Improvement of Power Density and COD Removal in a Sediment Microbial Fuel Cell with α-FeOOH Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nanoparticle Characterization
2.1.1. XRD Analysis
2.1.2. SEM-EDS Analysis
2.2. Biofilm Characterization
2.2.1. EIS Analysis
2.2.2. Cyclic Voltammetry
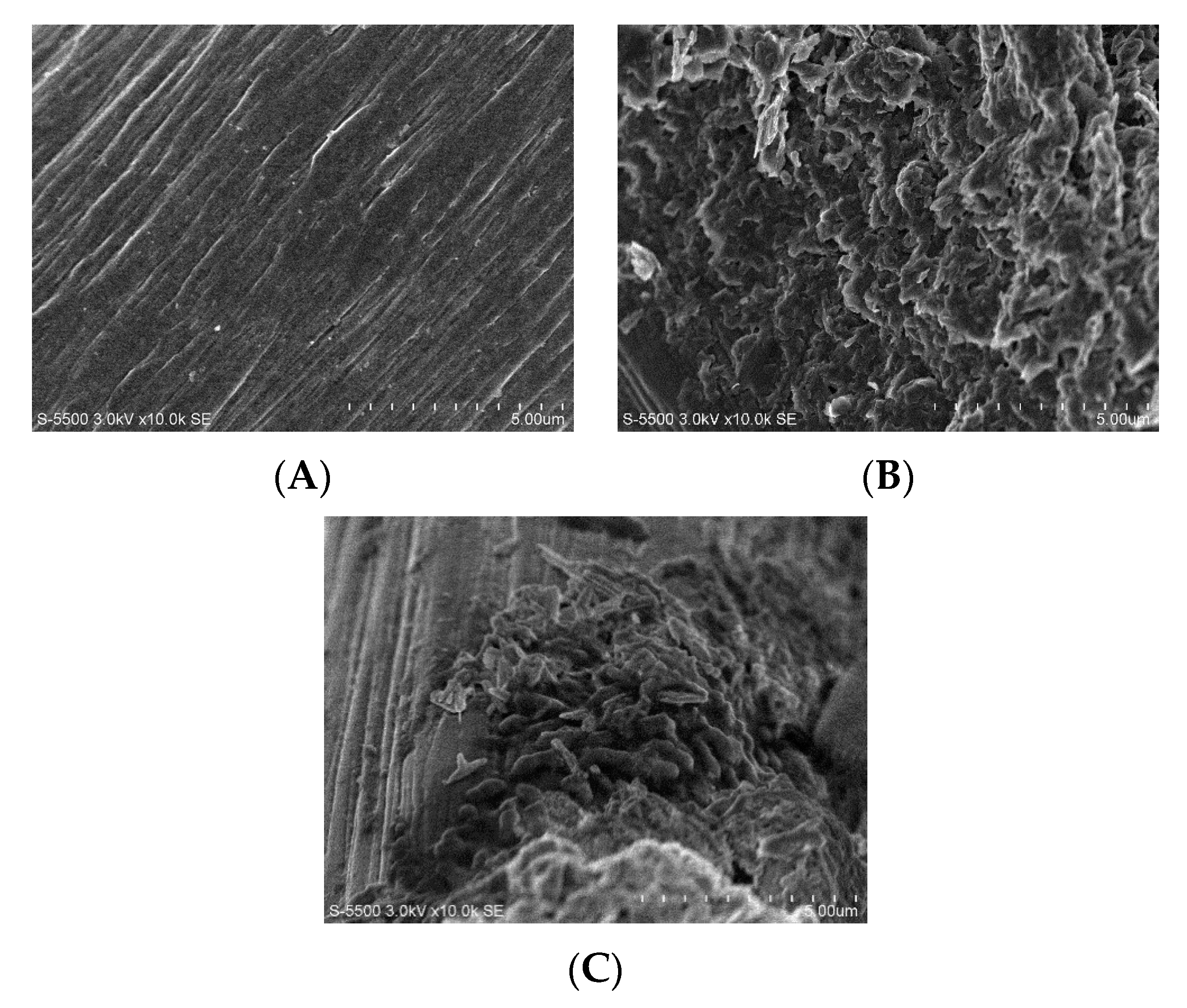
2.2.3. Surface Biofilm Analysis
2.3. Sediment Microbial Fuel Cell Performance
2.3.1. Polarization Curve and Maximum Power Density
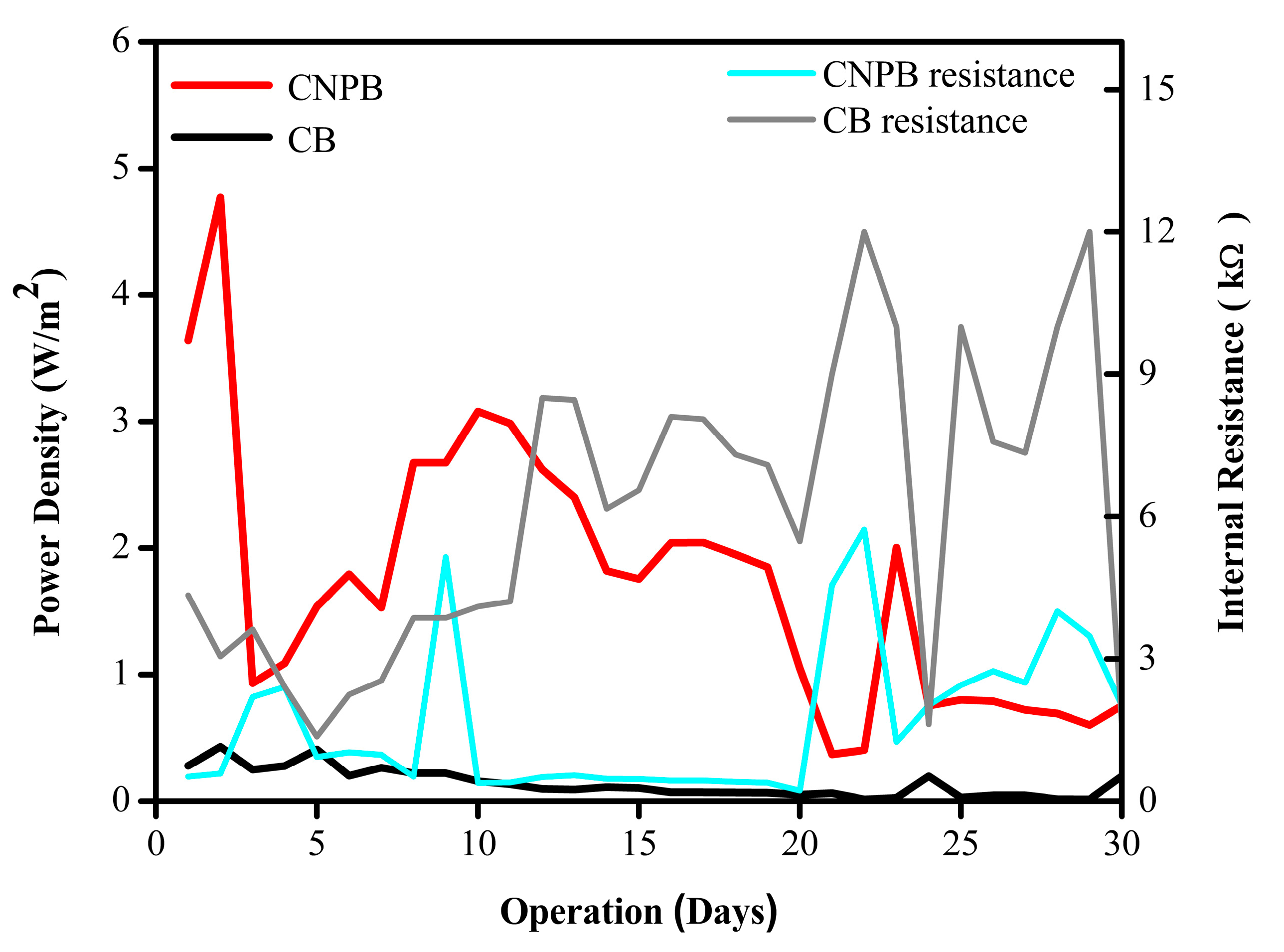
2.3.2. Long-Term Testing of α-FeOOH NPs in the SMFC
| Electrode Material | Cell Type | Nanoparticle | Maximum Power Density (W/m2) | TCOD (%) | Ref. |
|---|---|---|---|---|---|
| Macroporous sugarcane carbon | MFC | Fe | 3 | _ | [7] |
| Carbon cloth | MFC | Fe3O4 | 4 | 94 | [51] |
| Carbon fiber felt | SMFC | Fe2O3 | 9.8 × 10−3 | _ | [13] |
| Carbon derived from wax | MFC | β-FeOOH | 1.4 | _ | [52] |
| Carbon paper | MFC | α-FeOOH | 0.1 | _ | [16] |
| Carbon felt | SMFC | α-FeOOH | 4.9 | 99 | This study |
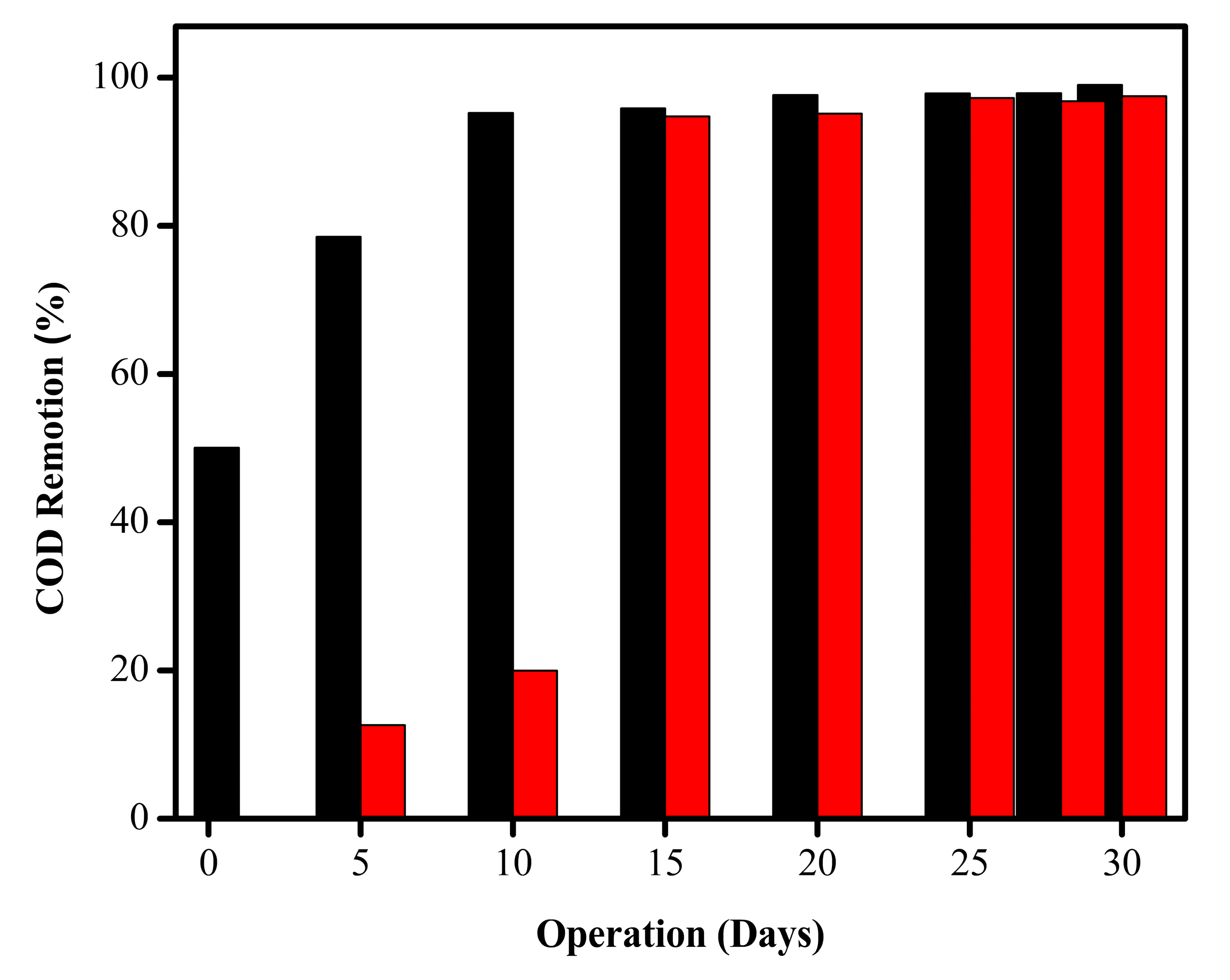
2.4. Total COD Removal
3. Materials and Methods
3.1. Hydrothermal Synthesis of α-FeOOH Nanoparticles
3.2. Fabrication of the Bioanode
3.3. α-FeOOH NPs and Bioanode Characterization
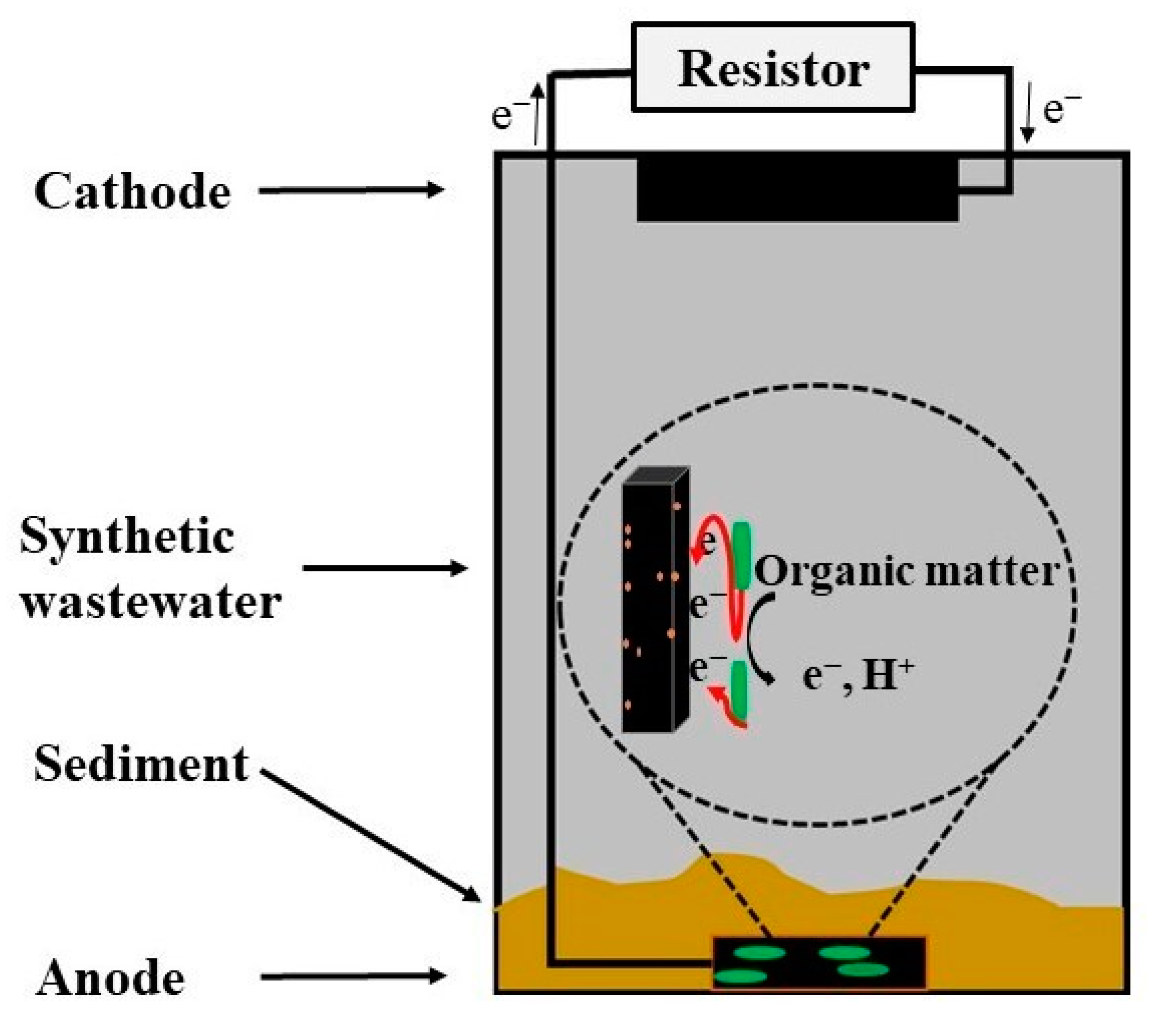
3.4. Sediment Microbial Fuel Cell Operation
3.5. Chemical Oxygen Demand (COD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feregrino-Rivas, M.; Ramirez-Pereda, B.; Estrada-Godoy, F.; Cuesta-Zedeño, L.F.; Rochín-Medina, J.J.; Bustos-Terrones, Y.A.; Gonzalez-Huitron, V.A. Performance of a Sediment Microbial Fuel Cell for Bioenergy Production: Comparison of Fluvial and Marine Sediments|. Biomass Bioenergy 2023, 168, 106657. [Google Scholar] [CrossRef]
- Abazarian, E.; Gheshlaghi, R.; Mahdavi, M.A. Interactions between Sediment Microbial Fuel Cells and Voltage Loss in Series Connection in Open Channels. Fuel 2023, 332, 126028. [Google Scholar] [CrossRef]
