IC80 and IC50 were defined as the lowest compound concentration that showed 80% or 50% reduction of fungal growth, respectively.
3.7. Computational Study
DFT calculations were performed in the gas phase to optimize the geometries and energies of the Schiff bases using B3LYP as functional with the basis set 6-311**G(d,p) and the Gaussian packet 09 [50] without any obstacle to geometry. The harmonic-vibration frequency studies for the palladium complexes were calculated by DFT using ωB97X-D as functional with the basis set LANL2DZ and the Gaussian packet 09. Theoretical vibrational spectra of [Pd2Cl4(5)2] and [Pd2Cl4(9)2] were interpreted through the potential energy distributions (PED) using the VEDA 4 program [78].
4. Conclusions
We report on the synthesis of a new series of Schiff bases 4–10 derived from nitrophenylpyrazole of hydroxyphenylimines, the new styrylimines 12–15 and the palladium complexes. The antifungal properties of these compounds against C. albicans and C. neoformans were evaluated. The structures of all ligands 3–10, their corresponding palladium complexes and styrylimines were determined by spectroscopic techniques.
Spectroscopic studies of the palladium complexes indicate that they are of the type [Pd2Cl4(N-het)2], where a dimer is formed with two palladium atoms linked by two bridging chlorine atoms and each palladium atom has a terminal chlorine and a pyrazolic ligand in a square planar geometry for each metal atom.
The prediction based on the theoretical data obtained with the Gaussian packet regarding the energy descriptors of the pyrazole compounds indicate that the best ligands will be those that have a smaller HOMO-LUMO difference and that are also softer. This prediction is consistent with the data obtained regarding the activity of the complexes in the C-C coupling reactions; those complexes exhibited higher activity and higher selectivity, even in aqueous media.
The palladium complexes exhibit high catalytic activity and high selectivity towards the formation of trans stilbene and styryilaldehydes in the model C-C Mizoroki–Heck coupling reaction. The mercury drop test confirmed that the complexes behaved as homogeneous catalysts, and it is believed that the catalytically active species is a palladium(=) complex with an N-heterocyclic ligand and three solvent molecules, which rapidly undergo the oxidative addition of aryl halides. This indicates that the lnitrophenylpyraolic imines ligands behave as excellent electron donors and acceptors, allowing the formation of stable complexes even at high temperatures and in aqueous media.
Although all imines 3–10 and 12–15 showed very low antifungal activity against C. albicans and C. neoformans, some of their Pd-complexes (i.e., [Pd2Cl4(3)2] [Pd2Cl4(5)2], [Pd2Cl4(8)2] and [Pd2Cl4(10)2]) showed comparatively higher antifungal effects, mainly against C. neoformans.