Abstract
In this paper, Au and Cu nanoparticles were successfully loaded onto porous g-C3N4 material through a hydrothermal synthesis method. By adjusting the proportion of Cu, Au-5%Cu/C3N4, Au-10%Cu/C3N4, and Au-15%Cu/C3N4, catalysts were prepared and used for the catalytic reduction of CO2 to methanol. Characterization analysis using high-resolution XPS spectra showed that with an increase in the doping amount of Cu, the electron cloud density on the Cu surface initially increased and then decreased. Electrons from Au atoms transferred to Cu atoms, leading to the accumulation of a more negative charge on the Cu surface, promoting the adsorption of partially positively charged C in CO2, which is more beneficial for catalyzing CO2. Among them, Au-10%Cu/C3N4 exhibited good reducibility and strong basic sites, as demonstrated by H2-TPR and CO2-TPD, with the conversion rates for CO2, methanol yield, and methanol selectivity being 11.58%, 41.29 g·kg−1·h−1 (0.39 μmol·g−1s−1), and 59.77%, respectively.
1. Introduction
With the rapid development of human society and the accelerated advancement of the industrial age, global carbon dioxide emissions have been continuously increasing, with only 1% of CO2 being able to be utilized resourcefully [1,2]. Research has found that the excessive emission of CO2 is the primary factor causing the greenhouse effect [3,4], leading to severe environmental threats such as ocean acidification, sea level rise, and soil erosion, which seriously endanger human survival. Therefore, mitigating global warming and reducing excessive CO2 emissions are current research hotspots in countries around the world [5,6].
Significantly, employing CO2 gas for the production of value-added products promotes sustainable utilization. Additionally, CO2 gas is currently the most abundant and accessible source of carbon, making it a raw material for synthesizing alternative fuels or additional products. This approach provides a more promising method by which to reduce the environmental impact of CO2 gas and utilize CO2 as a carbon source [7,8]. Currently, methods for converting CO2 gas into products mainly involve biotechnology, particularly its use as a carbon source [9]. Other techniques include electrocatalysis [10] and the chemical catalytic method [11,12]. The biological method mainly involves plants and microorganisms efficiently absorbing and converting CO2 into organic matter through photosynthesis. In recent years, afforestation has been used to mitigate the greenhouse effect. However, this approach has drawbacks, such as the long growth cycle of plants and certain geographical limitations [13,14,15].
In the current research, the chemical catalytic method is regarded as the most effective approach for CO2 fixation due to its clean and environmentally friendly attributes. Therefore, exploring efficient materials with which to convert CO2 into carbon-containing fuels is a promising approach to reducing CO2 levels in the atmosphere and producing fuel [16,17]. Catalytic reduction of CO2 mainly refers to the use of high-efficiency catalyst materials to convert CO2 into other valuable products. Its principle is mainly a process of energy conversion [18,19]. The catalytic CO2 reduction process mitigates the greenhouse effect resulting from excessive CO2 emission. Therefore, catalytic CO2 reduction represents a sustainable approach to addressing the current energy crisis and greenhouse effect [20,21].
The graphite-like carbon nitride (g-C3N4) is a carbon-nitrogen heterocyclic material with a heptazine ring as its basic unit. The stable-layered structure of g-C3N4 is formed by connecting the nitrogen atoms at the ends of each ring [22]. g-C3N4 exhibits excellent chemical stability and is currently used in various applications in adsorption and catalysis involving amorphous carbons. Studies have shown that the mesoporous spaces present in g-C3N4 are beneficial for the adsorption of various substances, facilitating subsequent catalytic reactions [23]. Research indicates that Cu2+ particles can be doped into the lattice of g-C3N4 by coordinating with the pyridinic nitrogen in g-C3N4, leading to the formation of highly dispersed copper species. g-C3N4 has shown promising prospects in various catalytic applications. g-C3N4 materials synthesized from urea through an alkaline hydrothermal method exhibit excellent performance in CO2 reduction. Characterization studies have indicated that –NH2 and –NH– functional groups play a major role in this process [24]. At present, the use of g-C3N4 to convert CO2 into fuel has become a current research hotspot. However, the original g-C3N4 has problems such as catalytic efficiency. In recent years, modifying g-C3N4 to optimize the catalytic performance of CO2 conversion and effectively improve the CO2 conversion has become a current research focus [25]. Modifications of g-C3N4 mainly involve doping with heteroatoms, constructing intra-molecular heterostructures, and introducing defects [26,27,28]. Anurag Kumar et al. immobilized molecular cobalt phthalocyanine tetra-carboxylic acid (CoPc-COOH) complexes on g-C3N4 and coupled them with triethylamine to fabricate a novel photocatalyst material. This material was used under visible light to convert CO2 into methanol. The results revealed that the enhanced efficiency in methanol production of the new material was mainly attributed to the binding ability of CoPc-COOH with CO2. Additionally, the material improved electron migration and charge separation [29]. Park et al. directly synthesized methanol by CO2 hydrogenation using g-C3N4 as a palladium catalyst carrier under neutral conditions. Compared with inert carriers such as carbon nanotubes, the high CO2 affinity of g-C3N4 is the reason for its enhanced catalytic activity and stability [30]. Deng et al. prepared Cu/g-C3N4-ZnO/Al2O3 catalyst material using a multi-step synthesis method. At 250 °C, the methanol space–time yield reached 5.73 mmol h−1 gCu−1 [31]. Various characterizations have demonstrated that strong interactions between the metal and the support promote the methanol space–time yield. Despite advancements in the conversion capability of CO2, the synthesis of these materials remains overly complex. Currently, several researchers have revealed that using Cu, Ag, and Au as metal single-atom particles dispersed on two-dimensional g-C3N4 carriers results in more effective CO2 conversion. Studies have indicated that depositing these metal single atoms addresses the limitation of pure g-C3N4, which requires a high potential to overcome the first proton–electron transfer. The deposition of single atoms enhances the catalytic activity of g-C3N4, generates intermediate species with moderate binding energy, and accelerates electron transfer [32].
Currently, researchers are mainly focused on utilizing single-metal elements to improve the defects of g-C3N4, while there is relatively limited research on the application of bimetallic element loading on g-C3N4. This study aimed to regulate the functional defects of carbon nitride by adding bimetallic elements Au and Cu to enhance its ability for catalytic hydrogenation of CO2 to methanol. The impact of the Cu element on catalytic activity was explored during the synthesis of g-C3N4 by adjusting the proportion of added Cu. Furthermore, this study assessed the CO2 conversion efficiency, methanol selectivity, and yield of various catalysts, elucidating the fundamental mechanism of bimetallic-modified g-C3N4 materials in the CO2 hydrogenation to methanol.
2. Results and Discussion
2.1. Textural and Structural Properties of the Catalysts
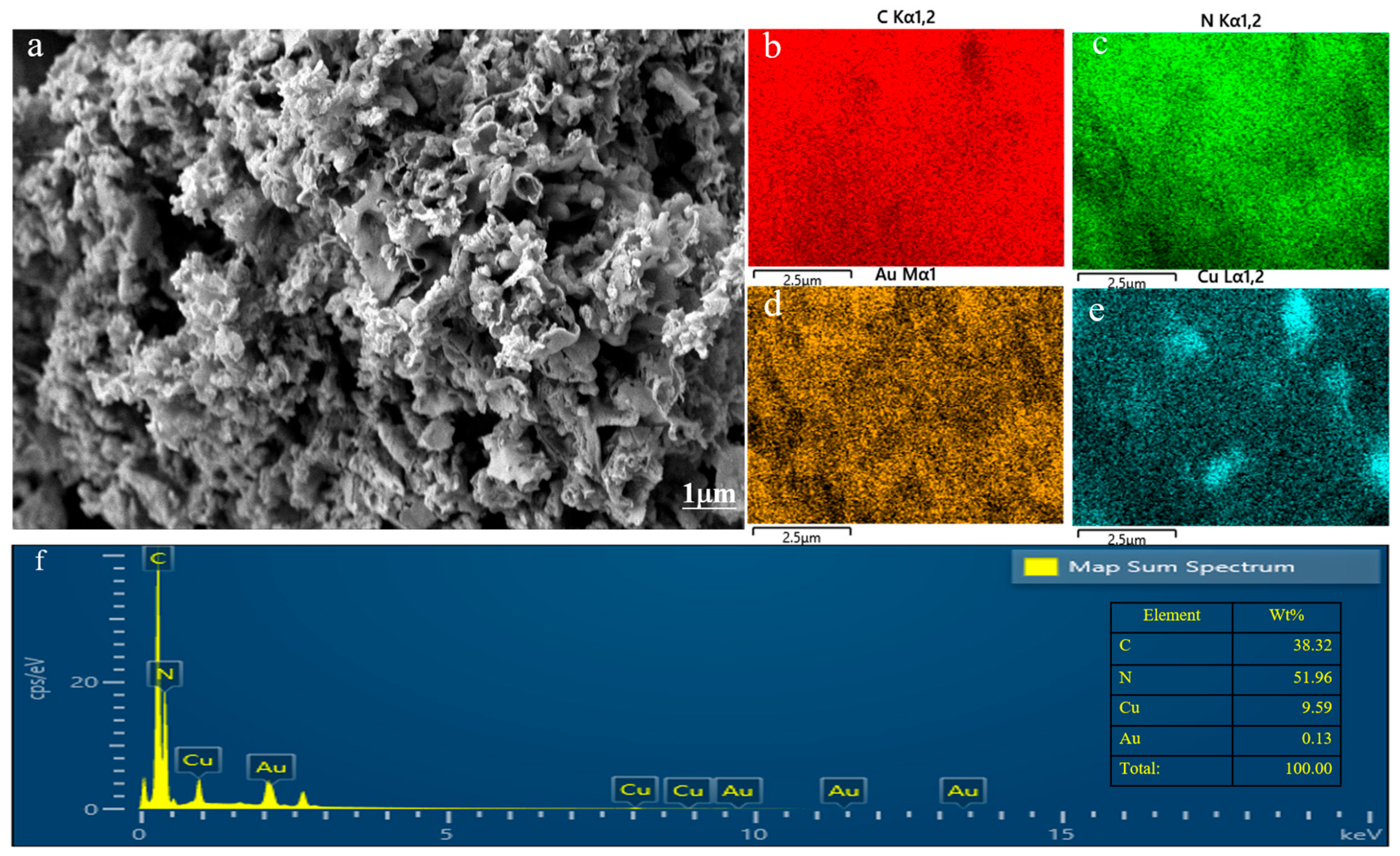
Figure 1 shows the SEM image of the Au–Cu/C3N4 composite material. The preparation method of the composite materials resulted in an irregular and porous structure in the catalyst, which provided abundant surface area and pore space that enhanced catalytic reactions within the catalyst. The elemental mapping of Au–Cu/C3N4 confirmed the successful loading of Au and Cu on the g-C3N4 material. Additionally, the uniform distribution of Au and Cu on g-C3N4 enhanced the catalytic effectiveness in subsequent stages. In the experiment, it is expected to load 0.1% Au and 10% Cu on g-C3N4. From Figure 1f, it can be seen that the actual composition ratio of Au-10%Cu/C3N4 almost meets the expected target.
