Abstract
As an environmentally friendly technology, enzymatic degradation of waste polyethylene terephthalate (PET) has great application potential. Mono (hydroxyethyl) terephthalate (MHET), an intermediate product of PET degradation, accumulates during the degradation process. MHET reduces the activity of PETase and influences further enzymatic degradation. The combined catalysis of MHETase and PETase is an effective strategy to solve this problem. However, the difference in thermostability between MHETase and PETase limits their combination. In our previous study, a PETase of muEst1 exhibited acceptable PET-degradation ability, but the abundant MHET accumulation in its degradation products limited its further application. In this study, MHETases with good thermostability were screened for combination with muEst1 for the cascade reaction of PET degradation, and a two-stage variable-temperature program was developed. The results of this investigation show that this approach results in a PET-degradation rate of 92.71% with a terephthalic acid content above 85.9%. This investigation provides an alternative method for scaled-up enzymatic PET degradation.
1. Introduction
Solid plastic-waste pollution is one of the most pressing environmental challenges today [1,2]. Among various commercial plastics, polyethylene terephthalate (PET) is particularly prominent due to its affordability, durability, and lightweight properties [3,4,5], and it has been widely applied in the fields of packaging [6], textiles [7], medical applications [8], and engineering materials [9]. However, statistics indicate that most PET wastes are left in the environment or in landfills, while less than 10% of them are effectively recycled [10,11]. The excessive use and improper disposal of PET has led to severe environmental pollution. Therefore, enhancing the recycling of PET waste and improving the efficiency of resource utilization are urgent concerns.
The mechanical method, which is based on crushing and melting, does not provide a valuable product with further applications. Chemical-degradation technology, including thermo-, electro-, and photocatalytic methods, are common methods for PET degradation. For instance, Kratish et al. successfully depolymerized PET into terephthalic acid (TPA) and ethylene at 260 °C in the presence of hydrogen [12]. Wang et al. degraded PET into bis(hydroxyethyl)terephthalate (BHET) using 1,3-dimethylimidazolium-2-carboxylate at 180 °C, achieving a recovery rate of 60% [13]. Cao et al. introduced an efficient PET-alcoholysis approach utilizing an oxygen-vacancy-rich catalyst under air, achieving space-time yields of 957.1 gBHET·gcat−1·h−1 at 180 °C [14]. Although chemical methods offer high degradation rates, the harsh degradation conditions result in high energy consumption, which remains a challenge to sustainable development [15]. In contrast, enzymatic degradation of PET offers significant sustainability advantages over traditional PET recycling methods. The degradation conditions for the enzymatic process are milder, and the lower degrees of energy consumption and environmental pollution associated with enzymatic processes make them more environmentally friendly. Additionally, PET can be broken down into its monomers through enzymatic degradation, yielding terephthalic acid (TPA) that can be reused for the reproduction of polymers [16] and ethylene glycol (EG) that can be utilized for the production of energy and chemicals [17]. However, the value of degradation products of traditional methods is low, which leads to some resource wasting. Thus, the enzymatic method enables closed-loop recycling of PET waste and is helpful for reducing reliance on virgin petrochemical resources.
Numerous studies have focused on the use of biocatalysts for PET biodegradation [18,19,20,21,22]. PETases are a family of cutinases that function in PET degradation. For example, LCC_ICCG [23], a well-studied PETase, can achieve greater than 90% depolymerization of PET in a short period. For this enzymatic-degradation process, most of the PETase converts PET into monomers of mono (hydroxyethyl) terephthalate (MHET) and TPA [23]. However, during the enzymatic degradation of PET, MHET accumulates due to the low activity of PETase towards this intermediate product [24,25]. In the enzymatic degradation of PET, MHET is not only an intermediate product, but also an inhibitor of PETase [26]. Thus, the accumulation of MHET hinders the catalytic activity of PETase, thereby further reducing the rate of the PET-degradation reaction. Fortunately, there are hydrolases with activity against MHET (MHETase), and a multi-enzyme-cascade strategy provides an effective solution to the problem of MHET accumulation. In 2016, Ideonella sakaiensis 201-F6, capable of secreting PETase and MHETase, was reported to achieve efficient degradation of PET to TPA at 30 °C through the cascade action of these two hydrolases within 6 weeks [27].
Normally, the active temperature of MHETase is around ambient temperature. Research endeavors have been carried out to find a PETase that could work under mild conditions [28,29,30]. However, since the enzymatic reaction for the PET degradation is a solid−liquid surface reaction, the number of reactive sites on the solid PET surface is the limiting factor. In the crystalline state of PET, there are limited reaction sites on its surface, which results in a low degradation efficiency. Related works indicate that degradation at a lower temperature would take many days [31]. Sun et al. [32] used the SpyTag/SpyCatcher system to immobilize DuraPETase and MHETase on calcium carbonate nanocrystals (CaP). This method increased the stability of dual enzymes capable of PET biodegradation at 40 °C and 50 °C, and the degradation rate of PET was around 50% after 10 days. It has been proven that the degradation rate primarily depends on the mobility of the polyester chains and the glass-transition temperature of PET [33]. A degradation temperature approaching the glass-transition temperature of PET (around 60 °C to 65 °C) is conducive to the loosening of the crystal structure of the polymer, thus greatly increasing the biodegradation rate. Therefore, developing a high-temperature enzymatic PET-degradation process at remains a research focus, and more attention has been focused on increasing the MHETase catalytic temperature to match the reaction temperature of PETase. Zhu et al. [26] designed MHETase using computational enzyme-design tools and further fused the designed protein with FAST-PETase to construct a dual-enzyme system for PET depolymerization. The fusion protein achieved 90% PET degradation at 50 °C for 72 h. Even though direct revolution could enhance the operational feasibility of dual-enzymatic degradation of PET, the process still falls short of achieving the reaction temperatures required by PETase. Since the optimal reaction conditions of PETase and MHETase are very different, the problem of how to optimize the combined cascade reactions remains key to obtaining a satisfactory TPA yield for PET degradation.
In our previous work, a cutinase Est1 from Thermobifida alba AHK119 [34] was studied, and its mutant with the specific changes N213M, T215P, S115P, Q93A, and L91W was shown to have PETase activity. However, the process of PET biodegradation with this mutant resulted in significant accumulation of MHET. It is necessary to find a suitable MHETase and to develop a dual-enzyme-cascade strategy to further improve PTA production from PET degradation.
A dual-enzyme-cascade strategy based on PETase and MHETase was investigated in this research. The strategies of protein fusion and a segmented degradation process were compared. The processing conditions, such as reaction time and temperature, were optimized. Furthermore, an efficient PET-depolymerization process with a variable-temperature-control program was developed. High-purity TPA could be obtained via this dual-enzyme-cascade. This work overcame the limitations associated with the different reaction conditions of the enzymes applied in the cascade-based degradation process, which provides a great potential for large-scale enzymatic PET degradation.
