Electrochemically Induced Cu-NiOOH/Cu2O/Cu Mesh Heteroarchitecture with Cu-Ni Dual Active Sites as Efficient Bifunctional Electrocatalyst for Urea-Assisted Energy-Saving Hydrogen Production in Alkaline Electrolyte
Abstract
1. Introduction
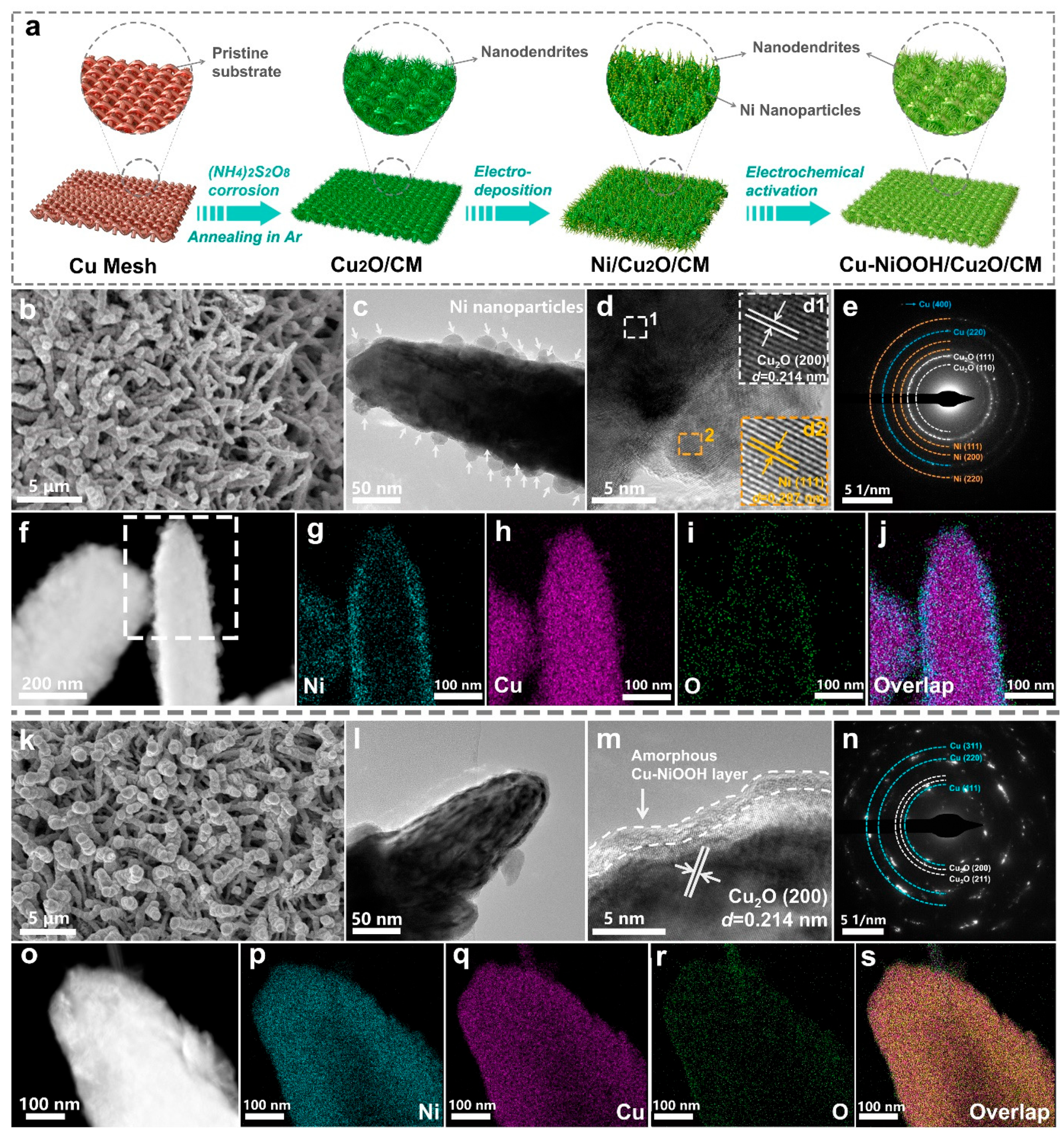
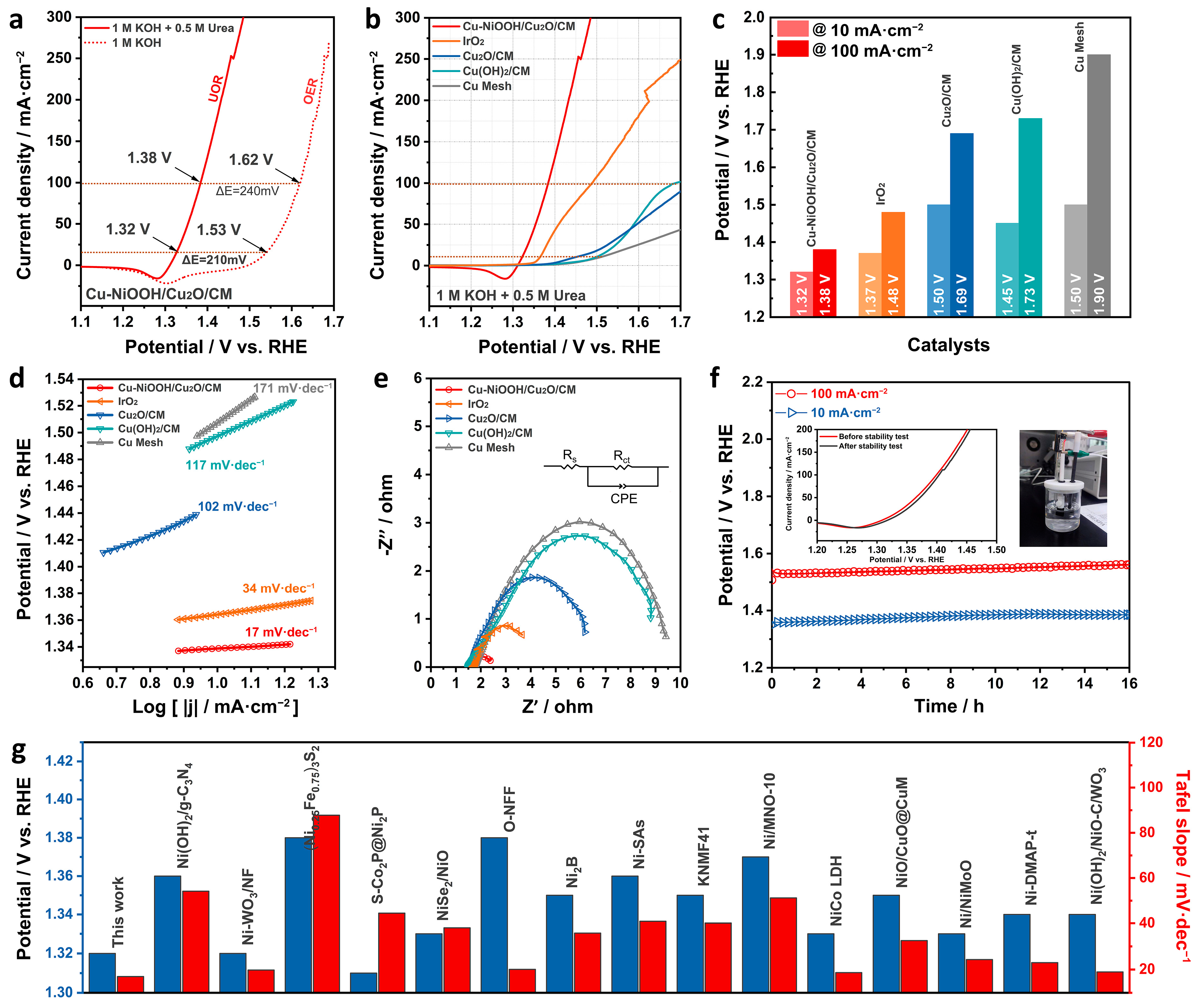
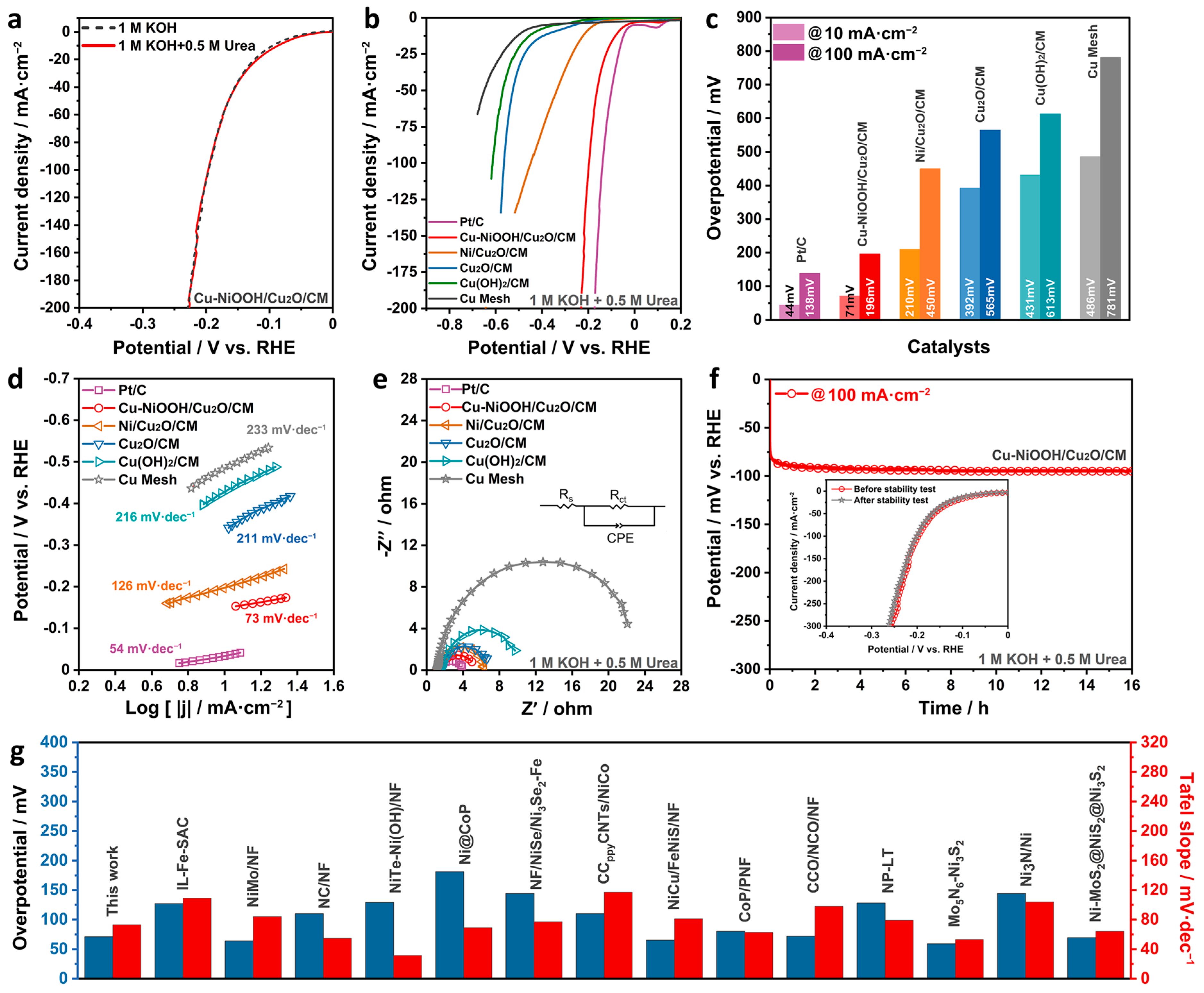
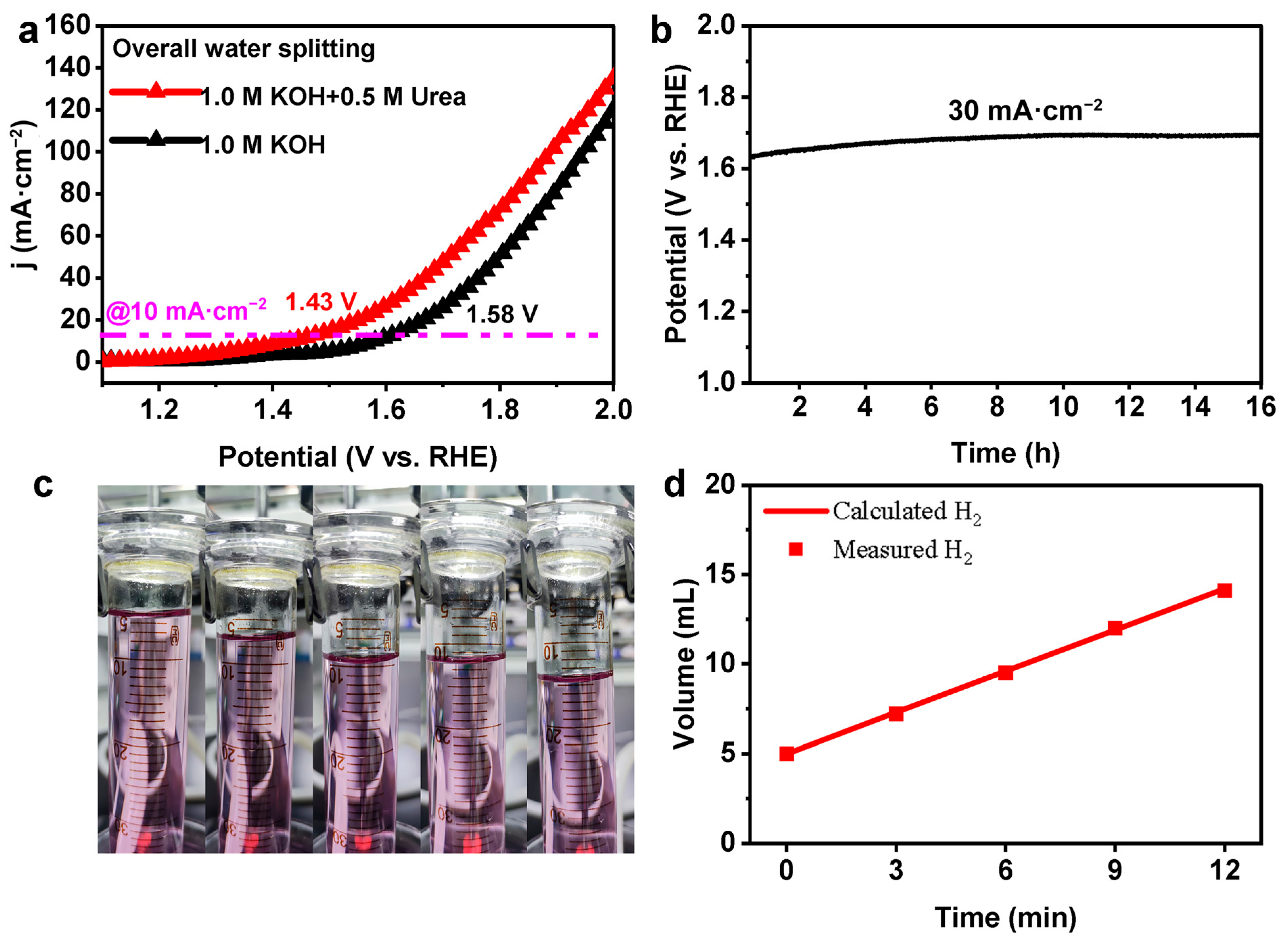
2. Results and Discussion
| Electrocatalyst | Urea Concentration in 1.0 M KOH Electrolyte | Cell Voltage at @10 mA cm−2 | Reference |
|---|---|---|---|
| Cu-NiOOH/Cu2O/CM | 0.5 M | 1.43 V | This work |
| Ni/NiO@CrOx | 0.33 M | 1.44 V | [56] |
| Ni/NiMoN | 0.5 M | 1.42 V | [57] |
| NiO/Ni2P/NF | 0.33 M | 1.45 V | [58] |
| CoSx/Ni(OH)2 | 0.5 M | 1.48 V | [59] |
| Mo-NiS | 0.5 M | 1.51 V | [60] |
| NiFeSbP | 0.5 M | 1.54 V | [61] |
| Fe2V-MOF | 0.5 M | 1.63 V | [62] |
3. Experimental Section
3.1. Chemicals and Materials
3.2. Synthesis Methods
3.3. Physical Characterizations and Electrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Xu, X.; Fei, L.; Zhou, W.; Shao, Z. Electrochemical oxidation of small molecules for energy-saving hydrogen production. Adv. Energy Mater. 2024, 2401242. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen society: From present to future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Fei, L.; Sun, H.; Ran, R.; Zhou, W.; Shao, Z. Self-supported nickel phosphide electrode for efficient alkaline water-to-hydrogen conversion via urea electrolysis. Ind. Eng. Chem. Res. 2021, 60, 1185–1193. [Google Scholar] [CrossRef]
- Yu, J.; Li, Z.; Wang, C.; Xu, X.; Liu, T.; Chen, D.; Shao, Z.; Ni, M. Engineering advanced noble-metal-free electrocatalysts for energy-saving hydrogen production from alkaline water via urea electrolysis. J. Colloid Interface Sci. 2024, 661, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.Y.; Lin, L.P.; Wang, S.Q.; Wang, Y.C.; Ma, X.W.; An, Q.; Zhao, L. Electrocatalytic urea oxidation: Advances in mechanistic insights, nanocatalyst design, and applications. J. Mater. Chem. A 2023, 11, 15100–15121. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Qin, Y.; Zhang, W.; Wang, L.; Luo, M.; Yang, H.; Guo, S. Recent advances on water-splitting electrocatalysis mediated by noble-metal-based nanostructured materials. Adv. Energy Mater. 2020, 10, 1903120. [Google Scholar] [CrossRef]
- Chao, T.; Hu, Y.; Hong, X.; Li, Y. Design of noble metal electrocatalysts on an atomic level. ChemElectroChem 2019, 6, 289–303. [Google Scholar] [CrossRef]
- Wang, K.; Wang, S.; Hui, K.S.; Gao, H.; Dinh, D.A.; Yuan, C.; Zha, C.; Shao, Z.; Tang, Z.; Hui, K.N. Synergistically boosting the elementary reactions over multiheterogeneous ordered macroporous Mo2C/NC-Ru for highly efficient alkaline hydrogen evolution. Carbon Energy 2022, 4, 856–866. [Google Scholar] [CrossRef]
- Wang, Z.L.; Liu, W.J.; Hu, Y.M.; Guan, M.L.; Xu, L.; Li, H.P.; Bao, J.; Li, H.M. Cr-doped CoFe layered double hydroxides: Highly efficient and robust bifunctional electrocatalyst for the oxidation of water and urea. Appl. Catal. B-Environ. 2020, 272, 118959. [Google Scholar] [CrossRef]
