Improved Microwave-Assisted Ethyl Levulinate Production Using Rice Husk-Derived Biobased Mesoporous Silica as Catalyst
Abstract
1. Introduction
2. Results and Discussion
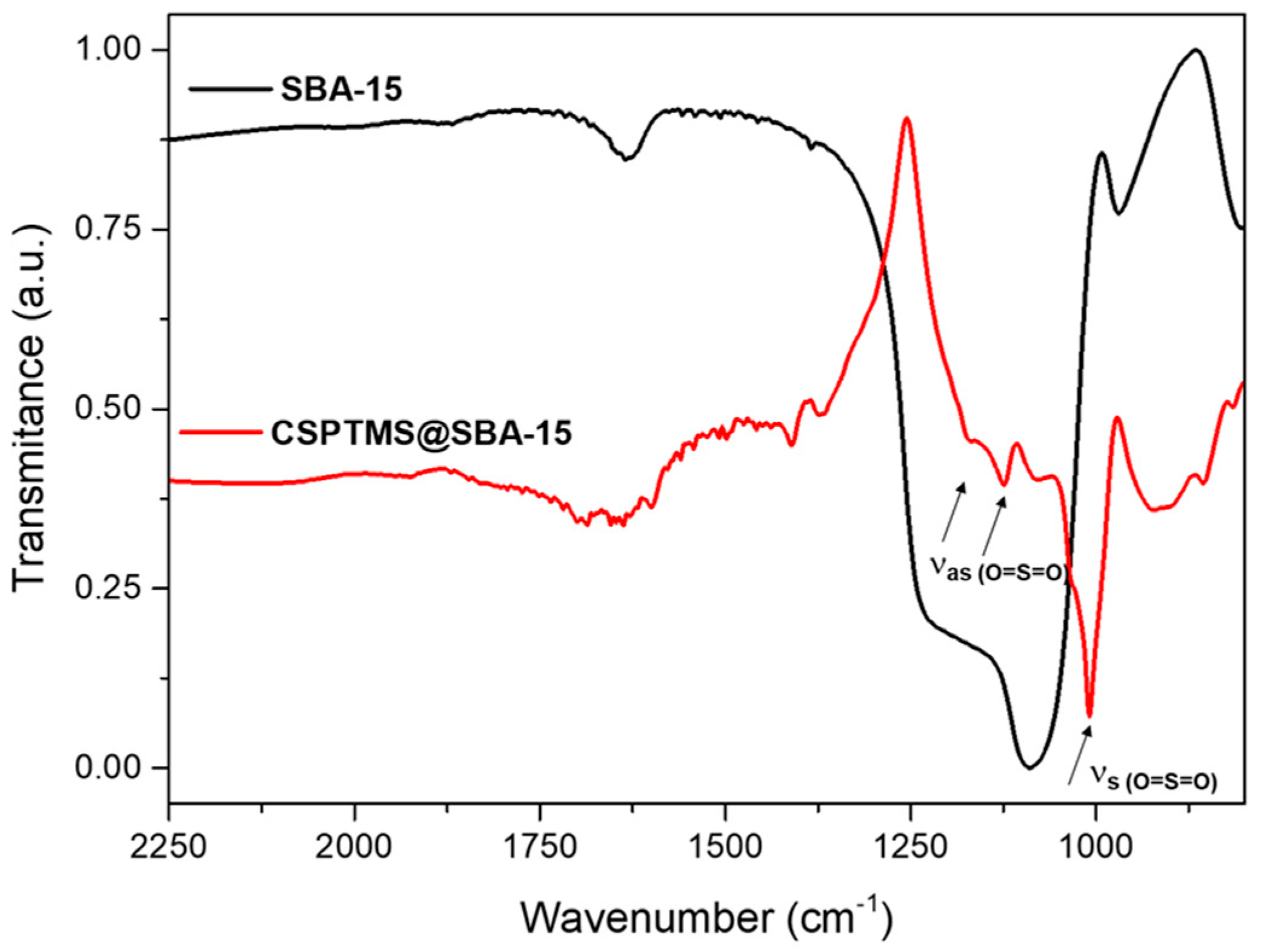
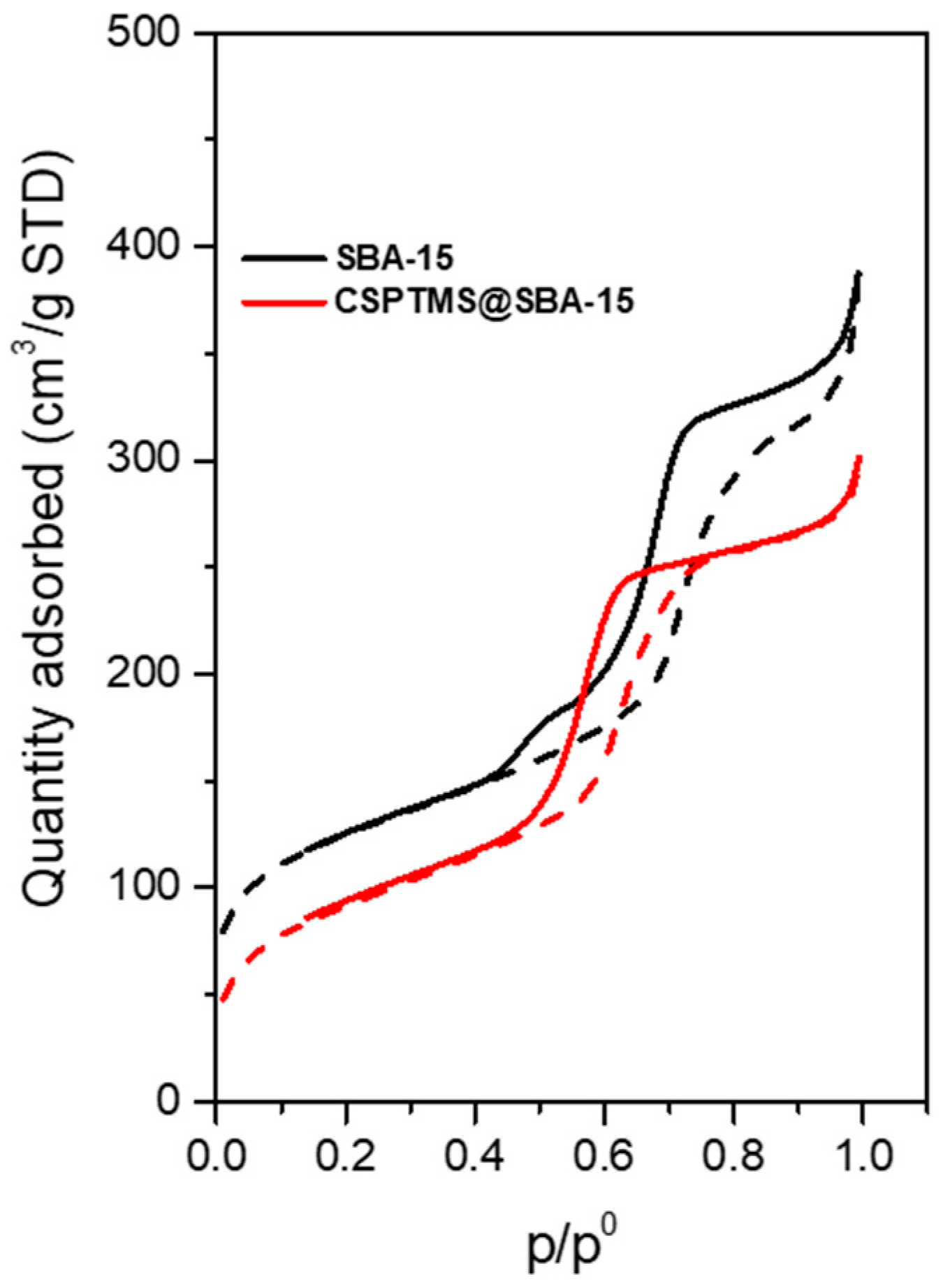
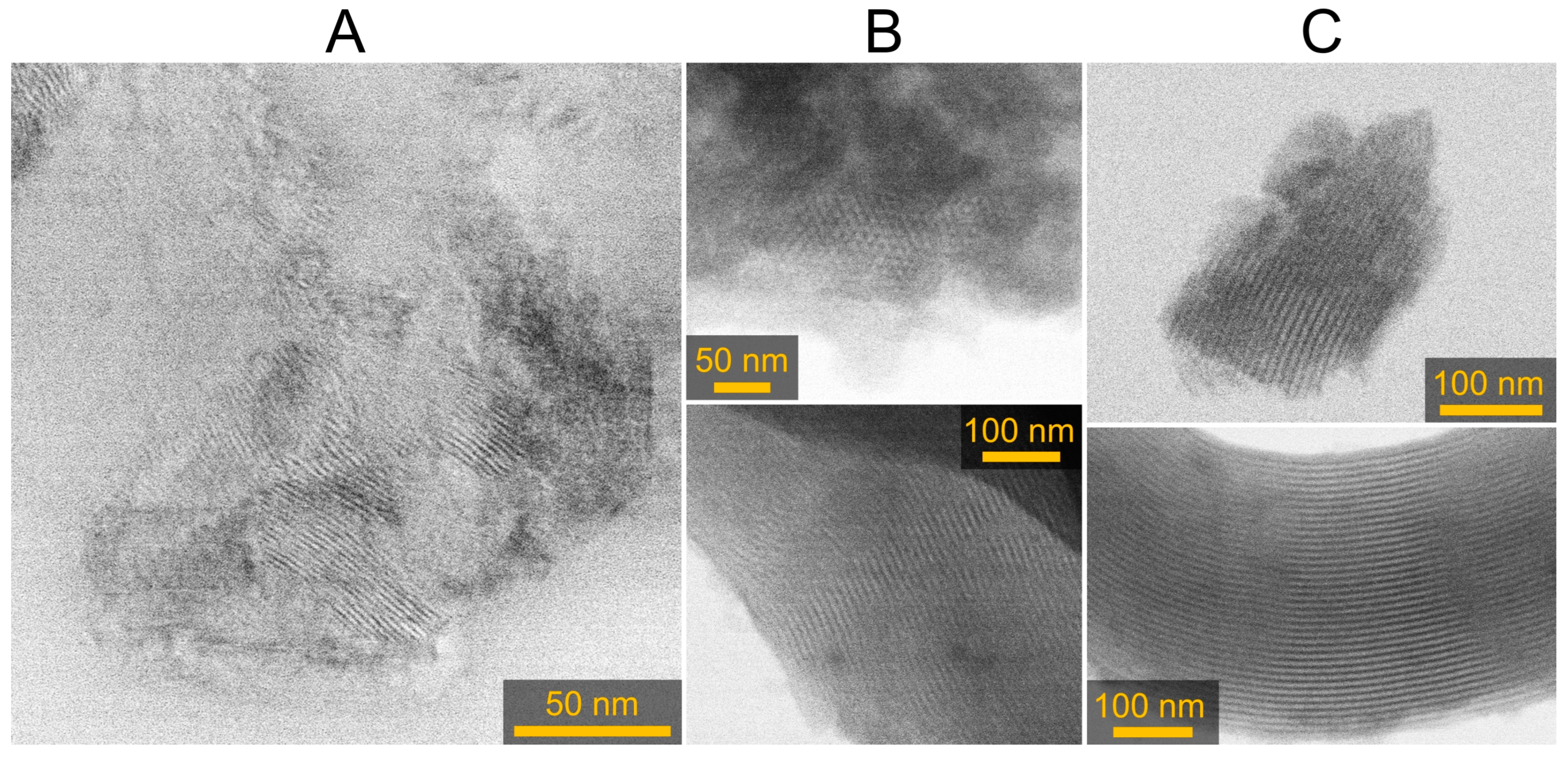
2.1. Material Characterization
2.2. Catalytic Results
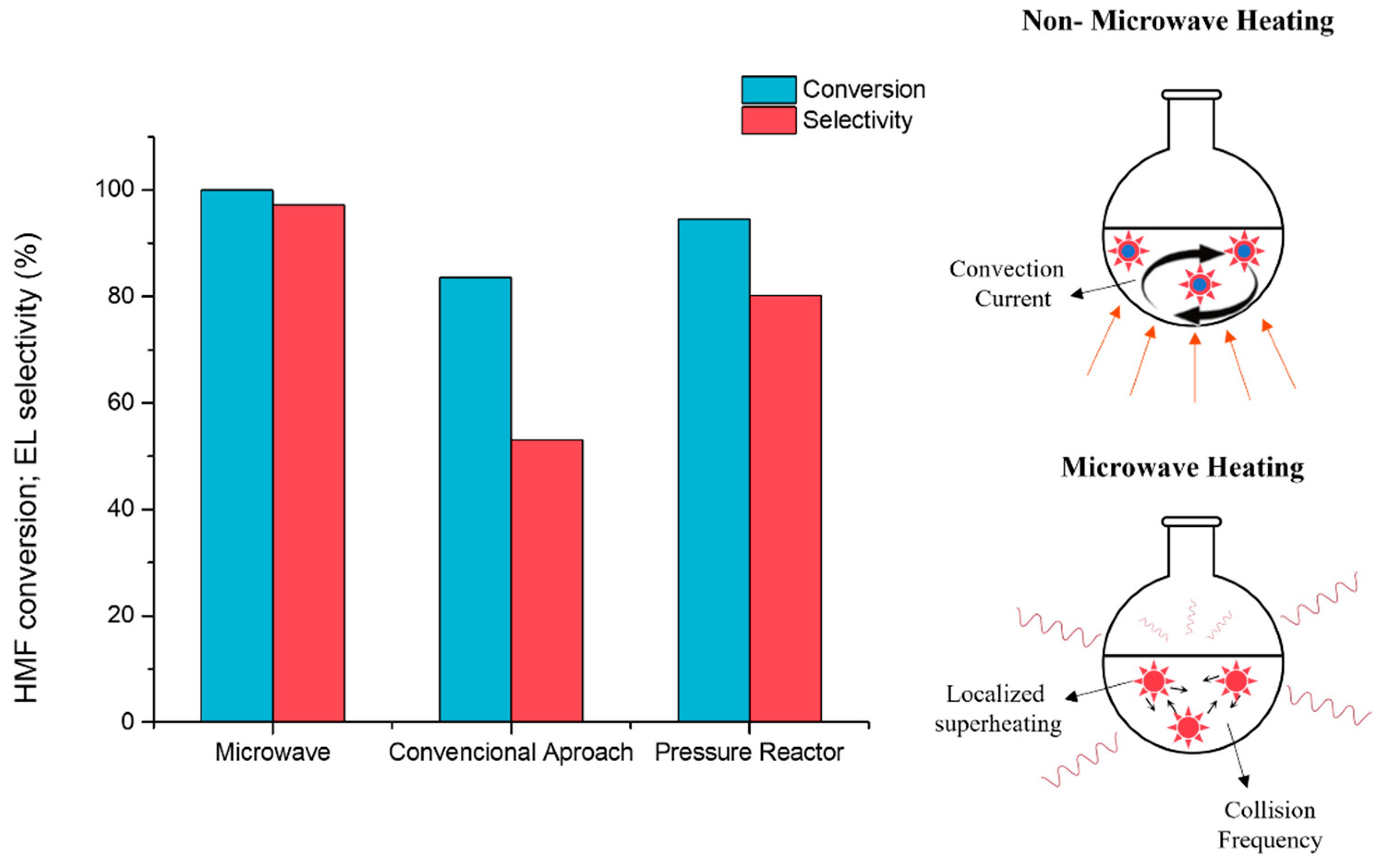
2.2.1. Microwave Irradiation vs. Non-MW Irradiation
2.2.2. Effect of Temperature
2.2.3. Effect of Reaction Time
2.2.4. Effect of the Solvent
2.2.5. Recyclability of the Catalyst
3. Experimental
3.1. Materials, Reagents, and Methods
3.2. Synthesis of SBA-15 Materials
3.3. SBA-15 Functionalization
3.4. Catalytic Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Appaturi, J.N.; Andas, J.; Ma, Y.K.; Phoon, B.L.; Batagarawa, S.M.; Khoerunnisa, F.; Hussin, M.H.; Ng, E.P. Recent Advances in Heterogeneous Catalysts for the Synthesis of Alkyl Levulinate Biofuel Additives from Renewable Levulinic Acid: A Comprehensive Review. Fuel 2022, 323, 124362. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Badgujar, V.C.; Bhanage, B.M. A Review on Catalytic Synthesis of Energy Rich Fuel Additive Levulinate Compounds from Biomass Derived Levulinic Acid. Fuel Process. Technol. 2020, 197, 106213. [Google Scholar] [CrossRef]
- Démolis, A.; Essayem, N.; Rataboul, F. Synthesis and Applications of Alkyl Levulinates. ACS Sustain. Chem. Eng. 2014, 2, 1338–1352. [Google Scholar] [CrossRef]
- Ahmad, E.; Alam, M.I.; Pant, K.K.; Haider, M.A. Catalytic and Mechanistic Insights into the Production of Ethyl Levulinate from Biorenewable Feedstocks. Green Chem. 2016, 18, 4804–4823. [Google Scholar] [CrossRef]