2. Results and Discussion
2.1. The Properties of MuEst1-Based PET Degradation
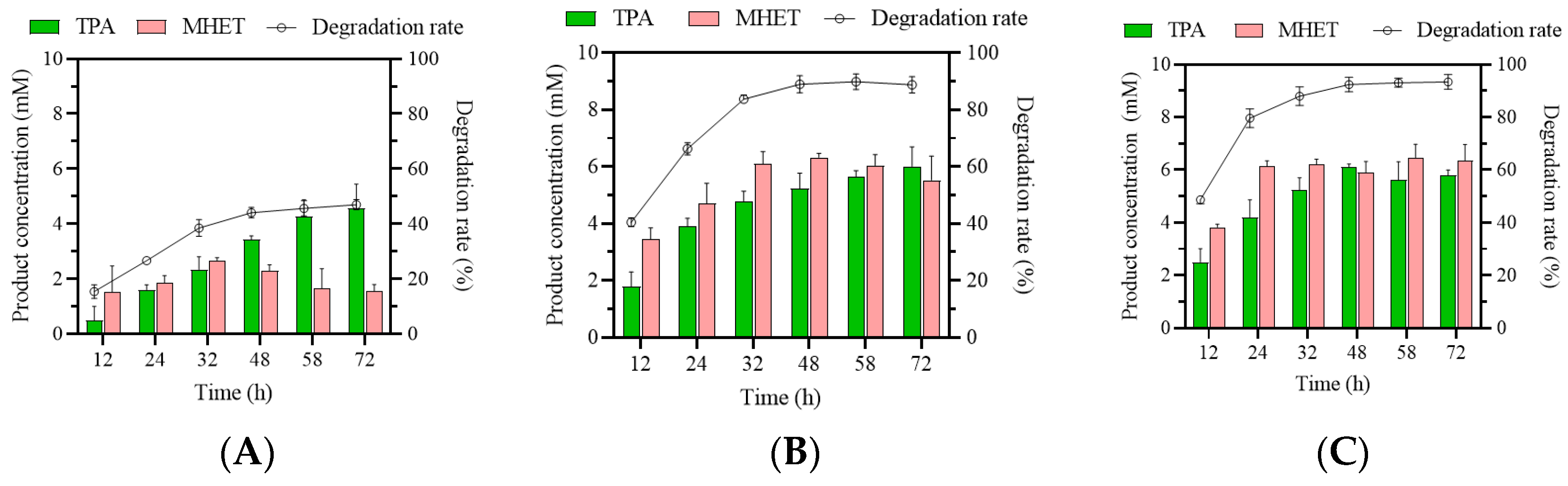
First, the efficiency of muEst1-based degradation of post-consumer PET particles was investigated. The degradation rate of PET and the yields of TPA and MHET from 72 h reactions with different enzyme dosages were evaluated (Figure 1). It could be found that at low enzyme concentrations (e.g., 3‰), the degradation rate of the PET was 25% within 24 h and reached only 40% after 72 h. It may be that there was insufficient enzyme present to provide enough substrate-binding sites for the PET. When the enzyme dosage was increased, the degradation rate significantly improved. With the addition of 3% enzyme, 68% of the PET was degraded within 24 h and the concentrations of MHET and TPA yields reached up to 4.7 mM and 3.9 mM, respectively. Subsequently, the degradation increased slowly and finally reached 90% at 72 h. A further increase in the enzyme dosage (5%) did not significantly increase PET degradation. Thus, an enzyme addition of 3% (w/w: enzyme/PET) was applied in subsequent experiments.
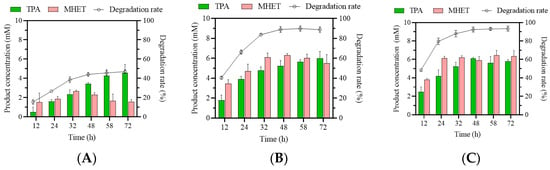
Figure 1.
Biodegradation of particles of a post-consumer commercial PET bottle. Product concentrations and degradation rates at 3‰ (A), 3% (B), and 5% (C) muEst1 dosages.
Moreover, the main degradation products of PET are TPA, MHET, and BHET. Typically, plenty of BHET was generated during the degradation of PET and was further hydrolyzed into MHET [35]. However, as shown in Figure 1, BHET was not detected during degradation by muEst1. Both TPA and MHET were observed throughout the biodegradation process, and MHET accounted for a large proportion of the final products. The accumulation of MHET greatly affects the purity of the desired product, TPA. Developing a cascade system that incorporates muEst1 and MHETase would be an efficient solution to this issue.
2.2. MHETase Selection
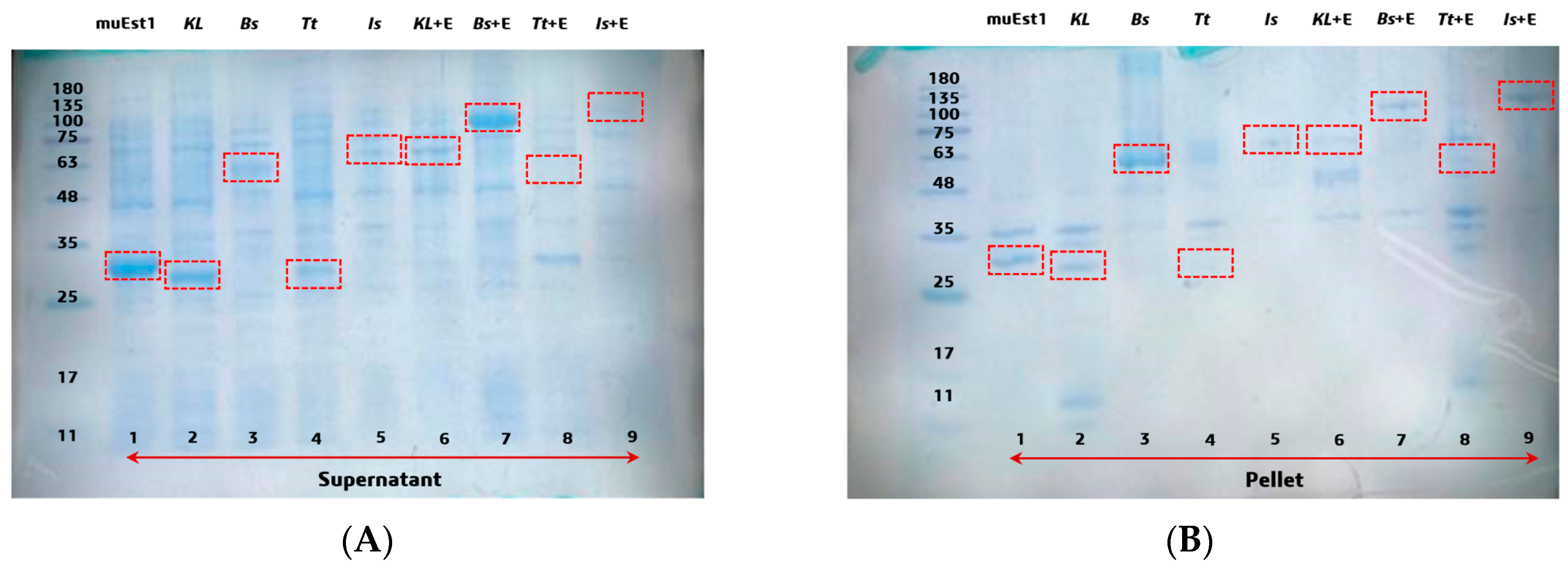
To find a suitable MHETase, four kinds of thermostable MHETases were investigated in this study. Firstly, the expression of individual proteins and fusion proteins with muEst1 was studied. For the fusion protein, tested MHETases were linked to the N-terminus of muEst1, with a flexible peptide fragment (GS) composed of 36 amino acids linking the two components. The expression levels were examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Figure 2). For the individual proteins, most of the tested MHETases could be expressed from Escherichia coli BL21(DE3) as soluble proteins, except for IsMHET (lane 5). The results indicated that a low temperature (18 °C) and a low concentration of IPTG (0.1 mM) facilitated the correct folding of MHETase proteins, thus improving the yield of soluble products [36,37]. However, the expression levels of those MHETases were different, and the highest level of soluble expression was obtained from KLMHETase (lane 2), which was expressed at a level similar to that of PETase muEst1 (lane 1). Among the fusion proteins, KL_muEst1 (lane 6) and Bs_muEst1 (lane 7) were obtained by soluble expression, but Tt_Est1 (lane 8) and Is_Est1 (lane 9) were expressed as inclusion bodies (Figure 2B). Consequently, correctly expressed MHETases and fusion proteins incorporating KL_muEst1 and Bs_muEst1 were further investigated.
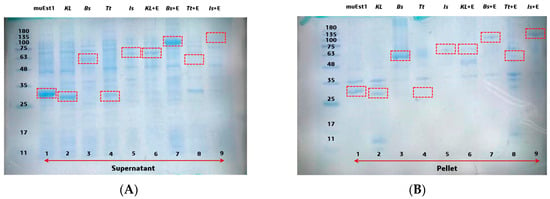
Figure 2.
SDS-PAGE of different hydrolases: (A) supernatant of the expression cell culture. (B) pellet of the expression cell culture. Lane 1: muEst1, lane 2: KLMHETase, lane 3: BsMHETase, lane 4: TtMHETase, lane 5: IsMHETase, lane 6: KL_muEst1, lane 7: Bs_muEst1, lane 8: Tt_muEst1, lane 9: Is-muEst1. The individual and fusion protein sizes were as follows: muEst1 29.4 kDa, KLMHETase 28.5 kDa, BsMHETase 54.4 kDa, TtMHETase 26.4 kDa, IsMHETase 63.8 kDa, KL_muEst1 60.1 kDa, Bs_muEst1 86.1 kDa, Tt_muEst1 58.1 kDa, and Is_muEst1 98.5 kDa. IsMHETase--MHETase from Ideonella sakaiensis, KLMHETase--redesigned from thermophilic carboxylesterase Est30 (Geobacillus stearothermophilus), TtMHETase--MHETase from Thermus thermophilus, and BsMHETase--MHETase from Bacillus subtilis.
