Research on Cu-Site Modification of g-C3N4/CeO2-like Z-Scheme Heterojunction for Enhancing CO2 Reduction and Mechanism Insight
Abstract
1. Introduction
2. Results and Discussion
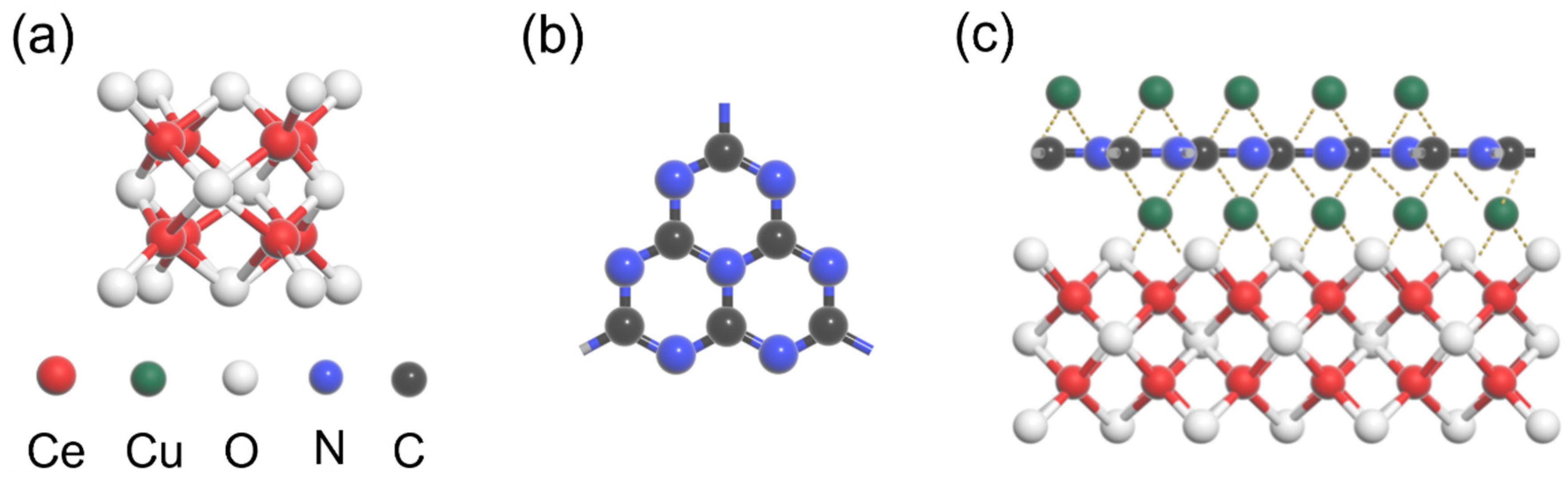
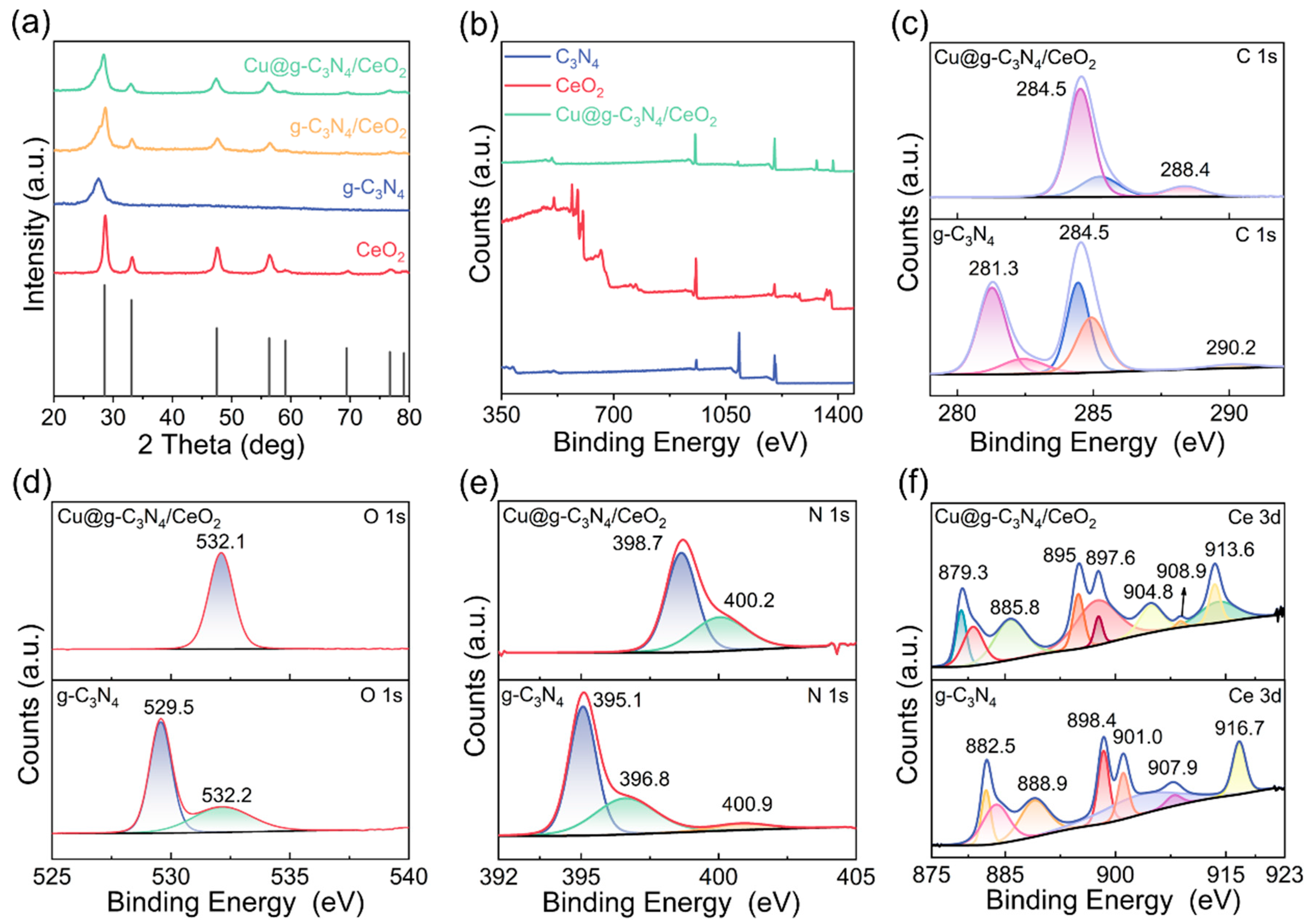
2.1. Photocatalyst Characterization
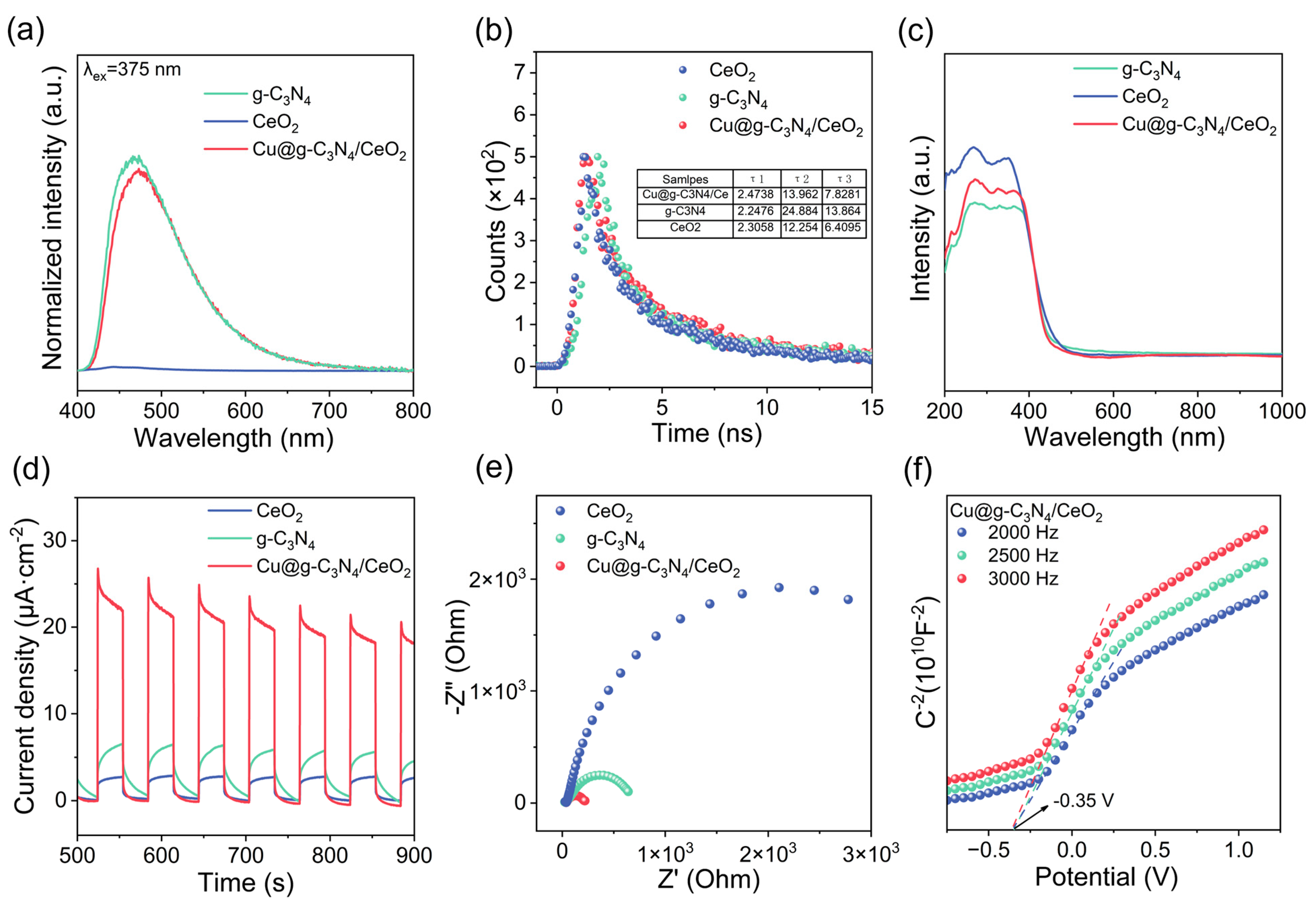
2.2. Surface Composition and Photoelectric Analysis
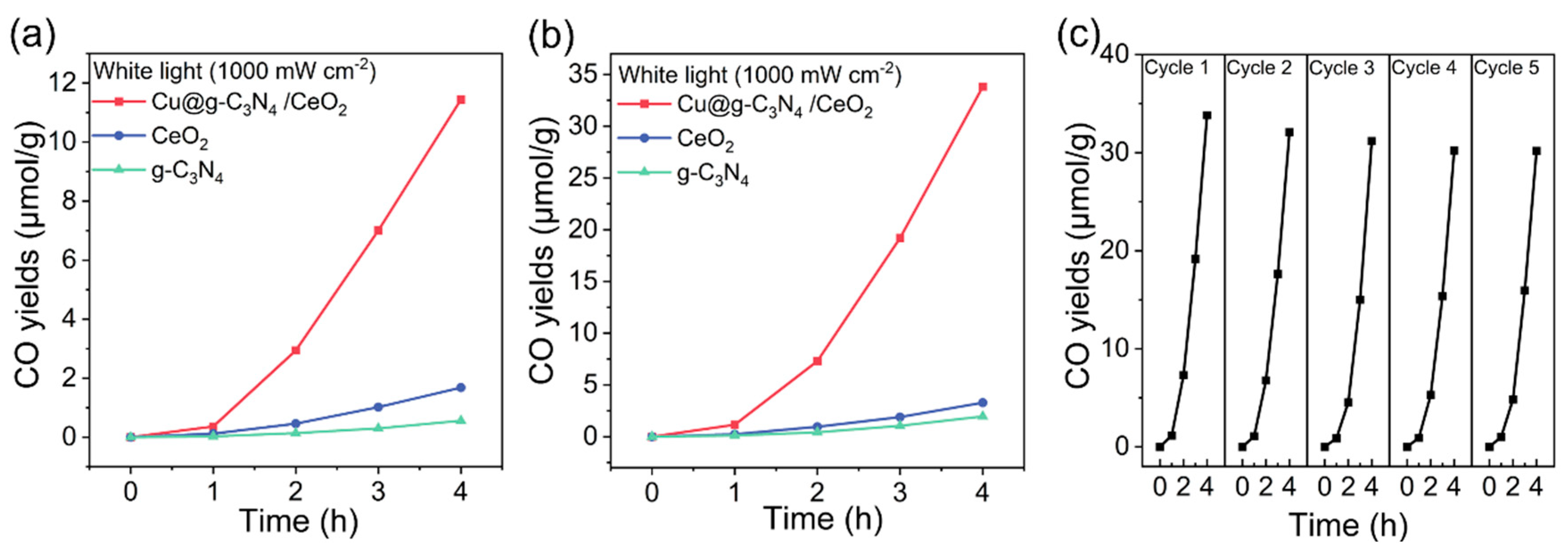
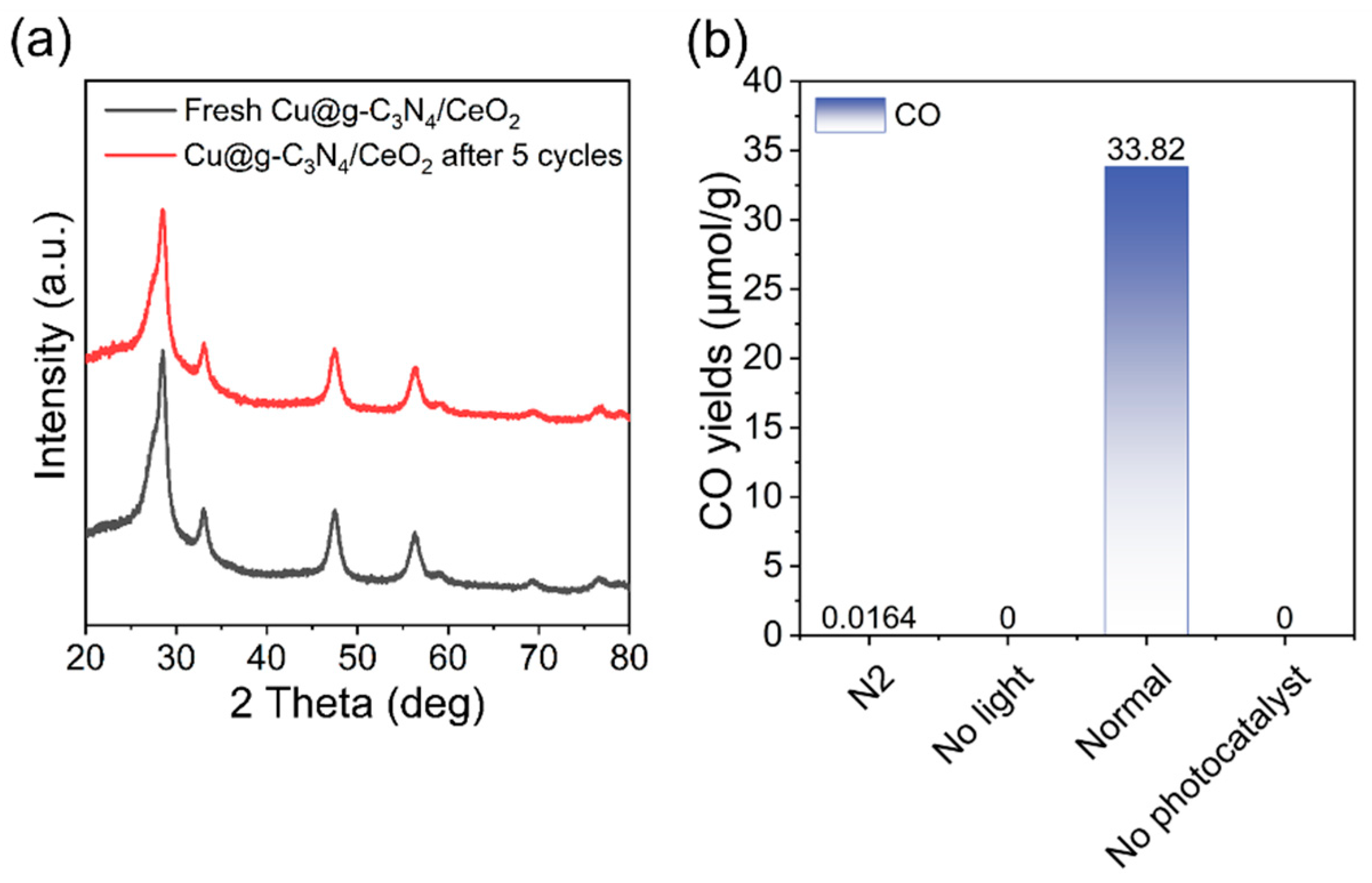
2.3. Photocatalytic Activity
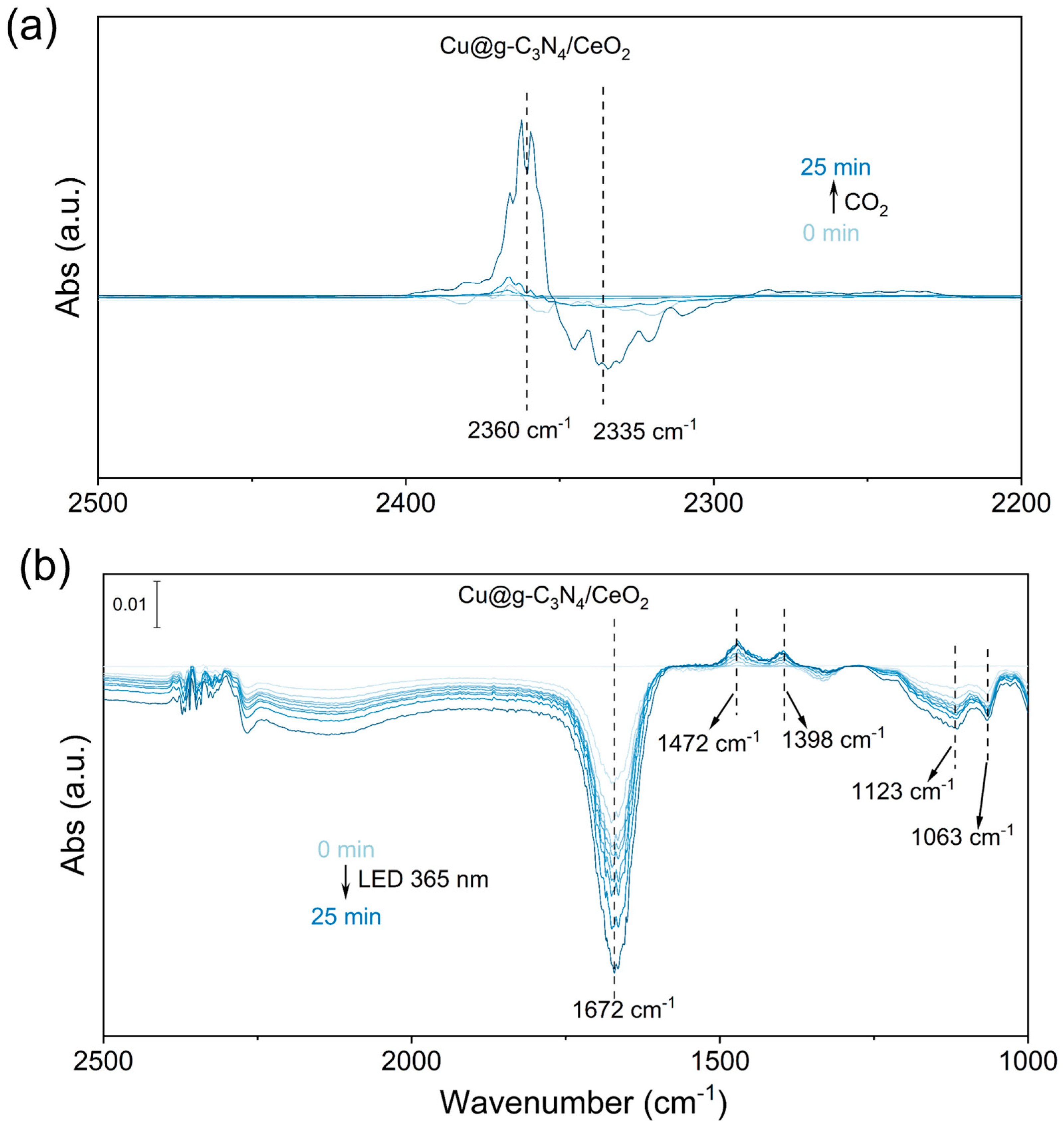
2.4. Mechanism of CO2 Photoreduction
3. Experimental Section
3.1. Methods
3.1.1. Materials
3.1.2. Synthesis of CeO2 Micro-Sheets
3.1.3. Synthesis of g-C3N4 Micro-Sheets
3.1.4. Preparation of g-C3N4/CeO2 Photocatalysts
3.1.5. Preparation of Cu@g-C3N4/CeO2 Photocatalysts
3.2. Characterizations
3.3. CO2 Photoreduction Experiments
3.4. Photo-Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced Photocatalytic CO2-Reduction Activity of Anatase TiO2 by Coexposed {001} and {101} Facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef]
- Li, K.; Peng, B.; Peng, T. Recent Advances in Heterogeneous Photocatalytic CO2 Conversion to Solar Fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Gong, E.; Ali, S.; Hiragond, C.B.; Kim, H.S.; Powar, N.S.; Kim, D.; Kim, H.; In, S.I. Solar fuels: Research and development strategies to accelerate photocatalytic CO2 conversion into hydrocarbon fuels. Energy Environ. Sci. 2022, 15, 880. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Bai, Z.; Lei, J.; Jin, H. Thermodynamic investigations of the supercritical CO2 system with solar energy and biomass. Appl. Energy 2017, 227, 108–118. [Google Scholar] [CrossRef]
