Catalyst Development for Biogas Dry Reforming: A Review of Recent Progress
Abstract
:1. Introduction
2. Basis of Biogas Dry Reforming Technology
3. Advances in Catalyst Research
3.1. Noble Metal Catalysts
3.2. Non-Noble Metal Catalysts
3.2.1. Nickel-Based Catalysts
3.2.2. Cobalt-Based Catalysts
3.2.3. Iron-Based Catalysts
4. Challenges and Future Trends
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atelge, M.R.; Krisa, D.; Kumar, G.; Eskicioglu, C.; Nguyen, D.D.; Chang, S.W.; Atabani, A.E.; Al-Muhtaseb, A.H.; Unalan, S. Biogas Production from Organic Waste: Recent Progress and Perspectives. Waste Biomass Valorization 2020, 11, 1019–1040. [Google Scholar] [CrossRef]
- Pavi, S.; Kramer, L.E.; Gomes, L.P.; Schiavo Miranda, L.A. Biogas production from co-digestion of organic fraction of municipal solid waste and fruit and vegetable waste. Bioresour. Technol. 2017, 228, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.P.; Jiang, X.; Jin, Z.H.; Yang, S.L.; Zhang, J. The performance and microbial community in a slightly alkaline biotrickling filter for the removal of high concentration H2S from biogas. Chemosphere 2020, 249, 126127. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yuan, J.; Li, T.; Jiang, X.; Ma, S.; Cen, W.; Jiang, W. A regenerable N-rich hierarchical porous carbon synthesized from waste biomass for H2S removal at room temperature. Sci. Total Environ. 2021, 768, 144452. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Basu, S.; Shetti, N.P.; Bhattu, M.; Alodhayb, A.N.; Pandiaraj, S. Biowaste to bioenergy nexus: Fostering sustainability and circular economy. Environ. Res. 2024, 250, 118503. [Google Scholar] [CrossRef] [PubMed]
- Obaideen, K.; Abdelkareem, M.A.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Maghrabie, H.M.; Olabi, A.G. Biogas role in achievement of the sustainable development goals: Evaluation, Challenges, and Guidelines. J. Taiwan Inst. Chem. Eng. 2022, 131, 104207. [Google Scholar] [CrossRef]
- Gupta, P.; Kurien, C.; Mittal, M. Biogas (a promising bioenergy source): A critical review on the potential of biogas as a sustainable energy source for gaseous fuelled spark ignition engines. Int. J. Hydrogen Energy 2023, 48, 7747–7769. [Google Scholar] [CrossRef]
- Di Micco, S.; Romano, F.; Jannelli, E.; Perna, A.; Minutillo, M. Techno-economic analysis of a multi-energy system for the co-production of green hydrogen, renewable electricity and heat. Int. J. Hydrogen Energy 2023, 48, 31457–31467. [Google Scholar] [CrossRef]
- Abanades, S.; Abbaspour, H.; Ahmadi, A.; Das, B.; Ehyaei, M.A.; Esmaeilion, F.; Assad, M.E.; Hajilounezhad, T.; Hmida, A.; Rosen, M.A.; et al. A conceptual review of sustainable electrical power generation from biogas. Energy Sci. Eng. 2022, 10, 630–655. [Google Scholar] [CrossRef]
- He, J.; Jin, Z.; Gan, F.; Xie, L.; Guo, J.; Zhang, S.; Jia, C.Q.; Ma, D.; Dai, Z.; Jiang, X. Liquefiable biomass-derived porous carbons and their applications in CO2 capture and conversion. Green Chem. 2022, 24, 3376–3415. [Google Scholar] [CrossRef]
- Kim, Y.; Kawahara, N.; Tsuboi, K.; Tomita, E. Combustion characteristics and NOx emissions of biogas fuels with various CO2 contents in a micro co-generation spark-ignition engine. Appl. Energy 2016, 182, 539–547. [Google Scholar] [CrossRef]