2.3. Dual-Enzymatic Combination for PET Degradation
The dual-enzymatic-combination process was based on a sequential-response mechanism, where the highly polymerized PET was first hydrolyzed into MHET. Subsequently, MHET was further hydrolyzed into TPA and glycol in a reaction catalyzed by MHETase.
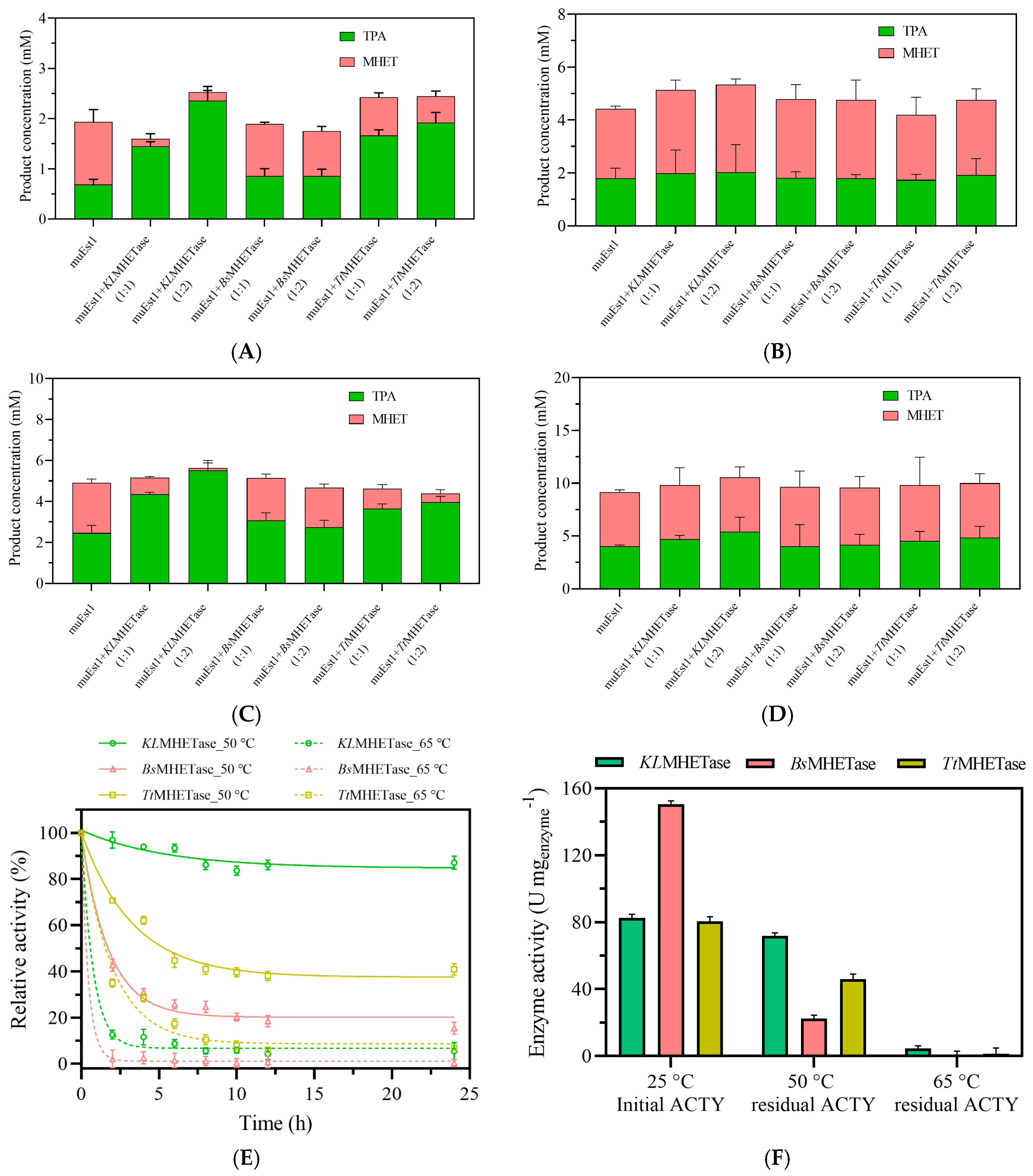
Combinations of muEst1 and different MHETases were tested first. As shown in Figure 3A,C, the combination of muEst1 and KLMHETase resulted in a notable amount of product release at 50 °C, with significantly improved TPA production compared to individual muEst1 catalysis. This indicates that KLMHETase has good catalytic performance against the intermediate product of MHET degradation. In contrast, the increase in the PET-degradation rate for the combination of BsMHETase/TtMHETase and muEst1 was not remarkable (Figure 3A–D). Although BsMHETase has been reported to have good performance in the degradation of PET films , Bååth et al. indicated that BsMHETase has a limited degradation effect on bis(2-(benzoyloxy) ethyl) terephthalate at 50 °C, suggesting that its thermostability and activity are still insufficient [38]. The TtMHETase samples yielded similar results; its thermostability was also insufficient to sustain functionality at high temperatures.
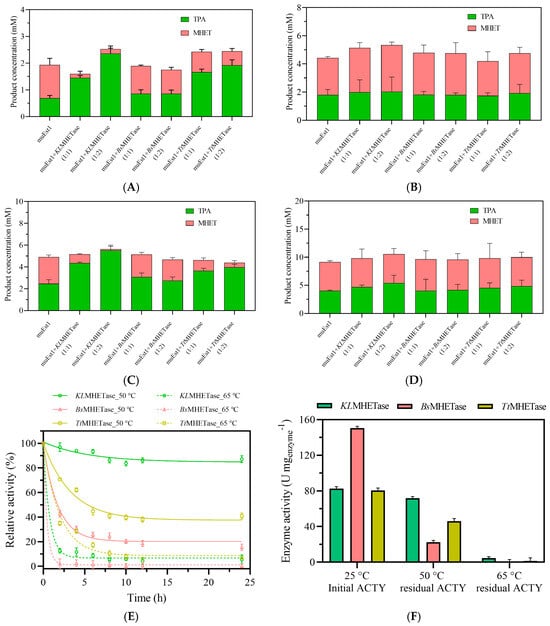
Figure 3.
The results of PET degradation by combinations of muEst1 and MHETases. (A) PET degradation at 50 °C for 8 h; (B) PET degradation at 65 °C for 8 h; (C) PET degradation at 50 °C for 24 h; (D) PET degradation at 65 °C for 24 h; (E) thermostability of enzymes at 50 °C and 65 °C; (F) comparison of enzymatic activity.
At the same time, the thermostabilities of those three MHETases were also evaluated. As shown in Figure 3E,F, KLMHETase maintained more than 85% relative activity for 24 h at 50 °C, but its activity rapidly decreased within the first 3 h at 65 °C. Meanwhile, the thermostability results for BsMHETase and TtMHETase (Figure 3E,F) confirmed their limitations in their application for PET/MHET degradation.
Since the fusion protein has the advantage of facilitating the transfer of intermediate products for the cascade enzymatic reaction [39,40], the degradation ability of fusion proteins derived from muEst1 and MHETases were investigated in the experiments described below.
2.4. Catalytic Performance of Fusion Protein
Although fusion proteins may facilitate the PET degradation by cascade reaction, the two components may influence each other, affecting the stability and function of the fusion protein. As illustrated in Figure 4, the PET-degradation capability of each fusion protein at the tested temperatures was significantly lower than that of the individual muEst1. The depolymerization of polyesters like PET requires a temperature above its glass-transition temperature. Therefore, 50 °C, being below the glass-transition temperature of PET, was insufficient as a reaction temperature, making the biodegradation process difficult. Meanwhile, due to the weak thermal tolerance of MHETase, harsh environments, like higher temperatures (e.g., 65 °C), destroy the functional conformation of the enzyme and lead to the loss of overall activity of the fusion protein.
