Unveiling the Synergistic Effect of Two-Dimensional Heterostructure NiFeP@FeOOH as Stable Electrocatalyst for Oxygen Evolution Reaction
Abstract
:1. Introduction
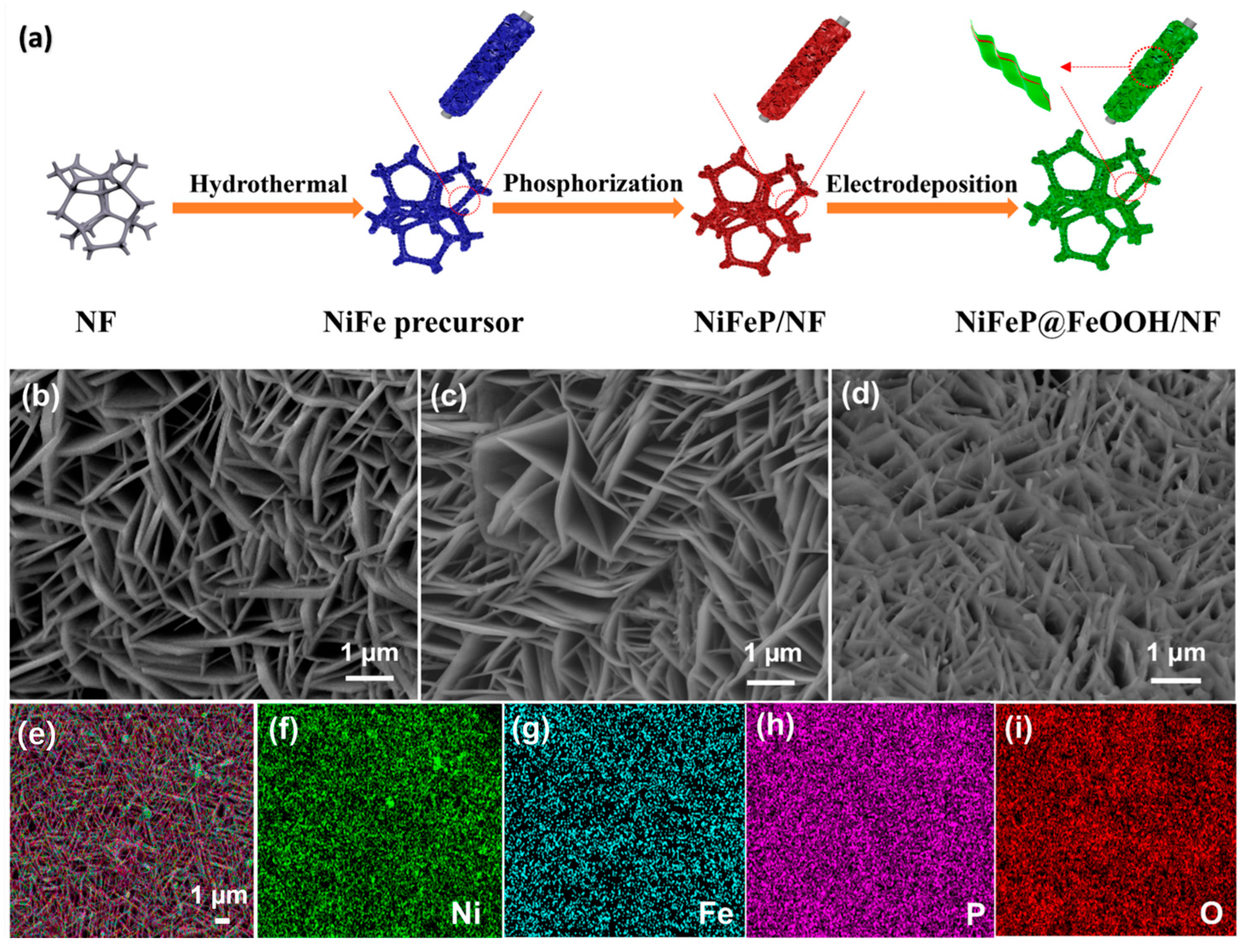
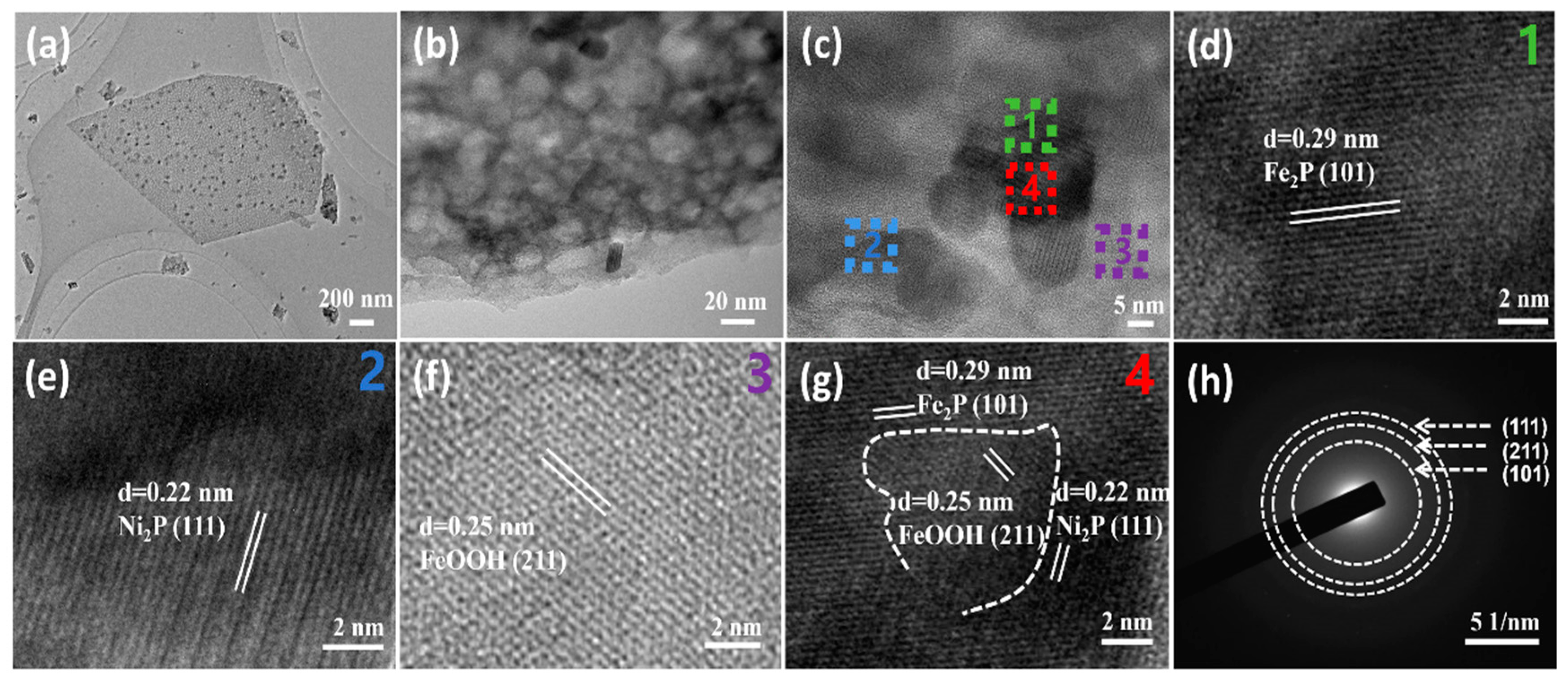
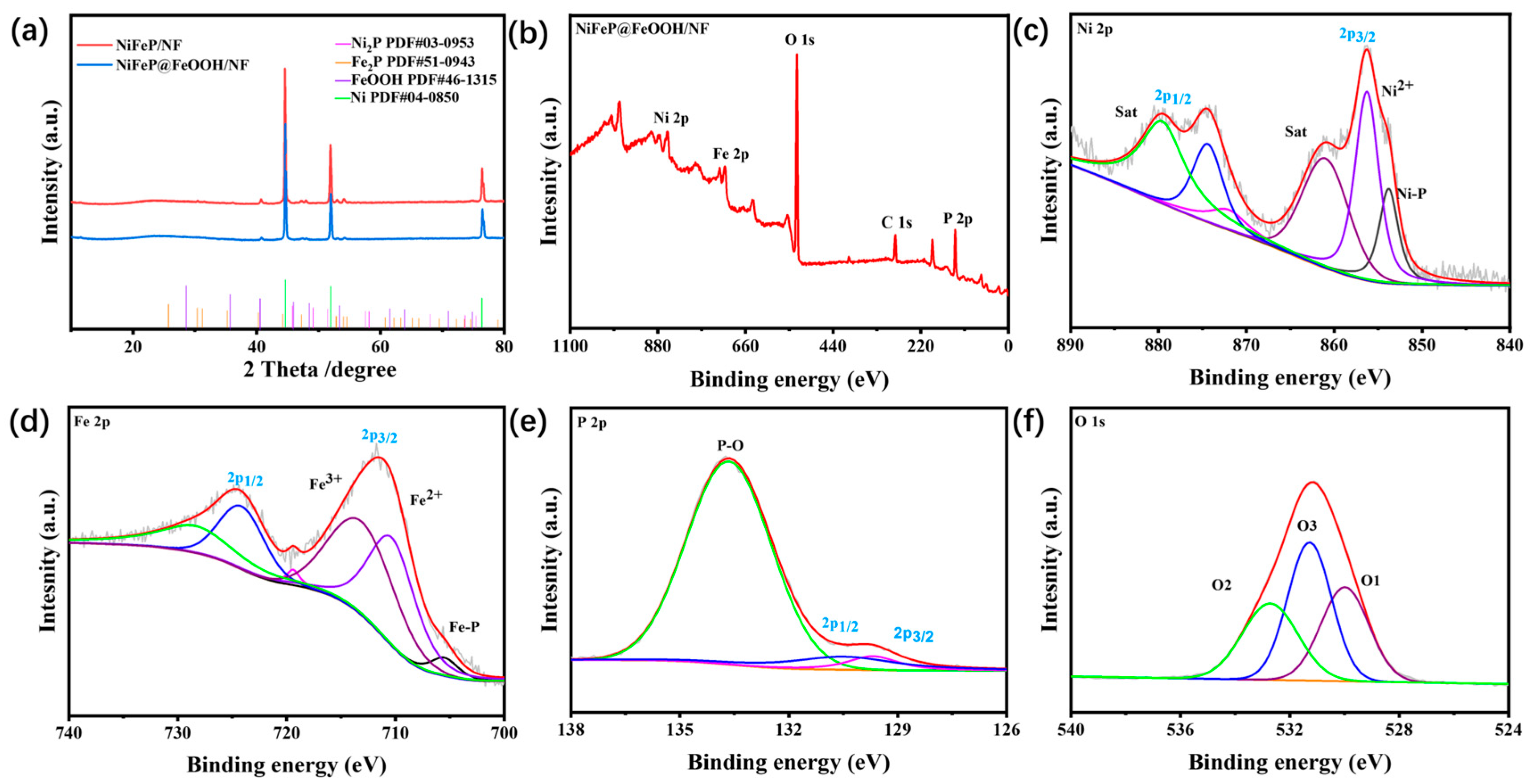
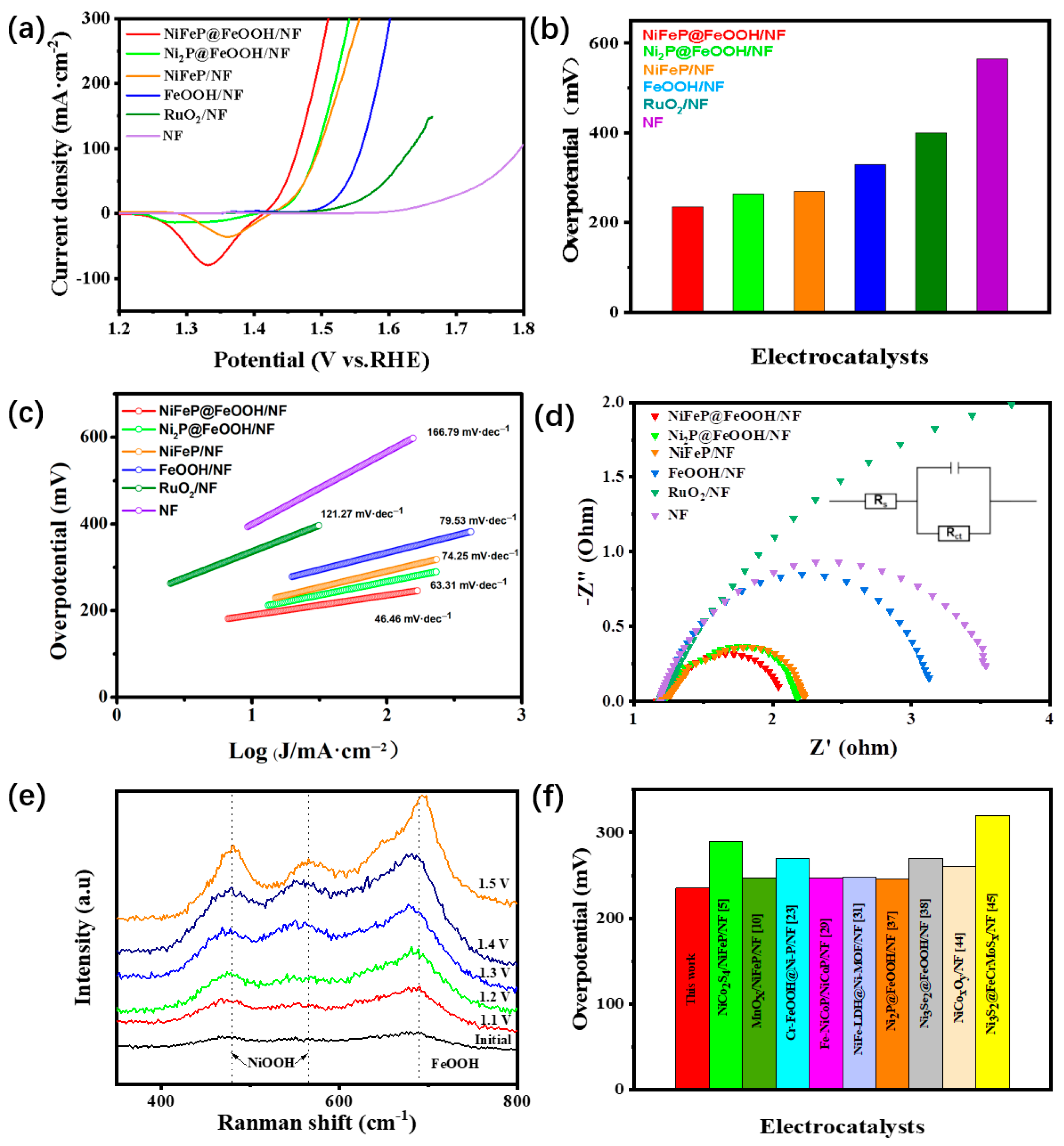
2. Results and Discussion
3. Experimental
3.1. Chemicals
3.2. Synthesis
3.2.1. Synthesis of NiFe Precursor/NF
3.2.2. Synthesis of NiFeP/NF
3.2.3. Synthesis of NiFeP@FeOOH/NF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gono, P.; Pasquarello, A. High-performance NiOOH/FeOOH electrode for OER catalysis. J. Chem. Phys. 2021, 154, 024706. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Qian, Y.; Jiang, H.L. Metal-Organic frameworks for photocatalytic water splitting and CO2 reduction. Angew. Chem. Int. Ed. 2023, 135, e202217565. [Google Scholar] [CrossRef]
- Li, L.; Cao, X.; Huo, J.; Qu, J.; Chen, W.; Liu, C.; Zhao, Y.; Liu, H.; Wang, G. High valence metals engineering strategies of Fe/Co/Ni-based catalysts for boosted OER electrocatalysis. J. Energy Chem. 2023, 76, 195–213. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Lee, S.J.; Murthy, A.P.; Madhavan, J.; Choi, M.Y. Fundamental aspects and recent advances in transition metal nitrides as electrocatalysts for hydrogen evolution reaction: A review. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100805. [Google Scholar] [CrossRef]
- Jiang, J.; Li, F.; Su, H.; Gao, Y.; Li, N.; Ge, L. Flower-like NiCo2S4/NiFeP/NF composite material as an effective electrocatalyst with high overall water splitting performance. Chin. Chem. Lett. 2022, 33, 4367–4374. [Google Scholar] [CrossRef]
- Li, L.; Guo, C.; Ning, J.; Zhong, Y.; Chen, D.; Hu, Y. Oxygen-vacancy-assisted construction of FeOOH/CdS heterostructure as an efficient bifunctional photocatalyst for CO2 conversion and water oxidation. Appl. Catal. B Environ. 2021, 293, 120203. [Google Scholar] [CrossRef]
