Abstract
Catalytic combustion is an effective strategy for alleviating volatile organic compounds (VOCs), including hydrocarbons and aromatic compounds, mostly derived from the petrochemical and pharmaceutical industries. We employed Pd/Al2O3 as a catalyst for combusting aromatic VOCs via hydrogen catalytic combustion. It differs from conventional approaches that do not necessitate additional electric heating. Briefly, when hydrogen (H2) is introduced below its lower explosive limit of 4% on the Pd/Al2O3 catalyst, it completely oxidizes important aromatic VOCs like benzene, toluene, ethyl benzene, and xylene to carbon dioxide and water. The catalytic performance of the integrated system remains stable even after long-term use. Therefore, hydrogen co-combustion on the Pd/Al2O3 catalyst can provide onsite heating for a facility without needing external electric heat. The catalytic performance shows no significant dependence on the sizes of Pd nanoparticles in both fresh and spent conditions, as demonstrated by XRD, XPS, and STEM analyses. Therefore, renewable green hydrogen can effectively reduce aromatic VOC pollutants, providing a more energy-efficient alternative. Our findings suggest that this integrated process is promising for converting aromatic VOCs into carbon dioxide and water without electric heating.
1. Introduction
Volatile organic compounds (VOCs), including aromatic compounds, originating from industrial activities have long been a pressing environmental concern, particularly in developing regions, and their presence can lead to the formation of photochemical smog and pose substantial risks to human health [1,2,3,4]. Moreover, many volatile organic compounds (VOCs) are extremely toxic and can cause cancer, even at low concentrations; thus, they must be removed completely [5,6,7]. Various control technologies have addressed these challenges, including condensation, adsorption, photodegradation, combustion, biodegradation, and photocatalysis [8,9]. In particular, catalytic combustion is considered a key technology for removing VOCs due to high combustion efficiency and relatively low demand for auxiliary fuel pose [10]. Briefly, catalytic combustion consists of different catalysts, including composite oxides, noble metals, and transition metal oxide catalysts [11,12,13,14]. However, platinum-group metal (PGM) catalysts like platinum (Pt) [15], palladium (Pd) [16], and rhodium (Rh) [17] have attracted more attention due to the complete oxidation of hydrocarbons [18,19]. For instance, Seshadri et al. observed the complete oxidation of hydrocarbons (non-aromatic) under mild conditions, involving the interaction between oxygen and hydrogen [20]. In addition, Moretti et al. conducted the full oxidation of aromatic VOCs through thermal oxidation at elevated temperatures (i.e., 1000 °C), which entails both the dissipation of high energy and the generation of additional by-products [21]. Therefore, scientists are interested in the catalytic combustion of aromatic VOCs at low or even room temperatures with no additional by-products [22].
Catalytic oxidation can convert VOCs or aromatic VOCs into CO2, water, and fewer by-products in the presence of a suitable catalyst at much lower operating temperatures (250–500 °C) [23,24,25] depending on the nature of the catalyst, nature of VOC mixtures, gas flow rate, type of reactor, etc. [26,27,28]. The main barrier to the oxidation of VOCs is its high energy consumption. Therefore, extensive research efforts have been devoted to decreasing the temperatures. However, heat energy is still needed either in thermal oxidation or catalytic oxidation processes to facilitate the complete oxidation of VOCs. Temperature is the critical factor that starts the VOC oxidation reaction rather than the amount of heat alone. Thus, creating novel approaches to improve heating efficiency appears to be advantageous in solving this fundamental problem. Overall, the destruction mechanisms involve the conversion of VOCs into carbon dioxide and water and the release of reaction heat, as shown in Equation (1), by thermal, catalytic, or biological oxidation. Furthermore, the degradation of small VOCs is a difficult obstacle, and most of the methods studied fall short of several environmental standards. However, few studies have focused on onsite heating that does not require extra electric heating.
This approach deviates from conventional methods by forgoing thermal recovery, resulting in a simpler system with reduced energy consumption and fewer by-products like NOx during the elimination of VOCs, contrasting notably with catalytic techniques. Catalytic hydrogen combustion (CHC) minimizes the catalyst bed area, facilitating reactions in the upper layer upon H2 addition and promoting hydrogen catalytic co-combustion. Utilizing hydrogen as a heating source offers greener and more efficient VOC oxidation compared to traditional methods, combining the advantages of thermal incineration and catalytic oxidation. Therefore, there is an urgent need to develop simple procedures that do not require extra electric heating to completely oxidize aromatic VOCs under mild conditions.
In this work, we investigated the combustion of aromatic VOCs consisting of hydrogen-assisted catalytic ignition with reduced energy consumption and fewer by-products on Pd/Al2O3. Pd/Al2O3 serves as a versatile catalyst not only for the combustion of aromatic benzene but also for the combustion of toluene, ethyl benzene, and xylene. Accordingly, the catalytic combustion of aromatic VOCs on the Pd/Al2O3 catalyst starts at room temperature and elevates the catalyst bed to temperatures conducive to the complete oxidation of aromatic VOCs. The Pd/Al2O3 catalyst continuously retains its ignition capability for hydrogen combustion at room temperature. Even the Pd particles grow by almost 23 nm due to the high-temperature reaction of 950 °C.
CxHyOz (g) + (x + y/4 − z/2) O2 → xCO2 + y/2 H2O + ∆H
2. Results and Discussion
2.1. Structure Evolution of Pd/Al2O3 Catalyst
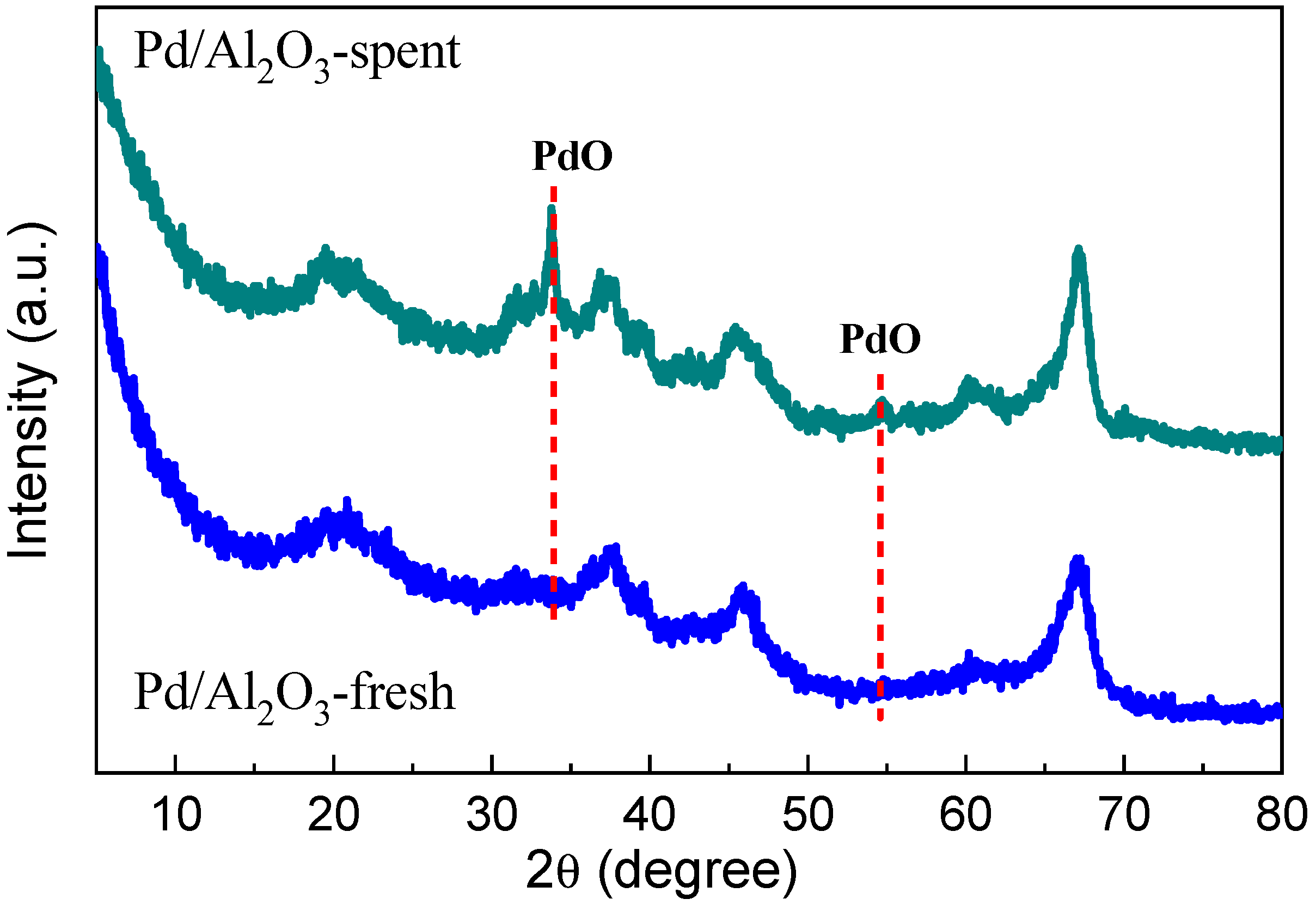
Catalytic oxidation of aromatic volatile organic compounds (VOCs) was carried out using Pd/Al2O3 catalysts labeled as fresh and spent before and after the catalytic oxidation of aromatic VOCs. The Brunauer–Emmett–Teller (BET) method was used to quantify the surface area of both fresh and spent Pd/Al2O3 samples. Due to surface covering, a common occurrence in catalytic combustion, the Pd/Al2O3-fresh catalyst had a greater surface area (212.3 m2/g) than the spent catalyst (174.4 m2/g) [29]. Further, to test the crystal structure and morphology of both samples (Pd/Al2O3-fresh and Pd/Al2O3-spent), XRD, TEM, and STEM analyses were performed. XRD patterns for both fresh and spent Pd/Al2O3 catalysts are shown in Figure 1. The Pd/Al2O3-fresh sample, as can be observed, only exhibits γ-Al2O3 diffraction peaks, and no Pd peaks were found, indicating that Pd was well distributed over the Al2O3 support. On the other hand, two more peaks for Pd/Al2O3-spent, corresponding to the PdO for the (101) and (112) planes (JCPDF = 85-0713), emerged at angles of 33.8° and 54.7° [30]. The apparent crystallite size of PdO on the (101) plane was approximately 23 nm, as determined by the Scherrer equation. The formation of PdO instead of metallic Pd is expected due to the catalyst being cooled in the air after undergoing high-temperature reaction tests. Remarkably, the Pd/Al2O3-spent catalyst exhibited the ability to catalyze hydrogen combustion at ambient temperature, even after the metallic Pd nanoparticles underwent oxidation and were transformed into bigger PdO particles.