- Chi-Wen, L.; Alfanti, L.K.; Cheng, Y.-S.; Liu, S.-H. Enhancing Bioelectricity Production and Copper Remediation in Constructed Single-Medium Plant Sediment Microbial Fuel Cells. Desalination 2022, 542, 116079. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.-G.; Liu, X.; Fu, Y. Anode Modification of Sediment Microbial Fuel Cells (SMFC) towards Bioremediating Mariculture Wastewater. Mar. Pollut. Bull. 2022, 182, 114013. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.O.; Sayed, E.T.; Obaid, M.; Choi, Y.-J.; Park, S.-G.; Al-Qaradawi, S.; Chae, K.-J. Transition Metal Nanoparticles Doped Carbon Paper as a Cost-Effective Anode in a Microbial Fuel Cell Powered by Pure and Mixed Biocatalyst Cultures. Int. J. Hydrogen Energy 2018, 43, 21560–21571. [Google Scholar] [CrossRef]
- Miran, F.; Mumtaz, M.W.; Mukhtar, H.; Akram, S. Iron Oxide–Modified Carbon Electrode and Sulfate-Reducing Bacteria for Simultaneous Enhanced Electricity Generation and Tannery Wastewater Treatment. Front. Bioeng. Biotechnol. 2021, 9, 747434. [Google Scholar] [CrossRef]
- Song, B.; Li, J.; Wang, Z.; Ali, J.; Wang, L.; Zhang, Z.; Liu, F.; Glebov, E.M.; Zhang, J.; Zhuang, X. N-Doped Fe Nanoparticles Anchored on 3D Carbonized Sugarcane Anode for High Power Density and Efficient Chromium(VI) Removal. J. Environ. Chem. Eng. 2022, 10, 108751. [Google Scholar] [CrossRef]
- Magotra, V.K.; Kang, T.W.; Aqueel Ahmed, A.T.; Inamdar, A.I.; Im, H.; Ghodake, G.; Choubey, R.K.; Kumar, V.; Kumar, S. Effect of Gold Nanoparticles Laced Anode on the Bio-Electro-Catalytic Activity and Power Generation Ability of Compost Based Microbial Fuel Cell as a Coin Cell Sized Device. Biomass Bioenergy 2021, 152, 106200. [Google Scholar] [CrossRef]
- Harshiny, M.; Samsudeen, N.; Kameswara, R.J.; Matheswaran, M. Biosynthesized FeO Nanoparticles Coated Carbon Anode for Improving the Performance of Microbial Fuel Cell. Int. J. Hydrogen Energy 2017, 42, 26488–26495. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Muthukumar, H.; Matheswaran, M. Amaranthus Spinosus Leaf Extract Mediated FeO Nanoparticles: Physicochemical Traits, Photocatalytic and Antioxidant Activity. ACS Sustain. Chem. Eng. 2015, 3, 3149–3156. [Google Scholar] [CrossRef]
