Defective TiO2/MIL-88B(Fe) Photocatalyst for Tetracycline Degradation: Characterization and Augmented Photocatalytic Efficiency
Abstract
1. Introduction
2. Results
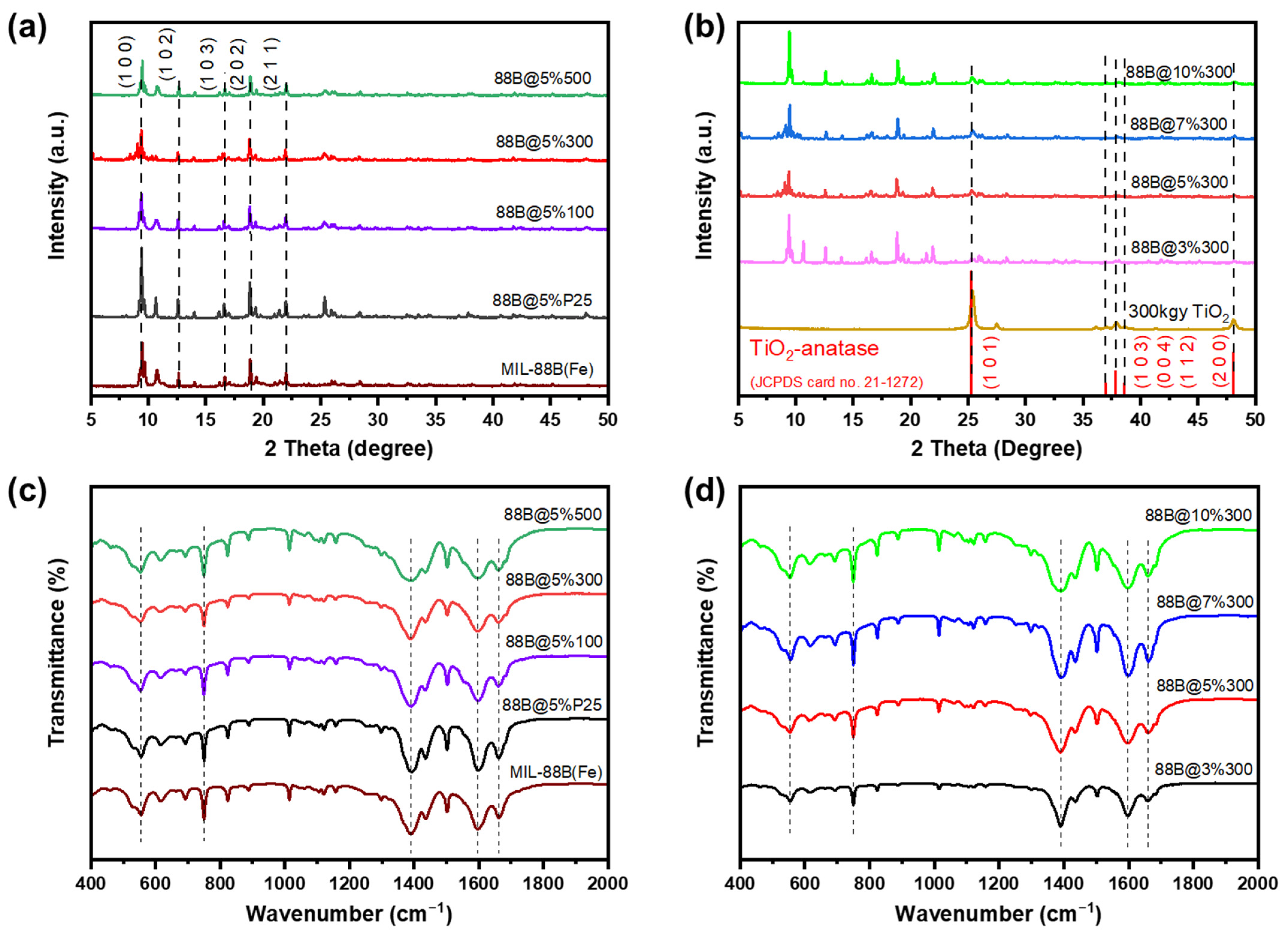
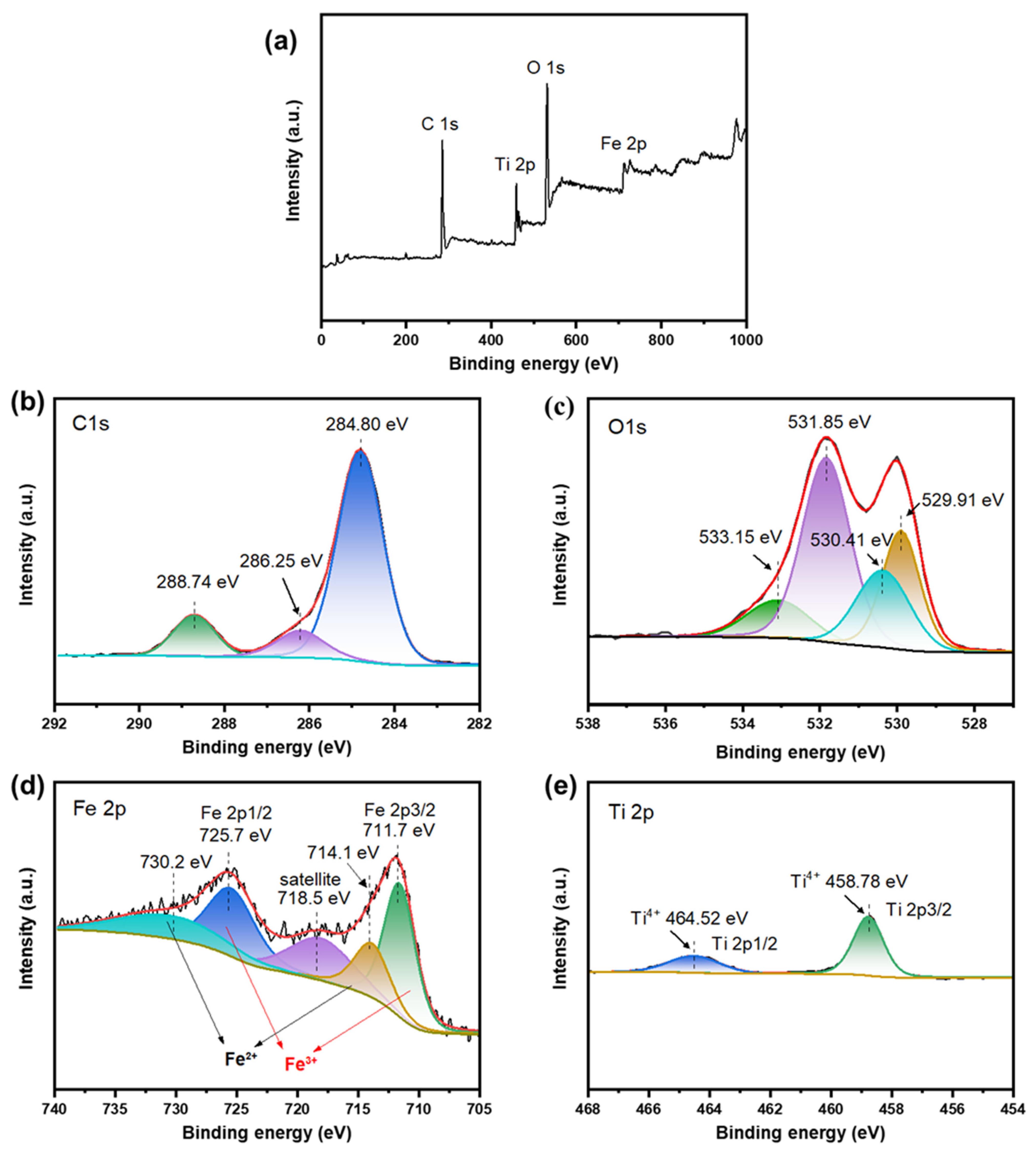
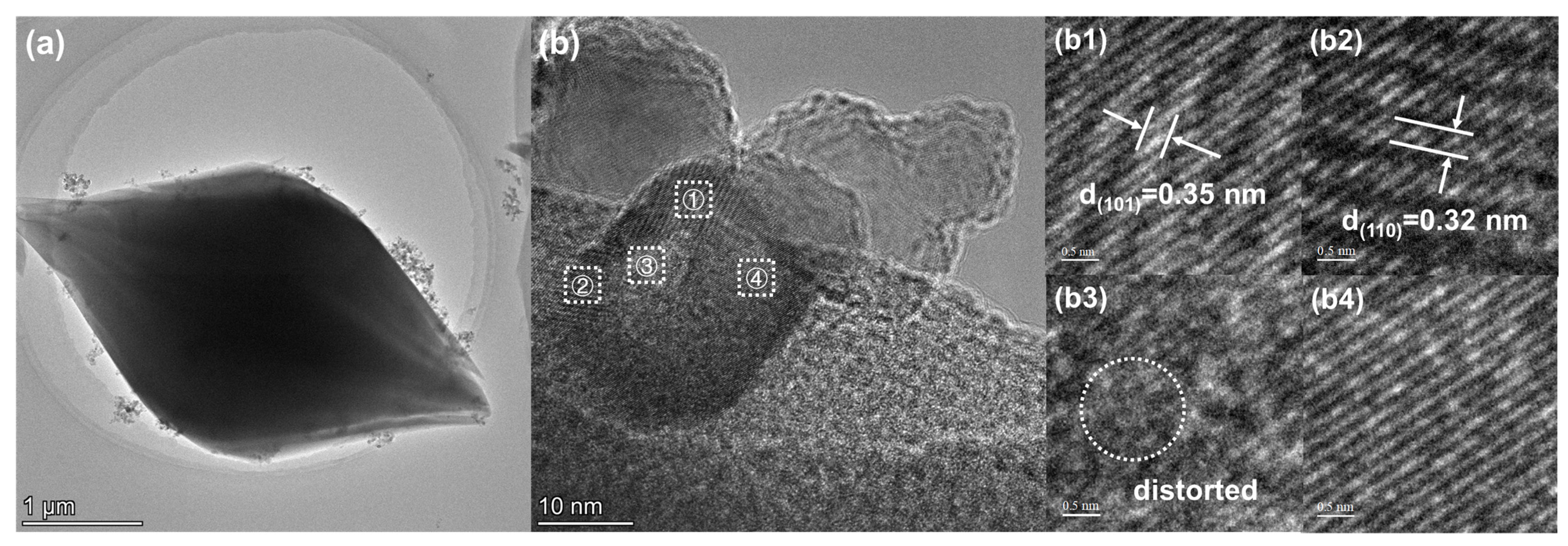
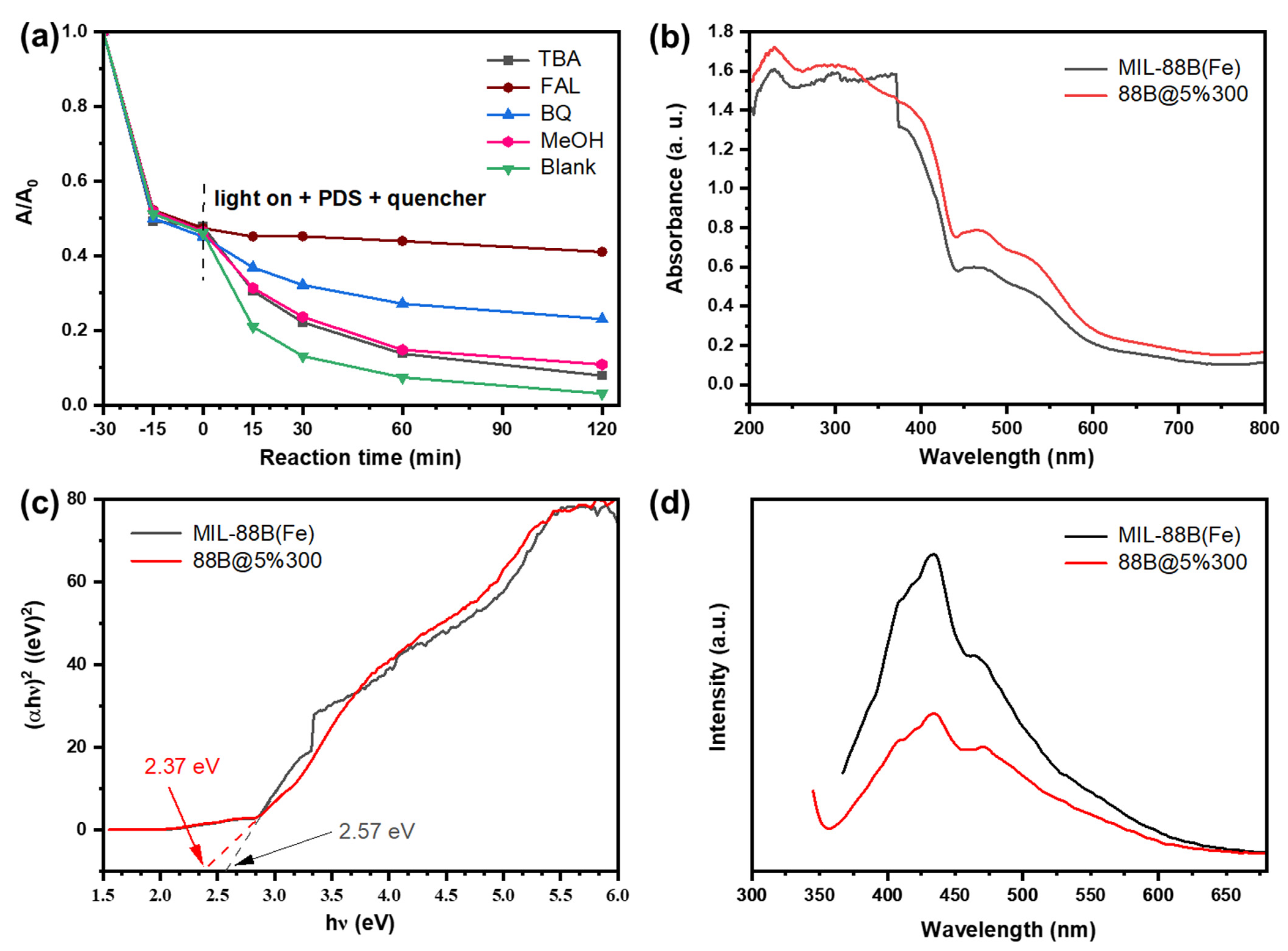
2.1. Characterizations
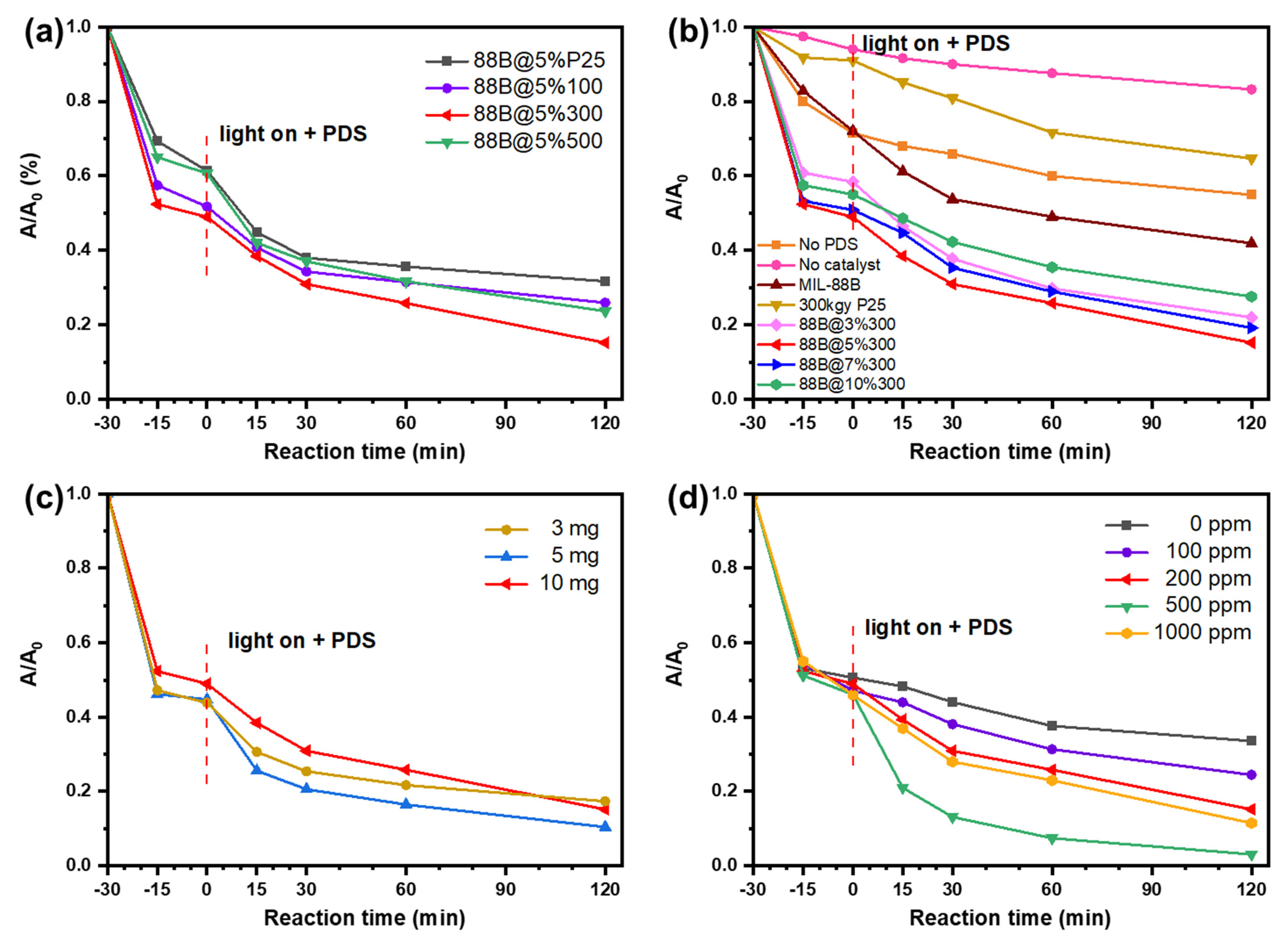
2.2. Photocatalysis Performance
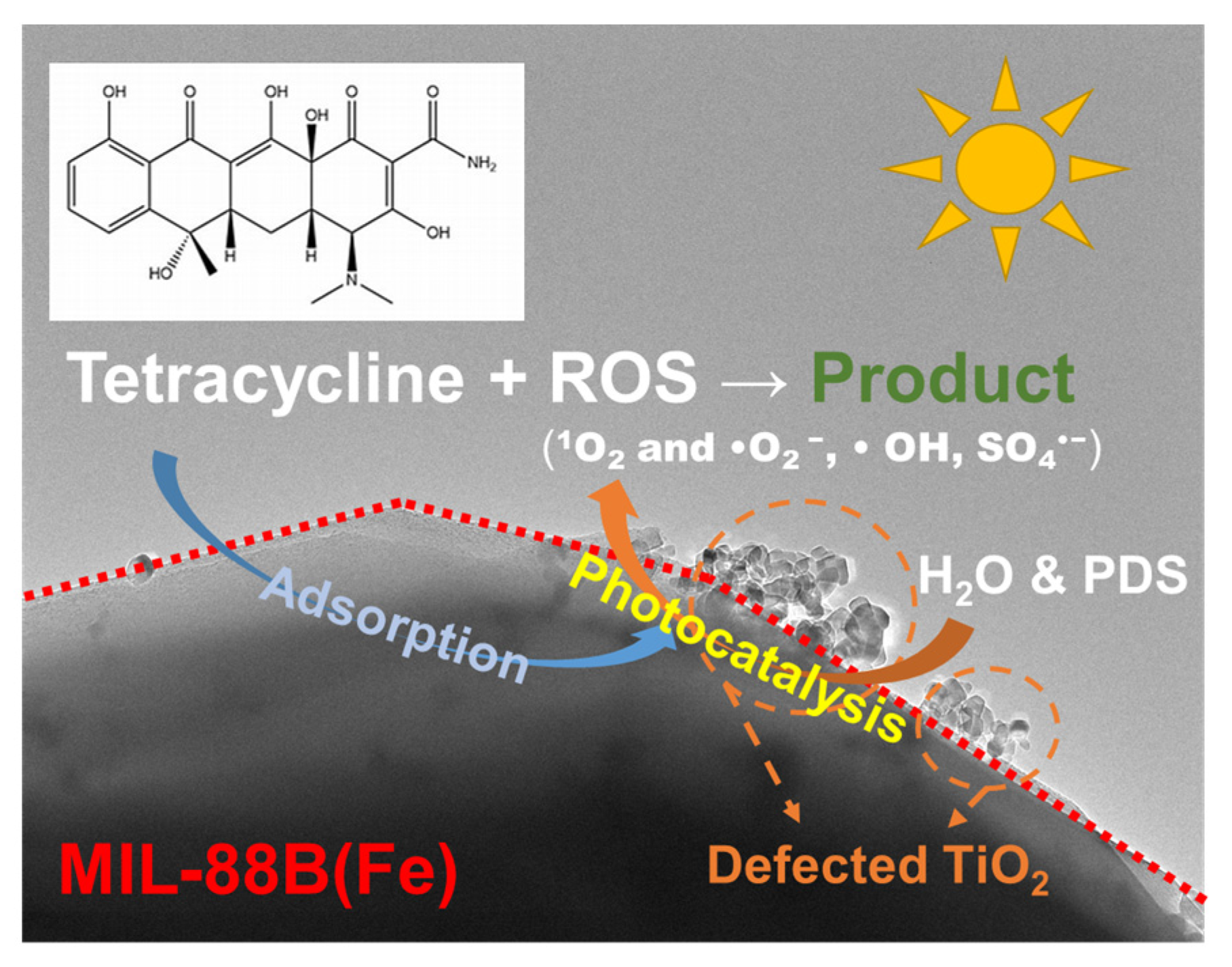
2.3. Mechanism
3. Materials and Methods
3.1. Materials
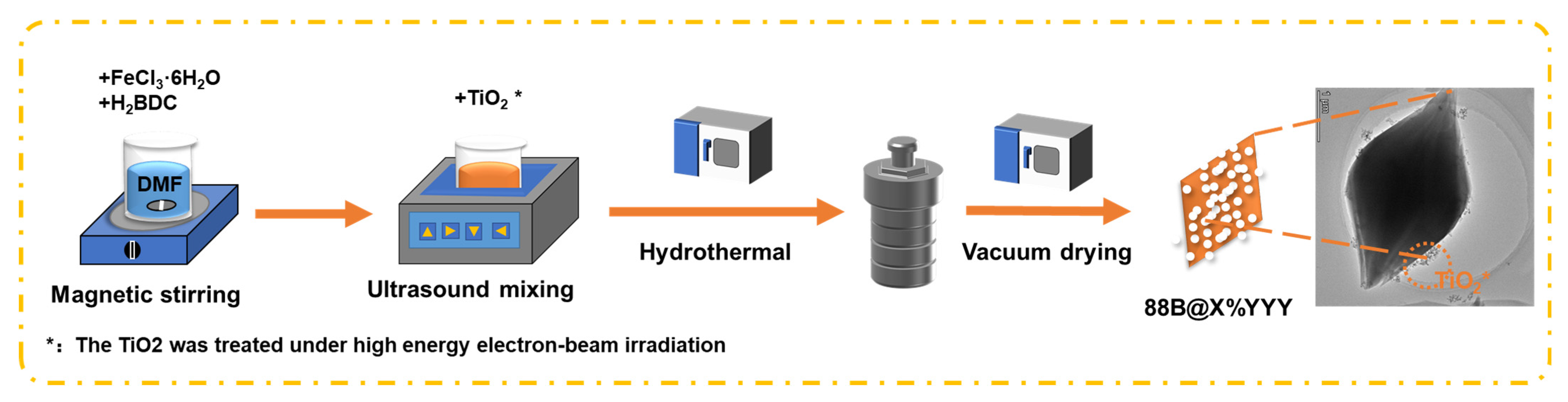
3.2. Catalyst Preparation
3.2.1. Electron-Beam Irradiation of TiO2
3.2.2. Synthesis of 88B@X%YYY Series Materials
3.3. Photocatalytic Experiments
3.4. Characterization Techniques
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, X.; Liu, A. Modification of Photocatalytic TiO2 Thin Films by Electron Beam Irradiation. Radiat. Phys. Chem. 2008, 77, 345–351. [Google Scholar] [CrossRef]
- Liang, R.; Wu, H.; Hu, Z.; Sun, J.; Fu, C.; Li, S.; Zhang, X.; Zhou, M. Novel Photoelectrocatalytic System of Oxygen Vacancy-Rich Black TiO2−x Nanocones Photoanode and Natural Air Diffusion Cathode for Efficient Water Purification and Simultaneous H2O2 Production. Appl. Catal. B 2024, 352, 124042. [Google Scholar] [CrossRef]
- Li, W.; Liao, G.; Duan, W.; Gao, F.; Wang, Y.; Cui, R.; Wang, X.; Wang, C. Synergistically Electronic Interacted PVDF/CdS/TiO2 Organic-Inorganic Photocatalytic Membrane for Multi-Field Driven Panel Wastewater Purification. Appl. Catal. B 2024, 354, 124108. [Google Scholar] [CrossRef]
