Design and Performance of CuNi-rGO and Ag-CuNi-rGO Composite Electrodes for Use in Fuel Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of the Nanocomposite Properties
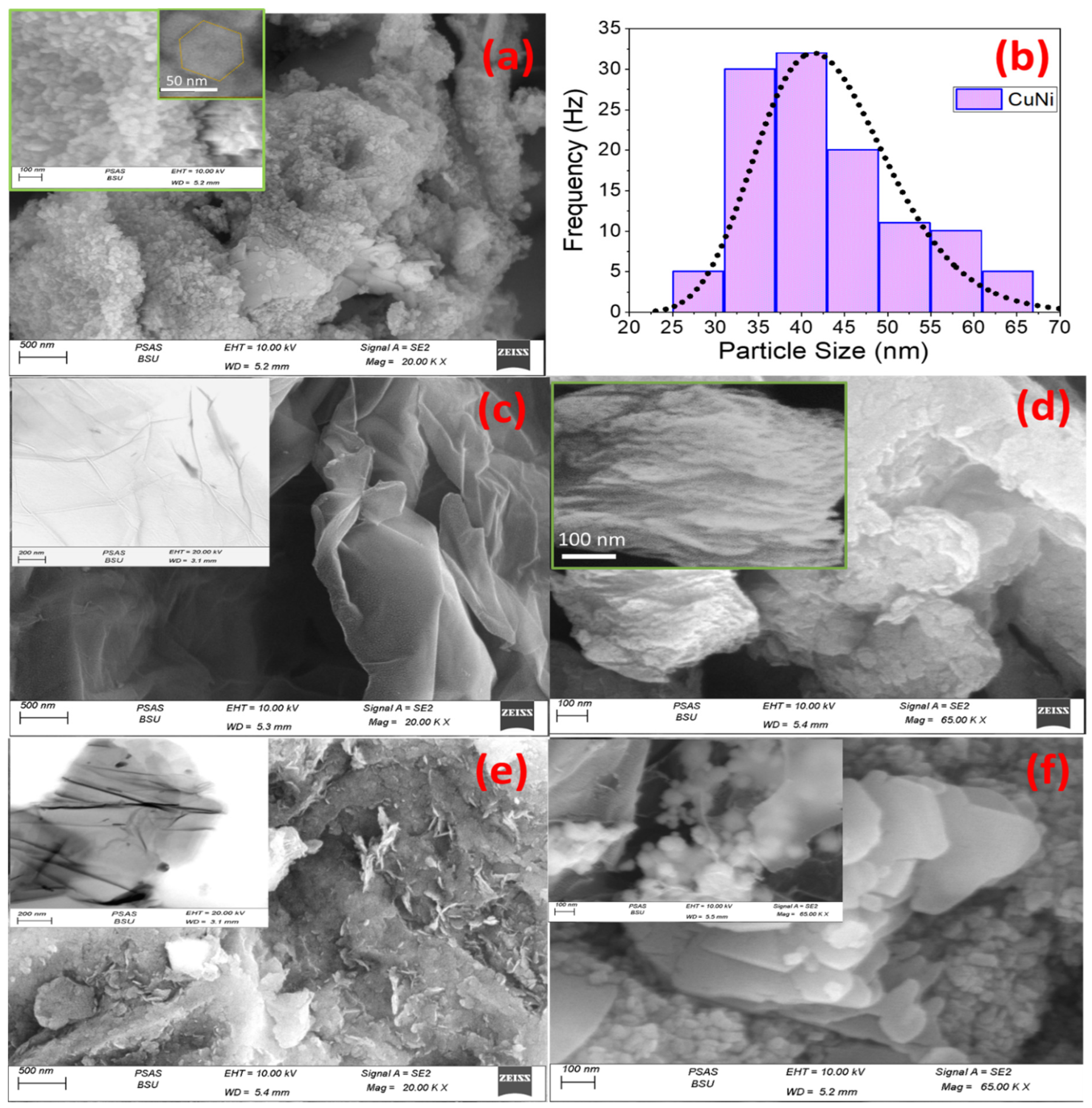
2.1.1. Nano-Morphological Analysis
2.1.2. Elemental Structure and Functional Entities
2.1.3. Structural Properties
2.1.4. Optical Band Gap
2.2. Electrooxidation Properties
2.2.1. Effect of Electrolytic and Sample Composition
2.2.2. Effect of Electrolytes Concentration
2.2.3. Scanning Rate Effect
2.2.4. LSV Measurements
2.2.5. Time-Dependent Current Analysis
2.2.6. EC Impedance (ECI) Spectral Analysis
3. Experimental Details
3.1. Materials
3.2. Synthesis of CuNi-GO and CuNi-rGO
3.3. CuNi-GO and CuNi-rGO Composite Characterization
3.4. Electrochemical (EC) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, R.; Feng, S.; Lauvaux, T. Country-Scale Trends in Air Pollution and Fossil Fuel CO2 Emissions during 2001–2018: Confronting the Roles of National Policies and Economic Growth. Environ. Res. Lett. 2020, 16, 014006. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid Oxide Fuel Cells (SOFCs): A Review of an Environmentally Clean and Efficient Source of Energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative Study of Different Fuel Cell Technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Lamy, C.; Lima, A.; LeRhun, V.; Delime, F.; Coutanceau, C.; Léger, J.-M. Recent Advances in the Development of Direct Alcohol Fuel Cells (DAFC). J. Power Sources 2002, 105, 283–296. [Google Scholar] [CrossRef]
- Wang, J.; Cao, X.; Fang, L.; You, X.; Wong, K.; Cao, S.; Xiao, C.; Cai, S.; Huang, Y.; Zhang, X. MoS2 Nanoflower Supported Pt Nanoparticle as an Efficient Electrocatalyst for Ethanol Oxidation Reaction. Int. J. Hydrogen Energy 2019, 44, 16411–16423. [Google Scholar] [CrossRef]
- Iwasita, T.; Dalbeck, R.; Pastor, E.; Xia, X. Progress in the Study of Electrocatalytic Reactions of Organic Species. Electrochim. Acta 1994, 39, 1817–1823. [Google Scholar] [CrossRef]
- Tran, L.T.; Nguyen, Q.M.; Nguyen, M.D.; Le, H.N.T.; Nguyen, T.T.; Vu, T.H.T. Preparation and Electrocatalytic Characteristics of the Pt-Based Anode Catalysts for Ethanol Oxidation in Acid and Alkaline Media. Int. J. Hydrogen Energy 2018, 43, 20563–20572. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, M.; Liu, H.; Wang, J.; Kim, M.J.; Yang, D.; Xie, Z.; Liu, J.; Xia, Y. Facile Synthesis of Pd–Pt Alloy Nanocages and Their Enhanced Performance for Preferential Oxidation of CO in Excess Hydrogen. ACS Nano 2011, 5, 8212–8222. [Google Scholar] [CrossRef]
- Hajian, A.; Rafati, A.A.; Yurchenko, O.; Urban, G.; Afraz, A.; Najafi, M.; Bagheri, A. Nanostructured Flower like Pt-Ru for Ethanol Oxidation and Determination. J. Electrochem. Soc. 2014, 162, B41. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A Review of Dry (CO 2) Reforming of Methane over Noble Metal Catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Li, M.; Hua, B.; Luo, J.; Pu, J.; Chi, B.; Jian, L. Carbon-Tolerant Ni-Based Cermet Anodes Modified by Proton Conducting Yttrium-and Ytterbium-Doped Barium Cerates for Direct Methane Solid Oxide Fuel Cells. J. Mater. Chem. A Mater. 2015, 3, 21609–21617. [Google Scholar] [CrossRef]
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of Current Development in Electrical Energy Storage Technologies and the Application Potential in Power System Operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef]
- Danaee, I.; Jafarian, M.; Forouzandeh, F.; Gobal, F.; Mahjani, M.G. Electrocatalytic Oxidation of Methanol on Ni and NiCu Alloy Modified Glassy Carbon Electrode. Int. J. Hydrogen Energy 2008, 33, 4367–4376. [Google Scholar] [CrossRef]
