Spartina alterniflora-Derived Carbons for High-Performance Oxygen Reduction Reaction (ORR) Catalysts
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of SAC and SANC
2.2. Electrocatalytic Performance of SAC and SANC
3. Materials and Methods
3.1. Reagents and Materials
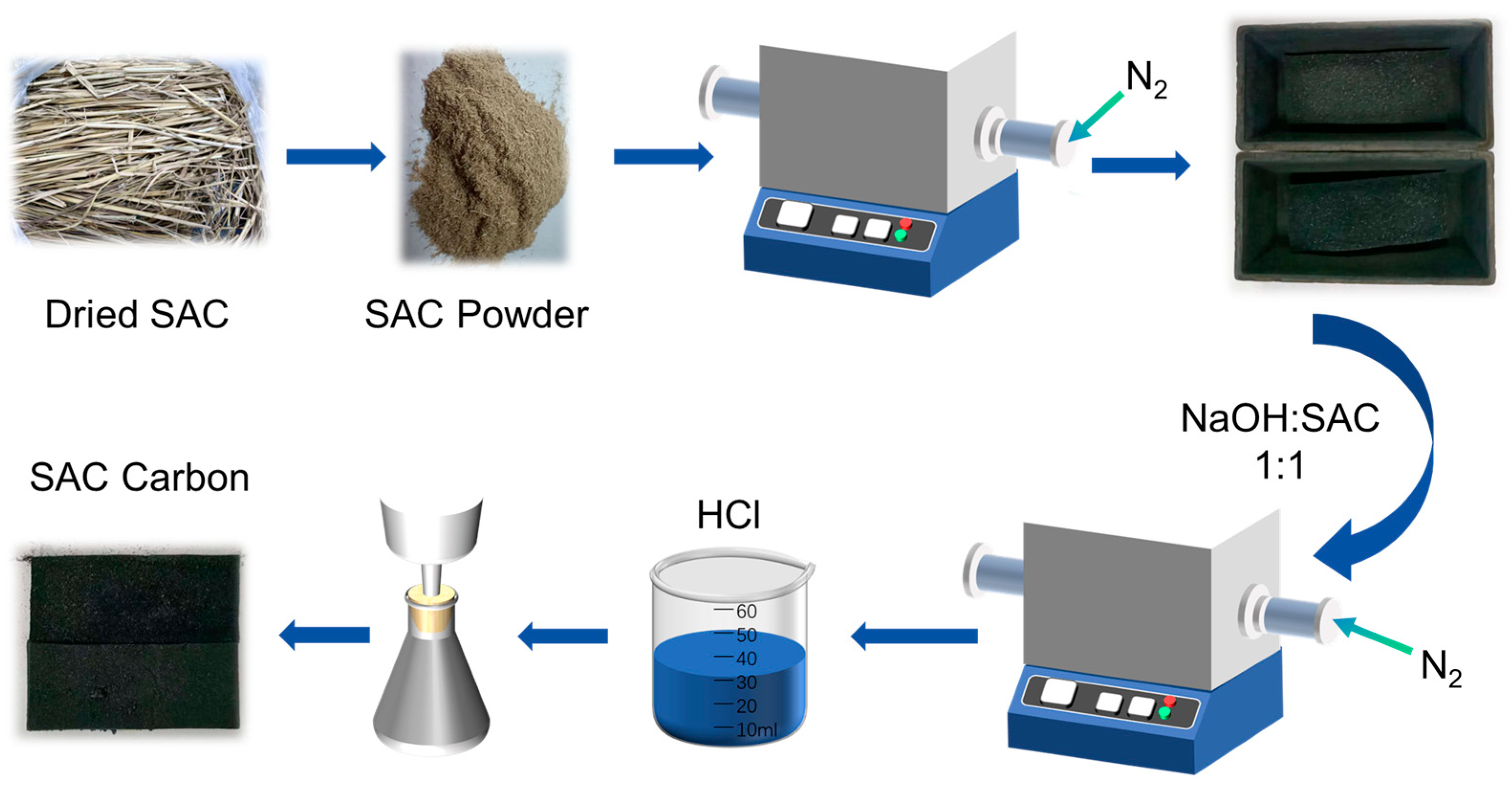
3.2. Material Synthesis
3.3. Characterization
3.4. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Omer, A.M. Energy, environment and sustainable development. Renew. Sustain. Energy Rev. 2008, 12, 2265–2300. [Google Scholar] [CrossRef]
- Bose, B.K. Global warming: Energy, environmental pollution, and the impact of power electronics. IEEE Ind. Electron. Mag. 2010, 4, 6–17. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y.; Liu, X.; Mao, M.; Li, K.; Li, Q.; Zhang, G.; Wang, C. Mobile energy storage technologies for boosting carbon neutrality. Innovation 2023, 4, 100518. [Google Scholar] [CrossRef]
- Chen, S.; Tao, R.; Guo, C.; Zhang, W.; Liu, X.; Yang, G.; Guo, P.; Sun, G.; Liang, J.; Lu, S. A new trick for an old technology: Ion exchange syntheses of advanced energy storage and conversion nanomaterials. Energy Storage Mater. 2021, 41, 758–790. [Google Scholar] [CrossRef]
- Amiri, A.; Shahbazian-Yassar, R. Recent progress of high-entropy materials for energy storage and conversion. J. Mater. Chem. A 2020, 9, 782–823. [Google Scholar] [CrossRef]
- Boudghene Stambouli, A.; Traversa, E. Fuel cells, an alternative to standard sources of energy. Renew. Sustain. Energy Rev. 2002, 6, 295–304. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Elsaid, K.; Wilberforce, T.; Kamil, M.; Sayed, E.T.; Olabi, A. Environmental aspects of fuel cells: A review. Sci. Total Environ. 2020, 752, 141803. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xu, G.; Dong, W.; Zhang, Y.; Zhao, Z.; Qiu, L.; Dong, J. Progress and perspective for in situ studies of oxygen reduction reaction in proton exchange membrane fuel cells. Adv. Sci. 2023, 10, 2300550. [Google Scholar] [CrossRef]
- Dey, S.; Mondal, B.; Chatterjee, S.; Rana, A.; Amanullah, S.; Dey, A. Molecular electrocatalysts for the oxygen reduction reaction. Nat. Rev. Chem. 2017, 0098, 2397–3358. [Google Scholar] [CrossRef]
- Luo, X.; Wu, W.; Wang, Y.; Li, Y.; Ye, J.; Wang, H.; Jiang, Q.; Zhou, Z.; Li, Y.C.; Wang, Y.; et al. Relay catalysis of multi-sites promotes oxygen reduction reaction. Adv. Funct. Mater. 2023, 33, 2215021. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Liu, Y.; Wei, Y.; Lan, F.; Wang, J.; Liu, X.; Wang, R.; Yang, Y.; Chen, J. Improving oxygen reduction reaction by cobalt iron-layered double hydroxide layer on nickel-metal organic framework as cathode catalyst in microbial fuel cell. Bioresour. Technol. 2023, 392, 130011. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, S.; Cui, C.; Miao, Q.; He, Y.; Li, X.; Xu, Q.; Zeng, G. Construction of catalytic covalent organic frameworks with redox-active sites for the oxygen reduction and the oxygen evolution reaction. Angew. Chem. Int. Ed. 2022, 61, e202213522. [Google Scholar] [CrossRef]