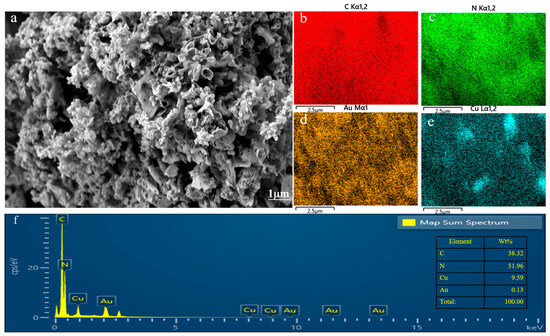
Figure 1.
(a) SEM images, (b–e) mapping images, and (f) EDS spectra of Au-10%Cu/C3N4.
In the first step of catalytic CO2 reduction, CO2 was adsorbed and reduced on the surface of the photocatalyst. The adsorption capacity and specific surface area of CO2 were evaluated. Brunauer–Emmett–Teller (BET) analysis was performed on composite catalyst materials with different doping ratios. The specific surface area and pore volume of each catalyst were obtained through N2 adsorption–desorption isotherms (Table 1). The specific surface areas of the various catalysts were comparable, indicating that increasing the doping ratio of Cu did not significantly affect the specific surface area of the composite material. Cu with 10% load has the largest specific surface area and average pore diameter. However, excessive Cu doping can lead to a decrease in specific surface area. The average pore size of the composite catalyst gradually decreased with increasing copper doping ratio.

Table 1.
Analysis of the catalyst-specific surface area.
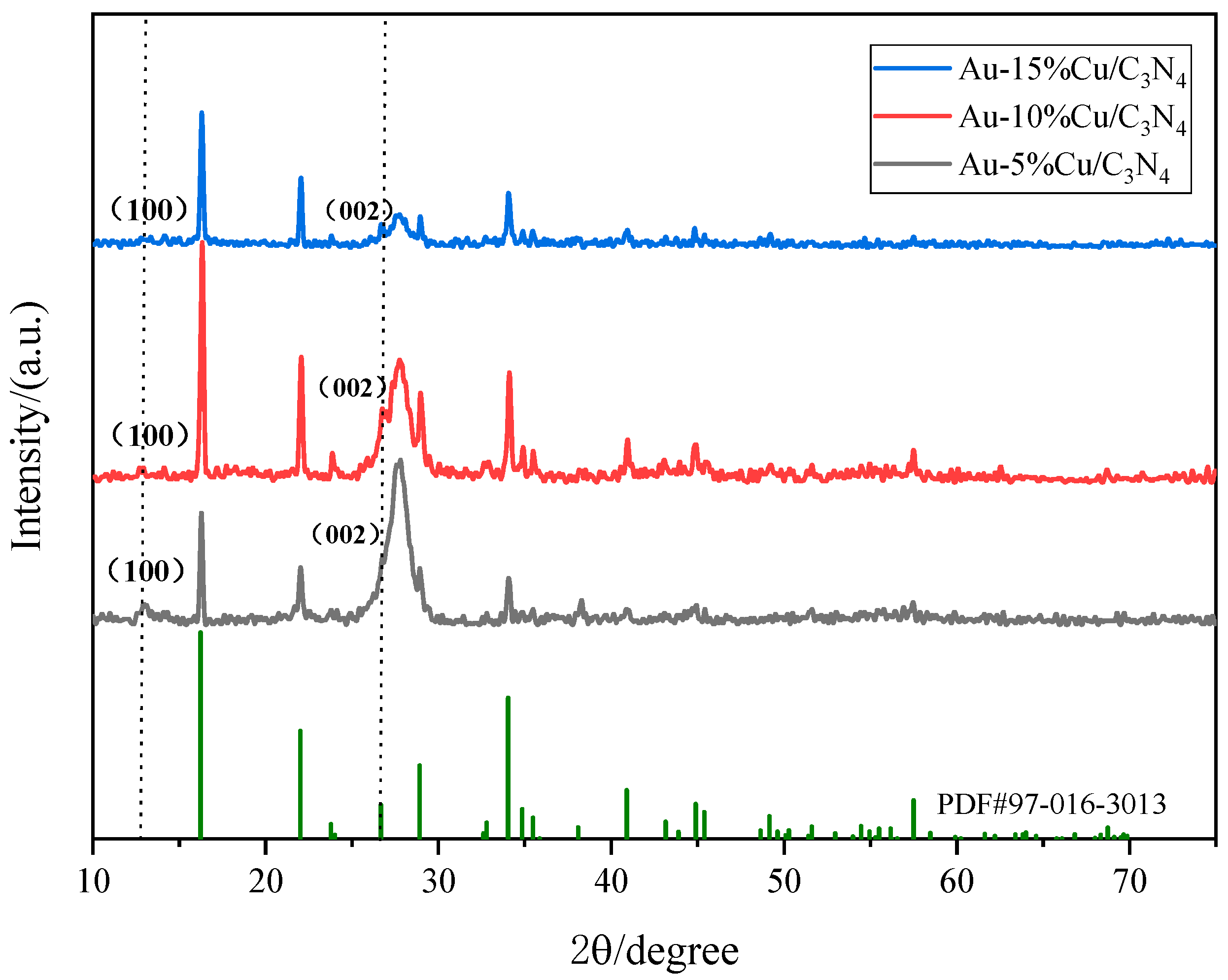
The XRD spectrum of the catalyst (Figure 2) exhibited prominent diffraction peaks corresponding to the (100) and (002) peaks of g-C3N4. The (002) diffraction peak at 27.32° was associated with the periodic stacking of conjugated aromatic ring C–N heterocycles [33,34]. As the doping ratio of Cu gradually increased, the (100) crystal plane diffraction peak of g-C3N4 gradually shifted to lower angles. Excessive doping of Cu can damage the layered structure of g-C3N4, leading to reduced crystallinity and weakened intensity of the diffraction peaks. However, the comparison of XRD results with SEM images confirmed the successful preparation of g-C3N4. Additionally, the spectrum of the Au–Cu/C3N4 composite material exhibited prominent diffraction peaks at ~2θ = 16.22°, 22.00°, and 34.03°, corresponding to the CuCl2·2H2O (101), (200), and (012) planes (PDF#97-016-3013), further confirming the successful loading of Cu into the g-C3N4 composite material. Particularly, the Au–10%Cu/C3N4 spectrum exhibited the most prominent diffraction peak, confirming that Au–10%Cu/C3N4 is the optimal catalyst material for subsequent use. Nevertheless, upon meticulous scrutiny, no prominent diffraction peaks of Au were detected in the composite material owing to the low doping amount of Au, resulting in the absence of visible diffraction peaks.
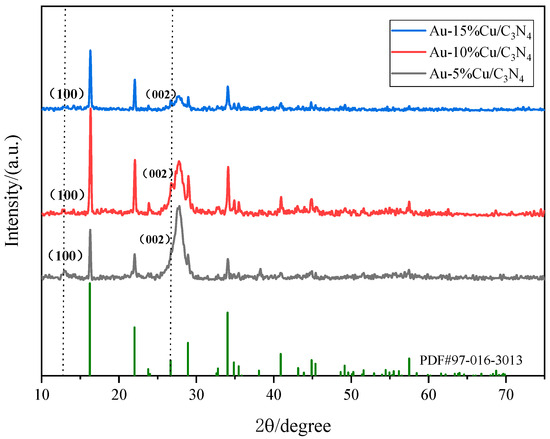
Figure 2.
X-ray diffraction (XRD) spectrum of the catalyst.
