Biodiesel Production from Waste Frying Oil (WFO) Using a Biomass Ash-Based Catalyst
Abstract
1. Introduction
2. Results
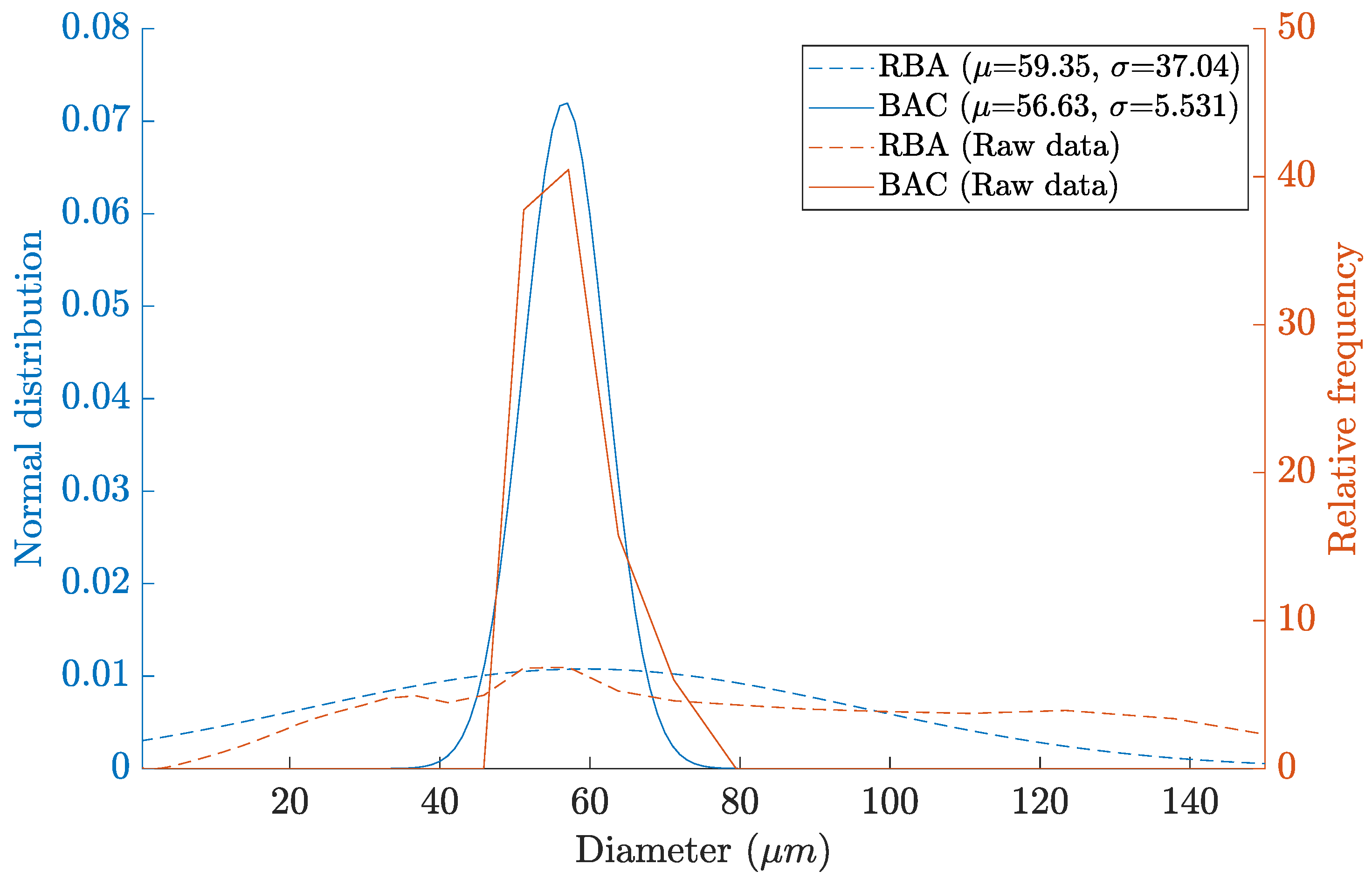
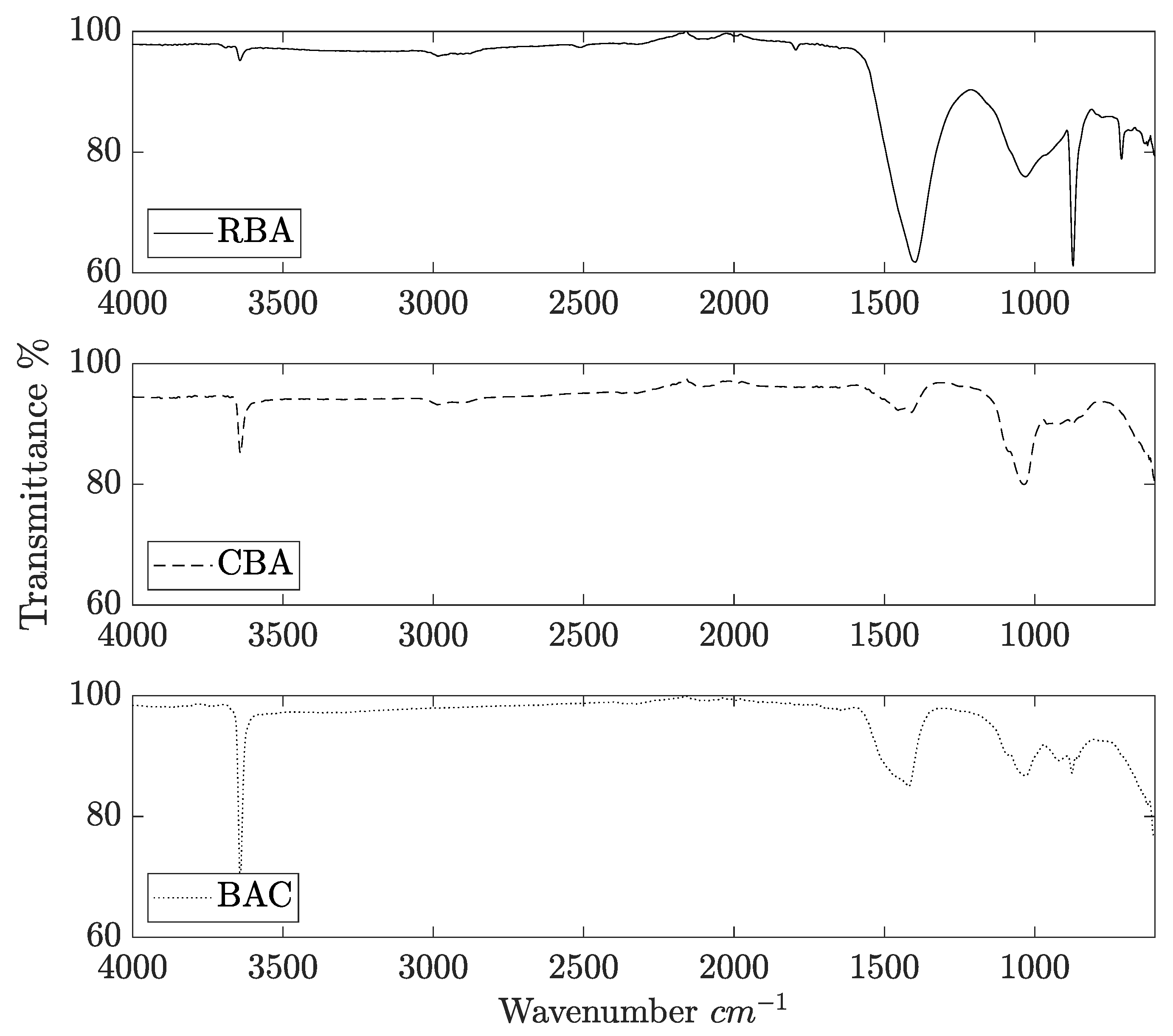
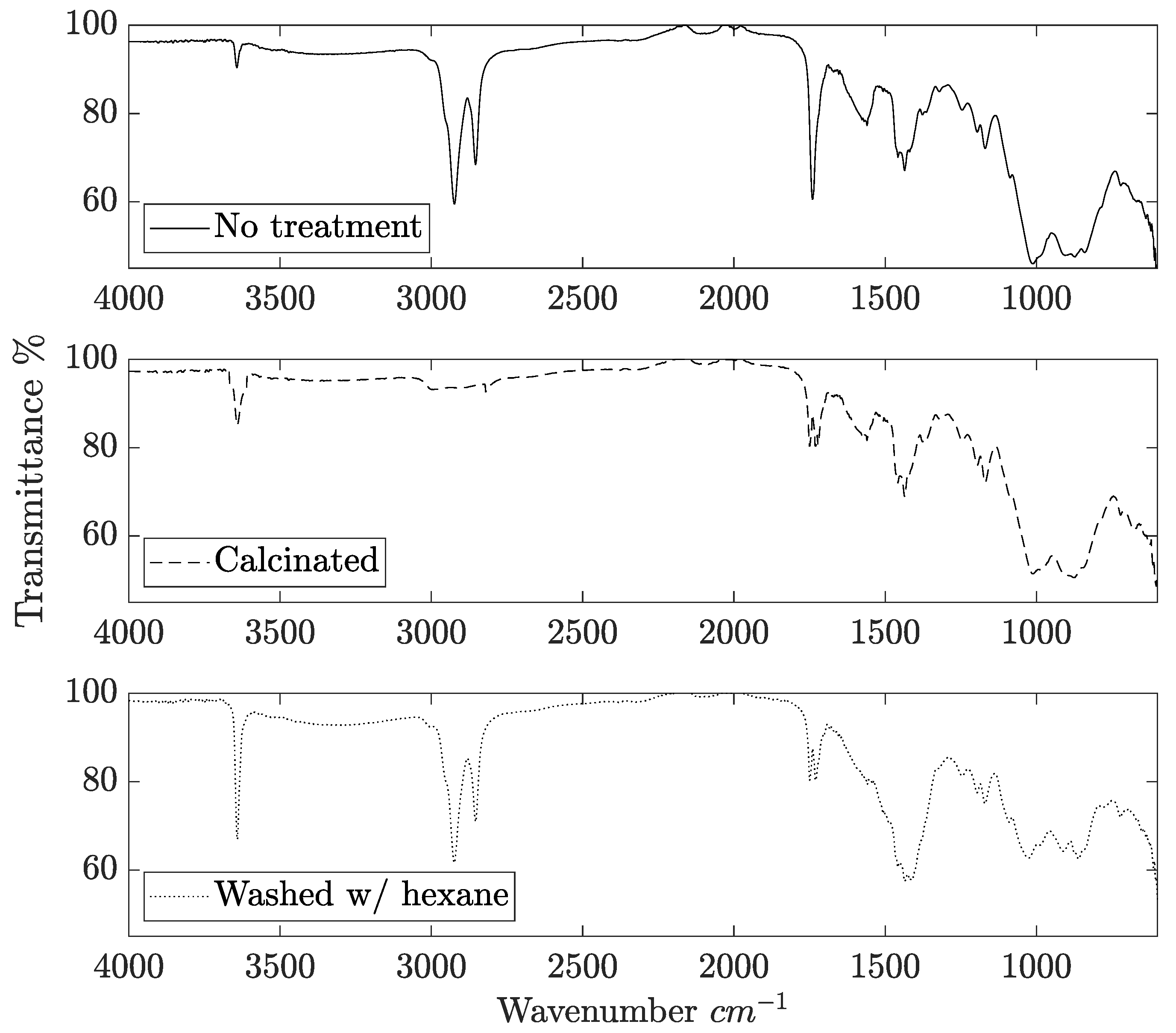
2.1. Catalyst Characterization
2.2. Biodiesel Production
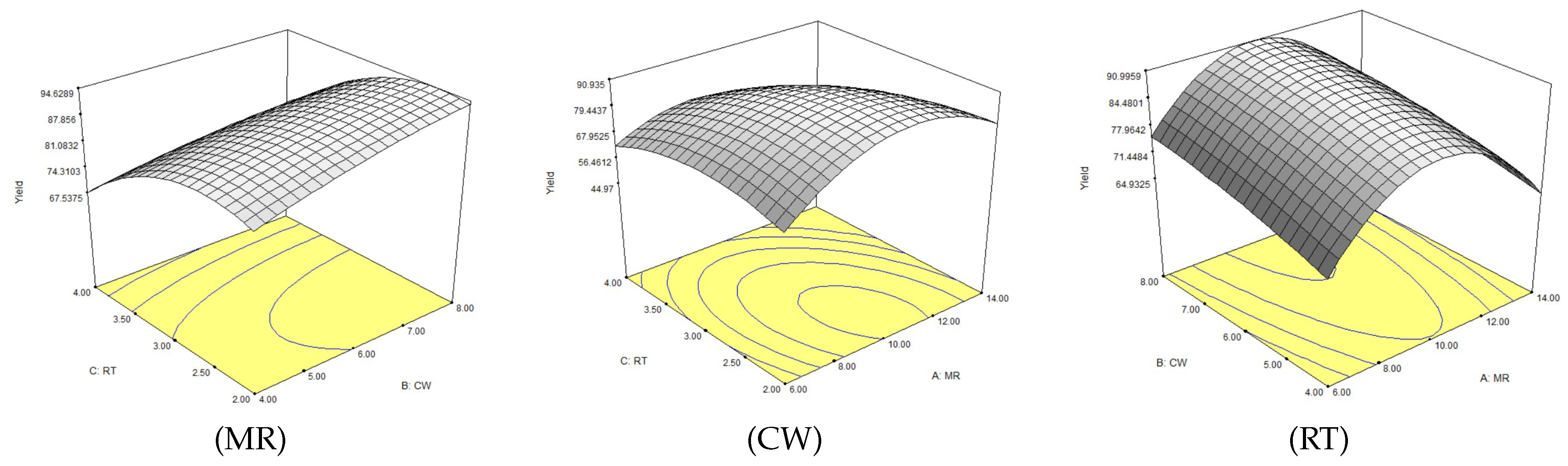
2.2.1. Experimental Design
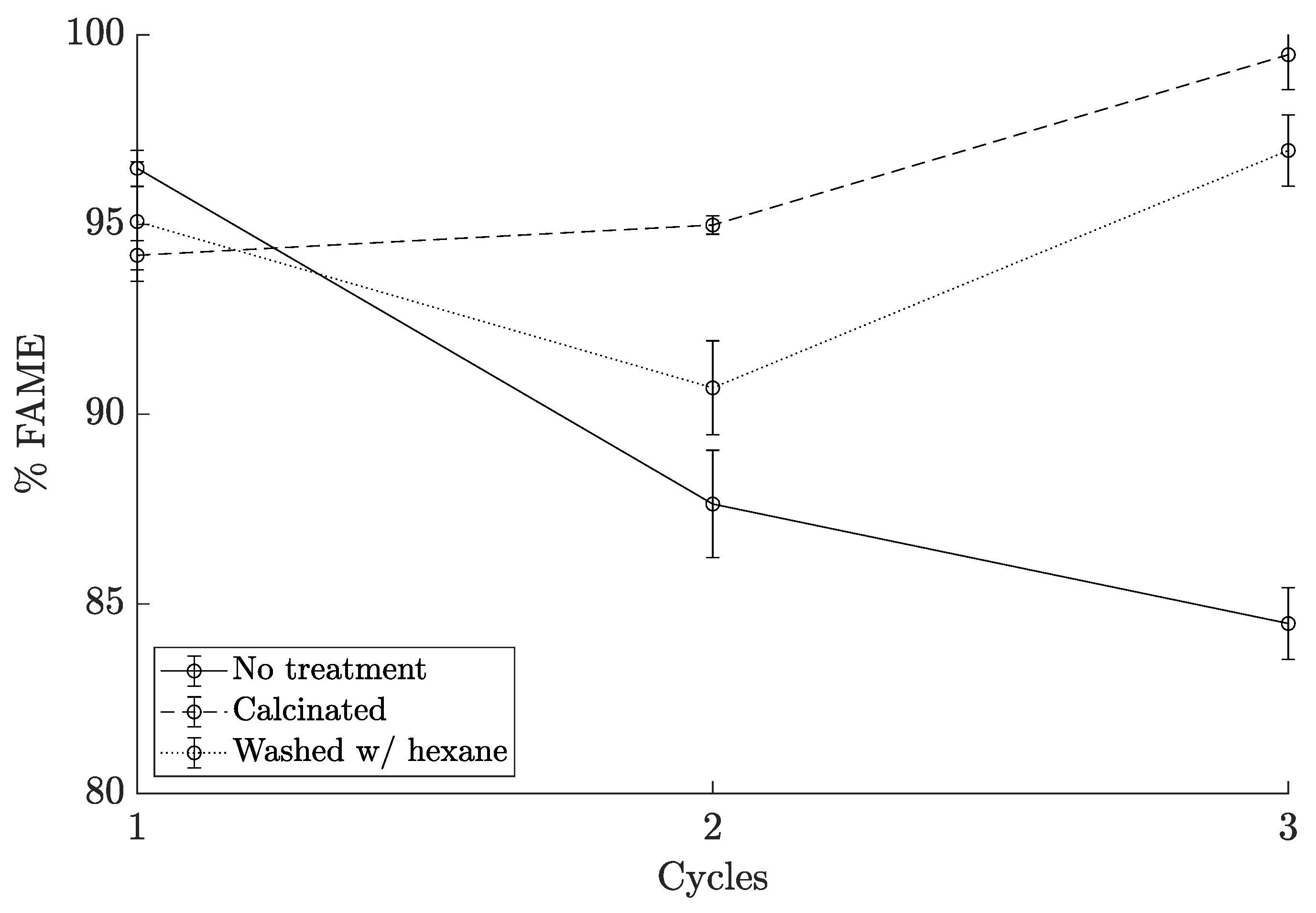
2.2.2. Catalyst Reuse
2.3. Biodiesel Properties
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Biodiesel Production
3.4.1. Experimental Design
3.4.2. Catalyst Reusability
3.4.3. Biodiesel Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- del Consuelo Ortiz Tapia, M.; Alamilla, P.G.; Gálvez, L.M.L.; Quezada, M.I.A.; Alamilla, R.G.; Chávez, M.A.L. Biodiesel production from crude palm oil (Elaeis guineensis Jacq). Ascending path method application. Acta Univ. 2016, 26, 3–10. [Google Scholar] [CrossRef]
- Yan, Y. Biodiesel. In Encyclopedia of Food Grains, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 3, pp. 245–250. [Google Scholar] [CrossRef]
- Vargas, E.M.; Neves, M.C.; Tarelho, L.A.; Nunes, M.I. Solid catalysts obtained from wastes for FAME production using mixtures of refined palm oil and waste cooking oils. Renew. Energy 2019, 136, 873–883. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from waste cooking oil via base-catalytic and supercritical methanol transesterification. Energy Convers. Manag. 2009, 50, 923–927. [Google Scholar] [CrossRef]
- Mumtaz, M.W.; Adnan, A.; Mukhtar, H.; Rashid, U.; Danish, M. Biodiesel Production Through Chemical and Biochemical Transesterification: Trends, Technicalities, and Future Perspectives. In Clean Energy for Sustainable Development: Comparisons and Contrasts of New Approaches; Academic Press: Cambridge, MA, USA, 2017; pp. 465–485. [Google Scholar] [CrossRef]
