The Influence of Acetone on the Kinetics of Water Electrolysis Examined at Polycrystalline Pt Electrode in Alkaline Solution
Abstract
:1. Introduction
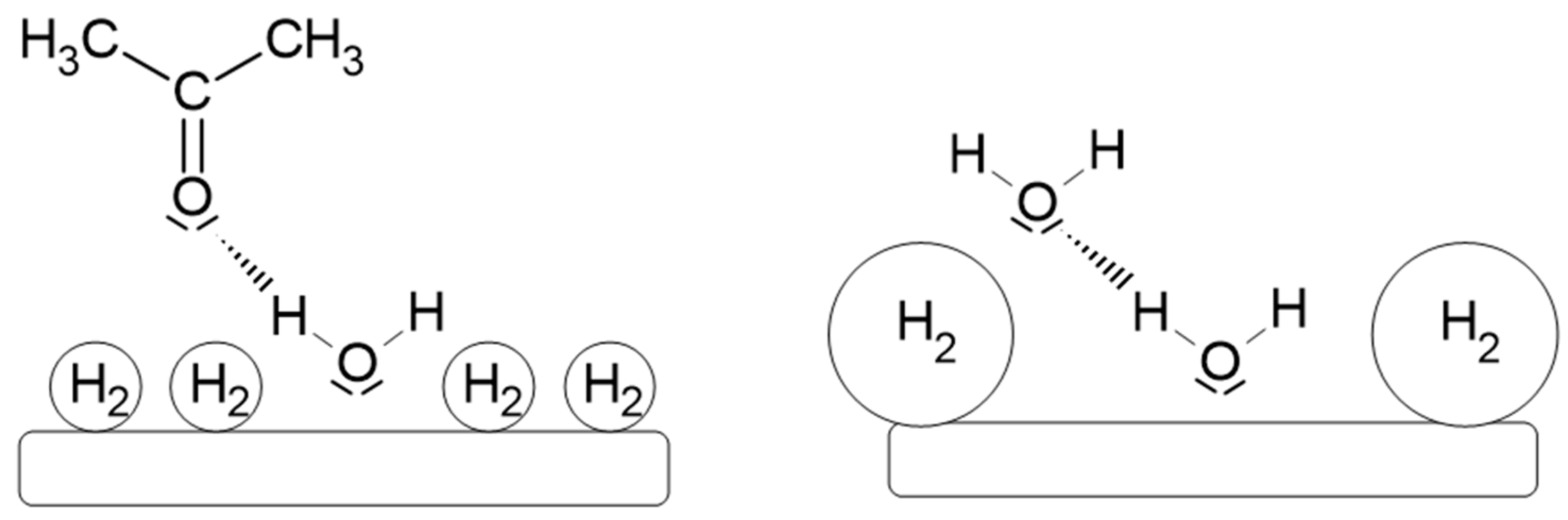
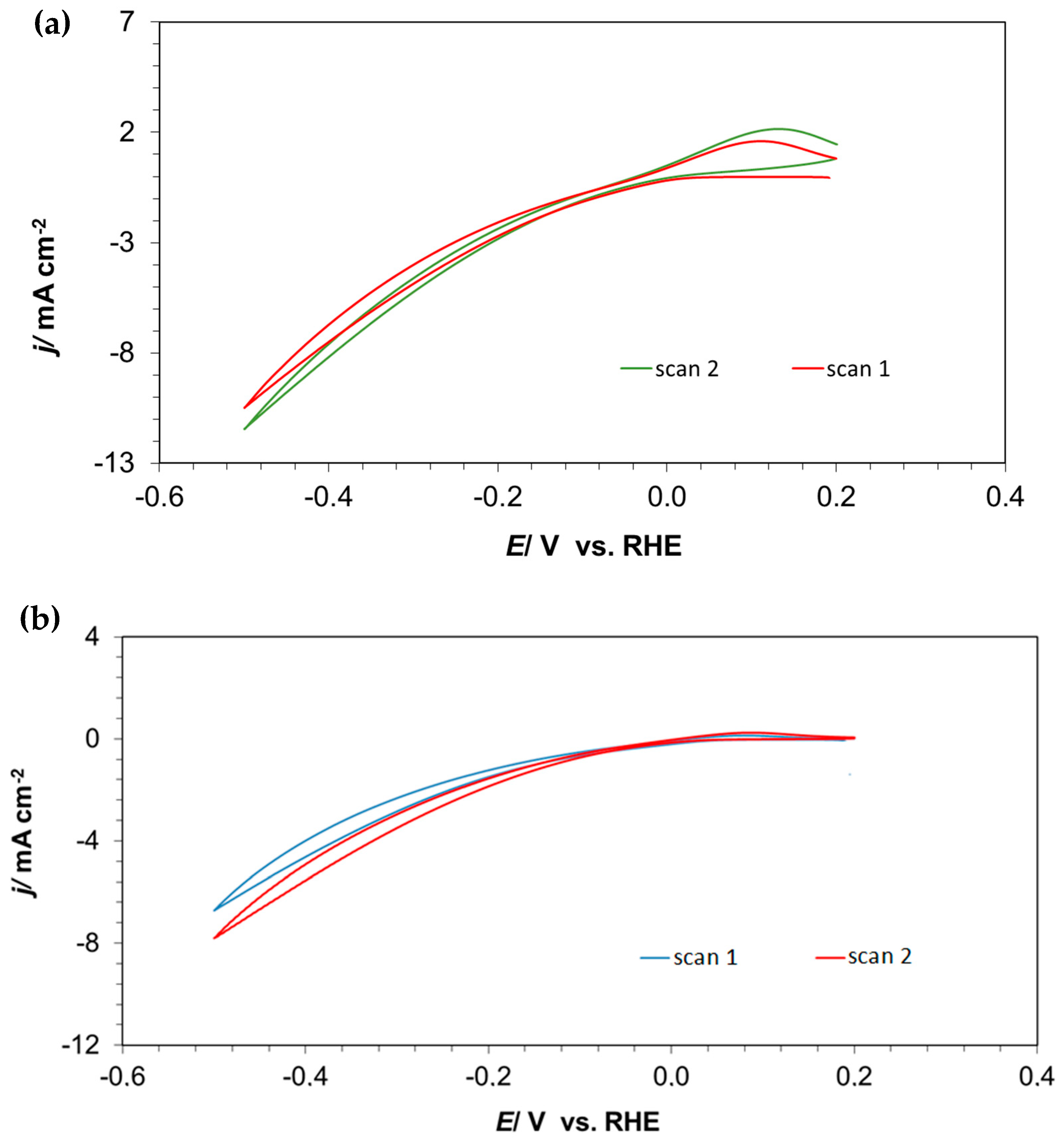
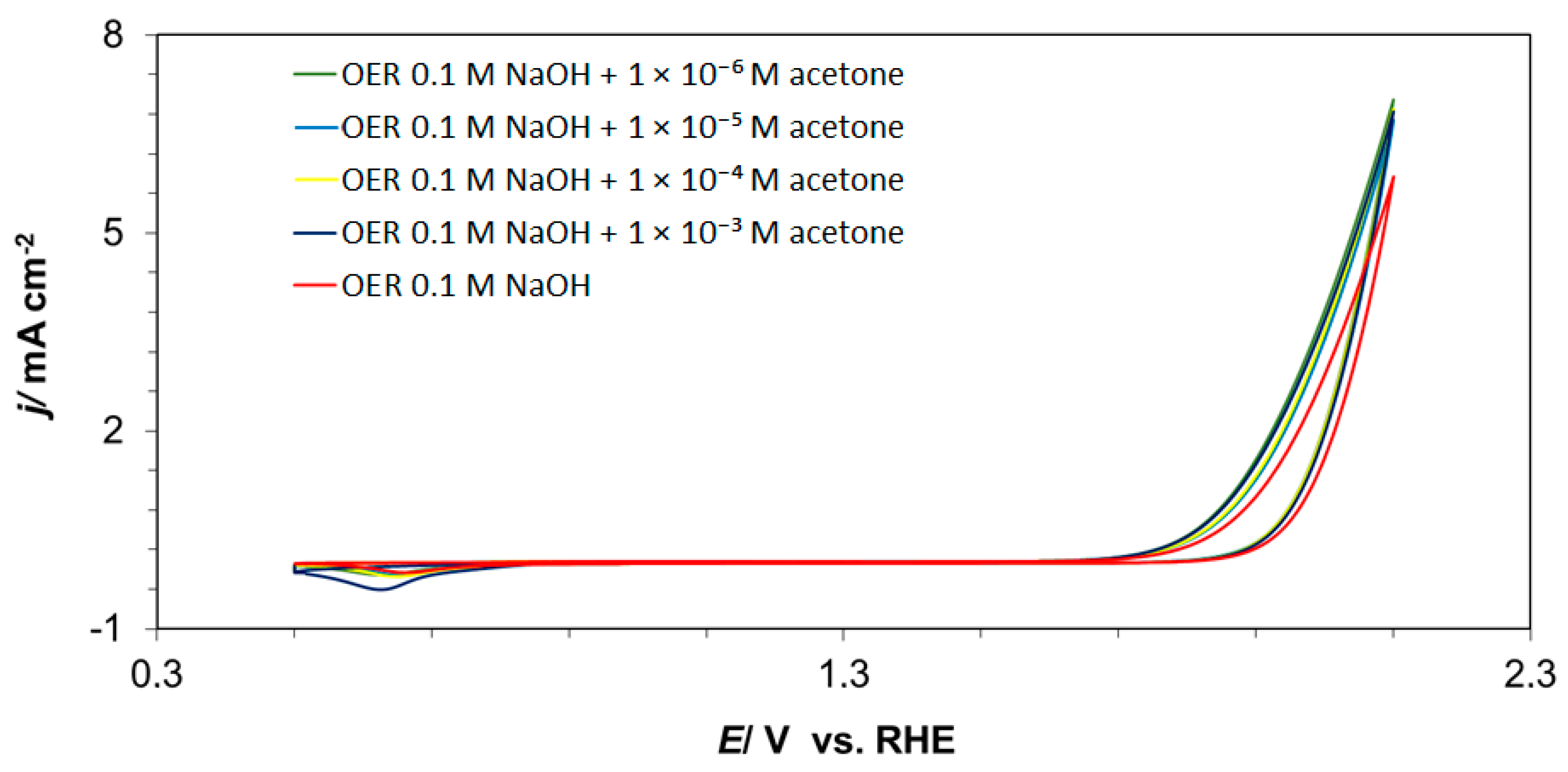
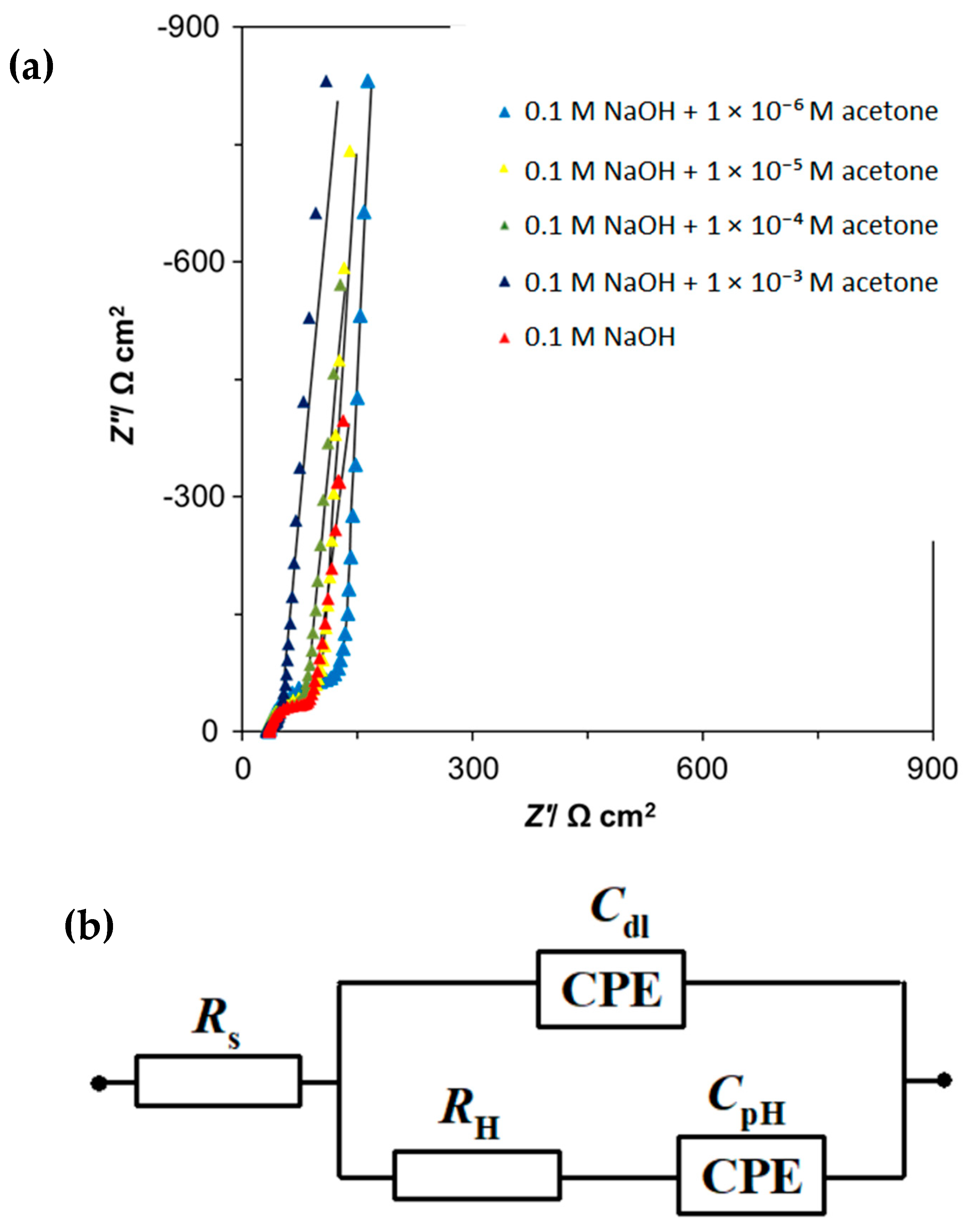
2. Results and Discussion
2.1. Cyclic Voltammetry
2.2. Ac. Impedance Spectroscopy
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Shao, Z.; Jiang, S.P. High-Entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Jiang, S.P.; Shao, Z. Modulating Metal-organic Frameworks for Catalyzing Acidic Oxygen Evolution for Proton Exchange Membrane Water Electrolysis. SusMat 2021, 1, 460–481. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen Society: From Present to Future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Becker, H.; Murawski, J.; Shinde, D.V.; Stephens, L.I.E.; Hinds, G.; Smith, G. Impact of Impurities on Water Electrolysis: A Review. Sustain. Energy Fuels 2023, 7, 1565–1603. [Google Scholar] [CrossRef]
- Barwe, S.; Mei, B.; Masa, J.; Schuhmann, W.; Ventosa, E. Overcoming Cathode Poisoning from Electrolyte Impurities in Alkaline Electrolysis by Means of Self-Healing Electrocatalyst Films. Nano Energy 2018, 53, 763–768. [Google Scholar] [CrossRef]
