Research Progress of Pt-Based Catalysts toward Cathodic Oxygen Reduction Reactions for Proton Exchange Membrane Fuel Cells
Abstract
:1. Introduction

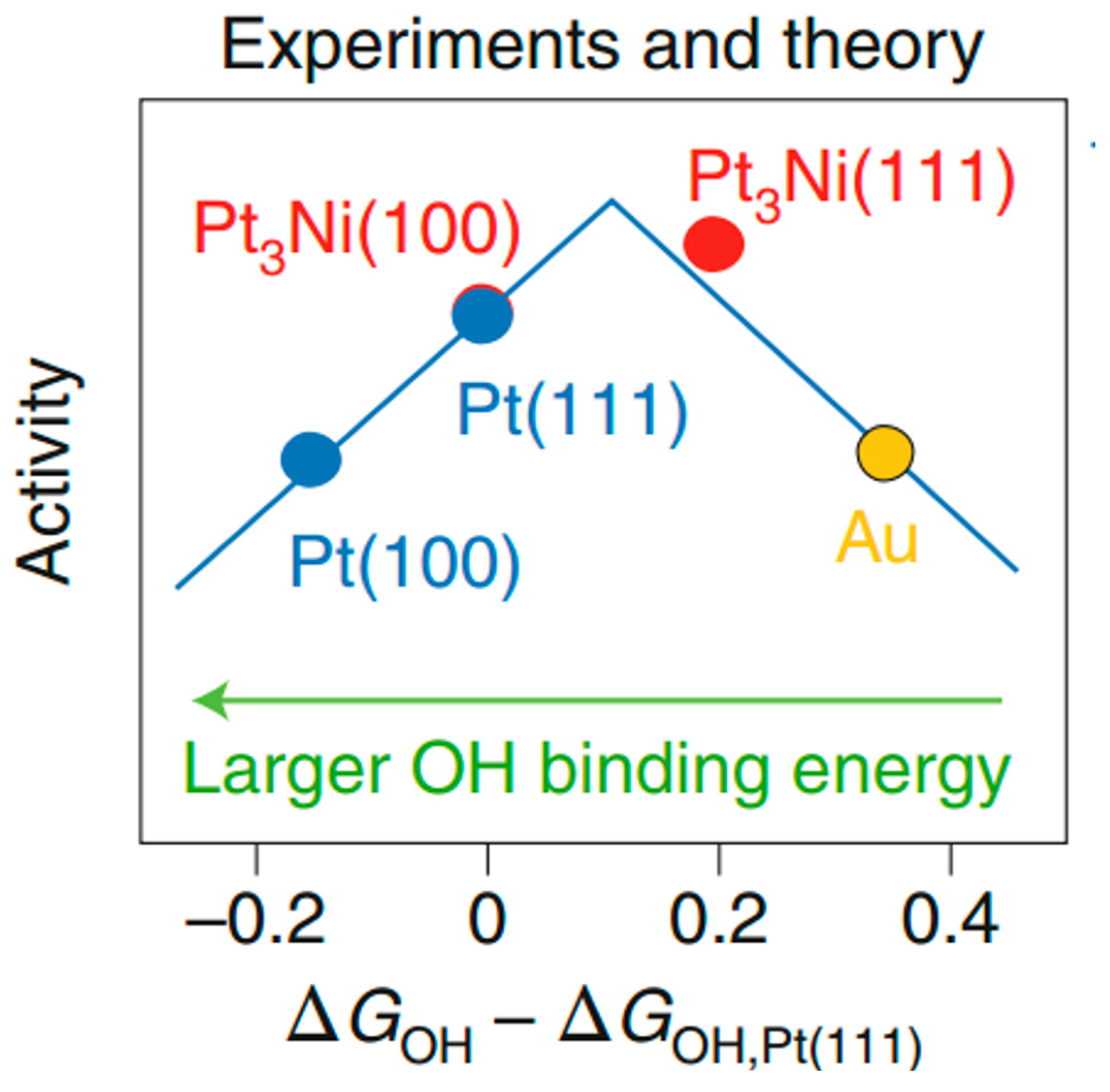
2. Pt-Based Alloy Catalysts
3. Pt-Based Intermetallic Catalysts

- During synthesis and preparation, there may be some problems in the high-temperature annealing process, such as the aggregation of nanoparticles, the difficulty in forming a core–shell structure for some transition metals (such as Fe), and trouble in controlling the shape of intermetallic compound nanocrystals during synthesis. Researchers need to explore the formation mechanism of nanocrystals from the perspective of reaction kinetics and thermodynamics, understand the relationship between surface structure and catalytic performance at the atomic scale, and develop more low-temperature annealing methods on this basis. In the future, adding low-melting metals may be the direction for structural ordering under milder conditions.
- Theoretical calculations can be used to further guide the determination of the morphology/structure of ordered intermetallic compounds and the study of the kinetic process of the catalyst surface.
- The reaction process can be monitored using advanced characterization techniques, such as in situ infrared spectroscopy (FTIRS), transmission electron microscopy (TEM), X-ray diffraction (XRD), and synchrotron radiation (XAFS) in in situ characterization techniques so as to further and more thoroughly study the reaction mechanism and deactivation mechanism.