- He, Y.T.; Yang, X.X.; Li, Y.S.; Liu, L.T.; Guo, S.W.; Shu, C.Y.; Liu, F.; Liu, Y.N.; Tan, Q.; Wu, G. Atomically dispersed Fe–Co dual metal sites as bifunctional oxygen electrocatalysts for rechargeable and flexible Zn–Air batteries. ACS Catal. 2022, 12, 1216–1227. [Google Scholar] [CrossRef]
- Patil, S.J.; Chodankar, N.R.; Hwang, S.K.; Rama Raju, G.S.; Huh, Y.S.; Han, Y.K. Fluorine engineered self-supported ultrathin 2D nickel hydroxide nanosheets as highly robust and stable bifunctional electrocatalysts for oxygen evolution and urea oxidation reactions. Small 2022, 18, 2103326. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Zhao, Z.; Shen, C.; Liu, H.P.; Pang, X.Y.; Gao, M.Q.; Mu, J.; Cao, F.; Li, G.Q. Ni/NiO heterostructures encapsulated in oxygen-doped graphene as multifunctional electrocatalysts for the HER, UOR and HMF oxidation reaction. Catal. Sci. Technol. 2021, 11, 2480–2490. [Google Scholar] [CrossRef]
- Liu, M.; Zou, W.; Qiu, S.; Su, N.; Cong, J.; Hou, L. Active site tailoring of Ni-based coordination polymers for high-efficiency dual-functional HER and UOR catalysis. Adv. Funct. Mater. 2024, 34, 2310155. [Google Scholar] [CrossRef]
- Liu, G.Q.; Sun, Z.T.; Liu, D.M.; Li, Y.T.; Zhang, W.X. Enhancing the surface polarization effect via Ni/NiMoOx heterojunction architecture for urea-assisted hydrogen generation. J. Colloid Interface Sci. 2023, 629, 1012–1020. [Google Scholar] [CrossRef]
- Wan, K.; Luo, J.S.; Zhou, C.; Zhang, T.; Arbiol, J.; Lu, X.; Mao, B.W.; Zhang, X.; Fransaer, J. Hierarchical porous Ni3S4 with enriched high-valence Ni sites as a robust electrocatalyst for efficient oxygen evolution reaction. Adv. Funct. Mater. 2019, 29, 1900315. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Chen, Y.; Kim, H.; Xu, X.; Guan, D.; Hu, Z.; Zhang, L.; Shao, Z.; Jung, W. Boosting ethanol oxidation by NiOOH-CuO nano-heterostructure for energy-saving hydrogen production and biomass upgrading. Appl. Catal. B-Environ. 2023, 325, 122388. [Google Scholar] [CrossRef]
- Xu, H.; Liao, Y.; Gao, Z.F.; Qing, Y.; Wu, Y.Q.; Xia, L.Y. A branch-like Mo-doped Ni3S2 nanoforest as a high-efficiency and durable catalyst for overall urea electrolysis. J. Mater. Chem. A 2021, 9, 3418–3426. [Google Scholar] [CrossRef]
- Li, D.; Wan, W.J.; Wang, Z.W.; Wu, H.Y.; Wu, S.X.; Jiang, T.; Cai, G.X.; Jiang, C.Z.; Ren, F. Self-derivation and surface reconstruction of Fe-doped Ni3S2 electrode realizing high-efficient and stable overall water and urea electrolysis. Adv. Energy Mater. 2022, 12, 2201913. [Google Scholar] [CrossRef]
- Wen, D.F.; Peng, W.D.; Zhang, W.T.; Xia, Y.; Ye, M.; Hu, W. Hetero-structured NiMoO4/Ni3S4/MoS2 pompons decorated nickel foam electrode for high-efficient urea and urine electrolysis. Appl. Surf. Sci. 2023, 608, 155166. [Google Scholar] [CrossRef]
- Chai, H.M.; Ma, X.; Dang, Y.C.; Zhang, Y.Q.; Yue, F.; Pang, X.X.; Wang, G.Q.; Yang, C.M. Triple roles of Ni(OH)2 promoting the electrocatalytic activity and stability of Ni3S4@Ni(OH)2 in anion exchange membrane water electrolyzers. J. Colloid Interface Sci. 2024, 654, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Guo, P.; Liu, M.; Guo, C.; Liu, H.; Wei, S.X.; Zhang, J.; Lu, X. Rational design of metallic NiTex (x = 1 or 2) as bifunctional electrocatalysts for efficient urea conversion. ACS Appl. Energy Mater. 2019, 2, 3363–3372. [Google Scholar] [CrossRef]