- Wang, A.; Harrhy, J.H.; Meng, S.; He, P.; Liu, L.; Song, H. Nonthermal plasma-catalytic conversion of biogas to liquid chemicals with low coke formation. Energy Convers. Manag. 2019, 191, 93–101. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Bi, G.C.; Zhang, S.L.; Tan, T.; Xie, J. CeMgOx-promoted Ni/boron nitride ultra-long stability bi-reforming for methanol-friendly syngas (H2/CO≈2): CO2/H2O activation and coke elimination mechanism. Fuel 2024, 364, 131122. [Google Scholar] [CrossRef]
- Bac, S.; Keskin, S.; Avci, A.K. Recent advances in sustainable syngas production by catalytic CO2 reforming of ethanol and glycerol. Sustain. Energy Fuels 2020, 4, 1029–1047. [Google Scholar] [CrossRef]
- Mondal, K.; Sasmal, S.; Badgandi, S.; Chowdhury, D.R.; Nair, V. Dry reforming of methane to syngas: A potential alternative process for value added chemicals-a techno-economic perspective. Environ. Sci. Pollut. Res. 2016, 23, 22267–22273. [Google Scholar] [CrossRef]
- Manfro, R.L.; Souza, M.M.V.M. Overview of Ni-Based Catalysts for Hydrogen Production from Biogas Reforming. Catalysts 2023, 13, 1296. [Google Scholar] [CrossRef]
- Zhao, X.; Joseph, B.; Kuhn, J.; Ozcan, S. Biogas Reforming to Syngas: A Review. Iscience 2020, 23, 101082. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Pal, A. Overview of hydrogen production from biogas reforming: Technological advancement. Int. J. Hydrogen Energy 2022, 47, 34831–34855. [Google Scholar] [CrossRef]
- Bak, C.-u.; Lim, C.-J.; Kim, Y.-D.; Kim, W.-S. Multi-stage adsorptive purification process for improving desulfurization performance of biogas. Sep. Purif. Technol. 2019, 227, 115702. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Sebastian, V.; Monzon, A.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. An in depth investigation of deactivation through carbon formation during the biogas dry reforming reaction for Ni supported on modified with CeO2 and La2O3 zirconia catalysts. Int. J. Hydrogen Energy 2018, 43, 18955–18976. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Douvartzides, S.L.; Siakavelas, G.I.; Tzounis, L.; Sebastian, V.; Stolojan, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. The Relationship between Reaction Temperature and Carbon Deposition on Nickel Catalysts Based on Al2O3, ZrO2 or SiO2 Supports during the Biogas Dry Reforming Reaction. Catalysts 2019, 9, 676. [Google Scholar] [CrossRef]
- Calgaro, C.O.; Perez-Lopez, O.W. Biogas dry reforming for hydrogen production over Ni-M-Al catalysts (M = Mg, Li, Ca, La, Cu, Co, Zn). Int. J. Hydrogen Energy 2019, 44, 17750–17766. [Google Scholar] [CrossRef]
- Rosset, M.; Feris, L.A.; Perez-Lopez, O.W. Biogas reforming to syngas over cu-modified Ni-Al-LDH catalysts. Int. J. Energy Res. 2022, 46, 22664–22678. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Abu Tarboush, B.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Wang, T.; Wu, G.; Ma, X.; Gong, J. Pt-based core-shell nanocatalysts with enhanced activity and stability for CO oxidation. Chem. Commun. 2013, 49, 10647–10649. [Google Scholar] [CrossRef]