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14060387/s1, Supp. Inf. Figure S1: Mass Spectrometry of compound 8. Supp. Inf. Figure S2: Mass Spectrometry of compound 9. Supp. Inf. Figure S3: Mass Spectrometry of compound 10. Supp. Inf. Figure S4: FT-IR spectrum of compound 8. Supp. Inf. Figure S5: FT-IR spectrum of compound 9. Supp. Inf. Figure S6: FT-IR spectrum of compound 10. Supp. Inf. Figure S7: FT-IR spectrum of [Pd2Cl4(3)2] complex. Supp. Inf. Figure S8: FT-IR spectrum of [Pd2Cl4(4)2] complex. Supp. Inf. Figure S9: FT-IR spectrum of [Pd2Cl4(5)2] complex. Supp. Inf. Figure S10: FT-IR spectrum of [Pd2Cl4(6)2] complex. Supp. Inf. Figure S11: FT-IR spectrum of [Pd2Cl4(7)2] complex. Supp. Inf. Figure S12: FT-IR spectrum of [Pd2Cl4(8)2] complex. Supp. Inf. Figure S13: FT-IR spectrum of [Pd2Cl4(9)2] complex. Supp. Inf. Figure S14: FT-IR spectrum of [Pd2Cl4(10)2] complex. Supp. Inf. Figure S15: Lower-frequencies region of the IR spectrum of [Pd2Cl4(3)2] complex. Supp. Inf. Figure S16: Lower-frequencies region of the IR spectrum of [Pd2Cl4(4)2] complex. Supp. Inf. Figure S17: Lower-frequencies region of the IR spectrum of [Pd2Cl4(6)2] complex. Supp. Inf. Figure S18: Lower-frequencies region of the IR spectrum of [Pd2Cl4(7)2] complex. Supp. Inf. Figure S19: Lower-frequencies region of the IR spectrum of [Pd2Cl4(8)2] complex. Supp. Inf. Figure S20: Lower-frequencies region of the IR spectrum of [Pd2Cl4(10)2] complex. Supp. Inf. Figure S21: 1H NMR for compound 8. Supp. Inf. Figure S22: 13C NMR for compound 8. Supp. Inf. Figure S23: DEPT-135 NMR for compound 8. Supp. Inf. Figure S24: HSQC NMR for compound 8. Supp. Inf. Figure S25: HMBC NMR for compound 8. Supp. Inf. Figure S26: 1H NMR for compound 9. Supp. Inf. Figure S27: 13C NMR of compound 9. Supp. Inf. Figure S28: DEPT-135 NMR of compound 9. Supp. Inf. Figure S29: HSQC of compound 9. Supp. Inf. Figure S30: HMBC of compound 9. Supp. Inf. Figure S31: 1H NMR for compound 10. Supp. Inf. Figure S32: 13C NMR for compound 10. Supp. Inf. Figure S33: DEPT-135 NMR for compound 10. Supp. Inf. Figure S34: HSQC NMR for compound 10. Supp. Inf. Figure S35: HMBC NMR for compound 10. Supp. Inf. Figure S36: 1H NMR of [Pd2Cl4(3)2] complex. Supp. Inf. Figure S37: 13C NMR of [Pd2Cl4(3)2] complex. Supp. Inf. Figure S38: 1H NMR of [Pd2Cl4(4)2] complex. Supp. Inf. Figure S39: 13C NMR of [Pd2Cl4(4)2] complex. Supp. Inf. Figure S40: 1H NMR of [Pd2Cl4(5)2] complex. Supp. Inf. Figure S41: 13C NMR of [Pd2Cl4(5)2] complex. Supp. Inf. Figure S42: 1H NMR of [Pd2Cl4(6)2] complex. Supp. Inf. Figure S43: 13C NMR of [Pd2Cl4(6)2] complex. Supp. Inf. Figure S44: 1H NMR of [Pd2Cl4(7)2] complex. Supp. Inf. Figure S45: 13C NMR of [Pd2Cl4(7)2] complex. Supp. Inf. Figure S46: 1H NMR of [Pd2Cl4(8)2] complex. Supp. Inf. Figure S47: 13C NMR of [Pd2Cl4(8)2] complex. Supp. Inf. Figure S48: 1H NMR of [Pd2Cl4(9)2] complex. Supp. Inf. Figure S49: 13C NMR of [Pd2Cl4(9)2] complex. Supp. Inf. Figure S50: 1H NMR of [Pd2Cl4(10)2] complex. Supp. Inf. Figure S51: 13C NMR of [Pd2Cl4(10)2] complex. Supp. Inf. Figure S52: FT-IR spectrum of trans-stilbene. Supp. Inf. Figure S53: 1H NMR of trans-stilbene. Supp. Inf. Figure S54: 13C NMR of trans-stilbene. Supp. Inf. Figure S55: DEPT-135 NMR of trans-stilbene. Supp. Inf. Figure S56: HSQC of trans-stilbene. Supp. Inf. Figure S57: HMBC of