- Shahzad, A.; Zulfiqar, F.; Nadeem, M.A. Cobalt containing bimetallic ZIFs and their derivatives as OER electrocatalysts: A critical review. Coord. Chem. Rev. 2023, 477, 214925. [Google Scholar] [CrossRef]
- Zeng, F.; Mebrahtu, C.; Liao, L.; Beine, A.K.; Palkovits, R. Stability and deactivation of OER electrocatalysts: A review. J. Energy Chem. 2022, 69, 301–329. [Google Scholar] [CrossRef]
- Wu, H.; Huang, Q.; Shi, Y.; Chang, J.; Lu, S. Electrocatalytic water splitting: Mechanism and electrocatalyst design. Nano Res. 2023, 16, 9142–9157. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Y.; Zhang, G.; Wu, M.; Chen, Z.; Sun, S.; Shi, Z. MnOx-decorated nickel-iron phosphides nanosheets: Interface modifications for robust overall water splitting at ultra-high current densities. Small 2022, 18, e2105803. [Google Scholar] [CrossRef]
- Song, J.; Wei, C.; Huang, Z.F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Meng, X.; Huang, H.; Zheng, L.; Chen, H.; Zhang, Y.; Yuan, W.; Zhang, L.Y. Controllable Synthesis of Web-Footed PdCu Nanosheets and Their Electrocatalytic Applications. Small 2022, 18, 2107623. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; He, J.; Wang, H.; Lin, J.; Chen, R.; Yi, K.; Huang, F.; Lin, Z.; Wang, M. Te-mediated electro-driven oxygen evolution reaction. Nano Res. Energy 2022, 1, e9120029. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, D.; Chen, G.; Zhang, N.; Wan, H.; Liu, X.; Ma, R. Microcrystallization and lattice contraction of NiFe LDHs for enhancing water electrocatalytic oxidation. Carbon Energy 2022, 4, 901–913. [Google Scholar] [CrossRef]
- Wu, B.; Gong, S.; Lin, Y.; Li, T.; Chen, A.; Zhao, M.; Chen, L. A unique NiOOH@FeOOH heteroarchitecture for enhanced oxygen evolution in saline water. Adv. Mater. 2022, 34, 2108619. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Mai, H.D.; Nam, K.H.; Park, C.M.; Jeon, K.J. Self-Healing Graphene-Templated Platinum–Nickel Oxide Heterostructures for Overall Water Splitting. ACS Nano 2022, 16, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, P.; Jose, V.; Lee, J.M. Design Strategies for Development of TMD-Based Heterostructures in Electrochemical Energy Systems. Matter 2020, 2, 526–553. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, J.; Xu, Z.; Wang, Q.; Wu, J.; Zhang, E.; Dou, S.; Sun, W.; Wang, D.; Li, Y. Ru–Co Pair Sites Catalyst Boosts the Energetics for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2022, 61, e202205946. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Wu, X. Realizing efficient electrochemical overall water electrolysis through hierarchical CoP@NiCo-LDH nanohybrids. Nano Energy 2023, 114, 108681. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Kwon, K. A review on biomass-derived N-doped carbons as electrocatalysts in electrochemical energy applications. Chem. Eng. J. 2022, 446, 137116. [Google Scholar] [CrossRef]
- De, A.; Karmakar, A.; Kundu, S. Enhancing the Surface-Active Sites of Bimetallic 2D Hydroxide Materials by Introducing Fe2+ Ions toward Effective Hydroxide Adsorption for the Water Oxidation Reaction. ACS Appl. Energy Mater. 2023, 6, 5761–5773. [Google Scholar] [CrossRef]
- Wang, D.; Umar, A.; Wu, X. Enhanced water electrolysis performance of bifunctional NiCoP electrocatalyst in alkaline media. J. Electroanal. Chem. 2023, 950, 117888. [Google Scholar] [CrossRef]
- Xu, S.; Du, Y.; Yu, X.; Wang, Z.; Wang, X.; Cheng, X.; Liu, Q.; Luo, Y.; Suo, X.; Wu, Q. A Cr-FeOOH@Ni-P/NF binder-free elec-trode as an excellent oxygen evolution reaction electrocatalyst. Nanoscale. 2021, 13, 17003–17010. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Y.; Yuan, M.; Hao, H.; Lv, Z.; Xu, L.; Wei, B. Operando spectroscopies unveil interfacial FeOOH induced highly reactive β-Ni(Fe)OOH for efficient oxygen evolution. Appl. Catal. B Environ. 2022, 318, 121825. [Google Scholar] [CrossRef]