- Tu, W.; Ghoussoub, M.; Singh, C.V.; Chin, Y.-H.C. Consequences of Surface Oxophilicity of Ni, Ni-Co, and Co Clusters on Methane Activation. J. Am. Chem. Soc. 2017, 139, 6928–6945. [Google Scholar] [CrossRef]
- Liu, Z.; Lustemberg, P.; Gutierrez, R.A.; Carey, J.J.; Palomino, R.M.; Vorokhta, M.; Grinter, D.C.; Ramirez, P.J.; Matolin, V.; Nolan, M.; et al. In Situ Investigation of Methane Dry Reforming on Metal/Ceria(111) Surfaces: Metal-Support Interactions and C-H Bond Activation at Low Temperature. Angew. Chem.-Int. Ed. 2017, 56, 13041–13046. [Google Scholar] [CrossRef]
- Niu, J.; Du, X.; Ran, J.; Wang, R. Dry (CO2) reforming of methane over Pt catalysts studied by DFT and kinetic modeling. Appl. Surf. Sci. 2016, 376, 79–90. [Google Scholar] [CrossRef]
- Chein, R.; Yang, Z.-W. H2S effect on dry reforming of biogas for syngas production. Int. J. Energy Res. 2019, 43, 3330–3345. [Google Scholar] [CrossRef]
- Acs, N.; Szuhaj, M.; Wirth, R.; Bagi, Z.; Maroti, G.; Rakhely, G.; Kovacs, K.L. Microbial Community Rearrangements in Power-to-Biomethane Reactors Employing Mesophilic Biogas Digestate. Front. Energy Res. 2019, 7, 132. [Google Scholar] [CrossRef]
- Kameya, Y.; Hanamura, K. Variation in Catalytic Activity of Carbon Black during Methane Decomposition: Active Site Estimations from Surface Structural Characteristics. Catal. Lett. 2012, 142, 460–463. [Google Scholar] [CrossRef]
- Oliveira, L.G.; Machado, B.; de Souza, L.P.; Correa, G.C.G.; Polinarski, M.A.; Cavalcanti Trevisan, S.V.; Borba, C.E.; Brackmann, R.; Alves, H.J. Dry reforming of biogas in a pilot unit: Scale-up of catalyst synthesis and green hydrogen production. Int. J. Hydrogen Energy 2022, 47, 35608–35625. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Vadodariya, D.M.; Alotibi, M.F.; Abu-Dahrieh, J.K.; Ibrahim, A.A.; Abasaeed, A.E.; Alarifi, N.; Kumar, R.; Al-Fatesh, A.S. Pd+Al2O3-Supported Ni-Co Bimetallic Catalyst for H2 Production through Dry Reforming of Methane: Effect of Carbon Deposition over Active Sites. Catalysts 2023, 13, 1374. [Google Scholar] [CrossRef]
- Moogi, S.; Ko, C.H.; Rhee, G.H.; Jeon, B.-H.; Khan, M.A.; Park, Y.-K. Influence of catalyst synthesis methods on anti-coking strength of perovskites derived catalysts in biogas dry reforming for syngas production. Chem. Eng. J. 2022, 437, 135348. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Aihemaiti, A.; Ju, T.; Chen, X.; Yan, F. A novel nickel catalyst supported on activated coal fly ash for syngas production via biogas dry reforming. Renew. Energy 2020, 149, 786–793. [Google Scholar] [CrossRef]
- Owgi, A.H.K.; Jalil, A.A.; Hussain, I.; Hassan, N.S.; Hambali, H.U.; Siang, T.J.; Vo, D.V.N. Catalytic systems for enhanced carbon dioxide reforming of methane: A review. Environ. Chem. Lett. 2021, 19, 2157–2183. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Wang, Y.; Zhao, Y. Comparative Studies of Non-noble Metal Modified Mesoporous M-Ni-CaO-ZrO2 (M=Fe, Co, Cu) Catalysts for Simulated Biogas Dry Reforming. Chin. J. Chem. 2017, 35, 113–120. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Akansu, H.; Arbag, H.; Tasdemir, H.M.; Yasyerli, S.; Yasyerli, N.; Dogu, G. Nickel-based alumina supported catalysts for dry reforming of biogas in the absence and the presence of H2S: Effect of manganese incorporation. Catal. Today 2022, 397, 37–49. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Papageridis, K.N.; Delimitis, A.; Pachatouridou, E.; Iliopoulou, E.F. Nickel on alumina catalysts for the production of hydrogen rich mixtures via the biogas dry reforming reaction: Influence of the synthesis method. Int. J. Hydrogen Energy 2015, 40, 9183–9200. [Google Scholar] [CrossRef]
- Vita, A.; Italiano, C.; Ashraf, M.A.; Pino, L.; Specchia, S. Syngas production by steam and oxy-steam reforming of biogas on monolith-supported CeO2-based catalysts. Int. J. Hydrogen Energy 2018, 43, 11731–11744. [Google Scholar] [CrossRef]
- Sadykov, V.A.A.; Eremeev, N.F.F.; Sadovskaya, E.; Fedorova, J.E.E.; Arapova, M.V.V.; Bobrova, L.N.N.; Ishchenko, A.V.V.; Krieger, T.A.A.; Melgunov, M.S.S.; Glazneva, T.S.S.; et al. Approaches to the design of efficient and stable catalysts for biofuel reforming into syngas: Doping the mesoporous MgAl2O4 support with transition metal cations. Dalton Trans. 2023, 52, 8756–8769. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Marin, G.B. Enhanced Carbon-Resistant Dry Reforming Fe-Ni Catalyst: Role of Fe. ACS Catal. 2015, 5, 3028–3039. [Google Scholar] [CrossRef]
- Oemar, U.; Hidajat, K.; Kawi, S. Pd-Ni catalyst over spherical nanostructured Y2O3 support for oxy-CO2 reforming of methane: Role of surface oxygen mobility. Int. J. Hydrogen Energy 2015, 40, 12227–12238. [Google Scholar] [CrossRef]
- Université, S.; University, A.; Université, S.; Université, S.; Université, S. Nickel Supported Modified Ceria Zirconia Lanthanum/Praseodymium/Yttrium Oxides Catalysts for Syngas Production through Dry Methane Reforming. Mater. Sci. Forum 2018, 941, 2214–2219. [Google Scholar]
- Usman, M.; Daud, W.M.A.W.; Abbas, H.F. Dry reforming of methane: Influence of process parameters—A review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
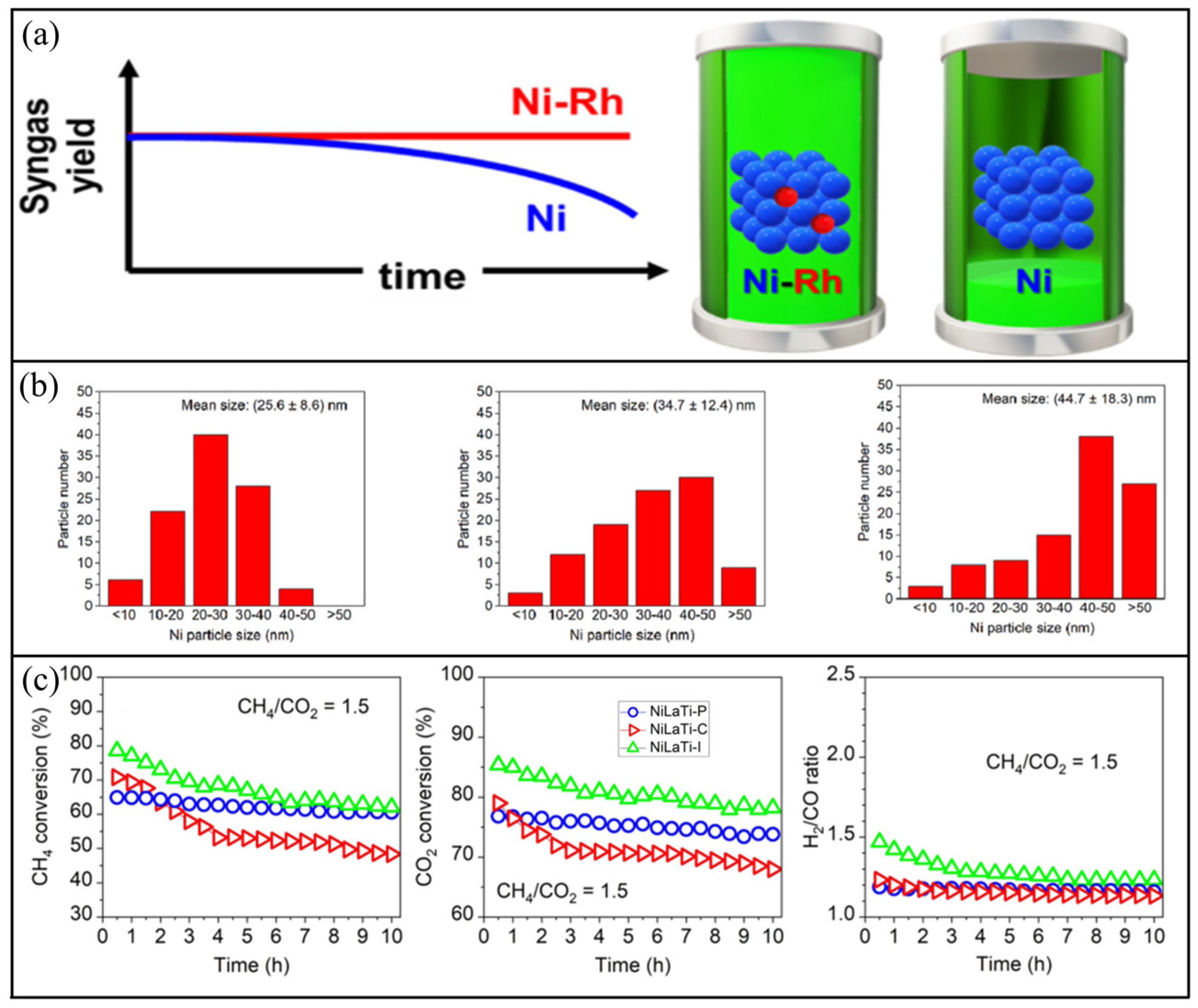
- Italiano, C.; Balzarotti, R.; Vita, A.; Latorrata, S.; Fabiano, C.; Pino, L.; Cristiani, C. Preparation of structured catalysts with Ni and Ni Rh/CeO2 catalytic layers for syngas production by biogas reforming processes. Catal. Today 2016, 273, 3–11. [Google Scholar] [CrossRef]
- Bereketidou, O.A.; Goula, M.A. Biogas reforming for syngas production over nickel supported on ceria-alumina catalysts. Catal. Today 2012, 195, 93–100. [Google Scholar] [CrossRef]
- Moral, A.; Reyero, I.; Alfaro, C.; Bimbela, F.; Gandia, L.M. Syngas production by means of biogas catalytic partial oxidation and dry reforming using Rh-based catalysts. Catal. Today 2018, 299, 280–288. [Google Scholar] [CrossRef]