- Yaou, B.; Maity, P. A Polymer-Au/TiO2 Nano-Composite Based Floating Catalyst for Photocatalytic Dye Degradation under Natural Sunlight. J. Photochem. Photobiol. C 2024, 449, 115405. [Google Scholar]
- Bibi, S.; Shah, S.; Muhammad, F.; Siddiq, M.; Kiran, L.; Aldossari, S.; Sheikh, M.; Sarwar, S. Cu-Doped Mesoporous TiO2 Photocatalyst for Efficient Degradation of Organic Dye via Visible Light Photocatalysis. Chemosphere 2023, 339, 139583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tang, P.; Yang, K.; Wang, Q.; Feng, W.; Tang, Y. PAA/TiO2@C Composite Hydrogels with Hierarchical Pore Structures as High Efficiency Adsorbents for Heavy Metal Ions and Organic Dyes Removal. Desalination 2023, 558, 116620. [Google Scholar] [CrossRef]
- Ali, F.; Ammar, S.; Ali, N.; Abdulmajeed, Y.; Jabbar, Z. Synthesis of AgCl/ZIF-8/C–TiO2 Heterojunction Photocatalysts for Enhanced Degradation of Levofloxacin under Visible-Light. Mater. Sci. Semicond. Process. 2024, 172, 108100. [Google Scholar] [CrossRef]
- Gu, J.; Li, S.; Xie, J.; Song, G.; Zhou, M. Degradation of Atrazine by Electro-Peroxone Enhanced by Fe and N Co-Doped Carbon Nanotubes with Simultaneous Catalysis of H2O2 and O3. Chemosphere 2024, 349, 140919. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Li, M.; An, Z.; Jiang, J.; Zhou, Y.; Ma, Y.; Xie, J.; Wei, F.; He, M. Effect of pH on UV/H2O2-Mediated Removal of Single, Mixed and Halogenated Parabens from Water. J. Hazard. Mater. 2024, 462, 132818. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Meng, H.; Wu, X.; Liu, Y.; Li, L. Cu/N Co-Doped Biochar Activating PMS for Selective Degrading Paracetamol via a Non-Radical Pathway Dominated by Singlet Oxygen and Electron Transfer. Chemosphere 2024, 357, 141858. [Google Scholar] [CrossRef]
