Palladium-Catalyzed Regioselective [3+2] Cycloadditions of α,β-Unsaturated Imines with Vinylethylene Carbonates: Access to Oxazolidines
Abstract
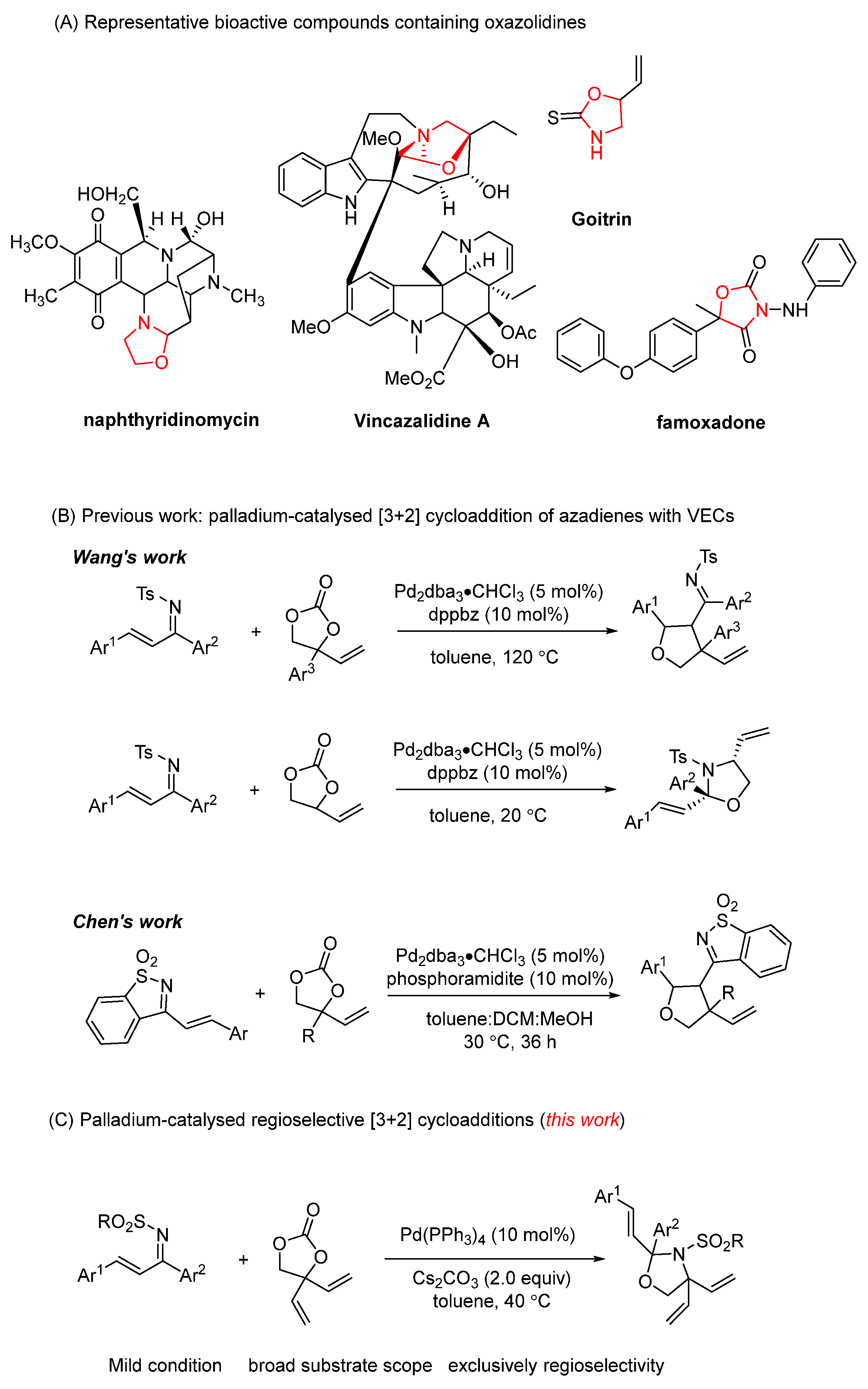
1. Introduction
2. Results
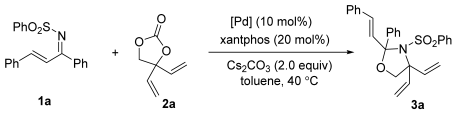
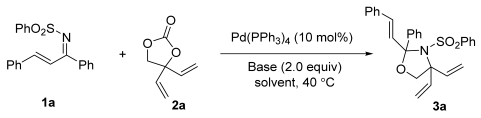
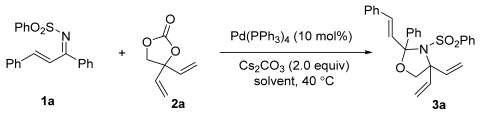
2.1. Studies on Various Palladium Catalysts
2.2. Screening of Different Bases
2.3. Screening of Solvents
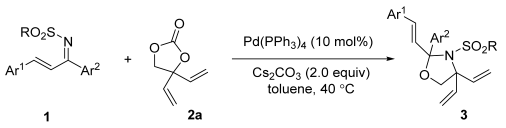
2.4. Substrate Scope
2.5. Gram-Scale Synthesis
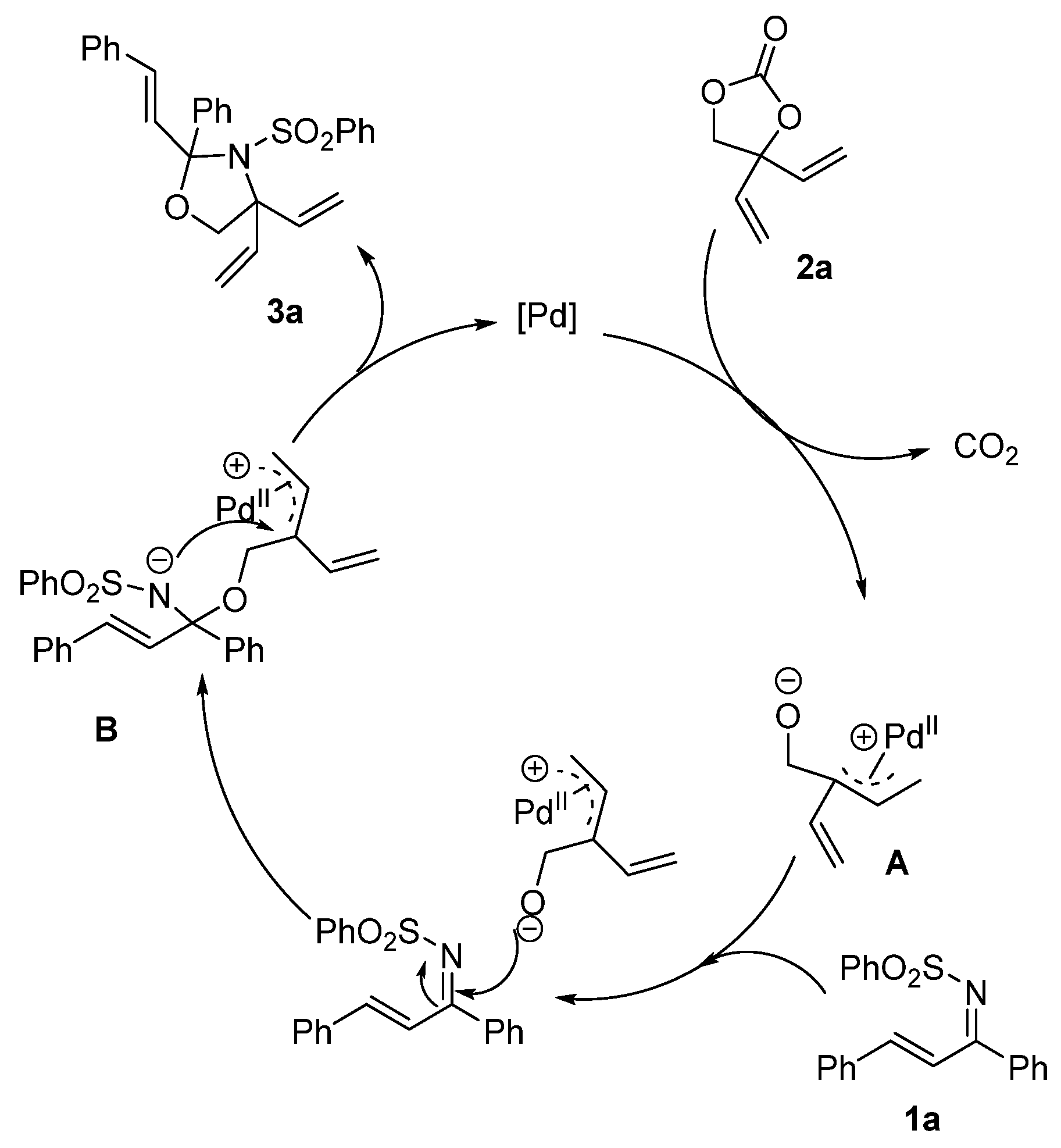
2.6. The Suggested Catalytic Mechanism
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scott, J.D.; Williams, R.M. Chemistry and Biology of the Tetrahydroisoquinoline Antitumor Antibiotics. Chem. Rev. 2002, 102, 1669–1730. [Google Scholar] [CrossRef] [PubMed]
- Kluepfel, D.; Baker, H.A.; Piattoni, G.; Sehgal, S.N.; Sidorowicz, A.; Singh, K.; Vézina, C. Naphthyridinomycin, a New Broad-spectrum Antibiotic. J. Antibiot. 1975, 28, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, Y.; Kase, A.; Okamoto, A.; Suzuki, K.; Hiroki, M.; Kaneda, T.; Uchiyama, N.; Morita, H. Vincazalidine A, a unique bisindole alkaloid from Catharanthus roseus. J. Nat. Med. 2024, 78, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Amorim de Souza, T.; Silva, J.P.R.; Rodrigues, D.F.; Herrera-Acevedo, C.; Barros de Menezes, R.P.; Borges, N.H.; Miranda de Melo, J.I.; Pinto de Siqueira-Júnior, J.; Scotti, M.T.; Abreu, L.S.; et al. Oxazolidines from Neocalyptrocalyx longifolium nhibit MsrA Protein in Methicillin Resistant Staphylococcus aureus. Rev. Bras. Farmacogn. 2023, 33, 1084–1088. [Google Scholar] [CrossRef]
- Liu, Y.; Esser, L.; Bai, H.; Fu, B.; Xia, D.; Zhou, Y.; Hong, S.; Yang, S.; Xiao, Y.; Qin, Z. Synthesis and Antiphytopathogenic Activity of Novel Oxazolidine-2,4-diones Bearing Phenoxypyridine Moiety. J. Agric. Food Chem. 2023, 71, 14199–24120. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Xu, H. Asymmetric catalysis with chiral oxazolidineligands. Chem. Commun. 