4. Pt-Based High-Entropy Alloy Catalysts
- The reaction mechanism and pathway of the catalyst have not been fully clarified; for example, the unique reaction mechanism of the cocktail effect and the connection between lattice distortion and activation barrier, etc., still need to be further studied. In addition, the reaction mechanism of a high-entropy alloy catalyst from a disordered to ordered structure has still not been explored. Current research often uses simple models to illustrate complex processes. Therefore, more advanced in situ/operational characterization methods, such as operando TEM and SEM techniques, are needed to understand the structure–activity relationship of HEA and to guide the design of catalysts [148,168].
- The rational design of high-entropy alloy catalysts is also a challenge. The active centers of Pt-based HEAs are composed of Pt and its surrounding atoms, but there are many possible atomic arrangements of Pt atoms. It is challenging to identify the active center of HEAs and calculate the ideal composition ratio due to the various coordination environment that each Pt atom has. Therefore, the design and preparation of a catalyst structure and composition require more advanced computer technology to assist in judging the reaction center and predicting the results. In the future, with the development of advanced methods such as machine learning and statistical analysis, it is expected that we can accurately and efficiently predict and design the composition and performance of high-entropy alloy catalysts [141,169,170].
- Common synthesis methods and routes have low efficiency. However, it is well known that the development of efficient synthesis methods and routes to improve the yield is the key to HEA catalysts from experimental research to large-scale industrial production. At present, the synthesis reaction of an HEA catalyst usually needs to be carried out under a high temperature, high pressure, and inert gas, which requires high experimental equipment and conditions. For example, the fast-moving bed pyrolysis method requires rapid heating and cooling in a specific instrument to uniformly disperse the catalyst at high temperatures with very small nanoparticles. Although electrochemical deposition synthesis can solve this problem well and control the reaction by adjusting the applied voltage at room temperature, the obtained materials are unevenly distributed and cannot be mass-produced. Therefore, efficient and controllable multi-component catalyst synthesis methods still need to be developed [171,172].
5. Coupled Low-Pt and PGM-Free Catalysts
- The complex structure makes it difficult to detect the number of active sites accurately and quantitatively. At present, there is a deviation in the number of active sites of PGM-free catalysts detected by Mössbauer spectroscopy, CO chemisorption, and other methods. Fourier transform alternating current voltammetry was reported by Rifael Z et al. [205]; it may be a promising method for accurately measuring the density of PGM-free active sites in the future. Additionally, the number of active sites can be determined by advanced artificial intelligence and computers to guide catalyst design [206,207,208,209].
- The coupling mechanism of coupled low-Pt and PGM-free catalysts has not been fully elucidated, especially the mechanism conducive to the selectivity of the four-electron ORR pathway. At present, these properties are mostly verified from experiments, and a comprehensive understanding of the coupling effect still needs to be obtained. In the future, advanced in situ characterization and operational characterization techniques can be used to accurately grasp the electrochemical reaction process of coupled low-Pt and PGM-free catalysts and construct the relationship between performance and structure [62,206].
6. Single-Atom Pt Catalysts
- Increasing the load of Pt while ensuring the uniform dispersion of atoms. In this regard, the development of techniques such as the atomic layer deposition, the photochemical reduction, and the wet chemical method can stabilize the defect sites of loaded metal atoms. Therefore, increasing the number of anchoring sites and the density of active sites through defect engineering is the key to single-atom Pt catalyst synthesis [226,227]. Recent studies have shown that Pt nanostructures rich in GB sites synthesized by GB engineering provide the best coordination environment for the reaction and can increase the residence time of oxygen, which is an effective strategy to improve the catalytic performance of ORR catalysts in the future. However, the grain boundary will accelerate the oxidation of Pt and reduce the stability of the catalyst, so further research is needed [41].
7. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Annual Energy Outlook. Energy information administration. Dep. Energy 2010, 92010, 1–15. [Google Scholar]
- Shukla, P.R.; Skeg, J.; Buendia, E.C.; Masson-Delmotte, V.; Pörtner, H.-O.; Roberts, D.; Zhai, P.; Slade, R.; Connors, S.; Van Diemen, S. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. 2019. Available online: https://philpapers.org/rec/SHUCCA-2 (accessed on 23 July 2024).
- Chai, Q.M.; Zhang, X.L. Technologies and policies for the transition to a sustainable energy system in china. Energy 2010, 35, 3995–4002. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Council, H. Hydrogen Insights 2022. 2022. Available online: https://hydrogencouncil.com/en/hydrogen-insights-2022/ (accessed on 23 July 2024).
- Wei, F.; Ren, X.; Gao, L.; Gao, G.; Zhou, C. Analysis on transformation and characteristics of American hydrogen energy strategy under carbon neutralization goal. Bull. Chin. Acad. Sci. (Chin. Version) 2021, 36, 1049–1057. [Google Scholar] [CrossRef]
- Thomas, J.M.; Edwards, P.P.; Dobson, P.J.; Owen, G.P. Decarbonising energy: The developing international activity in hydrogen technologies and fuel cells. J. Energy Chem. 2020, 51, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Neef, H.J. International overview of hydrogen and fuel cell research. Energy 2009, 34, 327–333. [Google Scholar] [CrossRef]
- Rahim Malik, F.; Yuan, H.-B.; Moran, J.C.; Tippayawong, N. Overview of hydrogen production technologies for fuel cell utilization. Eng. Sci. Technol. Int. J. 2023, 43, 101452. [Google Scholar] [CrossRef]
- U.S. Department of Education. US National Clean Hydrogen Strategy and Roadmap. 2021. Available online: https://www.hydrogen.energy.gov/library/roadmaps-vision/clean-hydrogen-strategy-roadmap (accessed on 23 July 2024).
