Electrochemical Detection of Dopamine: Novel Thin-Film Ti-Nanocolumnar Arrays/Graphene Monolayer-Cufoil Electrodes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology and Surface Characterization
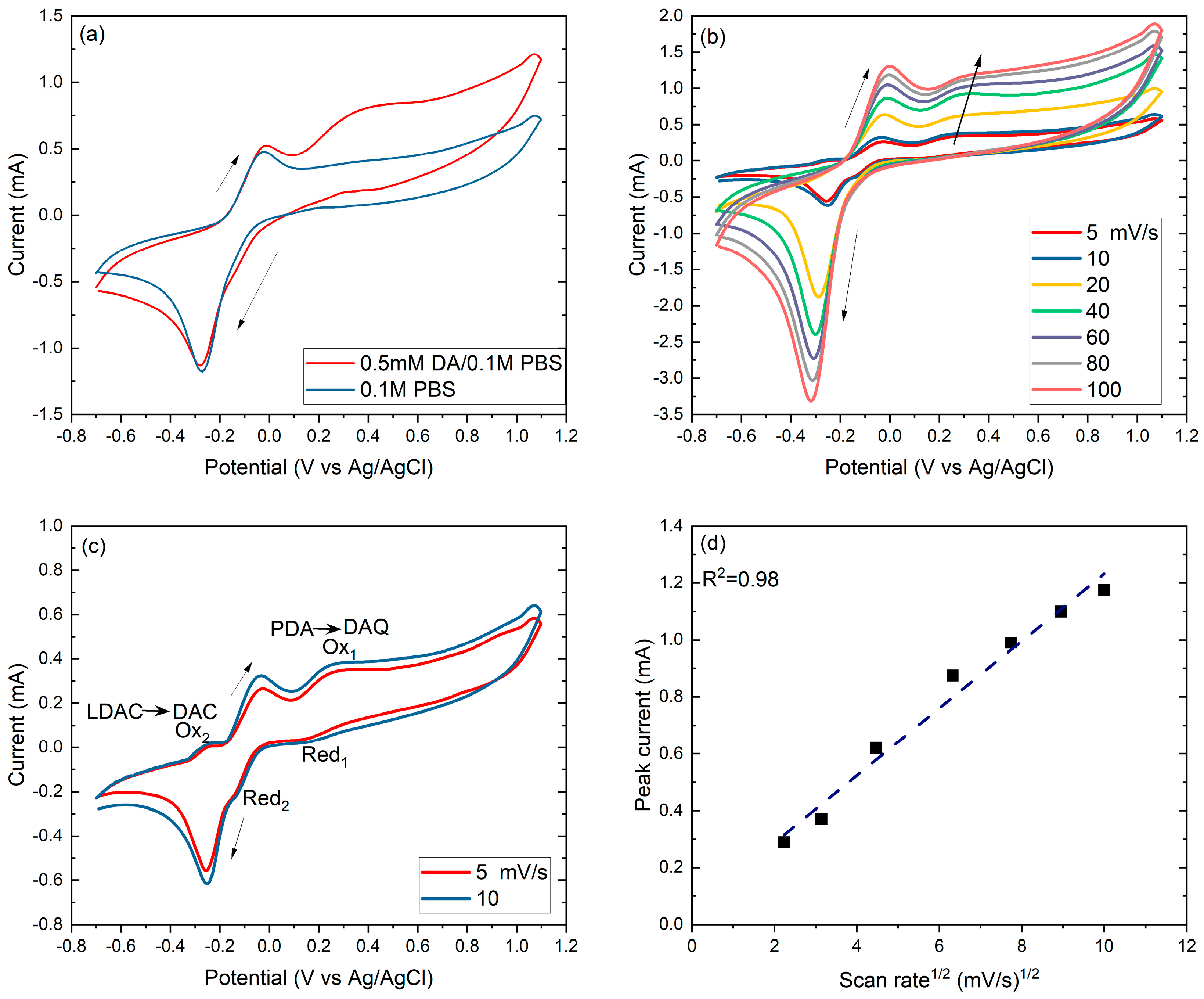
2.2. Electrochemical Performance of the TiNCs/Gm-Cufoil
2.2.1. Dopamine Electrooxidation Reaction Mechanism
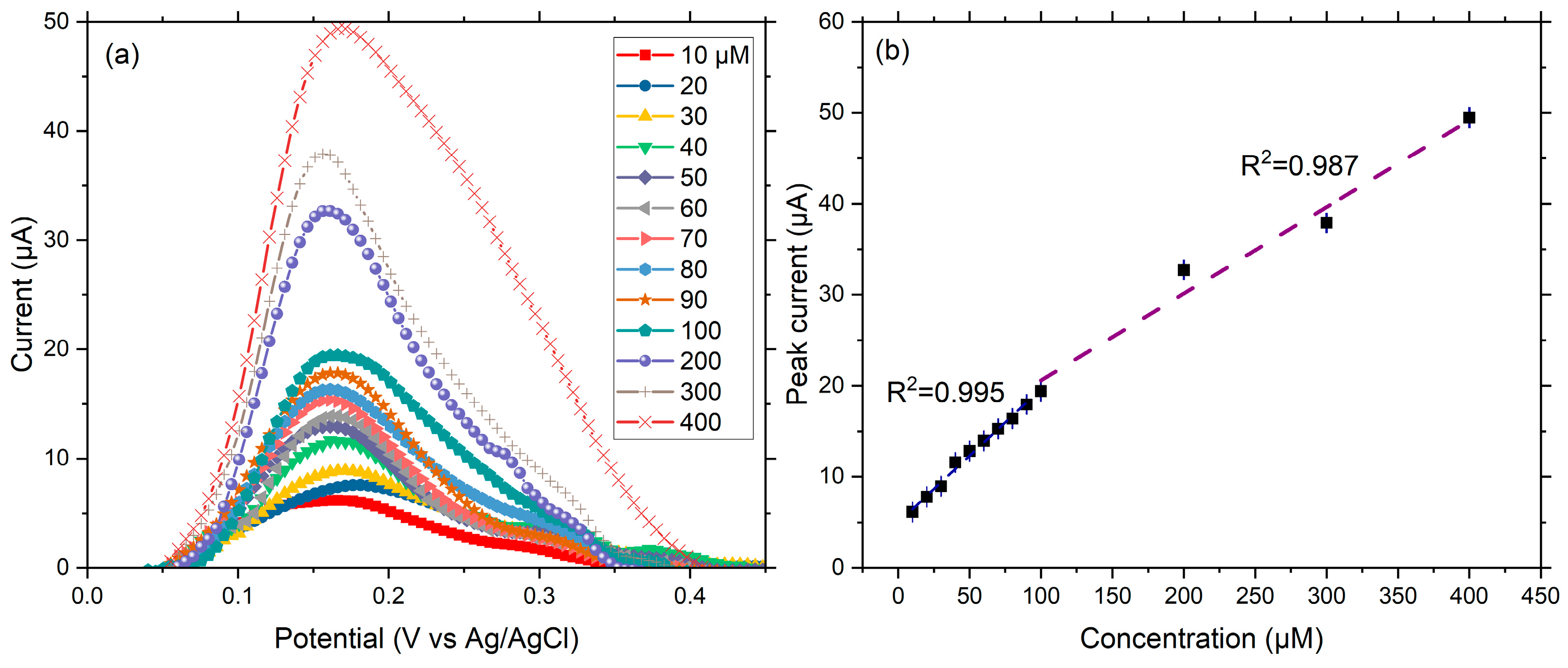
2.2.2. Dopamine Calibration Curve and Sensitivity
2.2.3. Selectivity, Long-Term Stability, and Repeatability
3. Experimental Part
3.1. Materials and Reagents
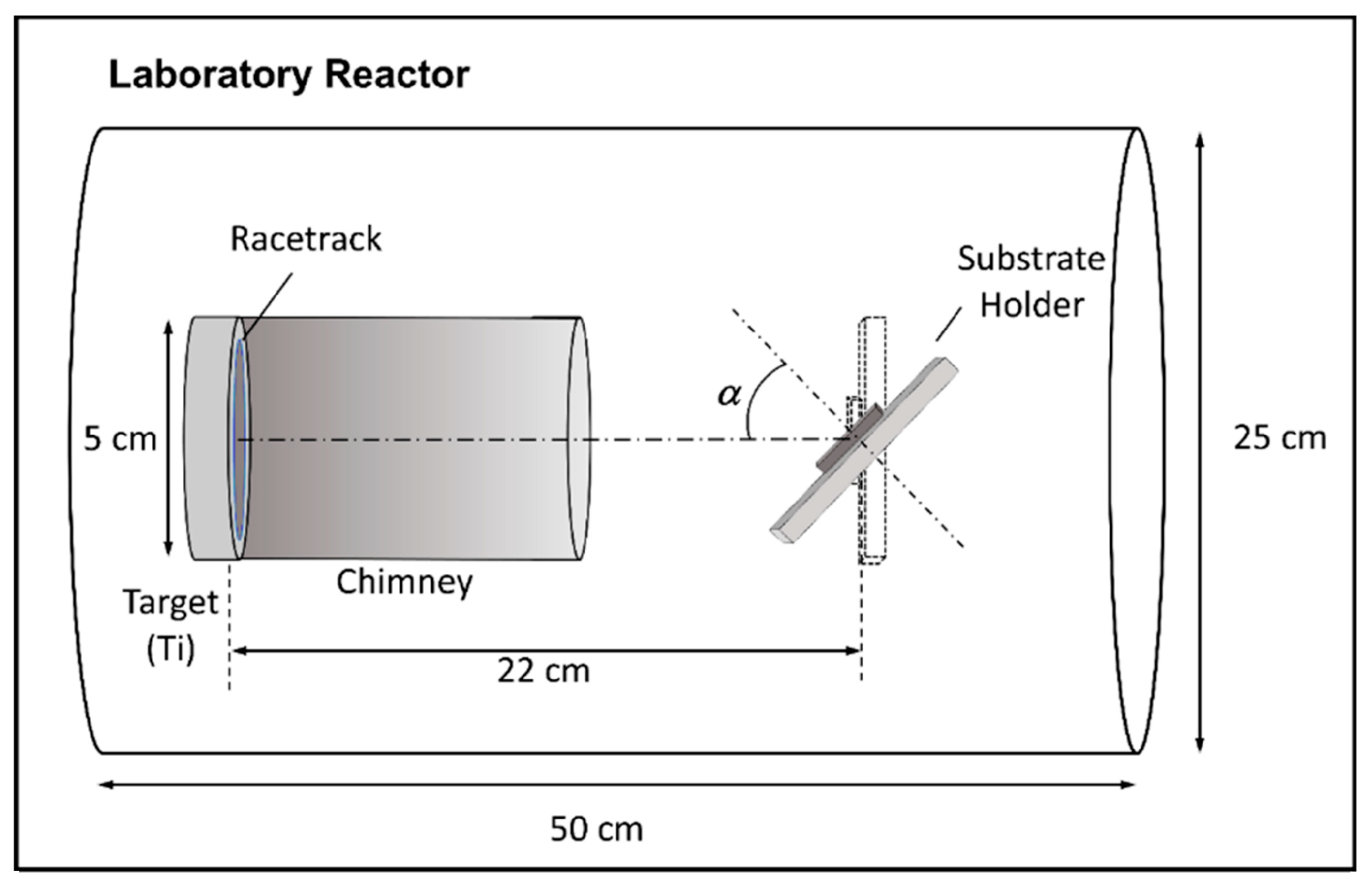
3.2. Synthesis of TiNCs/Gm-Cufoil Electrode
3.3. Structural Characterization
3.4. Electrochemical Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Channer, B.; Matt, S.M.; Nickoloff-Bybel, E.A.; Pappa, V.; Agarwal, Y.; Wickman, J.; Gaskill, P.J. Dopamine, Immunity, and Disease. Pharmacol. Rev. 2023, 75, 62–158. [Google Scholar] [CrossRef] [PubMed]
- Vayenas, C.G.; Bebelis, S.; Yentekakis, I.V.; Tsiakaras, P.; Karasali, H. Non-Faradaic Electrochemical Modification of Catalytic Activity: Reversible Promotion of Platinum Metals Catalysts. Platin. Met. Rev. 1990, 34, 122–130. [Google Scholar] [CrossRef]
- Liu, D.; Barbar, A.; Najam, T.; Javed, M.S.; Shen, J.; Tsiakaras, P.; Cai, X. Single noble metal atoms doped 2D materials for catalysis. Appl. Catal. B Environ. 2021, 297, 120389. [Google Scholar] [CrossRef]
- Volkov, A.; Gorbova, E.; Vylkov, A.; Medvedev, D.; Demin, A.; Tsiakaras, P. Design and applications of potentiometric sensors based on proton-conducting ceramic materials. A brief review. Sens. Actuators B Chem. 2017, 244, 1004–1015. [Google Scholar] [CrossRef]
- Gorbova, E.; Balkourani, G.; Molochas, C.; Sidiropoulos, D.; Brouzgou, A.; Demin, A.; Tsiakaras, P. Brief Review on High-Temperature Electrochemical Hydrogen Sensors. Catalysts 2022, 12, 1647. [Google Scholar] [CrossRef]
- Kalyakin, A.; Demin, A.K.; Gorbova, E.; Volkov, A.; Tsiakaras, P.E. Sensor Based on a Solid Oxide Electrolyte for Measuring the Water-Vapor and Hydrogen Content in Air. Catalysts 2022, 12, 1558. [Google Scholar] [CrossRef]