- Wang, X.; Xiang, R.; Li, S.; Song, K.; Huang, W. Self-standing 2D/2D Co3O4@FeOOH nanosheet arrays as promising catalysts for the oxygen evolution reaction. Dalton Trans. 2023, 52, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, F.; Li, X.; Wang, S.; Wang, Y.; Zha, Q.; Ni, Y. Connected design of Ni foam-supported porous Ni and FeOOH/porous Ni electrocatalysts for overall water splitting in alkaline media. J. Alloy. Compd. 2023, 942, 169014. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Long, X.; Chen, L.; Cao, Q.; Wang, J.; Qiu, C.; Lim, J.; Yang, S. Formation of FeOOH nanosheets induces sub-stitutional doping of CeO2-x with high-valence Ni for efficient water oxidation. Adv. Energy Mater. 2021, 11, 2002731. [Google Scholar] [CrossRef]
- Dai, M.; Wang, R. Synthesis and Applications of Nanostructured Hollow Transition Metal Chalcogenides. Small 2021, 17, 2006813. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, Y.; Liu, C.; Cheng, T.; Xu, S.; Li, D.; Jiang, D. Template confined construction of Fe-NiCoP/NiCoP/NF hetero-structures for highly efficient electrocatalytic oxygen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 37746–37756. [Google Scholar] [CrossRef]
- Chowdhury, P.R.; Medhi, H.; Bhattacharyya, K.G.; Hussain, C.M. Recent progress in the design and functionalization strategies of transition metal-based layered double hydroxides for enhanced oxygen evolution reaction: A critical review. Coord. Chem. Rev. 2023, 483, 215083. [Google Scholar] [CrossRef]
- Zeng, X.; Cai, Z.; Zhang, V.; Wang, D.; Xu, J.; Wang, X. Novel NiFe-LDH@Ni-MOF/NF heterostructured electrocatalysts for efficient oxygen evolution. Mater. Res. Lett. 2022, 10, 88–96. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, M.; Song, L.; Zhang, M. Research Advances in Amorphous-Crystalline Heterostructures Toward Efficient Electrochemical Applications. Small 2022, 19, e2206081. [Google Scholar] [CrossRef]