- Yang, G.; Lee, C.; Qiao, X.; Babu, S.K.; Martinez, U.; Spendelow, J.S. Advanced Electrode Structures for Proton Exchange Membrane Fuel Cells: Current Status and Path Forward. Electrochem. Energy Rev. 2024, 7, 9. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts-A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.S.; Li, Y.D. A fundamental comprehension and recent progress in advanced Pt-based ORR nanocatalysts. Smartmat 2021, 2, 56–75. [Google Scholar] [CrossRef]
- Pollet, B.G.; Kocha, S.S.; Staffell, I. Current status of automotive fuel cells for sustainable transport. Curr. Opin. Electrochem. 2019, 16, 90–95. [Google Scholar] [CrossRef]
- Ferriday, T.B.; Middleton, P.H. Alkaline fuel cell technology-A review. Int. J. Hydrogen Energy 2021, 46, 18489–18510. [Google Scholar] [CrossRef]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kaer, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrogen Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Küngas, R. Review-Electrochemical CO2 Reduction for CO Production: Comparison of Low- and High-Temperature Electrolysis Technologies. J. Electrochem. Soc. 2020, 167, 044508. [Google Scholar] [CrossRef]
- Li, M.R.; Zhao, M.W.; Li, F.; Zhou, W.; Peterson, V.K.; Xu, X.Y.; Shao, Z.P.; Gentle, I.; Zhu, Z.H. A niobium and tantalum co-doped perovskite cathode for solid oxide fuel cells operating below 500 °C. Nat. Commun. 2017, 8, 13990. [Google Scholar] [CrossRef]
- Qu, E.L.; Hao, X.F.; Xiao, M.; Han, D.M.; Huang, S.; Huang, Z.H.; Wang, S.J.; Meng, Y.Z. Proton exchange membranes for high temperature proton exchange membrane fuel cells: Challenges and perspectives. J. Power Sources 2022, 533, 231386. [Google Scholar] [CrossRef]
- Aralekallu, S.; Hadimane, S.; Shantharaja; Nemakal, M.; Koodlur Sannegowda, L. Organic hybrid of cobalt phthalocyanine embedded graphene as an efficient catalyst for oxygen reduction reaction. Fuel 2024, 361, 130736. [Google Scholar] [CrossRef]
- Aminudin, M.A.; Kamarudin, S.K.; Lim, B.H.; Majilan, E.H.; Masdar, M.S.; Shaari, N. An overview: Current progress on hydrogen fuel cell vehicles. Int. J. Hydrogen Energy 2023, 48, 4371–4388. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef]
- Huang, J.; Sementa, L.; Liu, Z.; Barcaro, G.; Feng, M.; Liu, E.; Jiao, L.; Xu, M.; Leshchev, D.; Lee, S.-J.; et al. Experimental Sabatier plot for predictive design of active and stable Pt-alloy oxygen reduction reaction catalysts. Nat. Catal. 2022, 5, 513–523. [Google Scholar] [CrossRef]