- Chauhan, D.; Ahn, Y.H. Alkaline Electrolysis of Wastewater and Low-Quality Water. J. Clean. Prod. 2023, 397, 136613. [Google Scholar] [CrossRef]
- Vigier, F.; Coutanceau, C.; Hahn, F.; Belgsir, E.M.; Lamy, C. On the Mechanism of Ethanol Electro-oxidation on Pt and PtSn Catalysts: Electrochemical and in situ IR Reflectance Spectroscopy Studies. J. Electroanal. Chem. 2004, 563, 81–89. [Google Scholar] [CrossRef]
- Rizo, R.; Ferre-Vilaplana, A.; Herrero, E.; Feliu, J.M. Ethanol Electro-oxidation Reaction Selectivity on Platinum in Aqueous Media. ACS Sustain. Chem. Eng. 2023, 11, 4960–4968. [Google Scholar] [CrossRef]
- Mekazni, D.S.; Arán-Ais, R.M.; Feliu, J.M.; Herrero, E. Understanding the Electrochemical Hydrogenation of Acetone on Pt Single Crystal Electrodes. J. Electroanal. Chem. 2022, 922, 116697. [Google Scholar] [CrossRef]
- Bondue, C.J.; Calle-Vallejo, F.; Figueiredo, M.C.; Koper, M.T.M. Structural Principles to Steer the Selectivity of the Electrocatalytic Reduction of Aliphatic Ketones on Platinum. Nat. Catal. 2019, 2, 243–250. [Google Scholar] [CrossRef]
- Bondue, C.J.; Koper, M.T.M. Electrochemical Reduction of the Carbonyl Functional Group: The Importance of Adsorption Geometry, Molecular Structure, and Electrode Surface Structure. J. Am. Chem. Soc. 2019, 141, 12071–12078. [Google Scholar] [CrossRef]