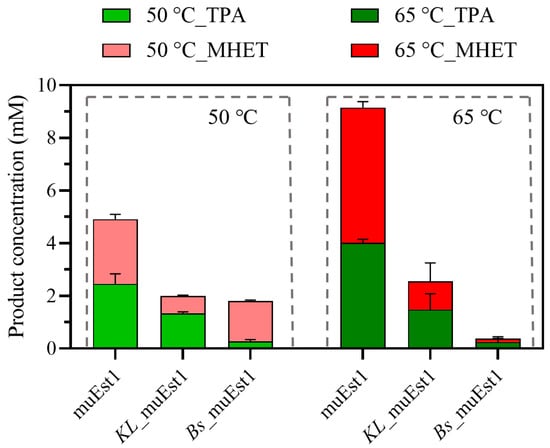
Figure 4.
PET degradation by fusion proteins.
The results of this research indicated that the weak thermostability of MHETase influences the activity of the fusion protein. The directed evolution of enzymes aimed at increasing the catalytic temperature and improving the enzyme’s thermostability remains key to the development of fusion proteins for PET degradation.
2.5. Optimization of Degradation by Enzymatic Cascade
From the results of individual enzymes and fusion-protein catalysis, it could be concluded that although the thermostability of KLMHETase is significantly better than that of other MHETases, it could still not function well at 65 °C, the optimal temperature for PET hydrolase activity, limiting its potential for combined application. The use of a segmented process could effectively sidestep the limitations associated with the different catalytic temperatures of multiple enzymes.
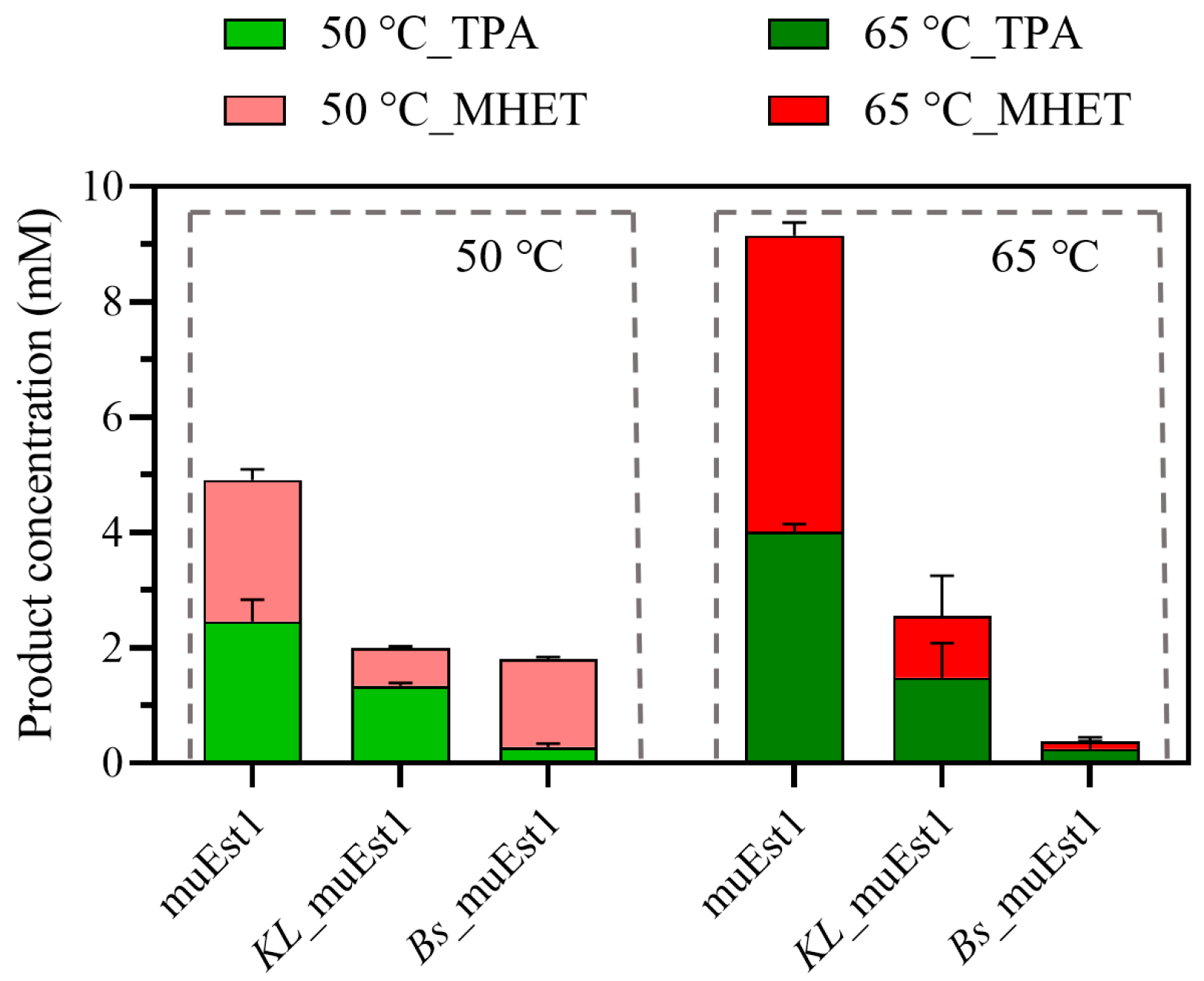
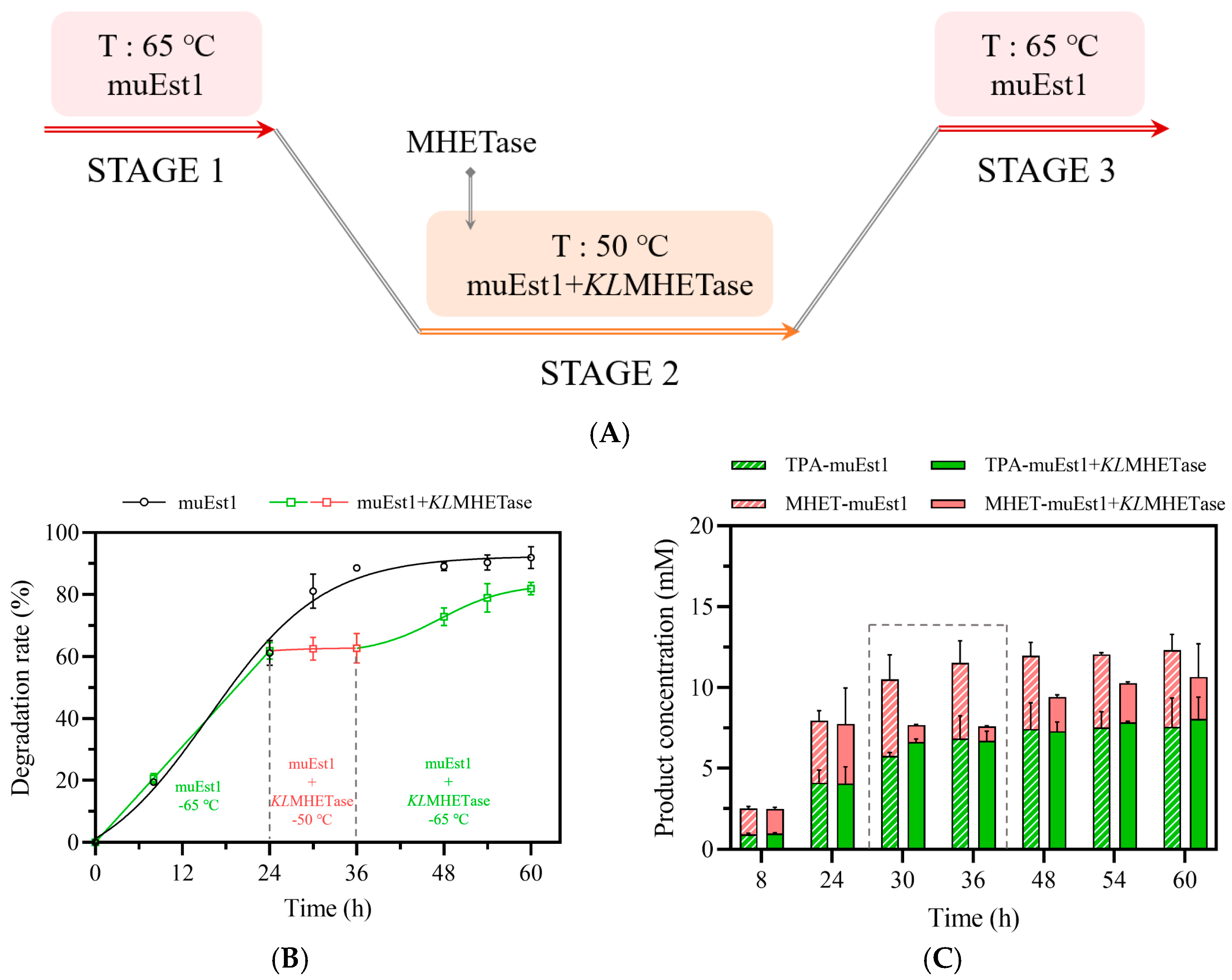
A three-stage process was thus designed for initial testing (Figure 5A). In this process, PET was first degraded by muEst1 at 65 °C for 24 h. Then, the temperature was decreased to 50 °C and KLMHETase was added for the degradation of MHET. After the second stage, it was assumed that more PET would be degraded since the accumulated MHET had been removed, shifting the balance of the hydrolysis reaction. Thus, in the third stage, the reaction temperature was reraised to 65 °C to facilitate residual PET degradation by muEst1.