- Baskar, G.; Kalavathy, G.; Aiswarya, R.; Selvakumari, I.A. Advances in bio-oil extraction from nonedible oil seeds and algal biomass. In Advances in Eco-Fuels for a Sustainable Environment; Woodhead Publishing: Sawston, UK, 2019; pp. 187–210. [Google Scholar] [CrossRef]
- Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Azid, A.; Umar, R.; Juahir, H.; Khatoon, H.; Endut, A. A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production. Renew. Sustain. Energy Rev. 2017, 70, 1040–1051. [Google Scholar] [CrossRef]
- Takagaki, A.; Toda, M.; Okamura, M.; Kondo, J.N.; Hayashi, S.; Domen, K.; Hara, M. Esterification of higher fatty acids by a novel strong solid acid. Catal. Today 2006, 116, 157–161. [Google Scholar] [CrossRef]
- Maroa, S.; Inambao, F. A review of sustainable biodiesel production using biomass derived heterogeneous catalysts. Eng. Life Sci. 2021, 21, 790–824. [Google Scholar] [CrossRef] [PubMed]
- Mierczynski, P.; Ciesielski, R.; Kedziora, A.; Maniukiewicz, W.; Shtyka, O.; Kubicki, J.; Albinska, J.; Maniecki, T.P. Biodiesel Production on MgO, CaO, SrO and BaO Oxides Supported on (SrO)(Al2O3) Mixed Oxide. Catal. Lett. 2015, 145, 1196–1205. [Google Scholar] [CrossRef]
- Sahu, O. Characterisation and utilization of heterogeneous catalyst from waste rice-straw for biodiesel conversion. Fuel 2021, 287, 119543. [Google Scholar] [CrossRef]
- Chen, G.Y.; Shan, R.; Shi, J.F.; Yan, B.B. Transesterification of palm oil to biodiesel using rice husk ash-based catalysts. Fuel Process. Technol. 2015, 133, 8–13. [Google Scholar] [CrossRef]
- Roschat, W.; Siritanon, T.; Yoosuk, B.; Promarak, V. Rice husk-derived sodium silicate as a highly efficient and low-cost basic heterogeneous catalyst for biodiesel production. Energy Convers. Manag. 2016, 119, 453–462. [Google Scholar] [CrossRef]
- Obadiah, A.; Swaroopa, G.A.; Kumar, S.V.; Jeganathan, K.R.; Ramasubbu, A. Biodiesel production from Palm oil using calcined waste animal bone as catalyst. Bioresour. Technol. 2012, 116, 512–516. [Google Scholar] [CrossRef]