- Pushkar, P.; Prakash, O.; Imran, M.; Mungray, A.A.; Kailasa, S.K.; Mungray, A.K. Effect of Cerium Oxide Nanoparticles Coating on the Electrodes of Benthic Microbial Fuel Cell. Sep. Sci. Technol. 2019, 54, 213–223. [Google Scholar] [CrossRef]
- Yang, C.; Xiao, N.; Chang, Z.; Huang, J.J.; Zeng, W. Biodegradation of TOC by Nano-Fe2O3 Modified SMFC and Its Potential Environmental Effects. ChemistrySelect 2021, 6, 5597–5602. [Google Scholar] [CrossRef]
- Lin, F.; Yuan, M.; Chen, Y.; Huang, Y.; Lian, J.; Qiu, J.; Xu, H.; Li, H.; Yuan, S.; Zhao, Y.; et al. Advanced Asymmetric Supercapacitor Based on Molybdenum Trioxide Decorated Nickel Cobalt Oxide Nanosheets and Three-Dimensional α-FeOOH/RGO. Electrochim. Acta 2019, 320, 134580. [Google Scholar] [CrossRef]
- Tian, C.; Yuan, P.; Huang, W.; Song, F.; Zhao, W. MoS2 Nanosheets Embedded in α-FeOOH as an Efficient Cathode for Enhanced MFC-Electro-Fenton Performance in Wastewater Treatment. Environ. Res. 2023, 216, 114818. [Google Scholar] [CrossRef] [PubMed]
- Xian, J.; Ma, H.; Li, Z.; Ding, C.; Liu, Y.; Yang, J.; Cui, F. α-FeOOH Nanowires Loaded on Carbon Paper Anodes Improve the Performance of Microbial Fuel Cells. Chemosphere 2021, 273, 129669. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.; Abdus-Salam, N.; Haleemat, A.I.; Bello, M.O.; Inyang, E.D.; Alkali, M.I.; Tripathy, B.C. High Performance Nanohybrid ZnO-α-FeOOH Nanocomposite Prepared for Toxic Metal Ions Removal from Wastewater: Combined Sorption and Desorption Studies. Inorg. Chem. Commun. 2022, 145, 109900. [Google Scholar] [CrossRef]
- Godwin, J.; Abdus-Salam, N.; Haleemat, A.I.; Panda, P.K.; Panda, J.; Tripathy, B.C. Facile Synthesis of Rod-like α-FeOOH Nanoparticles Adsorbent and Its Mechanism of Sorption of Pb(II) and Indigo Carmine in Batch Operation. Inorg. Chem. Commun. 2022, 140, 109346. [Google Scholar] [CrossRef]