- Zhao, M.; Cheng, X.; Xiao, H.; Gao, J.; Xue, S.; Wang, X.; Wu, H.; Jia, J.; Yang, N. Cobalt-iron oxide/black phosphorus nanosheet heterostructure: Electrosynthesis and performance of (photo-)electrocatalytic oxygen evolution. Nano Res. 2022, 16, 6057–6066. [Google Scholar] [CrossRef]
- Liang, H.; Gandi, A.; Xia, C.; Hedhili, M.; Anjum, D.; Schwingenschloogl, U.; Alshareef, H. Amorphous NiFe-OH/NiFeP electrocatalyst fabricated at low temperature for water oxidation applications. ACS Energy Lett. 2017, 2, 1035–10426. [Google Scholar] [CrossRef]
- Feng, J.; Chen, M.; Zhou, P.; Liu, D.; Chen, Y.; He, B.; Bai, H.; Liu, D.; Ip, W.; Chen, D.; et al. Recon-struction optimization of distorted FeOOH/Ni hydroxide for enhanced oxygen evolution reaction. Mater. Today Energy. 2022, 27, 101005. [Google Scholar] [CrossRef]
- Huang, C.; Qin, P.; Luo, Y.; Ruan, Q.; Liu, L.; Wu, Y.; Li, Q.; Xu, Y.; Liu, R.; Chu, P. Recent progress and perspective of cobalt-based catalysts for water splitting: Design and nanoarchitectonics. Mater. Today Energy. 2022, 23, 100911. [Google Scholar] [CrossRef]
- Zhang, Y.; You, L.; Liu, Q.; Li, Y.; Li, T.; Xue, Z.; Li, G. Interfacial Charge Transfer in a Hierarchical Ni2P/FeOOH Heterojunction Facilitates Electrocatalytic Oxygen Evolution. ACS Appl. Mater. Interfaces 2021, 13, 2765–2771. [Google Scholar] [CrossRef]
- Gao, J.; Ma, H.; Zhang, L.; Luo, X.; Yu, L. Interface engineering of Ni3Se2@FeOOH heterostructure nanoforests for highly-efficient overall water splitting. J. Alloys Compd. 2022, 893, 162244. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Ni, Y. Grass-like Ni/Cu nanosheet arrays grown on copper foam as efficient and non-precious catalyst for hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 268, 118392. [Google Scholar] [CrossRef]
- Luo, R.; Li, Y.; Xing, L.; Wang, N.; Zhong, R.; Qian, Z.; Du, C.; Yin, G.; Wang, Y.; Du, L. A dynamic Ni(OH)2-NiOOH/NiFeP heterojunction enabling high-performance E-upgrading of hydroxymethylfurfural. Appl. Catal. B Environ. 2022, 311, 121357. [Google Scholar] [CrossRef]
