Study on the Catalytic Activity and Selectivity of Manganese Dioxide-Modified Nickel–Iron-Based Hydroxide Electrodes for Initiating the Oxygen Evolution Reaction in Natural Seawater
Abstract
:1. Introduction
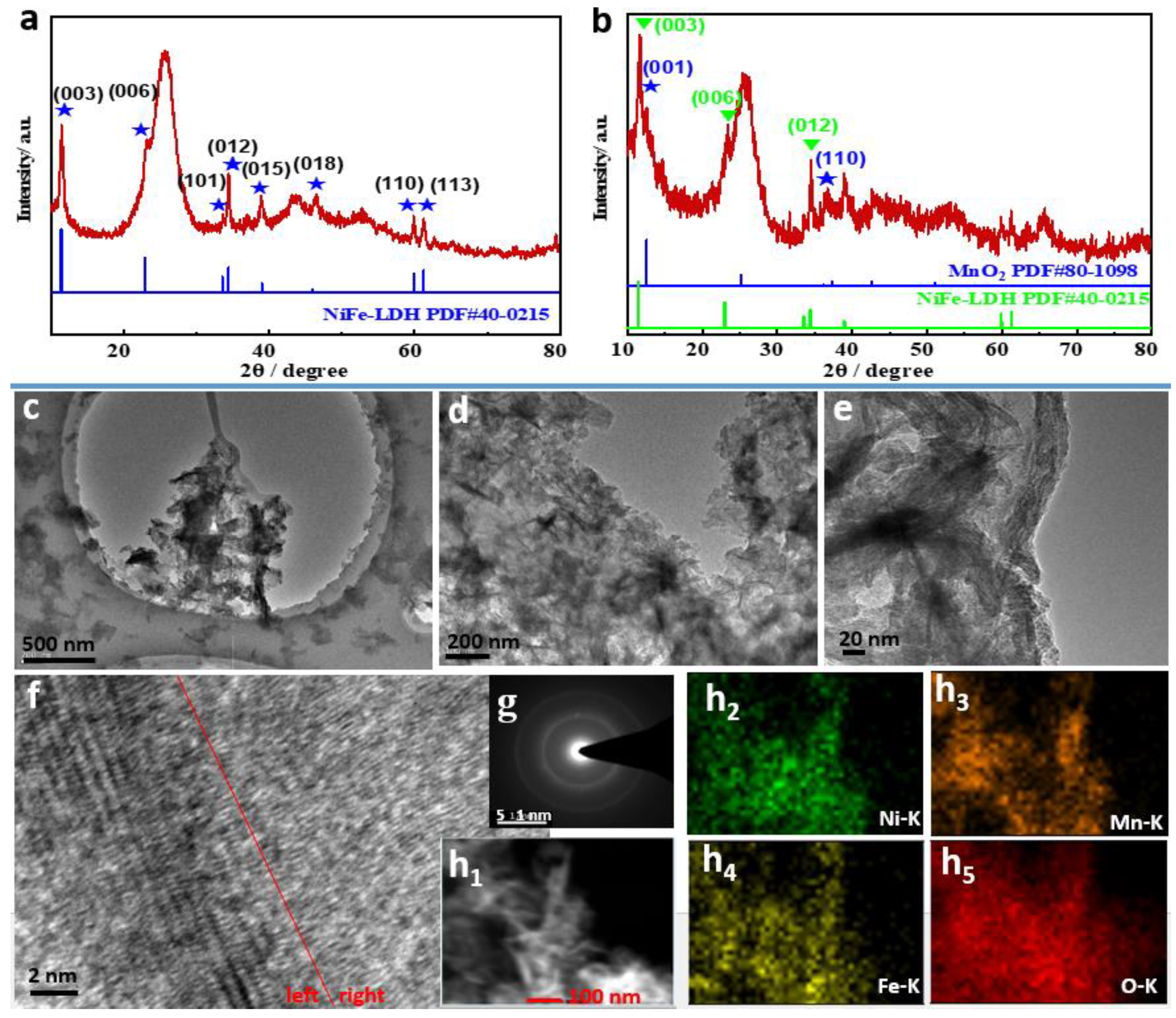
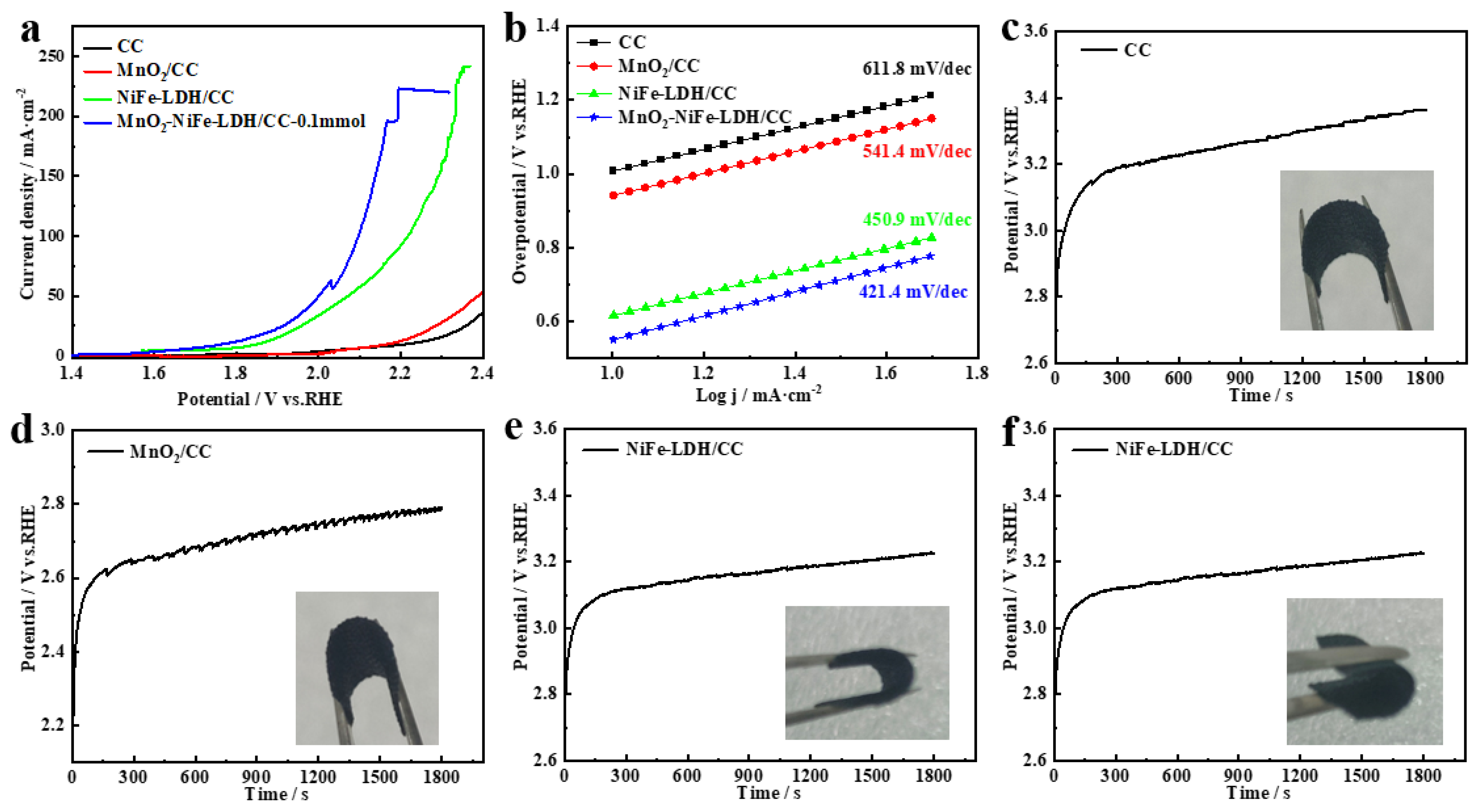
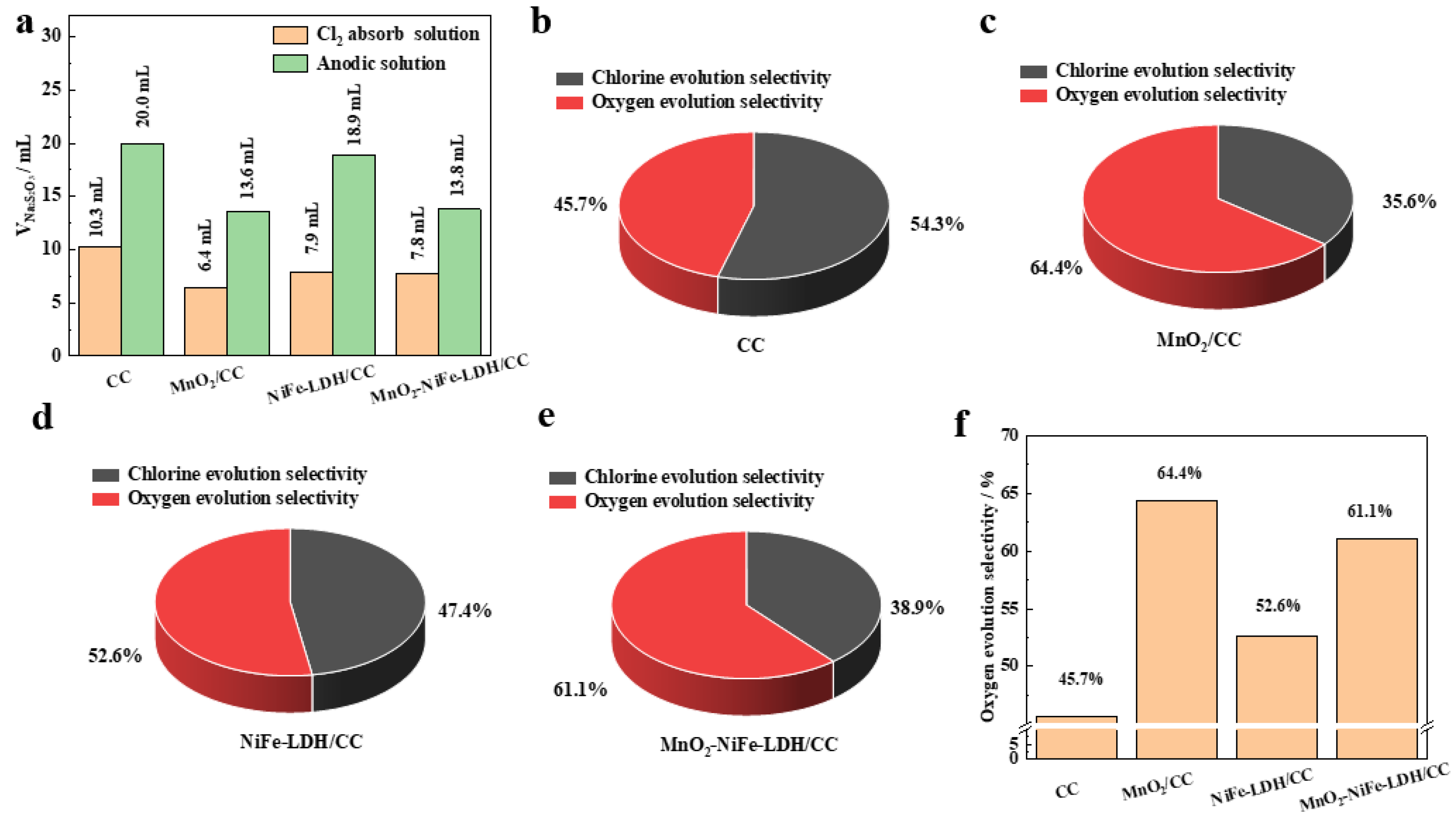
2. Results and Discussion
3. Experiments
3.1. Preparation of the NiFe-LDH/CC Sample
3.2. Preparation of the MnO2-NiFe-LDH/CC Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Li, Z.; Liu, T.; Zhao, S.; Guan, D.; Chen, D.; Shao, Z.; Ni, M. Morphology control and electronic tailoring of CoxAy (A = P, S, Se) electrocatalysts for water splitting. Chem. Eng. J. 2023, 460, 141674–141701. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, Y.; Qiu, H.; Su, C.; Shao, Z. High selectivity electrocatalysts for oxygen evolution reaction and anti-chlorine corrosion strategies in seawater splitting. Catalysts 2022, 12, 261. [Google Scholar] [CrossRef]
- Jiang, S.; Suo, H.; Zhang, T.; Liao, C.; Wang, Y.; Zhao, Q.; Lai, W. Recent advances in seawater electrolysis. Catalysts 2022, 12, 123. [Google Scholar] [CrossRef]
- Sha, Q.; Shen, J.; Yang, G.; Li, T.; Liu, W.; Kuang, Y.; Sun, X. A single-atom Au catalyst boosts high-efficiency electrochemical seawater oxidation. Catalysts 2024, 14, 348. [Google Scholar] [CrossRef]
- Lei, F.; Ma, X.; Shao, X.; Fang, Z.; Wang, Y.; Hao, W. Reasonable regulation of flexible sulfur-based bifunctional catalytic electrodes for efficient seawater splitting. Inorg. Chem. Front. 2024, 11, 2152–2163. [Google Scholar] [CrossRef]
- Khan, I. Pluronic-123 Assisted synthesis of cobalt vanadate microparticles (µ-CoV MPs) for durable electrochemical oxygen evolution reaction in seawater and connate water. Catalysts 2023, 13, 636. [Google Scholar] [CrossRef]