- Hassan, A.H.; Zainol, M.M.; Samion, M.A.; Azlan, M.A.; Asmadi, M.; Mohamad Daud, A.R.; Saad, I.; Mohd Nor Azman, N.A.N. Synthesis of Ethyl Levulinate over Sulfonated Lignin-Based Carbon Catalyst as a Fuel Additive to Biodiesel-Diesel Blends towards Engine Emissions. J. Clean Prod. 2023, 418, 138101. [Google Scholar] [CrossRef]
- Pasquale, G.; Vázquez, P.; Romanelli, G.; Baronetti, G. Catalytic Upgrading of Levulinic Acid to Ethyl Levulinate Using Reusable Silica-Included Wells-Dawson Heteropolyacid as Catalyst. Catal. Commun. 2012, 18, 115–120. [Google Scholar] [CrossRef]
- Vinod, N.; Dutta, S. Production of Alkyl Levulinates from Carbohydrate-Derived Chemical Intermediates Using Phosphotungstic Acid Supported on Humin-Derived Activated Carbon (PTA/HAC) as a Recyclable Heterogeneous Acid Catalyst. Chemistry 2023, 5, 800–812. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Niphadkar, P.S.; Deshpande, S.S.; Bokade, V.V. Esterification of Renewable Levulinic Acid to Ethyl Levulinate Biodiesel Catalyzed by Highly Active and Reusable Desilicated H-ZSM-5. J. Chem. Technol. Biotechnol. 2014, 89, 1507–1515. [Google Scholar] [CrossRef]
- Yadav, G.D.; Nair, J.J. Sulfated Zirconia and Its Modified Versions as Promising Catalysts for Industrial Processes. Microporous Mesoporous Mater. 1999, 33, 1–48. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Corma, A.; Llabrés i Xamena, F.X. Conversion of Levulinic Acid into Chemicals: Synthesis of Biomass Derived Levulinate Esters over Zr-Containing MOFs. Chem. Eng. Sci. 2015, 124, 52–60. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z.; Wang, R. Functionalized Metal-Organic Framework Catalysts for Sustainable Biomass Valorization. Adv. Polym. Technol. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Melero, J.A.; Morales, G.; Iglesias, J.; Paniagua, M.; Hernández, B.; Penedo, S. Efficient Conversion of Levulinic Acid into Alkyl Levulinates Catalyzed by Sulfonic Mesostructured Silicas. Appl. Catal. A Gen. 2013, 466, 116–122. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, L.; Xu, Z.; Wang, L. Direct Production of Ethyl Levulinate from Carbohydrates and Biomass Waste Catalyzed by Modified Porous Silica with Multiple Acid Sites. Process Biochem. 2022, 121, 152–162. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Badgujar, V.C.; Bhanage, B.M. Synthesis of Alkyl Levulinate as Fuel Blending Agent by Catalytic Valorization of Carbohydrates via Alcoholysis: Recent Advances and Challenges. Catal. Today 2023, 408, 9–21. [Google Scholar] [CrossRef]
- Hu, X.; Lievens, C.; Larcher, A.; Li, C.Z. Reaction Pathways of Glucose during Esterification: Effects of Reaction Parameters on the Formation of Humin Type Polymers. Bioresour. Technol. 2011, 102, 10104–10113. [Google Scholar] [CrossRef]
- Jia, S.; Wang, M.; Ma, J.; Liu, X.; Zhang, Y.; Xu, Z. Metal Chloride Mediated Efficient Conversion of Hydroxymethylfurfural (HMF) into Long-Chain Levulinate Ester. Bioresources 2021, 17, 849–861. [Google Scholar] [CrossRef]
- Singh, B. Rice Husk Ash. In Waste and Supplementary Cementitious Materials in Concrete: Characterisation, Properties and Applications; Woodhead Publishing: Sawston, UK, 2018; pp. 417–460. ISBN 9780081021569. [Google Scholar]