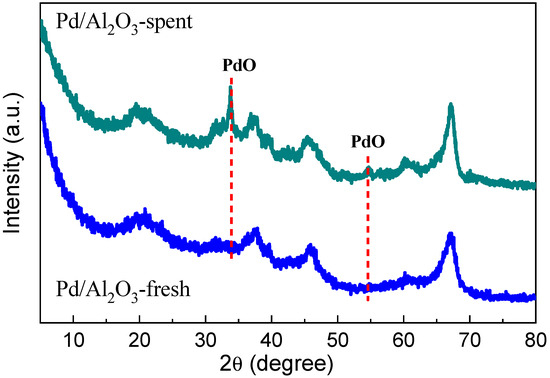
Figure 1.
XRD patterns for fresh Pd/Al2O3 and spent Pd/Al2O3 catalysts.
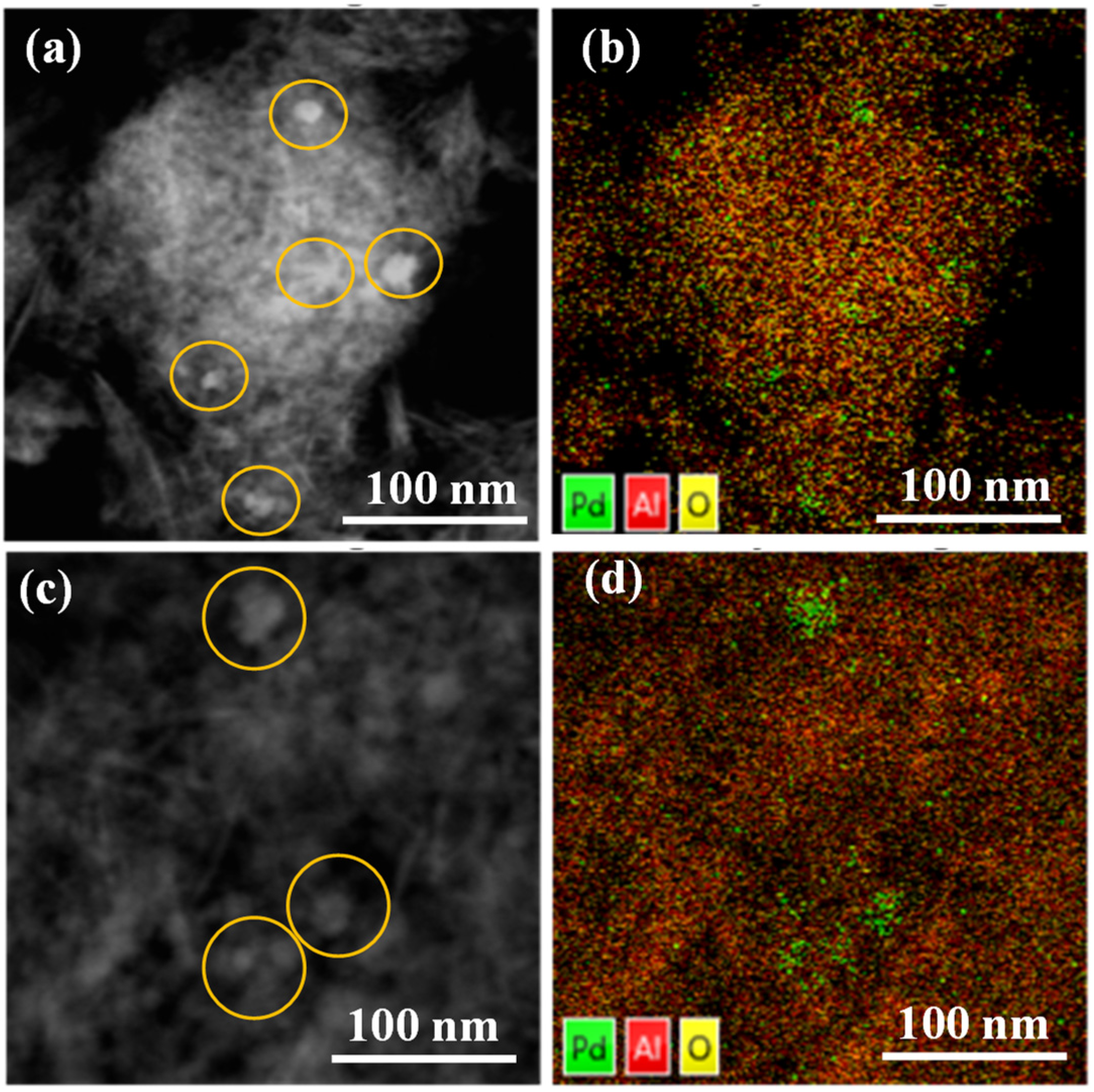
In addition, HAADF analysis and element mapping were also conducted to confirm the formation and dispersion of Pd nanoparticles in fresh and spent samples. The fresh Pd/Al2O3 sample exhibits a significant abundance of tiny Pd nanoparticles (Figure 2a). The element mapping image presented in Figure 2b demonstrates the remarkable distribution of Pd throughout the Al2O3 support, indicating the existence of smaller Pd species located beyond the visible Pd particles. These species are indicated by scattered green dots. On the other hand, in the Pd/Al2O3-spent catalyst, a few large Pd particles (10–40 nm) can only be observed (Figure 2c), and there are occasional green aggregations (Figure 2d). Moreover, TEM images unambiguously reveal that both catalysts exhibit Pd/PdO nanoparticles (Figure S1). Upon closer inspection of the TEM images, the crystal fringes of the particles become distinctly evident. Notably, when observed at even higher magnifications, these crystal fringes become remarkably clear, allowing for the measurement of an inter-fringe distance of 0.22 nanometers for the pristine catalyst (Figure S1c). This distance corresponds to the Pd (111) crystal plane, consistent with the XRD results. Interestingly, the Pd/Al2O3-spent catalyst demonstrates a slightly increased crystal fringe spacing of 0.24 nanometers but maintains the integrity of the (111) crystal plane (Figure S1f). This observation also aligns well with the XRD findings.
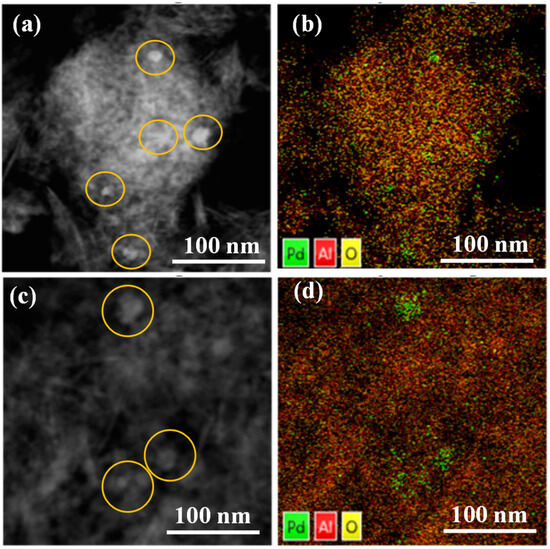
Figure 2.
HAADF and element mapping images for Pd/Al2O3-fresh (a,b) and Pd/Al2O3-spent (c,d) catalysts.