- Li, J.; Song, M.; Hu, Y.; Zhu, Y.; Zhang, J.; Wang, D. Hybrid heterostructure Ni3N|NiFeP/FF self-supporting electrode for high-current-density alkaline water Electrolysis. Small Methods 2023, 7, e2201616. [Google Scholar] [CrossRef]
- Chen, T.; Li, B.; Song, K.; Wang, C.; Ding, J.; Liu, E.; Chen, B.; He, F. Defect-activated surface reconstruction: Mechanism for triggering the oxygen evolution reaction activity of NiFe phosphide. J. Mater. Chem. A 2022, 10, 22750–22759. [Google Scholar] [CrossRef]
- Zhu, T.; Han, J.; Sun, T.; Chen, J.; Wang, S.; Ren, S.; Pi, X.; Xu, J.; Chen, K. Interface-Enhanced SiOx/Ru Heterocatalysts for Efficient Electrochemical Water Splitting. ACS Appl. Mater. Interfaces 2023, 15, 8200–8207. [Google Scholar] [CrossRef]
- Guo, G.; Zhong, D.; Zhao, T.; Liu, G.; Li, J.; Zhao, Q. NiCo-BDC derived Co3+ enriched NiCoxOy/NF nanosheets for oxygen evolution reaction. Int. J. Hydrogen Energy. 2022, 47, 23094–23105. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Zhong, H.; Ma, X.; Cao, Z. Morphology restructuring: MOFs derived Ni3S2@FeCrMoSx/NF as a highly efficient and stable catalyst for water oxidation. Int. J. Hydrogen Energy 2023, 48, 1852–1862. [Google Scholar] [CrossRef]
- Hao, H.; Li, Y.; Wu, Y.; Wang, Z.; Yuan, M.; Miao, J.; Lv, Z.; Xu, L.; Wei, B. In-situ probing the rapid reconstruction of FeOOH-decorated NiMoO4 nanowires with boosted oxygen evolution activity. Mater. Today Energy. 2022, 23, 100887. [Google Scholar] [CrossRef]
- Zhuang, S.; Tong, S.; Wang, H.; Xiong, H.; Gong, Y.; Tang, Y.; Liu, J.; Chen, Y.; Wan, P. The P/NiFe doped NiMoO4 micro-pillars arrays for highly acreaction towards overall water splitting. Int. J. Hydrogen Energy. 2019, 44, 24546–24558. [Google Scholar] [CrossRef]