- Xu, L.; Dong, Y.; Xu, W.; Zhang, W. Ultrafast and facile synthesis of (Ni/Fe/Mo)OOH on Ni foam for oxygen evolution reaction in seawater electrolysis. Catalysts 2023, 13, 924. [Google Scholar] [CrossRef]
- Das, D.; Santra, S.; Nanda, K.K. In Situ fabrication of a nickel/molybdenum carbide-anchored N-doped graphene/CNT hybrid: An efficient (Pre)catalyst for OER and HER. ACS Appl. Mater. Interfaces 2018, 10, 35025–35038. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Hua, Q.; Li, C.; Zhu, H.; Gao, L.; Ren, X.; Yang, P.; Liu, A. Enhancing electrochemical performance and corrosion resistance of nickel-based catalysts in seawater electrolysis: Focusing on OER and HER. J. Mater. Chem. A 2024. [Google Scholar] [CrossRef]
- 46th ESAO Congress 3–7 September 2019 Hannover, Germany Abstracts. Int. J. Artif. Organs 2019, 42, 386–474. [CrossRef]
- Wu, H.; Zhao, Z.; Wang, M.; Zheng, W.; Zhang, Y.; Wang, Y.; Ma, T.; Zeng, Z.; Cheng, C.; Li, S. Alkaline-earth-metal regulated metal carbides with bioinspired gradient OH spillover for efficient and long-lasting direct seawater electrolysis. J. Mater. Chem. A 2024, 12, 10755–10763. [Google Scholar] [CrossRef]
- Abdul Razzaq, A.; Chen, G.; Zhao, X.; Yuan, X.; Hu, J.; Li, Z.; Chen, Y.; Xu, J.; Shah, R.; Zhong, J.; et al. Cobalt coordination with pyridines in sulfurized polyacrylonitrile cathodes to form conductive pathways and catalytic M-N4S sites for accelerated Li-S kinetics. J. Energy Chem. 2021, 61, 170–178. [Google Scholar] [CrossRef]
- Wang, C.; Humayun, M.; Debecker, D.P.; Wu, Y. Electrocatalytic water oxidation with layered double hydroxides confining single atoms. Coord. Chem. Rev. 2023, 478, 214973. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, X.Y.; Xiong, B.; Chen, J.F.; Zou, W.; Fu, Z.P.; Lu, Y.L. Exploring the mechanism of the excellent catalytic activity of NiFe-layered double hydroxides in oxygen evolution reactions by modifying the iron content. J. Chem. Res. 2022, 46, 17475198221103519. [Google Scholar] [CrossRef]