- Tian, Z.; Li, Y.; Wang, L.; Xu, R.; Wei, A.; He, J.; Wang, C. Insights into the Generation of Multiple Reactive Species in UV254/PMS/I−System and Their Roles for Degradation of Phenolic and Non-Phenolic Pollutants. J. Water Process Eng. 2024, 61, 105345. [Google Scholar] [CrossRef]
- Zheng, W.; Sun, Y.; Gu, Y. Synergism of Adsorption and ROS-Dominated Catalytic Oxidation in Activating Peroxymonosulfate by Magnetic Hybrid MOFs for Selective Removal of Organophosphorus Pesticides. Chem. Eng. J. 2023, 459, 141668. [Google Scholar] [CrossRef]
- Nithyaa, N.; Muralidharan, M.; Namagal, S.; Victor, N. Defect Mediated Magnetism in Pr/TiO2-Reduced Graphene Oxide Nanocomposites. Surf. Interfaces 2024, 44, 103556. [Google Scholar] [CrossRef]
- Kandasamy, M.; Seetharaman, A.; Lakshmy, S.; Arjunan, N.; Manickavasakam, K.; Shetty, M.; Kanchana, S.; Qin, J.; Jothivenkatachalam, K.; Chakraborty, B. Defect Engineered N-S Codoped TiO2 Nanoparticles for Photocatalytic and Optical Limiting Applications: Experimental and DFT Insights. Spectrochimica. Acta A 2024, 310, 123846. [Google Scholar] [CrossRef]
- Choi, G.; Mandal, M.; Jung, H.; Panda, J.; Kwon, Y.; Zhang, K.; Vivek, E.; Shon, M.; Ravi, K.; Baek, K. Post-Synthetic Modifications (PSM)-Induced Defects in Hybrid Metal-Organic Frameworks (MOFs) to Unleash Potential in Gas Separation Membrane Applications. J. Mater. Sci. Technol. 2024, 201, 95–118. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Q.; Li, H.; Wu, Q.; Zhang, L.; Li, Y.; Qiao, L.; Yuan, Y.; Ma, J.; Wang, P. Crystal Defect Engineering to Construct Oxygen Vacancies in MXene-Derived TiO2 Nanocomposites for Boosting Photocatalytic Degradation of 2,4,6-Trichlorophenol. Chem. Eng. J. 2024, 481, 148855. [Google Scholar] [CrossRef]
- Kwon, Y.; Cho, H.; Na, H.; Lee, B.; Kim, S.; Kim, H. Improvement of Gas Sensing Behavior in Reduced Graphene Oxides by Electron-Beam Irradiation. Sens. Actuators B 2014, 203, 143–149. [Google Scholar] [CrossRef]
- Ma, X.; Yuan, H.; Qiao, Q.; Zhang, S.; Tao, H. Enhanced Catalysis for Degradation of Rhodamine B by Amino-Functionalized Fe-MOFs with High Adsorption Capacity. Colloid Surf. A 2023, 664, 131099. [Google Scholar] [CrossRef]
- Surblé, S.; Serre, C.; Mellot-Draznieks, C.; Millange, F.; Férey, G. A New Isoreticular Class of Metal-Organic-Frameworks with the MIL-88 Topology. Chem. Commun. 2006, 3, 284–286. [Google Scholar] [CrossRef]
- Liu, N.; Fei, F.; Dai, W.; Lei, J.; Bi, F.; Wang, B.; Quan, G.; Zhang, X.; Tang, L. Visible-Light-Assisted Persulfate Activation by SnS2/MIL-88B(Fe) Z-Scheme Heterojunction for Enhanced Degradation of Ibuprofen. J. Colloid Interface Sci. 2022, 625, 965–977. [Google Scholar] [CrossRef]
- Hou, L.; Catherine, H.; Harada, K.; Yoshida, M.; Chen, Y.; Hu, C. Reactive Seeding Growth of Cobalt-Doped MIL-88B(Fe) on Al2O3 Membrane for Phenol Removal in a Photocatalytic Membrane Reactor. J. Membr. Sci. 2023, 680, 121730. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, W.; Lanzalaco, S.; Zhao, L.; Sirés, I.; Xia, P.; Zhai, J.; He, Q. Ultra-Uniform MIL-88B(Fe)/Fe3S4 Hybrids Engineered by Partial Sulfidation to Boost Catalysis in Electro-Fenton Treatment of Micropollutants: Experimental and Mechanistic Insights. Chem. Eng. J. 2023, 455, 140757. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Shahsavari, S.; Gholami, M. Green Synthesis of Ternary Carbon Dots (CDs)/MIL-88B(Fe)/Bi2S3 Nanocomposite via MOF Templating as a Reusable Heterogeneous Nanocatalyst and Nano-Photocatalyst. Mater. Res. Bull. 2021, 138, 111204. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Granadeiro, C.; Loureiro, F.; Shaula, A.; Mikhalev, S.; Gonçalves, G.; Fagg, D. Comparative Analyses of MIL-88B(Fe) and MIL-100(Fe) Metal Organic Frameworks as Active Anode Materials for Li Ion Batteries. Electrochim. Acta 2023, 465, 142989. [Google Scholar] [CrossRef]
- An, Y.; Li, X.; Liu, Z.; Li, Y.; Zhou, Z.; Liu, X. Constant Oxidation of Atrazine in Fe(III)/PDS System by Enhancing Fe(III)/Fe(II) Cycle with Quinones: Reaction Mechanism, Degradation Pathway and DFT Calculation. Chemosphere 2023, 317, 137883. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Gu, Y.; He, B.; Chen, Z.; Hu, H.; Liu, H.; Ding, W. Biochar-Supported FeCo-MOF Derivative Catalyzes PDS-Mediated Degradation of Tetracycline. Sep. Purif. Technol. 2024, 349, 127841. [Google Scholar] [CrossRef]