- Aleman-Ramirez, J.; Moreira, J.; Torres-Arellano, S.; Longoria, A.; Okoye, P.U.; Sebastian, P. Preparation of a heterogeneous catalyst from moringa leaves as a sustainable precursor for biodiesel production. Fuel 2021, 284, 118983. [Google Scholar] [CrossRef]
- Thomas, M.; Jewell, R.; Jones, R. Coal Fly Ash as a Pozzolan; Elsevier: Amsterdam, The Netherlands, 2017; pp. 121–154. [Google Scholar] [CrossRef]
- Cabrera, M.; Galvin, A.P.; Agrela, F.; Carvajal, M.D.; Ayuso, J. Characterisation and technical feasibility of using biomass bottom ash for civil infrastructures. Constr. Build. Mater. 2014, 58, 234–244. [Google Scholar] [CrossRef]
- Elliott, A.; Mahmood, T.; Kamal, A. Boiler ash utilization in the Canadian pulp and paper industry. J. Environ. Manag. 2022, 319, 115728. [Google Scholar] [CrossRef] [PubMed]
- Adepoju, T. Synthesis of biodiesel from Annona muricata—Calophyllum inophyllum oil blends using calcined waste wood ash as a heterogeneous base catalyst. MethodsX 2021, 8, 101188. [Google Scholar] [CrossRef]
- Misra, M.K.; Ragland, K.W.; Baker, A.J. Wood ash composition as a function of furnace temperature. Biomass Bioenergy 1993, 4, 103–116. [Google Scholar] [CrossRef]
- Chouhan, A.P.S.; Sarma, A.K. Biodiesel production from Jatropha curcas L. oil using Lemna perpusilla Torrey ash as heterogeneous catalyst. Biomass Bioenergy 2013, 55, 386–389. [Google Scholar] [CrossRef]
- Deka, D.C.; Basumatary, S. High quality biodiesel from yellow oleander (Thevetia peruviana) seed oil. Biomass Bioenergy 2011, 35, 1797–1803. [Google Scholar] [CrossRef]
- Mutalib, A.A.A.; Ibrahim, M.L.; Matmin, J.; Kassim, M.F.; Mastuli, M.S.; Taufiq-Yap, Y.H.; Shohaimi, N.A.M.; Islam, A.; Tan, Y.H.; Kaus, N.H.M. SiO2-Rich Sugar Cane Bagasse Ash Catalyst for Transesterification of Palm Oil. BioEnergy Res. 2020, 13, 986–997. [Google Scholar] [CrossRef]
- Chakraborty, R.; Bepari, S.; Banerjee, A. Transesterification of soybean oil catalyzed by fly ash and egg shell derived solid catalysts. Chem. Eng. J. 2010, 165, 798–805. [Google Scholar] [CrossRef]
