Catalytic Valorization of Organic Solid Waste: A Pilot-Scale Run of Sugarcane Bagasse
Abstract
1. Introduction
2. Results and Discussion
2.1. Properties of Feedstock
2.2. Overall Reaction Performance
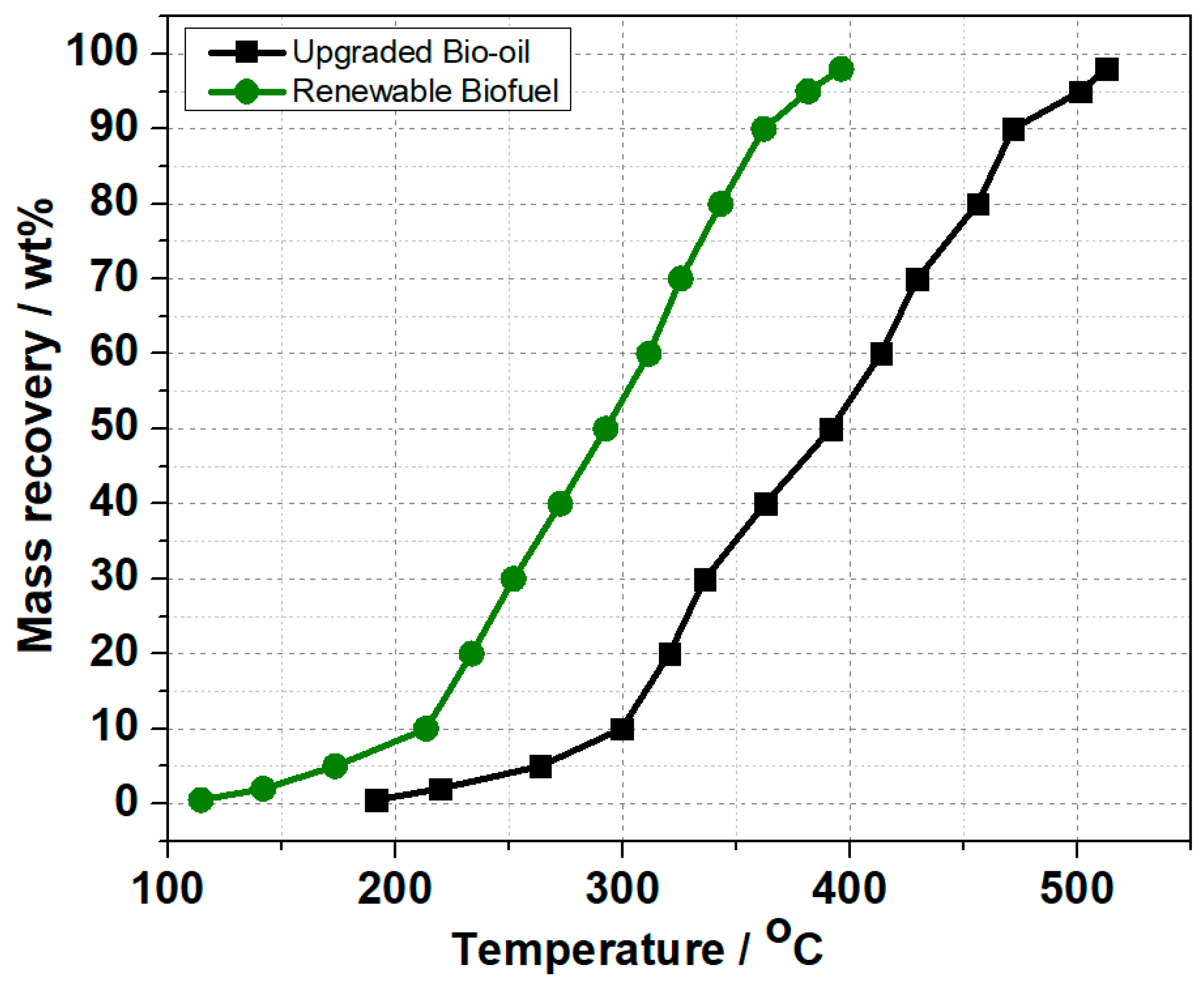
2.3. Comprehensive Analysis of the Upgraded Bio-Oil and Renewable Biofuel
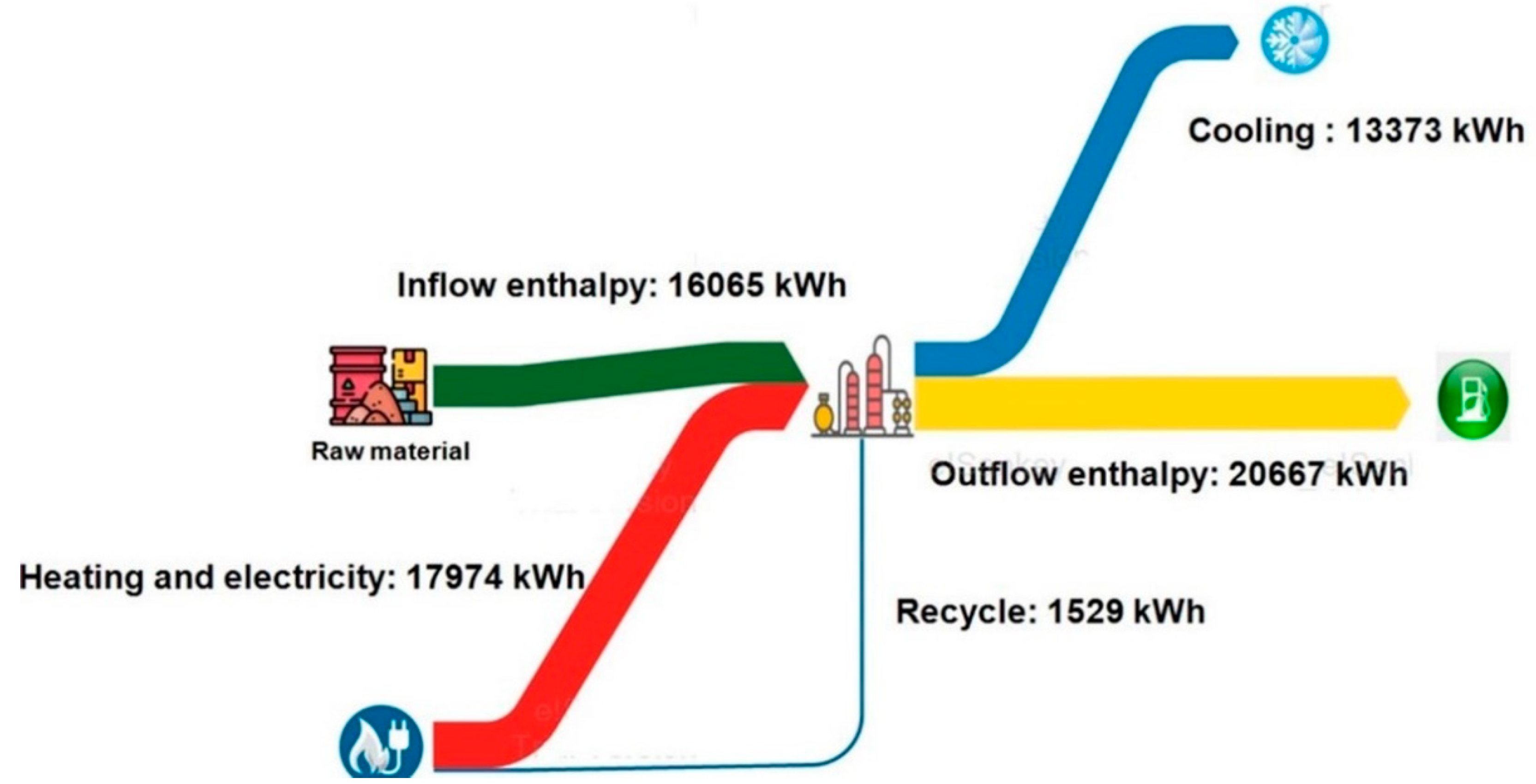
2.4. Techno-Economic Analysis and Energy Assessment
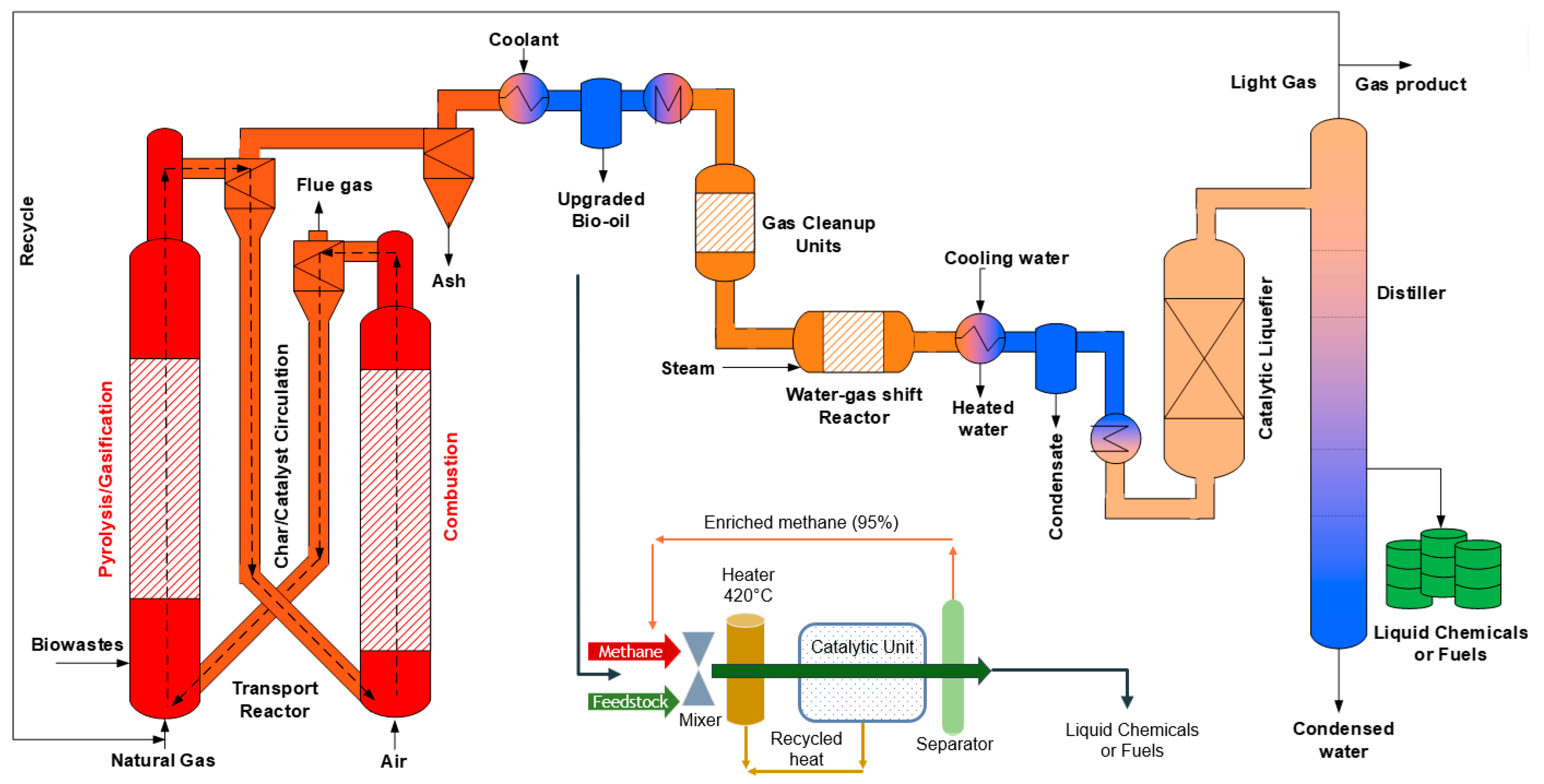
3. Experimental Process
3.1. Process Unit
3.2. Catalysts Preparation
3.3. Reaction Process
3.4. Feedstock and Products Characterizations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.K.; Liu, T.; Duan, Y.; et al. Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, H.; Yi, W.; Lei, H.; Liu, H.; Wang, W.; Ruan, R. Biocomposites from Organic Solid Wastes Derived Biochars: A Review. Materials 2020, 13, 3923. [Google Scholar] [CrossRef]
- Soobhany, N. Insight into the recovery of nutrients from organic solid waste through biochemical conversion processes for fertilizer production: A review. J. Clean. Prod. 2019, 241, 118413. [Google Scholar] [CrossRef]
- Xu, H.; Song, Y.; Zhang, Y.; Song, H. Organic solid waste upgrading under natural gas for valuable liquid products formation: Pilot demonstration of a highly integrated catalytic process. Bioresour. Technol. 2022, 346, 126645. [Google Scholar] [CrossRef] [PubMed]
- Gaeta-Bernardi, A.; Parente, V. Organic municipal solid waste (MSW) as feedstock for biodiesel production: A financial feasibility analysis. Renew. Energy 2016, 86, 1422–1432. [Google Scholar] [CrossRef]