- Jin, C.L.; Hou, M.Y.; Li, X.; Liu, D.; Qu, D.; Dong, Y.L.; Xie, Z.Z.; Zhang, C.C. Rapid electrodeposition of Fe-doped nickel selenides on Ni foam as a bifunctional electrocatalyst for water splitting in alkaline solution. J. Electroanal. Chem. 2022, 906, 116014. [Google Scholar] [CrossRef]
- Xu, X.C.; Liao, H.J.; Huang, L.; Chen, S.J.; Wang, R.; Wu, S.; Wu, Y.X.; Sun, Z.P.; Huang, H.T. Surface reconstruction and directed electron transport in NiSe2/MoSe2 Mott-Schottky heterojunction catalysts promote urea-assisted water splitting. Appl. Catal. B-Environ. 2024, 341, 123312. [Google Scholar] [CrossRef]
- Jia, X.; Kang, H.J.; Yang, X.X.; Li, Y.L.; Cui, K.; Wu, X.H.; Qin, W.; Wu, G. Amorphous Ni(III)-based sulfides as bifunctional water and urea oxidation anode electrocatalysts for hydrogen generation from urea-containing water. Appl. Catal. B-Environ. 2022, 312, 121389. [Google Scholar] [CrossRef]
- Tu, Z.T.; Liu, X.; Xiong, D.; Wang, J.Y.; Gong, S.Q.; Xu, C.; Wu, D.; Chen, Z.F. Ultrafast room-temperature activation of nickel foams as highly efficient electrocatalysts. Chem. Eng. J. 2023, 475, 146253. [Google Scholar] [CrossRef]
- Yang, W.L.; Yang, X.P.; Hou, C.; Li, B.; Gao, H.T.; Lin, J.; Luo, X.L. Rapid room-temperature fabrication of ultrathin Ni(OH)2 nanoflakes with abundant edge sites for efficient urea oxidation. Appl. Catal. B-Environ. 2019, 259, 118020. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.; Zhang, W.; Zhao, Y.; Dong, Z.; Wu, Z.; Xu, G.; Wang, L. Self-supported PdNi dendrite on Ni foam for improving monohydric alcohol and polyhydric alcohols electrooxidation. Fuel 2022, 326, 125083. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Huang, Y.; Tang, M.; Zou, X.; Pan, Z.; Huo, X.; Wu, J.; Lin, C.; Sun, Z.; et al. Electrochemical nitrate reduction to ammonia on CuCo nanowires at practical level. Adv. Funct. Mater. 2024, 2405179. [Google Scholar] [CrossRef]
- Wang, Q.X.; Cai, J.L.; Sun, Y.; Chen, W.Z.; Huang, H.; Wang, S.; Fu, L.; Xie, S. Urchin-like Au/Pd nanobranches for the oxygen reduction electrocatalysis. ACS Appl. Nano Mater. 2024, 7, 5534–5542. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, F.; Huang, A.; Wang, Y.; Chu, W.; Luo, W. A copper interface promotes the transformation of nickel hydroxide into high-valent nickel for an efficient oxygen evolution reaction. Inorg. Chem. Front. 2023, 10, 5111–5116. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, S.K.; Cho, S.K.; Kim, J.J. Enhanced catalytic activity of electrodeposited Ni-Cu-P toward oxygen evolution reaction. Appl. Catal. B-Environ. 2018, 237, 409–415. [Google Scholar] [CrossRef]
- Li, X.L.; Ma, Y.J.; Yang, Z.; Xu, S.S.; Wei, L.M.; Huang, D.; Wang, T.; Hu, N.; Zhang, Y. Hierarchical heterostructures based on prickly Ni nanowires/Cu2O nanoparticles with enhanced photocatalytic activity. Dalton Trans. 2016, 45, 7258–7266. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Liu, Y.; Liu, Q.; Xiang, W. Uniform and dense copper nanoparticles directly modified indium tin oxide electrode for non-enzymatic glucose sensing. J. Electroanal. Chem. 2019, 835, 273–280. [Google Scholar] [CrossRef]