- Mrozek, M.F.; Luo, H.; Weaver, M.J. Formic Acid Electrooxidation on Platinum Group Metals: Is Adsorbed Carbon Monoxide Solely a Catalytic Poison. Langmuir 2000, 16, 8463–8469. [Google Scholar] [CrossRef]
- Pierozynski, B. Electrochemical Reactivity of Urea at Pt(100) Surface in 0.5 M H2SO4 by AC Impedance Spectroscopy. J. Solid State Electrochem. 2013, 17, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Enders, S.; Kahl, H.; Winkelmann, J. Surface Tension of the Ternary System Water + Acetone + Toluene. J. Chem. Eng. Data 2007, 52, 1072–1079. [Google Scholar] [CrossRef]
- Howard, K.S.; McAllister, R.A. Surface Tension of Acetone-water Solutions up to Their Normal Boiling Points. AIChE. J. 1957, 3, 325–329. [Google Scholar] [CrossRef]
- Wu, N.; Li, X.; Liu, S.; Zhang, M.; Ouyang, S. Effect of Hydrogen Bonding on the Surface Tension Properties of Binary Mixture (Acetone-Water) by Raman Spectroscopy. Appl. Sci. 2019, 9, 1235. [Google Scholar] [CrossRef]
- Kuczyński, M.; Łuba, M.; Mikołajczyk, T.; Pierożyński, B. The Influence of Resorcinol on the Kinetics of Underpotentially Deposited Hydrogen, Cathodic Hydrogen and Anodic Oxygen Evolution Reactions, Examined at Polycrystalline Pt Electrode in 0.1 M NaOH Solution. Int. J. Hydrogen Energy 2023, 48, 10755–10764. [Google Scholar] [CrossRef]