- Paes, L.A.B.; Bezerra, B.S.; Deus, R.M.; Jugend, D.; Battistelle, R.A.G. Organic solid waste management in a circular economy perspective–A systematic review and SWOT analysis. J. Clean. Prod. 2019, 239, 118086. [Google Scholar] [CrossRef]
- Trivedi, P.; Malina, R.; Barrett, S.R.H. Environmental and economic tradeoffs of using corn stover for liquid fuels and power production. Energy Environ. Sci. 2015, 8, 1428–1437. [Google Scholar] [CrossRef]
- He, Z.; Zhao, A.; Liu, S.; Chen, Y.; Liu, J.; Zhao, W.; Yin, M.; Dong, Q.; Zhang, J.; Zhang, G.; et al. Preparation of nitrogen-containing chemicals from lignocellulosic biomass and nitrogen-rich organic solid waste by pyrolysis: Characteristics, reaction mechanisms, and feedstock interactions. Chem. Eng. J. 2024, 496, 153793. [Google Scholar] [CrossRef]
- Nogueira, K.M.V.; Mendes, V.; Carraro, C.B.; Taveira, I.C.; Oshiquiri, L.H.; Gupta, V.K.; Silva, R.N. Sugar transporters from industrial fungi: Key to improving second-generation ethanol production. Renew. Sustain. Energy Rev. 2020, 131, 109991. [Google Scholar] [CrossRef]
- Kumar, R.; Ghosh, A.K.; Pal, P. Synergy of biofuel production with waste remediation along with value-added co-products recovery through microalgae cultivation: A review of membrane-integrated green approach. Sci. Total Environ. 2020, 698, 134169. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Progress of the applications of bio-oil. Renew. Sustain. Energy Rev. 2020, 134, 110124. [Google Scholar] [CrossRef]
- Castro, R.E.N.; Alves, R.M.B.; Nascimento, C.A.O. Assessing the sugarcane bagasse and straw as a biofuel to propel light vehicles. Sustain. Energy Fuels 2021, 5, 2563. [Google Scholar] [CrossRef]
- Konde, K.S.; Nagarajan, S.; Kumar, V.; Patil, S.V.; Ranade, V.V. Sugarcane bagasse based biorefineries in India: Potential and challenges. Sustain. Energy Fuels 2021, 5, 52–78. [Google Scholar] [CrossRef]