- Brouzgou, A.; Tsiakaras, P. Electrocatalysts for Glucose Electrooxidation Reaction: A Review. Top. Catal. 2015, 58, 1311–1327. [Google Scholar] [CrossRef]
- Brouzgou, A.; Gorbova, E.; Wang, Y.; Jing, S.; Seretis, A.; Liang, Z.; Tsiakaras, P. Nitrogen-doped 3D hierarchical ordered mesoporous carbon supported palladium electrocatalyst for the simultaneous detection of ascorbic acid, dopamine, and glucose. Ionics 2019, 25, 6061–6070. [Google Scholar] [CrossRef]
- Brouzgou, A.; Lo Vecchio, C.; Baglio, V.; Aricò, A.S.; Liang, Z.X.; Demin, A.; Tsiakaras, P. Glucose electrooxidation reaction in presence of dopamine and uric acid over ketjenblack carbon supported PdCo electrocatalyst. J. Electroanal. Chem. 2019, 855, 113610. [Google Scholar] [CrossRef]
- Alvarez, R.; Muñoz-Piña, S.; González, M.U.; Izquierdo-Barba, I.; Fernández-Martínez, I.; Rico, V.; Arcos, D.; García-Valenzuela, A.; Palmero, A.; Vallet-Regi, M. Antibacterial nanostructured Ti coatings by magnetron sputtering: From laboratory scales to industrial reactors. Nanomaterials 2019, 9, 1217. [Google Scholar] [CrossRef]
- Sajjan, V.A.; Mohammed, I.; Nemakal, M.; Aralekallu, S.; Hemantha Kumar, K.R.; Swamy, S.; Sannegowda, L.K. Synthesis and electropolymerization of cobalt tetraaminebenzamidephthalocyanine macrocycle for the amperometric sensing of dopamine. J. Electroanal. Chem. 2019, 838, 33–40. [Google Scholar] [CrossRef]
- Brouzgou, A. Graphene-based Nanomaterials as Organocatalyst. In Graphene-Based Nanomaterial Catalysis; Singh, M., Rai, V.K., Rai, A., Eds.; Bentham Science Publishers Pte.: Singapore, 2022; Chapter 2; pp. 24–42. [Google Scholar]
- Melníková-Komínková, Z.; Valeš, V.; Frank, O.; Kalbáč, M. Evolution of the Raman 2D’ mode in monolayer graphene during electrochemical doping. Microchem. J. 2022, 181, 107739. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Gu, Y.T. Mechanical properties of graphene: Effects of layer number, temperature and isotope. Comput. Mater. Sci. 2013, 71, 197–200. [Google Scholar] [CrossRef]