- Jia, J.; O’Brien, P.G.; He, L.; Qiao, Q.; Fei, T.; Reyes, L.M.; Burrow, T.E.; Dong, Y.; Liao, K.; Varela, M.; et al. Carbon Dioxide Reduction: Visible and Near-Infrared Photothermal Catalyzed Hydrogenation of Gaseous CO2 over Nanostructured Pd@Nb2O5. Adv. Sci. 2016, 3, 10. [Google Scholar] [CrossRef]
- Hou, S.; Gao, X.; Lv, X.; Zhao, Y.; Yin, X.; Liu, Y.; Fang, J.; Yu, X.; Ma, X.; Ma, T.; et al. Decade Milestone Advancement of Defect-Engineered g-C3N4 for Solar Catalytic Applications. Nano-Micro Lett. 2024, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, N.; Guo, Z.; Wang, C.; Zhu, Z.; Tang, X.; Andrew Penyikie, G.; Wang, X.; Huo, P. Understanding the effect of multiple configurations in polymeric carbon nitride for efficient solar-driven hydrogen peroxide photosynthesis. Chem. Eng. J. 2024, 491, 152022. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, Y.; Xu, Y.; Zhang, Q.; Shi, X.; Li, D.; Tian, D.; Jiang, D. Improved charge transfer in polymeric carbon nitride synergistically induced by the aromatic rings modification and Schottky junctions for efficient photocatalytic CO2 reduction. Chem. Eng. J. 2023, 463, 142395. [Google Scholar] [CrossRef]
- Song, X.; Wu, Y.; Zhang, X.; Li, X.; Zhu, Z.; Ma, C.; Yan, Y.; Huo, P.; Yang, G. Boosting charge carriers separation and migration efficiency via fabricating all organic van der Waals heterojunction for efficient photoreduction of CO2. Chem. Eng. J. 2020, 408, 127292. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.; Guo, Y.; Yang, L.; Lv, M.; Tang, X.; Li, S. Enhanced photoreduction CO2 activity on g-C3N4: By synergistic effect of nitrogen defective-enriched and porous structure, and mechanism insights. Chem. Eng. J. 2020, 388, 124288. [Google Scholar] [CrossRef]
- Jun, W.; Bi, J.; Chuan, J.; Cheng, J. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 1603938. [Google Scholar]