- Miura, T.; Lee, S.H.; Inoue, S.; Endo, T. Improvement of enzymatic saccharification of sugarcane bagasse by dilute-alkali-catalyzed hydrothermal treatment and subsequent disk milling. Bioresour. Technol. 2012, 105, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.; Xu, H.; Li, Y.; Li, Z.; Li, W.; Meng, S.; Chang, L.Y.; Liu, L.; Song, H. Methane-assisted Selective Bio-oil Deoxygenation for High-Quality Renewable Fuel Production: A Rational Catalyst Design and Mechanistic Study. Chem. Eng. J. 2023, 475, 146052. [Google Scholar] [CrossRef]
- Jarvis, J.; Li, Z.; Meng, S.; Song, H. Methane Assisted Catalyst Synthesis and Catalytic Conversion of Fatty Acids. J. Mater. Chem. A 2022, 10, 18671–18678. [Google Scholar] [CrossRef]
- Meng, S.; Li, W.; Li, Z.; Song, H. Non-thermal Plasma Assisted Non-oxidative Methane Liquefaction for Fuel Production at Near Ambient Conditions. Catal. Sci. Technol. 2023, 13, 4665–4672. [Google Scholar] [CrossRef]
- Xu, H.; Cao, M.; Li, Z.; Li, W.; Meng, S.; Song, H. Production of Low Carbon Number Olefins from Natural Gas: Methane-involved Catalytic Non-Oxidative Propane Dehydrogenation. Chem. Eng. J. 2023, 454, 140508. [Google Scholar] [CrossRef]
- Shimizu, K.; Ueda, W.; Song, H. Introduction to integrated approaches for methane activation. Catal. Sci. Technol. 2024, 14, 2972. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Li, Z.; Song, Y.; Zhang, Y.; Song, H. Methane-assisted waste cooking oil conversion for renewable fuel production. Fuel 2022, 311, 122613. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Li, Z.; Meng, S.; Song, H. Catalytic methanotreating of vegetable oil: A pathway to Second-generation biodiesel. Fuel 2022, 311, 122504. [Google Scholar] [CrossRef]