- Sundaram, M.; Kamaraj, A.B.; Lillie, G. Experimental Study of Localized Electrochemical Deposition of Ni-Cu Alloy Using a Moving Anode. Procedia CIRP 2018, 68, 227–231. [Google Scholar] [CrossRef]
- Casati, R.; Vedani, M. Metal Matrix Composites Reinforced by Nano-Particles—A Review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Naseer, A.; Ahmad, F.; Aslam, M.; Guan, B.H.; Harun, W.S.W.; Muhamad, N.; Raza, M.R.; German, R.M. A Review of Processing Techniques for Graphene-Reinforced Metal Matrix Composites. Mater. Manuf. Process. 2019, 34, 957–985. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science (1979) 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent Advances in Graphene Based Polymer Composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas Barrier Performance of Graphene/Polymer Nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef]
- Wala, M.; Szewczyk, M.; Leśniak–Ziółkowska, K.; Kazek–Kęsik, A.; Simka, W. Preparation of NiCuGO Composite and Investigation of Its Electrocatalytic Properties in Methanol Oxidation. Electrochim. Acta 2022, 425, 140743. [Google Scholar] [CrossRef]
- Wala, M.; Blacha–Grzechnik, A.; Stolarczyk, A.; Bajkacz, S.; Dydo, P.; Simka, W. Unexpected Electrochemical Oxidation of Urea on a New NiCuGO Composite Catalyst. Int. J. Hydrogen Energy 2023, 48, 34229–34243. [Google Scholar] [CrossRef]
- Thamer, B.M.; Hameed, M.M.A.; Abdo, H.S.; El-Newehy, M.H. Bimetallic NiCu Alloy Nanoparticles Supported on Porous Carbon Nanosheets as an Efficient Electrocatalyst for Methanol and Urea Electrooxidation. Ionics 2023, 29, 4203–4215. [Google Scholar] [CrossRef]
- Gupta, S.; De, M. Role of Metal (Cu/Ni/Fe/Co)-Carbon Composite in Enhancing Electro-Oxidation of Ethylene Glycol. J. Appl. Electrochem. 2023, 53, 1795–1809. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Gu, J.; Su, L.; Cheng, L. An overview of metal oxide materials as electrocatalysts and supports for polymer electrolyte fuel cells. Energy Environ. Sci. 2014, 7, 2535–2558. [Google Scholar] [CrossRef]
- Tucker, M.C.; Ying, A.S. Metal-supported solid oxide fuel cells operated in direct-flame configuration. Int. J. Hydrogen Energy 2017, 42, 24426–24434. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Cassayre, L. Mixed Metal Oxide Systems Applied to Thermochemical Storage of Solar Energy: Benefits of Secondary Metal Addition in Co and Mn Oxides and Contribution of Thermodynamics. Appl. Sci. 2018, 8, 2618. [Google Scholar] [CrossRef]
- Hidayah, N.M.S.; Liu, W.-W.; Lai, C.-W.; Noriman, N.Z.; Khe, C.-S.; Hashim, U.; Lee, H.C. Comparison on Graphite, Graphene Oxide and Reduced Graphene Oxide: Synthesis and Characterization. AIP Conf. Proc. 2017, 1892, 150002. [Google Scholar]
- Pareek, A.; Sravan, J.S.; Mohan, S.V. Exploring Chemically Reduced Graphene Oxide Electrode for Power Generation in Microbial Fuel Cell. Mater. Sci. Energy Technol. 2019, 2, 600–606. [Google Scholar] [CrossRef]
- Fajardo, S.; Ocón, P.; Rodríguez, J.L.; Pastor, E. Co Supported on N and S Dual-Doped Reduced Graphene Oxide as Highly Active Oxygen-Reduction Catalyst for Direct Ethanol Fuel Cells. Chem. Eng. J. 2023, 461, 142053. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Fateev, V.N.; Pushkarev, A.S.; Pushkareva, I.V.; Ivanova, N.A.; Kalinichenko, V.N.; Yu Presnyakov, M.; Wei, X. Reduced Graphene Oxide and Its Modifications as Catalyst Supports and Catalyst Layer Modifiers for PEMFC. Materials 2018, 11, 1405. [Google Scholar] [CrossRef]
- Xu, M.; Wu, L.; Zhu, M.; Wang, Z.; Huang, Z.-H.; Wang, M.-X. Self-Supporting Nitrogen-Doped Reduced Graphene Oxide@ Carbon Nanofiber Hybrid Membranes as High-Performance Integrated Air Cathodes in Microbial Fuel Cells. Carbon 2022, 193, 242–257. [Google Scholar] [CrossRef]