- Jiang, T.; Wang, Z.; Wei, G.; Wu, S.; Huang, L.; Li, D.; Ruan, X.; Liu, Y.; Jiang, C.; Ren, F. Defective High-Crystallinity g-C3N4 Heterostructures by Double-End Modulation for Photocatalysis. ACS Energy Lett. 2024, 9, 1915–1922. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, X.; Song, X.; Liu, X.; Zhou, W.; Wang, H.; Huo, P. Pd Nanosheet-Decorated 2D/2D g-C3N4/WO3·H2O S-Scheme Photocatalyst for High Selective Photoreduction of CO2 to CO. Inorg. Chem. 2022, 61, 4171–4183. [Google Scholar] [CrossRef]
- Hu, H.; Hu, J.; Wang, X.; Gan, J.; Su, M.; Ye, W.; Zhang, W.; Ma, X.; Wang, H. Enhanced reduction and oxidation capability over the CeO2/g-C3N4 hybrid through surface carboxylation: Performance and mechanism. Catal. Sci. Technol. 2020, 10, 4712–4725. [Google Scholar] [CrossRef]
- Gao, W.; Li, Y.; Xiao, D.; Ma, D. Advances in photothermal conversion of carbon dioxide to solar fuels. J. Energy Chem. 2023, 83, 62–78. [Google Scholar] [CrossRef]
- Yang, X.; Peng, J.; Zhao, L.; Zhang, H.; Li, J.; Yu, P.; Fan, Y.; Wang, J.; Liu, H.; Dou, S. Insights on advanced g-C3N4 in energy storage: Applications, challenges, and future. Carbon Energy 2024, 9, 490. [Google Scholar] [CrossRef]
- Yin, S.; Sun, L.; Zhou, Y.; Li, X.; Li, J.; Song, X.; Huo, P.; Wang, H.; Yan, Y. Enhanced electron–hole separation in SnS2/Au/g-C3N4 embedded structure for efficient CO2 photoreduction. Chem. Eng. J. 2020, 406, 126776. [Google Scholar] [CrossRef]
- Ji, H.; Lin, L.; Chang, K. Plasma-assisted CO2 decomposition catalyzed by CeO2 of various morphologies. J. CO2 Util. 2022, 68, 102351. [Google Scholar] [CrossRef]
- Tran, D.; Pham, M.; Bui, X.; Wang, Y.; You, S. CeO2 as a photocatalytic material for CO2 conversion: A review. Sol. Energy 2022, 240, 443–466. [Google Scholar] [CrossRef]