trans-stilbene. Supp. Inf. Figure S58: FT-IR spectrum of 3-styrylbenzaldehyde. Supp. Inf. Figure S59: 1H NMR of 3-styrylbenzaldehyde. Supp. Inf. Figure S60: 13C NMR of 3-styrylbenzaldehyde. Supp. Inf. Figure S61: DEPT-135 NMR of 3-styrylbenzaldehyde. Supp. Inf. Figure S62: HSQC of 3-styrylbenzaldehyde. Supp. Inf. Figure S63: HMBC of 3-styrylbenzaldehyde. Supp. Inf. Figure S64: FT-IR spectrum of 4-styrylbenzaldehyde. Supp. Inf. Figure S65: 1H NMR of 4-styrylbenzaldehyde. Supp. Inf. Figure S66: 13C NMR of 4-styrylbenzaldehyde. Supp. Inf. Figure S67: DEPT-135 NMR of 4-styrylbenzaldehyde. Supp. Inf. Figure S68: HSQC of 4-styrylbenzaldehyde. Supp. Inf. Figure S69: HMBC of 4-styrylbenzaldehyde. Supp. Inf. Figure S70: Mass Spectrometry of compound 12. Supp. Inf. Figure S71: Mass Spectrometry of compound 13. Supp. Inf. Figure S72: Mass Spectrometry of compound 14. Supp. Inf. Figure S73: Mass Spectrometry of compound 15. Supp. Inf. Figure S74: FT-IR spectrum of compound 12. Supp. Inf. Figure S75: FT-IR spectrum of compound 13. Supp. Inf. Figure S76: FT-IR spectrum of compound 14. Supp. Inf. Figure S77: FT-IR spectrum of compound 15. Supp. Inf. Figure S78: 1H NMR for compound 12. Supp. Inf. Figure S79: 13C NMR for compound 12. Supp. Inf. Figure S80: 1H NMR for compound 13. Supp. Inf. Figure S81: 13C NMR for compound 13. Supp. Inf. Figure S82: 1H NMR for compound 14. Supp. Inf. Figure S83: 13C NMR for compound 14. Supp. Inf. Figure S84: 1H NMR for compound 15. Supp. Inf. Figure S85: 13C NMR for compound 15. Supp. Inf. Figure S86: Physicochemical data of Schiff bases 3–10. Supp. Inf. Figure S87: Percentages of growth inhibition of Schiff bases 3–10 and their [Pd2Cl4(3)2]-[Pd2Cl4(10)2] complexes against Candida albicans ATCC 10231 and Cryptococcus neoformans ATCC 32264. Supp. Inf. Figure S88: Percent inhibition vs. concentration against C. albicans of Schiff bases. Supp. Inf. Figure S89: Percent inhibition vs. concentration against C. neoformans of Schiff bases. Supp. Inf. Figure S90: Percent inhibition vs. concentration of Schiff bases 3, 5, 8. Supp. Inf. Figure S91: Enumeration of the new Schiff base-ligands 3–10. Supp. Inf. Figure S92: Enumeration of new Pd-Schiff base complexes type [Pd2Cl4(N-het)2]. Supp. Inf. Figure S93: Enumeration of new Styrylimines 12–15.
Author Contributions
J.E.T., J.L.-S. and A.R.-A.; conceptualization, methodology, investigation, writing of original draft and visualization, J.L.-S., A.R.-A., R.A., L.S., S.A.Z., R.L.L. and F.C.-C.; methodology, J.L.-S., A.R.-A., R.L.L. and F.C.-C.; software, J.E.T., J.L.-S., A.R.-A., R.A., L.S., S.A.Z., R.L.L. and F.C.-C.; validation, J.E.T., J.L.-S., A.R.-A., R.A., L.S., S.A.Z., R.L.L. and F.C.-C.; writing—review and editing, J.L.-S., A.R.-A., R.A., L.S., S.A.Z., R.L.L. and F.C.-C.; visualization, S.A.Z. and L.S.; planned and performed the antifungal assays, S.A.Z., R.L.L. and F.C.-C.; supervision, R.L.L. and F.C.-C.; project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universidad del Quindío (Colombia) through the project 1050.
Data Availability Statement
Data available within the article or its Supplementary Materials.