- He, P.; Song, H. Catalytic Conversion of Biomass by Natural Gas for Oil Quality Upgrading. Ind. Eng. Chem. Res. 2014, 53, 15862–15870. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Kinage, A.K.; Choudhary, T.V. Low-temperature nonoxidative activation of methane over H-galloaluminosilicate (MFI) zeolite. Science 1997, 275, 1286–1288. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Sawada, H. Conversion of methane into higher hydrocarbons in the presence of ethylene over H-ZSM-5 loaded with silver cations. Phys. Chem. Chem. Phys. 2002, 4, 3919–3923. [Google Scholar] [CrossRef]
- Anunziata, O.A.; Gonzalez, G.V.; Pierella, L.B. Catalytic activation of methane using n-pentane as co-reactant over Zn/H-ZSM-11 zeolite. Catal. Lett. 2003, 87, 167–171. [Google Scholar] [CrossRef]
- Anunziata, O.A.; Mercado, G.G.; Pierella, L.B. Improvement of methane activation using n-hexane as co-reactant over Zn/HZSM-11 zeolite. Catal. Commun. 2004, 5, 401–405. [Google Scholar] [CrossRef]
- Guo, A.; Zhou, Y.; Chen, K.; Xue, Z.; Wang, Z.; Song, H. Co-processing of Vacuum Residue/Fraction Oil Blends: Effect of Fraction Oils Recycle on the Stability of Coking Feedstock. J. Anal. Appl. Pyrolysis 2014, 109, 109–115. [Google Scholar] [CrossRef]
- He, P.; Shan, W.; Xiao, Y.; Song, H. Performance of Zn/ZSM-5 for In Situ Catalytic Upgrading of Pyrolysis Bio-oil by Methane. Top. Catal. 2016, 59, 86–93. [Google Scholar] [CrossRef]
- Xiao, Y.; He, P.; Cheng, W.; Liu, J.; Shan, W.; Song, H. Converting Solid Wastes into Liquid Fuel using a Novel Methanolysis Process. Waste Manag. 2016, 49, 304–310. [Google Scholar] [CrossRef]