2011, 47, 3339–3350. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, W. Renaissance of pyridine-oxazolines as chiral ligands for asymmetric catalysis. Chem. Soc. Rev. 2018, 47, 1783–1810. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wang, Q.; Yao, X.; Wei, D.; Liu, Y.-G.; Li, E.-Q.; Duan, Z. Rigid P-Chiral Phosphorus Ligands for Highly Selective Palladium-Catalyzed (4 + 2) and (4 + 4) Annulations. Org. Lett. 2022, 24, 9205–9209. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.-C.; Wang, Y.; Li, E.-Q.; Cui, H.; Duan, Z. Enantio- and Diastereoselective Synthesis of β-Aryl-β-pyrazolyl α-Amino Acid Esters via Copper-Catalyzed Reaction of Azomethine Ylides with Benzylidenepyrazolones. Adv. Synth. Catal. 2019, 361, 1389. [Google Scholar] [CrossRef]
- Guo, W.; Gómez, J.E.; Cristòfol, À.; Xie, J.; Kleij, A.W. Catalytic Transformations of Functionalized Cyclic Organic Carbonates. Angew. Chem. Int. Ed. 2018, 57, 13735–13747. [Google Scholar] [CrossRef]
- Allen, B.D.W.; Lakeland, C.P.; Harrity, J.P.A. Utilizing Palladium-Stabilized Zwitterions for the Construction of N-Heterocycles. Chem.-Eur. J. 2017, 23, 13830–13857. [Google Scholar] [CrossRef]
- Zhang, Y.; Khan, A. Palladium-Catalyzed Asymmetric Decarboxylative Cycloaddition of Vinylethylene Carbonates with Electrophiles: Construction of Quaternary Stereocenters. Synlett 2015, 26, 853–860. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, P.; Li, E.-Q. Recent Advances in Palladium-Catalyzed [4 + n] Cycloaddition of Lactones, Benzoxazinanones, Allylic Carbonates, and Vinyloxetanes. Top. Curr. Chem. 2023, 381, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Meng, Y.; Wu, L.; Li, E.-Q. Recent advances in annulations enabled by nucleophilic Lewis base/metal dual catalysis. Chin. Chem. Lett. 2023, 34, 108544. [Google Scholar] [CrossRef]
- Du, J.; Li, Y.-F.; Ding, C.-H. Recent advances of Pd-π-allyl zwitterions in cycloaddition reactions. Chin. Chem. Lett. 2023, 34, 108401. [Google Scholar] [CrossRef]