- Kaur, J.; Sharma, V.; Kumar Das, D.; Pandit, B.; Shahzad Samdani, M.; Shkir, M.; Aslam Manthrammel, M.; Nangan, S.; Jagadeesha Angadi, V.; Ubaidullah, M. Single-Atom catalysts for oxygen reduction reaction and methanol oxidation reaction. Fuel 2024, 358, 130241. [Google Scholar] [CrossRef]
- Zhao, J.J.; Fu, C.H.; Ye, K.; Liang, Z.; Jiang, F.L.; Shen, S.Y.; Zhao, X.R.; Ma, L.; Shadike, Z.; Wang, X.M.; et al. Manipulating the oxygen reduction reaction pathway on Pt-coordinated motifs. Nat. Commun. 2022, 13, 685. [Google Scholar] [CrossRef]
- Ma, N.; Xiong, Y.; Wang, Y.; Zhang, Y.; Wang, Q.; Luo, S.; Zhao, J.; Huang, C.; Fan, J. A review of advancements in theoretical simulation of oxygen reduction reaction and oxygen evolution reaction single-atom catalysts. Mater. Today Sustain. 2024, 27, 100876. [Google Scholar] [CrossRef]
- Montemore, M.M.; van Spronsen, M.A.; Madix, R.J.; Friend, C.M. O2 Activation by Metal Surfaces: Implications for Bonding and Reactivity on Heterogeneous Catalysts. Chem. Rev. 2018, 118, 2816–2862. [Google Scholar] [CrossRef]
- Jiang, K.; Zhao, J.; Wang, H. Catalyst Design for Electrochemical Oxygen Reduction toward Hydrogen Peroxide. Adv. Funct. Mater. 2020, 30, 2003321. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Wang, X.X.; Sokolowski, J.; Liu, H.; Wu, G. Pt alloy oxygen-reduction electrocatalysts: Synthesis, structure, and property. Chin. J. Catal. 2020, 41, 739–755. [Google Scholar] [CrossRef]
- Du, L.; Zhang, S.; Chen, G.; Yin, G.; Du, C.; Tan, Q.; Sun, Y.; Qu, Y.; Gao, Y. Polyelectrolyte Assisted Synthesis and Enhanced Oxygen Reduction Activity of Pt Nanocrystals with Controllable Shape and Size. ACS Appl. Mater. Interfaces 2014, 6, 14043–14049. [Google Scholar] [CrossRef]
- Yu, X.; Ye, S. Recent advances in activity and durability enhancement of Pt/C catalytic cathode in PEMFC: Part II: Degradation mechanism and durability enhancement of carbon supported platinum catalyst. J. Power Sources 2007, 172, 145–154. [Google Scholar] [CrossRef]
- Mo, S.; Du, L.; Huang, Z.; Chen, J.; Zhou, Y.; Wu, P.; Meng, L.; Wang, N.; Xing, L.; Zhao, M.; et al. Recent Advances on PEM Fuel Cells: From Key Materials to Membrane Electrode Assembly. Electrochem. Energy Rev. 2023, 6, 28. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhang, W.Y.; Han, G.K.; Zhou, Y.W.; Li, L.F.; Kong, F.P.; Gao, Y.Z.; Du, C.Y.; Wang, J.J.; Du, L.; et al. Deactivated Pt Electrocatalysts for the Oxygen Reduction Reaction: The Regeneration Mechanism and a Regenerative Protocol. ACS Catal. 2021, 11, 9293–9299. [Google Scholar] [CrossRef]