- Yoko, A.; Wang, H.; Furuya, K.; Takahashi, D.; Seong, G.; Tomai, T.; Frenkel, A.I.; Saito, M.; Inoue, K.; Ikuhara, Y.; et al. Reduction of (100)-Faceted CeO2 for Effective Pt Loading. Chem. Mater. 2024, 36, 5611–5620. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, C.Y.; Wu, R.J. Hydrocarbon production by addition of Cu-ZnO on g-C3N4 for CO2 conversion. J. Chin. Chem. Soc. 2020, 67, 1654–1660. [Google Scholar] [CrossRef]
- Tang, X.; Shen, W.; Li, D.; Li, B.; Wang, Y.; Song, X.; Zhu, Z.; Huo, P. Research on cobalt-doping sites in g-C3N4 framework and photocatalytic reduction CO2 mechanism insights. J. Alloys Compd. 2023, 954, 170044. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, L.; Li, C.; Mo, Z.; Ye, M.; Zhu, Z.; Sun, S.; Wong, J.W.C. Triclocarban transformation and removal in sludge conditioning using chalcopyrite–triggered percarbonate treatment. J. Hazard. Mater. 2024, 463, 132944. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Heil, T.; Zhu, B.; Tung, C.-W.; Yu, J.; Chen, H.M.; Antonietti, M.; Cao, S. A Single Cu-Center Containing Enzyme-Mimic Enabling Full Photosynthesis under CO2 Reduction. ACS Nano 2020, 14, 8584–8593. [Google Scholar] [CrossRef]
- Zhu, Z.; Ye, J.; Tang, X.; Chen, Z.; Yang, J.; Huo, P.; Ng, Y.H.; Crittenden, J. Vacancy-Rich CoSx@LDH@Co-NC Catalytic Membrane for Antibiotic Degradation with Mechanistic Insights. Environ. Sci. Technol. 2023, 57, 16131–16140. [Google Scholar] [CrossRef]
- Zhu, Z.; Xing, X.; Qi, Q.; Shen, W.; Wu, H.; Li, D.; Li, B.; Liang, J.; Tang, X.; Zhao, J.; et al. Fabrication of Graphene Modified CeO2/g-C3N4 Heterostructures for Photocatalytic Degradation of Organic Pollutants. Chin. J. Struct. Chem. 2023, 42, 100194. [Google Scholar] [CrossRef]