- Nikolaraki, E.; Goula, G.; Panagiotopoulou, P.; Taylor, M.J.; Kousi, K.; Kyriakou, G.; Kondarides, D.I.; Lambert, R.M.; Yentekakis, I.V. Support Induced Effects on the Ir Nanoparticles Activity, Selectivity and Stability Performance under CO2 Reforming of Methane. Nanomaterials 2021, 11, 2880. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xie, K. Active Exsolved Metal-Oxide Interfaces in Porous Single-Crystalline Ceria Monoliths for Efficient and Durable CH4/CO2 Reforming. Angew. Chem.-Int. Ed. 2022, 61, 13079. [Google Scholar] [CrossRef] [PubMed]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Katsoni, A.; Diamadopoulos, E.; Mantzavinos, D.; Delimitis, A. Dry Reforming of Methane: Catalytic Performance and Stability of Ir Catalysts Supported on γ-Al2O3, Zr0.92Y0.08O2−δ (YSZ) or Ce0.9Gd0.1O2−δ (GDC) Supports. Top. Catal. 2015, 58, 1228–1241. [Google Scholar] [CrossRef]
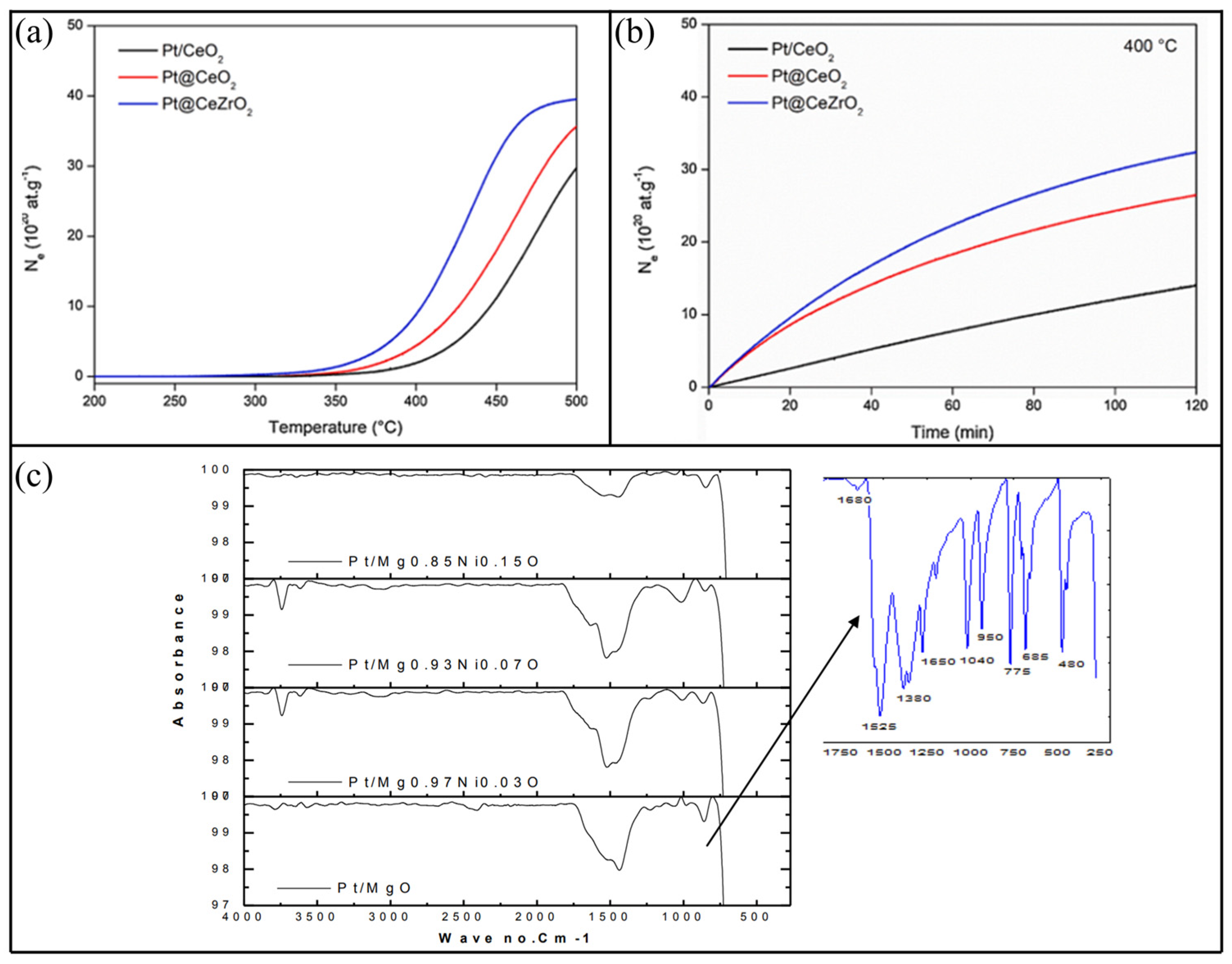
- Marinho, A.L.A.; Rabelo-Neto, R.C.; Epron, F.; Bion, N.; Noronha, F.B.; Toniolo, F.S. Pt nanoparticles embedded in CeO2 and CeZrO2 catalysts for biogas upgrading: Investigation on carbon removal mechanism by oxygen isotopic exchange and DRIFTS. J. CO2 Util. 2021, 49, 101572. [Google Scholar] [CrossRef]
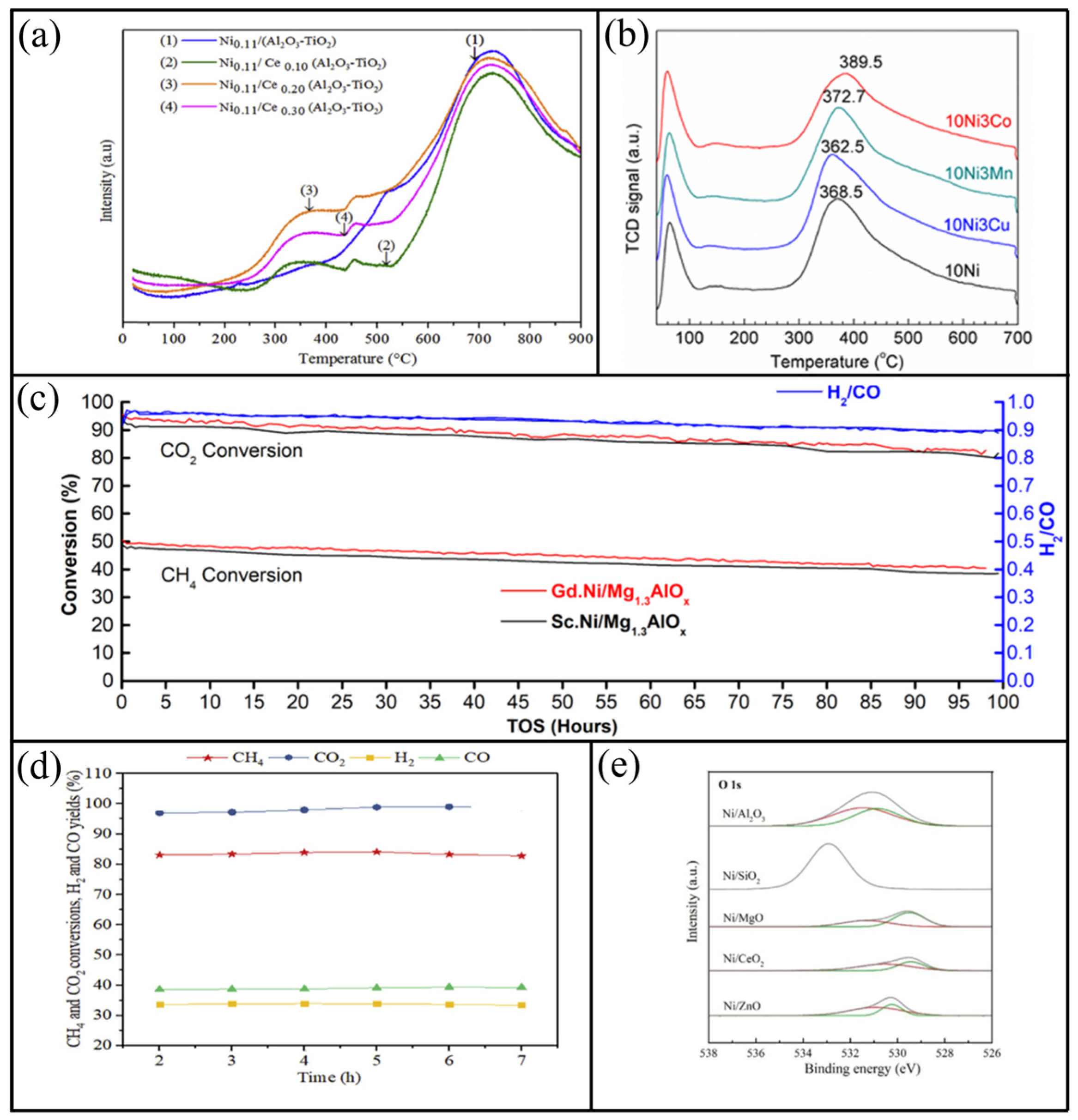
- Al-Doghachi, F.J.; Zainal, Z.; Saiman, M.I.; Embong, Z.; Taufiq-Yap, Y.H. Hydrogen Production from Dry-Reforming of Biogas over Pt/Mg1-xNixO Catalysts. Energy Procedia 2015, 79, 18–25. [Google Scholar] [CrossRef]
- Rosha, P.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Biogas reforming for hydrogen enrichment by ceria decorated over nickel catalyst supported on titania and alumina. Int. J. Hydrogen Energy 2018, 43, 21246–21255. [Google Scholar] [CrossRef]
- Navarro-Puyuelo, A.; Reyero, I.; Moral, A.; Bimbela, F.; Banares, M.A.; Gandia, L.M. Effect of oxygen addition, reaction temperature and thermal treatments on syngas production from biogas combined reforming using Rh/alumina catalysts. J. Ind. Eng. Chem. 2019, 80, 217–226. [Google Scholar] [CrossRef]
- Itkulova, S.S.; Zakumbaeva, G.D.; Mukazhanova, A.A.; Nurmakanov, Y.Y. Syngas production by biogas reforming over the Co-based multicomponent catalysts. Cent. Eur. J. Chem. 2014, 12, 1255–1261. [Google Scholar] [CrossRef]
- le Sache, E.; Johnson, S.; Pastor-Perez, L.; Horri, B.A.; Reina, T.R. Biogas Upgrading Via Dry Reforming Over a Ni-Sn/CeO2-Al2O3 Catalyst: Influence of the Biogas Source. Energies 2019, 12, 1007. [Google Scholar] [CrossRef]
- Abdullah, B.; Ghani, N.A.A.; Vo, D.-V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef]
- Gao, Y.; Golmakani, A.; Nabavi, S.A.; Jiang, J.; Manovic, V. Simulative optimization of catalyst configuration for biogas dry reforming. Int. J. Hydrogen Energy 2021, 46, 12835–12845. [Google Scholar] [CrossRef]
- Zhumabek, M.; Xanthopoulou, G.; Tungatarova, S.A.; Baizhumanova, T.S.; Vekinis, G.; Murzin, D.Y. Biogas Reforming over Al-Co Catalyst Prepared by Solution Combustion Synthesis Method. Catalysts 2021, 11, 274. [Google Scholar] [CrossRef]
- Veiga, S.; Romero, M.; Faccio, R.; Segobia, D.; Apesteguia, C.; Perez, A.L.; Brondino, C.D.; Bussi, J. Biogas dry reforming over Ni-La-Ti catalysts for synthesis gas production: Effects of preparation method and biogas composition. Fuel 2023, 346, 128300. [Google Scholar] [CrossRef]
- Vo, C.-M.; Cao, A.N.T.; Qazaq, A.S.; Pham, C.Q.; Nguyen, D.L.T.; Alsaiari, M.; Vu, T.V.; Sharma, A.; Phuong, P.T.T.; Van, T.T.; et al. Toward syngas production from simulated biogas dry reforming: Promotional effect of calcium on cobalt-based catalysts performance. Fuel 2022, 326, 125106. [Google Scholar] [CrossRef]