- Wang, X.X.; Li, L.; Wang, Z.; Tan, L.; Wu, Z.; Liu, Z.; Gai, S.; Yang, P. NiS2/MoS2 on carbon cloth as a bifunctional electrocatalyst for overall water splitting. Electrochim. Acta 2019, 326, 134983. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Jiang, W.; Zhang, Y.; Huang, L.; Chen, Y.; Yang, Y.; Li, L.; Hu, J. In situ transformation of Cu2O@MnO2 to Cu@Mn(OH)2 nanosheet-on-nanowire arrays for efficient hydrogen evolution. Nano Res. 2018, 11, 1798–1809. [Google Scholar] [CrossRef]
- Deng, Y.L.; Handoko, A.D.; Du, Y.H.; Xi, S.; Yeo, B.S. In situ Raman spectroscopy of copper and copper oxide surfaces during electrochemical oxygen evolution reaction: Identification of CuIII oxides as catalytically active species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Fedorov, A.V.; Kukushkin, R.G.; Yeletsky, P.M.; Bulavchenko, O.A.; Chesalov, Y.A.; Yakovlev, V.A. Temperature-programmed reduction of model CuO, NiO and mixed CuO–NiO catalysts with hydrogen. J. Alloys Compd. 2020, 844, 156135. [Google Scholar] [CrossRef]
- Lu, Y.; Qin, Y.H.; Chen, M.Y.; Liu, G.F.; Qi, P.C.; Wu, H.; Tang, Y.W. Multi-dimensional Ni(OH)2/(Ni(OH)2(NiOOH).167).857@Ni3S2 hierarchical structure for high-performance asymmetric supercapacitor. Appl. Surf. Sci. 2023, 611, 155625. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, Z.; Huang, X.; Qin, Y.; Yang, H.; Ren, X.; Zhang, Q.; Liu, J.; Shao, M.; He, C. Integrating well-controlled core-shell structures into “superaerophobic” electrodes for water oxidation at large current densities. Appl. Catal. B-Environ. 2021, 286, 119920. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.C.; Gou, L.C.; Wei, S.C.; Hou, X.D.; Wu, L. High-performance methanol electrolysis towards energy-saving hydrogen production: Using Cu2O-Cu decorated Ni2P nanoarray as bifunctional monolithic catalyst. Chem. Eng. J. 2023, 454, 140292. [Google Scholar] [CrossRef]
- Wang, H.; Ying, J.; Xiao, Y.X.; Chen, J.B.; Li, J.H.; He, Z.Z.; Yang, H.J.; Yang, X.Y. Ultrafast synthesis of Cu2O octahedrons inlaid in Ni foam for efficient alkaline water/seawater electrolysis. Electrochem. Commun. 2022, 134, 107177. [Google Scholar] [CrossRef]
- Luo, J.; Tang, L.; Song, J.; Zhou, J.; Liu, S.; Chang, T.; Fang, Y. Synergistic engineering of doped Ni2P-Ni12P5 heterostructure electrocatalysts for urea oxidation reaction. Mol. Catal. 2024, 564, 114276. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, Y.Y.; Li, L.; Luo, S.; Qing, Y.; Tian, C.H.; Xu, H.; Zhang, J.X.; Wu, Y.Q. Ultrafine homologous Ni2P–Co2P heterostructures via space-confined topological transformation for superior urea electrolysis. Adv. Funct. Mater. 2023, 33, 2303300. [Google Scholar] [CrossRef]
- Kim, M.J.; Min, K.; Ko, D.; Seong, H.; Eun Shim, S.; Baeck, S.H. Regulating the electronic structure of Ni2P by one-step Co, N dual-doping for boosting electrocatalytic performance toward oxygen evolution reaction and urea oxidation reaction. J. Colloid Interface Sci. 2023, 650, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Rui, K.; Dou, S.X.; Sun, W. Boosting electrochemical water oxidation: The merits of heterostructured electrocatalysts. J. Mater. Chem. A 2020, 8, 6393–6405. [Google Scholar] [CrossRef]
- Xu, S.; Ruan, X.; Ganesan, M.; Wu, J.; Ravi, S.K.; Cui, X. Transition metal-based catalysts for urea oxidation reaction (UOR): Catalyst design strategies, applications, and future perspectives. Adv. Funct. Mater. 2024, 34, 2313309. [Google Scholar] [CrossRef]