- Peng, L.S.; Yang, N.; Yang, Y.Q.; Wang, Q.; Xie, X.Y.; Sun-Waterhouse, D.; Shang, L.; Zhang, T.R.; Waterhouse, G.I.N. Atomic cation-vacancy engineering of NiFe-layered double hydroxides for improved activity and stability towards the oxygen evolution reaction. Angew. Chem. Int. Ed. 2021, 60, 24612–24619. [Google Scholar] [CrossRef] [PubMed]
- An, X.Y.; Hu, Q.B.; Zhu, W.L.; Liu, L.N.; Zhang, Y.S.; Zhao, J.G. Partially amorphous NiFe-based bimetallic hydroxide nanocatalyst for efficient oxygen evolution reaction. Appl. Phys. A-Mater. Sci. Process. 2021, 127, 865. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Liu, S.; Chen, G.; Wu, W.; Li, Y.; Jin, P.J.; Xu, C.L. Amorphous NiFe-layered double hydroxides nanosheets for oxygen evolution reaction. Electrochim. Acta 2020, 356, 136827. [Google Scholar] [CrossRef]
- Wei, H.S.; Liu, J.; Deng, Y.D.; Hu, W.B.; Zhong, C. Studies on the effect of the substrate on the electrocatalytic performance of electrodeposited NiFe hydroxides for oxygen evolution reaction. Int. J. Electrochem. Sci. 2019, 14, 4173–4184. [Google Scholar] [CrossRef]
- Park, K.R.; Jeon, J.; Choi, H.; Lee, J.; Lim, D.H.; Oh, N.; Han, H.; Ahn, C.; Kim, B.; Mhin, S. NiFe layered double hydroxide electrocatalysts for an efficient oxygen evolution reaction. ACS Appl. Energy Mater. 2022, 5, 8592–8600. [Google Scholar] [CrossRef]
- Dionigi, F.; Reier, T.; Pawolek, Z.; Gliech, M.; Strasser, P. Design criteria, operating conditions, and nickel–iron hydroxide catalyst materials for selective seawater electrolysis. ChemSusChem 2016, 9, 962–972. [Google Scholar] [CrossRef]