- Malathi, A.; Arunachalam, P.; Madhavan, J.; Al-Mayouf, A.M.; Ghanem, M.A. Rod-on-Flake α-FeOOH/BiOI Nanocomposite: Facile Synthesis, Characterization and Enhanced Photocatalytic Performance. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 435–445. [Google Scholar] [CrossRef]
- Mineo, G.; Bruno, L.; Bruno, E.; Mirabella, S. WO3 Nanorods Decorated with Very Small Amount of Pt for Effective Hydrogen Evolution Reaction. Nanomaterials 2023, 13, 1071. [Google Scholar] [CrossRef]
- Barai, H.R.; Banerjee, A.N.; Hamnabard, N.; Joo, S.W. Synthesis of Amorphous Manganese Oxide Nanoparticles-to-Crystalline Nanorods through a Simple Wet-Chemical Technique Using K+ Ions as a ‘Growth Director’ and Their Morphology-Controlled High Performance Supercapacitor Applications. RSC Adv. 2016, 6, 78887–78908. [Google Scholar] [CrossRef]
- Liu, H.; Zou, J.; Ding, Y.; Liu, B.; Wang, Y. Novel α-FeOOH Corner-Truncated Tetragonal Prisms: Crystal Structure, Growth Mechanism and Lithium Storage Properties. J. Appl. Electrochem. 2019, 49, 657–669. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, S.; Schaller, R.; Jiao, J.; Chaplen, F.; Liu, H. Nanoparticle Decorated Anodes for Enhanced Current Generation in Microbial Electrochemical Cells. Biosens. Bioelectron. 2011, 26, 1908–1912. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Nakamura, R.; Kai, F.; Watanabe, K.; Hashimoto, K. Respiratory Interactions of Soil Bacteria with (Semi)Conductive Iron-Oxide Minerals. Environ. Microbiol. 2010, 12, 3114–3123. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Xie, D.; Yue, X.; Feng, C.; Wei, C. Ruthenium Oxide-Coated Carbon Felt Electrode: A Highly Active Anode for Microbial Fuel Cell Applications. J. Power Sources 2012, 210, 26–31. [Google Scholar] [CrossRef]
- Yin, Y.; Huang, G.; Zhou, N.; Liu, Y.; Zhang, L. Increasing Power Generation of Microbial Fuel Cells with a Nano-CeO2 Modified Anode. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 1212–1218. [Google Scholar] [CrossRef]
- Muthukumar, H.; Mohammed, S.N.; Chandrasekaran, N.; Sekar, A.D.; Pugazhendhi, A.; Matheswaran, M. Effect of Iron Doped Zinc Oxide Nanoparticles Coating in the Anode on Current Generation in Microbial Electrochemical Cells. Int. J. Hydrogen Energy 2019, 44, 2407–2416. [Google Scholar] [CrossRef]