- Song, H.; Zhang, X.; Liu, Y.; Su, Z. Developing Graphene-Based Nanohybrids for Electrochemical Sensing. Chem. Rec. 2019, 19, 534–549. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, L.; Wang, B.; Ruoff, R.S. Chemical vapor deposition of graphene on thin-metal films. Cell Rep. Phys. Sci. 2021, 2, 100372. [Google Scholar] [CrossRef]
- Kuntoji, G.; Kousar, N.; Gaddimath, S.; Koodlur Sannegowda, L. Macromolecule–Nanoparticle-Based Hybrid Materials for Biosensor Applications. Biosensors 2024, 14, 277. [Google Scholar] [CrossRef]
- Khoshroo, A.; Sadrjavadi, K.; Taran, M.; Fattahi, A. Electrochemical system designed on a copper tape platform as a nonenzymatic glucose sensor. Sens. Actuators B Chem. 2020, 325, 128778. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Cui, Y.-W.; Zhang, L.-C. Recent development in beta titanium alloys for biomedical applications. Metals 2020, 10, 1139. [Google Scholar] [CrossRef]
- Sarraf, M.; Rezvani Ghomi, E.; Alipour, S.; Ramakrishna, S.; Liana Sukiman, N. A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical applications. Bio-Des. Manuf. 2022, 5, 371–395. [Google Scholar] [CrossRef]
- Bertel, L.; Miranda, D.A.; García-Martín, J.M. Nanostructured titanium dioxide surfaces for electrochemical biosensing. Sensors 2021, 21, 6167. [Google Scholar] [CrossRef]
- Pandey, L.M. Design of biocompatible and self-antibacterial titanium surfaces for biomedical applications. Curr. Opin. Biomed. Eng. 2023, 25, 100423. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Long, Y. Molten salt-assisted synthesis of C-TiN nanocomposites for sensitive dopamine determination. Mater. Lett. 2022, 329, 133234. [Google Scholar] [CrossRef]
- Feng, J.; Li, Q.; Cai, J.; Yang, T.; Chen, J.; Hou, X. Electrochemical detection mechanism of dopamine and uric acid on titanium nitride-reduced graphene oxide composite with and without ascorbic acid. Sens. Actuators B Chem. 2019, 298, 126872. [Google Scholar] [CrossRef]