- Sheikh-Mohseni, M.A.; Hassanzadeh, V.; Habibi, B. Reduced Graphene Oxide Supported Bimetallic Ni-Co Nanoparticles Composite as an Electrocatalyst for Oxidation of Methanol. Solid State Sci. 2019, 98, 106022. [Google Scholar] [CrossRef]
- Rahmani, K.; Habibi, B. NiCo Alloy Nanoparticles Electrodeposited on an Electrochemically Reduced Nitrogen-Doped Graphene Oxide/Carbon-Ceramic Electrode: A Low Cost Electrocatalyst towards Methanol and Ethanol Oxidation. RSC Adv. 2019, 9, 34050–34064. [Google Scholar] [CrossRef] [PubMed]
- Noor, T.; Pervaiz, S.; Iqbal, N.; Nasir, H.; Zaman, N.; Sharif, M.; Pervaiz, E. Nanocomposites of NiO/CuO Based MOF with rGO: An Efficient and Robust Electrocatalyst for Methanol Oxidation Reaction in DMFC. Nanomaterials 2020, 10, 1601. [Google Scholar] [CrossRef]
- Sonia Theres, G.; Velayutham, G.; Santhana Krishnan, P.; Shanthi, K. Synergistic Impact of Ni–Cu Hybrid Oxides Deposited on Ordered Mesoporous Carbon Scaffolds as Non-Noble Catalyst for Methanol Oxidation. J. Mater. Sci. 2019, 54, 1502–1519. [Google Scholar] [CrossRef]
- Yuan, L.-S.; Zheng, Y.-X.; Jia, M.-L.; Zhang, S.-J.; Wang, X.-L.; Peng, C. Nanoporous Nickel-Copper-Phosphorus Amorphous Alloy Film for Methanol Electro-Oxidation in Alkaline Medium. Electrochim. Acta 2015, 154, 54–62. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, W.; Cheng, D. Facile Synthesis of Cu/NiCu Electrocatalysts Integrating Alloy, Core–Shell, and One-Dimensional Structures for Efficient Methanol Oxidation Reaction. ACS Appl. Mater. Interfaces 2017, 9, 19843–19851. [Google Scholar] [CrossRef]
- Mardani, Z.; Gholivand, M.B. Improvement of Electrocatalytic Activity of Bimetal Sulfide of Ni-Cu by α-MnO2 for Methanol Oxidation. J. Electroanal. Chem. 2022, 923, 116822. [Google Scholar] [CrossRef]
- Urbańczyk, E.; Wala, M.; Blacha-Grzechnik, A.; Stolarczyk, A.; Maciej, A.; Simka, W. Electrocatalytic Methanol Oxidation Using Ni–Co–Graphene Composite Electrodes. Int. J. Hydrogen Energy 2021, 46, 33272–33286. [Google Scholar] [CrossRef]
- Shojaeenezhad, S.S.; Farbod, M.; Kazeminezhad, I. Effects of Initial Graphite Particle Size and Shape on Oxidation Time in Graphene Oxide Prepared by Hummers’ Method. J. Sci. Adv. Mater. Devices 2017, 2, 470–475. [Google Scholar] [CrossRef]
- Mohamed, A.; Shaban, M.; Kordy, M.G.M.; Al-Senani, G.M.; Eissa, M.F.; Hamdy, H. Fabrication and Characterization of NiCu/GO and NiCu/RGO Nanocomposites for Fuel Cell Application. RSC Adv. 2024, 14, 6776–6792. [Google Scholar] [CrossRef]
- Alfryyan, N.; Kordy, M.G.M.; Abdel-Gabbar, M.; Soliman, H.A.; Shaban, M. Characterization of the Biosynthesized Intracellular and Extracellular Plasmonic Silver Nanoparticles Using Bacillus Cereus and Their Catalytic Reduction of Methylene Blue. Sci. Rep. 2022, 12, 12495. [Google Scholar] [CrossRef]
- Almarashi, J.Q.M.; Gadallah, A.-S.; Shaban, M.; Ellabban, M.A.; Hbaieb, K.; Kordy, M.G.M.; Zayed, M.; Mohamed, A.-A.H. Quick Methylene Blue Dye Elimination via SDS-Ag Nanoparticles Catalysts. Sci. Rep. 2024, 14, 15227. [Google Scholar] [CrossRef] [PubMed]
- Dandia, A.; Bansal, S.; Sharma, R.; Rathore, K.S.; Parewa, V. Microwave-assisted nanocatalysis: A CuO NPs/rGO composite as an efficient and recyclable catalyst for the Petasis-borono–Mannich reaction. RSC Adv. 2018, 8, 30280. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-C.; Yeh, H.-H.; Hu, C.-P.; Lecaros, R.L.G.; Cheng, C.-C.; Hung, W.-S.; Tsai, H.-A.; Lee, K.-R.; Lai, J.-Y. The Influence of Intermediate Layer and Graphene Oxide Modification on the CO2 Capture Efficiency of Pebax-GO/PDMS/PSf Mixed Matrix Composite Membranes. J. Taiwan Inst. Chem. Eng. 2022, 135, 104379. [Google Scholar] [CrossRef]
- Lee, A.Y.; Yang, K.; Anh, N.D.; Park, C.; Lee, S.M.; Lee, T.G.; Jeong, M.S. Raman Study of D* Band in Graphene Oxide and Its Correlation with Reduction. Appl. Surf. Sci. 2021, 536, 147990. [Google Scholar] [CrossRef]