- Lakshmipriya, T.; Gopinath, S.C.B. Introduction to Nanoparticles and Analytical Devices. In Nanoparticles in Analytical and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–29. [Google Scholar]
- Sahu, R.; Parkhey, P. Advancements in Bioelectrochemical System-Based Wastewater Treatment: A Review on Nanocatalytic Approach. Sustain. Energy Technol. Assess. 2021, 48, 101558. [Google Scholar] [CrossRef]
- Guo, H.; Barnard, A.S. Thermodynamic Modelling of Nanomorphologies of Hematite and Goethite. J. Mater. Chem. 2011, 21, 11566. [Google Scholar] [CrossRef]
- Savla, N.; Anand, R.; Pandit, S.; Prasad, R. Utilization of Nanomaterials as Anode Modifiers for Improving Microbial Fuel Cells Performance. J. Renew. Mater. 2020, 8, 1581–1605. [Google Scholar] [CrossRef]
- Yao, Y.; Hou, C.; Zhang, X. Construct α-FeOOH-Reduced Graphene Oxide Aerogel as a Carrier for Glucose Oxidase Electrode. Membranes 2022, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Meng, K.; Hu, X.; Zhang, Y.; Zeng, T.; Wan, Q.; Lai, G.; Chen, X.; Yang, N. A Versatile Sensing Platform Based on FeOOH Nanorod/Expanded Graphite for Electrochemical Quantification of Bioanalytes. J. Electroanal. Chem. 2021, 902, 115803. [Google Scholar] [CrossRef]
- Dai, K.; Yan, Y.; Wang, Q.-T.; Zheng, S.-J.; Huang, Z.-Q.; Sun, T.; Zeng, R.J.; Zhang, F. Electricity Production and Key Exoelectrogens in a Mixed-Culture Psychrophilic Microbial Fuel Cell at 4 °C. Appl. Microbiol. Biotechnol. 2022, 106, 4801–4811. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yuan, Y.; Liu, T.; Zhou, S. High-Capacity Carbon-Coated Titanium Dioxide Core–Shell Nanoparticles Modified Three Dimensional Anodes for Improved Energy Output in Microbial Fuel Cells. J. Power Sources 2015, 274, 170–176. [Google Scholar] [CrossRef]