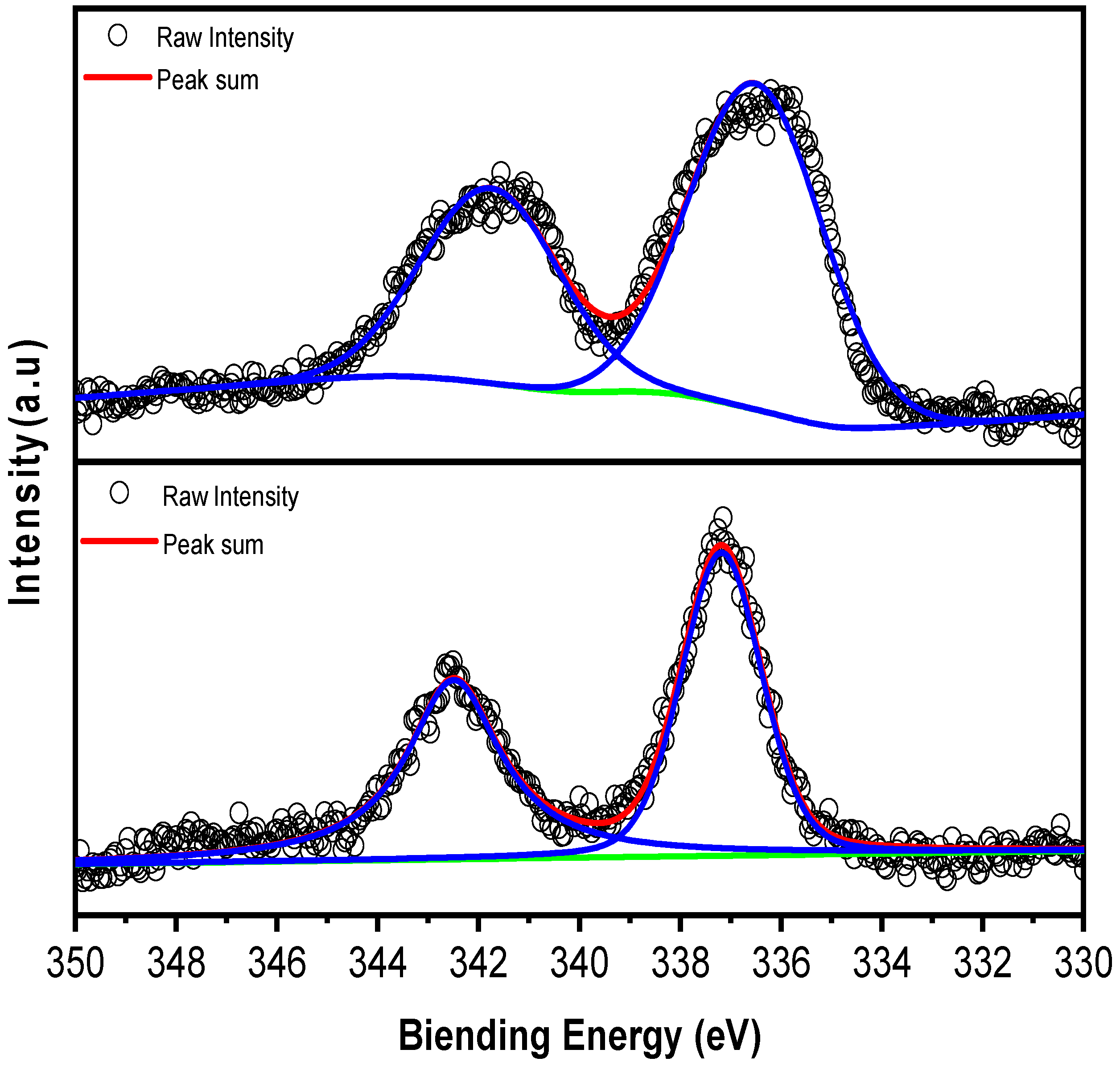
To further study the surface oxidation of the Pd element, X-ray photoelectron spectroscopy (XPS) was performed on both fresh and spent samples (Figure 3). Figure 3 displays the XPS spectra for the Pd 3d region of both fresh and spent Pd/Al2O3 catalysts, including the fitting results for these signals. X-ray photoelectron spectroscopy (XPS) was conducted on both the fresh and spent samples to investigate the surface oxidation state of Pd. The spectra in Figure 3 reveal positive shifts in binding energies, which indicate partial oxidation of Pd to PdO, a finding that aligns with the results from X-ray diffraction (XRD) [29]. The partial oxidation of Pd can also be observed from the shift in binding energy, which further demonstrates the consistency of the XRD and STEM results.
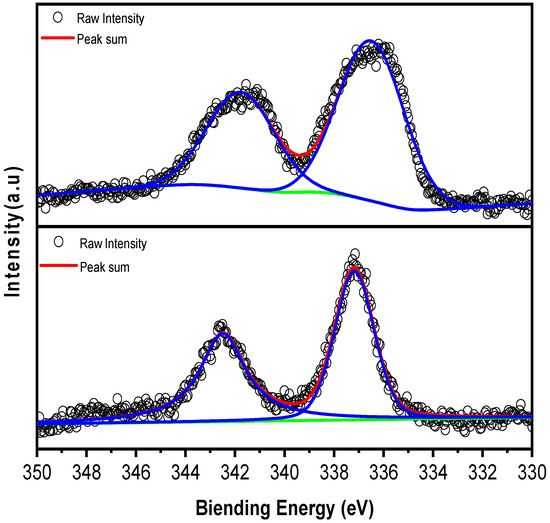
Figure 3.
XPS spectra of Pd for fresh Pd/Al2O3 (top) and spent Pd/Al2O3 (down).
2.2. Catalytic Performance Test
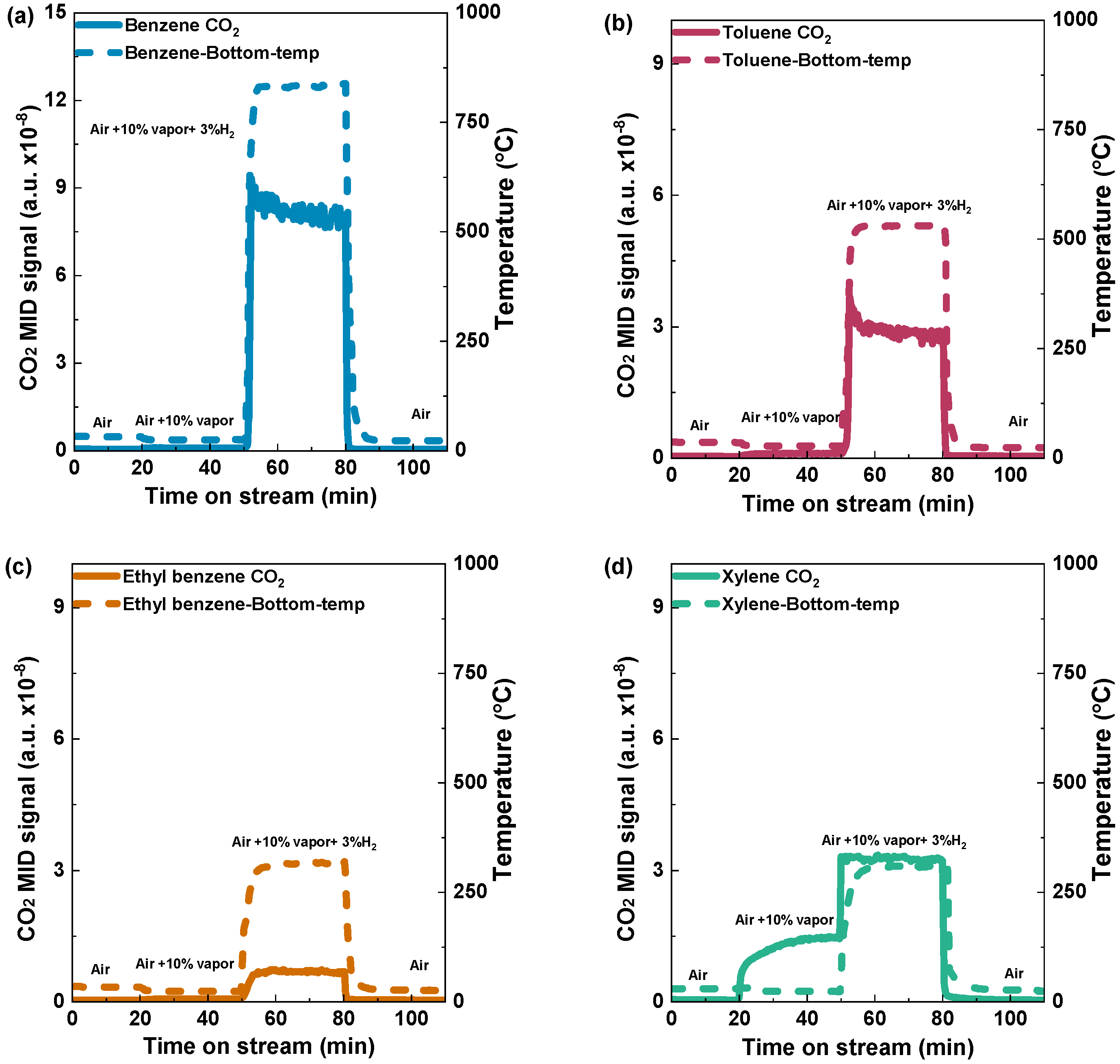
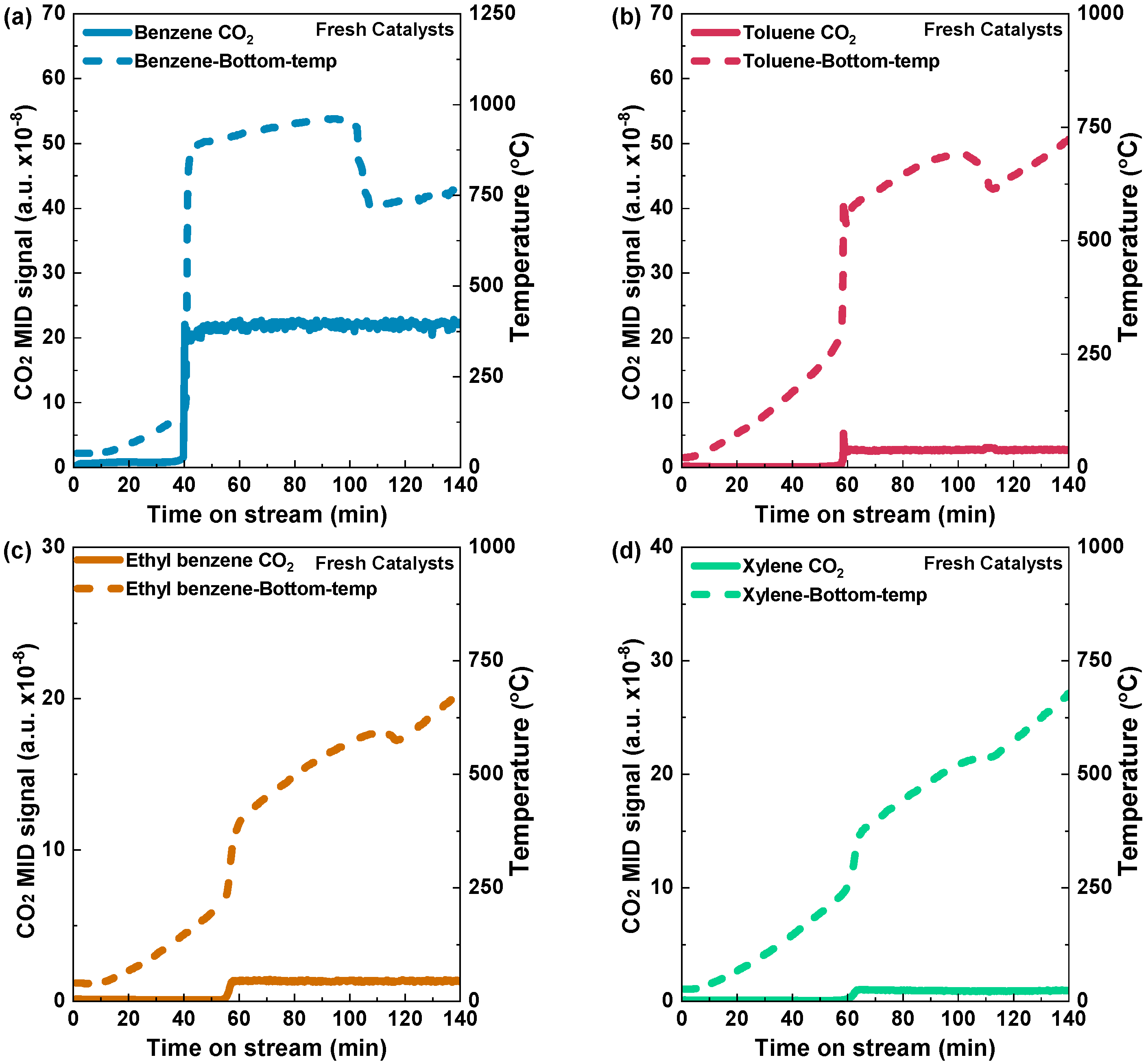
To assess the impact of hydrogen ignition on the oxidation of aromatic VOCs, we first established the optimal amounts of hydrogen and organic VOCs. Before optimization, air was employed to purge the channel at the first stage of the reaction to ensure an unobstructed flow, followed by the controlled introduction of a specified quantity of benzene, constituting 10% of the total volume, into the system. Initially, the reaction was started by a hydrogen concentration of 3%, rapidly increasing the bed temperature, reaching 839 °C (Figure 4a). With the rise in temperature, the