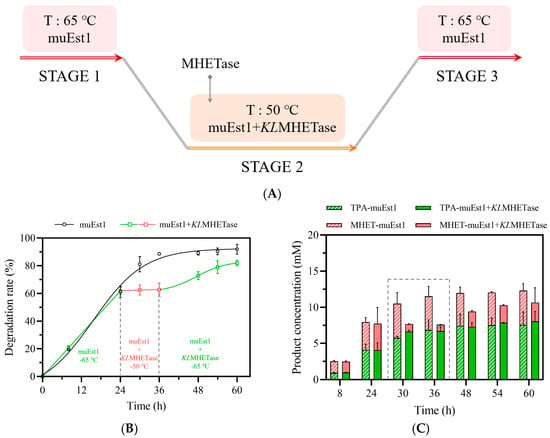
Figure 5.
PET degradation by the three-stage process. (A) Scheme of the three-stage process; (B) Comparison of PET degradation by different processes; (C) Analysis of product composition and release.
The results of this three-stage process ae shown in Figure 5B,C. Unlike the process with muEst1 alone, this process produced TPA as the major product of the degradation after 30 h (6 h after KLMHETase addition and temperature decrease). However, the total degradation rate of PET was quite low (60%) and remained around 70–80% even after the temperature was raised again to 65 °C. The degradation rate is thus significantly lower than that obtained with catalysis by the cutinase muEst1 alone (90%). The limited PET-degradation rate may be attributable to the high concentration of TPA, which may influence the balance of the hydrolysis reaction catalyzed by muEst1. Additionally, the temperature increase would have denatured KLMHETase and the precipitate enzyme would hinder the interaction between muEst1 and PET particles, thus limiting the of PET-degradation reaction.
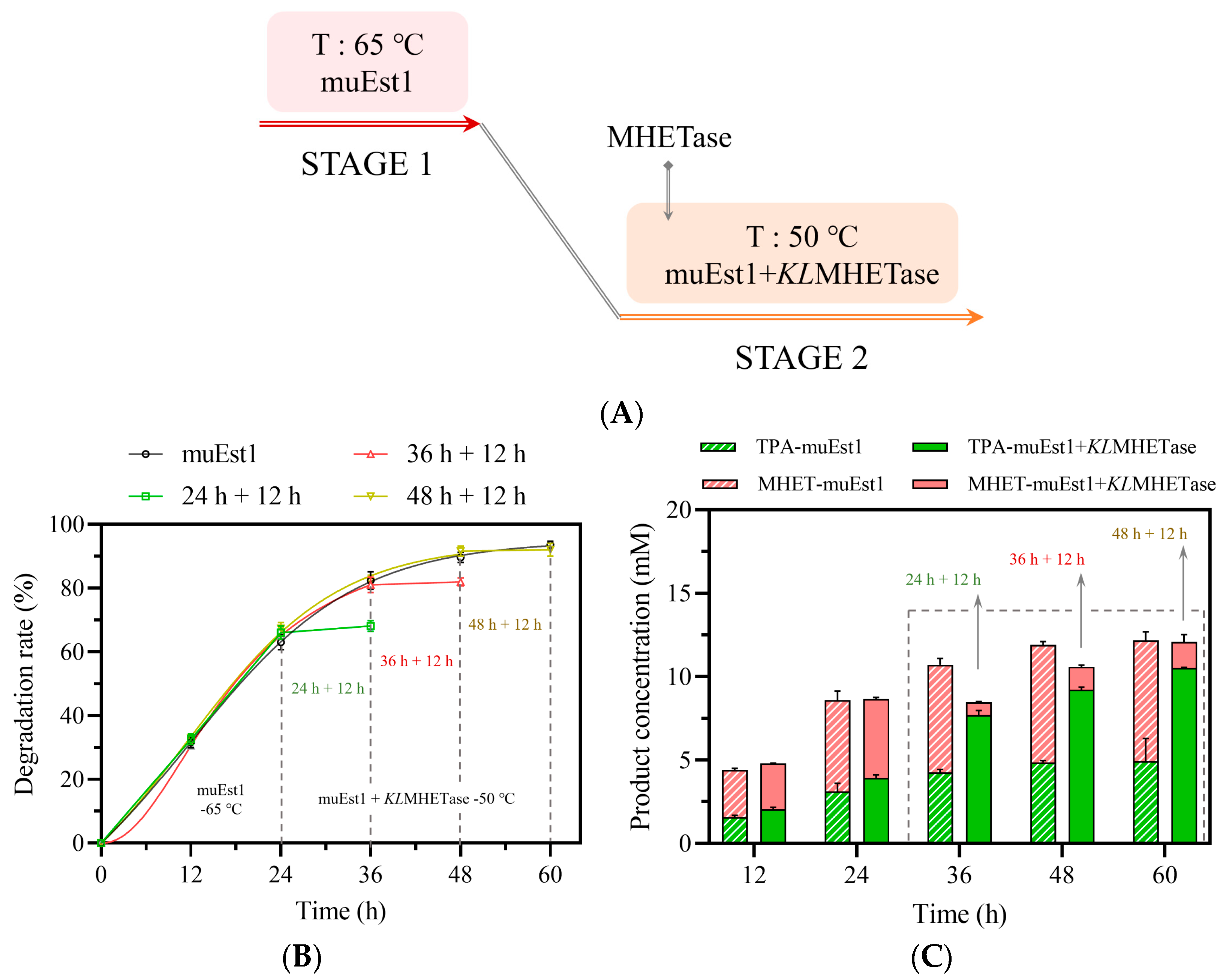
To further simplify this process, a two-stage approach was developed to replace the three-stage scheme (Figure 6). First, cutinase muEst1 was used to fully degrade PET at 65 °C. Then, the reaction system was cooled to 50 °C and KLMHETase was added to fully degrade the accumulated MHET. The duration of the first stage was optimized first. As shown in Figure 6, the first stage (65 °C, muEst1) was terminated at 24 h, 36 h, and 48 h, respectively. Then, the second stage (50 °C, muEst1 + KLMHETase) was run for another 12 h. It was found that the optimal PET degradation rate was obtained with muEst1 catalysis for 48 h, followed by 12 h of KLMHETase-mediated degradation (Figure 6B,C). Under the optimal program, a PET-degradation rate of 92.92% was achieved, with a yield of 87.2% for the desired TPA, an improvement of 46.7% compared to muEst1-mediated catalysis alone. It should be noticed that in the second stage, the PET degradation was limited because the reaction temperature of this stage was set at 50 °C to promote catalysis by MHETase. However, 50 °C is much lower than the glass-transition temperature of PET (about 65 °C) and is not suitable for muEst1 activity. The low temperature resulted in limited PET degradation during the second stage.
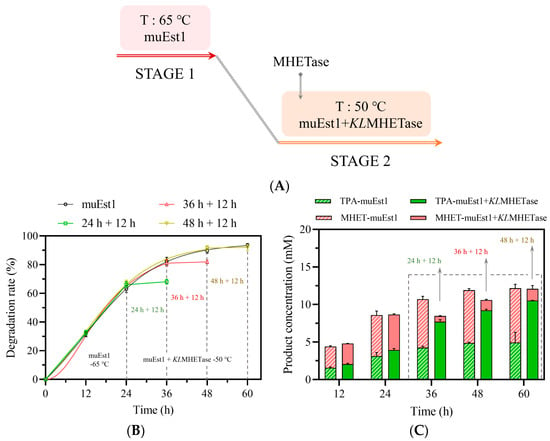
Figure 6.