- Ahmad, S.; Nawaz, T.; Ali, A.; Orhan, M.F.; Samreen, A.; Kannan, A.M. An overview of proton exchange membranes for fuel cells: Materials and manufacturing. Int. J. Hydrogen Energy 2022, 47, 19086–19131. [Google Scholar] [CrossRef]
- Banham, D.; Kishimoto, T.; Zhou, Y.J.; Sato, T.; Bai, K.; Ozaki, J.; Imashiro, Y.; Ye, S.Y. Critical advancements in achieving high power and stable nonprecious metal catalyst-based MEAs for real-world proton exchange membrane fuel cell applications. Sci. Adv. 2018, 4, eaar7180. [Google Scholar] [CrossRef]
- Zeng, J.; Francia, C.; Dumitrescu, M.A.; Videla, A.H.A.M.; Ijeri, V.S.; Specchia, S.; Spinelli, P. Electrochemical Performance of Pt-Based Catalysts Supported on Different Ordered Mesoporous Carbons (Pt/OMCs) for Oxygen Reduction Reaction. Ind. Eng. Chem. Res. 2012, 51, 7500–7509. [Google Scholar] [CrossRef]
- Giddaerappa; Manjunatha, N.; Shantharaja; Hojamberdiev, M.; Sannegowda, L.K. Tetraphenolphthalein Cobalt(II) Phthalocyanine Polymer Modified with Multiwalled Carbon Nanotubes as an Efficient Catalyst for the Oxygen Reduction Reaction. ACS Omega 2022, 7, 14291–14304. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffatc, S.; Liu, C.-J. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z. Grain boundary engineering: An emerging pathway toward efficient electrocatalysis. InfoMat 2024, 6, e12608. [Google Scholar] [CrossRef]
- Xia, J.; Wang, B.; Di, J.; Li, Y.; Yang, S.-Z.; Li, H.; Guo, S. Construction of single-atom catalysts for electro-, photo- and photoelectro-catalytic applications: State-of-the-art, opportunities, and challenges. Mater. Today 2022, 53, 217–237. [Google Scholar] [CrossRef]
- Ren, X.; Lv, Q.; Liu, L.; Liu, B.; Wang, Y.; Liu, A.; Wu, G. Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustain. Energy Fuels 2020, 4, 15–30. [Google Scholar] [CrossRef]
- Li, Z.; Shen, T.; Hu, Y.; Chen, K.; Lu, Y.; Wang, D. Progress on Ordered Intermetallic Electrocatalysts for Fuel Cells Application. Acta Phys.-Chim. Sin. 2021, 37, 2010029. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, S.; Cheng, K.W.; Wen, Z.Y.; Zhang, X.Y.; Yi, S.J.; Pan, M. Study on Nitrogen-doped PtCo/C Alloy Catalyst. Chem. J. Chin. Univ.-Chin. 2023, 44, 20230016. [Google Scholar] [CrossRef]