- Ranjbar-Azad, M.; Behpour, M. Facile in situ co-precipitation synthesis of CuO–NiO/rGO nanocomposite for lithium-ion battery anodes. J. Mater. Sci. Mater. Electron. 2021, 32, 18043–18056. [Google Scholar] [CrossRef]
- Haywood, B.C.G.; Collins, M.F. Lattice dynamics of MnO. J. Phys. C Solid State Phys. 1969, 2, 46. [Google Scholar] [CrossRef]
- He, R.; Chung, T.F.; Delaney, C.; Keiser, C.; Jauregui, L.A.; Shand, P.M.; Chancey, C.C.; Wang, Y.; Bao, J.; Chen, Y.P. Observation of Low Energy Raman Modes in Twisted Bilayer Graphene. Nano Lett. 2013, 13, 3594–3601. [Google Scholar] [CrossRef]
- Ramnani, P.; Neupane, M.R.; Ge, S.; Balandin, A.A.; Lake, R.K.; Mulchandani, A. Raman Spectra of Twisted CVD Bilayer Graphene. Carbon 2017, 123, 302–306. [Google Scholar] [CrossRef]
- Hembram, K.P.S.S.; Kim, J.G.; Lee, S.G.; Park, J.; Lee, J.-K. Radial-Tangential Mode of Single-Wall Carbon Nanotubes Manifested by Landau Regulation: Reinterpretation of Low- and Intermediate-Frequency Raman Signals. Sci. Rep. 2023, 13, 5012. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Song, J.S.; Liu, P.; Xu, W.W.; Fang, T.; Jiao, Z. Monolayer graphene/NiO nanosheets with two−dimension structure for supercapacitors. J. Mater. Chem. 2011, 21, 18792–18798. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, D.W.; Zhao, S.Q.; Wu, J.J.; Xu, K.; Zhu, Q. Room temperature NO2 sensing: What advantage does the rGO−NiO Nanocomposite have over pristine NiO. Phys. Chem. Chem. Phys. 2015, 17, 14903–14911. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Song, Z.; Miao, Z.; Zhao, J. Ni-N-Doped Carbon-Modified Reduced Graphene Oxide Catalysts for Electrochemical CO2 Reduction Reaction. Catalysts 2021, 11, 561. [Google Scholar] [CrossRef]
- Li, S.; Niu, Z.; Jiao, Y.; Jin, P.; Yang, D.; Bai, C.; Liu, J.; Li, G.; Luo, Y. Preparation of Different Morphology Cu/GO Nanocomposites and Their Catalytic Performance for Thermal Decomposition of Ammonium Perchlorate. RSC Adv. 2022, 12, 22806–22814. [Google Scholar] [CrossRef] [PubMed]
- Luan, D.; Zhang, X.; Yu, Y.; Chen, Y.; Ma, Y.; Bi, C.; Zhao, D. Fabrication and electrochemical properties of graphene/copper–nickel solid solution reinforced polyaniline composite. J. Mater. Sci. Mater. Electron. 2017, 28, 14738–14746. [Google Scholar] [CrossRef]
- Kumari, V.; Kaushal, S.; Singh, P.P. Green Synthesis of a CuO/rGO Nanocomposite Using a Terminalia Arjuna Bark Extract and Its Catalytic Activity for the Purification of Water. Mater. Adv. 2022, 3, 2170–2184. [Google Scholar] [CrossRef]
- Vuong Hoan, N.T.; Anh Thu, N.T.; Van Duc, H.; Cuong, N.D.; Quang Khieu, D.; Vo, V. Fe 3 O 4/Reduced Graphene Oxide Nanocomposite: Synthesis and Its Application for Toxic Metal Ion Removal. J. Chem. 2016, 2016, 2418172. [Google Scholar] [CrossRef]
- Volanti, D.P.; Orlandi, M.O.; Andrés, J.; Longo, E. Efficient Microwave-Assisted Hydrothermal Synthesis of CuO Sea Urchin-like Architectures via a Mesoscale Self-Assembly. CrystEngComm 2010, 12, 1696–1699. [Google Scholar] [CrossRef]
- Zhao, Q.; Kulik, H.J. Where Does the Density Localize in the Solid State? Divergent Behavior for Hybrids and DFT+ U. J. Chem. Theory Comput. 2018, 14, 670–683. [Google Scholar] [CrossRef]
- Liu, H.B.; Xiang, L.; Jin, Y. Hydrothermal Modification and Characterization of Ni(OH)2 with High Discharge Capability. Cryst. Growth Des. 2006, 6, 283–286. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; Hu, Z.; Duan, W.; Cheng, F.; Chen, J. Porous CuO Nanowires as the Anode of Rechargeable Na-Ion Batteries. Nano. Res. 2014, 7, 199–208. [Google Scholar] [CrossRef]
- Gao, T.; Jelle, B.P. Paraotwayite-Type α-Ni (OH) 2 Nanowires: Structural, Optical, and Electrochemical Properties. J. Phys. Chem. C 2013, 117, 17294–17302. [Google Scholar] [CrossRef]