- Wang, A.; He, P.; Yung, M.; Zeng, H.; Qian, H.; Song, H. Catalytic Co-Aromatization of Ethanol and Methane. Appl. Catal. B Environ. 2016, 198, 480–492. [Google Scholar] [CrossRef]
- He, P.; Zhao, L.; Song, H. Bitumen Partial Upgrading over Mo/ZSM-5 under Methane Environment: Methane Participation Investigation. Appl. Catal. B Environ. 2017, 201, 438–450. [Google Scholar] [CrossRef]
- Austin, D.; Wang, A.; He, P.; Qian, H.; Zeng, H.; Song, H. Catalytic Valorization of Biomass Derived Glycerol under Methane: Effect of Catalyst Synthesis Method. Fuel 2018, 216, 218–226. [Google Scholar] [CrossRef]
- Wang, A.; Austin, D.; Karmakar, A.; Bernard, G.; Michaelis, V.; Yung, M.; Zeng, H.; Song, H. Methane Upgrading of Acetic Acid as a Model Compound for Biomass Derived Liquid over Modified Zeolite Catalyst. ACS Catal. 2017, 7, 3681–3692. [Google Scholar] [CrossRef]
- Wang, A.; Austin, D.; Qian, H.; Zeng, H.; Song, H. Catalytic Valorization of Furfural Under Methane Environment. ACS Sustain. Chem. Eng. 2018, 6, 8891–8903. [Google Scholar] [CrossRef]
- Wang, A.; Austin, D.; Song, H. Catalytic Upgrading of Biomass and its Model Compounds for Fuel Production. Curr. Org. Chem. 2019, 23, 517–529. [Google Scholar] [CrossRef]
- Wang, A.; Austin, D.; He, P.; Mao, X.; Zeng, H.; Song, H. Direct Catalytic Co-conversion of Cellulose and Methane to Renewable Petrochemicals. Catal. Sci. Technol. 2018, 8, 5632–5645. [Google Scholar] [CrossRef]
- Austin, D.; Wang, A.; Harrhy, J.; Mao, X.; Zeng, H.; Song, H. Catalytic Aromatization of Acetone as a Model Compound for Biomass Derived Oil under Methane Environment. Catal. Sci. Technol. 2018, 8, 5104–5114. [Google Scholar] [CrossRef]