- Wang, Y.L.; Yin, H.B.; Dong, F.; Zhao, X.G.; Qu, Y.K.; Wang, L.X.; Peng, Y.; Wang, D.S.; Fang, W.; Li, J.H. N-coordinated Cu–Ni dual-single-atom catalyst for highly selective electrocatalytic reduction of nitrate to ammonia. Small 2023, 19, 2207695. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhao, J.; Huang, L.; Lu, Y.R.; Malek, A.; Gao, G.; Zhuang, Z.; Wang, D.; Yavuz, C.T.; Lu, X. Stabilizing ruthenium dioxide with cation-anchored sulfate for durable oxygen evolution in proton-exchange membrane water electrolyzers. Nat. Commun. 2023, 14, 8093. [Google Scholar] [CrossRef]
- Du, W.; Feng, Y.; Jiang, J.; Zhao, T.; Xu, G.; Zhang, L. Preparation of hollow nanorod CoxCu2−xSe/CF electrode assembled from nanoparticles and its urea-assisted hydrogen production performance. J. Alloys Compd. 2024, 970, 172517. [Google Scholar] [CrossRef]
- Xu, X.J.; Guo, T.; Xia, J.Y.; Zhao, B.L.; Su, G.; Wang, H.; Huang, M.; Toghan, A. Modulation of the crystalline/amorphous interface engineering on Ni-P-O-based catalysts for boosting urea electrolysis at large current densities. Chem. Eng. J. 2021, 425, 130514. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, N.; Yang, C.; Zhu, X.; Jiang, Y.; Shen, X.; Li, C.; Sun, Q. Surface wettability engineering: CoSx-Ni3S2 nanoarray electrode for improving overall water splitting. Appl. Catal. B-Environ. 2020, 269, 118780. [Google Scholar] [CrossRef]
- Xu, X.J.; Zhang, C.H.; Li, J.Y.; Liu, H.; Su, G.; Shi, Z.C.; Huang, M.H. Redistributing interfacial charge density of Ni12P5/Ni3P via Fe doping for ultrafast urea oxidation catalysis at large current densities. Chem. Eng. J. 2023, 452, 139362. [Google Scholar] [CrossRef]
- Liu, M.Y.; Zou, W.H.; Cong, J.; Su, N.; Qiu, S.; Hou, L. Identifying and unveiling the role of multivalent metal states for bidirectional UOR and HER over Ni, Mo-trithiocyanuric based coordination polymer. Small 2023, 19, 2302698. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, X.; Li, Y.; Zou, Z.; Ling, Y.; Wang, Q. KCl-assisted rapid and low-cost synthesis of antiperovskite (Fe1−xCux)4N anchored on Cu2O nanosheets for highly efficient electrochemical overall water splitting. Chem. Eng. J. 2023, 460, 141854. [Google Scholar] [CrossRef]
- Xu, H.W.; Zhang, W.D.; Yao, Y.; Yang, J.; Liu, J.; Gu, Z.G.; Yan, X. Amorphous chromium oxide confined Ni/NiO nanoparticles-assembled nanosheets for highly efficient and stable overall urea splitting. J. Colloid Interface Sci. 2023, 629, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Gu, Y.; Wang, D.; Jiao, Y.; Wu, A.; Tian, C. Hollow NiMo-based nitride heterojunction with super-hydrophilic/aerophobic surface for efficient urea-assisted hydrogen production. J. Energy Chem. 2024, 95, 428–439. [Google Scholar] [CrossRef]