- Pierożyński, B.; Kuczyński, M.; Mikołajczyk, T. Electro-Reactivity of Resorcinol on Pt(111) Single-Crystal Plane and Its Influence on the Kinetics of Underpotentially Deposited Hydrogen and Hydrogen Evolution Reaction Processes in 0.1 M NaOH Solution. Crystals 2024, 14, 545. [Google Scholar] [CrossRef]
- Li, C.; Sallee, A.M.; Zhang, X.; Kumar, S. Electrochemical Hydrogenation of Acetone to Produce Isopropanol Using a Polymer Electrolyte Membrane Reactor. Energies 2018, 11, 2691. [Google Scholar] [CrossRef]
- Jaksic, M.M.; Johansen, B.; Tunold, R. Electrochemical Behaviour of Platinum in Alkaline and Acidic Solutions of Heavy and Regular Water. Int. J. Hydrogen Energy 1993, 18, 817–837. [Google Scholar] [CrossRef]
- Birss, V.I.; Damjanovic, A. Oxygen Evolution at Platinum Electrodes in Alkaline Solutions: I. Dependence on Solution pH and Oxide Film Thickness. J. Electrochem. Soc. 1987, 134, 113. [Google Scholar] [CrossRef]
- Modestov, A.D.; Tarasevich, M.R.; Pu, H. Study of 2-Propanol, 1-Propanol, and Acetone Electrochemical Oxidation on Pt in Gelled Phosphoric Acid at 170 °C. Electrocatalysis 2016, 7, 42–49. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy, Emphasizing Solid Materials and Systems; John Wiley & Sons: New York, NY, USA, 1987; pp. 1–368. ISBN 0-471-83122-0. [Google Scholar]







| 0.1 M NaOH | |||
|---|---|---|---|
| E/mV | RH/Ω cm2 | Cdl/μF cm−2 | CpH/μF cm−2 |
| 100 | 60.0 ± 1.6 | 23.2 ± 0.7 | 164 ± 4 |