- Dong, L.; Chu, Y.; Sun, W. Controllable Synthesis of Nickel Hydroxide and Porous Nickel Oxide Nanostructures with Different Morphologies. Chem.–A Eur. J. 2008, 14, 5064–5072. [Google Scholar] [CrossRef]
- Askari, S.; Mariotti, D.; Brunet, P.; Vahl, A.; Benedikt, J. Iron-doped nanoflakes of layered double hydroxide of nickel for high-performance hybrid zinc batteries. Mater. Today Energy 2021, 22, 100879. [Google Scholar] [CrossRef]
- Zayed, M.; Ahmed, A.M.; Shaban, M. Synthesis and Characterization of Nanoporous ZnO and Pt/ZnO Thin Films for Dye Degradation and Water Splitting Applications. Int. J. Hydrogen Energy 2019, 44, 17630–17648. [Google Scholar] [CrossRef]
- Akbari, B.; Tavandashti, M.P.; Zandrahimi, M. Particle Size Characterization of Nanoparticles–A Practical Approach. Iran. J. Mater. Sci. Eng. 2011, 8, 48–56. [Google Scholar]
- Zayed, M.; Nasser, N.; Shaban, M.; Alshaikh, H.; Hamdy, H.; Ahmed, A.M. Effect of Morphology and Plasmonic on Au/ZnO Films for Efficient Photoelectrochemical Water Splitting. Nanomaterials 2021, 11, 2338. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Abdelkarem, K.; El Sayed, A.M. Structural, Optical and Gas Sensing Properties of Cu2O/CuO Mixed Phase: Effect of the Number of Coated Layers and (Cr+ S) Co-Doping. Phase Transit. 2019, 92, 347–359. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; El-Gohary, Z.; Soliman, H.S. Structural and Optical Studies of Thermally Evaporated CoPc Thin Films. Opt. Laser Technol. 2003, 35, 523–531. [Google Scholar] [CrossRef]
- Bayansal, F.; Şahin, B.; Yüksel, M.; Biyikli, N.; Çetinkara, H.A.; Güder, H.S. Influence of Coumarin as an Additive on CuO Nanostructures Prepared by Successive Ionic Layer Adsorption and Reaction (SILAR) Method. J. Alloys Compd. 2013, 566, 78–82. [Google Scholar] [CrossRef]
- Abid; Sehrawat, P.; Islam, S.S.; Mishra, P.; Ahmad, S. Reduced Graphene Oxide (rGO) Based Wideband Optical Sensor and the Role of Temperature, Defect States and Quantum Efficiency. Sci. Rep. 2018, 8, 3537. [Google Scholar] [CrossRef] [PubMed]
- Abdelkarem, K.; Saad, R.; Ahmed, A.M.; Fathy, M.I.; Shaban, M.; Hamdy, H. Efficient Room Temperature Carbon Dioxide Gas Sensor Based on Barium Doped CuO Thin Films. J. Mater. Sci. 2023, 58, 11568–11584. [Google Scholar] [CrossRef]
- Ngetich, C.C.; Mutua, J.M.; Kareru, P.G.; Murimi, E.; Karanja, K. Synthesis and Characterization of Graphene Oxide and Graphene Nanomaterial for Fuel Cell Applications. In Proceedings of the Sustainable Research and Innovation Conference, JKUAT Main Campus, Kenya, 6–7 October 2021; pp. 92–96. [Google Scholar]
- Sawicki, B.; Tomaszewicz, E.; Piątkowska, M.; Groń, T.; Duda, H.; Górny, K. Correlation between the Band-Gap Energy and the Electrical Conductivity in MPr2W2O10 Tungstates (Where M= Cd, Co, Mn). Acta Phys. Pol. A 2016, 129, A94–A96. [Google Scholar] [CrossRef]
- Raslan, A.; Del Burgo, L.S.; Ciriza, J.; Pedraz, J.L. Graphene Oxide and Reduced Graphene Oxide-Based Scaffolds in Regenerative Medicine. Int. J. Pharm. 2020, 580, 119226. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Ubaidullah, M.; Ahmed, J.; Alrobei, H.; Alshehri, S.M. Copper Nickel@ Reduced Graphene Oxide Nanocomposite as Bifunctional Electro-Catalyst for Excellent Oxygen Evolution and Oxygen Reduction Reactions. Mater. Lett. 2020, 260, 126969. [Google Scholar]
- Chaturvedi, K.R.; Sharma, T. Carbonated Polymeric Nanofluids for Enhanced Oil Recovery from Sandstone Reservoir. J. Pet. Sci. Eng. 2020, 194, 107499. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.; Kong, Q.; Tong, X.; Wu, S.; Zong, N.; Xu, R.; Yang, L. One-Step Electrosynthesis of Bifunctional NiCu Nanosheets on Iron Foam for Remarkably Enhanced Alkaline Water Splitting. Sustainability 2023, 15, 12240. [Google Scholar] [CrossRef]