- Chen, X.; Yu, Y.; Han, X.; Wang, H.; Hua, Y.; Wu, D.; Deng, P.; Xiao, J.; Tian, X.; Li, J. Introducing sulfur to nickel-iron selenide for high-efficiency alkaline seawater electrolysis. Sci. China Chem. 2024, 67, 2747–2754. [Google Scholar] [CrossRef]
- Tang, M.; Du, K.; Yu, R.; Shi, H.; Wang, P.; Guo, Y.; Wei, Q.; Yin, H.; Wang, D. Microzone-acidification-driven degradation mechanism of the NiFe-based anode in seawater electrolysis. ACS Appl. Mater. Interfaces 2024, 16, 3260–3269. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, L.; Li, J.; He, X.; Zheng, Y.; Sun, S.; Fang, X.; Zheng, D.; Luo, Y.; Wang, Y.; et al. High-efficiency overall alkaline seawater splitting: Using a nickel–iron sulfide nanosheet array as a bifunctional electrocatalyst. J. Mater. Chem. A 2023, 11, 1116–1122. [Google Scholar] [CrossRef]
- Sang, S.L.; Meng, H.M.; Shi, Y.H.; Yu, H.Y.; Fan, Z.S.; Sun, D.B. Preparation of oxygen evolution anodes for seawater electrolysis. In Proceedings of the 7th National Surface Engineering Academic Conference and the 2nd Surface Engineering Youth Academic Forum, Wuhan, China, 27–30 April 2015. [Google Scholar]
- Vos, J.G.; Wezendonk, T.A.; Jeremiasse, A.W.; Koper, M.T.M. MnOx/IrOx as selective oxygen evolution electrocatalyst in acidic chloride solution. J. Am. Chem. Soc. 2018, 140, 10270–10281. [Google Scholar] [CrossRef]
- Yan, H.; Wang, X.; Linkov, V.; Ji, S.; Wang, R. Selectivity of oxygen evolution reaction on carbon cloth-supported delta-MnO2 nanosheets in electrolysis of real seawater. Molecules 2023, 28, 854. [Google Scholar] [CrossRef]
- Chang, J.; Zang, S.; Song, F.; Wang, W.; Wu, D.; Xu, F.; Jiang, K.; Gao, Z. Heterostructured nickel, iron sulfide@nitrogen, sulfur co-doped carbon hybrid with efficient interfacial charge redistribution as bifunctional catalyst for water electrolysis. Appl. Catal. A Gen. 2022, 630, 118459–118468. [Google Scholar] [CrossRef]
