Impact of Oxygen-Containing Groups on Pd/C in the Catalytic Hydrogenation of Acetophenone and Phenylacetylene
Abstract
:1. Introduction
2. Results and Discussion
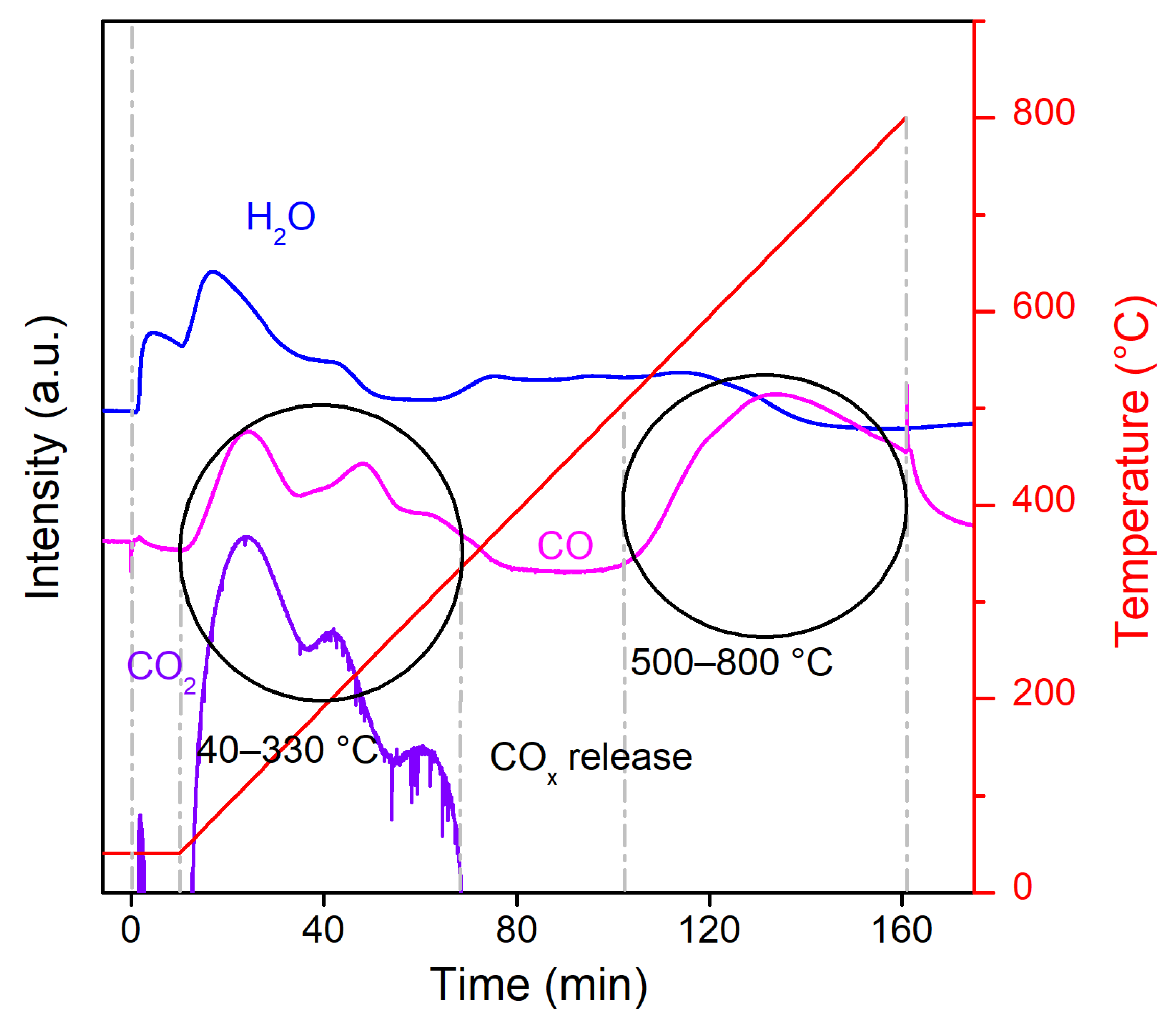
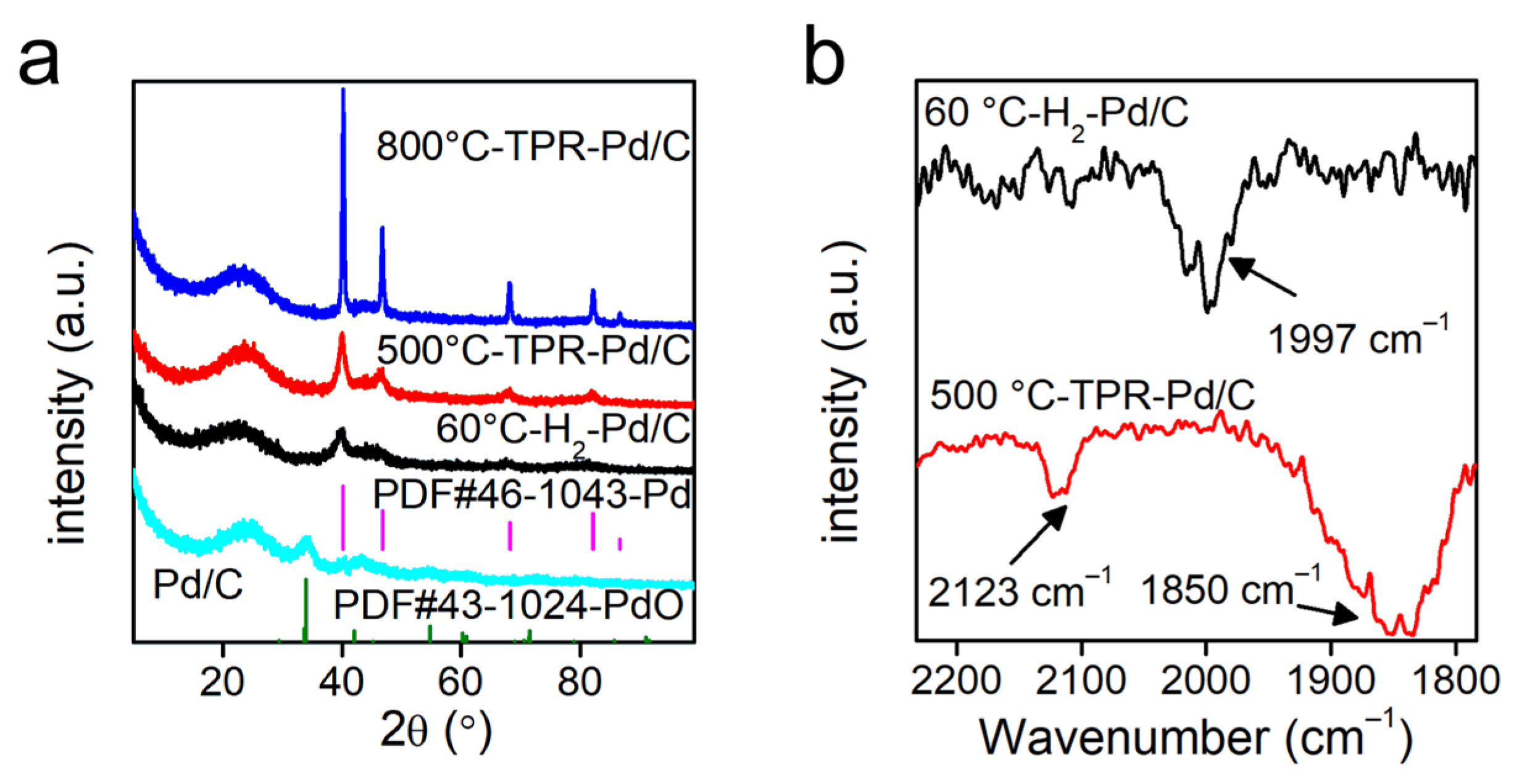
2.1. Catalyst Characterization
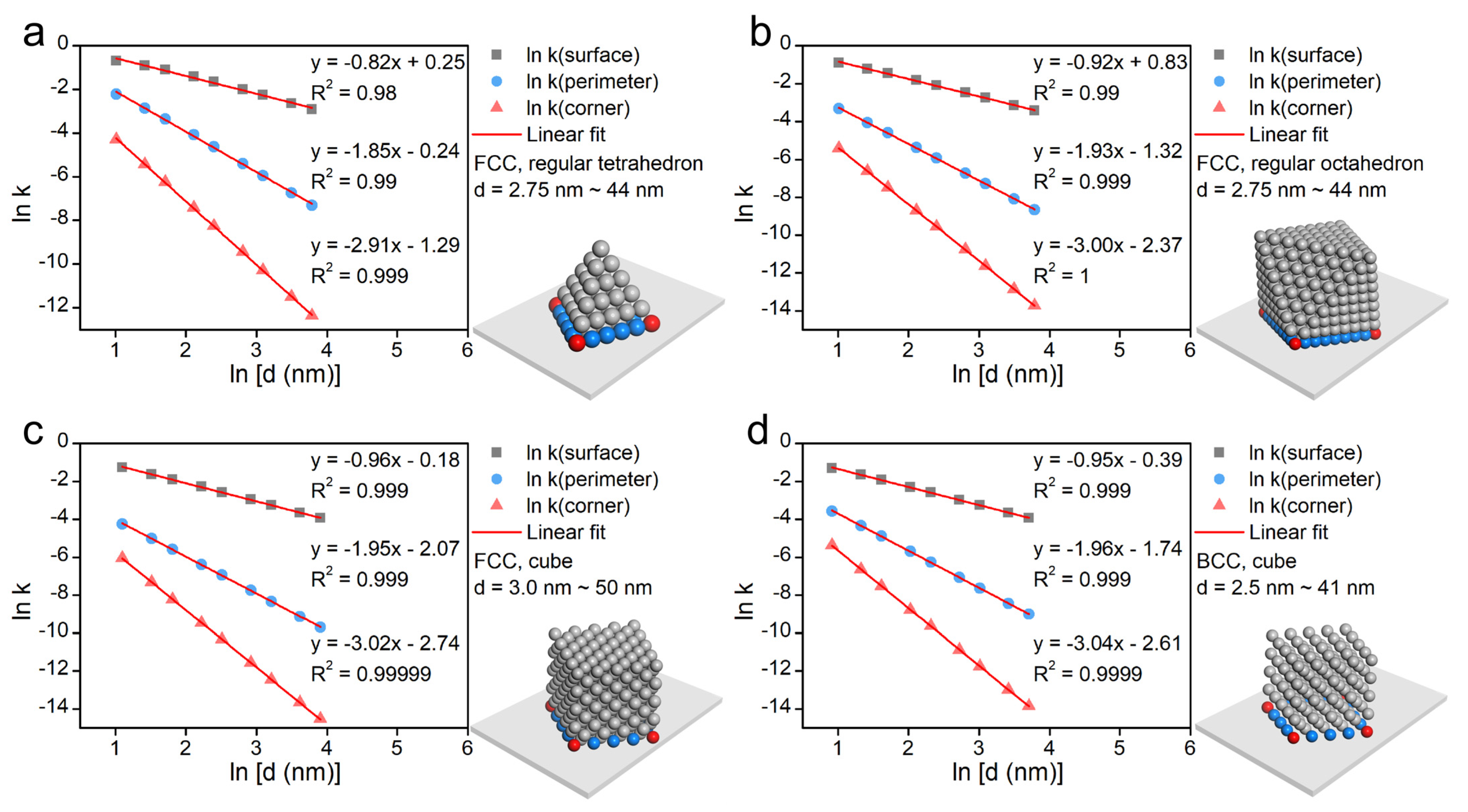
2.2. Mathematical Analysis of the Size Effect
2.3. Relationship between Oxygen-Containing Groups in Pd/C and the Hydrogenation Activity of Acetophenone and Phenylacetylene
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, W.; Yan, L.; Wang, B.; Zhang, Q.; Song, H.; Wang, S.; Zhang, Q.; Wang, Y. Efficient Catalysts for the Green Synthesis of Adipic Acid from Biomass. Angew. Chem. Int. Ed. 2021, 60, 4712–4719. [Google Scholar]
- Takahashi, S.; Sekiya, R.; Haino, T. Metal Nanoparticles on Lipophilic Nanographenes. Angew. Chem. Int. Ed. 2022, 61, e202205514. [Google Scholar]
- Huang, F.; Deng, Y.; Chen, Y.; Cai, X.; Peng, M.; Jia, Z.; Ren, P.; Xiao, D.; Wen, X.; Wang, N.; et al. Atomically Dispersed Pd on Nanodiamond/Graphene Hybrid for Selective Hydrogenation of Acetylene. J. Am. Chem. Soc. 2018, 140, 13142–13146. [Google Scholar]
- Zugic, B.; Zhang, S.; Bell, D.C.; Tao, F.F.; Flytzani-Stephanopoulos, M. Probing the Low-Temperature Water-Gas Shift Activity of Alkali-Promoted Platinum Catalysts Stabilized on Carbon Supports. J. Am. Chem. Soc. 2014, 136, 3238–3245. [Google Scholar] [PubMed]
- Salazar, A.; Moreno-Simoni, M.; Kumar, S.; Labella, J.; Torres, T.; de la Torre, G. Supramolecular Subphthalocyanine Cage as Catalytic Container for the Functionalization of Fullerenes in Water. Angew. Chem. Int. Ed. 2023, 62, e202311255. [Google Scholar]
- Huang, X.; Akdim, O.; Douthwaite, M.; Wang, K.; Zhao, L.; Lewis, R.J.; Pattisson, S.; Daniel, I.T.; Miedziak, P.J.; Shaw, G.; et al. Au-Pd Separation Enhances Bimetallic Catalysis of Alcohol Oxidation. Nature 2022, 603, 271–275. [Google Scholar] [PubMed]