- Bhagiyalakshmi, M.; Yun, L.J.; Anuradha, R.; Jang, H.T. Utilization of Rice Husk Ash as Silica Source for the Synthesis of Mesoporous Silicas and Their Application to CO2 Adsorption through TREN/TEPA Grafting. J. Hazard. Mater. 2010, 175, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.J.; Fernandes, F.A.N.; Rodrigues, M.G.F. Study of Co/SBA-15 Catalysts Prepared by Microwave and Conventional Heating Methods and Application in Fischer–Tropsch Synthesis. Appl. Catal. A Gen. 2013, 468, 32–37. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Deon, M.; Benvenutti, E.V.; Barros, V.A.; de Melo, D.C.; Franceschi, E.; Egues, S.M.; De Conto, J.F. Effect of Microwave Irradiation on the Structural, Chemical, and Hydrophilicity Characteristics of Ordered Mesoporous Silica SBA-15. J. Solgel. Sci. Technol. 2020, 94, 708–718. [Google Scholar] [CrossRef]
- Babaei, Z.; Najafi Chermahini, A.; Dinari, M. Synthesis of N-Butyl Levulinate as a Fuel Additive Using Bimetallic Zr/Al Catalysts Supported on Mesoporous Silica: Applying Experimental Design to Optimize the Reaction Conditions. Colloids. Surf. A Physicochem. Eng. Asp. 2021, 625, 126885. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, G.; Yang, X.; Yin, H.; Fu, X.; Liao, J.; Tu, J.; Huang, X.; Qin, F.G.F.; Xu, Y. Effects of P-Toluenesulfonic Acid in the Conversion of Glucose for Levulinic Acid and Sulfonated Carbon Production. Energy Fuels 2017, 31, 2847–2854. [Google Scholar] [CrossRef]
- Usai, E.M.; Sini, M.F.; Meloni, D.; Solinas, V.; Salis, A. Sulfonic Acid-Functionalized Mesoporous Silicas: Microcalorimetric Characterization and Catalytic Performance toward Biodiesel Synthesis. Microporous Mesoporous Mater. 2013, 179, 54–62. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, S.; Chan, J.C.C.; Chao, J.C.H. Template-Free Synthesis of Mesoporous Phenylsulfonic Acid Functionalized Silica. Microporous Mesoporous Mater. 2006, 96, 321–330. [Google Scholar] [CrossRef]