PET degradation in the two-stage process. (A) Scheme of the two-stage process; (B) Product release in the single-enzyme muEst1 samples; (C) Product release in the dual-enzyme samples.
Thus, temperature-programmed biodegradation of PET could effectively increase the yield of the TPA, reducing MHET generation. As an enzymatic-degradation product, TPA is easy to purify and is easily reused to produce polymers, which makes it a better product than MHET. In the single-enzyme biodegradation process, MHET is easily accumulated, affecting the separation and reuse of TPA. The dual-enzyme-cascade system is designed to ensure that each enzyme can perform under its optimal conditions. The strategy not only enhanced the catalytic efficiency of the process, but also increased the concentration of TPA in the final degradation products. This strategy would be beneficial for the sustainability of waste PET recycling and increase the economic feasibility of enzymatic PET degradation.
2.6. Application of the Two-Stage Variable-Temperature Program in a Scaled-Up System
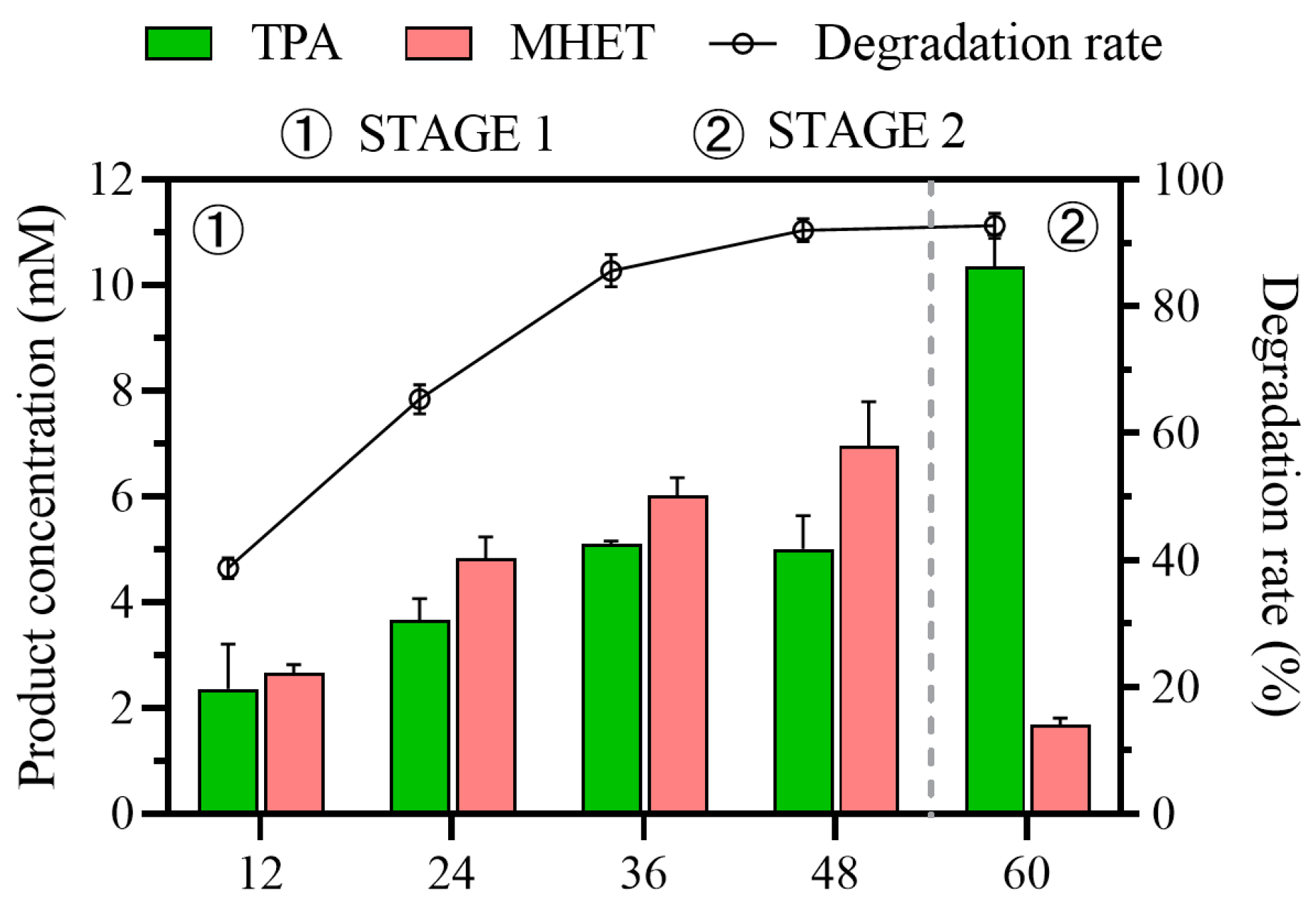
To verify the application potential of the process, a 2 L reaction was conducted with 5 g of waste PET particles as degradation substrate. As illustrated in Figure 7, the degradation rate of PET and the TPA yield were satisfactory. Due to factors such as mass-transfer intensity, the TPA yield slightly decreased to 85.9%, while the degradation rate remained consistent at 92.71%. This further demonstrated the potential applicability of this two-stage, multi-enzyme, variable-temperature process.
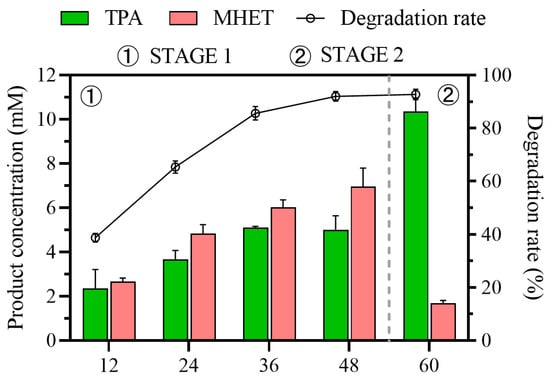
Figure 7.
PET degradation in a 5 L bioreactor.
3. Materials and Methods
3.1. Strains, Plasmids, and Reagents
Competent cells of E. coli BL21(DE3) and E. coli Trans 10 were purchased from Transgen (Beijing, China). The cutinase (Est1) sequence from the strain Thermobifida alba AHK119, with a His-tag at the C-terminus, was cloned into the pET-22b(+) plasmid. Cutinase of IsMHETase [41] (GenBank code: GAP38911.1, PDB ID: 6QZ3) from Ideonella sakaiensis, KLMHETase (redesigned from thermophilic carboxylesterase Est30 (Geobacillus stearothermophilus, PDB ID: 1TQH) [26], TtMHETase [42] (PDB ID: 1UFO) from Thermus thermophilus, and BsMHETase [38] (GenBank code: P37967.2, PDB ID: 1QE3) from Bacillus subtilis were chosen as MHETases for investigation, and their genes were synthesized by Azenta (Waltham, MA, USA). Tryptone and yeast extract were purchased from Oxoid (Basingstoke, UK). Terephthalic acid and mono (hydroxyethyl) terephthalate were purchased from GlpBio (Montclair, NJ, USA). 4-Nitrophenyl butyrate (pNPB) was purchased from Yuanye (Shanghai, China). Other chemicals were of analytical grade and were obtained from Beijing Chemical Works (Beijing, China).
3.2. Plasmid Construction
The gene for the cutinase Est1 was manually mutated to muEst1 (Est1 with the mutations N213M, T215P, S115P, Q93A, and L91W), and the this mutant gene was cloned into the plasmid pET-22b(+). Four target MHETase genes were cloned into pET-22b(+), each with a C-terminal His-tag. The fusion protein was constructed by linking MHETase to the N-terminus of muEst1, and a flexible linker composed of 36 amino acids (GS) was applied to connect the two enzymes. All the plasmids were transferred into competent E. coli BL21(DE3) through electroporation.