| 150 | 82.6 ± 3.9 | 26.4 ± 4.6 | 182 ± 7 |
| 200 | 67.2 ± 1.9 | 43.8 ± 1.2 | 427 ± 14 |
| 250 | 212.9 ± 13.9 | 59.1 ± 1.2 | 209 ± 10 |
| 300 | 696.0 ± 73.6 | 61.2 ± 4.3 | 291 ± 31 |
| 350 | 4294.0 ± 112.0 | 76.2 ± 0.9 | 344 ± 19 |
| 0.1 M NaOH + 1.0 × 10−6 M acetone | |||
| 100 | 52.9 ± 1.9 | 33.6 ± 1.6 | 172 ± 3 |
| 150 | 88.6 ± 3.4 | 32.6 ± 1.2 | 141 ± 6 |
| 200 | 138.0 ± 5.3 | 39.7 ± 1.2 | 163 ± 8 |
| 250 | 91.6 ± 3.9 | 73.3 ± 2.7 | 458 ± 28 |
| 300 | 224.0 ± 19.0 | 78.3 ± 2.2 | 231 ± 22 |
| 350 | 1050.0 ± 57.3 | 76.8 ± 1.3 | 352 ± 23 |
| 0.1 M NaOH + 1.0 × 10−5 M acetone | |||
| 100 | 41.3 ± 2.2 | 38.5 ± 2.5 | 186 ± 8 |
| 150 | 62.6 ± 2.9 | 37.2 ± 1.8 | 152 ± 7 |
| 200 | 98.4 ± 4.3 | 45.9 ± 1.7 | 188 ± 10 |
| 250 | 77.6 ± 3.7 | 77.8 ± 3.2 | 449 ± 27 |
| 300 | 274.0 ± 25.4 | 75.1 ± 2.0 | 523 ± 31 |
| 350 | 1029.0 ± 59.9 | 87.5 ± 1.5 | 411 ± 12 |
| 0.1 M NaOH + 1.0 × 10−4 M acetone | |||
| 100 | 30.3 ± 1.8 | 34.5 ± 2.7 | 197 ± 9 |
| 150 | 67.4 ± 3.2 | 46.9 ± 2.1 | 266 ± 13 |
| 200 | 75.0 ± 2.1 | 45.5 ± 4.4 | 175 ± 9 |
| 250 | 91.1 ± 5.3 | 64.6 ± 2.5 | 421 ± 26 |
| 300 | 349.0 ± 25.9 | 50.3 ± 1.2 | 487 ± 17 |
| 350 | 855.0 ± 50.2 | 115.0 ± 2.2 | 352 ± 19 |
| 0.1 M NaOH + 1.0 × 10−3 M acetone | |||
| 100 | 14.5 ± 1.4 | 46.6 ± 6.9 | 187 ± 10 |
| 150 | 19.3 ± 1.6 | 43.6 ± 5.1 | 162 ± 8 |
| 200 | 25.0 ± 2.1 | 45.5 ± 4.4 | 175 ± 9 |