- Balula, S.S.; Cunha-Silva, L.; Santos, I.C.M.S.; Estrada, A.C.; Fernandes, A.C.; Cavaleiro, J.A.S.; Pires, J.; Freire, C.; Cavaleiro, A.M.V. Mono-Substituted Silicotungstates as Active Catalysts for Sustainable Oxidations: Homo- and Heterogeneous Performance. New J. Chem. 2013, 37, 2341–2350. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, R.; Yue, B.; Gu, M.; Pei, S.; He, H. Synthesis, Characterization and Catalytic Application of SBA-15 Immobilized Rare Earth Metal Sandwiched Polyoxometalates. J. Mol. Catal. A Chem. 2007, 270, 50–55. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Nogueira, L.S.; Gago, S.; Almeida, P.L.; Corvo, M.C.; de Castro, B.; Granadeiro, C.M.; Balula, S.S. Desulfurization Process Conciliating Heterogeneous Oxidation and Liquid Extraction: Organic Solvent or Centrifugation/Water? Appl. Catal. A Gen. 2017, 542, 359–367. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the Porous Structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Konwar, L.J.; Mäki-Arvela, P.; Salminen, E.; Kumar, N.; Thakur, A.J.; Mikkola, J.P.; Deka, D. Towards Carbon Efficient Biorefining: Multifunctional Mesoporous Solid Acids Obtained from Biodiesel Production Wastes for Biomass Conversion. Appl. Catal. B Environ. 2015, 176, 20–35. [Google Scholar] [CrossRef]
- Peixoto, A.F.; Soliman, M.M.A.; Pinto, T.V.; Silva, S.M.; Costa, P.; Alegria, E.C.B.A.; Freire, C. Highly Active Organosulfonic Aryl-Silica Nanoparticles as Efficient Catalysts for Biomass Derived Biodiesel and Fuel Additives. Biomass Bioenergy 2021, 145, 105936. [Google Scholar] [CrossRef]