- Zallouz, S.; Pronkin, S.N.; Le Meins, J.-M.; Pham-Huu, C.; Matei Ghimbeu, C. New Development in Carbon-Based Electrodes and Electrolytes for Enhancement of Supercapacitor Performance and Safety. In Renewable Energy Production and Distribution; Elsevier: Amsterdam, The Netherlands, 2023; Volume 2, pp. 353–408. [Google Scholar]
- Kurzweil, P. CAPACITORS|Electrochemical Metal Oxides Capacitors. In Encyclopedia of Electrochemical Power Sources; Elsevier: Amsterdam, The Netherlands, 2009; pp. 665–678. [Google Scholar]
- Kandpal, R.; Nara, S.; Shahadat, M.; Ansari, M.O.; Alshahrie, A.; Ali, S.W.; Ahammad, S.Z.; Malhotra, B.D. Impedance Spectroscopic Study of Biofilm Formation on Pencil Lead Graphite Anode in Microbial Fuel Cell. J. Taiwan Inst. Chem. Eng. 2021, 128, 114–123. [Google Scholar] [CrossRef]
- Roy, H.; Rahman, T.U.; Tasnim, N.; Arju, J.; Rafid, M.M.; Islam, M.R.; Pervez, M.N.; Cai, Y.; Naddeo, V.; Islam, M.S. Microbial Fuel Cell Construction Features and Application for Sustainable Wastewater Treatment. Membranes 2023, 13, 490. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Li, B.; Jia, W.; Lei, Y. Effect of Inoculum Types on Bacterial Adhesion and Power Production in Microbial Fuel Cells. Appl. Biochem. Biotechnol. 2010, 160, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Salar-Garcia, M.J.; Obata, O.; Kurt, H.; Chandran, K.; Greenman, J.; Ieropoulos, I.A. Impact of Inoculum Type on the Microbial Community and Power Performance of Urine-Fed Microbial Fuel Cells. Microorganisms 2020, 8, 1921. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.B. Microbial Fuel Cells; Wiley: Hoboken, NJ, USA, 2008; ISBN 9780470239483. [Google Scholar]
- Simeon, M.I.; Asoiro, F.U.; Aliyu, M.; Raji, O.A.; Freitag, R. Polarization and Power Density Trends of a Soil-based Microbial Fuel Cell Treated with Human Urine. Int. J. Energy Res. 2020, 44, 5968–5976. [Google Scholar] [CrossRef]
- Liang, P.; Huang, X.; Fan, M.-Z.; Cao, X.-X.; Wang, C. Composition and Distribution of Internal Resistance in Three Types of Microbial Fuel Cells. Appl. Microbiol. Biotechnol. 2007, 77, 551–558. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Li, X.F.; Ren, Y.P.; Wang, X.H. Effect of Fe(III) on the Performance of Sediment Microbial Fuel Cells in Treating Waste-Activated Sludge. RSC Adv. 2016, 6, 47974–47980. [Google Scholar] [CrossRef]