- Huang, L.; Wei, M.; Qi, R.; Dong, C.; Dang, D.; Yang, C.; Xia, C.; Chen, C.; Zaman, S.; Li, F.; et al. An integrated platinum-nanocarbon electrocatalyst for efficient oxygen reduction. Nat. Commun. 2022, 13, 6703. [Google Scholar] [CrossRef]
- Tian, X.L.; Xu, Y.Y.; Zhang, W.; Wu, T.; Xia, B.Y.; Wang, X. Unsupported platinum-based electrocatalysts for oxygen reduction reaction. ACS Energy Lett. 2017, 2, 2035–2043. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, M.; Lu, L.; Barkholtz, H.M.; Li, Y.; Wang, Y.; Jiang, L.; Wu, Z.; Liu, D.; Zhuang, L.; et al. High performance platinum single atom electrocatalyst for oxygen reduction reaction. Nat. Commun. 2017, 8, 15938. [Google Scholar] [CrossRef]
- Sivaraman, R.; Opulencia, M.J.C.; Majdi, A.; Patra, I.; Kadhem Abid, M.; Hammid, A.T.; Derakhshandeh, M. Design a promising non-precious electro-catalyst for oxygen reduction reaction in fuel cells. Int. J. Hydrogen Energy 2022, 48, 6308–6316. [Google Scholar] [CrossRef]
- Friedman, A.; Mizrahi, M.; Levy, N.; Zion, N.; Zachman, M.; Elbaz, L. Application of molecular catalysts for the oxygen reduction reaction in alkaline fuel cells. ACS Appl. Mater. Interfaces 2021, 13, 58532–58538. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Sepunaru, L.; Compton, R.G. Innovative catalyst design for the oxygen reduction reaction for fuel cells. Chem. Sci. 2016, 7, 3364–3369. [Google Scholar] [CrossRef]
- Xiang, Q.; Yin, W.; Liu, Y.; Yu, D.; Wang, X.; Li, S.; Chen, C. A study of defect-rich carbon spheres as a metal-free electrocatalyst for an efficient oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 24314–24320. [Google Scholar] [CrossRef]
- Long, X.; Li, D.; Wang, B.; Jiang, Z.; Xu, W.; Wang, B.; Yang, D.; Xia, Y. Heterocyclization strategy for construction of linear conjugated polymers: Efficient metal-free electrocatalysts for oxygen reduction. Angew. Chem. Int. Ed. 2019, 58, 11369–11373. [Google Scholar] [CrossRef]
- Hu, C.; Paul, R.; Dai, Q.; Dai, L. Carbon-based metal-free electrocatalysts: From oxygen reduction to multifunctional electrocatalysis. Chem. Soc. Rev. 2021, 50, 11785–11843. [Google Scholar] [CrossRef]
- Fu, A.; Xiao-qing, B.; Xiao-yang, D.; Zi-zai, M.; Xiao-guang, W. Carbon-based metal-free oxygen reduction reaction electrocatalysts: Past, present and future. New Carbon Mater. 2022, 37, 338–354. [Google Scholar]
- Ren, G.; Chen, S.; Zhang, J.; Zhang, N.; Jiao, C.; Qiu, H.; Liu, C.; Wang, H. N-doped porous carbon spheres as metal-free electrocatalyst for oxygen reduction reaction. J. Mater. Chem. A 2021, 9, 5751–5758. [Google Scholar] [CrossRef]
- Oh, J.; Park, S.; Jang, D.; Shin, Y.; Lim, D.; Park, S. Metal-free N-doped carbon blacks as excellent electrocatalysts for oxygen reduction reactions. Carbon 2019, 145, 481–487. [Google Scholar] [CrossRef]