volatile organic compounds (VOCs) underwent combustion, producing carbon dioxide (CO2). After the temperature and CO2 levels reached a steady state, the provision of both hydrogen and VOCs was halted. During the concluding stage of the studies, as the air was supplied, both peaks exhibited a progressive decline and eventually reverted to the baseline. The experimental methods employed demonstrate the ability to initiate and regulate the combustion process with precision, facilitating a comprehensive examination of the impact of hydrogen ignition on benzene catalytic combustion.
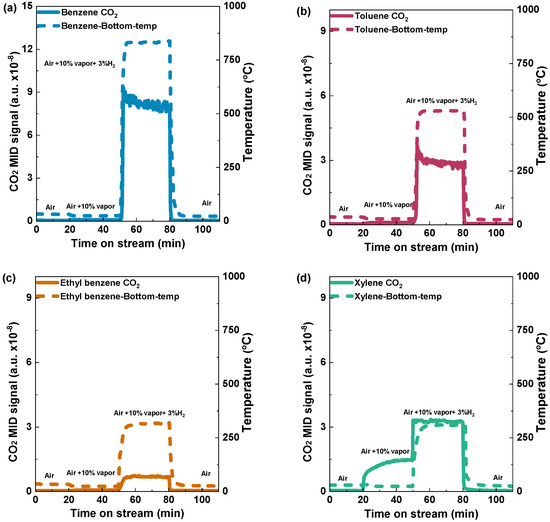
Figure 4.
Hydrogen catalytic combustion reaction of (a) benzene, (b) toluene, (c) ethyl benzene, and (d) xylene over the Pd/Al2O3 catalyst. Condition: 0.5 g catalyst; VOC concentration: 10% vapor; and Total flow rate: 1000 mL min−1 (GHSV: 120,000 mL h−1g−1).
Similarly, a 10% volume of toluene was used, which resulted in a sharp increase in the bed temperature, reaching a notable temperature of 545 °C (Figure 4b). In addition, a comparable occurrence was noted for ethyl benzene and xylene, with approximate temperatures of 330 °C and 340 °C, respectively (Figure 4c,d). Due to its higher calorific value, benzene reached a higher bed temperature than toluene, ethylbenzene, and xylene. This implies that the combustion of benzene can release more heat energy compared to the combustion of the other mentioned VOCs. To study the effect of bed temperature due to the presence of VOCs on the Pd/Al2O3 catalyst, the bed temperatures of the catalyst with H2 concentrations of 2%, 3%, and 4% were measured (Figure S2).