- Yousaf, A.B.; Alsaydeh, S.A.M.; Zavahir, F.S.; Kasak, P.; Zaidi, S.J. Ultra-Low Pt-Decorated NiCu Bimetallic Alloys Nanoparticles Supported on Reduced Graphene Oxide for Electro-Oxidation of Methanol. MRS Commun. 2018, 8, 1050–1057. [Google Scholar] [CrossRef]
- Ballarin, B.; Seeber, R.; Tonelli, D.; Vaccari, A. Electrocatalytic Properties of Nickel (II) Hydrotalcite-Type Anionic Clay: Application to Methanol and Ethanol Oxidation. J. Electroanal. Chem. 1999, 463, 123–127. [Google Scholar] [CrossRef]
- Abdel-Hady, E.E.; Shaban, M.; Abdel-Hamed, M.O.; Gamal, A.; Yehia, H.; Ahmed, A.M. Synthesis and Characterization of NiCoPt/CNFs Nanoparticles as an Effective Electrocatalyst for Energy Applications. Nanomaterials 2022, 12, 492. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Zhao, T.S.; Liang, Z.X. Performance of Alkaline Electrolyte-Membrane-Based Direct Ethanol Fuel Cells. J. Power Sources 2009, 187, 387–392. [Google Scholar] [CrossRef]
- Deng, Z.; Yi, Q.; Zhang, Y.; Nie, H. NiCo/CN/CNT Composite Catalysts for Electro-Catalytic Oxidation of Methanol and Ethanol. J. Electroanal. Chem. 2017, 803, 95–103. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Yassin, M.A.; Al-Mubaddel, F.S.; Amen, M.T. New Electrooxidation Characteristic for Ni-Based Electrodes for Wide Application in Methanol Fuel Cells. Appl. Catal. A Gen. 2018, 555, 148–154. [Google Scholar] [CrossRef]
- Yang, J.; Ju, Z.; Jiang, Y.; Xing, Z.; Xi, B.; Feng, J.; Xiong, S. Enhanced Capacity and Rate Capability of Nitrogen/Oxygen Dual-doped Hard Carbon in Capacitive Potassium-ion Storage. Adv. Mater. 2018, 30, 1700104. [Google Scholar] [CrossRef] [PubMed]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Sood, A.; Poletayev, A.D.; Cogswell, D.A.; Csernica, P.M.; Mefford, J.T.; Fraggedakis, D.; Toney, M.F.; Lindenberg, A.M.; Bazant, M.Z.; Chueh, W.C. Electrochemical Ion Insertion from the Atomic to the Device Scale. Nat. Rev. Mater. 2021, 6, 847–867. [Google Scholar] [CrossRef]
- Tamang, S.; Rai, S.; Bhujel, R.; Bhattacharyya, N.K.; Swain, B.P.; Biswas, J. A Concise Review on GO, rGO and Metal Oxide/rGO Composites: Fabrication and Their Supercapacitor and Catalytic Applications. J. Alloys Compd. 2023, 947, 169588. [Google Scholar] [CrossRef]
- Stratakes, B.M.; Dempsey, J.L.; Miller, A.J.M. Determining the Overpotential of Electrochemical Fuel Synthesis Mediated by Molecular Catalysts: Recommended Practices, Standard Reduction Potentials, and Challenges. ChemElectroChem 2021, 8, 4161–4180. [Google Scholar] [CrossRef]
- Li, Z.; Yan, Y.; Xu, S.-M.; Zhou, H.; Xu, M.; Ma, L.; Shao, M.; Kong, X.; Wang, B.; Zheng, L. Alcohols Electrooxidation Coupled with H2 Production at High Current Densities Promoted by a Cooperative Catalyst. Nat. Commun. 2022, 13, 147. [Google Scholar] [CrossRef]
- Parsa, A.; Amanzadeh-Salout, S. Electrocatalytic Activity and Electrochemical Impedance Spectroscopy of Poly (Aniline-Co-Ortho-Phenylenediamine) Modified Electrode on Ascorbic Acid. Orient. J. Chem. 2016, 32, 2051. [Google Scholar] [CrossRef]
- Feng, L.-J.; Zhang, X.-H.; Zhao, D.-M.; Wang, S.-F. Electrochemical Studies of Bovine Serum Albumin Immobilization onto the Poly-o-Phenylenediamine and Carbon-Coated Nickel Composite Film and Its Interaction with Papaverine. Sens. Actuators B Chem. 2011, 152, 88–93. [Google Scholar] [CrossRef]
- Dhillon, S.; Kant, R. Theory for Electrochemical Impedance Spectroscopy of Heterogeneous Electrode with Distributed Capacitance and Charge Transfer Resistance. J. Chem. Sci. 2017, 129, 1277–1292. [Google Scholar] [CrossRef]
- Helal, N.H. Corrosion Inhibition and Adsorption Behavior of Methionine on Mg-Al-Zn Alloy. J. Chem. Eng. Mater. Sci. 2011, 2, 28–38. [Google Scholar]