Acknowledgments
The authors are grateful to Universidad del Quindío project 1050, Universidad del Valle, and DGAPA-UNAM (PAPIIT project IN-211522) for financial support. SZ and LS acknowledge Agencia Nacional de Promoción Científica y Tecnológica de la Argentina PICT2014-1170. LS is a researcher of CONICET. SZ and LS are teaching members of the Pharmacognosy Area, School of Chemical and Pharmaceutical Sciences, National University of Rosario, Argentina.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Gangurde, K.B.; More, R.A.; Adole, V.A.; Ghotekar, D.S. Design, synthesis and biological evaluation of new series of benzotriazole-pyrazole clubbed thiazole hybrids as bioactive heterocycles: Antibacterial, antifungal, antioxidant, cytotoxicity study. J. Mol. Struct. 2024, 1299, 136760. [Google Scholar] [CrossRef]
- Li, M.; Huang, H.; Pu, Y.; Tian, W.; Deng, Y.; Lu, J. A close look into the biological and synthetic aspects of fused pyrazole derivatives. Eur. J. Med. Chem. 2022, 243, 114739. [Google Scholar] [CrossRef]
- Thakur, S.; Jaryal, A.; Bhalla, A. Recent advances in biological and medicinal profile of schiff bases and their metal complexes: An updated version (2018–2023). Results Chem. 2024, 7, 101350. [Google Scholar] [CrossRef]
- Ahmad, S.; Nadeem; Anwar, A.; Hameed, A.; Tirmizi, S.A.; Zierkiewicz, W.; Abbas, A.; Isab, A.A.; Alotaibi, M.A. Synthesis, characterization, DFT calculations and antibacterial activity of palladium(II) cyanide complexes with thioamides. J. Mol. Struct. 2017, 1141, 204–212. [Google Scholar] [CrossRef]
- Shen, L.; Huang, S.; Nie, Y.; Lei, F. An Efficient Microwave-Assisted Suzuki Reaction using a New Pyridine-Pyrazole/Pd(II) Species as Catalyst in Aqueous Media. Molecules 2013, 18, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Afrin, A.A.; Jayaraj, M.S.; Gayathri, C.; Swamy, P. An overview of Schiff base-based fluorescent turn-on probes: A potential candidate for tracking live cell imaging of biologically active metal ions. Sens. Diagn. 2023, 2, 988–1076. [Google Scholar] [CrossRef]
- Mehandi, R.; Twala, C.; Ali, A.; Ahmedi, S.; Rana, M.; Sultana, R.; Manzoor, N.; Abid, M.; Javed, S.; Nishat, R.N. Tuning emission of luminescent 2-7 disubstituted sila-and germafluorenes with –(trifluoromethyl)phenyl, -(malononitrile)phenyl, and -nitrobenzene substituents. J. Organomet. Chem. 2024, 1005, 122994. [Google Scholar] [CrossRef]
- Ache, H.J. Chemical Aspects of Fusion Technology. Chem. Int. Ed. Engl. 1989, 28, 1–20. [Google Scholar] [CrossRef]
- Chen, X.; Wu, T.; Du, Z.; Kang, W.; Xu, R.; Meng, F.; Liu, C.; Chen, Y.; Bao, Q.; Shen, J.; et al. Discovery of a brain-permeable bromodomain and extra terminal domain (BET) inhibitor with selectivity for BD1 for the treatment of multiple sclerosis. Eur. J. Med. Chem. 2024, 265, 116080. [Google Scholar] [CrossRef]
- Singh, P.; Paul, K.; Holzer, W. Synthesis of pyrazole-based hybrid molecules: Search for potent multidrug resistance modulators. Bioorg. Med. Chem. 2006, 14, 5061–5071. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Kariuki, B.M.; Mohamed, H.A.; Bekheit, M.S.; Awad, H.M.; El-Hiti, G.A. Synthesis and anticancer activity of 3-(1-aryl-5-methyl-1H-1,2,3-triazol-4-yl)-1-phenyl-1H-pyrazole-4-carbaldehydes. J. Mol. Struct. 2023, 1294, 136528. [Google Scholar] [CrossRef]
- Chandrasekharan, S.P.; Dhami, A.; Kumar, S.; Mohanan, K. Recent advances in pyrazole synthesis employing diazo compounds and synthetic analogues. Recent advances in pyrazole synthesis employing diazo compounds and synthetic analogues. Org. Biomol. Chem. 2022, 20, 8787–8817. [Google Scholar] [CrossRef] [PubMed]