- Pan, X.; Wang, W.; Chen, Y.; Wen, Q.; Li, X.; Lin, C.; Wang, J.; Xu, H.; Yang, L. Bio-Electrocatalyst Fe3O4/Fe@C Derived from MOF as a High-Performance Bioanode in Single-Chamber Microbial Fuel Cell. Biochem. Eng. J. 2022, 187, 108611. [Google Scholar] [CrossRef]
- Vilajeliu-Pons, A.; Puig, S.; Salcedo-Dávila, I.; Balaguer, M.D.; Colprim, J. Long-Term Assessment of Six-Stacked Scaled-up MFCs Treating Swine Manure with Different Electrode Materials. Environ. Sci. Water Res. Technol. 2017, 3, 947–959. [Google Scholar] [CrossRef]
- Jiang, D.; Curtis, M.; Troop, E.; Scheible, K.; McGrath, J.; Hu, B.; Suib, S.; Raymond, D.; Li, B. A Pilot-Scale Study on Utilizing Multi-Anode/Cathode Microbial Fuel Cells (MAC MFCs) to Enhance the Power Production in Wastewater Treatment. Int. J. Hydrogen Energy 2011, 36, 876–884. [Google Scholar] [CrossRef]
- Hidalgo, D.; Tommasi, T.; Velayutham, K.; Ruggeri, B. Long Term Testing of Microbial Fuel Cells: Comparison of Different Anode Materials. Bioresour. Technol. 2016, 219, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Oguz Koroglu, E.; Civelek Yoruklu, H.; Demir, A.; Ozkaya, B. Scale-Up and Commercialization Issues of the MFCs. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 565–583. [Google Scholar]
- Chen, H.; Zhao, C.; Song, Y.; Wang, X.; Zhu, L.; Ai, T. Performance Improvement of Microbial Fuel Cells by Fermentation Gas Driven Fluidization of Magnetite Nanoparticles and Carbon Particles. J. Power Sources 2023, 555, 232365. [Google Scholar] [CrossRef]
- Tang, X.; Liu, L.; Cui, Y. β-FeOOH Modified Carbon Monolith Anode Derived from Wax Gourd for Microbial Fuel Cells. Int. J. Hydrogen Energy 2022, 47, 4838–4845. [Google Scholar] [CrossRef]