- Chang, J.; Wang, G.; Wang, M.; Wang, Q.; Li, B.; Zhou, H.; Zhu, Y.; Zhang, W.; Omer, M.; Orlovskaya, N.; et al. Improving Pd–N–C Fuel Cell Electrocatalysts through Fluorination-Driven Rearrangements of Local Coordination Environment. Nat. Energy 2021, 6, 1144–1153. [Google Scholar]
- Huang, F.; Peng, M.; Chen, Y.; Cai, X.; Qin, X.; Wang, N.; Xiao, D.; Jin, L.; Wang, G.; Wen, X.D.; et al. Low-Temperature Acetylene Semi-Hydrogenation over the Pd1-Cu1 Dual-Atom Catalyst. J. Am. Chem. Soc. 2022, 144, 18485–18493. [Google Scholar] [PubMed]
- Min, X.Q.; Kanan, M.W. Pd-Catalyzed Electrohydrogenation of Carbon Dioxide to Formate: High Mass Activity at Low Overpotential and Identification of the Deactivation Pathway. J. Am. Chem. Soc. 2015, 137, 4701–4708. [Google Scholar]
- Cao, K.; Skowron, S.T.; Stoppiello, C.T.; Biskupek, J.; Khlobystov, A.N.; Kaiser, U. Direct Imaging of Atomic Permeation through a Vacancy Defect in the Carbon Lattice. Angew. Chem. Int. Ed. 2020, 59, 22922–22927. [Google Scholar]
- Rao, R.G.; Blume, R.; Hansen, T.W.; Fuentes, E.; Dreyer, K.; Moldovan, S.; Ersen, O.; Hibbitts, D.D.; Chabal, Y.J.; Schlogl, R.; et al. Interfacial Charge Distributions in Carbon-Supported Palladium Catalysts. Nat. Commun. 2017, 8, 340. [Google Scholar]
- Pang, S.; Zhang, Y.; Huang, Y.; Yuan, H.; Shi, F. N/O-Doped Carbon as a “Solid Ligand” for Nano-Pd Catalyzed Biphenyl- and Triphenylamine Syntheses. Catal. Sci. Technol. 2017, 7, 2170–2182. [Google Scholar]
- Huang, X.; Gonzalez, O.M.M.; Zhu, J.; Korányi, T.I.; Boot, M.D.; Hensen, E.J.M. Reductive Fractionation of Woody Biomass into Lignin Monomers and Cellulose by Tandem Metal Triflate and Pd/C Catalysis. Green Chem. 2017, 19, 175–187. [Google Scholar]
- Lu, C.; Wang, M.; Feng, Z.; Qi, Y.; Feng, F.; Ma, L.; Zhang, Q.; Li, X. A Phosphorus–Carbon Framework over Activated Carbon Supported Palladium Nanoparticles for the Chemoselective Hydrogenation of Para-Chloronitrobenzene. Catal. Sci. Technol. 2017, 7, 1581–1589. [Google Scholar]