- Ho, W.W.S.; Ng, H.K.; Gan, S. Development and characterisation of novel heterogeneous palm oil mill boiler ash-based catalysts for biodiesel production. Bioresour. Technol. 2012, 125, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.W.S.; Ng, H.K.; Gan, S.; Tan, S.H. Evaluation of palm oil mill fly ash supported calcium oxide as a heterogeneous base catalyst in biodiesel synthesis from crude palm oil. Energy Convers. Manag. 2014, 88, 1167–1178. [Google Scholar] [CrossRef]
- Maneerung, T.; Kawi, S.; Wang, C.H. Biomass gasification bottom ash as a source of CaO catalyst for biodiesel production via transesterification of palm oil. Energy Convers. Manag. 2015, 92, 234–243. [Google Scholar] [CrossRef]
- Yoosuk, B.; Udomsap, P.; Puttasawat, B.; Krasae, P. Modification of calcite by hydration–dehydration method for heterogeneous biodiesel production process: The effects of water on properties and activity. Chem. Eng. J. 2010, 162, 135–141. [Google Scholar] [CrossRef]
- Hoyos-Montilla, A.A.; Puertas, F.; Mosquera, J.M.; Tobón, J.I. Infrared spectra experimental analyses on alkali-activated fly ash-based binders. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 269, 120698. [Google Scholar] [CrossRef]
- Ren, F.; Ding, Y.; Leng, Y. Infrared spectroscopic characterization of carbonated apatite: A combined experimental and computational study. J. Biomed. Mater. Res. Part A 2014, 102, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Haq, E.U.; Padmanabhan, S.K.; Licciulli, A. Synthesis and characteristics of fly ash and bottom ash based geopolymers–A comparative study. Ceram. Int. 2014, 40, 2965–2971. [Google Scholar] [CrossRef]
- Fleet, M.E. Infrared spectra of carbonate apatites v2 Region bands. Biomaterials 2009, 30, 1473–1481. [Google Scholar] [CrossRef]
- Cienkosz-Stepańczak, B.; Szostek, K.; Lisowska-Gaczorek, A. Optimizing FTIR method for characterizing diagenetic alteration of skeletal material. J. Archaeol. Sci. Rep. 2021, 38, 103059. [Google Scholar] [CrossRef]