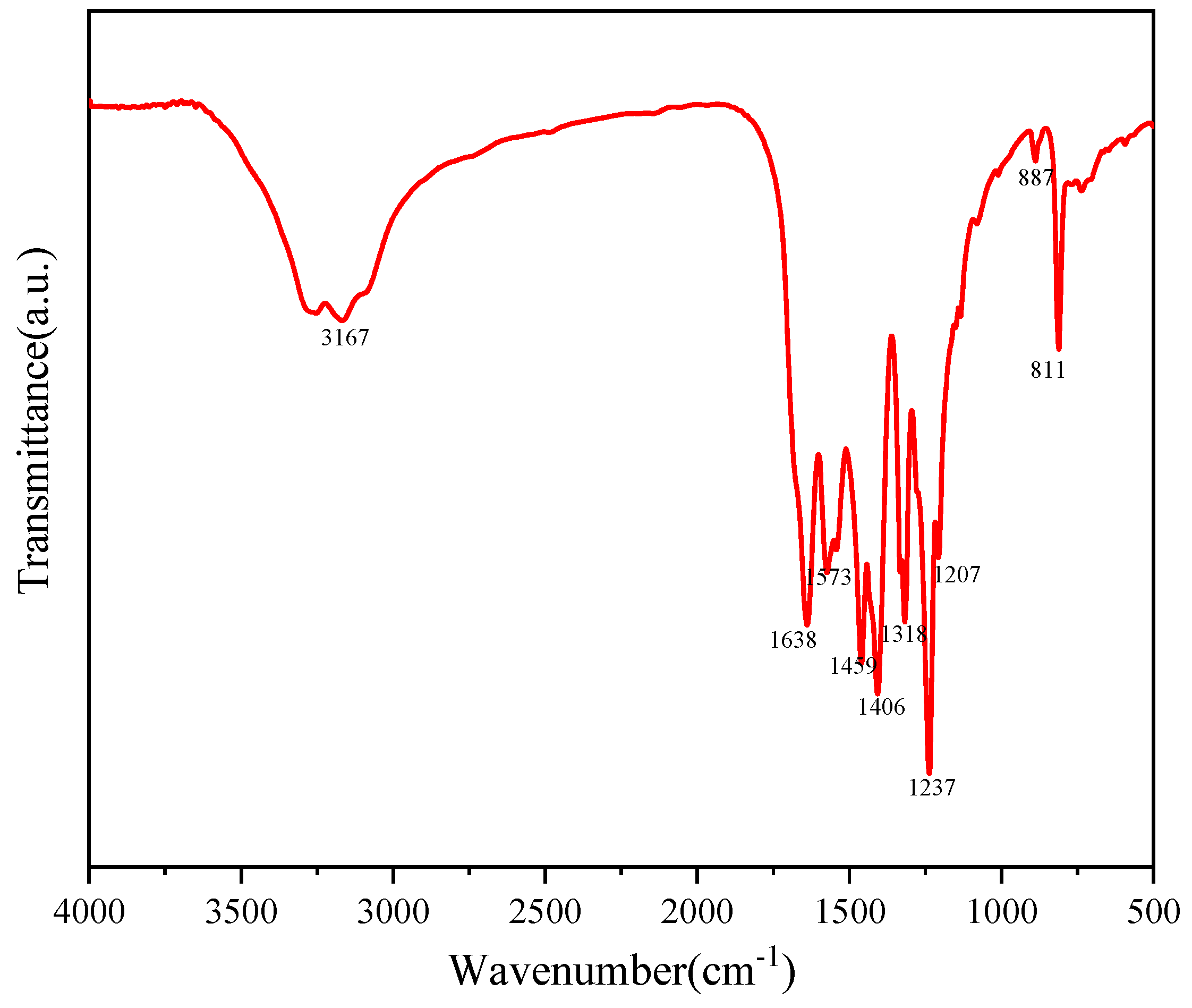
The molecular structure of Au-10%Cu/C3N4 was examined using FTIR analysis. The infrared spectrum of Au-10%Cu/C3N4 (Figure 3) exhibited three main characteristic peaks, i.e., above 2900 cm−1, in the range of 1200–1700 cm−1, and below 1000 cm−1 [35,36]. These characteristic peaks were mainly attributed to the stretching vibrations of N–H and O–H bonds, CN heterocycles, and triazine rings. The broad feature peak at 3167 cm−1 was mainly attributed to surface H2O molecules, indicating that the prepared nanosheets exhibited a mesoporous structure. This structure proved advantageous in boosting catalytic activity [37]. The sharp peak at 811 cm−1 corresponded to the out-of-plane bending vibration of triazine ring features [38,39]. The peaks observed in the range of 1200–1700 cm−1 can be attributed to the vibrations of CN in the aromatic system. The comparison of Au-10%Cu/C3N4 with traditional g-C3N4 materials revealed that upon the incorporation of Au and Cu, the characteristic peaks of g-C3N4 shifted. This shift indicates that the surface hybridization of metals with g-C3N4 altered the main framework structure of g-C3N4, suggesting that metal doping enhanced photocatalytic activity. The redshift observed in g-C3N4 within the complex indicates a weakening of the C–N bonds in g-C3N4, leading to an expanded conjugated system [40].
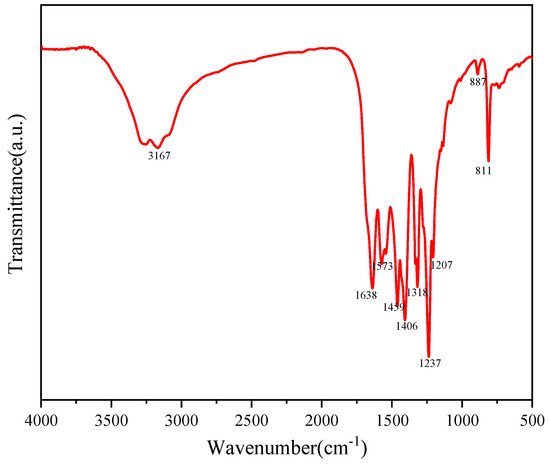
Figure 3.
Fourier transform infrared spectroscopy (FTIR) of Au–10%Cu/C3N4.
2.2. X-ray Photoelectron Spectroscopy (XPS) Analysis
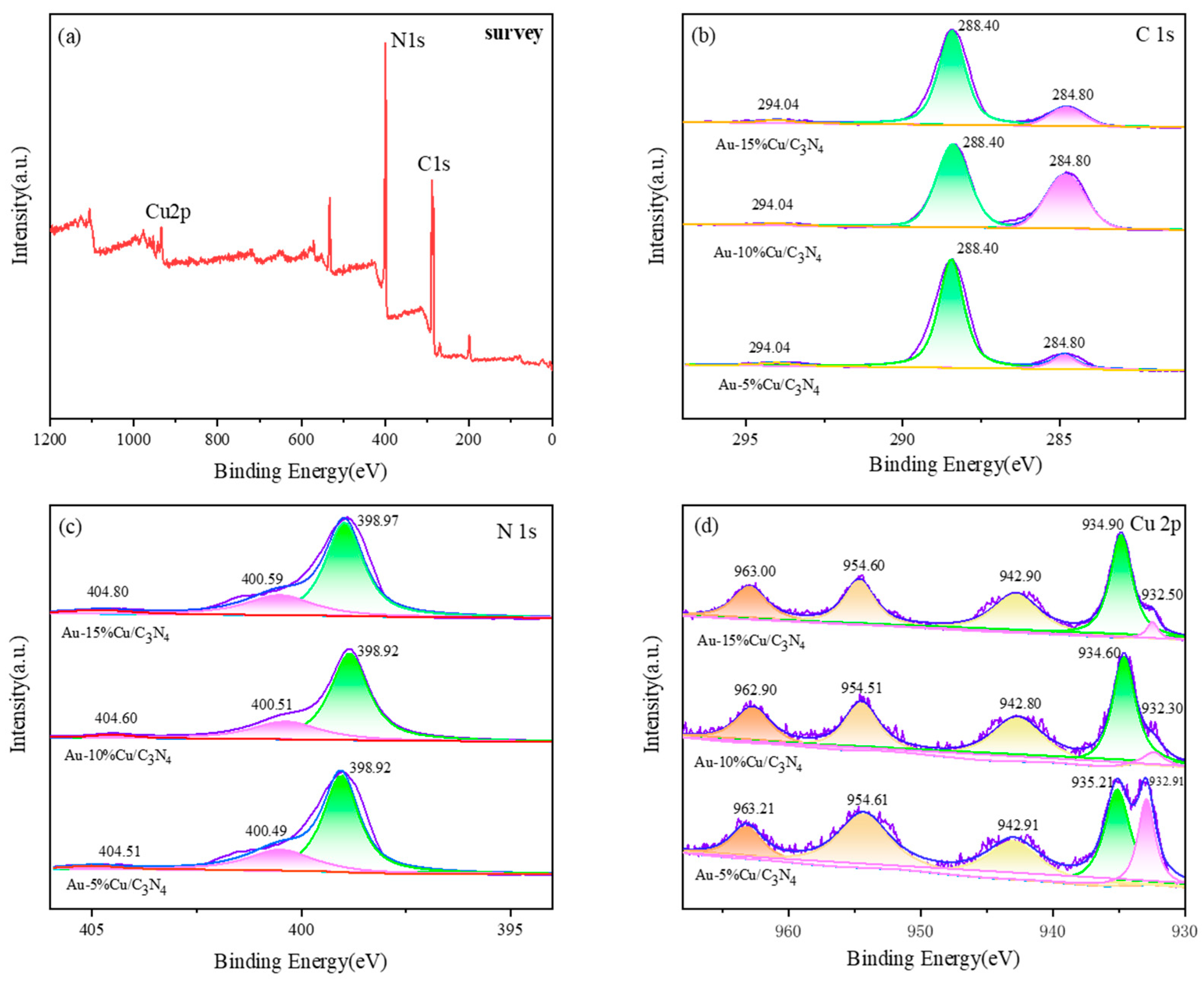
The chemical composition and oxidation state of elements in the composite catalyst were analyzed via XPS (Figure 4), confirming the successful loading of the catalyst; however, due to the small amount of Au doping in the material, the relatively obvious peak spectrum cannot be detected in the XPS high-resolution map. The full spectrum in (a) exhibited peaks corresponding to elements such as C, N, and Cu, indicating the successful preparation of the composite material. The C1s XPS spectrum of the catalyst (Figure 1b) exhibited three peaks around 284.2, 288.4, and 294.0 eV. The peak at 284.7 eV corresponded to external carbon (sp3 C–C) or C=C double bond. The peak at 288.4 eV was associated with sp2 hybridized N = C-N [41]. However, with an increasing Cu doping amount, the intensity of the peak at 288.4 eV decreased, indicating a strong interaction between nitrogen–carbon and the metal. Owing to the presence of aromatic rings in g-C3N4, the peak at 294.0 eV can be attributed to the π–π satellite peak [42,43]. The XPS fitting data for N 1s (Figure 1c) revealed three peaks at ~398.9, 400.5, and 404 eV, corresponding to the three composite materials. The peaks at 398.9 and 400.5 eV corresponded to sp2 hybridized nitrogen in g-C3N4 (C=N-C) and tertiary nitrogen atoms in N-(C)3 groups, respectively [44]. The peak at 404 eV corresponded to amino groups (NHx) and π–π* excitation bonds [45,46,47]. As the doping amount increased, the position of the N1s spectral peak shifted toward higher binding energy positions, indicating the formation of coordination bonds between copper ions and nitrogen in the composite material. Moreover, a small fraction of the lone pair electrons from nitrogen atoms moved toward copper ions to maintain charge balance, thereby reducing the electron density of nitrogen atoms. This observation confirms the successful doping of Cu into g-C3N4. Figure 4d shows the high-resolution XPS spectrum of Cu 2p, where the characteristic peaks of Cu 2p3/2 and Cu 2p1/2 are located at around 932.30, 934.60, and 954.51, with satellite peaks appearing at 942.80 and 962.90. The presence of these peaks indicates the existence of Cu2+ in the internal structure of the material, consistent with the results shown by XRD [48,49]. With an increase in the doping amount of Cu, a phenomenon of initially negative and then positive binding energy shift was observed on the Au-Cu/C3N4 material, indicating a density change in the electron cloud of Cu from an initial increase to a subsequent decrease. The variation in binding energy of Au-Cu/C3N4 materials suggests an electron transfer from Au atoms to Cu atoms. This electron transfer results in the accumulation of more negative charges on the surface of Cu, leading to a more stable form of Cu in the catalyst. Moreover, this electron transfer facilitates the adsorption of positively charged C in CO2 and transfers excess negative charges to CO2, thereby achieving the catalytic purpose of CO2 conversion [40].