- Vikatakavi, A.; Mauri, S.; Rivera-Salazar, M.L.; Dobovičnik, E.; Pelatti, S.; D’Addato, S.; Torelli, P.; Luches, P.; Benedetti, S. Role of Metal Dopants in Hydrogen Dissociation on Cu:CeO2 and Fe:CeO2 Surfaces Studied by Ambient-Pressure X-ray Absorption Spectroscopy. ACS Appl. Energy Mater. 2024, 7, 2746–2754. [Google Scholar] [CrossRef]
- Li, S.; Lu, J.; Wang, G.; Li, X.; Liu, W.; Xin, C.; Zhan, H. Study on the performance of photothermal catalyzed carbon dioxide hydrogenation to CH4 over doped sodium tantalate-based perovskite catalysts. J. Environ. Chem. Eng. 2024, 12, 113108. [Google Scholar] [CrossRef]
- Pan, D.; Wang, Y.; Lin, L.; Zhang, Y.; Zhou, M.; Li, Z.; Xu, S. Cu/Ni-loaded oxygen vacancy-rich CeO2 rod derived from metal organic framework for efficient photothermal CO2 hydrogenation. J. Mater. Sci. Mater. Electron. 2024, 35, 791. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Li, L.; Chen, L. Carbon nanotubes @Cu/Ni loaded BFO for photothermal catalytic conversion of CO2 by H2O. Mater. Chem. Phys. 2023, 309, 128417. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, J.; Yin, Q.; Zhang, C.; Zeng, Y.; Xu, S.; Liang, Q.; Zhou, M.; Li, Z. Reinforcing the efficiency of photocatalytic CO2 conversion with H2O vapor through the integration of photothermal Cu/C@Bi/C supported on 3D g-C3N4 under full-spectrum solar irradiation. J. Environ. Chem. Eng. 2024, 12, 113008. [Google Scholar] [CrossRef]
- Kong, N.; Han, B.; Li, Z.; Fang, Y.; Feng, K.; Wu, Z.; Wang, S.; Xu, A.-B.; Yu, Y.; Li, C.; et al. Ruthenium Nanoparticles Supported on Mg(OH)2 Microflowers as Catalysts for Photothermal Carbon Dioxide Hydrogenation. ACS Appl. Energy Mater. 2020, 3, 3028–3033. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, L.; Long, H.; Li, J.; Tan, W.; Ji, J.; Sun, J.; Tang, C.; Dong, L. Influence of CeO2 loading on structure and catalytic activity for NH3-SCR over TiO2-supported CeO2. J. Rare Earths 2020, 38, 883–890. [Google Scholar] [CrossRef]