- Ha, Q.L.M.; Atia, H.; Kreyenschulte, C.; Lund, H.; Bartling, S.; Lisak, G.; Wohlrab, S.; Armbruster, U. Effects of modifier (Gd, Sc, La) addition on the stability of low Ni content catalyst for dry reforming of model biogas. Fuel 2022, 312, 122823. [Google Scholar] [CrossRef]
- Rosha, P.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Catalytic reforming of synthetic biogas for hydrogen enrichment over Ni supported on ZnO-CeO2 mixed catalyst. Biomass Bioenergy 2019, 125, 70–78. [Google Scholar] [CrossRef]
- Gao, Y.; Aihemaiti, A.; Jiang, J.; Meng, Y.; Ju, T.; Han, S.; Chen, X.; Liu, J. Inspection over carbon deposition features of various nickel catalysts during simulated biogas dry reforming. J. Clean. Prod. 2020, 260, 120944. [Google Scholar] [CrossRef]
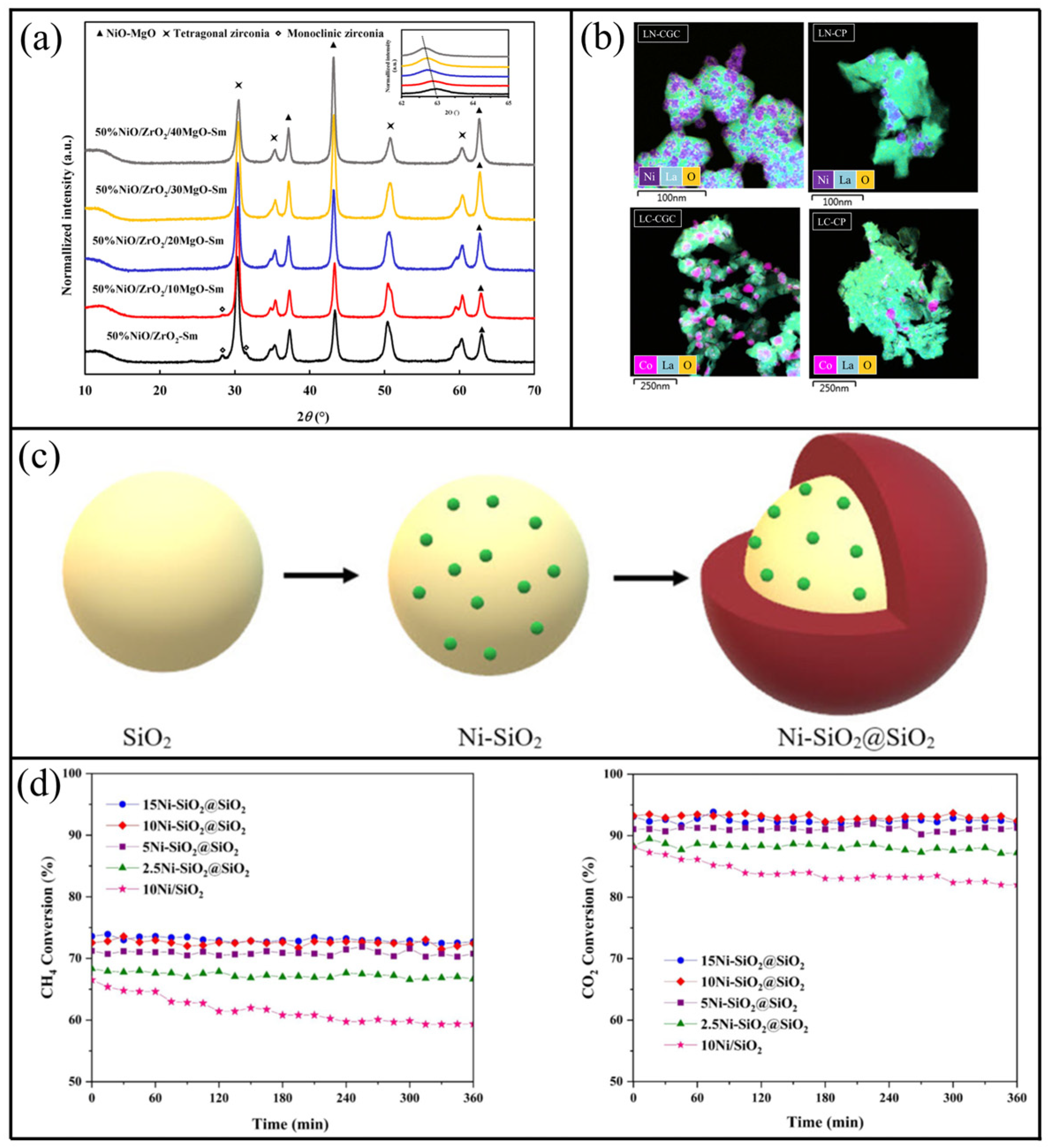
- Chuenjai, M.; Wongsakulphasatch, S.; Yong, N.; Maneeprakorn, W.; Sudsakorn, K.; Tongnan, V.; Hartley, U.W.; Ratchahat, S.; Kiatkittipong, W.; Assabumrungrat, S. Synthesis of NiO/MgO/ZrO2 catalyst for syngas production from partial oxidation and dry reforming of biogas. Int. J. Hydrogen Energy 2022, 47, 41386–41396. [Google Scholar] [CrossRef]
- Kaviani, M.; Rezaei, M.; Alavi, S.M.; Akbari, E. High coke resistance Ni-SiO2@SiO2 core-shell catalyst for biogas dry reforming: Effects of Ni loading and calcination temperature. Fuel 2022, 330, 125609. [Google Scholar] [CrossRef]
- Jabbour, K.; Saad, A.; Inaty, L.; Davidson, A.; Massiani, P.; El Hassan, N. Ordered mesoporous Fe-Al2O3 based-catalysts synthesized via a direct “one-pot” method for the dry reforming of a model biogas mixture. Int. J. Hydrogen Energy 2019, 44, 14889–14907. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Syahri, K.M.; Jia, Q.; Shan, S. Production of hydrogen-rich syngas and multiwalled carbon nanotubes by biogas decomposition over zirconia supported iron catalysts. J. Ind. Eng. Chem. 2020, 84, 150–166. [Google Scholar] [CrossRef]
- Rattanaamonkulchai, R.; Kludpantanapan, T.; Srifa, A.; Koo-Amornpattana, W.; Chaiwat, W.; Sakdaronnarong, C.; Charinpanitkul, T.; Assabumrungrat, S.; Wongsakulphasatch, S.; Show, P.L.; et al. Simultaneous production of hydrogen and carbon nanotubes from biogas over mono- and bimetallic catalyst. J. Environ. Chem. Eng. 2022, 10, 107910. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, S.; Mao, D.; Hu, C. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: A review. Fuel Process. Technol. 2018, 169, 199–206. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Ramirez, P.J.; Liu, Z.; Gutierrez, R.A.; Grinter, D.G.; Carrasco, J.; Senanayake, S.D.; Rodriguez, J.A.; Veronica Ganduglia-Pirovano, M. Room-Temperature Activation of Methane and Dry Reforming with CO2 on Ni-CeO2(111) Surfaces: Effect of Ce3+ Sites and Metal-Support Interactions on C-H Bond Cleavage. ACS Catal. 2016, 6, 8184–8191. [Google Scholar] [CrossRef]
- Qin, Z.; Chen, J.; Xie, X.; Luo, X.; Su, T.; Ji, H. CO2 reforming of CH4 to syngas over nickel-based catalysts. Environ. Chem. Lett. 2020, 18, 997–1017. [Google Scholar] [CrossRef]