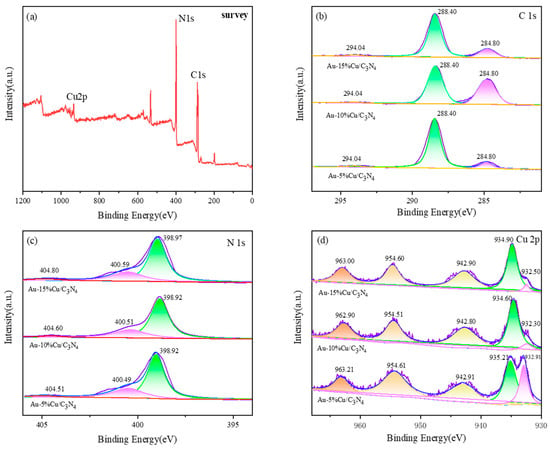
Figure 4.
XPS spectrum of the catalyst (a) survey (b) C 1s (c) N 1s (d) Cu 2p.
2.3. Catalyst Reducibility
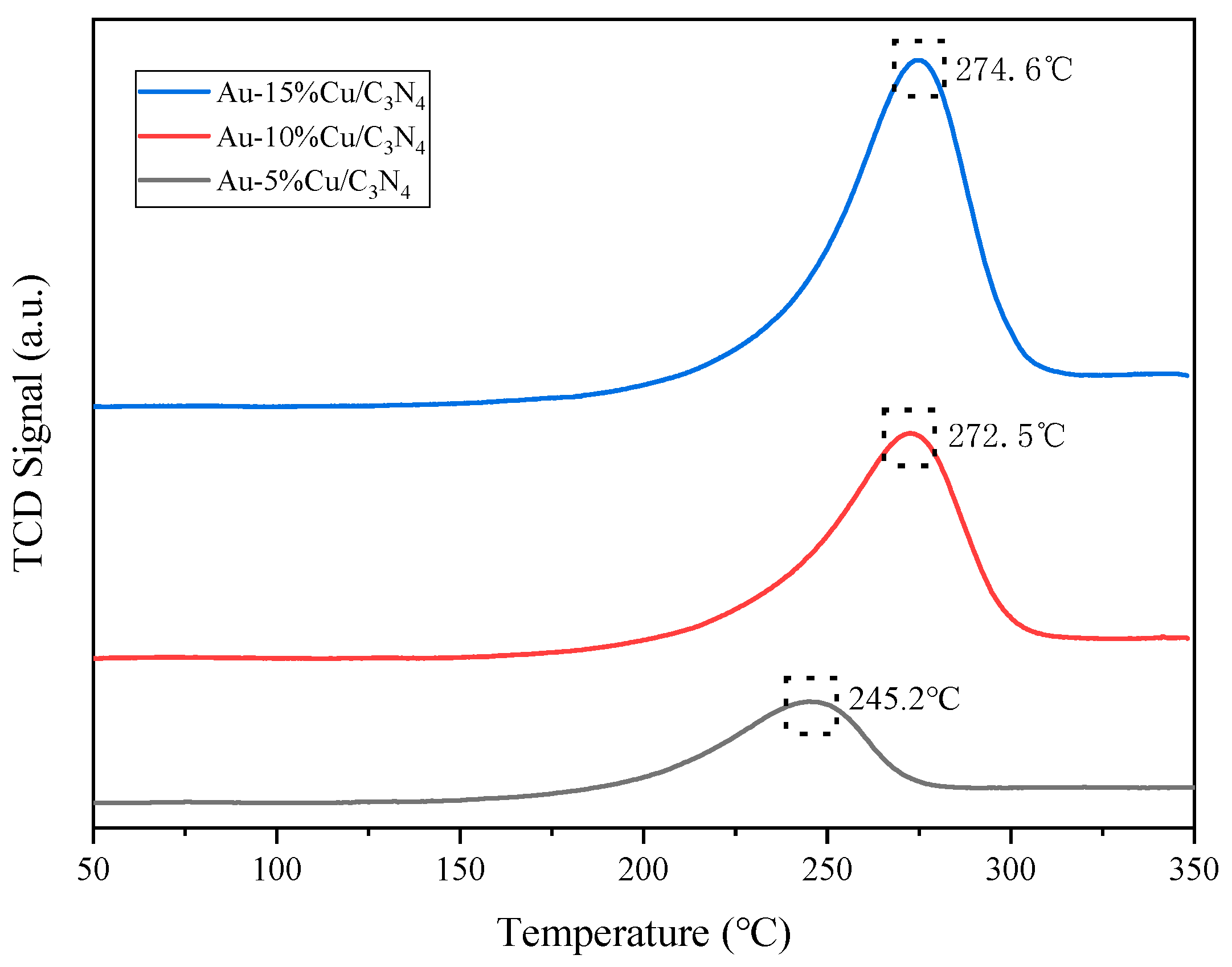
The H2-TPR curves of each catalyst (Figure 5) indicated a clear single reduction peak in the H2-TPR spectrum of all catalysts. With the increasing Cu doping amount, the reduction peak first increased and then decreased, owing to the reduction of large Cu2+ species (190–270 °C). The reduction peak of highly dispersed Cu2+ ranged from 240 to 280 °C. This suggests the presence of highly dispersed Cu2+ in the composite catalysts, with all reduction peaks attributed to Cu2+ reduction. Additionally, the peak temperatures of the catalyst reduction peaks shifted toward higher temperatures, owing to the enhanced interaction between the metal (Cu) and the support [50,51]. Increasing the Cu doping enhanced the reduction performance of the catalyst. However, excessive Cu addition should be avoided to prevent the aggregation of the metal component (Cu) on the catalyst surface, which could affect CO2 conversion.
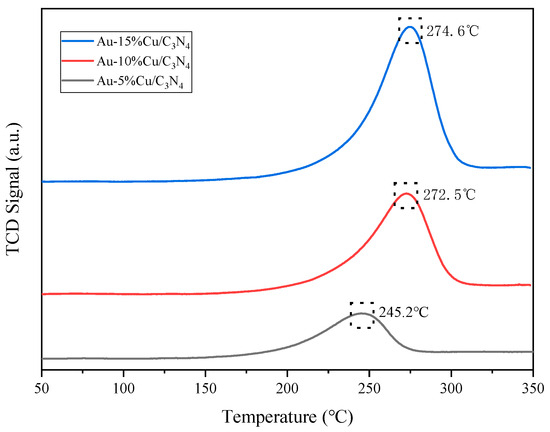
Figure 5.
H2-temperature-programmed reduction (H2-TPR) curve of the catalyst.