- Joseph, T.; Varghese, H.T.; Panicker, C.Y.; Viswanathan, K.; Dolezal, M.; Van Alsenoy, C. Spectroscopic (FT-IR, FT-Raman), First Order Hyperpolarizability, NBO Analysis, HOMO and LUMO Analysis of N-[(4-(Trifluoromethyl)Phenyl]Pyrazine-2-Carboxamide by Density Functional Methods. Arab. J. Chem. 2017, 10, S2281–S2294. [Google Scholar] [CrossRef]
- Luan, Z.; Fournier, J.A.; Wooten, J.B.; Miser, D.E. Preparation and Characterization of (3-Aminopropyl) Triethoxysilane-Modified Mesoporous SBA-15 Silica Molecular Sieves. Microporous Mesoporous Mater. 2005, 83, 150–158. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Lapkin, A.; Bozkaya, B.; Mays, T.; Borello, L.; Edler, K.; Crittenden, B. Preparation and Characterisation of Chemisorbents Based on Heteropolyacids Supported on Synthetic Mesoporous Carbons and Silica. Catal. Today 2003, 81, 611–621. [Google Scholar] [CrossRef]
- Hamoudi, S.; Royer, S.; Kaliaguine, S. Propyl- and Arene-Sulfonic Acid Functionalized Periodic Mesoporous Organosilicas. Microporous Mesoporous Mater. 2004, 71, 17–25. [Google Scholar] [CrossRef]
- Sow, B.; Hamoudi, S.; Hassan Zahedi-Niaki, M.; Kaliaguine, S. 1-Butanol Etherification over Sulfonated Mesostructured Silica and Organo-Silica. Microporous Mesoporous Mater. 2005, 79, 129–136. [Google Scholar] [CrossRef]
- Pizzolitto, C.; Ghedini, E.; Menegazzo, F.; Signoretto, M.; Giordana, A.; Cerrato, G.; Cruciani, G. Effect of Grafting Solvent in the Optimisation of Sba-15 Acidity for LevulinIc Acid Production. Catal. Today 2020, 345, 183–189. [Google Scholar] [CrossRef]
- Shan, J.; Wang, Q.; Hao, H.; Guo, H. Critical Review on the Synthesis of Levulinate Esters from Biomass-Based Feedstocks and Their Application. Ind. Eng. Chem. Res. 2023, 62, 17135–17147. [Google Scholar] [CrossRef]