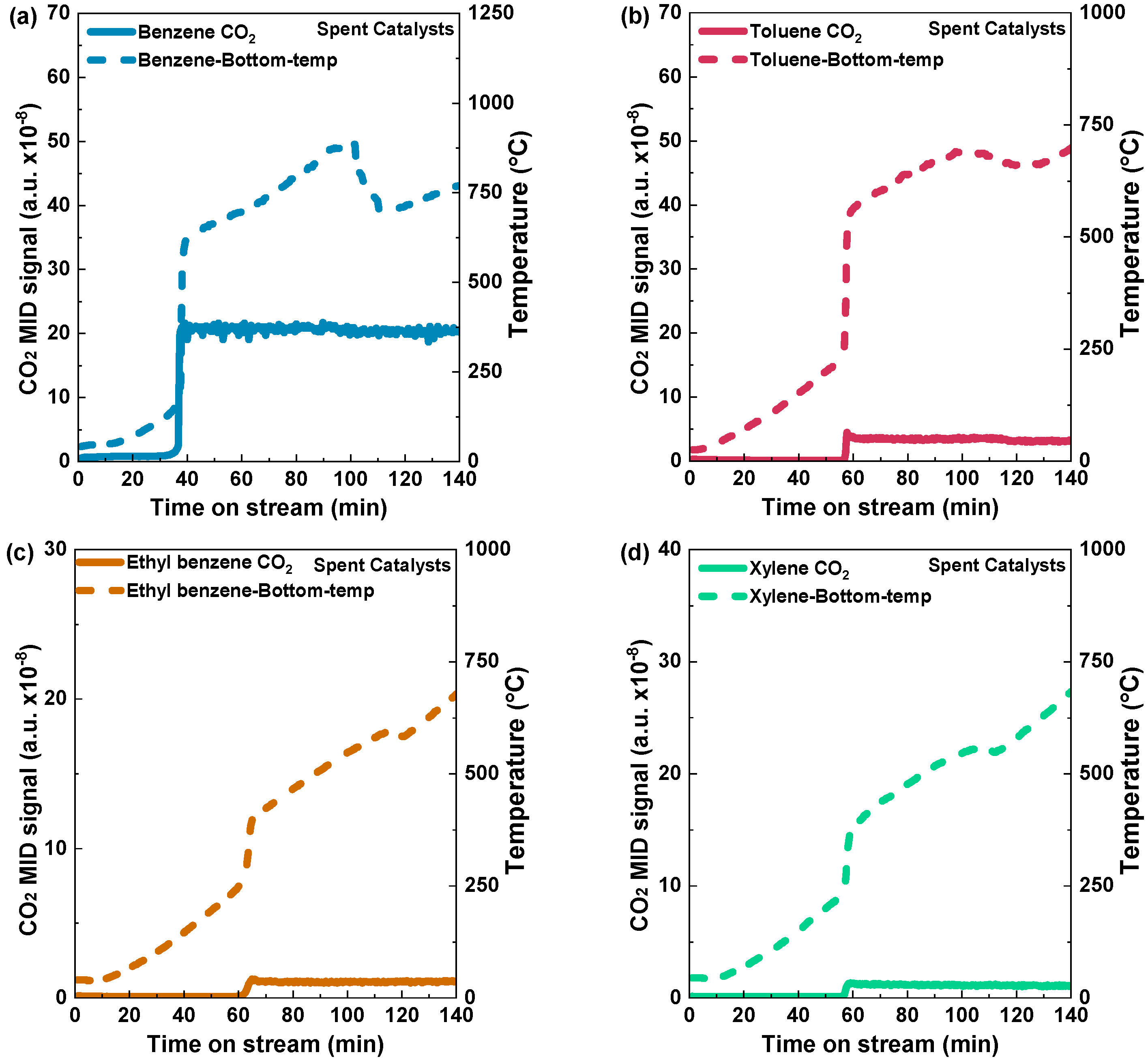
In order to determine the light-off temperatures of various aromatic VOCs on Pd/Al2O3-fresh and Pd/Al2O3-spent catalysts, temperature-programed reactions were conducted via an electric heating furnace. The temperature rise profiles of Pd/Al2O3-fresh and the light-off curves resulting in CO2 production can be seen in Figure 5. During the determination of light-off temperatures for both fresh and spent catalysts using this electric heating furnace method, no H2 was introduced. The carbon dioxide (CO2) signal for benzene was detected at a temperature of 187 °C after a stream time of 40 min. When the furnace temperature was raised over 400 °C, the CO2 signal reached a stable level, indicating complete oxidation of benzene (Figure 5a). In the case of toluene, the carbon dioxide (CO2) signal exhibited a slight increase after 56 min, with a corresponding temperature of 276 °C, and a temperature peak at 300 °C became stable with an increase in the furnace temperature, indicating complete oxidation (Figure 5b). For ethyl benzene and xylene, a CO2 peak was observed at 54 and 60 min, respectively (Figure 5c,d). The temperature was raised from 200 °C to 400 °C for ethyl benzene and 250 °C to 360 °C for xylene, respectively, in Figure 5c,d. In short, for the Pd/Al2O3-fresh catalysts, the light-off temperatures of benzene, toluene, ethyl benzene, and xylene were 187 °C, 276 °C, 260 °C, and 265 °C, respectively. On the other hand, Figure 6a–d show the light-off temperatures for benzene, toluene, ethyl benzene, and xylene on the Pd/Al2O3-spent catalyst, which were 177 °C, 257 °C, 258 °C, and 249 °C, respectively.
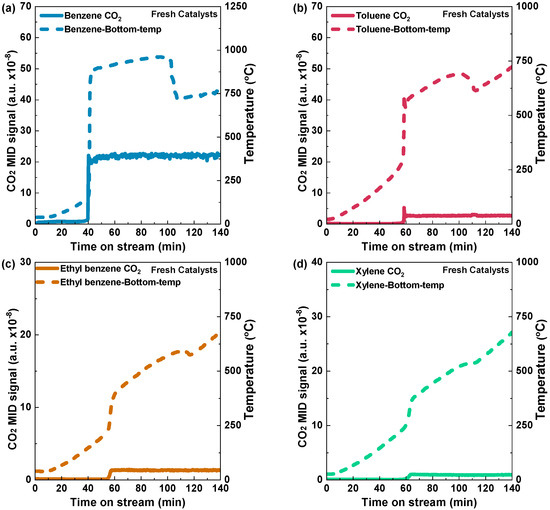
Figure 5.
Light-off curves illustrated by CO2 formation and catalyst bed temperature raising profiles during the temperature-programed reactions of (a) benzene, (b) toluene, (c) ethyl benzene, and (d) xylene over fresh Pd/Al2O3 using an electric heating furnace. Condition: 0.5 g catalyst; VOC concentration: 10% vapor; Temperature: 0–725 °C: 5 °C min−1; and Total flow rate: 1000 mL min−1 (GHSV: 120,000 mL h−1g−1).
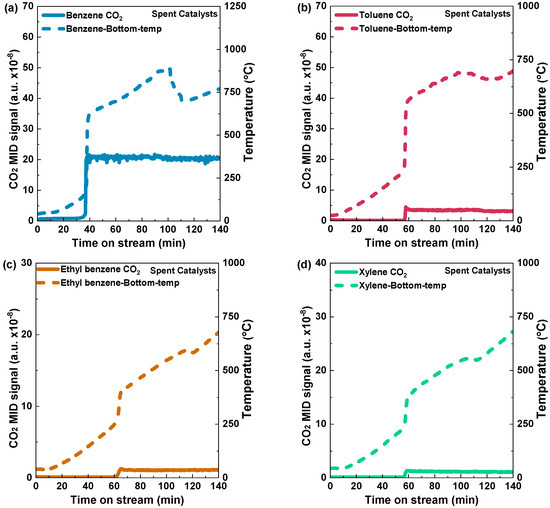
Figure 6.
Light-off curves illustrated by CO2 formation and catalyst bed temperature raising profiles during the temperature-programed reactions of (a) benzene, (b) toluene, (c) ethyl benzene, and (d) xylene over spent Pd/Al2O3 catalyst using an electric heating furnace. Condition: 0.5 g catalyst; VOC concentration: 10% vapor; Temperature: 0–725 °C: 5 °C min−1, and Total flow rate: 1000 mL min−1 (GHSV: 120,000 mL h−1·g−1).
The findings highlight the dependability and consistency of the catalysts, even with extended usage. The existence of CO2 peaks in all the experiments is a definitive indicator of these materials’ catalytic activity and efficiency (Figure 6a–d). The most significant observation is the similar performance of the spent catalysts and their fresh counterparts regarding the conversion efficiency. The spent catalysts exhibit enduring catalytic activity, which offers favorable prospects for their utilization in various catalytic reactions. This improves the overall efficiency and helps to enhance economic sustainability in different industrial applications.
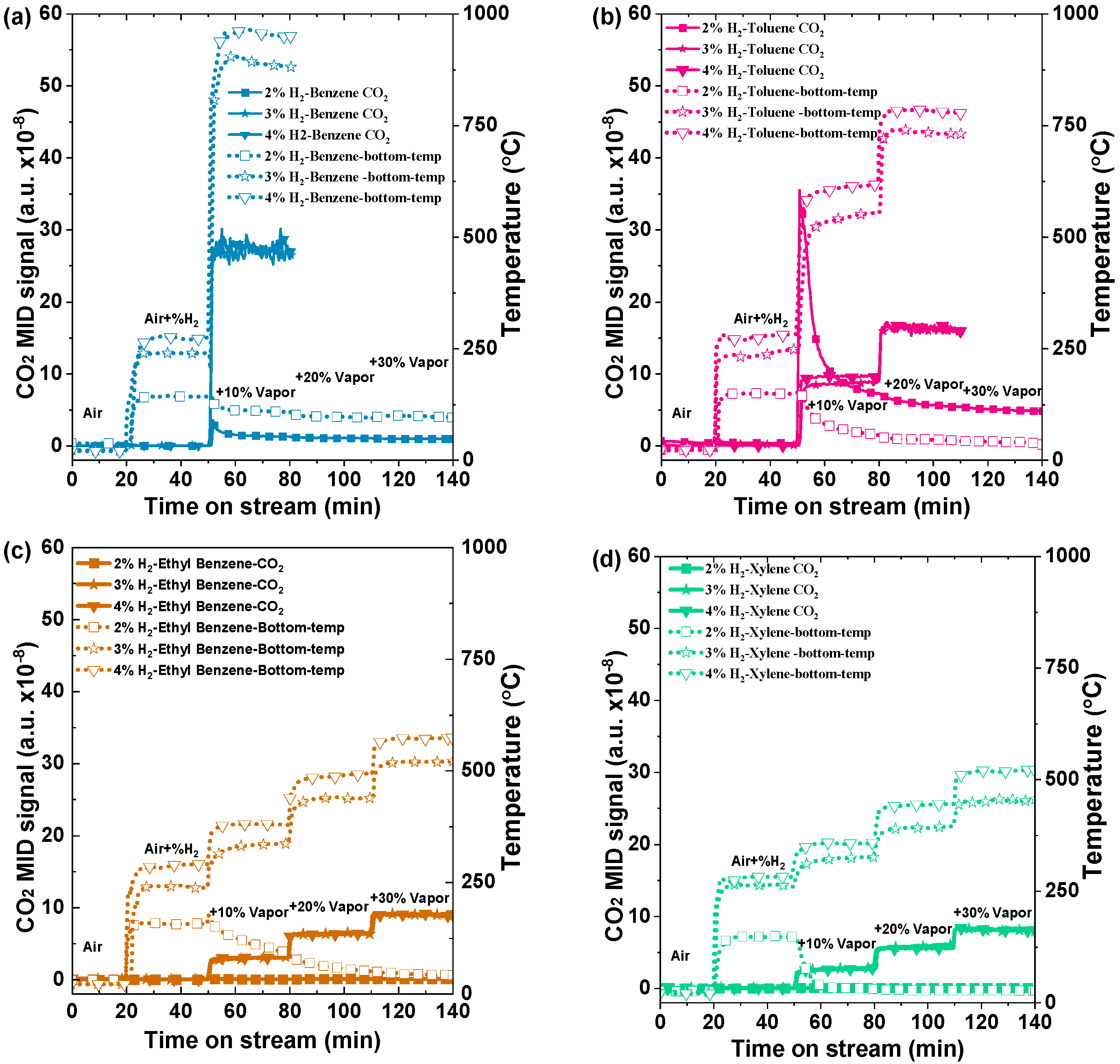
Furthermore, to investigate the impact of hydrogen gas on the aromatic volatile organic compounds (VOCs), various quantities of hydrogen gas (specifically, 2%, 3%, and 4%) were employed, as depicted in Figure 7. The evolution profiles of catalyst bed temperatures and CO2 signals with co-feeding of H2 and VOCs are shown in Figure 7a–d. In all experiments, the background CO2 signals could be obtained within the initial 20 min of airflow at a rate of 1000 mL/min. Initially, the effect of 2% H2 was studied for benzene with 10%, 20%, and 30% of VOCs, and the temperature gradually dropped towards room temperature from the initial 10% concentration insertion to the last 30% insertion. The CO2 signal intensities in stepwise mode also declined at the same time. The unexpected reduction in temperature with increasing benzene content may be attributed to insufficient surface coverage and hydrogen ignition. The presence of these variables may hinder the desired exothermic process, leading to the observed reduction in temperature instead of the anticipated increase. A similar pattern was observed for the other VOCs, including toluene, ethylbenzene, and xylene.