2.4. Surface Basicity Analysis of the Catalyst
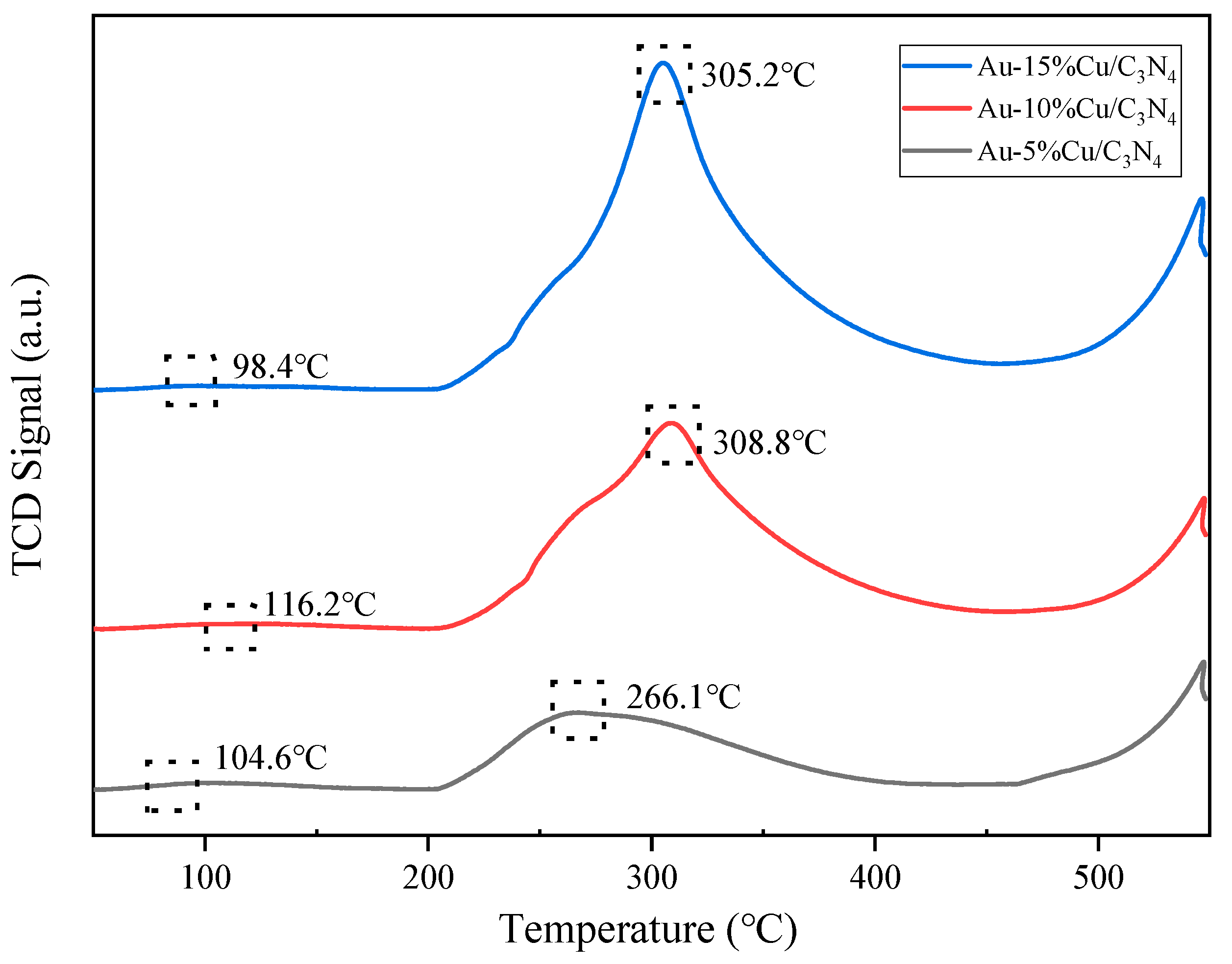
The CO2 adsorption capacity of catalysts was assessed using CO2-TPD analysis. Figure 6 shows the CO2-TPD curves of catalysts with different amounts of Cu addition. Through data analysis and processing, the curves can be categorized into two regions. The range of 50–200 °C corresponded to the α region (weak adsorption sites). This region was mainly associated with physical adsorption, particularly the adsorption of CO2 on the weak basic hydroxyl (–OH) sites on the catalyst sample surface. The range of 200–550 °C corresponded to the β region (medium strength adsorption sites), which was related to chemical adsorption. This region was mainly influenced by metal–oxygen electron pairs on the catalyst surface (Cu-O and Au-O), the presence of surface low-coordination oxygen ions, and oxygen defects that affected CO2 adsorption [51,52,53]. The data indicated that with increasing Cu doping levels, the area of the β peak in the composite catalyst also increased. Particularly, the Au–10%Cu/C3N4 catalyst exhibited the largest peak area and a higher peak temperature. This indicates that the catalyst featured abundant and stronger medium-strength basic sites, suggesting stronger interactions between the metal and the support. These interactions potentially led to an increase in Cu–O bonds, low-coordination oxygen ions, and surface defects, which enhanced the CO2 adsorption capacity of the catalyst. Moreover, the adsorption of CO2 on medium-strength basic sites also increased. In experiments on the hydrogenation of CO2 reaction, surface basic sites and defect sites of the catalyst were beneficial for CO2 adsorption and H2 overflow, thereby facilitating faster CO2 activation and methanol production [54,55]. Hence, in Cu-based catalysts supported on g-C3N4, the incorporation of a small quantity of Au proved advantageous for enhancing CO2 conversion. However, Cu played a crucial role in this catalyst, and increasing the Cu doping level regulated the surface acidity, basicity, and defect structures of the catalyst, which are key factors in promoting CO2 conversion and accelerating CO2 activation [56].
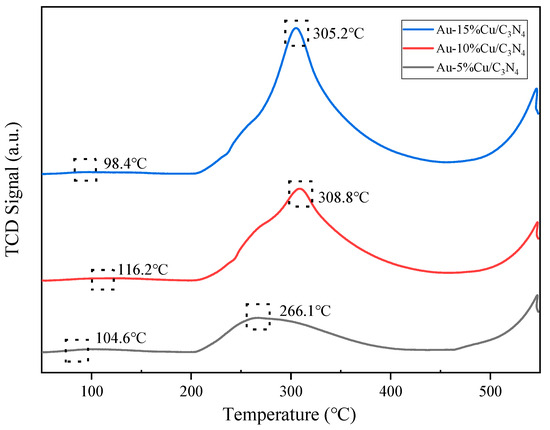
Figure 6.
CO2-temperature-programmed desorption (CO2-TPD) curve of the catalyst.
2.5. Evaluation of Catalyst Activity
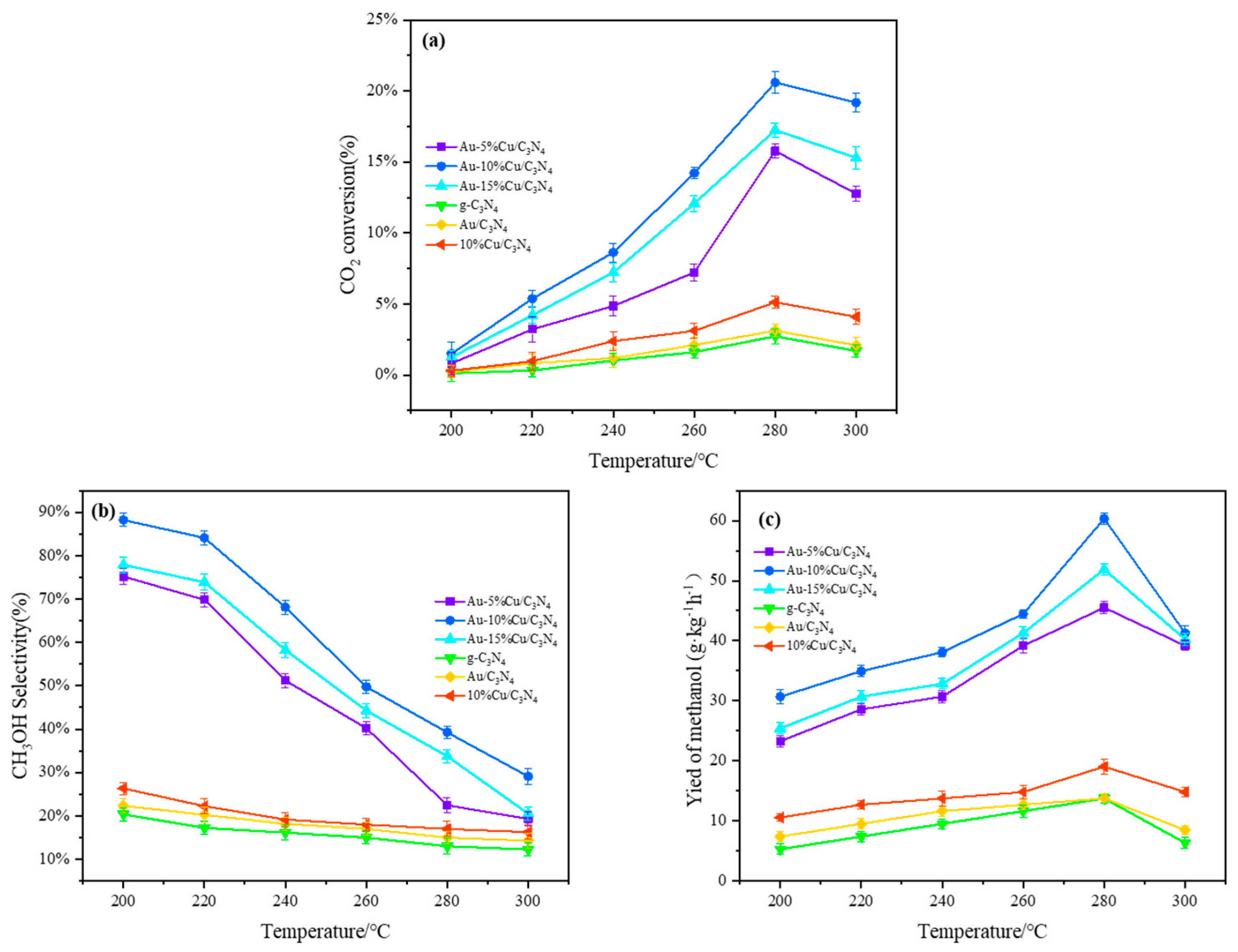
The catalytic performance of catalysts with different doping ratios was evaluated under the corresponding reaction temperatures and pressures. Figure 7 shows the activity parameters of different catalysts. By comparing the blank sample and single-metal sample, it can be observed that both exhibit relatively low catalytic activity. Data analysis indicated a significant improvement in the conversion rate of CO2, methanol selectivity, and methanol yield after doping with Cu. Incorporating a small amount of Cu on the surface of Au-C3N4 was effective. As the Cu loading ratio increased from 5% to 10%, the conversion rate of CO2 increased from 7.44% to 11.58%. However, at a loading amount of 15%, the conversion rate of CO2 significantly decreased to 9.55%. Mechanism analysis of CO2 hydrogenation to synthesize methanol suggested that this phenomenon may be due to atomic hydrogen sources provided by Cu for the hydrogenation of carbon-containing substances through spill-over [57]. Typically, the generation of atomic hydrogen in this process proceeded in two steps: first, molecular H2 was adsorbed onto the active sites of Cu as it passed through the catalyst, and then the molecular H2 was dissociated on the adsorbent to generate atomic hydrogen [58]. Therefore, an increase in Cu resulted in more active sites for H2 molecule adsorption. The dissociation of H2 molecules was influenced by Cu activity, which was mainly affected by Au-C3N4. A stronger interaction between the bimetallic components resulted in a higher dissociation rate of H2. According to characterization results, this phenomenon may be attributed to the increased Cu content, which modified the pore structure and specific surface area of the catalyst, thereby enhancing interactions between Cu-Au-C3N4. However, excessively high loading of Cu can cause aggregation on the catalyst surface, thereby reducing dispersion and affecting the CO2 conversion rate. Therefore, a moderate increase in the co-catalyst Cu is beneficial for improving hydrogenation performance [40]. Methanol selectivity increased with increasing Cu loading ratios, reaching 59.77% on the catalyst containing 10% Cu (Figure 7b). As the temperature increased, the conversion rate of CO2 increased, while methanol selectivity decreased. This trend can be elucidated through the principles of thermodynamics and kinetics [59]. With increasing loading amount, the methanol yield and yield reached up to 41.29g·kg−1·h−1 (0.39 μmol·g−1s−1) and 6.9% on the catalyst with 10% Cu loading (Figure 7c). By comparing the Cu-based catalysts reported in most studies, it is found in Table 2 that the performance of the prepared bimetallic Au-Cu-supported g-C3N4 catalyst is higher than that of most Cu-based catalysts in terms of CO2 conversion, methanol selectivity, and yield. It is further proved that bimetal doping is beneficial in improving the performance of the catalyst.