- Ganesan, P.; Sivanantham, A.; Shanmugam, S. Inexpensive electrochemical synthesis of nickel iron sulphides on nickel foam: Super active and ultra-durable electrocatalysts for alkaline electrolyte membrane water electrolysis. J. Mater. Chem. A 2016, 4, 16394–16402. [Google Scholar] [CrossRef]
- Han, C.; Li, W.; Shu, C.; Guo, H.; Liu, H.; Dou, S.; Wang, J. Catalytic activity boosting of nickel sulfide toward oxygen evolution reaction via confined overdoping engineering. ACS Appl. Energy Mater. 2019, 2, 5363–5372. [Google Scholar] [CrossRef]
- Han, Y.; Zeng, X.; Liu, Y.; Shi, S.; Xiong, P.; Wang, T.; Pan, X.; Li, J.; Hu, W.; Deng, Y. Crystalline–amorphous Ni4.5Fe4.5S8/NiFeS heterostructure for alkaline water oxidation electrocatalysis. Mater. Today Energy 2023, 38, 101442–101450. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, J.-P.; Tang, X.-Q.; Yang, X.; Han, Y.-W.; Lan, M.-J.; Tang, X.; Shen, Y. Highly efficient sulfur-doped Ni3Fe electrocatalysts for overall water splitting: Rapid synthesis, mechanism and driven by sustainable energy. J. Colloid Interface Sci. 2024, 653, 1423–1431. [Google Scholar] [CrossRef]
- Zhang, M.; Chang, S.; Chen, X.; Zhang, Y.; Zhang, Z.; Xue, H.; Deng, Y.; Jiang, Y. Regulating electron-spin state enables enhanced electrocatalytic water splitting properties in bimetallic sulfides. Fuel 2024, 362, 130941–130950. [Google Scholar] [CrossRef]
- Meng, X.-y.; Wang, M.; Zhang, Y.; Li, Z.; Ding, X.; Zhang, W.; Li, C.; Li, Z. Superimposed OER and UOR performances by the interaction of each component in an Fe–Mn electrocatalyst. Dalton Trans. 2022, 51, 16605–16611. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Ma, Y.; Chen, W.; Tang, Y.; Li, L.; Wang, J. Facile one-step electrodeposition of two-dimensional nickel-iron bimetallic sulfides for efficient electrocatalytic oxygen evolution. J. Alloys Compd. 2022, 894, 162533–162541. [Google Scholar] [CrossRef]