- Paul, J.; Kim, J. Reticular synthesis of a conductive composite derived from metal-organic framework and Mxene for the electrochemical detection of dopamine. Appl. Surf. Sci. 2023, 613, 156103. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Spiro, R.C.; Jain, H.; Falk, M.M. Effects of titanium implant surface topology on bone cell attachment and proliferation in vitro. Med. Devices Evid. Res. 2022, 15, 103–119. [Google Scholar] [CrossRef]
- Zhang, J.; Siddiqui, M.K.; Rauf, A.; Ishtiaq, M. On ve-degree and ev-degree based topological properties of single walled titanium dioxide nanotube. J. Clust. Sci. 2021, 32, 821–832. [Google Scholar] [CrossRef]
- Alvarez, R.; Garcia-Martin, J.M.; Garcia-Valenzuela, A.; Macias-Montero, M.; Ferrer, F.J.; Santiso, J.; Rico, V.; Cotrino, J.; Gonzalez-Elipe, A.R.; Palmero, A. Nanostructured Ti thin films by magnetron sputtering at oblique angles. J. Phys. D Appl. Phys. 2016, 49, 045303. [Google Scholar] [CrossRef]
- Mobini, S.; González, M.U.; Caballero-Calero, O.; Patrick, E.E.; Martín-González, M.; García-Martín, J.M. Effects of nanostructuration on the electrochemical performance of metallic bioelectrodes. Nanoscale 2022, 14, 3179–3190. [Google Scholar] [CrossRef]
- Rocha, J.F.; Hasimoto, L.H.; Santhiago, M. Recent progress and future perspectives of polydopamine nanofilms toward functional electrochemical sensors. Anal. Bioanal. Chem. 2023, 415, 3799–3816. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; García-Martín, J.M.; Álvarez, R.; Palmero, A.; Esteban, J.; Pérez-Jorge, C.; Arcos, D.; Vallet-Regí, M. Nanocolumnar coatings with selective behavior towards osteoblast and Staphylococcus aureus proliferation. Acta Biomater. 2015, 15, 20–28. [Google Scholar] [CrossRef]
- Peláez-Abellán, E.; Rocha-Sousa, L.; Müller, W.-D.; Guastaldi, A.C. Electrochemical stability of anodic titanium oxide films grown at potentials higher than 3 V in a simulated physiological solution. Corros. Sci. 2007, 49, 1645–1655. [Google Scholar] [CrossRef]
- Azumi, K.; Seo, M. Changes in electrochemical properties of the anodic oxide film formed on titanium during potential sweep. Corros. Sci. 2001, 43, 533–546. [Google Scholar] [CrossRef]