- Khan, A.; Zheng, R.; Kan, Y.; Ye, J.; Xing, J.; Zhang, Y.J. Palladium-Catalyzed Decarboxylative Cycloaddition of Vinylethylene Carbonates with Formaldehyde: Enantioselective Construction of Tertiary Vinylglycols. Angew. Chem. Int. Ed. 2014, 53, 6439–6442. [Google Scholar] [CrossRef]
- Singha, S.; Patra, T.; Daniliuc, C.G.; Glorius, F. Highly Enantioselective [5+2] Annulations through Cooperative N-Heterocyclic Carbene (NHC) Organocatalysis and Palladium Catalysis. J. Am. Chem. Soc. 2018, 140, 3551–3554. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Khan, A.; Zheng, R.; Jin, L.Y.; Zhang, Y.J. Pd-Catalyzed Asymmetric Decarboxylative Cycloaddition of Vinylethylene Carbonates with Imines. Org. Lett. 2015, 17, 6230–6233. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-U.; Ahn, H.-I.; Cho, H.-J.; Xuan, Z.; Kim, J.H. Asymmetric Synthesis of N-Fused 1,3-Oxazolidines via Pd-Catalyzed Decarboxylative (3 + 2) Cycloaddition. Adv. Synth. Catal. 2020, 362, 1836–1840. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, D.; Jiang, F.; Liao, J.; Wang, W.; Wu, Y.; Zheng, L.; Guo, H. Palladium-catalyzed stereoselective (3 + 2) cycloaddition of vinylethylene carbonates with cyclic N-sulfonyl ketimines. Org. Biomol. Chem. 2021, 19, 4877–4881. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, Y.; Wang, D.; Zhang, Z.; Wang, C.; Zhou, L.; Zhang, C.; Song, B.; Guo, H. Formal [5+3] Cycloaddition of Zwitterionic Allylpalladium Intermediates with Azomethine Imines for Construction of N,O-Containing Eight-Membered Heterocycles. Adv. Synth. Catal. 2018, 360, 652–658. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, D.; Chen, Y.; Deng, H.; Jiang, F.; Wang, W.; Wu, Y.; Guo, H. Palladium-Catalyzed [5+2] Annulation of Vinylethylene Carbonates with Barbiturate-Derived Alkenes. Org. Lett. 2020, 22, 7158–7163. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Chatani, N.; Murai, S. The First Catalytic Carbonylative [4+1] Cycloaddition Using a 1,3-Conjugated System. A New Transformation of α,β-Unsaturated Imines to Unsaturated γ-Lactams Catalyzed by Ru3(CO)12. J. Am. Chem. Soc. 1999, 121, 1758–1759. [Google Scholar] [CrossRef]