- Di, L.; Zhang, J.; Craven, M.; Wang, Y.; Wang, H.; Zhang, X.; Tu, X. Dehydrogenation of Formic Acid over Pd/C Catalysts: Insight into the Cold Plasma Treatment. Catal. Sci. Technol. 2020, 10, 6129–6138. [Google Scholar]
- Kaushik, M.; Basu, K.; Benoit, C.; Cirtiu, C.M.; Vali, H.; Moores, A. Cellulose Nanocrystals as Chiral Inducers: Enantioselective Catalysis and Transmission Electron Microscopy 3d Characterization. J. Am. Chem. Soc. 2015, 137, 6124–61247. [Google Scholar] [PubMed]
- Kim, K.D.; Wang, Z.; Tao, Y.; Ling, H.; Yuan, Y.; Zhou, C.; Liu, Z.; Gaborieau, M.; Huang, J.; Yu, A. The Comparative Effect of Particle Size and Support Acidity on Hydrogenation of Aromatic Ketones. ChemCatChem 2019, 11, 4810–4817. [Google Scholar]
- Qiao, M.; Wang, Y.; Gao, S.; Fu, Q.; Wang, X.; Qin, R.; Zheng, N. Ligand Modification-Induced Electronic Effects and Synergistic Protic Solvent Effects Promote C—O Bond Hydrogenation. ACS Catal. 2024, 14, 11468–11476. [Google Scholar]
- Paul, R.; Shit, S.C.; Fovanna, T.; Ferri, D.; Srinivasa Rao, B.; Gunasooriya, G.K.K.; Dao, D.Q.; Le, Q.V.; Shown, I.; Sherburne, M.P.; et al. Realizing Catalytic Acetophenone Hydrodeoxygenation with Palladium-Equipped Porous Organic Polymers. ACS Appl. Mater. Interfaces 2020, 12, 50550–50565. [Google Scholar]
- Xiang, Y.-z.; Lv, Y.-a.; Xu, T.-y.; Li, X.-n.; Wang, J.-g. Selectivity Difference between Hydrogenation of Acetophenone over Cnts and Acs Supported Pd Catalysts. J. Mol. Catal. A Chem. 2011, 351, 70–75. [Google Scholar]
- Al-Wadhaf, H.A.; Karpov, V.M.; Katsman, E.A. Activity and Selectivity of Carbon Supported Palladium Catalysts Prepared from Bis(η3-Allyl)Palladium Complexes in Phenylacetylene Hydrogenation. Catal. Commun. 2018, 116, 67–71. [Google Scholar]