- Muguruma, H.; Inoue, Y.; Inoue, H.; Ohsawa, T. Electrochemical study of dopamine at electrode fabricated by cellulose-assisted aqueous dispersion of long-length carbon nanotube. J. Phys. Chem. C 2016, 120, 12284–12292. [Google Scholar] [CrossRef]
- Biswas, B.; Singh, P.C. Protonation State of Dopamine Neurotransmitter at the Aqueous Interface: Vibrational Sum Frequency Generation Spectroscopy Study. Langmuir 2022, 38, 1380–1385. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Zhu, Z. A novel electrochemical sensor based on carbon nanotubes array for selective detection of dopamine or uric acid. Talanta 2019, 201, 295–300. [Google Scholar] [CrossRef]
- Balkourani, G.; Brouzgou, A.; Tsiakaras, P. A review on recent advancements in electrochemical detection of dopamine using carbonaceous nanomaterials. Carbon 2023, 213, 118281. [Google Scholar] [CrossRef]
- Schindler, S.; Bechtold, T. Mechanistic insights into the electrochemical oxidation of dopamine by cyclic voltammetry. J. Electroanal. Chem. 2019, 836, 94–101. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Ivanov, I.N.; Nguyen, M.D.; Zestos, A.G.; Venton, B.J. High temporal resolution measurements of dopamine with carbon nanotube yarn microelectrodes. Anal. Chem. 2014, 86, 5721–5727. [Google Scholar] [CrossRef] [PubMed]
- Nazari, Z.; Hadi Nematollahi, M.; Zareh, F.; Pouramiri, B.; Mehrabani, M. An electrochemical sensor based on carbon quantum dots and ionic liquids for selective detection of dopamine. ChemistrySelect 2023, 8, e202203630. [Google Scholar] [CrossRef]
- Selvolini, G.; Lazzarini, C.; Marrazza, G. Electrochemical nanocomposite single-use sensor for dopamine detection. Sensors 2019, 19, 3097. [Google Scholar] [CrossRef] [PubMed]
- Neupane, M.P.; Park, I.S.; Lee, S.J.; Kim, K.A.; Lee, M.H.; Bae, T.S. Study of Anodic Oxide Films of Titanium Fabricated by Voltammetric Technique in Phosphate Buffer Media. Int. J. Electrochem. Sci. 2009, 4, 197–207. [Google Scholar] [CrossRef]