| 250 | 61.5 ± 5.4 | 43.6 ± 2.5 | 178 ± 10 |
| 300 | 189.0 ± 13.9 | 39.7 ± 1.2 | 200 ± 13 |
| 350 | 506.0 ± 27.3 | 51.6 ± 1.1 | 143 ± 5 |
| 0.1 M NaOH | ||
|---|---|---|
| E/mV | Rct/Ω cm2 | Cdl/μF cm−2 |
| −100 | 104.2 ± 1.1 | 42.1 ± 0.9 |
| −150 | 72.8 ± 1.0 | 43.5 ± 1.3 |
| −200 | 52.2 ± 0.7 | 44.9 ± 1.6 |
| −300 | 34.1 ± 0.6 | 48.1 ± 2.3 |
| −400 | 25.2 ± 0.5 | 51.5 ± 3.1 |
| −500 | 20.2 ± 0.5 | 54.4 ± 4.1 |
| 0.1 M NaOH + 1.0 × 10−4 M acetone | ||
| −100 | 102.7 ± 1.1 | 44.7 ± 1.0 |
| −150 | 78.5 ± 0.9 | 45.5 ± 1.2 |
| −200 | 52.1 ± 0.7 | 47.5 ± 1.6 |
| −300 | 31.2 ± 0.6 | 51.2 ± 2.6 |
| −400 | 22.3 ± 0.5 | 55.3 ± 3.6 |
| −500 | 17.9 ± 0.5 | 58.7 ± 4.6 |
| 0.1 M NaOH + 1.0 × 10−3 M acetone | ||
| −100 | 91.1 ± 1.19 | 49.5 ± 1.3 |
| −150 | 73.2 ± 1.0 | 49.8 ± 1.5 |
| −200 | 49.6 ± 0.7 | 51.9 ± 1.9 |
| −300 | 29.0 ± 0.6 | 56.2 ± 3.1 |
| −400 | 20.5 ± 0.5 | 60.9 ± 4.4 |
| −500 | 16.3 ± 0.5 | 65.2 ± 5.7 |
| 0.1 M NaOH | ||
|---|---|---|
| E/mV | Rct/Ω cm2 | Cdl/μF cm−2 |
| 1600 | 16,976 ± 1645 | 23.3 ± 1.2 |
| 1700 | 1644 ± 20 | 22.9 ± 0.3 |
| 1800 | 260 ± 2 | 25.7 ± 0.6 |
| 1900 | 45 ± 1 | 24.3 ± 1.5 |
| 2000 | 14 ± 0 | 24.3 ± 2.6 |
| 0.1 M NaOH + 1.0 × 10−6 M acetone | ||
| 1600 | 14,805 ± 169 | 24.4 ± 0.2 |
| 1700 | 1446 ± 9 | 24.5 ± 0.3 |
| 1800 | 99 ± 1 | 30.0 ± 0.8 |
| 1900 | 45 ± 0 | 27.6 ± 1.4 |