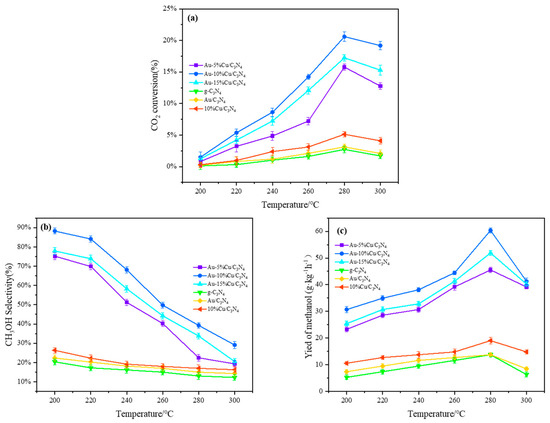
Figure 7.
Performance of the catalysts in CO2 hydrogenation to methanol ((a) CO2 conversion rate; (b) methanol selectivity; (c) methanol yield) (catalyst weight = 0.5 g; V(H2):V(CO2):V(N2) = 69:2:8; T = 200–300 °C; P = 3.0 MPa; WHSV = 3600 mL·g−1·h−1).

Table 2.
Comparison of catalytic performance of Cu-based catalysts.
3. Materials and Methods
3.1. Preparation of Catalysts
3.1.1. Preparation of g-C3N4
First, 10 g of urea was dispersed in 15 mL of deionized water, and the pH of the solution was adjusted using 0.1 M hydrochloric acid. Subsequently, the solution was dried at 60 °C for 24 h. The obtained powder was placed in a crucible and rapidly heated to 550 °C at a rate of 5 °C/min and held for 2 h. After the crucible was cooled to room temperature, the resulting g-C3N4 material was dissolved in an isopropanol–water mixture and stirred to obtain the g-C3N4 catalyst.
3.1.2. Preparation of Au Nanoparticles
First, 0.1 g of AuCl4·3H2O was added to a mixture containing 10 mL of tetralin and 10 mL of ethanolamine. The mixture was magnetically stirred to ensure uniform mixing. Subsequently, 0.04 g of the tert-butylamine borane complex was dissolved in a mixture of 1 mL of tetralin and 1 mL of oleylamine. The precursor solution obtained was then added to the mixed solvent and reacted at 40 °C for 1 h to synthesize Au nanoparticles. The resulting precipitate of Au nanoparticles was washed with acetone.
3.1.3. Preparation of Au–Cu Nanoparticle Composites
First, a certain amount of Cu(CH3COO)2·H2O was dissolved in a mixture containing 0.5 mL of Oleic acid amide and 2.2 mL of trioctylamine. The mixture was stirred and heated to 70 °C until the solution became clear. Subsequently, the obtained Au nanoparticles were added to the precursor solution with varying amounts of Cu(CH3COO)2·H2O added in proportions of 5%, 10%, and 15%. The resulting mixed solution was evaporated at 120 °C, transferred into a crucible, heated to 300 °C at a rate of 25 °C per minute, and maintained at this temperature for 1 h. After cooling, the powdered material was dispersed in ethanol, and the solution was allowed to settle to obtain Au–Cu nanoparticles. Finally, the composite metal particles were washed with acetone.
3.1.4. Preparation of Au–Cu/C3N4 Composite Materials
A certain amount of g-C3N4 was ultrasonically dispersed in a mixture of isopropanol and water solvent. Subsequently, different ratios of Au–Cu nanoparticles (containing Au-5% Cu, Au-10% Cu, and Au-15% Cu) were added to the dispersion, and the solution was adjusted to acidic condition with 0.1M HCl and stirred for 120 min. The resulting solution was transferred into a high-pressure reactor vessel composed of polytetrafluoroethylene and maintained at 180 °C for 12 h. After cooling to room temperature, the obtained nano-composite material was washed three times alternately with deionized water and ethanol, dried in a vacuum, and then heated at 200 °C in a N2 atmosphere for 13 h to obtain the final Au–Cu/C3N4 composite material.
3.2. Characterization of the Catalyst
Characterization of the Au–Cu/C3N4 composite material: The specific surface area and pore size of the Au–Cu/C3N4 composite material were evaluated through BET surface area and pore size analysis (Micromeritics JW-BK200B, Beijing, China). N2 was used as the adsorbate, and the sample was degassed at −196 °C for 4 h. The microscopic morphology, structure, and size of the Au–Cu/C3N4 composite material were examined via SEM (model S4800, Qingzhou, China). Using the Bruker instrument model D8 Advance, X-ray diffraction (XRD) was performed on Au-Cu/C3N4 composite materials with a copper target as the anode and the diffractometer in BB geometry to characterize the characteristic crystal planes. The Au-Cu/C3N4 composite material was analyzed using X-ray photoelectron spectroscopy (XPS) with C1s standard peak correction, and the measurements were conducted using a Shimadzu (Tokyo, Japan) AXIS ULTRA DLD instrument. Fourier transform infrared spectroscopy (FT-IR) was employed to characterize the functional group structures of the Au-Cu/C3N4 composite material, with experiments conducted using a Shimadzu IRTracer100 FT-IR spectrometer from Japan in the wavenumber range of 400–4000 cm−1, utilizing potassium bromide dilution, a resolution of 4, and no degassing. Additionally, Raman spectroscopy analysis was conducted on the Au–Cu/C3N4 composite material using a single-frequency excitation line at 532 nm. The CO2-TPD analysis was performed using a chemisorption analyzer (Micromeritics 2950, Beijing, China). First, the sample underwent a reduction process at 300 °C for 4 h, followed by cooling to 50 °C. Subsequently, the sample was exposed to 20% CO2/Ar atmosphere for 1 h, purged with He, desorbed, and ramped up to the desired temperature. The desorption of CO2 molecules was monitored using online instrumentation. H2-TPR testing was conducted using a chemisorption analyzer (Micromeritics 2950, China). First, the sample was purged at 300 °C for 30 min and cooled to 60 °C. Subsequently, the reaction gas was switched to 10% H2/Ar reaction gas, and the temperature was ramped from 60 °C to 800 °C at a rate of 10 °C/min. During this process, reaction gases were analyzed, and the results were recorded. The gaseous products were analyzed online by an Agilent (Santa Clara, CA, USA) GC 7820A gas chromatograph, equipped with a thermal conductivity detector (TCD) and a flame ionization detector (FID). The chromatographic columns used were Porapak Q columns (two), a 6ft PQ/8ft 5A column (one), and a Restek Rt-Q-Bond capillary column.
3.3. Catalyst Activity Evaluation
The CO2 hydrogenation reaction was carried out on a high-pressure fixed-bed continuous flow reactor–gas chromatograph (Shimadzu GC-2014C) combined system. A total of 0.5 g of catalyst was loaded in the temperature-controlled zone of a stainless steel reaction tube, with the remaining space filled and fixed with quartz sand. The flow rate of the 5% H2/Ar gas was set at 30 mL·min−1 and ramped up to 300 °C at a rate of 2 °C·min−1 for a 4-h reduction; then it was cooled to room temperature and switched to the reaction gas for pressurized reaction. The reaction conditions were as follows: WHSV = 3600 mL·g−1·h−1; V(H2):V(CO2):V(N2) = 69:23:8; gas flow rate at 30 mL·min−1; and reaction temperature ranging from 200 to 300 °C (in this paper, the selection of the reaction gas ratio references [31]). The formula for the conversion rate of CO2 is as follows:
where (CO2)b and (CO2)a, respectively, represent the moles of CO2 before and after the reaction.
4. Conclusions
The g-C3N4 modified-Au–Cu alloy nanoparticles were successfully prepared for catalyzing the hydrogenation of CO2 to methanol. The g-C3N4 nanosheets, synthesized with varying amounts of Cu, provided abundant active sites for CO2 adsorption and activation. The electronic properties of Au enhanced the interaction between the metal and the catalyst support, thereby enhancing the stability of Cu on the catalyst surface. Therefore, the formation of additional active sites at the interface between the metal and support in the Au–Cu/C3N4 catalyst enhanced its capability for CO2 adsorption and activation, leading to excellent performance. Through exploring the catalyst’s activity, it was found that Au-10%Cu/C3N4 exhibited higher CO2 selectivity, methanol yield, yield and methanol selectivity of 11.6%, 41.29g·kg−1·h−1 (0.39 μmol·g−1s−1), 6.9% and 60%.