- Han, J.W.; Kim, C.; Park, J.S.; Lee, H. Highly Coke-Resistant Ni Nanoparticle Catalysts with Minimal Sintering in Dry Reforming of Methane. Chemsuschem 2014, 7, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; van Veen, A.C. Tuning the catalytic performance of Ni-catalysed dry reforming of methane and carbon deposition via Ni-CeO2-x interaction. Appl. Catal. B-Environ. 2018, 237, 641–648. [Google Scholar] [CrossRef]
- Mei, D.H.; Shen, X.Q.; Liu, S.Y.; Zhou, R.S.; Yuan, X.C.; Rao, Z.Q.; Sun, Y.F.; Fang, Z.; Du, X.S.; Zhou, Y.; et al. Plasma-catalytic reforming of biogas into syngas over Ni-based bimetallic catalysts. Chem. Eng. J. 2023, 462, 142044. [Google Scholar] [CrossRef]
- De, S.; Zhang, J.; Luque, R.; Yan, N. Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef]
- Carrasco-Ruiz, S.; Zhang, Q.; Gandara-Loe, J.; Pastor-Perez, L.; Odriozola, J.A.; Reina, T.R.; Bobadilla, L.F. H2-rich syngas production from biogas reforming: Overcoming coking and sintering using bimetallic Ni-based catalysts. Int. J. Hydrogen Energy 2023, 48, 27907–27917. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Khan, M.R.; Cheng, C.K. Catalytic performance of ceria-supported cobalt catalyst for CO-rich hydrogen production from dry reforming of methane. Int. J. Hydrogen Energy 2016, 41, 198–207. [Google Scholar] [CrossRef]
- Park, J.-H.; Yeo, S.; Kang, T.-J.; Shin, H.-R.; Heo, I.; Chang, T.-S. Effect of Zn promoter on catalytic activity and stability of Co/ZrO2 catalyst for dry reforming of CH4. J. CO2 Util. 2018, 23, 10–19. [Google Scholar] [CrossRef]
- Phuong, D.H.L.; Alsaiari, M.; Pham, C.Q.; Hieu, N.H.; Pham, T.-P.T.; Rajamohan, N.; Pham, D.D.; Vo, D.-V.N.; Trinh, T.H.; Setiabudi, H.D.; et al. Carbon dioxide reforming of methane over modified iron-cobalt alumina catalyst: Role of promoter. J. Taiwan Inst. Chem. Eng. 2024, 155, 105253. [Google Scholar] [CrossRef]
- Budiman, A.W.; Song, S.-H.; Chang, T.-S.; Shin, C.-H.; Choi, M.-J. Dry Reforming of Methane Over Cobalt Catalysts: A Literature Review of Catalyst Development. Catal. Surv. Asia 2012, 16, 183–197. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Al Fatesh, A.S.; Ibrahim, A.A.; Kurdi, A.N.; Abasaeed, A.E. Yttria Modified ZrO2 Supported Ni Catalysts for CO2 Reforming of Methane: The Role of Ce Promoter. ACS Omega 2021, 6, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, Z.; Chen, X.; Rui, N.; Betancourt, L.E.; Lin, L.; Xu, W.; Sun, C.-J.; Abeykoon, A.M.M.; Rodriguez, J.A.; et al. Effects of Zr Doping into Ceria for the Dry Reforming of Methane over Ni/CeZrO2 Catalysts: In Situ Studies with XRD, XAFS, and AP-XPS. ACS Catal. 2020, 10, 3274–3284. [Google Scholar] [CrossRef]
- Wu, P.; Tao, Y.; Ling, H.; Chen, Z.; Ding, J.; Zeng, X.; Liao, X.; Stampfl, C.; Huang, J. Cooperation of Ni and CaO at Interface for CO2 Reforming of CH4: A Combined Theoretical and Experimental Study. ACS Catal. 2019, 9, 10060–10069. [Google Scholar] [CrossRef]
- Ngoc Thang, T.; Quyet Van, L.; Nguyen Van, C.; Trinh Duy, N.; Nguyen Huu Huy, P.; Phuong, P.T.T.; Monir, M.U.; Abd Aziz, A.; Quang Duc, T.; Abidin, S.Z.; et al. La-doped cobalt supported on mesoporous alumina catalysts for improved methane dry reforming and coke mitigation. J. Energy Inst. 2020, 93, 1571–1580. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, G.; Liu, J.; Zhang, Y.; Li, T.; Zhang, X.; Wang, Y.; Zhao, Y.; Li, G. Exploring the role of promoters (Y, Tb and Gd) in the performance of Co-based catalyst for dry reforming of methane. Int. J. Hydrogen Energy 2023, 48, 38251–38265. [Google Scholar] [CrossRef]