- Audevard, J.; Navarro-Ruiz, J.; Bernardin, V.; Tison, Y.; Corrias, A.; Del Rosal, I.; Favre-Réguillon, A.; Philippe, R.; Gerber, I.C.; Serp, P. Adjustment of the Single Atom/Nanoparticle Ratio in Pd/Cnt Catalysts for Phenylacetylene Selective Hydrogenation. ChemCatChem 2023, 15, e202300036. [Google Scholar]
- Yu, W.; Xin, Z.; Niu, S.; Lin, T.-W.; Guo, W.; Xie, Y.; Wu, Y.; Ji, X.; Shao, L. Nanosized Palladium on Phosphorus-Incorporated Porous Carbon Frameworks for Enhanced Selective Phenylacetylene Hydrogenation. Catal. Sci. Technol. 2017, 7, 4934–4939. [Google Scholar]
- Chung, J.; Kim, C.; Jeong, H.; Yu, T.; Binh, D.H.; Jang, J.; Lee, J.; Kim, B.M.; Lim, B. Selective Semihydrogenation of Alkynes on Shape-Controlled Palladium Nanocrystals. Chem. Asian J. 2013, 8, 919–925. [Google Scholar] [PubMed]
- Dominguez-Dominguez, S.; Berenguer-Murcia, A.; Pradhan, B.K.; Linares-Solano, A.; Cazorla-Amoros, D. Semihydrogenation of Phenylacetylene Catalyzed by Palladium Nanoparticles Supported on Carbon Materials. J. Phys. Chem. C 2008, 112, 3827–3834. [Google Scholar]
- Mironenko, R.M.; Likholobov, V.A.; Belskaya, O.B. Nanoglobular Carbon and Palladium–Nanoglobular Carbon Catalysts for Liquid-Phase Hydrogenation of Organic Compounds. Russ. Chem. Rev. 2022, 91, RCR501. [Google Scholar]
- Mao, Z.; Gu, H.; Lin, X. Recent Advances of Pd/C-Catalyzed Reactions. Catalysts 2021, 11, 1078. [Google Scholar] [CrossRef]
- Iwanow, M.; Gartner, T.; Sieber, V.; Konig, B. Activated Carbon as Catalyst Support: Precursors, Preparation, Modification and Characterization. Beilstein J. Org. Chem. 2020, 16, 1188–1202. [Google Scholar] [PubMed]
- Mironenko, R.M.; Belskaya, O.B.; Likholobov, V.A. Likholobov. Approaches to the Synthesis of Pd/C Catalysts with Controllable Activity and Selectivity in Hydrogenation Reactions. Catal. Today 2020, 357, 152–165. [Google Scholar]