- Wang, A.; Song, H. Maximizing the Production of Aromatic Hydrocarbons from Lignin Conversion by Coupling Methane Activation. Bioresour. Technol. 2018, 268, 505–513. [Google Scholar] [CrossRef]
- Wang, A.; Austin, D.; He, P.; Ha, M.; Michaelis, V.; Liu, L.; Qian, H.; Zeng, H.; Song, H. Mechanistic Investigation on Catalytic Deoxygenation of Phenol as a Model Compound of Biocrude Under Methane. ACS Sustain. Chem. Eng. 2019, 7, 1512–1523. [Google Scholar] [CrossRef]
- Wang, A.; Austin, D.; Song, H. Investigations of thermochemical upgrading of biomass and its model compounds: Opportunities for methane utilization. Fuel 2019, 246, 443–453. [Google Scholar] [CrossRef]
- Peng, H.; Wang, A.; He, P.; Harrhy, J.; Meng, S.; Song, H. Solvent-free catalytic conversion of xylose with methane to aromatics over Zn-Cr modified zeolite catalyst. Fuel 2019, 253, 988–996. [Google Scholar] [CrossRef]

| Proximate Analysis (wt.%) | Heating Value (MJ/kg) | Ultimate Analysis (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Moisture | Volatile Matter | Fixed Carbon | Ash | C | H | N | S | O | |
| 5.22 | 82.64 | 8.35 | 3.79 | 24.5 | 46.55 | 5.92 | 0.12 | 0.09 | 47.32 |
| Overall Mass Balance (%) | Char Yield (%) | Gas Yield (%) | Liquid Yield (%) |
|---|---|---|---|
| 94.5 | 12.5 | 21.5 | 60.5 |
| Properties | Upgraded Bio-Oil | Renewable Fuel |
|---|---|---|
| Basic Kinematic viscosity (mm2/s, 40 °C) | 22.23 | 7.126 |
| Kinematic viscosity (mm2/s, 100 °C) | 3.98 | 2.256 |
| Viscosity index | 50 | 134 |
| Density (kg/m3, 20 °C) | 915.6 | 855.4 |
| API (o) | 22.6 | 33.4 |
| Flashpoint (open cup, °C) | 168 | 94 |
| Freezing point (°C) | −2 | −23 |
| Pour point (°C) | 3 | −18 |