- Lamberth, C. Pyrazoie Chemistry in Crop Protection. Heterocycles 2007, 71, 1467–1502. [Google Scholar] [CrossRef]
- Alsafy, S.M.; Alrazzak, N.A. Synthesis, Characterization, and Biological Activity Study of New Heterocyclic Compounds. Eng. Proc. 2023, 59, 178. [Google Scholar]
- Hwang, S.H.; Wagner, K.M.; Morisseau, C.; Liu, J.Y.; Dong, H.; Wecksler, A.T.; Hammock, B.D. Synthesis and Structure−Activity Relationship Studies of Urea-Containing Pyrazoles as Dual Inhibitors of Cyclooxygenase-2 and Soluble Epoxide Hydrolase. J. Med. Chem. 2011, 54, 3037–3050. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.H.; Gaffer, H.E.; Elattar, K.M. Insights into the medicinal chemistry of heterocycles integrated with a pyrazolo[1,5-a]pyrimidine scaffold. RSC. Med. Chem. 2022, 13, 1150–1196. [Google Scholar]
- Jian, J.; An, W.; Min, C.; Mengqi, W.; Chunlong, Y. Novel 5-chloro-pyrazole derivatives containing a phenylhydrazone moiety as potent antifungal agents: Synthesis, crystal structure, biological evaluation and a 3D-QSAR study. New J. Chem. 2019, 43, 6350–6360. [Google Scholar]
- Zhang, Y.; Wu, C.; Zhang, N.; Fan, R.; Ye, Y.; Xu, J. Recent Advances in the Development of Pyrazole Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 12724. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.X.; Stepan, A.F.; Plummer, M.S.; Zhang, Y.H.; Yu, J.Q. Divergent C–H Functionalizations Directed by Sulfonamide Pharmacophores: Late-Stage Diversification as a Tool for Drug Discovery. J. Am. Chem. Soc. 2011, 133, 7222–7228. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Shayanfar, A.; Soltani, B. Copper pyrazole complexes as potential anticancer agents: Evaluation of cytotoxic response against cancer cells and their mechanistic action at the molecular level. Coord. Chem. Rev. 2024, 498, 215459. [Google Scholar] [CrossRef]
- Ghatak, T.; Shah, C.; Althagafi, I.; Giri, N.G.; Gopal, N.; Nath, M.; Pratap, R. Iodine/DMSO-mediated one-pot access towards 1-aryl-2-(pyrazol-5-yl)ethane-1,2-diones via a domino reaction from functionalized pent-2-ene-1,5-diones. Biomol. Chem. 2024, 22, 1859–1870. [Google Scholar] [CrossRef]
- Zhu, L.; Cheng, L.; Zhang, Y.; Xie, R.; You, J. Highly Efficient Copper-Catalyzed N-Arylation of Nitrogen-Containing Heterocycles with Aryl and Heteroaryl Halides. J. Org. Chem. 2007, 72, 2737–2743. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.K.; Lunagariya, K.S.; Khunt, R.C. Multi-step Synthesis of Novel Pyrazole Derivatives as Anticancer Agents. Polycycl. Aromat. Compd. 2023, 1–14. [Google Scholar] [CrossRef]
- Pan, X.; Luo, Y.; Wu, J. Route to Pyrazolo[5,1-a]isoquinolines via a Copper-Catalyzed Tandem Reaction of 2-Alkynylbromobenzene with Pyrazole. J. Org. Chem. 2013, 78, 5756–5760. [Google Scholar] [CrossRef]
- Parshad, M.; Kumar, D.; Verma, V. A mini review on applications of pyrazole ligands in coordination compounds and metal organic frameworks. Inorg. Chim. Acta 2024, 560, 121789. [Google Scholar] [CrossRef]
- Bhanuchandra, M.; Kuram, M.R.; Sahoo, A.K. Silver(I)-Catalyzed Reaction between Pyrazole and Propargyl Acetates: Stereoselective Synthesis of the Scorpionate Ligands (E)-Allyl-gem-dipyrazoles (ADPs). J. Org. Chem. 2013, 78, 11824–11834. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Hasan, N.; Bhat, S.A.; Ali, R.; Alam, S.D.; Jain, A.K.; Khan, S.A. Color tuning and violet light emission via coordination of pyrazole in tris(benzoyltrifluoroacetonate)Sm(III). Opt. Mater. 2024, 148, 114877. [Google Scholar] [CrossRef]
- Jiang, B.; Ning, Y.; Fan, W.; Tu, S.J.; Li, G. Oxidative Dehydrogenative Couplings of Pyrazol-5-amines Selectively Forming Azopyrroles. J. Org. Chem. 2014, 79, 4018–4024. [Google Scholar] [CrossRef]