- Yin, Y.; Yin, H.; Wu, Z.; Qi, C.; Tian, H.; Zhang, W.; Hu, Z.; Feng, L. Characterization of Coals and Coal Ashes with High Si Content Using Combined Second-Derivative Infrared Spectroscopy and Raman Spectroscopy. Crystals 2019, 9, 513. [Google Scholar] [CrossRef]
- Naemchan, K.; Meejoo, S.; Onreabroy, W.; Limsuwan, P. Temperature Effect on Chicken Egg Shell Investigated by XRD, TGA and FTIR. Adv. Mater. Res. 2008, 55–57, 333–336. [Google Scholar] [CrossRef]
- de Oliveira Romano, R.C.; Bernardo, H.M.; Maciel, M.H.; Pileggi, R.G.; Cincotto, M.A. Using isothermal calorimetry, X-ray diffraction, thermogravimetry and FTIR to monitor the hydration reaction of Portland cements associated with red mud as a supplementary material. J. Therm. Anal. Calorim. 2019, 137, 1877–1890. [Google Scholar] [CrossRef]
- Uprety, B.K.; Chaiwong, W.; Ewelike, C.; Rakshit, S.K. Biodiesel production using heterogeneous catalysts including wood ash and the importance of enhancing byproduct glycerol purity. Energy Convers. Manag. 2016, 115, 191–199. [Google Scholar] [CrossRef]
- Gualtieri, M.L.; Romagnoli, M.; Miselli, P.; Cannio, M.; Gualtieri, A.F. Full quantitative phase analysis of hydrated lime using the Rietveld method. Cem. Concr. Res. 2012, 42, 1273–1279. [Google Scholar] [CrossRef]
- Kumar, K.V.; Subha, T.J.; Ahila, K.; Ravindran, B.; Chang, S.; Mahmoud, A.H.; Mohammed, O.B.; Rathi, M. Spectral characterization of hydroxyapatite extracted from Black Sumatra and Fighting cock bone samples: A comparative analysis. Saudi J. Biol. Sci. 2021, 28, 840–846. [Google Scholar] [CrossRef]
- Suchanek, K.; Bartkowiak, A.; Perzanowski, M.; Marszałek, M. From monetite plate to hydroxyapatite nanofibers by monoethanolamine assisted hydrothermal approach. Sci. Rep. 2018, 8, 15408. [Google Scholar] [CrossRef]
- Sharma, M.; Khan, A.A.; Puri, S.; Tuli, D. Wood ash as a potential heterogeneous catalyst for biodiesel synthesis. Biomass Bioenergy 2012, 41, 94–106. [Google Scholar] [CrossRef]