- Xu, X.; Ji, S.; Wang, H.; Wang, X.; Linkov, V.; Wang, R. Porous hetero-structured nickel oxide/nickel phosphide nanosheets as bifunctional electrocatalyst for hydrogen production via urea electrolysis. J. Colloid Interface Sci. 2022, 615, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Sang, B.; Xue, C.; Guo, S.; Wng, X.; Xie, S.; Wang, C.; Li, R.Q. A hierarchical CoSx/Ni(OH)2 heterostructure as a bifunctional electrocatalyst for urea-assisted energy-efficient hydrogen production. Chem. Commun. 2024, 60, 6643–6646. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Kong, D.; Zhao, Q.; Zhao, L.; Zhang, J.; Chen, X.; Li, Y.; Xu, Y.; Meng, C. Revealing the reactant mediation role of low-valence Mo for accelerated urea-assisted water splitting. Adv. Funct. Mater. 2023, 33, 2210656. [Google Scholar] [CrossRef]
- Shooshtari Gugtapeh, H.; Rezaei, M. Hierarchical flower-like NiFeSbP electrocatalyst toward efficient and stable urea-assisted electrolytic hydrogen production. ACS Appl. Energy Mater. 2022, 5, 15689–15700. [Google Scholar] [CrossRef]
- Chai, N.; Kong, Y.; Jiang, Q.; Guo, Q.; Chen, T.; Ma, X.; Yi, F.Y. Vanadium-doped bimetallic nanoporous metal–organic frameworks as bifunctional electrocatalysts for urea-assisted hydrogen production. ACS Appl. Nano Mater. 2024, 7, 14392–14405. [Google Scholar] [CrossRef]
- Du, H.; Hu, H.; Wang, X.; Ran, N.; Chen, W.; Zhu, H.; Zhou, Y.; Yang, M.; Wang, J.; Liu, J. Vertical cross-alignments of 2D semiconductors with steered internal electric field for urea electrooxidation via balancing intermediates adsorption. Small 2024, 2401053. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, L.; Ren, J.T.; Wang, H.Y.; Tian, W.W.; Sun, M.L.; Yuan, Z.Y. Artificial heterointerfaces with regulated charge distribution of Ni active sites for urea oxidation reaction. Small Methods 2024, 2400108. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, F.; Xue, S.; Lin, H.; Sun, Y.; Cao, J.; Chen, S. Fe doped Ni3S2 nanosheet arrays for efficient and stable electrocatalytic overall urea splitting. ACS Appl. Energy Mater. 2022, 5, 1183–1192. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, T.; Fang, X.; Fan, Y.; Qian, S.; Gao, Y.; Cheng, N.; Xue, H.; Tian, J. Interface engineering of S-doped Co2P@Ni2P core–shell heterostructures for efficient and energy-saving water splitting. Chem. Eng. J. 2022, 439, 135743. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Liu, H.; Feng, L. Efficient synergism of NiSe2 nanoparticle/NiO nanosheet for energy-relevant water and urea electrocatalysis. Appl. Catal. B-Environ. 2020, 276, 119165. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.C.; Han, S.S.; Cho, K. Accessible Ni-Fe-oxalate framework for electrochemical urea oxidation with radically enhanced kinetics. Adv. Funct. Mater. 2024, 34, 2315625. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Yin, H.; He, H.; Wang, Z.; Yu, D.; Liang, J.; Huang, Y.; Qin, L.; Chen, D. Understanding the promotion mechanism of boron during the surface reconstruction of Ni2B nanoflakes for efficient urea electrocatalytic oxidation. Chem. Eng. J. 2023, 477, 146885. [Google Scholar] [CrossRef]
- Luo, F.; Pan, S.; Xie, Y.; Li, C.; Yu, Y.; Yang, Z. Atomically dispersed Ni electrocatalyst for superior urea-assisted water splitting. J. Energy Chem. 2024, 90, 1–6. [Google Scholar] [CrossRef]