- Han, B.; Li, J.-L.; Ma, C.; Zhang, S.-J.; Chen, Y.-C. Organocatalytic Asymmetric Inverse-Electron-Demand Aza-Diels–Alder Reaction of N-Sulfonyl-1-aza-1,3-butadienes and Aldehydes. Angew. Chem. Int. Ed. 2008, 47, 9971–9974. [Google Scholar] [CrossRef]
- Lu, L.Q.; Zhang, J.J.; Li, F.; Cheng, Y.; An, J.; Chen, J.R.; Xiao, W.J. Tuning Electronic and Steric Effects: Highly Enantioselective [4+1] Pyrroline Annulation of Sulfur Ylides with α,β-Unsaturated Imines. Angew. Chem. Int. Ed. 2010, 49, 4495–4498. [Google Scholar] [CrossRef]
- Tian, J.; Zhou, R.; Sun, H.; Song, H.; He, Z. Phosphine-Catalyzed [4+1] Annulation between α,β-Unsaturated Imines and Allylic Carbonates: Synthesis of 2-Pyrrolines. J. Org. Chem. 2011, 76, 2374–2378. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Jia, P.; Liang, L.; Huang, Y. Phosphine-Catalyzed Sequential [2+3] and [3+2] Annulation Domino Reaction of γ-Benzyl-Substituted Allenoates with α,β-Unsaturated Ketimines To Construct aza-Bicyclo[3,3,0]octane Derivatives. ACS Catal. 2014, 4, 600–603. [Google Scholar] [CrossRef]
- Tan, W.-W.; Ong, Y.-J.; Yoshikai, N. Synthesis of Highly Substituted Pyridines through Copper-Catalyzed Condensation of Oximes and α,β-Unsaturated Imines. Angew. Chem. 2017, 129, 8352–8356. [Google Scholar] [CrossRef]
- Rong, Z.-Q.; Yang, L.-C.; Liu, S.; Yu, Z.; Wang, Y.-N.; Tan, Z.Y.; Huang, R.-Z.; Lan, Y.; Zhao, Y. Nine-Membered Benzofuran-Fused Heterocycles: Enantioselective Synthesis by Pd-Catalysis and Rearrangement via Transannular Bond Formation. J. Am. Chem. Soc. 2017, 139, 15304–15307. [Google Scholar] [CrossRef]
- Li, Q.-Z.; Guan, Y.-L.; Huang, Q.-W.; Qi, T.; Xiang, P.; Zhang, X.; Leng, H.-J.; Li, J.-L. Temperature-Controlled Divergent Asymmetric Synthesis of Indole-Based Medium-Sized Heterocycles through Palladium Catalysis. ACS Catal. 2023, 13, 1164–1172. [Google Scholar] [CrossRef]