- Tian, H.; Zhao, G.; Zhang, Y.; Wang, Y.; Cao, T. Hierarchical (0 0 1) Facet Anatase/Rutile TiO2 Heterojunction Photoanode with Enhanced Photoelectrocatalytic Performance. Electrochim. Acta 2013, 96, 199–205. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, T.; Pan, Q.; Zhang, X.; Guo, J. PtFe/Nitrogen-Doped Graphene for High-Performance Electrooxidation of Formic Acid with Composition Sensitive Electrocatalytic Activity. RSC Adv. 2015, 5, 60237–60245. [Google Scholar] [CrossRef]
- Khan, I.A.; Sofian, M.; Badshah, A.; Khan, M.A.; Imran, M.; Nadeem, M.A. Stable and Efficient PtRu Electrocatalysts Supported on Zn-BTC MOF Derived Microporous Carbon for Formic Acid Fuel Cells Application. Front. Chem. 2020, 8, 367. [Google Scholar] [CrossRef]
- Li, F.-M.; Kang, Y.-Q.; Liu, H.-M.; Zhai, Y.-N.; Hu, M.-C.; Chen, Y. Atoms Diffusion-Induced Phase Engineering of Platinum-Gold Alloy Nanocrystals with High Electrocatalytic Performance for the Formic Acid Oxidation Reaction. J. Colloid Interface Sci. 2018, 514, 299–305. [Google Scholar] [CrossRef]
- Shen, S.Y.; Zhao, T.S.; Xu, J.B.; Li, Y.S. Synthesis of PdNi Catalysts for the Oxidation of Ethanol in Alkaline Direct Ethanol Fuel Cells. J. Power Sources 2010, 195, 1001–1006. [Google Scholar] [CrossRef]
- Chen, F.-K.; Ho, Y.-H.; Chang, H.-W.; Tsai, Y.-C. Nanocomposite Integrating Tube-like NiCo2S4 and Carbon Nanotubes for Electrooxidation of Methanol. Electrochem. Commun. 2020, 117, 106783. [Google Scholar] [CrossRef]
- Hu, J.; Li, R.; Wang, X.; Wang, S.; Fang, C. Strain-Induced Porous Pd@ PdPt Core/Shell Nanocubes as Effective All-in-One Electrocatalysts toward Multialcohol Oxidation. ACS Appl. Nano Mater. 2023, 7, 10213–10222. [Google Scholar] [CrossRef]
- Higareda, A.; Mares-Briones, F.; Rosas, G.; Pérez, R.; Esparza, R. Enhanced Durability of Pdpt/c Electrocatalyst during the Ethanol Oxidation Reaction in Alkaline Media. J. Solid State Electrochem. 2022, 26, 2143–2151. [Google Scholar] [CrossRef]
- Xia, Q.Q.; Zhang, L.Y.; Zhao, Z.L.; Li, C.M. Growing Platinum-Ruthenium-Tin Ternary Alloy Nanoparticles on Reduced Graphene Oxide for Strong Ligand Effect toward Enhanced Ethanol Oxidation Reaction. J. Colloid Interface Sci. 2017, 506, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, K.; Ye, J.; Xue, F.; Cheng, Y.; Lyu, Z.; Liao, X.; Wang, W.; Zhang, Q.; Chen, X. Facilitating the C–C Bond Cleavage on Sub-10 Nm Concavity-Tunable Rh@ Pt Core–Shell Nanocubes for Efficient Ethanol Electrooxidation. J. Mater. Chem. A Mater. 2019, 7, 17987–17994. [Google Scholar] [CrossRef]
- Han, Z.; Wang, A.-J.; Zhang, L.; Wang, Z.-G.; Fang, K.-M.; Yin, Z.-Z.; Feng, J.-J. 3D Highly Branched PtCoRh Nanoassemblies: Glycine-Assisted Solvothermal Synthesis and Superior Catalytic Activity for Alcohol Oxidation. J. Colloid Interface Sci. 2019, 554, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.Y.; Li, T.T.; Yin, Z.H.; Chen, L.; Wang, J.J. Remarkably Enhanced Acidic Photoelectrochemical Glycerol Oxidation Achieving the Theoretical Maximum Photocurrent of BiVO4 through Anion Modulation. Chem. Eng. J. 2024, 493, 152461. [Google Scholar] [CrossRef]
- Javan, H.; Asghari, E.; Ashassi-Sorkhabi, H. Design of New Anodic Bimetallic Nanocatalyst Composed of Ni–Cu Supported by Reduced Carbon Quantum Dots for the Methanol Oxidation Reaction. Diam. Relat. Mater. 2021, 115, 108348. [Google Scholar] [CrossRef]
- Mao, Y.H.; Chen, C.Y.; Fu, J.X.; Lai, T.Y.; Lu, F.H.; Tsai, Y.C. Electrodeposition of Nickel copper on Titanium Nitride for Methanol Electrooxidation. Surf. Coat. Technol. 2018, 350, 949–953. [Google Scholar] [CrossRef]