- Wang, Z.; Su, J.; Huang, T.; Liu, Y.; Zhao, T.; Li, J.; Zhang, L. Biocrystal-Encased Manganese Ferrite Coupling with Peroxydisulfate: Synergistic Mechanism of Adsorption and Catalysis towards Tetracycline Removal. Chem. Eng. J. 2023, 468, 143580. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, P.; Liu, L.; Ren, B.; Yuan, S.; Chen, S.; Liu, L.; Chen, Y. MOF-Derived FeCo Carbon Nanomaterials with Abundant Oxygen Vacancies as Highly Efficient Peroxymonosulfate Catalyst for Ciprofloxacin Degradation. J. Environ. Chem. Eng. 2023, 11, 110603. [Google Scholar] [CrossRef]
- Luo, Y.; Shi, G.; Yu, S.; Liu, Z.; Yin, J.; Xue, M.; Sun, Q.; Shen, F.; Li, X.; Yin, Z. Novel MIL-88B(Fe)/ZnTi-LDH High-Low Junctions for Adsorption and Photodegradation of Tetracycline: Characteristics, Performance, and Mechanisms. Chem. Eng. J. 2023, 473, 145198. [Google Scholar] [CrossRef]
- Jiao, Y.; Peng, C.; Guo, F.; Bao, Z.; Yang, J.; Schmidt-Mende, L.; Dunbar, R.; Qin, Y.; Deng, Z. Facile Synthesis and Photocatalysis of Size-Distributed TiO2 Hollow Spheres Consisting of {116} Plane-Oriented Nanocrystallites. J. Phys. Chem. C 2011, 115, 6405–6409. [Google Scholar] [CrossRef]
- Wu, P.; Jin, X.; Qiu, Y.; Ye, D. Recent Progress of Thermocatalytic and Photo/Thermocatalytic Oxidation for VOCs Purification over Manganese-Based Oxide Catalysts. Environ. Sci. Technol. 2021, 55, 4268–4286. [Google Scholar] [CrossRef]
- Yang, S.; Lu, Q.; Wang, F.; Zhi, Y.; Chen, J.; Wang, Y.; Zhang, H.; Yin, H.; Sun, P.; Cao, W. S-Scheme SnO/TiO2 Heterojunction with High Hole Mobility for Boosting Photocatalytic Degradation of Gaseous Benzene. Chem. Eng. J. 2023, 478, 147345. [Google Scholar] [CrossRef]