- Liu, W.; Zang, M.; Zhang, J.; Wang, Q.; Liu, Y.-Z.; Deng, W.-P. Pd-catalyzed exclusively regioselective [5+4] cycloaddition for the construction of 1,5-di/ox-azonanes. Org. Chem. Front. 2023, 10, 1680–1685. [Google Scholar] [CrossRef]
- Xie, X.; Yuan, D.; Ma, B.; Jin, J.; Wang, E.; Zhou, W.; Hu, Y.; Hu, L.; Wang, J. Sterically and Temperature Controlled Divergent Cycloadditions of α,β-Unsaturated Imines with Vinylethylene Carbonates: Insights from Experimental and DFT Studies. Adv. Synth. Catal. 2022, 364, 1168–1178. [Google Scholar] [CrossRef]
- Ke, M.; Qiao, B.; Yu, Y.; Li, X.; Xiao, X.; Li, S.-J.; Lan, Y.; Chen, F. Palladium-Catalyzed Asymmetric [3+2] Annulation of Vinylethylene Carbonates with Alkenes Installed on Cyclic N-Sulfonyl Imines: Highly Enantio- and Diastereoselective Construction of Chiral Tetrahydrofuran Scaffolds Bearing Three Vicinal and Quaternary Stereocenters. J. Org. Chem. 2022, 87, 5166–5177. [Google Scholar] [PubMed]
- Shi, B.; Liu, J.-B.; Wang, Z.-T.; Wang, L.; Lan, Y.; Lu, L.-Q.; Xiao, W.-J. Synthesis of Chiral Endocyclic Allenes by Palladium-Catalyzed Asymmetric Annulation Followed by Cope Rearrangement. Angew. Chem. Int. Ed. 2022, 61, e202117215. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Kleij, A.W.; Yang, W. Diversity-Orientated Stereoselective Synthesis through Pd-Catalyzed Switchable Decarboxylative C−N/C−S Bond Formation in Allylic Surrogates. Chem. Eur. J. 2018, 24, 19156. [Google Scholar] [CrossRef] [PubMed]

 | ||
|---|---|---|
| Entry | [Pd] | Yield (%) b |
| 1 | Pd(PPh3)4 | 82 |
| 2 | Pd2dba3 | 82 |
| 3 | Pd(dba)2 | 67 |
| 4 | PdCl2 | 20 |
| 5 | Pd2dba3·CHCl3 | 78 |
| 6 | Pd(OAc)2 | 9 |
| 7 | Pd(CH3CN)2Cl2 | 75 |
 | ||
|---|---|---|
| Entry | Base | Yield (%) b |
| 1 | Cs2CO3 | 82 |
| 2 | Na2CO3 | 57 |
| 3 | K2CO3 | 76 |
| 4 | LiOH | 64 |
| 5 | NaHCO3 | 57 |
| 6 | K2HPO4 | 59 |
| 7 | DMAP | 24 |
| 8 | DBU | - |
 | ||
|---|---|---|
| Entry | Solvent | Yield (%) b |
| 1 | Toluene | 82 |
| 2 | CH2Cl2 | 72 |
| 3 | CHCl3 | 45 |
| 4 | MeCN | 49 |