- Zaman, S.; Su, Y.Q.; Dong, C.L.; Qi, R.J.; Huang, L.; Qin, Y.Y.; Huang, Y.C.; Li, F.M.; You, B.; Guo, W.; et al. Scalable Molten Salt Synthesis of Platinum Alloys Planted in Metal-Nitrogen-Graphene for Efficient Oxygen Reduction. Angew. Chem.-Int. Ed. 2022, 61, e202115835. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Nagai, T.; Kuwaki, A.; Jinnouchi, R.; Morimoto, Y. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles. Nat. Nanotechnol. 2021, 16, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, M.; Zhao, Z.; Zhang, Z.; Ye, S.; Xu, S.; Wang, H.; Li, H. Bridging the gap between highly active oxygen reduction reaction catalysts and effective catalyst layers for proton exchange membrane fuel cells. Nat. Energy 2021, 6, 475–486. [Google Scholar] [CrossRef]
- Ma, Y.B.; Miao, L.Q.; Guo, W.H.; Yao, X.Z.; Qin, F.; Wang, Z.X.; Du, H.D.; Li, J.; Kang, F.Y.; Gan, L. Modulating Surface Composition and Oxygen Reduction Reaction Activities of Pt-Ni Octahedral Nanoparticles by Microwave-Enhanced Surface Diffusion during Solvothermal Synthesis. Chem. Mater. 2018, 30, 4355–4360. [Google Scholar] [CrossRef]
- Wang, G.; Van Hove, M.A.; Ross, P.N.; Baskes, M. Monte Carlo simulations of segregation in Pt-Ni catalyst nanoparticles. J. Chem. Phys. 2005, 122, 024706. [Google Scholar] [CrossRef]
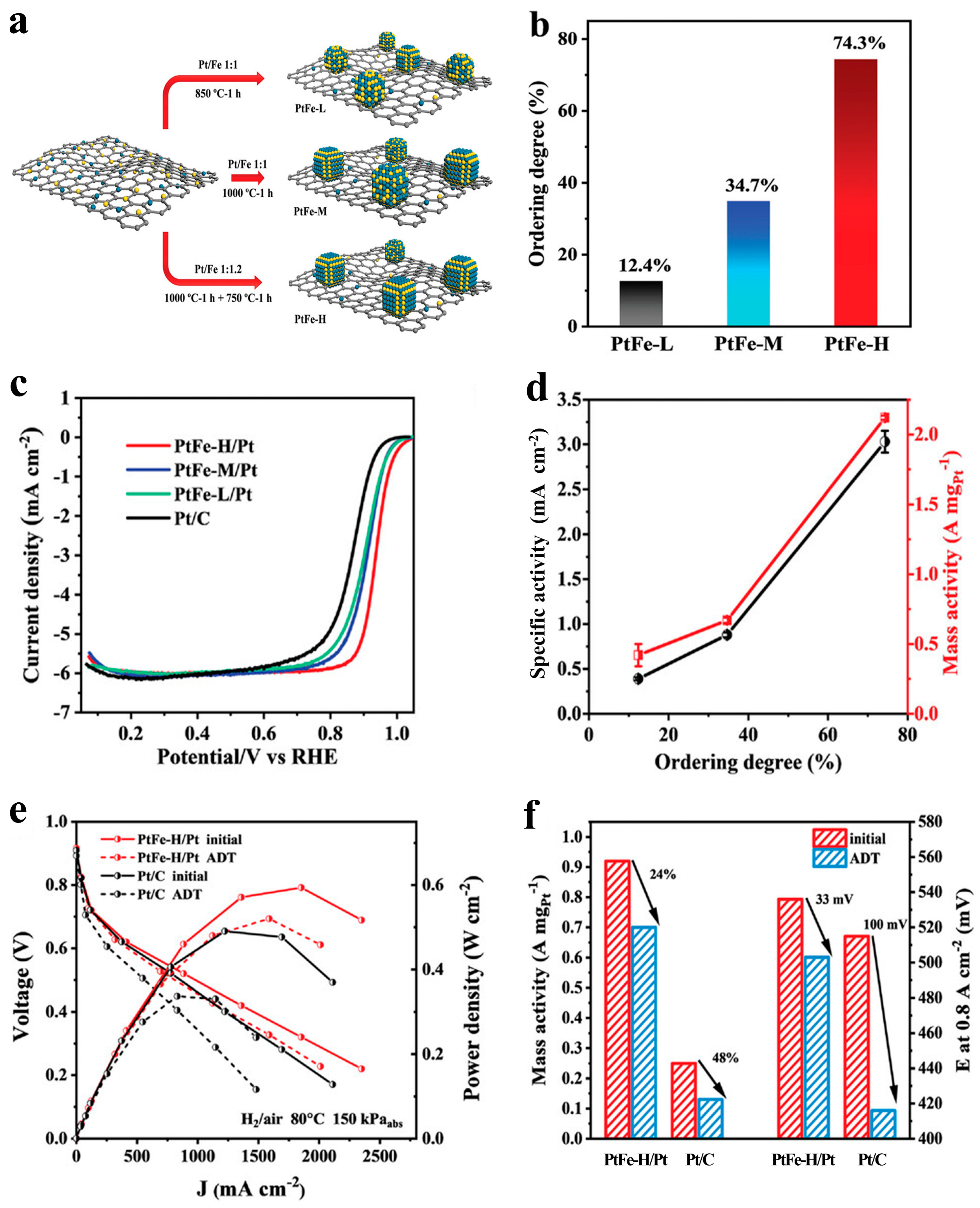
- Song, T.-W.; Chen, M.-X.; Yin, P.; Tong, L.; Zuo, M.; Chu, S.-Q.; Chen, P.; Liang, H.-W. Intermetallic PtFe Electrocatalysts for the Oxygen Reduction Reaction: Ordering Degree-Dependent Performance. Small 2022, 18, 2202916. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Shen, T.; Deng, Z.; Yang, H.; Li, Z.; Zhang, J.; Zhang, R.; Hu, Y.; Zhao, X.; Xin, H. Surface engineering of PdFe ordered intermetallics for efficient oxygen reduction electrocatalysis. Chem. Eng. J. 2021, 408, 127297. [Google Scholar] [CrossRef]
- Song, T.-W.; Xu, C.; Sheng, Z.-T.; Yan, H.-K.; Tong, L.; Liu, J.; Zeng, W.-J.; Zuo, L.-J.; Yin, P.; Zuo, M.; et al. Small molecule-assisted synthesis of carbon supported platinum intermetallic fuel cell catalysts. Nat. Commun. 2022, 13, 6521. [Google Scholar] [CrossRef]
- Zeng, W.-J.; Wang, C.; Yan, Q.-Q.; Yin, P.; Tong, L.; Liang, H.-W. Phase diagrams guide synthesis of highly ordered intermetallic electrocatalysts: Separating alloying and ordering stages. Nat. Commun. 2022, 13, 7654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Wang, S. High-Entropy Alloys for Electrocatalysis: Design, Characterization, and Applications. Small 2022, 18, 2104339. [Google Scholar] [CrossRef]
- Yeh, J.-W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Jien-Wei, Y. Recent progress in high entropy alloys. Ann. Chim. Sci. Mat 2006, 31, 633–648. [Google Scholar] [CrossRef]