- Cabiac, A.; Delahay, G.; Durand, R.; Trens, P.; Plée, D.; Medevielle, A.; Coq, B. The Influence of Textural and Structural Properties of Pd/Carbon on the Hydrogenation of Cis,Trans,Trans-1,5,9-Cyclododecatriene. Appl. Catal. A 2007, 318, 17–21. [Google Scholar]
- Cabiac, A.; Delahay, G.; Durand, R.; Trens, P.; Coq, B.; Plee, D. Controlled Preparation of Pd/Ac Catalysts for Hydrogenation Reactions. Carbon 2007, 45, 3–10. [Google Scholar]
- Kang, M.; Song, M.W.; Kim, K.L. Palladium Catalysts Supported on Activated Carbon with Different Textural and Surface Chemical Properties. React. Kinet. Catal. Lett. 2002, 76, 207–212. [Google Scholar]
- Gu, Y.; Li, Y.; Zhang, J.; Zhang, H.; Wu, C.; Lin, J.; Zhou, J.; Fan, Y.; Murugadoss, V.; Guo, Z. Effects of Pretreated Carbon Supports in Pd/C Catalysts on Rosin Disproportionation Catalytic Performance. Chem. Eng. Sci. 2020, 216, 115588. [Google Scholar]
- Calvo, L.; Gilarranz, M.A.; Casas, J.A.; Mohedano, A.F.; Rodríguez, J.J. Hydrodechlorination of 4-Chlorophenol in Aqueous Phase Using Pd/Ac Catalysts Prepared with Modified Active Carbon Supports. Appl. Catal. B 2006, 67, 68–76. [Google Scholar]
- Calvo, L.; Gilarranz, M.A.; Casas, J.A.; Mohedano, A.F.; Rodríguez, J.J. Effects of Support Surface Composition on the Activity and Selectivity of Pd/C Catalysts in Aqueous-Phase Hydrodechlorination Reactions. Ind. Eng. Chem. Res. 2005, 44, 6661–6667. [Google Scholar]
- Li, J.; Ma, L.; Li, X.; Lu, C.; Liu, H. Effect of Nitric Acid Pretreatment on the Properties of Activated Carbon and Supported Palladium Catalysts. Ind. Eng. Chem. Res. 2005, 44, 5478–5482. [Google Scholar]
- Kosydar, R.; Szewczyk, I.; Natkański, P.; Duraczyńska, D.; Gurgul, J.; Kuśtrowski, P.; Drelinkiewicz, A. New Insight into the Effect of Surface Oxidized Groups of Nanostructured Carbon Supported Pd Catalysts on the Furfural Hydrogenation. Surf. Interfaces 2019, 17, 100379. [Google Scholar]