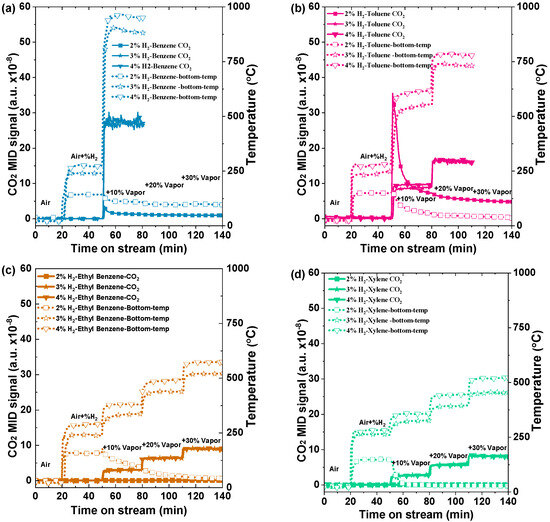
Figure 7.
CO2 formation curves and corresponding catalyst bed temperature profiles of various VOCs with co-combustion of H2 of 2% and 4% (a) benzene, (b) toluene, (c) ethyl benzene, and (d) xylene with a total flow of 1000 mL min−1 over 0.5 g of Pd/Al2O3 catalyst (GHSV: 120,000 mL h−1g−1) and 10%, 20%, and 30% vapor concentrations of VOCs.
The CO2 signal intensities in stepwise mode also decreased simultaneously. For benzene, Figure 7a displays the changes in the catalyst bed temperature and CO2 production as the co-feeding concentration of 3% H2 increases. Preheating to a temperature of 200 °C was achieved by using a concentration of 3% H2, which is higher than 2% H2. It is evident that the addition of 10% benzene vapor and 3% H2 caused the temperature for benzene oxidation to rise to 900 °C, leading to the total combustion of benzene. After infusing 4% H2 with 10% vol benzene, the temperature rose significantly to 950 °C. The sharp increase in temperature and carbon dioxide signals suggest that benzene is undergoing complete co-combustion over the Pd/Al2O3 catalyst [31]. For the 3% and 4% H2 toluene cases, there are distinct variations in both the CO2 levels and temperature profiles, as shown in Figure 7b. The temperatures measured were 550 °C and 720 °C, and 619 °C and 782 °C, which corresponded to hydrogen concentrations of 10% and 20%, respectively, for 3% H2 and 4% H2. These results indicate that the temperature of toluene is slightly lower than that of benzene, presumably due to its lower calorific value. On the other hand, the effect of hydrogen combustion with ethyl benzene and xylene was also conducted. The temperatures 345 °C, 440 °C, and 520 °C correspond to VOC concentrations of 10%, 20%, and 30%, respectively, for 3% H2. Similarly, the temperatures 380 °C, 493 °C, and 573 °C correspond to the same VOC concentrations for 4% H2, as shown in Figure 7c [32]. For xylene, with the effect of 3% and 4% H2, the temperatures associated with the 10%, 20%, and 30% VOC concentrations were about 326 °C, 394 °C, 453 °C and 371 °C, 445 °C, and 522 °C, respectively in (Figure 7d).
Overall, it can be seen that the preheating temperature achieved with 2% hydrogen is below the respective light-off temperatures of benzene (180 °C), toluene (250 °C), ethyl benzene, and xylene (250 °C). Consequently, the initiation of oxidation for these volatile organic compounds is effectively catalyzed through hydrogen co-combustion. Each experiment followed a well-structured protocol to ensure accurate initiation and precise combustion process monitoring. The stability of temperature and CO2 levels confirmed steady-state conditions, and the system returned to baseline values after air was introduced, indicating the successful completion of the catalytic reaction. The same trend was observed for all other aromatic VOCs. These studies demonstrated that hydrogen has the potential to be an efficient enhancer for the catalytic combustion of VOCs [33].
The hydrogen co-combustion on Pd/Al2O3 provides the advantage of onsite heating, which makes hydrogen catalytic combustion possible at room temperature, and the performance of the Pd/Al2O3-spent catalyst can be preserved in the same manner as a fresh sample despite having been subjected to cyclic operation for the combustion of different organic VOCs at very high temperatures. These findings indicate that the fresh and used catalysts exhibit comparable performance, even at temperatures exceeding 900 °C. Thus, the size of the Pd particles does not seem to impact the catalytic activity as the spent catalyst exhibits considerably larger particles than the fresh catalyst. Another benefit of onsite heating is allowing the co-feed volatile organic molecules to actively engage in catalytic combustion when the catalyst bed is heated with flowing hydrogen. It is reasonable to infer that the heat generated played a crucial role in increasing the bed temperature, which in turn facilitated the oxidation reaction of the substance [34,35].
3. Experimental Section
3.1. Materials
Palladium chloride powder (Tianjin Fengchuan Chemical Reagent Technologies Co., Ltd., Tianjin China) > 99.9%, alumina spherical shape powder and hydrochloric acid 36.0~38.0%, benzene 99.9% (Tianjin Fuyu Fine Chemicals, Tianjin, China), toluene 99.9% (Tianjin Fuyu Fine Chemicals), ethyl benzene 99.9% (Tianjin Fuyu Fine Chemicals), and xylene 99.9% (Tianjin Fuyu Fine Chemicals) were used in the experiments.
3.2. Catalyst Preparation
To prepare Pd/Al2O3, 100 g of alumina (spherical shape, 2–3 mm, Tianjin Xidian Chemical Technology, Tianjin, China) was impregnated with 100 mL of PdCl2 (Tianjin Fengchuan chemical reagent Technologies Co., Ltd.; >99.9%) solution containing 10 g/L of Pd. This resulted in a Pd loading of ~1 wt.% on alumina. After drying for 8 h at 120 °C, the impregnated sample was calcined for 5 h at 500 °C at a heating rate of 5 °C/min. The mixture was then reduced in H2 for 2 h at 500 °C at a flow rate of 300 mL/min.
3.3. Characterization
Brunauer–Emmett–Teller (BET) analysis was used to determine the specific surface areas of the fresh and spent catalysts using N2 adsorption at −196 °C on a Micromeritics ASAP 2460 instrument (Norcross, GA, USA). Before evaluation, the samples were degassed at 350 °C for 5 h under vacuum to eliminate any adsorbed species. X-ray diffraction (XRD) patterns were measured by a PANalytical PW 3040/60 X’Pert PRO diffractometer (Malvern, UK), which was powered by a Cu K radiation source (=1.5406 Å) and ran at 40 kV and 40 mA. JEOL-JEM-2100F (Tokyo, Japan) at 200 keV for both scanning and transmission electron microscopy (TEM and STEM) was used to test the morphology and distribution of the elements. An Oxford Instruments ISIS/INCA (Abingdon, UK) energy dispersive X-ray spectroscopy (EDS) system equipped with an Oxford Pentafet Ultrathin Window (UTW) detector was used for energy dispersive X-ray analysis. Using a lacey carbon-coated copper grid, specimens were developed by suspending the powdered sample. X-ray photoelectron spectroscopy (XPS) measurements were performed using an ESCALAB 250 instrument equipped with Al Kα radiation (hν = 1486.6 eV) (Waltham, MA, USA). The binding energy values were calibrated with the C 1s peak set at 284.6 eV.