- Pavlović, S.M.; Marinković, D.M.; Kostić, M.D.; Janković-Častvan, I.M.; Mojović, L.V.; Stanković, M.V.; Veljković, V.B. A CaO/zeolite-based catalyst obtained from waste chicken eggshell and coal fly ash for biodiesel production. Fuel 2020, 267, 117171. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Sugimoto, Y.; Yamanaka, S.; Hidaka, J. Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel production. Fuel 2008, 87, 2798–2806. [Google Scholar] [CrossRef]
- Cvengroš, J.; Cvengrošová, Z. Used frying oils and fats and their utilization in the production of methyl esters of higher fatty acids. Biomass Bioenergy 2004, 27, 173–181. [Google Scholar] [CrossRef]
- Usta, N.; Ozturk, E.; Can, O.; Conkur, E.; Nas, S.; Çon, A.; Can, A.; Topcu, M. Combustion of biodiesel fuel produced from hazelnut soapstock/waste sunflower oil mixture in a Diesel engine. Energy Convers. Manag. 2005, 46, 741–755. [Google Scholar] [CrossRef]
- Benjumea, P.; Agudelo, J.; Agudelo, A. Basic properties of palm oil biodiesel–diesel blends. Fuel 2008, 87, 2069–2075. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F. Production of biodiesel through optimized alkaline-catalyzed transesterification of rapeseed oil. Fuel 2008, 87, 265–273. [Google Scholar] [CrossRef]
- Viriya-empikul, N.; Krasae, P.; Nualpaeng, W.; Yoosuk, B.; Faungnawakij, K. Biodiesel production over Ca-based solid catalysts derived from industrial wastes. Fuel 2012, 92, 239–244. [Google Scholar] [CrossRef]
- Jitjamnong, J.; Thunyaratchatanon, C.; Luengnaruemitchai, A.; Kongrit, N.; Kasetsomboon, N.; Sopajarn, A.; Chuaykarn, N.; Khantikulanon, N. Response surface optimization of biodiesel synthesis over a novel biochar-based heterogeneous catalyst from cultivated (Musa sapientum) banana peels. Biomass Convers. Biorefinery 2021, 11, 2795–2811. [Google Scholar] [CrossRef]