- Chenari, H.M.; Riasvand, L.; Khalili, S. The synthesis condition and characterization of thermally treated CeO2 and ZnO CeO2 composite fibers. Ceram. Int. 2019, 45, 14223–14228. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, W.; Yang, F.; Bao, X. Interface-controlled synthesis of CeO2(111) and CeO2(100) and their structural transition on Pt(111). Chin. J. Catal. 2019, 40, 204–213. [Google Scholar] [CrossRef]
- Smeekens, S.P.; Malireddi, R.K.; Plantinga, T.S.; Buffen, K.; Netea, M.G. Autophagy is redundant for the host defense against systemic Candida albicans infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Qiu, G.; Xiao, Y.; Tao, X.; Li, B. Optimal construction of WO3·H2O/Pd/CdS ternary Z-scheme photocatalyst with remarkably enhanced performance for oxidative coupling of benzylamines. J. Catal. 2019, 374, 378–390. [Google Scholar] [CrossRef]
- Zhang, N.; Li, X.; Ye, H.; Chen, S.; Ju, H.; Liu, D.; Lin, Y.; Ye, W.; Wang, C.; Xu, Q. Oxide Defect Engineering Enables to Couple Solar Energy into Oxygen Activation. J. Am. Chem. Soc. 2016, 138, 8928–8935. [Google Scholar] [CrossRef]
- Tong, R.; Sun, Z.; Zhong, X.; Wang, X.; Xu, J.; Yang, Y.; Xu, B.; Wang, S.; Pan, H. Enhancement of Visible-Light Photocatalytic Hydrogen Production by CeCO3OH in g-C3N4/CeO2 System. ChemCatChem 2019, 11, 1069–1075. [Google Scholar] [CrossRef]
- Saravanan, V.; Lakshmanan, P.; Palanisami, N.; Amalraj, J.; Pyarasani, R.D.; Ramalingan, C. 2D/3D- C3N4/CeO2 S-scheme heterojunctions with enhanced photocatalytic performance. Inorg. Chem. Commun. 2022, 291, 898. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Fan, X.; Wu, M.; Du, Y.; Wang, M.; Kong, Q.; Zhang, L.; Shi, J. Dual synergetic effects in MoS2/pyridine-modified g-C3N4 composite for highly active and stable photocatalytic hydrogen evolution under visible light. Appl. Catal. B Environ. 2016, 190, 36–43. [Google Scholar] [CrossRef]