- Quereshi, S.; Ahmad, E.; Pant, K.K.; Dutta, S. Insights into the Metal Salt Catalyzed Ethyl Levulinate Synthesis from Biorenewable Feedstocks. Catal. Today 2017, 291, 187–194. [Google Scholar] [CrossRef]
- Ge, X.; Li, H.; Liu, M.; Zhao, Z.; Jin, X.; Fan, X.; Gao, X. Microwave-Assisted Catalytic Alcoholysis of Fructose to Ethoxymethylfurfural (EMF) over Carbon-Based Microwave-Responsive Catalyst. Fuel Process. Technol. 2022, 233, 107305. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Q. Variations of Major Product Derived from Conversion of 5-Hydroxymethylfurfural over a Modified Mofs-Derived Carbon Material in Response to Reaction Conditions. Nanomaterials 2018, 8, 492. [Google Scholar] [CrossRef] [PubMed]
- Velasco Calderón, J.C.; Arora, J.S.; Mushrif, S.H. Mechanistic Investigation into the Formation of Humins in Acid-Catalyzed Biomass Reactions. ACS Omega 2022, 7, 44786–44795. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.F.; Ramos, R.; Moreira, M.M.; Soares, O.S.G.P.; Ribeiro, L.S.; Pereira, M.F.R.; Delerue-Matos, C.; Freire, C. Production of Ethyl Levulinate Fuel Bioadditive from 5-Hydroxymethylfurfural over Sulfonic Acid Functionalized Biochar Catalysts. Fuel 2021, 303, 121227. [Google Scholar] [CrossRef]
- Kamegawa, T.; Mizuno, A.; Yamashita, H. Hydrophobic Modification of SO3H-Functionalized Mesoporous Silica and Investigations on the Enhanced Catalytic Performance. Catal. Today 2015, 243, 153–157. [Google Scholar] [CrossRef]
- Pereira, J.R.; Corvo, M.C.; Peixoto, A.F.; Aguiar-Ricardo, A.; Marques, M.M.B. Sulfonic Acid-Functionalized (Bio)Materials as Catalysts for Efficient Amide Bond Synthesis. ChemCatChem 2023, 15, e202201318. [Google Scholar] [CrossRef]