- Volli, V.; Purkait, M.K.; Shu, C.M. Preparation and characterization of animal bone powder impregnated fly ash catalyst for transesterification. Sci. Total Environ. 2019, 669, 314–321. [Google Scholar] [CrossRef]
- Mukhametov, A.; Mamayeva, L.; Kazhymurat, A.; Akhlan, T.; Yerbulekova, M. Study of vegetable oils and their blends using infrared reflectance spectroscopy and refractometry. Food Chem. X 2023, 17, 100386. [Google Scholar] [CrossRef] [PubMed]
- González, M.; Cea, M.; Reyes, D.; Romero-Hermoso, L.; Hidalgo, P.; Meier, S.; Benito, N.; Navia, R. Functionalization of biochar derived from lignocellulosic biomass using microwave technology for catalytic application in biodiesel production. Energy Convers. Manag. 2017, 137, 165–173. [Google Scholar] [CrossRef]
- Silva, G.A.M.; Rós, P.C.M.D.; Souza, L.T.A.; Costa, A.P.O.; de Castro, H.F. Physico-chemical, spectroscopical and thermal characterization of biodiesel obtained by enzymatic route as a tool to select the most efficient immobilized lipase. Braz. J. Chem. Eng. 2012, 29, 39–47. [Google Scholar] [CrossRef]
- Gerpen, J.V.; Knothe, G.; Haas, M.J.; Schultz, A.K.; Banavali, R.; Topp, K.D.; Vandersall, M.T. Biodiesel Production. In The Biodiesel Handbook, 2nd ed.; AOCS Press: Urbana, IL, USA, 2010; pp. 31–96. [Google Scholar] [CrossRef]
- Dharma, S.; Masjuki, H.; Ong, H.C.; Sebayang, A.; Silitonga, A.; Kusumo, F.; Mahlia, T. Optimization of biodiesel production process for mixed Jatropha curcas–Ceiba pentandra biodiesel using response surface methodology. Energy Convers. Manag. 2016, 115, 178–190. [Google Scholar] [CrossRef]
- ISO 660; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. International Organization for Standardization: Geneva, Switzerland, 2020.
- AOCS. AOCS Methods for Biodiesel Feedstock Quality, 6th ed.; AOCS Press: Urbana, IL, USA, 2012. [Google Scholar]
- EN14214; Liquid Petroleum Products. Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications. Requirements and Test Methods. European Standard: Brussel, Belgium, 2021.