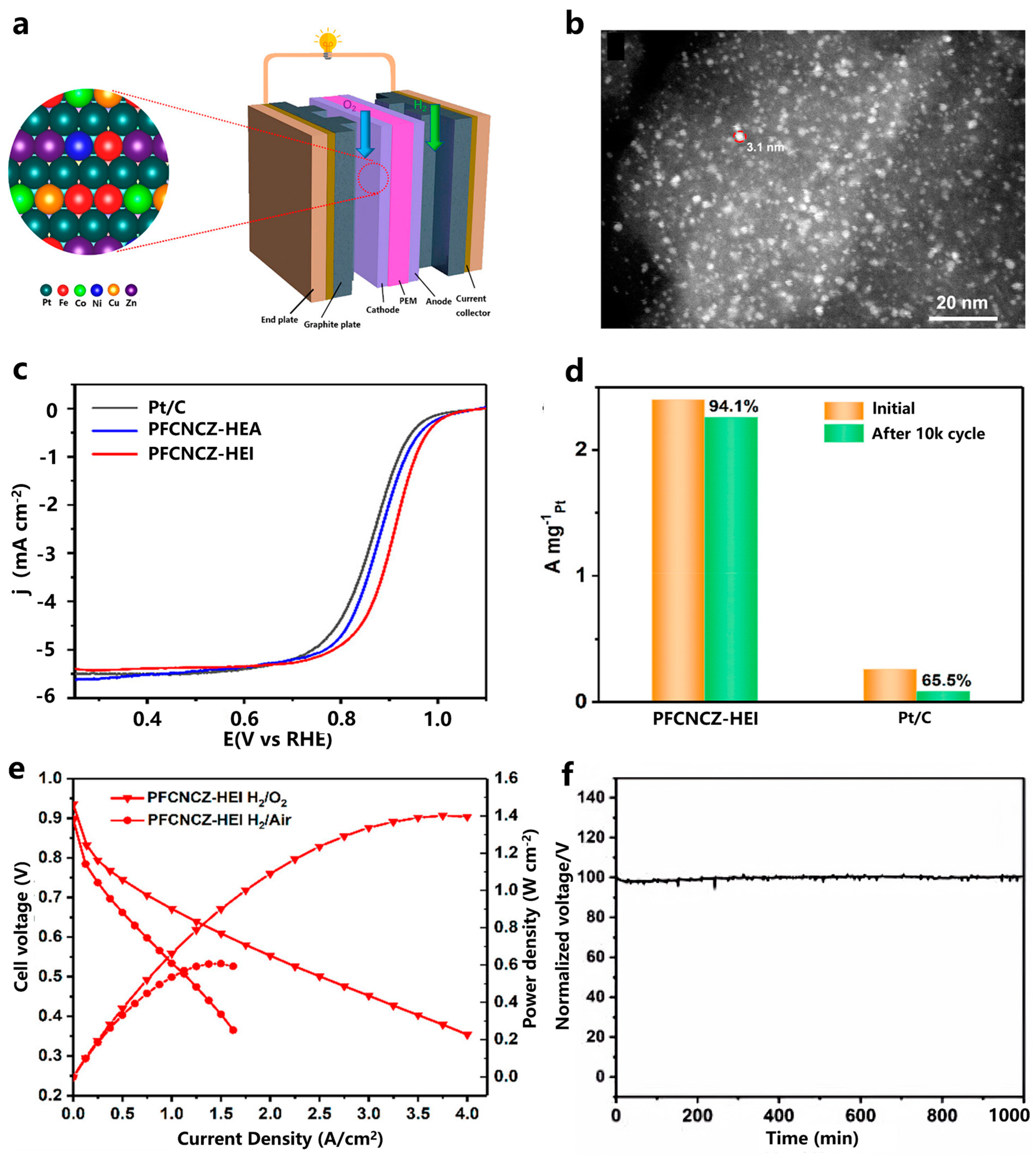
- Chen, T.; Qiu, C.Y.; Zhang, X.K.; Wang, H.C.; Song, J.; Zhang, K.; Yang, T.H.; Zuo, Y.X.; Yang, Y.L.; Gao, C.; et al. An Ultrasmall Ordered High-Entropy Intermetallic with Multiple Active Sites for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2023, 146, 1174–1184. [Google Scholar] [CrossRef]
- Feng, G.; Ning, F.; Pan, Y.; Chen, T.; Song, J.; Wang, Y.; Zou, R.; Su, D.; Xia, D. Engineering Structurally Ordered High-Entropy Intermetallic Nanoparticles with High-Activity Facets for Oxygen Reduction in Practical Fuel Cells. J. Am. Chem. Soc. 2023, 145, 11140–11150. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.; Wen, J.; Kubal, J.; Sen, F.G.; Zou, J.; Greeley, J.; Chan, M.; Barkholtz, H.; Ding, W.; Liu, D.-J. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 2018, 362, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
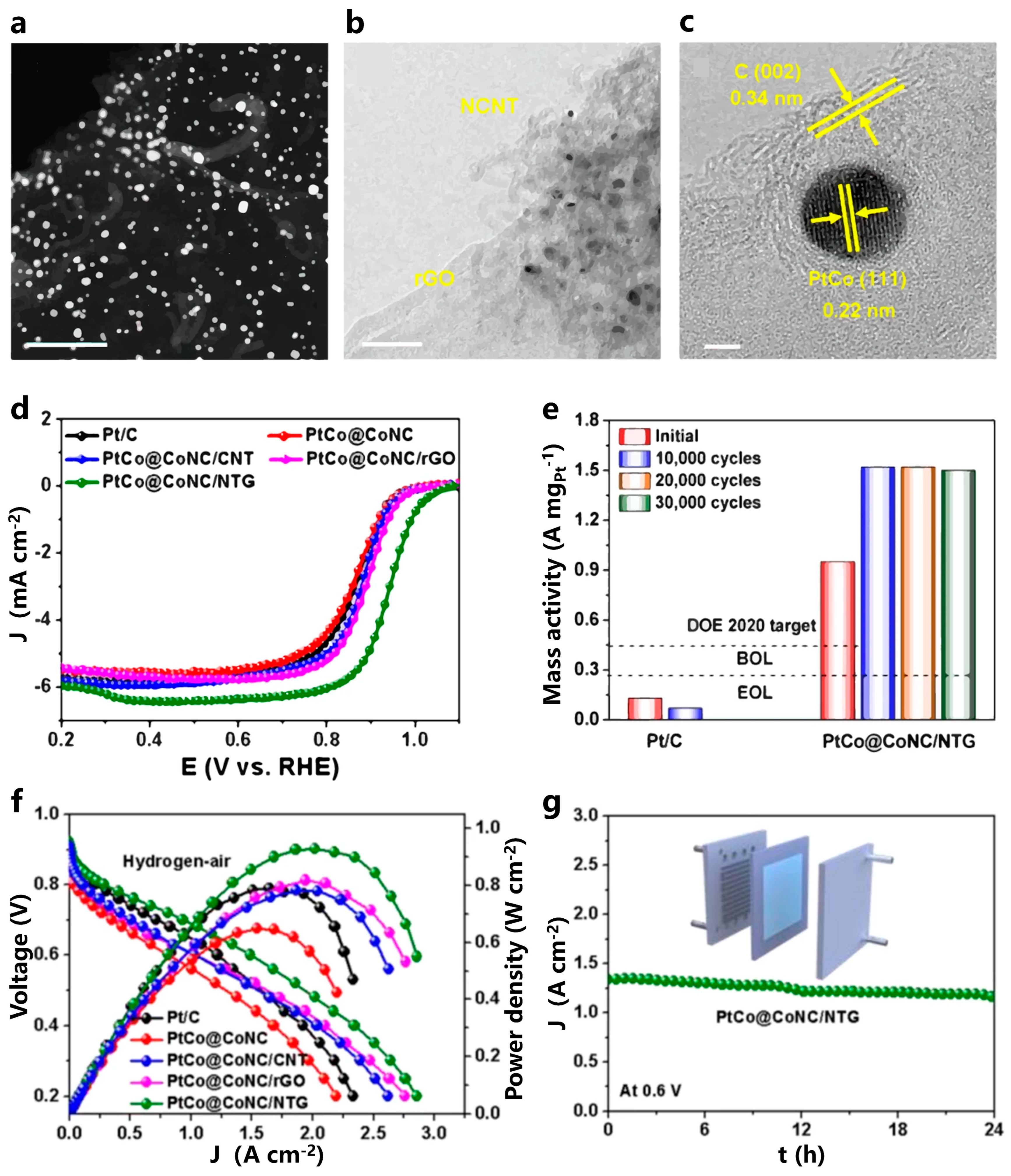
- Zhou, Y.D.; Wang, N.; Xing, L.X.; Zhang, X.T.; Zhong, R.Y.; Peng, Y.Q.; Chen, Y.; Ye, S.Y.; Xie, X.H.; Du, L. The emerging coupled low-PGM and PGM-free catalysts for oxygen reduction reaction. Chem Catal. 2023, 3, 100484. [Google Scholar] [CrossRef]
- Huang, L.; Wei, M.; Qi, R.J.; Dong, C.L.; Dang, D.; Yang, C.C.; Xia, C.F.; Chen, C.; Zaman, S.; Li, F.M.; et al. An integrated platinum-nanocarbon electrocatalyst for efficient oxygen reduction. Nat. Commun. 2022, 13, 6703. [Google Scholar] [CrossRef]
- Wang, Y.F.; Su, Y.Q.; Hensen, E.J.M.; Vlachos, D.G. Finite-Temperature Structures of Supported Subnanometer Catalysts Inferred via Statistical Learning and Genetic Algorithm-Based Optimization. ACS Nano 2020, 14, 13995–14007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Q.; Li, L.; Banis, M.N.; Li, J.; Adair, K.; Sun, Y.; Li, R.; Zhao, Z.-J.; Gu, M.; et al. Single atom surface engineering: A new strategy to boost electrochemical activities of Pt catalysts. Nano Energy 2022, 93, 106813. [Google Scholar] [CrossRef]
- Wang, F.Z.; Yang, J.; Li, J.; Han, Y.Y.; Li, A.; Xu, R.; Feng, X.; Wang, T.; Tong, C.; Li, J.W.; et al. Which is Best for ORR: Single Atoms, Nanoclusters, or Coexistence? ACS Energy Lett. 2023, 9, 93–101. [Google Scholar] [CrossRef]