- Wan, M.; Jimu, A.; Yang, H.; Zhou, J.; Dai, X.; Zheng, Y.; Ou, J.; Yang, Y.; Liu, J.; Wang, L. MXene quantum dots enhanced 3D-printed electrochemical sensor for the highly sensitive detection of dopamine. Microchem. J. 2023, 184, 108180. [Google Scholar] [CrossRef]
- Sriram, B.; Kogularasu, S.; Wang, S.-F.; Sheu, J.-K. Deep eutectic solvent-mediated synthesis of spinel zinc chromite nanoparticles: A simple label-free electrochemical sensor for dopamine and ascorbic acid. ACS Appl. Nano Mater. 2023, 6, 17593–17602. [Google Scholar] [CrossRef]
- Ahmed, J.; Faisal, M.; Alsareii, S.; Jalalah, M.; Harraz, F.A. A novel gold-decorated porous silicon-poly (3-hexylthiophene) ternary nanocomposite as a highly sensitive and selective non-enzymatic dopamine electrochemical sensor. J. Alloys Compd. 2023, 931, 167403. [Google Scholar] [CrossRef]
- Niu, B.; Liu, M.; Li, X.; Guo, H.; Chen, Z. Vein-like Ni-BTC@ Ni3S4 with sulfur vacancy and Ni3+ fabricated in situ etching vulcanization strategy for an electrochemical sensor of dopamine. ACS Appl. Mater. Interfaces 2023, 15, 13319–13331. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, J.; Li, J.; Li, Y.; Yang, P.; Zhao, P.; Fei, J.; Xie, Y. A novel dopamine electrochemical sensor based on 3D flake nickel oxide/cobalt oxide@ porous carbon nanosheets/carbon nanotubes/electrochemical reduced of graphene oxide composites modified glassy carbon electrode. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131284. [Google Scholar] [CrossRef]
- Saha, S.; Sarkar, P.; Turner, A.P.F. Interference-Free Electrochemical Detection of Nanomolar Dopamine Using Doped Polypyrrole and Silver Nanoparticles. Electroanalysis. 2014, 26, 2197–2206. [Google Scholar] [CrossRef]
- Golrokh Amin, B.; De Silva, U.; Masud, J.; Nath, M. Ultrasensitive and Highly Selective Ni3Te2 as a Nonenzymatic Glucose Sensor at Extremely Low Working Potential. ACS Omega 2019, 4, 11152–11162. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency, S.M.H., ICH Q2(R2) Guideline on Validation of Analytical Procedures. 2023. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q2r2-guideline-validation-analytical-procedures-step-5-revision-1_en.pdf (accessed on 30 June 2024).