| Sample | Composition | Specific Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cc/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Mg | Na | K | Al | P | Si | C | ||||
| RBA | 77.80 | 2.48 | - | 2.85 | 0.95 | 1.12 | - | 15.82 | - | - | - |
| CBA | 85.69 | 5.52 | - | 3.16 | - | 2.07 | - | 3.42 | 11.75 | 3.83 | 0.019 |
| BAC | 89.05 | 3.38 | - | - | - | 0.88 | - | 6.33 | 10.60 | 4.52 | 0.011 |
| N° Run | N° Experiment | MR | CW | RT | % FAME | % FAME Model |
|---|---|---|---|---|---|---|
| 1 | 8 | 14 | 6 | 4 | 42.13 | 43.39 |
| 2 | 15 | 10 | 6 | 3 | 89.00 | 88.22 |
| 3 | 6 | 14 | 6 | 2 | 74.66 | 78.09 |
| 4 | 5 | 6 | 6 | 2 | 68.30 | 67.50 |
| 5 | 2 | 14 | 4 | 3 | 69.57 | 64.95 |
| 6 | 10 | 10 | 8 | 2 | 95.61 | 92.89 |
| 7 | 12 | 10 | 8 | 4 | 73.69 | 73.04 |
| 8 | 7 | 6 | 6 | 4 | 65.10 | 60.92 |
| 9 | 3 | 6 | 8 | 3 | 70.77 | 75.02 |
| 10 | 1 | 6 | 4 | 3 | 64.00 | 64.10 |
| 11 | 14 | 10 | 6 | 3 | 89.12 | 88.22 |
| 12 | 11 | 10 | 4 | 4 | 65.74 | 69.52 |
| 13 | 9 | 10 | 4 | 2 | 80.67 | 81.24 |
| 14 | 4 | 14 | 8 | 3 | 72.43 | 72.33 |
| 15 | 13 | 10 | 6 | 3 | 86.40 | 88.22 |
| GL | SC Adjst. | F-Value | p-Value | |
|---|---|---|---|---|
| Model | 9 | 48.115917 | 13.10 | 0.006 |
| Linear | 3 | 16.054225 | 13.11 | 0.008 |
| MR | 1 | 0.041089 | 0.10 | 0.764 |
| CW | 1 | 3.203072 | 7.85 | 0.038 |
| RT | 1 | 12.810064 | 31.38 | 0.003 |
| Quadratic | 3 | 28.573184 | 23.33 | 0.002 |
| MR2 | 1 | 25.663634 | 62.88 | 0.001 |
| CW2 | 1 | 0.450301 | 1.10 | 0.342 |
| RT2 | 1 | 4.337852 | 10.63 | 0.022 |
| 2-factor interactions | 3 | 3.488507 | 2.85 | 0.144 |
| MR × CW | 1 | 0.064078 | 0.16 | 0.708 |
| MR × RT | 1 | 2.841467 | 6.96 | 0.046 |
| CW × RT | 1 | 0.582963 | 1.43 | 0.286 |
| Error | 5 | 2.040844 | ||
| Lack of fit | 3 | 1.888895 | 8.29 | 0.110 |
| Pure error | 2 | 0.15195 | ||
| Total | 14 | 50.156761 |
| Properties | Washed w/Hexane (Third Cycle) | EN 14214 Std. |
|---|---|---|
| Kinematic viscosity at 40 C (mm2/s) | 4.87 | Max. 5.00 |
| Density at 15 C (kg/m3) | 862.0 | Max. 900 |
| Water content (mg/kg) | 0.25 | Max. 5.00 |
| Biodiesel yield (wt%) | 96.95 | Min. 96.50 |
| Acid value (mg KOH/g) | 1.27 | Max. 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahuelcura, B.; González, M.E.; Gutierrez, N.; Ñanculeo, J.; Romero-García, J.M. Biodiesel Production from Waste Frying Oil (WFO) Using a Biomass Ash-Based Catalyst. Catalysts 2024, 14, 553. https://doi.org/10.3390/catal14080553
Nahuelcura B, González ME, Gutierrez N, Ñanculeo J, Romero-García JM. Biodiesel Production from Waste Frying Oil (WFO) Using a Biomass Ash-Based Catalyst. Catalysts. 2024; 14(8):553. https://doi.org/10.3390/catal14080553
Chicago/Turabian StyleNahuelcura, Benjamín, María Eugenia González, Nicolas Gutierrez, Jaime Ñanculeo, and Juan Miguel Romero-García. 2024. "Biodiesel Production from Waste Frying Oil (WFO) Using a Biomass Ash-Based Catalyst" Catalysts 14, no. 8: 553. https://doi.org/10.3390/catal14080553
APA StyleNahuelcura, B., González, M. E., Gutierrez, N., Ñanculeo, J., & Romero-García, J. M. (2024). Biodiesel Production from Waste Frying Oil (WFO) Using a Biomass Ash-Based Catalyst. Catalysts, 14(8), 553. https://doi.org/10.3390/catal14080553