- Shi, X.; Li, J.; Zhang, X.; Guo, J. MOF derived NiCo-LDH hollow structure for oxygen evolution reaction. J. Phys. Chem. Solids. 2023, 172, 11105. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, X.; Yang, F.; Liu, Z. Synergistic effect of Co and Fe bimetallic oxides/hydroxides composite structure as a bifunctional electrocatalyst for enhancing overall water splitting performance. J. Alloys Compd. 2022, 895, 162614. [Google Scholar] [CrossRef]
- Liang, D.; Mao, J.; Liu, P.; Li, J.; Yan, J.; Song, W. In-situ doping of Co in nickel selenide nanoflower for robust electrocatalysis towards oxygen evolution. Int. J. Hydrogen Energy. 2020, 45, 27047–27055. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, K.; Yao, Q.; Wu, C.; Huang, M.; Guan, L. Heterogeneous Ni-Fe-P integrated with nickel foam as an efficient and durable electrocatalyst for water oxidation. Int. J. Hydrogen Energy. 2019, 44, 11684–11694. [Google Scholar] [CrossRef]
- Bian, J.; Song, Z.; Li, X.; Zhang, Y.; Cheng, C. Nickel iron phosphide ultrathin nanosheets anchored on nitrogen-doped carbon nanoflake arrays as a bifunctional catalyst for efficient overall water splitting. Nanoscale. 2020, 12, 8443–8452. [Google Scholar] [CrossRef]
- Read, C.G.; Callejas, J.F.; Holder, C.F.; Schaak, R.E. General Strategy for the Synthesis of transition metal phosphide films for electrocatalytic hydrogen and oxygen evolution. ACS Appl. Mater. Interfaces. 2016, 8, 12798–12803. [Google Scholar] [CrossRef]
- Lin, J.; Yan, Y.; Li, C.; Si, X.; Wang, H.; Qi, J.; Cao, J.; Zhong, Z.; Fei, W.; Feng, J. Bifunctional electrocatalysts based on Mo-doped NiCoP nanosheet arrays for overall water splitting. Nano-Micro Lett. 2019, 11, 55. [Google Scholar] [CrossRef]
- Lin, Y.; Pan, Y.; Liu, S.; Sun, K.; Cheng, Y.; Liu, M.; Wang, Z.; Li, X.; Zhang, J. Construction of multi-dimensional core/shell Ni/NiCoP nano-heterojunction for efficient electrocatalytic water splitting. Appl. Catal. B Environ. 2019, 259, 118039. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Vasileff, A.; Qiao, S.Z. Anion and cation modulation in metal compounds for bifunctional overall water splitting. ACS nano. 2016, 10, 8738–8745. [Google Scholar] [CrossRef]