- Zhao, D.; Yu, G.; Tian, K.; Xu, C. A highly sensitive and stable electrochemical sensor for simultaneous detection towards ascorbic acid, dopamine, and uric acid based on the hierarchical nanoporous PtTi alloy. Biosens. Bioelectron. 2016, 82, 119–126. [Google Scholar] [CrossRef]
- Zou, M.-Y.; Nie, S.-P.; Yin, J.-Y.; Xie, M.-Y. Ascorbic acid induced degradation of polysaccharide from natural products: A review. Int. J. Biol. Macromol. 2020, 151, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Josephine, D.S.R.; Babu, K.J.; Gnana Kumar, G.P.; Sethuraman, K. Titanium dioxide anchored graphene oxide nanosheets for highly selective voltammetric sensing of dopamine. Microchim. Acta 2017, 184, 781–790. [Google Scholar] [CrossRef]
- Babaei, A.; Taheri, A.R. Nafion/Ni(OH)2 nanoparticles-carbon nanotube composite modified glassy carbon electrode as a sensor for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid. Sens. Actuators B Chem. 2013, 176, 543–551. [Google Scholar] [CrossRef]
- Mani, V.; Govindasamy, M.; Chen, S.-M.; Karthik, R.; Huang, S.-T. Determination of dopamine using a glassy carbon electrode modified with a graphene and carbon nanotube hybrid decorated with molybdenum disulfide flowers. Microchim. Acta 2016, 183, 2267–2275. [Google Scholar] [CrossRef]
- Amir, H.; Ponpandian, N.; Viswanathan, C. An electrochemical dopamine sensor based on RF magnetron sputtered TiO2/SS thin film electrode. Mater. Lett. 2021, 300, 130175. [Google Scholar] [CrossRef]
- Po, H.N.; Senozan, N.M. The Henderson-Hasselbalch Equation: Its History and Limitations. J. Chem. Educ. 2001, 78, 1499. [Google Scholar] [CrossRef]

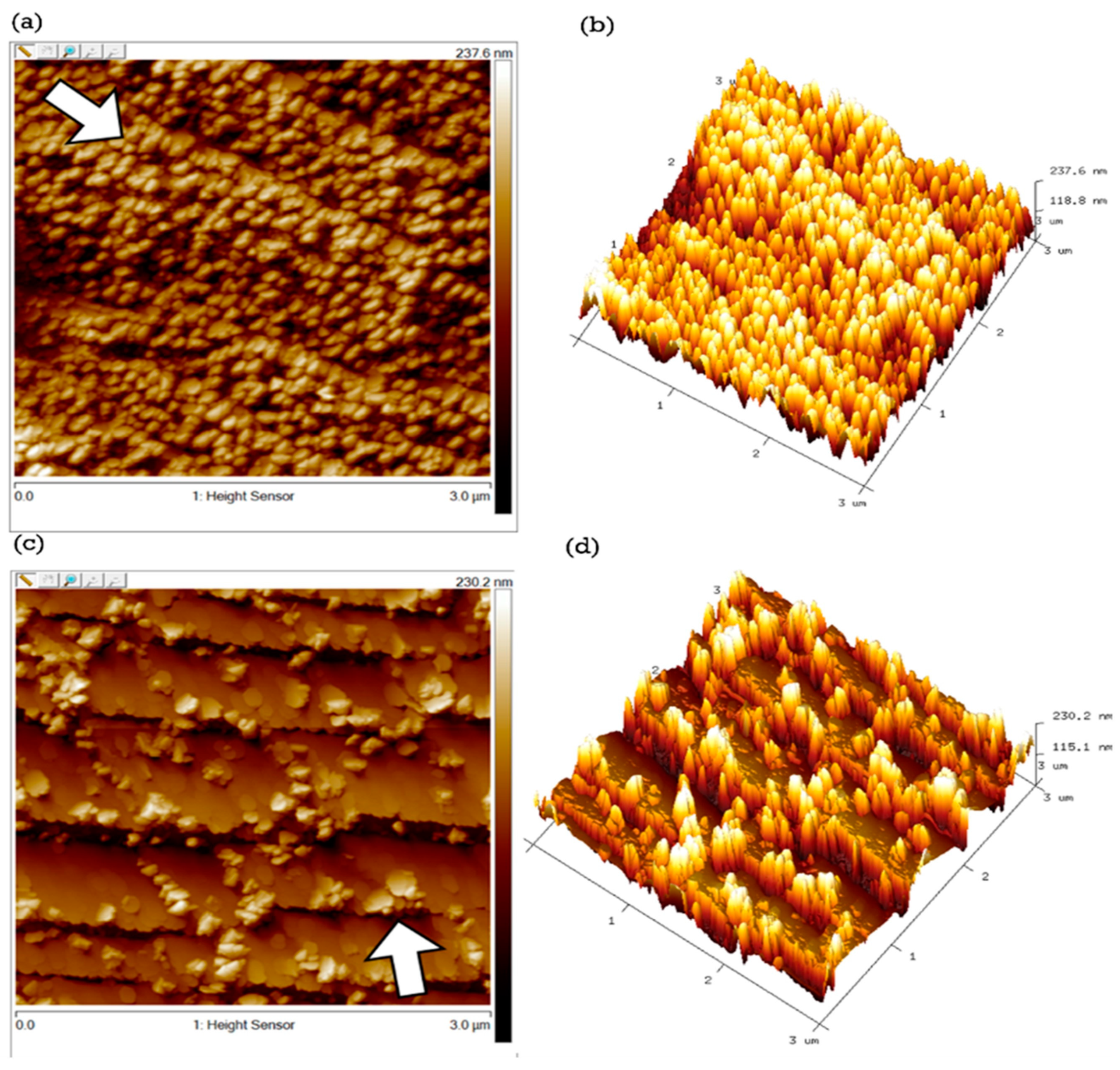



| Element | Weight % | Atomic % |
|---|---|---|
| C | 3.8 | 15.6 |
| N | 2.8 | 9.7 |
| O | 0.3 | 1.7 |