- Konovalov, D.I.; Novikova, E.D.; Ivanov, A.A.; Yanshole, V.V.; Kuratieva, N.V.; Berezin, A.S.; Shestopalov, M.A. The route to Re6Te8 cluster complexes with organic ligands. Polyhedron 2024, 251, 116874. [Google Scholar] [CrossRef]
- Maeda, H.; Haketa, Y.; Nakanishi, T. Aryl-Substituted C<sub>3</sub>-Bridged Oligopyrroles as Anion Receptors for Formation of Supramolecular Organogels. J. Am. Chem. Soc. 2007, 129, 13661–13674. [Google Scholar]
- Burling, S.; Field, L.D.; Messerle, B.A.; Rumble, S.L. Late Transition Metal Catalyzed Intramolecular Hydroamination: The Effect of Ligand and Substrate Structure. Organometallics 2007, 26, 4335–4343. [Google Scholar] [CrossRef]
- Olguin, J.; Brooker, S. Spin crossover active iron(II) complexes of selected pyrazole-pyridine/pyrazine ligands. Coord. Chem. Rev. 2011, 255, 203–240. [Google Scholar] [CrossRef]
- Fustero, S.; Sanchez-Rosello, M.; Barrio, P.; Simon-Fuentes, A. From 2000 to Mid-2010: A Fruitful Decade for the Synthesis of Pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Kim, H.S.; Kurasawa, Y. Synthesis of pyrazoles and condensed pyrazoles. J. Heterocycl. Chem. 1999, 36, 321–332. [Google Scholar] [CrossRef]
- Salazar-Muñoz, J.; Zarate, X.; Sotomayor-Jaramillo, J.; Bustos, C.; Schott, E. Synthesis and theoretical study of a new family of pyrazole derivative. J. Mol. Struct. 2024, 1301, 137267. [Google Scholar] [CrossRef]
- Makino, K.; Kim, H.S.; Kurasawa, Y. Synthesis of pyrazoles. J. Heterocycl. Chem. 1998, 35, 489–497. [Google Scholar] [CrossRef]
- Ocansey, E.; Darkwa, J.; Makhubela, B.C.E. Bis(pyrazolyl)palladium(II) complexes as catalysts for Mizoroki–Heck cross-coupling reactions. Polyhedron 2019, 166, 52–59. [Google Scholar] [CrossRef]
- Bulger, A.S.; Nasrallah, D.J.; Meza, A.T.; Garg, N.K. Enantioselective nickel-catalyzed Mizoroki–Heck cyclizations of amide electrophiles. Chem. Sci. 2024, 15, 2593–2600. [Google Scholar] [CrossRef]
- Heck, R.F.; Herrmann, W.A. N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis. Angew. Chem. Int. 2002, 41, 1290–1309. [Google Scholar]
- Zhu, Y.; Dong, W.; Tang, W. Palladium-catalyzed cross-couplings in the synthesis of agrochemicals. Adv. Agrochem 2022, 1, 125–138. [Google Scholar] [CrossRef]
- Hegde, S.; Nizam, A.; Vijayan, A. Furaldehyde-based magnetic supported palladium nanoparticles as an efficient heterogeneous catalyst for Mizoroki–Heck cross-coupling reaction. New J. Chem. 2024, 48, 1121–1129. [Google Scholar] [CrossRef]
- Townley, C.; Branduardi, D.; Chessari, G.; Cons, B.D.; Griffiths-Jones, C.; Hall, R.J.; Johnson, C.N.; Ochi, Y.; Whibley, S.; Grainge, R. Enabling synthesis in fragment-based drug discovery (FBDD): Microscale high-throughput optimisation of the medicinal chemist’s toolbox reactions. RSC Med. Chem. 2023, 14, 2699–2713. [Google Scholar] [CrossRef] [PubMed]
- Bräse, S.; de Meijere, A. Metal-Catalyzed Cross-Coupling Reactions, 2nd ed.; de Meijere, A., Diederich, F., Eds.; Wiley-VCH: Weinheim, Germany, 2004; pp. 218–225. [Google Scholar]
- Mathew, B.; Nath, M. Recent Approaches to Antifungal Therapy for Invasive Mycoses. Chem. Med. Chem. 2009, 4, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Wright, G. New Targets and Screening Approaches in Antimicrobial Drug Discovery. Chem. Rev. 2005, 105, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Playford, E.; Sorrell, T. Antifungal therapy in invasive fungal infections. Curr. Opin. Pharmacol. 2010, 10, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Leidich, S.; Isham, N.; Leitner, I.; Ryder, N.S.; Ghannoum, M.A. Clinical Trichophyton rubrum Strain Exhibiting Primary Resistance to Terbinafine. Antimicrob. Agents Chemother. 