- Dong, G.; Chen, T.; Xie, F.; Xue, D.; Liu, T.; Chen, L.; Xia, J.; Du, S.; Wang, F.; Xie, F.; et al. NiFeP composites supported on Ni foam as an efficient and robust bifunctional electrocatalyst for overall water splitting in alkaline solution. J. Alloys Compd. 2023, 968, 171764. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Deng, H.; Zhang, C.; Su, J.W.; Dong, Y.; Lin, J. Ternary nickel iron phosphide supported on nickel foam as a high-efficiency electrocatalyst for overall water splitting. Int. J. Hydrogen Energy. 2018, 43, 7299–7306. [Google Scholar] [CrossRef]
- Wang, X.D.; Chen, H.Y.; Xu, Y.F.; Liao, J.F.; Chen, B.X.; Rao, H.S.; Kuang, D.B.; Su, C.Y. Self-supported NiMoP2 nanowires on carbon cloth as an efficient and durable electrocatalyst for overall water splitting. J. Mater. Chem. A. 2017, 5, 7191–7199. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Q.; Jiang, Z.; Wang, C.; Yang, S.; Liu, H.; Xing, B.; Cheng, H.; Wang, K. Unveiling the Synergistic Effect of Two-Dimensional Heterostructure NiFeP@FeOOH as Stable Electrocatalyst for Oxygen Evolution Reaction. Catalysts 2024, 14, 511. https://doi.org/10.3390/catal14080511
Hou Q, Jiang Z, Wang C, Yang S, Liu H, Xing B, Cheng H, Wang K. Unveiling the Synergistic Effect of Two-Dimensional Heterostructure NiFeP@FeOOH as Stable Electrocatalyst for Oxygen Evolution Reaction. Catalysts. 2024; 14(8):511. https://doi.org/10.3390/catal14080511
Chicago/Turabian StyleHou, Qinglong, Zhigang Jiang, Chen Wang, Shuhan Yang, Haizhen Liu, Bo Xing, Honghui Cheng, and Kuikui Wang. 2024. "Unveiling the Synergistic Effect of Two-Dimensional Heterostructure NiFeP@FeOOH as Stable Electrocatalyst for Oxygen Evolution Reaction" Catalysts 14, no. 8: 511. https://doi.org/10.3390/catal14080511