3.4. Reactor Setup and Performance Test
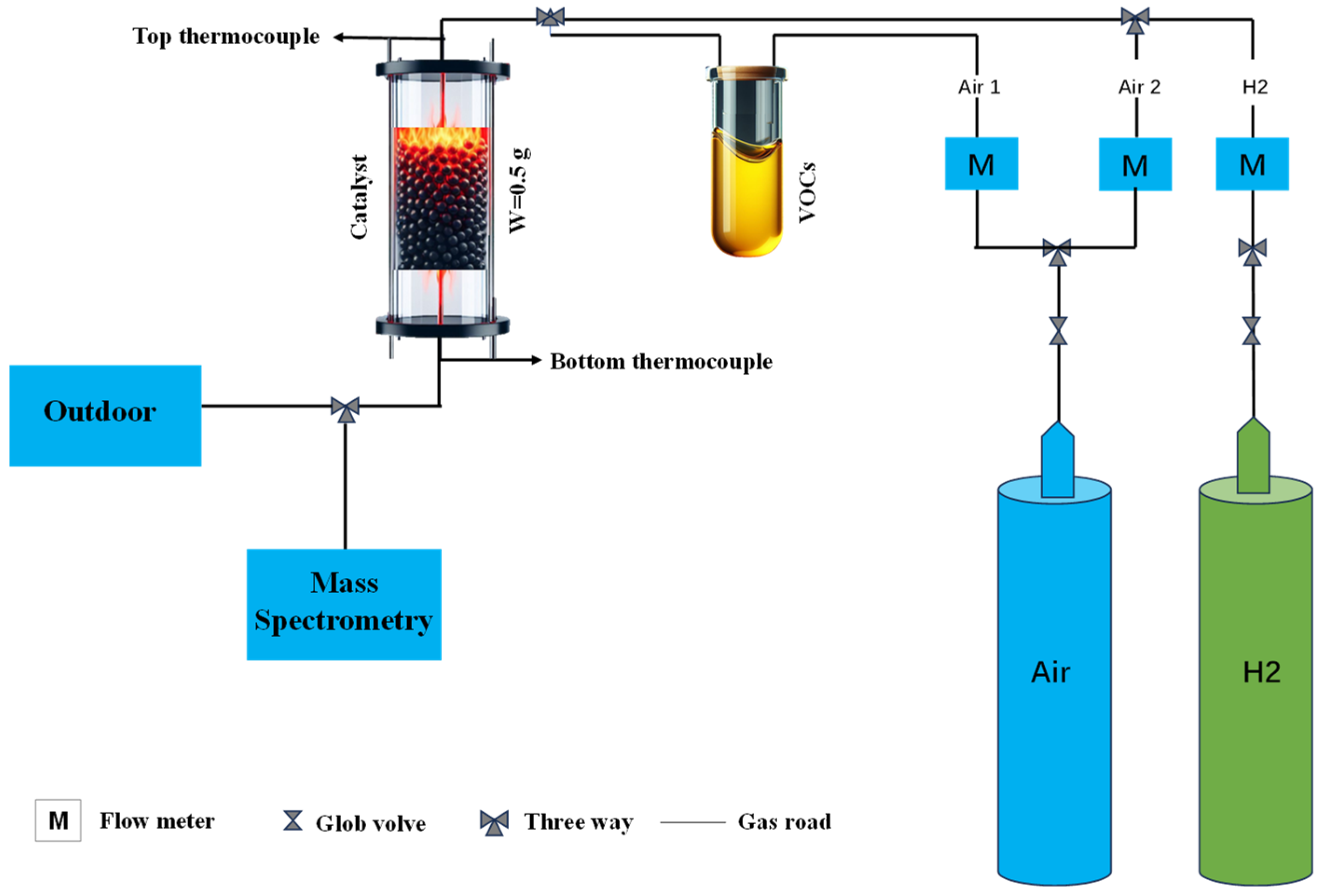
The fixed-bed reactor consisted of a quartz tube 30 cm in length and 10 mm in inner diameter. In the center of the quartz tube, 0.5 g of Pd/Al2O3 catalyst with a volume of 0.78 cm3 was supported by 200 mesh ceramic honeycombs. Scheme 1 shows the packing specifications for the k-type thermocouple used to measure the temperature inside the catalyst bed. The typical molecules for aromatic compounds were determined to be benzene, toluene, ethyl benzene, and xylene. By bubbling the chemical vapors (benzene 99.7%, toluene 99.7%, ethyl benzene 99.7%, and xylene 99.7%; Tianjin Fuyu Fine Chemicals) at flow rates of 100, 200, and 300 mL/min, respectively, VOC emissions were replicated. In light of this, the vapor concentrations in a 1000 mL/min total flow were designated as 10%, 20%, and 30% vapor, respectively (for details, please see Table 1). Hydrogen was supplied from a high-purity hydrogen generator (Beijing Zhonghuipu, SPH-300A, Beijing, China). The flow rates of air and H2 were controlled by calibrated mass flow controllers, and the total flow rate was maintained at 1000 mL/min, i.e., giving an hourly gas space velocity of 120,000 mL/g-h or 77,000 h−1. The reactants and products were analyzed and recorded using a Hiden Analytical mass spectrometer (HPR-20, R&D) (Warrington, UK) by monitoring the signals of H2 (m/z = 2), benzene (m/z = 78), toluene (m/z = 91), ethyl benzene (m/z = 91), xylene (m/z = 91), and CO2 (m/z = 44). Temperature-programed catalytic reactions of VOCs and air over Pd/Al2O3 were conducted to identify the light-off temperatures using an electric heating furnace at a ramp rate of 5 °C/min from room temperature to 750 °C.
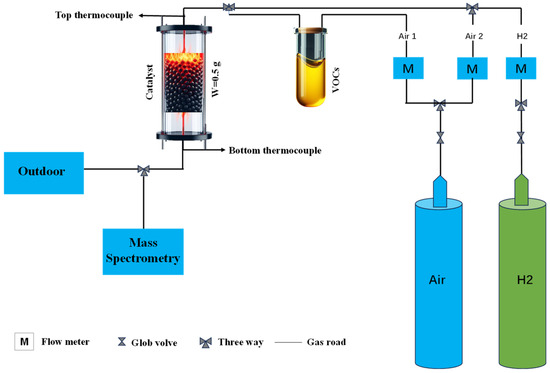
Scheme 1.
Schematic diagram of hydrogen co-combustion of aromatic volatile organic compounds.

Table 1.
Vapor pressures and heat of combustion for various aromatic VOCs and the nominal concentrations and calorific values for mixtures of VOCs and air in different compositions.
4. Conclusions
In conclusion, a detailed analysis has been conducted on the catalytic oxidation of VOCs over a Pd/Al2O3 catalyst, specifically using hydrogen co-combustion as a heating source, which is completely different from the conventional electric heating approach. Remarkably, the catalytic combustion of hydrogen over the Pd/Al2O3 catalyst exhibits the capability to initiate at room temperature and elevate the catalyst bed to temperatures conducive to the complete oxidation of aromatic VOCs, such as benzene, toluene, ethyl benzene, and xylene. Notably, this process achieves oxidation with hydrogen concentrations below the low explosive limit of 4%. Consequently, this streamlined and efficient VOC elimination system obviates the need for energy-intensive external electric heating, offering a sustainable and environmentally conscious route for using green hydrogen derived from renewable energy sources. Our dual investigation into the catalytic combustion of aromatic VOCs with hydrogen enhancement and electric heating catalytic combustion using fresh and spent catalysts demonstrates two critical findings. First, hydrogen proves to be a highly effective combustion enhancer, offering cleaner and more efficient combustion processes for aromatic VOCs, contributing significantly to emission reduction and industrial sustainability. Second, the enduring performance of spent catalysts, comparable to their fresh counterparts, reveals a remarkable potential for cost-effective and sustainable industrial applications. These findings not only enhance our understanding of catalytic combustion but also offer tangible solutions for reducing aromatic VOC emissions and improving the sustainability of industrial processes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14090563/s1, Figure S1: HRTEM images for fresh (a–c) and spent (d–f) Pd/Al2O3 catalysts. Figure S2: Temperature of catalyst bed for 2%, 3%, and 4% H2.
Author Contributions
L.U. and S.M. synthesized the catalysts, performed most of the experiments, and collected and analyzed the data. L.C. and J.-C.Z. established the reaction setup and validated the concept. P.R.M. revised the manuscript. W.-Z.L. designed this study and supervised the project. All authors contributed to the general discussion and co-wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the “Transformational Technologies for Clean Energy and Demonstration”, Strategic Priority Research Program of the Chinese Academy of Sciences (XDA21040200).