- Ma, Z.H.; Han, Y.; Wang, X.; Sun, G.W.; Li, Y. Lignin-derived hierarchical porous flower-like carbon nanosheets decorated with biomass carbon quantum dots for efficient oxygen reduction. Colloids Surf. A 2022, 652, 129818. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Zhao, S.S.; Zhao, C.K.; Wang, G.X.; Wu, M.B. Nitrogen-doped hierarchical porous carbons derived from biomass for oxygen reduction reaction. Front. Chem. 2023, 11, 1218451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jiang, S.; Huang, B.; Shen, X.; Chen, W.; Zhou, T.; Cheng, H.; Cheng, B.; Wu, C.; Li, W.; et al. Sustainable production of value-added carbon nanomaterials from biomass pyrolysis. Nat. Sustain. 2020, 3, 753–760. [Google Scholar] [CrossRef]
- Hu, X.; Nango, K.; Bao, L.; Li, T.; Hasan, M.D.M.; Li, C. High yields of solid carbonaceous materials from biomass. Green Chem. 2019, 21, 1128–1140. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Shetti, N.P.; Reddy, K.R.; Kwon, E.E.; Nadagouda, M.N.; Aminabhavi, T.M. Biomass utilization and production of biofuels from carbon neutral materials. Environ. Pollut. 2021, 276, 116731. [Google Scholar] [CrossRef]
- Yuancheng, H.; Zheng, T.; Siyu, Z.; Hong, W.; Yougen, T.; Dan, S.; Haiyan, W. Renewable waste biomass-derived carbon materials for energy storage. J. Phys. D Appl. Phys. 2022, 55, 1250834. [Google Scholar]
- Elyasi, S.; Saha, S.; Hameed, N.; Mahon, P.J.; Juodkazis, S.; Salim, N. Emerging trends in biomass-derived porous carbon materials for hydrogen storage. Int. J. Hydrogen Energy 2024, 62, 272–306. [Google Scholar] [CrossRef]
- Wang, X.; Yan, D.; Liu, L.; Xu, K.; Zhong, J. Biomass-derived activated carbon nanoarchitectonics with hibiscus flowers for high-performance supercapacitor electrode applications. Chem. Eng. Technol. 2022, 45, 649–657. [Google Scholar]
- Zheng, L.; Chen, M.; Liang, S.; Lü, Q. Oxygen-rich hierarchical porous carbon derived from biomass waste-kapok flower for supercapacitor electrode. Diam. Relat. Mater. 2021, 113, 108267. [Google Scholar] [CrossRef]
- Nagaraj, M.; Sadhasivam, T.; Sol, B.S.; Athibala, M.; Yu, R.C.; Tae, H.O.; Yoong, A.K. N-doped defect-rich porous carbon nanosheets framework from renewable biomass as efficient metal-free bifunctional electrocatalysts for HER and OER application. Renew. Energy 2023, 222, 119801. [Google Scholar]
- Zhao, J.; Wu, W.; Jia, X.; Xia, T.; Li, Q.; Zhang, J.; Wang, Q.; Zhang, W.; Lu, C. High-value utilization of biomass waste: From garbage floating on the ocean to high-performance rechargeable Zn–MnO2 batteries with superior safety. J. Mater. Chem. A 2020, 8, 18198–18206. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Peng, H.; Tang, C.; Lieder, M.; Zhang, Q.; Titirici, M. Porous carbon derived from rice husks as sustainable bioresources: Insights into the role of micro-/mesoporous hierarchy in hosting active species for lithium-sulphur batteries. Green Chem. 2016, 18, 5169–5179. [Google Scholar] [CrossRef]
- Deli, L.; Qidong, Y.; Zhe, C.; Fengtian, Z.; Chen, L.; Sheng, H. High-efficiency phenol removal by novel biomass-based alginate composite hydrogel. Chem. Phys. Lett. 2023, 826, 140676. [Google Scholar]
- Susanto, S.; Nurtono, T.; Widiyastuti, W.; Yeh, M.H.; Setyawan, H. Controlling N-doping nature at carbon aerogels from biomass for enhanced oxygen reduction in seawater batteries. ACS Omega 2024, 9, 13994–14004. [Google Scholar] [CrossRef]