| Ti | 6.2 | 6.3 |
| Cu | 86.9 | 66.7 |
| Electrode | LOD (μM) | Linear Range (μM) | Sensitivity (μAμM−1cm−2) | Refs |
|---|---|---|---|---|
| TiO2/SS (stainless steel) | 0.023 | 50.0–450.0 | 0.0219 | [57] |
| C-TiN/GCE (glassy carbon electrode) | 0.03 | 0.1–5.0 and 5.0–250.0 | 9.6 | [23] |
| TiN-rGO/GCE | 0.159 | 5.0–175.0 | - | [24] |
| MOF-Ti3C2/GCE | 0.11 | 0.09–0.3 | - | [25] |
| TiO2 /GO/GCE | 0.027 | 0.2–10.0 | 1.55 | [54] |
| TiNCs /Gm-Cufoil | 6.63 | 10.0–90.0 and 100.0–400.0 | 0.14 and 0.095 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balkourani, G.; García-Martín, J.M.; Gorbova, E.; Lo Vecchio, C.; Baglio, V.; Brouzgou, A.; Tsiakaras, P. Electrochemical Detection of Dopamine: Novel Thin-Film Ti-Nanocolumnar Arrays/Graphene Monolayer-Cufoil Electrodes. Catalysts 2024, 14, 478. https://doi.org/10.3390/catal14080478
Balkourani G, García-Martín JM, Gorbova E, Lo Vecchio C, Baglio V, Brouzgou A, Tsiakaras P. Electrochemical Detection of Dopamine: Novel Thin-Film Ti-Nanocolumnar Arrays/Graphene Monolayer-Cufoil Electrodes. Catalysts. 2024; 14(8):478. https://doi.org/10.3390/catal14080478
Chicago/Turabian StyleBalkourani, Georgia, José Miguel García-Martín, Elena Gorbova, Carmelo Lo Vecchio, Vincenzo Baglio, Angeliki Brouzgou, and Panagiotis Tsiakaras. 2024. "Electrochemical Detection of Dopamine: Novel Thin-Film Ti-Nanocolumnar Arrays/Graphene Monolayer-Cufoil Electrodes" Catalysts 14, no. 8: 478. https://doi.org/10.3390/catal14080478
APA StyleBalkourani, G., García-Martín, J. M., Gorbova, E., Lo Vecchio, C., Baglio, V., Brouzgou, A., & Tsiakaras, P. (2024). Electrochemical Detection of Dopamine: Novel Thin-Film Ti-Nanocolumnar Arrays/Graphene Monolayer-Cufoil Electrodes. Catalysts, 14(8), 478. https://doi.org/10.3390/catal14080478