| 2000 | 14 ± 0 | 23.8 ± 2.3 |
| 0.1 M NaOH + 1.0 × 10−3 M acetone | ||
| 1600 | 12,266 ± 126 | 23.9 ± 0.2 |
| 1700 | 1157 ± 5 | 23.7 ± 0.3 |
| 1800 | 161 ± 1 | 28.0 ± 0.6 |
| 1900 | 30 ± 0 | 26.0 ± 1.8 |
| 2000 | 11 ± 0 | 27.6 ± 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamicka, A.; Mikołajczyk, T.; Kuczyński, M.; Pierożyński, B. The Influence of Acetone on the Kinetics of Water Electrolysis Examined at Polycrystalline Pt Electrode in Alkaline Solution. Catalysts 2024, 14, 488. https://doi.org/10.3390/catal14080488
Adamicka A, Mikołajczyk T, Kuczyński M, Pierożyński B. The Influence of Acetone on the Kinetics of Water Electrolysis Examined at Polycrystalline Pt Electrode in Alkaline Solution. Catalysts. 2024; 14(8):488. https://doi.org/10.3390/catal14080488
Chicago/Turabian StyleAdamicka, Aleksandra, Tomasz Mikołajczyk, Mateusz Kuczyński, and Bogusław Pierożyński. 2024. "The Influence of Acetone on the Kinetics of Water Electrolysis Examined at Polycrystalline Pt Electrode in Alkaline Solution" Catalysts 14, no. 8: 488. https://doi.org/10.3390/catal14080488
APA StyleAdamicka, A., Mikołajczyk, T., Kuczyński, M., & Pierożyński, B. (2024). The Influence of Acetone on the Kinetics of Water Electrolysis Examined at Polycrystalline Pt Electrode in Alkaline Solution. Catalysts, 14(8), 488. https://doi.org/10.3390/catal14080488