- Klemeyer, L.; Park, H.; Huang, J. Geometry-Dependent Thermal Reduction of Graphene Oxide Solid. ACS Mater. Lett. 2021, 3, 511–515. [Google Scholar]
- Zhang, C.; Yang, G.; Jiang, H.; Liu, Y.; Chen, R.; Xing, W. Phenol Hydrogenation to Cyclohexanone over Palladium Nanoparticles Loaded on Charming Activated Carbon Adjusted by Facile Heat Treatment. Chin. J. Chem. Eng. 2020, 28, 2600–2606. [Google Scholar]
- Chen, Y.; Ding, X.; Qiu, W.; Song, J.; Nan, J.; Bai, G.; Pang, S. Effects of Surface Oxygen-Containing Groups of the Flowerlike Carbon Nanosheets on Palladium Dispersion, Catalytic Activity and Stability in Hydrogenolytic Debenzylation of Tetraacetyldibenzylhexaazaisowurtzitane. Catalysts 2021, 11, 441. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, L.; Lu, C.; Xu, X.; Lyu, J.; Li, X. Solvent-Free Selective Hydrogenation of O-Chloronitrobenzene over Large Palladium Particles on Oxygen-Poor Activated Carbon. React. Kinet. Mech. Catal. 2015, 114, 629–638. [Google Scholar]
- You, P.; Zhan, S.; Ruan, P.; Qin, R.; Mo, S.; Zhang, Y.; Liu, K.; Zheng, L.; Zheng, N. Interfacial Oxidized Pd Species Dominate Catalytic Hydrogenation of Polar Unsaturated Bonds. Nano Res. 2023, 17, 228–234. [Google Scholar]
- Cargnello, M.; Doan-Nguyen, V.V.; Gordon, T.R.; Diaz, R.E.; Stach, E.A.; Gorte, R.J.; Fornasiero, P.; Murray, C.B. Control of Metal Nanocrystal Size Reveals Metal-Support Interface Role for Ceria Catalysts. Science 2013, 341, 771–773. [Google Scholar] [PubMed]
- López, G.P.; Castner, D.G.; Ratner, B.D. Xps O 1s Binding Energies for Polymers Containing Hydroxyl, Ether, Ketone and Ester Groups. Surf. Interface Anal. 2004, 17, 267–272. [Google Scholar]
- Xu, T.-y.; Zhang, Q.-f.; Yang, H.-f.; Li, X.-n.; Wang, J.-g. Role of Phenolic Groups in the Stabilization of Palladium Nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 9783–9789. [Google Scholar]