- Aboelhassan, M.M.; Peixoto, A.F.; Freire, C. Sulfonic Acid Functionalized Silica Nanoparticles as Catalysts for the Esterification of Linoleic Acid. New J. Chem. 2017, 41, 3595–3605. [Google Scholar] [CrossRef]













| Catalyst | Textural Properties (a) | |
|---|---|---|
| SBET (m2/g) | Vp (cm3/g) | |
| SBA-15 MW 2 h | 355.66 | 0.206 |
| SBA-15 MW 4 h | 318.21 | 0.382 |
| SBA-15 MW 6 h | 449.05 | 0.571 |
| SBA-15 | 574.40 | 0.823 |
| Catalyst | Textural Properties (a) | |
|---|---|---|
| SBET (m2/g) | Vp (cm3/g) | |
| SBA-15 | 449.05 | 0.571 |
| SBA-15@CSPTMS | 334.63 | 0.421 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, S.O.; Marques, I.; Bamburov, A.; Yaremchenko, A.A.; Peixoto, A.F.; Leite, A. Improved Microwave-Assisted Ethyl Levulinate Production Using Rice Husk-Derived Biobased Mesoporous Silica as Catalyst. Catalysts 2024, 14, 482. https://doi.org/10.3390/catal14080482
Ribeiro SO, Marques I, Bamburov A, Yaremchenko AA, Peixoto AF, Leite A. Improved Microwave-Assisted Ethyl Levulinate Production Using Rice Husk-Derived Biobased Mesoporous Silica as Catalyst. Catalysts. 2024; 14(8):482. https://doi.org/10.3390/catal14080482
Chicago/Turabian StyleRibeiro, Susana O., Inês Marques, Aleksandr Bamburov, Aleksey A. Yaremchenko, Andreia F. Peixoto, and Andreia Leite. 2024. "Improved Microwave-Assisted Ethyl Levulinate Production Using Rice Husk-Derived Biobased Mesoporous Silica as Catalyst" Catalysts 14, no. 8: 482. https://doi.org/10.3390/catal14080482
APA StyleRibeiro, S. O., Marques, I., Bamburov, A., Yaremchenko, A. A., Peixoto, A. F., & Leite, A. (2024). Improved Microwave-Assisted Ethyl Levulinate Production Using Rice Husk-Derived Biobased Mesoporous Silica as Catalyst. Catalysts, 14(8), 482. https://doi.org/10.3390/catal14080482