3.3. Preparation of Enzymes
Recombinant cells of E. coli BL21(DE3) were cultured at 37 °C in LB medium containing 0.1 mg/mL ampicillin (Amp) until the OD600 reached 0.6–0.8. Subsequently, 0.1 mM of isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to induce protein expression at 18 °C for 14 h. The cells were then collected by centrifugation (4000 rpm × 5 min, 4 °C). Afterwards, harvested cells were resuspended in 50 mM Tris-HCl buffer (pH 8.0) and then lysed by sonication. The crude enzyme solution was collected by centrifugation (8000 rpm × 5 min, 4 °C), and subjected to Ni+ affinity chromatography [43]. The target protein was eluted with a solution containing 250 mM imidazole and filtered through an ultrafiltration membrane (10 kDa, Millipore, Burlington, VT, USA) (3200 rpm × 60 min, 4 °C) to remove the imidazole. Finally, the target protein was identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and its concentration was determined using the Bradford method (Coomassie Brilliant Blue (CBB) dye binding method) [44].
3.4. Preparation of Substrate of PET Particles
PET particles were obtained from post-consumer Coca-Cola bottles (Coca Cola, Atlanta, GA, USA). After the top neck and bottom of the bottle were removed, the rest of the bottle was cut into small squares of 1 × 1 cm. Then, the small pieces of PET were further smashed into particles approximately 0.5 mm in diameter via a high-speed grinder (AM500, Ants Scientific Instruments, Beijing, China). The crystallinity of the PET particles was measured by differential scanning calorimetry (DSC) under nitrogen conditions (DSC1, Mettler Toledo, Greifensee, Switzerland).
3.5. Enzymatic-Activity Assay
The activity of the target PETase and MHETases were assayed by hydrolysis pNPB (1 mM), with absorbance changes measured at 415 nm [45]. Based on the protein concentration, different purified enzyme solutions were proportionally added to 50 mM Tris-HCl buffer to obtain a final volume of 100 µL, then 100 µL of 2 mM pNPB was added. Absorbance changes in the reactant at 415 nm were recorded through a Microplate Reader (Multiskan FC, Thermo Fisher, Waltham, MA, USA) over a period of 1 min. One unit of enzyme activity (U) was defined as the amount of enzyme that releases 1 mM of p-nitrophenol per minute. Relative activity was expressed as the percentage activity of the experimental group compared to that of the untreated blank group.
3.6. PET-Degradation Process
The PET-degradation process was carried out in a 4 mL glass reaction vial. The reaction system contained 5 mg of PET particles and 3% free enzyme (w/w = 1:1 or 1:2, PETase/MHETase) or fusion protein (w/w, Enzyme/PET). A buffer (50 mM Tris-HCl, 300 mM CaCl2, pH 8.0) was added to bring the total reaction volume to 2 mL without the addition of extra water. The reaction was conducted at different temperatures (50 °C and 65 °C) with periodic sampling.
The scaled-up system was tested in a 5L bioreactor. A 2 L system was tested with 5 g of waste PET particles as degradation substrate, and 3% (enzyme: PET, w/w) muEst1 or KLMHETase was applied in each stage. The reaction proceeded at 280 rpm at 65 °C for 48 h, then was cooled to 50 °C for 12 h.
For the segmented PET-degradation process, the first stage was set at 65 °C for catalysis by muEst1 PETase and the second stage was set at 50 °C and involved the addition of MHETase. For the three-stage process samples, the third-stage temperature was readjusted to 65 °C.
Finally, the reaction was terminated by boiling and the reactant solution was filtered through a 0.22 µm nylon filter. The contents of TPA and MHET in the sample were quantified by high-performance liquid chromatography (HPLC, Shimadzu LC-20a, Kyoto, Japan) according to methods established in previous studies [46].
4. Conclusions
In this study, a two-stage multi-enzyme-reaction process was developed to achieve highly efficient degradation of PET and to obtain a high yield of TPA in the degradation products. Initially, MHETases with good thermostability were expressed and fused with PETase of muEst1. Among all the investigated MHETases, KLMHETase showed the best thermostability, but it still could not function at the temperature required for catalysis by muEst1. The weak thermostability of MHETase prevents its application in combination with muEst1 in the form of free enzyme or as a fusion protein. Thus, a two-stage, multi-enzyme, variable-temperature process for PET degradation was developed in which muEst1 was applied as a single catalyst at 65 °C for 48 h, following which the reaction temperature was reduced to 50 °C and KLMHETase was added and allowed to react for 12 h. The PET-degradation rate was 92.71%, and the TPA yield was 85.9%. This two-stage process overcomes the issue of reaction-temperature differences between the two enzymes and demonstrates the potential of PET degradation by enzymatic cascade for industrial applications.
Author Contributions
Data curation, J.W. and Q.W.; Funding acquisition, K.N.; Investigation, J.W. and S.J.; Methodology, D.L.; Software, D.L.; Supervision, F.W. and K.N.; Writing–original draft, D.L.; Writing–review & editing, D.L., Q.W., F.W., L.D. and K.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National key R&D program of China (No. 2021YFC2103600).
Data Availability Statement
The data that have been used are confidential.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Iroegbu, A.O.C.; Ray, S.S.; Mbarane, V.; Bordado, J.C.; Sardinha, J.P. Plastic pollution: A perspective on matters arising: Challenges and opportunities. ACS Omega 2021, 6, 19343–19355. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Norton, M. Green chemistry and the plastic pollution challenge: Towards a circular economy. Green Chem. 2020, 22, 6310–6322. [Google Scholar] [CrossRef]
- Sarda, P.; Hanan, J.C.; Lawrence, J.G.; Allahkarami, M. Sustainability performance of polyethylene terephthalate, clarifying challenges and opportunities. J. Polym. Sci. 2022, 60, 7–31. [Google Scholar] [CrossRef]
- Carr, C.M.; Clarke, D.J.; Dobson, A.D. Microbial polyethylene terephthalate hydrolases: Current and future perspectives. Front. Microbiol. 2020, 11, 571265. [Google Scholar] [CrossRef] [PubMed]
- Mudondo, J.; Lee, H.-S.; Jeong, Y.; Kim, T.H.; Kim, S.; Sung, B.H.; Park, S.-H.; Park, K.; Cha, H.G.; Yeon, Y.J. Recent advances in the chemobiological upcycling of polyethylene terephthalate (PET) into value-added chemicals. J. Microbiol. Biotechnol. 2023, 33, 1. [Google Scholar] [CrossRef]
- Malik, N.; Kumar, P.; Shrivastava, S.; Ghosh, S.B. An overview on PET waste recycling for application in packaging. Int. J. Plast. Technol. 2017, 21, 1–24. [Google Scholar] [CrossRef]
- Sousa, A.F.; Patrício, R.; Terzopoulou, Z.; Bikiaris, D.N.; Stern, T.; Wenger, J.; Loos, K.; Lotti, N.; Siracusa, V.; Szymczyk, A. Recommendations for replacing PET on packaging, fiber, and film materials with biobased counterparts. Green Chem. 2021, 23, 8795–8820. [Google Scholar] [CrossRef]
- Darie-Niță, R.N.; Râpă, M.; Frąckowiak, S. Special features of polyester-based materials for medical applications. Polymers 2022, 14, 951. [Google Scholar] [CrossRef]
- Makvandi, P.; Iftekhar, S.; Pizzetti, F.; Zarepour, A.; Zare, E.N.; Ashrafizadeh, M.; Agarwal, T.; Padil, V.V.; Mohammadinejad, R.; Sillanpaa, M. Functionalization of polymers and nanomaterials for water treatment, food packaging, textile and biomedical applications: A review. Environ. Chem. Lett. 2021, 19, 583–611. [Google Scholar] [CrossRef]