- Patten, K.; O’Casey, C.; Metzger, C. Large-scale chemical control of smooth cordgrass (spartina alterniflora) in Willapa Bay, WA: Towards eradication and ecological restoration. Invasive Plant Sci. Manag. 2017, 10, 284–292. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, Y.; Yuan, L.; Li, W.; Zhu, X.; Zhang, L. Emergency control of spartina alterniflora re-invasion with a chemical method in Chongming Dongtan, China. Water Sci. Eng. 2020, 13, 24–33. [Google Scholar] [CrossRef]
- Qiu, Z.Q.; Mao, D.H.; Feng, K.D.; Wang, M.; Xiang, H.X.; Wang, Z.M. High-Resolution Mapping Changes in the Invasion of Spartina Alterniflora in the Yellow River Delta. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2022, 15, 6445–6455. [Google Scholar] [CrossRef]
- Cheng, H.; Tang, Z.; Luo, X.; Zheng, Z. Spartina alterniflora-derived porous carbon using as anode material for sodium-ion battery. Sci. Total Environ. 2021, 777, 146120. [Google Scholar] [CrossRef]
- Wang, S.T.; Chen, Y.; Zhao, Y.L.; Wei, G.Y.; Li, D.L.; Liu, X.P. Mesopore-dominated N, S co-doped carbon as advanced oxygen reduction reaction electrocatalysts for Zn-air battery. J. Mater. Sci. 2022, 57, 19431–19446. [Google Scholar] [CrossRef]
- Zhan, Y.; Yu, X.; Cao, L.; Zhang, B.; Wu, X.; Xie, F.; Zhang, W.; Chen, J.; Xie, W.; Mai, W.; et al. The influence of nitrogen source and doping sequence on the electrocatalytic activity for oxygen reduction reaction of nitrogen doped carbon materials. Int. J. Hydrogen Energy 2016, 41, 13493–13503. [Google Scholar] [CrossRef]
- Dong, X.; Huayang, Y.; Heng, L.; Peng, H.; Qun, L.; Yuanpeng, W. Carbon-based and carbon-supported nanomaterials for the catalytic conversion of biomass: A review. Environ. Chem. Lett. 2022, 20, 1719–1744. [Google Scholar]
- Zhou, X.; Wang, P.; Zhang, Y.; Wang, L.; Zhang, L.; Zhang, L.; Xu, L.; Liu, L. Biomass based nitrogen-doped structure-tunable versatile porous carbon materials. J. Mater. Chem. A 2017, 25, 12958–12968. [Google Scholar] [CrossRef]
- Sunwen, X.; Wenhan, G.; Ning, C.; Lin, S.; Hewen, Z.; Wang, L.; Xu, C.; Jian, Z.; Yingquan, C.; Haiping, Y.; et al. Synthesis and application in oxygen reduction reaction of N-doping porous graphitic carbon from biomass waste. Fuel Process. Technol. 2021, 224, 107028. [Google Scholar]
- Zhang, P.; Fan, J.; Wang, Y.; Dang, Y.; Heumann, S.; Ding, Y. Insights into the role of defects on the Raman spectroscopy of carbon nanotube and biomass-derived carbon. Carbon 2024, 222, 118998. [Google Scholar] [CrossRef]
- Jiang, L.; van Dijk, B.; Wu, L.; Maheu, C.; Hofmann, J.P.; Tudor, V.; Koper, M.T.M.; Hetterscheid, D.G.H.; Schneider, G.F. Predoped oxygenated defects activate nitrogen-doped graphene for the oxygen reduction reaction. ACS Catal. 2021, 12, 173–182. [Google Scholar] [CrossRef]