- Qin, R.; Wang, P.; Liu, P.; Mo, S.; Gong, Y.; Ren, L.; Xu, C.; Liu, K.; Gu, L.; Fu, G.; et al. Carbon Monoxide Promotes the Catalytic Hydrogenation on Metal Cluster Catalysts. Research 2020, 2020, 4172794. [Google Scholar]


| Name | Reduction Temperature | Metal Dispersion | Active Particle Diameter (Hemisphere) | TEM Diameter |
|---|---|---|---|---|
| 60 °C-H2-Pd/C | 60 °C | 20.2% | 5.5 nm | 3.2 nm |
| 500 °C-TPR-Pd/C | 500 °C | 9.7% | 12 nm | 7.0 nm |
| 800 °C-TPR-Pd/C | 800 °C | 3.6% | 31 nm | 22 nm |
| Model | FCC, Regular Tetrahedron | FCC, Regular Octahedron | FCC, Cube | BCC, Cube | Regular Polyhedron |
|---|---|---|---|---|---|
| D | D | D | D | D | D → ∞ D = αd |
| N | D(D + 1)(D + 2)/6 | (2D − 1)(2D)(2D + 1)/6 − 4(D − 1)D(D + 1)/6 | D[D2 + (D − 1)2] + (D − 1)[D(D − 1) + D(D − 1)] | D3 + (D − 1)3 | βD3 |
| X(surface) | 3(D − 2)(D − 1)/2 + 1 | 7(D − 2)(D − 1)/2 + (2D − 1) | 5 × 2(D − 1)2 − (2D − 3) | 5(D − 1)2 − (2D − 3) | γD2 |
| X(perimeter) | 3(D − 2) | 3(D − 2) | 4(D − 2) | 4(D − 2) | δD1 |
| X(corner) | 3 | 3 | 4 | 4 | εD0 |
| ln k | ln (X/N) | ln (X/N) | ln (X/N) | ln (X/N) | k(surface) = γβ−1α−1d−1 k(perimeter) = δβ−1α−2d−2 k(corner) = εβ−1α−3d−3 |
| Δ | D | D | + 1 | + 1 | |
| d (nm) | 0.275 Δ | 0.275 Δ | 0.275 Δ | 0.275 Δ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, P.; Wu, L.; Zhou, L.; Xu, Y.; Qin, R. Impact of Oxygen-Containing Groups on Pd/C in the Catalytic Hydrogenation of Acetophenone and Phenylacetylene. Catalysts 2024, 14, 545. https://doi.org/10.3390/catal14080545
You P, Wu L, Zhou L, Xu Y, Qin R. Impact of Oxygen-Containing Groups on Pd/C in the Catalytic Hydrogenation of Acetophenone and Phenylacetylene. Catalysts. 2024; 14(8):545. https://doi.org/10.3390/catal14080545
Chicago/Turabian StyleYou, Pengyao, Liming Wu, Lu Zhou, Yong Xu, and Ruixuan Qin. 2024. "Impact of Oxygen-Containing Groups on Pd/C in the Catalytic Hydrogenation of Acetophenone and Phenylacetylene" Catalysts 14, no. 8: 545. https://doi.org/10.3390/catal14080545