- Siddique, R.; Khatib, J.; Kaur, I. Use of recycled plastic in concrete: A review. Waste Manag. 2008, 28, 1835–1852. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U.; Ahmed, W.; Arshad, H. Recent trends in recycling and reusing techniques of different plastic polymers and their composite materials. Sustain. Mater. Technol. 2022, 31, e00382. [Google Scholar] [CrossRef]
- Kratish, Y.; Li, J.; Liu, S.; Gao, Y.; Marks, T.J. Polyethylene Terephthalate Deconstruction Catalyzed by a Carbon-Supported Single-Site Molybdenum-Dioxo Complex. Angew. Chem. 2020, 132, 20029–20033. [Google Scholar] [CrossRef]
- Wang, L.; Nelson, G.A.; Toland, J.; Holbrey, J.D. Glycolysis of PET using 1, 3-dimethylimidazolium-2-carboxylate as an organocatalyst. ACS Sustain. Chem. Eng. 2020, 8, 13362–13368. [Google Scholar] [CrossRef]
- Cao, J.; Liang, H.; Yang, J.; Zhu, Z.; Deng, J.; Li, X.; Elimelech, M.; Lu, X. Depolymerization mechanisms and closed-loop assessment in polyester waste recycling. Nat. Commun. 2024, 15, 6266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, Y.; Ren, Y.; Li, Z.; Kong, X.; Shao, M.; Duan, H. Plastic waste valorization by leveraging multidisciplinary catalytic technologies. ACS Catal. 2022, 12, 9307–9324. [Google Scholar] [CrossRef]
- Zimmermann, W. Biocatalytic recycling of polyethylene terephthalate plastic. Philos. Trans. R. Soc. A 2020, 378, 20190273. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, Y.; Ma, X.; Gong, J. Ethylene glycol: Properties, synthesis, and applications. Chem. Soc. Rev. 2012, 41, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zou, H. Biotechnological application of cutinase: A powerful tool in synthetic biology. SynBio 2022, 1, 54–64. [Google Scholar] [CrossRef]
- Müller, R.J.; Schrader, H.; Profe, J.; Dresler, K.; Deckwer, W.D. Enzymatic degradation of poly(ethylene terephthalate): Rapid hydrolyse using a hydrolase from T. fusca. Macromol. Rapid Commun. 2005, 26, 1400–1405. [Google Scholar] [CrossRef]
- Su, L.; Woodard, R.W.; Chen, J.; Wu, J. Extracellular location of Thermobifida fusca cutinase expressed in Escherichia coli BL21 (DE3) without mediation of a signal peptide. Appl. Environ. Microbiol. 2013, 79, 4192–4198. [Google Scholar] [CrossRef] [PubMed]
- Herrero Acero, E.; Ribitsch, D.; Steinkellner, G.; Gruber, K.; Greimel, K.; Eiteljoerg, I.; Trotscha, E.; Wei, R.; Zimmermann, W.; Zinn, M. Enzymatic surface hydrolysis of PET: Effect of structural diversity on kinetic properties of cutinases from Thermobifida. Macromolecules 2011, 44, 4632–4640. [Google Scholar] [CrossRef]
- Sulaiman, S.; Yamato, S.; Kanaya, E.; Kim, J.-J.; Koga, Y.; Takano, K.; Kanaya, S. Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. Appl. Environ. Microbiol. 2012, 78, 1556–1562. [Google Scholar] [CrossRef]
- Tournier, V.; Topham, C.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.-L.; Texier, H.; Gavalda, S. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef]
- Pinto, A.V.; Ferreira, P.; Neves, R.P.; Fernandes, P.A.; Ramos, M.J.; Magalhaes, A.L. Reaction mechanism of MHETase, a PET degrading enzyme. ACS Catal. 2021, 11, 10416–10428. [Google Scholar] [CrossRef]
- Sagong, H.-Y.; Seo, H.; Kim, T.; Son, H.F.; Joo, S.; Lee, S.H.; Kim, S.; Woo, J.-S.; Hwang, S.Y.; Kim, K.-J. Decomposition of the PET film by MHETase using Exo-PETase function. ACS Catal. 2020, 10, 4805–4812. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Luo, Z.; Yang, Z.; Zhang, Z.; Wang, P.; Li, M.; Zhang, Y.; Feng, Y.; Lu, D. Computational design of highly efficient thermostable MHET hydrolases and dual enzyme system for PET recycling. Commun. Biol. 2023, 6, 1135. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Sun, J.; Pang, Y.; Lei, Z.; OuYang, B.; Lai, W.; Wang, Y.; Lan, D. Enzymatic depolymerization of plastic materials by a highly efficient two-enzyme system. Biochem. Eng. J. 2024, 204, 109222. [Google Scholar] [CrossRef]
- Shi, L.; Zhu, L. Recent advances and challenges in enzymatic depolymerization and recycling of PET wastes. ChemBioChem 2024, 25, e202300578. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liu, P.; Tan, Z.; Zhao, W.; Gao, J.; Gu, Q.; Ma, H.; Liu, H.; Zhu, L. Complete depolymerization of PET wastes by an evolved PET hydrolase from directed evolution. Angew. Chem. 2023, 135, e202218390. [Google Scholar] [CrossRef]
- Chen, K.; Hu, Y.; Dong, X.; Sun, Y. Molecular insights into the enhanced performance of EKylated PETase toward PET degradation. ACS Catal. 2021, 11, 7358–7370. [Google Scholar] [CrossRef]
- Chen, K.; Dong, X.; Sun, Y. Sequentially co-immobilized PET and MHET hydrolases via Spy chemistry in calcium phosphate nanocrystals present high-performance PET degradation. J. Hazard. Mater. 2022, 438, 129517. [Google Scholar] [CrossRef]
- Kawai, F.; Oda, M.; Tamashiro, T.; Waku, T.; Tanaka, N.; Yamamoto, M.; Mizushima, H.; Miyakawa, T.; Tanokura, M. A novel Ca2+-activated, thermostabilized polyesterase capable of hydrolyzing polyethylene terephthalate from Saccharomonospora viridis AHK190. Appl. Microbiol. Biotechnol. 2014, 98, 10053–10064. [Google Scholar] [CrossRef]
- Thumarat, U.; Kawabata, T.; Nakajima, M.; Nakajima, H.; Sugiyama, A.; Yazaki, K.; Tada, T.; Waku, T.; Tanaka, N.; Kawai, F. Comparison of genetic structures and biochemical properties of tandem cutinase-type polyesterases from Thermobifida alba AHK119. J. Biosci. Bioeng. 2015, 120, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Barnard, E.; Arias, J.J.R.; Thielemans, W. Chemolytic depolymerisation of PET: A review. Green Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Vera, A.; González-Montalbán, N.; Arís, A.; Villaverde, A. The conformational quality of insoluble recombinant proteins is enhanced at low growth temperatures. Biotechnol. Bioeng. 2007, 96, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alonso, M.; García-Fruitós, E.; Villaverde, A. Yield, solubility and conformational quality of soluble proteins are not simultaneously favored in recombinant Escherichia coli. Biotechnol. Bioeng. 2008, 101, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Bååth, J.A.; Borch, K.; Jensen, K.; Brask, J.; Westh, P. Comparative biochemistry of four polyester (PET) hydrolases. ChemBioChem 2021, 22, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hess, H. Toward Rational Design of High-efficiency Enzyme Cascades. ACS Catal. 2017, 7, 6018–6027. [Google Scholar] [CrossRef]
- Cai, X.; Jiao, L.; Yan, H.; Wu, Y.; Gu, W.; Du, D.; Lin, Y.; Zhu, C. Nanozyme-involved biomimetic cascade catalysis for biomedical applications. Mater. Today 2021, 44, 211–228. [Google Scholar] [CrossRef]
- Knott, B.C.; Erickson, E.; Allen, M.D.; Gado, J.E.; Graham, R.; Kearns, F.L.; Pardo, I.; Topuzlu, E.; Anderson, J.J.; Austin, H.P. Characterization and engineering of a two-enzyme system for plastics depolymerization. Proc. Natl. Acad. Sci. USA 2020, 117, 25476–25485. [Google Scholar] [CrossRef] [PubMed]
- Mrigwani, A.; Thakur, B.; Guptasarma, P. Conversion of polyethylene terephthalate into pure terephthalic acid through synergy between a solid-degrading cutinase and a reaction intermediate-hydrolysing carboxylesterase. Green Chem. 2022, 24, 6707–6719. [Google Scholar] [CrossRef]
- Porath, J. Immobilized metal ion affinity chromatography. Protein Expr. Purif. 1992, 3, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, K.-E.; Kovacic, F. Determination of lipolytic enzyme activities. Pseudomonas Methods Protoc. 2014, 1149, 111–134. [Google Scholar]
- Lu, D.; Wu, J.; Jin, S.; Wu, Q.; Deng, L.; Wang, F.; Nie, K. The enhancement of waste PET particles enzymatic degradation with a rotating packed bed reactor. J. Clean. Prod. 2024, 434, 140088. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).