2003, 47, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Vicente, M.F.; Basilio, A.; Cabello, A.; Peláez, F. Microbial natural products as a source of antifungals. Clin. Microbiol. Infect. 2003, 9, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Quirante, J.; Ruiz, D.; Gonzalez, A.; López, C.; Cascante, M.; Cortés, R.; Messeguer, R.; Calvis, C.; Baldomà, L.; Pascual, A.; et al. Platinum(II) and palladium(II) complexes with (N,N′) and (C,N,N′)− ligands derived from pyrazole as anticancer and antimalarial agents: Synthesis, characterization and in vitro activities. J. Inorg. Biochem. 2011, 105, 1720–1728. [Google Scholar] [CrossRef]
- Abu-Surrah, A.S.; Safieh, K.A.A.; Ahmad, I.M.; Abdalla, M.Y.; Ayoub, M.T.; Qaroush, A.K.; Abu-Mahtheieh, A.M. New palladium(II) complexes bearing pyrazole-based Schiff base ligands: Synthesis, characterization and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 471–475. [Google Scholar] [CrossRef]
- Dahm, G.; Bailly, C.; Karmazin, L.; Bellemin-Laponnaz, S. Synthesis, structural characterization and in vitro anti-cancer activity of functionalized N-heterocyclic carbene platinum and palladium complexes. J. Organomet. Chem. 2015, 794, 115–124. [Google Scholar] [CrossRef]
- Restrepo-Acevedo, A.; Osorio, N.; Giraldo-López, L.E.; D’Vries, R.F.; Zacchino, S.; Abonia, R.; Le Lagadec, R.; Cuenú-Cabezas, F. Synthesis and antifungal activity of nitrophenyl-pyrazole substituted Schiff bases. J. Mol. Struc. 2022, 1253, 132289. [Google Scholar] [CrossRef]
- Cuenú, F.; Londoño-Salazar, J.; Torres, J.E.; Abonia, R.; D‘Vries, R. Synthesis, structural characterization and theoretical studies of a new Schiff base 4-(((3-(tert-Butyl)-(1-phenyl)pyrazol-5-yl) imino)methyl)phenol. J. Mol. Struct. 2018, 1152, 163–176. [Google Scholar]
- Moreno-Fuquen, R.; Cuenú, F.; Torres, J.E.; De la Vega, G.; Galarza, E.; Abonia, R.; Kennedy, A.R. Presence of π…π and Csingle bondH…π interactions in the new Schiff base 2-{(E)-[(3-tert-butyl-1-phenyl-1H-pyrazol-5-yl)imino]methyl}phenol: Experimental and DFT computational studies. J. Mol. Struct. 2017, 1150, 366–373. [Google Scholar] [CrossRef]
- Cuenú, F.; Abonia, R.; Bolaños, A.; Cabrera, A. Synthesis, structural elucidation and catalytic activity toward a model Mizoroki–Heck C–C coupling reaction of the pyrazolic Tröger’s base Pd4Cl8(PzTB)2 complex. J. Organomet. Chem. 2011, 696, 1834–1839. [Google Scholar] [CrossRef]
- Toka, F.; Kaymakçıoğlua, B.K.; Sağlıkb, B.N.; Leventb, S.; Özkayb, Y.; Kaplancıklı, Z.A. Synthesis and biological evaluation of new pyrazolone Schiff bases as monoamine oxidase and cholinesterase inhibitors. Bioorg. Chem. 2019, 84, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, S.L.; Agrahari, B.; Pathak, D.D. Synthesis and characterization of a new Pd(II)-Schiff base complex [Pd(APD)2]: An efficient and recyclable catalyst for Heck-Mizoroki and Suzuki-Miyaura reactions. J. Organomet. Chem. 2017, 846, 105–112. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part A: Theory and Applications in Inorganic Chemistry. Part A, 6th ed.; Wiley, S.J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; p. 193. [Google Scholar]
- Goodfellow, R.J.; Goggin, P.L.; Venanzi, L.M. The skeletal stretching frequencies of some bridged platinum and palladium chloro-complexes. J. Chem. Soc. A. 1967, 1897–1900. [Google Scholar] [CrossRef]
- Nelana, S.M.; Kumar, K.; Guzei, I.A.; Mahamo, T.; Darkwa, J. Electronic effects on the structures and catalytic properties of (pyrazol-1-yl)phenylmethanone palladium(II) complexes. J. Organomet. Chem. 2017, 848, 159–165. [Google Scholar] [CrossRef]