- Ding, R.; Zhang, S.; Chen, Y.; Rui, Z.; Hua, K.; Wu, Y.; Li, X.; Duan, X.; Wang, X.; Li, J. Application of Machine Learning in Optimizing Proton Exchange Membrane Fuel Cells: A Review. Energy AI 2022, 9, 100170. [Google Scholar] [CrossRef]
- Zhao, C.; Yuan, S.; Cheng, X.; Zheng, Z.; Liu, J.; Yin, J.; Shen, S.; Yan, X.; Zhang, J. The Effect of Catalyst Layer Design on Catalyst Utilization in PEMFC Studied via Stochastic Reconstruction Method. Energy AI 2023, 13, 100245. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.-W.; Voon, C.H. Synthesis of Graphene Oxide Using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Liu, J.; Li, H.; Zhang, H. Improved Mechanical Properties of Ni-rGO/Cu Composites Prepared by Molecular-Level Mixing. Appl. Phys. A 2022, 128, 165. [Google Scholar] [CrossRef]
- Sadiq, M.; Arya, A.; Sharma, A.L. Dielectric Study of Polymer Nanocomposite Films for Energy Storage Applications. In Proceedings of the Recent Trends in Materials and Devices: Proceedings ICRTMD 2015; Springer: Berlin/Heidelberg, Germany, 2017; pp. 389–396. [Google Scholar]







| Nanocatalysts | Rs/Ω | Rct/Ω | CdI/µF | ZW | θmax | fmax/s−1 | /ms |
|---|---|---|---|---|---|---|---|
| Ag-CuNi-rGO | 72.9 ± 0.1 | 8.3 ± 0.1 | 23.18 ± 0.11 | 4.5 ± 0.04 | 86.4° ± 0.2 | 13.48 ± 0.40 | 74.2 ± 2.2 |
| 84.9 ± 0.2 | 1.09 ± 0.11 | 918.3 ± 91.8 | |||||
| CuNi-rGO | 36.4 ± 0.6 | 7.5 ± 0.2 | 3.53 ± 0.15 | 1.34 ± 0.03 | 72.0° ± 0.2 | 33.85 ± 0.50 | 29.5 ± 0.4 |
| 69.4° ± 0.2 | 1.38 ± 0.15 | 722.6 ± 77.4 | |||||
| CuNi-GO | 37.2 ± 0.8 | 1.2 ± 0.1 | 2.09 ± 0.13 | 1.57 ± 0.02 | 78.3° ± 0.3 | 2.57 ± 0.20 | 388.4 ± 30.0 |
| CuNi | 35.4 ± 0.5 | 3.3 ± 0.2 | 0.78 ± 0.07 | 6.36 ± 0.04 | 81.8° ± 0.3 | 13.39 ± 0.30 | 74.7 ± 1.7 |
| Catalysts | Nanomorphologies | Electrolytes | Onset Potentials (mV) | Current Density | Catalyst Dose (mg) | Ref. |
|---|---|---|---|---|---|---|
| Pt79Fe21/N-doped graphene | Nanoparticles/sheets | 1 M Formic + 0.5 M sulfuric acids | 110 mV vs. RHE | 186 mA/g @ 0.4 V vs. RHE | 2 | [97] |
| Pt1Ru2/microporous carbon-950 | Nanoparticles/microporous | 0.5 M H2SO4 + 1 M CH2O2 | 110 mV vs. Ag/AgCl | 9.5 mA/cm2 @ 700 mV | 10 | [98] |
| PtAu/Au | Nanotexture | 0.5 M Formic + 0.5 M H2SO4 | - | 228 mA/g @ 0.51 V | 8 | [99] |
| Pd2Ni3/C | Nanopowder | 1 M ethanol + 1 M KOH | −650 mV vs. MMO | 217 mA/cm2 | 10 | [100] |
| NiCo2S4/CNT(3:1) | Nanocomposites | 1 M KOH + 1 M methanol | - | 160 mA/cm2 @ 600 mV | 4 | [101] |
| Pd@PdPt | Porous nanocubes | 0.5 M KOH + 1 M ethanol | 467 mV vs. RHE | 3.4 mA/cm2 | - | [102] |
| PdPt/C | Nanoparticles | 0.3 M KOH + 1 M ethanol | −370 mV | 3.3 mA/cm2 | 1 | [103] |
| Pt1Ru0.5Sn0.5-RGO | Nanoparticles | 1 M KOH + 1 M ethanol | −633 mV | 2.5 mA/cm2 | 3 | [104] |
| Rh@Pt | Nanocubes | 0.1 M HClO4 + 0.2 M ethanol | 460 mV vs. RHE | 2.6 mA/cm2 | - | [105] |
| PtCoRh | Nano-assemblies | 1 M KOH + 1 M ethanol | 250 mV vs. RHE | 40.3 mA/cm2 | 2 | [106] |
| CuNi-rGO | Nanospheres/sheets | 1 M KOH + 2 M methanol | −315 mV | 267.2 mA/g | 25 | This study |
| Ag- CuNi-rGO | −548 mV vs. Hg/HgCl | 679.3 mA/g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaban, M.; Mohamed, A.; Kordy, M.G.M.; AlMohamadi, H.; Eissa, M.F.; Hamdy, H. Design and Performance of CuNi-rGO and Ag-CuNi-rGO Composite Electrodes for Use in Fuel Cells. Catalysts 2024, 14, 551. https://doi.org/10.3390/catal14080551
Shaban M, Mohamed A, Kordy MGM, AlMohamadi H, Eissa MF, Hamdy H. Design and Performance of CuNi-rGO and Ag-CuNi-rGO Composite Electrodes for Use in Fuel Cells. Catalysts. 2024; 14(8):551. https://doi.org/10.3390/catal14080551
Chicago/Turabian StyleShaban, Mohamed, Aya Mohamed, Mohamed G. M. Kordy, Hamad AlMohamadi, M. F. Eissa, and Hany Hamdy. 2024. "Design and Performance of CuNi-rGO and Ag-CuNi-rGO Composite Electrodes for Use in Fuel Cells" Catalysts 14, no. 8: 551. https://doi.org/10.3390/catal14080551