- Kim, J.; Mirzaei, A.; Woo, H.; Kim, S. Combination of Pd Loading and Electron Beam Irradiation for Superior Hydrogen Sensing of Electrospun ZnO Nanofibers. Sens. Actuators B 2019, 284, 628–637. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, Y.; Chu, P.; Lv, L.; Tao, J.; Wang, Z.; Liu, Y.; Dai, H.; Deng, J. Catalytic Combustion of Toluene over Highly Dispersed Pt NPs Embedded in Porous TiO2 via in Situ Pyrolysis of MOFs. Catal. Today 2024, 437, 114792. [Google Scholar] [CrossRef]
- Nishino, M.; Inagaki, K.; Morikawa, Y.; Yamamura, K.; Ohkubo, Y. Identification of Chemical Species on Plasma-Treated Polytetrafluoroethylene Surface by Ab-Initio Calculations of Core-Energy-Level Shift in X-Ray Photoelectron Spectra. Appl. Surf. Sci. 2024, 655, 159369. [Google Scholar] [CrossRef]
- Guo, R.; Qin, W.; Wang, B.; Li, L.; Chen, Q.; Tan, Y.; Zhong, Y.; Zhao, Z.; Liu, N.; Mo, Z. NH2-MIL-88B(Fe)/TiO2/PAN Electrostatically Spun Nanofiber Membrane for Photocatalytic Degradation of Tetracycline and Oil–Water Separation. Sep. Purif. Technol. 2024, 351, 128059. [Google Scholar] [CrossRef]
- Qahtan, T.; Owolabi, T.; Saleh, T. X-Ray Photoelectron Spectroscopy of Surface-Treated TiO2 Mesoporous Film by 500 eV Argon Ion Beam. J. Mol. Liq. 2024, 393, 123556. [Google Scholar] [CrossRef]
- Sun, F.; Chen, T.; Liu, H.; Zou, X.; Zhai, P.; Chu, Z.; Shu, D.; Wang, H.; Chen, D. The pH-Dependent Degradation of Sulfadiazine Using Natural Siderite Activating PDS: The Role of Singlet Oxygen. Sci. Total Environ. 2021, 784, 147117. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, G.; Zhang, X.; He, S.; Xu, W.; Zhang, X.; Zhong, S. Resource Utilization of Medical Waste Incineration Fly Ash to Activate Peroxydisulfate for Tetracycline Degradation: Synergy between Adsorption and PDS Activation. Environ. Res. 2024, 258, 119488. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, Y.; Zhou, Z.; Bi, F.; Qiao, R.; Jiang, S.; Wang, J.; Zhang, X. PVP-Modified Spindle-Shaped MIL-88B(Fe) to Enhance the Degradation of Tetracycline by Activated Peroxodisulfate: A Comparative Study and Mechanistic Investigation. Prog. Nat. Sci. 2023, 33, 872–880. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, D.; Wang, Z.; Xu, J.; Shen, H.; Zhang, X.; Liu, N. Defective TiO2/MIL-88B(Fe) Photocatalyst for Tetracycline Degradation: Characterization and Augmented Photocatalytic Efficiency. Catalysts 2024, 14, 528. https://doi.org/10.3390/catal14080528
Xiang D, Wang Z, Xu J, Shen H, Zhang X, Liu N. Defective TiO2/MIL-88B(Fe) Photocatalyst for Tetracycline Degradation: Characterization and Augmented Photocatalytic Efficiency. Catalysts. 2024; 14(8):528. https://doi.org/10.3390/catal14080528
Chicago/Turabian StyleXiang, Dongsheng, Zhihao Wang, Jingwen Xu, Hongdan Shen, Xiaodong Zhang, and Ning Liu. 2024. "Defective TiO2/MIL-88B(Fe) Photocatalyst for Tetracycline Degradation: Characterization and Augmented Photocatalytic Efficiency" Catalysts 14, no. 8: 528. https://doi.org/10.3390/catal14080528
APA StyleXiang, D., Wang, Z., Xu, J., Shen, H., Zhang, X., & Liu, N. (2024). Defective TiO2/MIL-88B(Fe) Photocatalyst for Tetracycline Degradation: Characterization and Augmented Photocatalytic Efficiency. Catalysts, 14(8), 528. https://doi.org/10.3390/catal14080528