- Wu, T.H.; Liu, Y.S.; Hong, C.T.; Hou, B.W. Binary and nanostructured NiMn perovskite fluorides as efficient electrocatalysts for urea oxidation reaction. J. Colloid Interface Sci. 2024, 653, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Maheskumar, V.; Min, A.; Moon, C.J.; Senthil, R.A.; Choi, M.Y. Modulating the electronic structure of Ni/NiO nanocomposite with high-valence Mo doping for energy-saving hydrogen production via boosting urea oxidation kinetics. Small Struct. 2023, 4, 2300212. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, D.; Chen, L.; Chen, S.; Wan, H.; Chen, G.; Zhang, N.; Liu, X.; Ma, R. Collaborative optimization of thermodynamic and kinetic for Ni-based hydroxides in electrocatalytic urea oxidation reaction. Appl. Catal. B-Environ. 2024, 340, 123214. [Google Scholar] [CrossRef]
- Yang, L.; He, R.; Wang, X.; Yang, T.; Zhang, T.; Zuo, Y.; Lu, X.; Liang, Z.; Li, J.; Arbiol, J.; et al. Self-supported NiO/CuO electrodes to boost urea oxidation in direct urea fuel cells. Nano Energy 2023, 115, 108714. [Google Scholar] [CrossRef]
- Jiang, H.; Bu, S.; Gao, Q.; Long, J.; Wang, P.; Lee, C.S.; Zhang, W. Ultrathin two-dimensional nickel-organic framework nanosheets for efficient electrocatalytic urea oxidation. Mater. Today Energy 2022, 27, 101024. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Guo, H.; Ren, J.; Zhang, H.; Wu, Y.; Song, R. Defect-rich Ni(OH)2/NiO regulated by WO3 as core–shell nanoarrays achieving energy-saving water-to-hydrogen conversion via urea electrolysis. Chem. Eng. J. 2022, 433, 134497. [Google Scholar] [CrossRef]
- Sathyaseelan, A.; Elumalai, V.; Perumalsamy, M.; Ali, H.L.; Sajeev, A.; Kim, S.J. Toward highly accessible Fe-N4 sites via rational design of metal chelated ionic liquids for ORR, OER and HER trifunctional electrocatalysis. Chem. Eng. J. 2024, 489, 151235. [Google Scholar] [CrossRef]
- Yuan, F.H.; Mohammadi, M.R.; Ma, L.L.; Cui, Z.D.; Zhu, S.L.; Li, Z.Y.; Wu, S.L.; Jiang, H.; Liang, Y.Q. Electrodeposition of self-supported NiMo amorphous coating as an efficient and stable catalyst for hydrogen evolution reaction. Rare Met. 2022, 41, 2624–2632. [Google Scholar] [CrossRef]
- Askari, N.; Tasviri, M.; Ghiasi, M.; Amiri, M.; Wark, M. One-pot synthesis of NiCe-LDH for efficient electrochemical water splitting: A comprehensive experimental and theoretical study. Mater. Today Sustain. 2024, 26, 100784. [Google Scholar] [CrossRef]
- Li, Q.; Huang, N.; Zhu, W.; Ma, H.; Du, J.; He, X.; Wang, S.; Li, C.; Wang, W.; Weng, Y. Preparation of NiTe-Ni(OH)2/NF active cathode material as an electrocatalyst for hydrogen evolution. J. Alloys Compd. 2024, 990, 174458. [Google Scholar] [CrossRef]
- Abahussain, A.A.M.; Alharbi, A.F.; Wazeer, W.; El-Deeb, H.; Nassr, A.B.A.A. Stainless steel as gas evolving electrodes in water electrolysis: Boosting the electrocatalytic hydrogen evolution reaction on electrodeposited Ni@CoP modified stainless steel electrodes. Fuel 2024, 368, 131605. [Google Scholar] [CrossRef]
- Deng, R.; Yao, H.; Wang, Y.; Wang, C.; Zhang, S.; Guo, S.; Li, Y.; Ma, S. Interface effect of Fe doped NiSe/Ni3Se2 heterojunction as highly efficient electrocatalysts for overall water splitting. Chem. Eng. J. 2024, 488, 150996. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, Y.; Yu, M.; Li, X.; Wang, Y.; Ma, Z.; Liu, S. Ni-Co-O-S derived catalysts on hierarchical N-doped carbon supports with strong interfacial interactions for improved hybrid water splitting performance. Small 2024, 20, 2310087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, D.; Yang, C.; Li, Y.; Yang, Z.; Xu, S.; Xiong, D.; Wang, L.; Chu, P.K. Reversible surface reconstruction of bifunctional NiCu/FeNi2S4 heterostructure catalyst for overall water splitting. Int. J. Hydrogen Energy 2024, 62, 418–428. [Google Scholar] [CrossRef]