Data Availability Statement
The data presented in this article are either available in the main manuscript or can be provided upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Wang, Y.; Yao, C.; Cao, Y.; Zhang, C.; Tang, W. Confined nanoreactor for synthesis of MnCe composite oxide nanowires with enhanced catalytic activity towards aromatic VOCs oxidation. Ceram. Int. 2023, 49, 1137–1147. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Xu, W.; Xu, Z.; Jia, H.; Chen, J. Homogeneous introduction of CeOy into MnOx-based catalyst for oxidation of aromatic VOCs. Appl. Catal. B Environ. 2018, 224, 825–835. [Google Scholar] [CrossRef]
- Tang, W.; Wang, S.; Xiao, W.; Du, S.; Lu, X.; Hoang, S.; Ding, J.; Gao, P.-X. Pre-surface leached cordierite honeycombs for MnxCo3-xO4 nano-sheet array integration with enhanced hydrocarbons combustion. Catal. Today 2019, 320, 196–203. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, S.; Huang, Z.; Xu, H.; Shen, W. Insights into enhancing SO2 tolerance for catalytic combustion of toluene over sulfated CeZrOx supported platinum catalysts. Colloids Surf. A Physicochem. Eng. Asp. 2023, 669, 131539. [Google Scholar] [CrossRef]
- Bi, F.; Zhao, Z.; Yang, Y.; Gao, W.; Liu, N.; Huang, Y.; Zhang, X. Chlorine-coordinated Pd single atom enhanced the chlorine resistance for volatile organic compound degradation: Mechanism study. Environ. Sci. Technol. 2022, 56, 17321–17330. [Google Scholar] [CrossRef] [PubMed]
- Odoom-Wubah, T.; Li, Q.; Adilov, I.; Huang, J.; Li, Q. Towards efficient Pd/Mn3O4 catalyst with enhanced acidic sites and low temperature reducibility for Benzene abatement. J. Mol. Catal. 2019, 477, 110558. [Google Scholar] [CrossRef]
- Yang, X.; Ma, X.; Yu, X.; Ge, M. Exploration of strong metal-support interaction in zirconia supported catalysts for toluene oxidation. Appl. Catal. B Environ. 2020, 263, 118355. [Google Scholar] [CrossRef]
- Kim, S.-I.; Im, M.; Cho, E.; Jang, H.; Jang, S.Y.; Kim, D.W.; Kim, K.W.; Heo, I.; Kim, Y.J.; Lee, J.H. Effects of thermal aging on the electronic and structural properties of Pt-Pd and toluene oxidation activity. Sci. Total Environ. 2022, 847, 157482. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Liang, C.; Li, S.; Liu, X.; Du, X.; Yang, K.; Zhao, J.; Yu, Q.; Zhai, Y. Regulating CeO2 morphologies on the catalytic oxidation of toluene at lower temperature: A study of the structure–activity relationship. J. Catal. 2023, 418, 151–162. [Google Scholar] [CrossRef]
- Sorrels, J.L.; Baynham, A.; Randall, D.; Hancy, C. Incinerators and Oxidizers; United States Environmental Protection Agency: Washington, DC, USA, 2017.
- Yang, D.; Dong, F.; Han, W.; Zhang, J.; Tang, Z. Significant Enhanced SO2 Resistance of Pt/SiO2 Catalysts by Building the Ultrathin Metal Oxide Shell for Benzene Catalytic Combustion. ACS Appl. Mater. Interfaces 2023, 15, 42541–42556. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; He, H.; Yu, Y.; Yuan, T.; Tian, Z.; Wang, J.; Li, Y. Effect of the pressure on the catalytic oxidation of volatile organic compounds over Ag/Al2O3 catalyst. Appl. Catal. B Environ. 2009, 89, 659–664. [Google Scholar] [CrossRef]
- Doggali, P.; Teraoka, Y.; Mungse, P.; Shah, I.K.; Rayalu, S.; Labhsetwar, N. Combustion of volatile organic compounds over Cu–Mn based mixed oxide type catalysts supported on mesoporous Al2O3, TiO2 and ZrO2. J. Mol. Catal. A Chem. 2012, 358, 23–30. [Google Scholar] [CrossRef]
- He, Z.; He, Z.; Wang, D.; Bo, Q.; Fan, T.; Jiang, Y. Mo-modified Pd/Al2O3 catalysts for benzene catalytic combustion. J. Environ. Sci. 2014, 26, 1481–1487. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Di, S.; Xu, L.; Wu, Z.; Yao, S. Construction of Pt-MnO2 interface with strong electron coupling effect for plasma catalytic oxidation of aromatic VOCs. Colloids Surf. A Physicochem. Eng. Asp. 2023, 665, 131248. [Google Scholar] [CrossRef]
- Lv, M.; Song, S.; Verma, P.; Wen, M. Hollow mesoporous aluminosilicate spheres imbedded with Pd nanoparticles for high performance toluene combustion. Catal. Today 2023, 410, 135–142. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Zhu, T.; Ning, R. Catalytic oxidation of chlorobenzene over noble metals (Pd, Pt, Ru, Rh) and the distributions of polychlorinated by-products. J. Hazard. Mater. 2019, 363, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Yang, W.; Liu, M.; Cen, K. Effects of hydrogen addition on methane catalytic combustion in a microtube. Int. J. Hydrogen Energy 2007, 32, 1286–1293. [Google Scholar] [CrossRef]
- Karagiannidis, S.; Mantzaras, J. Numerical investigation on the hydrogen-assisted start-up of methane-fueled, catalytic microreactors. Flow Turbul. Combus. 2012, 89, 215–230. [Google Scholar] [CrossRef]
- Seshadri, V.; Kaisare, N.S. Simulation of hydrogen and hydrogen-assisted propane ignition in pt catalyzed microchannel. Combust. Flame 2010, 157, 2051–2062. [Google Scholar] [CrossRef]
- Moretti, E.C. Reduce VOC and HAP emissions. Chem. Eng. Prog. 2002, 98, 30–40. [Google Scholar]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B Environ. 2021, 281, 119447. [Google Scholar] [CrossRef]
- Carabineiro, S.; Chen, X.; Martynyuk, O.; Bogdanchikova, N.; Avalos-Borja, M.; Pestryakov, A.; Tavares, P.; Órfão, J.; Pereira, M.F.R.; Figueiredo, J.L. Gold supported on metal oxides for volatile organic compounds total oxidation. Catal. Today 2015, 244, 103–114. [Google Scholar] [CrossRef]
- Chen, M.; Qi, L.; Fan, L.; Zhou, R.; Zheng, X. Zirconium-pillared montmorillonite and their application in supported palladium catalysts for volatile organic compounds purification. Mater. Lett. 2008, 62, 3646–3648. [Google Scholar] [CrossRef]
- Santos, V.P.; Carabineiro, S.A.; Tavares, P.B.; Pereira, M.F.; Órfão, J.J.; Figueiredo, J.L. Oxidation of CO, ethanol and toluene over TiO2 supported noble metal catalysts. Appl. Catal. B Environ. 2010, 99, 198–205. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Peng, J.; Wang, S. Performance and characterization of supported metal catalysts for complete oxidation of formaldehyde at low temperatures. Appl. Catal. B Environ. 2007, 73, 282–291. [Google Scholar] [CrossRef]
- Li, J.W.; Pan, K.L.; Yu, S.J.; Yan, S.Y.; Chang, M.B. Removal of formaldehyde over MnxCe1−xO2 catalysts: Thermal catalytic oxidation versus ozone catalytic oxidation. J. Environ. Sci. 2014, 26, 2546–2553. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Ge, S.; Yang, H.; Liu, M.; Liu, M. Study on anti-sulfur dioxide poisoning of palladium-based catalyst for toluene catalytic combustion. Int. J. Hydrogen Energy 2021, 46, 6329–6340. [Google Scholar] [CrossRef]
- Murata, K.; Ohyama, J.; Yamamoto, Y.; Arai, S.; Satsuma, A. Methane combustion over Pd/Al2O3 catalysts in the presence of water: Effects of Pd particle size and alumina crystalline phase. ACS Catal. 2020, 10, 8149–8156. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Properties and performance of Pd based catalysts for catalytic oxidation of volatile organic compounds. Appl. Catal. B Environ. 2009, 9, 429–436. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Deng, J.; Jing, L.; Hao, X.; Zhang, X.; Yu, X.; Dai, H. PdPtVOx/CeO2−ZrO2: Highly efficient catalysts with good sulfur dioxide-poisoning reversibility for the oxidative removal of ethylbenzene. J. Environ. Sci. 2024, 138, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yu, J.; Lee, S.; Tahmasebi, A.; Jeon, C.H.; Lucas, J. Advances in catalytic hydrogen combustion research: Catalysts, mechanism, kinetics, and reactor designs. Int. J. Hydrogen Energy 2021, 46, 40073–40104. [Google Scholar] [CrossRef]
- Kozhukhova, A.; Du Preez, S.; Shuro, I.; Bessarabov, D. Development of a low purity aluminum alloy (Al6082) anodization process and its application as a platinum-based catalyst in catalytic hydrogen combustion. Surf. Coat. Technol. 2020, 404, 126483. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Malakhov, A.A.; Bessarabov, D.G. A thermally conductive Pt/AAO catalyst for hydrogen passive autocatalytic recombination. Catalysts 2021, 11, 491. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).