- Sekar, A.D.; Jayabalan, T.; Muthukumar, H.; Chandrasekaran, N.I.; Mohamed, S.N.; Matheswaran, M. Enhancing Power Generation and Treatment of Dairy Waste Water in Microbial Fuel Cell Using Cu-Doped Iron Oxide Nanoparticles Decorated Anode. Energy 2019, 172, 173–180. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Obaid, M.; Poo, K.-M.; Ali Abdelkareem, M.; Talas, S.A.; Fadali, O.A.; Kim, H.Y.; Chae, K.-J. Fe/Fe2O3 Nanoparticles as Anode Catalyst for Exclusive Power Generation and Degradation of Organic Compounds Using Microbial Fuel Cell. Chem. Eng. J. 2018, 349, 800–807. [Google Scholar] [CrossRef]
- Mejía-López, M.; Verea, L.; Sebastian, P.J.; Verde, A.; Perez-Sarinana, B.Y.; Campos, J.; Hernández-Romano, J.; Moeller-Chávez, G.E.; Lastres, O.; Monjardín-Gámez, J.d.J. Improvement of Biofilm Formation for Application in a Single Chamber Microbial Electrolysis Cell. Fuel Cells 2021, 21, 317–327. [Google Scholar] [CrossRef]






| EXP | R1 (Ω) | C1 (F) | R2 (Ω) | W (Ω) |
|---|---|---|---|---|
| Anode | 18 | 6.9 × 10−3 | 4.6 | 0.3 |
| Bioanode | 18 | 3.8 × 10−3 | 4.2 | 0.3 |
| Bioanode with FeOOH NPs | 13 | 2.1 × 10−3 | 1.5 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejía-López, M.; Lastres, O.; Alemán-Ramírez, J.L.; Verde, A.; Alvarez, J.C.; Torres-Arellano, S.; Trejo-Díaz, G.N.; Sebastian, P.J.; Verea, L. Improvement of Power Density and COD Removal in a Sediment Microbial Fuel Cell with α-FeOOH Nanoparticles. Catalysts 2024, 14, 561. https://doi.org/10.3390/catal14090561
Mejía-López M, Lastres O, Alemán-Ramírez JL, Verde A, Alvarez JC, Torres-Arellano S, Trejo-Díaz GN, Sebastian PJ, Verea L. Improvement of Power Density and COD Removal in a Sediment Microbial Fuel Cell with α-FeOOH Nanoparticles. Catalysts. 2024; 14(9):561. https://doi.org/10.3390/catal14090561
Chicago/Turabian StyleMejía-López, Monica, Orlando Lastres, José Luis Alemán-Ramírez, Antonio Verde, José Campos Alvarez, Soleyda Torres-Arellano, Gabriela N. Trejo-Díaz, Pathiyamattom J. Sebastian, and Laura Verea. 2024. "Improvement of Power Density and COD Removal in a Sediment Microbial Fuel Cell with α-FeOOH Nanoparticles" Catalysts 14, no. 9: 561. https://doi.org/10.3390/catal14090561