- Quan, H.; Fan, X.; Wang, W.; Gao, W.; Dong, Y.; Chen, D. Hierarchically porous carbon derived from biomass: Effect of mesopore and heteroatom-doping on electrochemical performance. Appl. Surf. Sci. 2018, 460, 8–16. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, Y.; Zheng, R.; Wang, Q.; Li, X.; Wei, H.; Wang, L.; Li, Z.; Wang, F.; Han, N. Biomass-derived carbon material as efficient electrocatalysts for the oxygen reduction reaction. Biomass Bioenergy 2022, 168, 106676. [Google Scholar] [CrossRef]
- Wei, W.; Ge, H.; Huang, L.; Kuang, M.; Al-Enizi, A.M.; Zhang, L.; Zheng, G. Hierarchically tubular nitrogen-doped carbon structures for the oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 13634–13638. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Pérez-Rodríguez, S.; Torres, D.; Canevesi, R.; Morallón, E.; Cazorla-Amorós, D.; Celzard, A.; Fierro, V. Nitrogen sites prevail over textural properties in N-doped carbons for the oxygen reduction reaction. J. Colloid Interface Sci. 2023, 654, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.M.; Mestre, A.S.; Martins, A.; Nunes, N.; Carvalho, A.P.; Freire, C. Biomass-derived nanoporous carbons as electrocatalysts for oxygen reduction reaction. Catal. Today 2019, 357, 269–278. [Google Scholar] [CrossRef]
- Liu, W.T.; Wu, X. Ligin-derived graphene-like ultrathin carbon materials for ORR catalysis. J. Phys. Conf. Ser. 2024, 2749, 012003. [Google Scholar] [CrossRef]
- Girimonte, A.; Stefani, A.; Mucci, C.; Giovanardi, R.; Marchetti, A.; Innocenti, M.; Fontanesi, C. Electrochemical performance of metal-free carbon-based catalysts from different hydrothermal carbonization treatments for oxygen reduction reaction. Nanomaterials 2024, 14, 173. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Dijk, B.V.; Wu, L.F.; Maheu, C.; Tudor, V.; Hofmann, J.P.; Jiang, L.; Hetterscheid, D.; Schneider, G.F. Role of vacancy defects and nitrogen dopants for the reduction of oxygen on graphene. ACS Catal. 2024, 14, 11065–11075. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, Y.; Zuo, L.L.; Long, C.; Yang, C.Y.; Zhang, X.F. Tailoring heteroatoms in conjugated microporous polymers for boosting oxygen electrochemical reduction to hydrogen peroxide. ACS Catal. 2023, 13, 4790–4798. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Zhou, Y.; Guo, L.; Li, H.; Shang, H.; Liu, X. Spartina alterniflora-Derived Carbons for High-Performance Oxygen Reduction Reaction (ORR) Catalysts. Catalysts 2024, 14, 555. https://doi.org/10.3390/catal14090555
Hao X, Zhou Y, Guo L, Li H, Shang H, Liu X. Spartina alterniflora-Derived Carbons for High-Performance Oxygen Reduction Reaction (ORR) Catalysts. Catalysts. 2024; 14(9):555. https://doi.org/10.3390/catal14090555
Chicago/Turabian StyleHao, Xinmeng, Yougui Zhou, Lihua Guo, Huipeng Li, Hong Shang, and Xuanhe Liu. 2024. "Spartina alterniflora-Derived Carbons for High-Performance Oxygen Reduction Reaction (ORR) Catalysts" Catalysts 14, no. 9: 555. https://doi.org/10.3390/catal14090555
APA StyleHao, X., Zhou, Y., Guo, L., Li, H., Shang, H., & Liu, X. (2024). Spartina alterniflora-Derived Carbons for High-Performance Oxygen Reduction Reaction (ORR) Catalysts. Catalysts, 14(9), 555. https://doi.org/10.3390/catal14090555