- Ceylan, Ü.; Tari, G.Ö.; Gökce, H.; Aʇar, E. Spectroscopic (FT–IR and UV–Vis) and theoretical (HF and DFT) investigation of 2-Ethyl-N-[(5-nitrothiophene-2-yl)methylidene]aniline. J. Mol. Struct. 2016, 1110, 1–10. [Google Scholar] [CrossRef]
- Temel, E.; Alasalvar, C.; Gökçe, H.; Güder, A.; Albayrak, Ç.; Alpaslan, Y.B.; Alpaslan, G.; Dilek, N. DFT calculations, spectroscopy and antioxidant activity studies on (E)-2-nitro-4-[(phenylimino)methyl]phenol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 534–546. [Google Scholar] [CrossRef]
- Ozdemir, M.; Sonmez, M.; Sen, F.; Dincer, M.; Ozdemir, N. A novel one-pot synthesis of heterocyclic compound (4-benzoyl-5-phenyl-2-(pyridin-2-yl)-3,3a-dihydropyrazolo[1,5-c]pyrimidin-7(6H)-one): Structural (X-ray and DFT) and spectroscopic (FT-IR, NMR, UV-Vis and Mass) characterization Studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Tüzün, B.; Kaya, C.; Obot, I.B. Determination of corrosion inhibition effects of amino acids: Quantum chemical and molecular dynamic simulation study. J. Taiwan Inst. Chem. Eng. 2015, 58, 528–535. [Google Scholar] [CrossRef]
- Taira, T.; Yanagimoto, T.; Sakai, K.; Sakai, H.; Endo, A.; Imura, T. Synthesis of surface-active N-heterocyclic carbene ligand and its Pd-catalyzed aqueous Mizoroki–Heck reaction. Tetrahedron 2016, 5, 053. [Google Scholar]
- Anton, D.R.; Crabtree, R.H. Dibenzo[a,e]cyclooctatetraene in a proposed test for heterogeneity in catalysts formed from soluble platinum-group metal complexes. Organometallics 1983, 2, 855–859. [Google Scholar] [CrossRef]
- Widegren, J.A.; Finke, R.G. The Problem of Distinguishing True Homogeneous Catalysis from Soluble or Other Metal-Particle Heterogeneous Catalysis under Reducing Conditions. J. Mol. Catal. A 2003, 198, 317–341. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Brossmer, C.; Öfele, K.; Reisinger, C.P.; Priermeier, T.; Beller, M.; Fischer, H. Palladacycles as Efficient Catalysts for Aryl Coupling Reactions. Chem. Int. Ed. Engl. 1995, 34, 1848–1849. [Google Scholar]
- Larhed, M.; Hallberg, A. Microwave-Promoted Palladium-Catalyzed Coupling Reactions. J. Org. Chem. 1996, 61, 9582–9584. [Google Scholar] [CrossRef]
- Buchmeiser, M.R.; Wurst, K. Access to Well-Defined Heterogeneous Catalytic Systems via Ring-Opening Metathesis Polymerization (ROMP): Applications in Palladium(II)-Mediated Coupling Reactions. J. Am. Chem. Soc. 1999, 121, 11101–11107. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Third Edition. CLSI document M27-A3 (ISBN 1-56238-666-2); Clinical and Laboratory Standards Institute: 950 West Valley Road, Suite 2500, Wayne, PA 19087, USA, 2008; pp. 1–25. [Google Scholar]
- Garber, G. An overview of fungal infections. Drugs 2001, 61 (Suppl. S1), 1–12. [Google Scholar] [CrossRef]
- Patterson, T. Advances and challenges in management of invasive mycoses. Lancet 2005, 366, 1013–1025. [Google Scholar] [CrossRef]
- Kontoyiannis, D.; Mantadakis, E.; Samonis, G. Systemic mycoses in the immunocompromised host: An update in antifungal therapy. J. Hosp. Infect. 2003, 53, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Singh, N. Treatment of opportunistic mycoses: How long is long enough? Lancet Infect. Dis. 2003, 3, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Galgiani, J.N.; Lewis, M.L. In vitro studies of activities of the antifungal triazoles SCH56592 and itraconazole against Candida albicans, Cryptococcus neoformans, and other pathogenic yeasts. Antimicrob. Agents Chemother. 1997, 41, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Jamroz, M.H. Vibrational energy distribution analysis (VEDA): Scopes and limitations. Spectrochim. Acta A 2013, 114, 220–230. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).