| 5 | DMF | 24 |
| 6 | Et2O | 73 |
| 7 | EtOAc | 66 |
| 8 | THF | 67 |
| 9 | 1,4-dioxane | 57 |
 | ||||
|---|---|---|---|---|
| Entry | R | Ar1 | Ar2 | Yield (%) b |
| 1 | Ph | Ph | Ph | 82 (3a) |
| 2 | 4-MeC6H4 | Ph | Ph | 56 (3b) |
| 3 | 4-BrC6H4 | Ph | Ph | 61 (3c) |
| 4 | 4-NO2C6H4 | Ph | Ph | 84 (3d) |
| 5 | 4-CF3C6H4 | Ph | Ph | 60 (3e) |
| 6 | 4-FC6H4 | Ph | Ph | 72 (3f) |
| 7 | 4-MeOC6H4 | Ph | Ph | 30 (3g) |
| 8 | 3-BrC6H4 | Ph | Ph | 32 (3h) |
| 9 | Ph | 4-MeC6H4 | Ph | 27 (3i) |
| 10 | Ph | 4-FC6H4 | Ph | 22 (3j) |
| 11 | Ph | 2,4,6-Me3C6H2 | Ph | 53 (3k) |
| 12 | Ph | 4-ClC6H4 | Ph | 63 (3l) |
| 13 | Ph | 2-FC6H4 | Ph | 68 (3m) |
| 14 | Ph | Ph | 4-ClC6H4 | 77 (3n) |
| 15 | Ph | Ph | 4-FC6H4 | 84 (3o) |
| 16 | Ph | Ph | 3-BrC6H4 | 32 (3p) |
| 17 | 4-MeC6H4 | Ph | 4-ClC6H4 | 37 (3q) |
| 18 | Ph | Ph | 3,5-MeC6H3 | 53 (3r) |
| 19 | Ph | 4-ClC6H4 | 3,5-MeC6H3 | 50 (3s) |
| 20 | Ph | 3-MeC6H4 | 4-ClC6H4 | 36 (3t) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Y.; Sun, T.; Liu, Q.; Li, E.-Q. Palladium-Catalyzed Regioselective [3+2] Cycloadditions of α,β-Unsaturated Imines with Vinylethylene Carbonates: Access to Oxazolidines. Catalysts 2024, 14, 508. https://doi.org/10.3390/catal14080508
Wang Y, Wang Y, Sun T, Liu Q, Li E-Q. Palladium-Catalyzed Regioselective [3+2] Cycloadditions of α,β-Unsaturated Imines with Vinylethylene Carbonates: Access to Oxazolidines. Catalysts. 2024; 14(8):508. https://doi.org/10.3390/catal14080508
Chicago/Turabian StyleWang, Yuanbo, Yue Wang, Tong Sun, Qinglin Liu, and Er-Qing Li. 2024. "Palladium-Catalyzed Regioselective [3+2] Cycloadditions of α,β-Unsaturated Imines with Vinylethylene Carbonates: Access to Oxazolidines" Catalysts 14, no. 8: 508. https://doi.org/10.3390/catal14080508
APA StyleWang, Y., Wang, Y., Sun, T., Liu, Q., & Li, E.-Q. (2024). Palladium-Catalyzed Regioselective [3+2] Cycloadditions of α,β-Unsaturated Imines with Vinylethylene Carbonates: Access to Oxazolidines. Catalysts, 14(8), 508. https://doi.org/10.3390/catal14080508