- Liao, Q.; You, T.; Liu, X.; Deng, K.; Liu, P.; Tian, W.; Ji, J. Coupled plasma etching and electrodeposition of CoP/NiO nanosheets with surface reconstruction for water-splitting. J. Mater. Chem. A 2024, 12, 9830–9840. [Google Scholar] [CrossRef]
- Saranya, V.; Anandha, B.G.; Navaneethan, M.; Archana, J. Hierarchical CuCo2O4/NiCo2O4 on self-standing Ni foam for high-performance water electrolysis. J. Alloys Compd. 2024, 988, 173894. [Google Scholar] [CrossRef]
- Jiang, X.; Kyriakou, V.; Song, C.; Wang, X.; Costil, S.; Deng, C.; Liu, T.; Jiang, T.; Liao, H. A novel multi-channel porous structure facilitating mass transport towards highly efficient alkaline water electrolysis. J. Energy Chem. 2024, 93, 511–518. [Google Scholar] [CrossRef]
- Fang, B.; Jin, J.; Li, Y.; Dang, H.; Shao, M.; Zhao, L.; Yin, N.; Wang, W. Interfacial electronic modulation of Mo5N6/Ni3S2 heterojunction array boosts electrocatalytic alkaline overall water splitting. Small 2024, 20, 2310825. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Qiu, Y.; Dong, H.; Gao, B.; Zhang, X.; Chu, P.K.; Peng, X. Metallic Ni3N/Ni heterostructure for efficient hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 59, 400–407. [Google Scholar] [CrossRef]
- Yu, H.; Pan, J.; Zhang, Y.; Wang, L.; Ji, H.; Xu, K.; Zhi, T.; Zhuang, Z. Designing multi-heterogeneous interfaces of Ni-MoS2@NiS2@Ni3S2 hybrid for hydrogen evolution. Nano Res. 2024, 17, 4782–4789. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Xu, X.; Wang, M.; Chen, T.; Ju, Q.; Hao, L.; Chen, Z.; Yu, X.; Li, C. Electrochemically Induced Cu-NiOOH/Cu2O/Cu Mesh Heteroarchitecture with Cu-Ni Dual Active Sites as Efficient Bifunctional Electrocatalyst for Urea-Assisted Energy-Saving Hydrogen Production in Alkaline Electrolyte. Catalysts 2024, 14, 496. https://doi.org/10.3390/catal14080496
Zhao K, Xu X, Wang M, Chen T, Ju Q, Hao L, Chen Z, Yu X, Li C. Electrochemically Induced Cu-NiOOH/Cu2O/Cu Mesh Heteroarchitecture with Cu-Ni Dual Active Sites as Efficient Bifunctional Electrocatalyst for Urea-Assisted Energy-Saving Hydrogen Production in Alkaline Electrolyte. Catalysts. 2024; 14(8):496. https://doi.org/10.3390/catal14080496
Chicago/Turabian StyleZhao, Kaige, Xinhao Xu, Manli Wang, Tao Chen, Qianlin Ju, Lulu Hao, Zelin Chen, Xiaolong Yu, and Changjiu Li. 2024. "Electrochemically Induced Cu-NiOOH/Cu2O/Cu Mesh Heteroarchitecture with Cu-Ni Dual Active Sites as Efficient Bifunctional Electrocatalyst for Urea-Assisted Energy-Saving Hydrogen Production in Alkaline Electrolyte" Catalysts 14, no. 8: 496. https://doi.org/10.3390/catal14080496
APA StyleZhao, K., Xu, X., Wang, M., Chen, T., Ju, Q., Hao, L., Chen, Z., Yu, X., & Li, C. (2024). Electrochemically Induced Cu-NiOOH/Cu2O/Cu Mesh Heteroarchitecture with Cu-Ni Dual Active Sites as Efficient Bifunctional Electrocatalyst for Urea-Assisted Energy-Saving Hydrogen Production in Alkaline Electrolyte. Catalysts, 14(8), 496. https://doi.org/10.3390/catal14080496