| Cetane number | 26 | 51 |
| Heating value (MJ/kg) | 29.85 | 44.57 |
| Compositional | ||
| Saturated (wt.%) | 80.25 | 75.48 |
| Aromatics (wt.%) | 18.5 | 24.51 |
| Polar (Resin + Asphaltene, wt.%) | 0.8 | 0.01 |
| TAN (mg KOH/g) | 8.2 | 0.01 |
| Water content (wt.%) | 1.17 | Trace amount |
| Carbon residue (wt.%) | 0.25 | 0.10 |
| Ash content (wt.%) | 0.15 | 0.001 |
| Organochlorine content (ppm) | <1.0 | <1.0 |
| Elemental analysis | ||
| N (wt.%) | 0.25 | 0.0036 |
| O (wt.%) | 9.7 | 0.02 |
| S (wt.%) | 0.17 | 0.06 |
| Ca (ppm) | 10.5 | 3.2 |
| K (ppm) | 41.2 | 5.7 |
| Na (ppm) | 6.5 | 0.5 |
| Mg (ppm) | 15.2 | 0.3 |
| P (ppm) | 1.5 | 1.0 |
| Distillates | Upgraded Bio-Oil | Renewable Biofuel |
|---|---|---|
| Naphtha (wt.%) (<180 °C) | 0 | 6 |
| Medium distillate (wt.%) (180–270 °C) | 6 | 33 |
| Gas oil (wt.%) (270–425 °C) | 61 | 61 |
| Heavy residue (wt.%) (>425 °C) | 33 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Omidkar, A.; Song, H. Catalytic Valorization of Organic Solid Waste: A Pilot-Scale Run of Sugarcane Bagasse. Catalysts 2024, 14, 568. https://doi.org/10.3390/catal14090568
Li Z, Omidkar A, Song H. Catalytic Valorization of Organic Solid Waste: A Pilot-Scale Run of Sugarcane Bagasse. Catalysts. 2024; 14(9):568. https://doi.org/10.3390/catal14090568
Chicago/Turabian StyleLi, Zhaofei, Ali Omidkar, and Hua Song. 2024. "Catalytic Valorization of Organic Solid Waste: A Pilot-Scale Run of Sugarcane Bagasse" Catalysts 14, no. 9: 568. https://doi.org/10.3390/catal14090568
APA StyleLi, Z., Omidkar, A., & Song, H. (2024). Catalytic Valorization of Organic Solid Waste: A Pilot-Scale Run of Sugarcane Bagasse. Catalysts, 14(9), 568. https://doi.org/10.3390/catal14090568