- Michalow-Mauke, K.A.; Lu, Y.; Kowalski, K.; Graule, T.; Nachtegaal, M.; Kröcher, O.; Ferri, D. Flame-Made WO3/CeOx-TiO2 Catalysts for Selective Catalytic Reduction of NOx by NH3. ACS Catal. 2015, 5, 5657–5672. [Google Scholar] [CrossRef]
- Feng, C.; Luo, J.; Chen, C.; Zuo, S.; Ren, Y.; Wu, Z.-P.; Hu, M.; Ould-Chikh, S.; Ruiz-Martínez, J.; Han, Y.; et al. Cooperative tungsten centers in polymeric carbon nitride for efficient overall photosynthesis of hydrogen peroxide. Energy Environ. Sci. 2024, 17, 1520–1530. [Google Scholar] [CrossRef]
- Feng, C.; Bo, T.; Maity, P.; Zuo, S.; Zhou, W.; Huang, K.W.; Mohammed, O.F.; Zhang, H. Regulating Photocatalytic CO2 Reduction Kinetics through Modification of Surface Coordination Sphere. Adv. Funct. Mater. 2023, 34, 2309761. [Google Scholar] [CrossRef]
- Wu, G.; He, Z.; Wang, Q.; Wang, H.; Wang, Z.; Sun, P.; Mo, Z.; Liu, H.; Xu, H. Non-metal inducing charge rearrangement in carbon nitride to promote photocatalytic hydrogen production. J. Mater. Sci. Technol. 2024, 195, 1–8. [Google Scholar] [CrossRef]
- Mo, Z.; Miao, Z.; Yan, P.; Sun, P.; Wu, G.; Zhu, X.; Ding, C.; Zhu, Q.; Lei, Y.; Xu, H. Electronic and energy level structural engineering of graphitic carbon nitride nanotubes with B and S co-doping for photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2023, 645, 525–532. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, D.; Yan, J.; Lee, L.Y.S. Photocatalytic CO2 Reduction Enabled by Interfacial S-Scheme Heterojunction between Ultrasmall Copper Phosphosulfide and g-C3N4. ACS Appl. Mater. Interfaces 2021, 13, 9762–9770. [Google Scholar] [CrossRef]