- Bahari, M.B.; Setiabudi, H.D.; Trinh Duy, N.; Phuong, P.T.T.; Quang Duc, T.; Jalil, A.A.; Ainirazali, N.; Vo, D.-V.N. Insight into the influence of rare-earth promoter (CeO2, La2O3, Y2O3, and Sm2O3) addition toward methane dry reforming over Co/mesoporous alumina catalysts. Chem. Eng. Sci. 2020, 228, 115967. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Zhang, Y.; Zhang, Y.; Zheng, S. Lithium adsorption from brine by iron-doped titanium lithium ion sieves. Particuology 2018, 41, 40–47. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Sabbe, M.; Poelman, H.; Detavernier, C.; Marin, G.B. Controlling the stability of a Fe-Ni reforming catalyst: Structural organization of the active components. Appl. Catal. B-Environ. 2017, 209, 405–416. [Google Scholar] [CrossRef]


| Entry | Catalysts | Reaction Conditions | CH4 Conversion (%) | CO2 Conversion (%) | Product Selectivity (H2/CO Ratio) | References |
|---|---|---|---|---|---|---|
| 1 | Ni/MgO | CH4/CO2/N2 = 2:2:1, 750 °C, 200 mg | 96.4 | 91.7 | ≈1.06 | [60] |
| 2 | Co(NO3)2 + Al(NO3)3 | CH4/CO2 = 1:1, 900 °C | 100 | 86.2 | ≈1.16 | [61] |
| 3 | NiLaTi-I | CH4/CO2 = 1.5:1, 800 °C, 200 mg | 79 | ≈79 | >1 | [62] |
| 4 | Ni0.11/Ce0.20 (Al2O3-TiO2) | CH4/CO2 = 1.5:1, 850 °C, 150 mg | 84.9 | 95.6 | 1.06 | [55] |
| 5 | LaNiO3 | CH4/CO2 = 1.5:1, 850 °C, 0.5 g | 97.2 | 93.8 | 1.6 | [34] |
| 6 | 0.2Ca-10Co/Al2O3 | CH4/CO2 = 1:1, 973 K, 100 mg | 84 | 89 | 0.74 | [63] |
| 7 | Gd.Ni/Mg1.3AlOx | CH4/CO2 = 2:1, 750 °C, 50 mg | 49 | 95 | >0.9 | [64] |
| 8 | Ni0.10/(Zn0.1-Ce0.9) | CH4/CO2 = 1.5:1, 900 °C, 150 mg | 83.1 | 97 | 1.04 | [65] |
| 9 | Ni/Al2O3 | CH4/CO2/N2 = 2:2:1, 750 °C, 200 mg | 82.7 | / | >0.9 | [66] |
| 10 | NiO/ZrO2/MgO | CH4/CO2 = 1.5:1, 900 °C, 150 mg | 97 | 100 | 1.7 | [67] |
| 11 | Ni-SiO2@SiO2 | CH4/CO2 = 1.5:1, 700 °C, 100 mg | 72.7 | 93 | 0.95 | [68] |
| 12 | Fe5%Ni5%Al2O3 | CH4/CO2 = 1.8:1, 700 °C, 100 mg | 22 | 49 | ≈1.16 | [69] |
| 13 | Fe/ZrO2 | CH4/CO2 = 1.5:1, 900 °C, 3.5 g | 92 | 89 | 2.25 | [70] |
| 14 | NiMo/MgO | CH4/CO2 = 1.5:1, 900 °C, 1 g | 95 | 100 | 3.1 | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Wu, J.; Huang, Z.; Tan, H.; Tang, Y.; Feng, Z.; Deng, R.; Zhang, H.; Zairov, R.; Pan, Z. Catalyst Development for Biogas Dry Reforming: A Review of Recent Progress. Catalysts 2024, 14, 494. https://doi.org/10.3390/catal14080494
Hu W, Wu J, Huang Z, Tan H, Tang Y, Feng Z, Deng R, Zhang H, Zairov R, Pan Z. Catalyst Development for Biogas Dry Reforming: A Review of Recent Progress. Catalysts. 2024; 14(8):494. https://doi.org/10.3390/catal14080494
Chicago/Turabian StyleHu, Wei, Jundao Wu, Zeai Huang, Hao Tan, Yifan Tang, Zilong Feng, Rui Deng, Hongwei Zhang, Rustem Zairov, and Zhicheng Pan. 2024. "Catalyst Development for Biogas Dry Reforming: A Review of Recent Progress" Catalysts 14, no. 8: 494. https://doi.org/10.3390/catal14080494