- Choi, D.; Ryu, S. Efficient and selective oxygen evolution reaction in seawater electrolysis with electrochemically synthesized amorphous-like NiFes. Electron. Mater. Lett. 2023, 20, 173–182. [Google Scholar] [CrossRef]
- Han, Y.; Shao, L.; Liu, Y.; Li, G.; Wang, T.; Zheng, X.; Li, J.; Han, X.; Hu, W.; Deng, Y. Sulfate-assisted Ni/Fe-based electrodes for anion exchange membrane saline splitting. Nano Res. 2024, 17, 5985–5995. [Google Scholar] [CrossRef]
- Jeung, Y.; Roh, H.; Yong, K. Co-anion exchange prepared 2D structure Ni(Co,Fe)PS for efficient overall water electrolysis. Appl. Surf. Sci. 2022, 576, 151720–151727. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Fan, M.; Yan, H.; Wang, Z.; Song, J.; Wang, H.; Ren, J. Study on the Catalytic Activity and Selectivity of Manganese Dioxide-Modified Nickel–Iron-Based Hydroxide Electrodes for Initiating the Oxygen Evolution Reaction in Natural Seawater. Catalysts 2024, 14, 502. https://doi.org/10.3390/catal14080502
Liu F, Fan M, Yan H, Wang Z, Song J, Wang H, Ren J. Study on the Catalytic Activity and Selectivity of Manganese Dioxide-Modified Nickel–Iron-Based Hydroxide Electrodes for Initiating the Oxygen Evolution Reaction in Natural Seawater. Catalysts. 2024; 14(8):502. https://doi.org/10.3390/catal14080502
Chicago/Turabian StyleLiu, Fangfang, Miaomiao Fan, Haofeng Yan, Zheng Wang, Jimei Song, Hui Wang, and Jianwei Ren. 2024. "Study on the Catalytic Activity and Selectivity of Manganese Dioxide-Modified Nickel–Iron-Based Hydroxide Electrodes for Initiating the Oxygen Evolution Reaction in Natural Seawater" Catalysts 14, no. 8: 502. https://doi.org/10.3390/catal14080502