- Cai, S.; Chen, J.; Li, Q.; Jia, H. Enhanced Photocatalytic CO2 Reduction with Photothermal Effect by Cooperative Effect of Oxygen Vacancy and Au Cocatalyst. ACS Appl. Mater. Interfaces 2021, 13, 14221–14229. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, X.; Li, W.; Sun, H.; Jia, S.; Zhou, Y.; Hao, Y.; Liu, Z.; Yin, S.; Guo, C.; et al. Harnessing Photo-to-Thermal Conversion in Sulfur-Vulcanized Mxene for High-Efficiency Solar-to-Carbon-Fuel Synthesis. Adv. Funct. Mater. 2024, 34, 2400121. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Cai, J.; Sun, Y.; Jia, S.; Liu, Z.; Tang, X.; Hu, B.; Zhang, Y.; Yan, Y.; Zhu, Z. Research on Cu-Site Modification of g-C3N4/CeO2-like Z-Scheme Heterojunction for Enhancing CO2 Reduction and Mechanism Insight. Catalysts 2024, 14, 546. https://doi.org/10.3390/catal14080546
Zhou Y, Cai J, Sun Y, Jia S, Liu Z, Tang X, Hu B, Zhang Y, Yan Y, Zhu Z. Research on Cu-Site Modification of g-C3N4/CeO2-like Z-Scheme Heterojunction for Enhancing CO2 Reduction and Mechanism Insight. Catalysts. 2024; 14(8):546. https://doi.org/10.3390/catal14080546
Chicago/Turabian StyleZhou, Yiying, Junxi Cai, Yuming Sun, Shuhan Jia, Zhonghuan Liu, Xu Tang, Bo Hu, Yue Zhang, Yan Yan, and Zhi Zhu. 2024. "Research on Cu-Site Modification of g-C3N4/CeO2-like Z-Scheme Heterojunction for Enhancing CO2 Reduction and Mechanism Insight" Catalysts 14, no. 8: 546. https://doi.org/10.3390/catal14080546
APA StyleZhou, Y., Cai, J., Sun, Y., Jia, S., Liu, Z., Tang, X., Hu, B., Zhang, Y., Yan, Y., & Zhu, Z. (2024). Research on Cu-Site Modification of g-C3N4/CeO2-like Z-Scheme Heterojunction for Enhancing CO2 Reduction and Mechanism Insight. Catalysts, 14(8), 546. https://doi.org/10.3390/catal14080546