Author Contributions
Conceptualization, C.L. (Chenyang Li) and J.Y.; methodology, C.L. (Chenyang Li) and J.Y.; software, J.Y.; validation, C.Z.; formal analysis, C.Z.; investigation, K.F.; resources, C.L. (Chen Lyu); writing—original draft preparation, J.Y.; writing—review and editing, C.L. (Chenyang Li); visualization, J.Y.; supervision, C.W.; project administration, C.L. (Chen Lyu); funding acquisition, C.L. (Chenyang Li). All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Department of Science and Technology of Jilin Province (grant no. 20210508050RQ) and by the Bureau of Science and Technology of Yulin.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
We acknowledge the Department of Science and Technology of Jilin Province and the Bureau of Science and Technology of Yulin.
Conflicts of Interest
Author Cong Wang was employed by the company China Municipal Engineering Northeast Design and Research Institute Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Chang. 2017, 7, 243–249. [Google Scholar] [CrossRef]
- Liu, R.L.; Chen, Z.X.; Yao, Y.; Li, Y.; Cheema, W.A.; Wang, D.W.; Zhu, S.M. Recent advancements in g-C3N4-based photocatalysts for photocatalytic CO2reduction: A mini review. Rsc Adv. 2020, 10, 29408–29418. [Google Scholar] [CrossRef]
- Jones, C.W.; Park, A.H.A.; Wright, P. The Complex Interplay of Separations, Reactions and Storage in Pursuit of Carbon Neutrality: A Central Role for Nanomaterials. Acc. Chem. Res. 2023, 56, 3545–3546. [Google Scholar] [CrossRef]
- Navarro-Jaén, S.; Virginie, M.; Bonin, J.; Robert, M.; Wojcieszak, R.; Khodakov, A.Y. Highlights and challenges in the selective reduction of carbon dioxide to methanol. Nat. Rev. Chem. 2021, 5, 564–579. [Google Scholar] [CrossRef]
- Nisar, A.; Khan, S.; Hameed, M.; Nisar, A.; Ahmad, H.; Mehmood, S.A. Bio-conversion of CO2 into biofuels and other value-added chemicals via metabolic engineering. Microbiol. Res. 2021, 251, 126813. [Google Scholar] [CrossRef]
- Idso, S.B. The search for global Co2 etc greenhouse effects. Environ. Conserv. 1985, 12, 29–35. [Google Scholar] [CrossRef]
- Garba, M.D.; Usman, M.; Khan, S.; Shehzad, F.; Galadima, A.; Ehsan, M.F.; Ghanem, A.S.; Humayun, M. CO2 towards fuels: A review of catalytic conversion of carbon dioxide to hydrocarbons. J. Environ. Chem. Eng. 2021, 9, 104756. [Google Scholar] [CrossRef]
- López, L.R.; Dessì, P.; Cabrera-Codony, A.; Rocha-Melogno, L.; Kraakman, B.; Naddeo, V.; Balaguer, M.D.; Puig, S. CO2 in indoor environments: From environmental and health risk to potential renewable carbon source. Sci. Total Environ. 2023, 856, 159088. [Google Scholar] [CrossRef]
- Hu, C.C.; Lin, C.W.; Hu, C.P.; Keshebo, D.L.; Huang, S.H.; Hung, W.S.; Lee, K.R.; Lai, J.Y. Carbon dioxide enrichment of PDMS/PSf composite membranes for solving the effect and food crisis. J. CO2 Util. 2022, 61, 102011. [Google Scholar] [CrossRef]
- Popovic, S.; Smiljanic, M.; Jovanovic, P.; Vavra, J.; Buonsanti, R.; Hodnik, N. Stability and Degradation Mechanisms of Copper-Based Catalysts for Electrochemical CO2 Reduction. Angew. Chem. Int. Edit. 2020, 59, 14736–14746. [Google Scholar] [CrossRef]
- Cao, S.W.; Li, Y.; Zhu, B.C.; Jaroniec, M.; Yu, J.G. Facet effect of Pd cocatalyst on photocatalytic CO2 reduction over g-C3N4. J. Catal. 2017, 349, 208–217. [Google Scholar] [CrossRef]
- Malati, M.A. Mitigation of CO2 greenhouse effect, combined disposal and utilisation by photocatalysis. Energy Convers. Manag. 1996, 37, 1345–1350. [Google Scholar] [CrossRef]
- Anwer, A.H.; Khan, N.; Khan, M.D.; Shakeel, S.; Khan, M.Z. Redox mediators as cathode catalyst to boost the microbial electro-synthesis of biofuel product from carbon dioxide. Fuel 2021, 302, 121124. [Google Scholar] [CrossRef]
- Zachariah, E.J.; Sabulal, B.; Nair, D.N.K.; Johnson, A.J.; Kumar, C.S.P. Carbon dioxide emission from bamboo culms. Plant Biol. 2016, 18, 400–405. [Google Scholar] [CrossRef]
- Zhang, S.P.; Liu, Z.R. Advances in the biological fixation of carbon dioxide by microalgae. J. Chem. Technol. Biotechnol. 2021, 96, 1475–1495. [Google Scholar] [CrossRef]
- Ran, J.R.; Jaroniec, M.; Qiao, S.Z. Cocatalysts in Semiconductor-based Photocatalytic CO2 Reduction: Achievements, Challenges, and Opportunities. Adv. Mater. 2018, 30, 1704649. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Cheng, B.; Yu, J.G. Graphene-Based Photocatalysts for Solar-Fuel Generation. Angew. Chem. Int. Edit. 2015, 54, 11350–11366. [Google Scholar] [CrossRef]
- Qin, D.Y.; Zhou, Y.; Wang, W.J.; Zhang, C.; Zeng, G.M.; Huang, D.L.; Wang, L.L.; Wang, H.; Yang, Y.; Lei, L.; et al. Recent advances in two-dimensional nanomaterials for photocatalytic reduction of CO2: Insights into performance, theories and perspective. J. Mater. Chem. A 2020, 8, 19156–19195. [Google Scholar] [CrossRef]
- Chen, X.; Jin, F.M. Photocatalytic reduction of carbon dioxide by titanium oxide-based semiconductors to produce fuels. Front. Energy 2019, 13, 207–220. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloy. Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Schwalbe, M.; Huang, H.; Li, G. Photocatalytic and Photoelectrochemical Carbon Dioxide Reduction. ChemPhotoChem 2022, 6, e202100217. [Google Scholar] [CrossRef]
- Cui, Y.J. In-situ synthesis of C3N4/CdS composites with enhanced photocatalytic properties. Chin. J. Catal. 2015, 36, 372–379. [Google Scholar] [CrossRef]
- Li, J.; Wu, D.D.; Iocozzia, J.; Du, H.W.; Liu, X.Q.; Yuan, Y.P.; Zhou, W.; Li, Z.; Xue, Z.M.; Lin, Z.Q. Achieving Efficient Incorporation of π-Electrons into Graphitic Carbon Nitride for Markedly Improved Hydrogen Generation. Angew. Chem. Int. Edit. 2019, 58, 1985–1989. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Q.; Wang, D.F.; Tang, J.T.; Song, S.; Chen, J.M.; Tao, X.Y. A quasi-hexagonal prism-shaped carbon nitride for photoreduction of carbon dioxide under visible light. Environ. Sci. Pollut. Res. 2017, 24, 8219–8229. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, L.X.; Shi, J.L. Converting CO2 into fuels by graphitic carbon nitride-based photocatalysts. Nanotechnology 2018, 29, 412001. [Google Scholar] [CrossRef]
- Cometto, C.; Ugolotti, A.; Grazietti, E.; Moretto, A.; Bottaro, G.; Armelao, L.; Di Valentin, C.; Calvillo, L.; Granozzi, G. Copper single-atoms embedded in 2D graphitic carbon nitride for the CO2 reduction. NPJ 2D Mater. Appl. 2021, 5, 63. [Google Scholar] [CrossRef]
- Wang, J.B.; Wang, Y.N.; Yu, M.Y.; Li, G.J.; Zhang, S.L.; Zhong, Q. Formation of flaky carbon nitride and beta-Indium sulfide heterojunction with efficient separation of charge carriers for enhanced photocatalytic carbon dioxide reduction. J. Colloid Interface Sci. 2022, 611, 71–81. [Google Scholar] [CrossRef]
- Chagoya, K.L.; Nash, D.J.; Jiang, T.; Le, D.; Alayoglu, S.; Idrees, K.B.; Zhang, X.; Farha, O.K.; Harper, J.K.; Rahman, T.S.; et al. Mechanically Enhanced Catalytic Reduction of Carbon Dioxide over Defect Hexagonal Boron Nitride. ACS Sustain. Chem. Eng. 2021, 9, 2447–2455. [Google Scholar] [CrossRef]
- Kumar, A.; Prajapati, P.K.; Aathira, M.S.; Bansiwal, A.; Boukherroub, R.; Jain, S.L. Highly improved photoreduction of carbon dioxide to methanol using cobalt phthalocyanine grafted to graphitic carbon nitride as photocatalyst under visible light irradiation. J. Colloid Interface Sci. 2019, 543, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, J.H.; Kim, E.H.; Kim, K.Y.; Choi, Y.H.; Youn, D.H.; Lee, J.S. A highly active and stable palladium catalyst on a g-C3N4 support for direct formic acid synthesis under neutral conditions. Chem. Commun. 2016, 52, 14302–14305. [Google Scholar] [CrossRef]
- Deng, K.X.; Hu, B.; Lu, Q.Y.; Hong, X.L. Cu/g-C3N4 modified ZnO/Al2O3 catalyst: Methanol yield improvement of CO2 hydrogenation. Catal. Commun. 2017, 100, 81–84. [Google Scholar] [CrossRef]
- Posada-Pérez, S.; Vidal-López, A.; Solá, M.; Poater, A. 2D carbon nitride as a support with single Cu, Ag, and Au atoms for carbon dioxide reduction reaction. Phys. Chem. Chem. Phys. 2023, 25, 8574–8582. [Google Scholar] [CrossRef]
- Cao, S.W.; Low, J.X.; Yu, J.G.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Jia, G.R.; Wang, Z.X.; Gong, M.; Wang, Y.; Li, L.H.; Dong, Y.L.; Liu, L.L.; Zhang, L.; Zhao, J.X.; Zheng, W.T.; et al. Ultrathin origami accordion-like structure of vacancy-rich graphitized carbon nitride for enhancing CO2 photoreduction. Carbon Energy 2023, 5, e270. [Google Scholar] [CrossRef]
- Li, C.M.; Yu, S.Y.; Dong, H.J.; Liu, C.B.; Wu, H.J.; Che, H.N.; Chen, G. Z-scheme mesoporous photocatalyst constructed by modification of Sn3O4 nanoclusters on g-C3N4 nanosheets with improved photocatalytic performance and mechanism insight. Appl. Catal. B-Environ. 2018, 238, 284–293. [Google Scholar] [CrossRef]
- Han, Q.; Wang, B.; Zhao, Y.; Hu, C.G.; Qu, L.T. A Graphitic-C3N4 “Seaweed” Architecture for Enhanced Hydrogen Evolution. Angew. Chem. Int. Edit. 2015, 54, 11433–11437. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhang, M.W.; Yang, C.; Wang, X.C. Nanospherical Carbon Nitride Frameworks with Sharp Edges Accelerating Charge Collection and Separation at a Soft Photocatalytic Interface. Adv. Mater. 2014, 26, 4121–4126. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, B.; Gao, J.; Cheng, Z.H.; Zhao, Y.; Zhang, Z.P.; Qu, L.T. Atomically Thin Mesoporous Nanomesh of Graphitic C3N4 for High-Efficiency Photocatalytic Hydrogen Evolution. ACS Nano 2016, 10, 2745–2751. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Leinenweber, K.; Bauer, M.; Garvie, L.A.J.; McMillan, P.F.; Wolf, G.H. High-pressure bulk synthesis of crystalline C6N9H3•HCl:: A novel C3N4 graphitic derivative. J. Am. Chem. Soc. 2001, 123, 7788–7796. [Google Scholar] [CrossRef]
- Li, P.Y.; Liu, L.; An, W.J.; Wang, H.; Guo, H.X.; Liang, Y.H.; Cui, W.Q. Ultrathin porous g-C3N4 nanosheets modified with AuCu alloy nanoparticles and C-C coupling photothermal catalytic reduction of CO2 to ethanol. Appl. Catal. B-Environ. 2020, 266, 118618. [Google Scholar] [CrossRef]
- Kapustin, G.I.; Brueva, T.R.; Klyachko, A.L.; Beran, S.; Wichterlova, B. Determination of the number and acid strength of acid sites in zeolites by ammonia adsorption—Comparison of calorimetry and temperature-programmed desorption of ammonia. Appl. Catal. 1988, 42, 239–246. [Google Scholar] [CrossRef]
- Faisal, M.; Ismail, A.A.; Harraz, F.A.; Al-Sayari, S.A.; El-Toni, A.M.; Al-Assiri, M.S. Synthesis of highly dispersed silver doped g-C3N4 nanocomposites with enhanced visible-light photocatalytic activity. Mater. Des. 2016, 98, 223–230. [Google Scholar] [CrossRef]
- Liu, C.Y.; Huang, H.W.; Cui, W.; Dong, F.; Zhang, Y.H. Band structure engineering and efficient charge transport in oxygen substituted g-C3N4 for superior photocatalytic hydrogen evolution. Appl. Catal. B-Environ. 2018, 230, 115–124. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Huang, S.Y.; Deng, J.J.; Gangadharan, D.T.; Yang, F.; Xu, Z.H.; Giorgi, G.; Palummo, M.; Chaker, M.; Ma, D.L. Ice-Assisted Synthesis of Black Phosphorus Nanosheets as a Metal-Free Photocatalyst: 2D/2D Heterostructure for Broadband H2 Evolution. Adv. Funct. Mater. 2019, 29, 1902486. [Google Scholar] [CrossRef]
- Hua, S.X.; Qu, D.; An, L.; Jiang, W.S.; Wen, Y.J.; Wang, X.Y.; Sun, Z.C. Highly efficient p-type Cu3P/n-type g-C3N4 photocatalyst through Z-scheme charge transfer route. Appl. Catal. B-Environ. 2019, 240, 253–261. [Google Scholar] [CrossRef]
- Shu, Z.; Wang, Y.; Wang, W.B.; Zhou, J.; Li, T.T.; Liu, X.Q.; Tan, Y.G.; Zhao, Z.L. A green one-pot approach for mesoporous g-C3N4 nanosheets with in situ sodium doping for enhanced photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2019, 44, 748–756. [Google Scholar] [CrossRef]
- Sun, Z.C.; Zhu, M.S.; Fujitsuka, M.; Wang, A.J.; Shi, C.; Majima, T. Phase Effect of NixPy Hybridized with g-C3N4 for Photocatalytic Hydrogen Generation. Acs Appl. Mater. Interfaces 2017, 9, 30583–30590. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, C.; Yang, Y.P.; Dun, X.J.; Gao, J.M.; Jin, X.J. A novel electrochemical sensor based on CuO/H-C3N4/rGO nanocomposite for efficient electrochemical sensing nitrite. J. Alloys Compd. 2019, 798, 764–772. [Google Scholar] [CrossRef]
- Karthik, P.; Kumar, T.R.N.; Neppolian, B. Redox couple mediated charge carrier separation in g-C3N4/CuO photocatalyst for enhanced photocatalytic H2 production. Int. J. Hydrog. Energy 2020, 45, 7541–7551. [Google Scholar] [CrossRef]
- Rui, N.; Wang, Z.Y.; Sun, K.H.; Ye, J.Y.; Ge, Q.F.; Liu, C.J. CO2 hydrogenation to methanol over Pd/In2O3: Effects of Pd and oxygen vacancy. Appl. Catal. B-Environ. 2017, 218, 488–497. [Google Scholar] [CrossRef]
- Wang, S.; Song, L.X.; Qu, Z.P. Cu/ZnAl2O4 catalysts prepared by ammonia evaporation method: Improving methanol selectivity in CO2 hydrogenation via regulation of metal-support interaction. Chem. Eng. J. 2023, 469, 144008. [Google Scholar] [CrossRef]
- Dong, X.S.; Li, F.; Zhao, N.; Xiao, F.K.; Wang, J.W.; Tan, Y.S. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts prepared by precipitation-reduction method. Appl. Catal. B-Environ. 2016, 191, 8–17. [Google Scholar] [CrossRef]
- Hu, Q.; Fan, G.L.; Yang, L.; Li, F. Aluminum-Doped Zirconia-Supported Copper Nanocatalysts: Surface Synergistic Catalytic Effects in the Gas-Phase Hydrogenation of Esters. Chemcatchem 2014, 6, 3501–3510. [Google Scholar] [CrossRef]
- Prinetto, F.; Ghiotti, G.; Durand, R.; Tichit, D. Investigation of acid-base properties of catalysts obtained from layered double hydroxides. J. Phys. Chem. B 2000, 104, 11117–11126. [Google Scholar] [CrossRef]
- León, M.; Díaz, E.; Bennici, S.; Vega, A.; Ordóñez, S.; Auroux, A. Adsorption of CO2 on Hydrotalcite-Derived Mixed Oxides: Sorption Mechanisms and Consequences for Adsorption Irreversibility. Ind. Eng. Chem. Res. 2010, 49, 3663–3671. [Google Scholar] [CrossRef]
- Scotti, N.; Dangate, M.; Gervasini, A.; Evangelisti, C.; Ravasio, N.; Zaccheria, F. Unraveling the Role of Low Coordination Sites in a Cu Metal Nanoparticle: A Step toward the Selective Synthesis of Second Generation Biofuels. ACS Catal. 2014, 4, 2818–2826. [Google Scholar] [CrossRef]
- Arena, F.; Italiano, G.; Barbera, K.; Bordiga, S.; Bonura, G.; Spadaro, L.; Frusteri, F. Solid-state interactions, adsorption sites and functionality of Cu-ZnO/ZrO2 catalysts in the CO2 hydrogenation to CH3OH. Appl. Catal. A-Gen. 2008, 350, 16–23. [Google Scholar] [CrossRef]
- Liu, C.H.; Guo, X.M.; Guo, Q.S.; Mao, D.S.; Yu, J.; Lu, G.Z. Methanol synthesis from CO2 hydrogenation over copper catalysts supported on MgO-modified TiO2. J. Mol. Catal. A-Chem. 2016, 425, 86–93. [Google Scholar] [CrossRef]
- Guo, X.M.; Mao, D.S.; Lu, G.Z.; Wang, S.; Wu, G.S. Glycine-nitrate combustion synthesis of CuO-ZnO-ZrO2 catalysts for methanol synthesis from CO2 hydrogenation. J. Catal. 2010, 271, 178–185. [Google Scholar] [CrossRef]
- Bansode, A.; Tidona, B.; von Rohr, P.R.; Urakawa, A. Impact of K and Ba promoters on CO2 hydrogenation over Cu/Al2O3 catalysts at high pressure. Catal. Sci. Technol. 2013, 3, 767–778. [Google Scholar] [CrossRef]
- Chang, K.; Wang, T.F.; Chen, J.G.G. Hydrogenation of CO2 to methanol over CuCeTiOx catalysts. Appl. Catal. B-Environ. 2017, 206, 704–711. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).