- Ni, B.X.; Shen, P.; Zhang, G.R.; Zhao, J.J.; Ding, H.H.; Ye, Y.F.; Yue, Z.Y.; Yang, H.; Wei, H.; Jiang, K. Second-Shell N Dopants Regulate Acidic O2 Reduction Pathways on Isolated Pt Sites. J. Am. Chem. Soc. 2024, 146, 11181–11192. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Hirata, A.; Tanabe, E.; Matsune, H.; Kishida, M. Preparation of supported Pt–Co alloy nanoparticle catalysts for the oxygen reduction reaction by coverage with silica. J. Catal. 2010, 274, 228–238. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Niu, Z.; Chen, P.; Zhou, G.; Li, Y. A strategy for designing a concave Pt–Ni alloy through controllable chemical etching. Angew. Chem. 2012, 124, 12692–12696. [Google Scholar] [CrossRef]
- Shui, J.I.; Chen, C.; Li, J.C.M. Evolution of Nanoporous Pt-Fe Alloy Nanowires by Dealloying and their Catalytic Property for Oxygen Reduction Reaction. Adv. Funct. Mater. 2011, 21, 3357–3362. [Google Scholar] [CrossRef]
- Yang, X.H.; Sun, W.T.; Chen, J.T.; Gao, Y.; Zhang, R.X.; Luo, Q.; Lyu, T.; Du, L. The degradation of cathodic Fe/N/C catalyst in PEMFCs: The evolution of remanent active sites after demetallation. J. Mater. Sci. Technol. 2024, 173, 100–106. [Google Scholar] [CrossRef]
- Ge, X.B.; Chen, L.Y.; Kang, J.L.; Fujita, T.; Hirata, A.; Zhang, W.; Jiang, J.H.; Chen, M.W. A Core-Shell Nanoporous Pt-Cu Catalyst with Tunable Composition and High Catalytic Activity. Adv. Funct. Mater. 2013, 23, 4156–4162. [Google Scholar] [CrossRef]
- Zuo, Y.P.; Rao, D.W.; Li, S.; Li, T.T.; Zhu, G.L.; Chen, S.M.; Song, L.; Chai, Y.; Han, H.Y. Atomic Vacancies Control of Pd-Based Catalysts for Enhanced Electrochemical Performance. Adv. Mater. 2018, 30, 1704171. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Han, C.; Xie, P.; Li, H.; Yao, P.F.; Cao, J.; Ruan, M.B.; Song, P.; Gong, X.; Lu, M.; et al. Highly dispersed PtNi nanoparticles modified carbon black as high-performanced electrocatalyst for oxygen reduction in acidic medium. J. Electroanal. Chem. 2022, 904, 115908. [Google Scholar] [CrossRef]
- Jia, Q.Y.; Zhao, Z.P.; Cao, L.; Li, J.K.; Ghoshal, S.; Davies, V.; Stavitski, E.; Attenkofer, K.; Liu, Z.Y.; Li, M.F.; et al. Roles of Mo Surface Dopants in Enhancing the ORR Performance of Octahedral PtNi Nanoparticles. Nano Lett. 2018, 18, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.H.; Jiang, Z.; Wu, R.F.; Liu, Y.; Huang, L.; Hu, N.; Tsiakaras, P.; Shen, P.K. Cross-double dumbbell-like Pt-Ni nanostructures with enhanced catalytic performance toward the reactions of oxygen reduction and methanol oxidation. Appl. Catal. B-Environ. 2019, 246, 277–283. [Google Scholar] [CrossRef]
- Min, M.K.; Cho, J.H.; Cho, K.W.; Kim, H. Particle size and alloying effects of Pt-based alloy catalysts for fuel cell applications. Electrochim. Acta 2000, 45, 4211–4217. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Nagar, R.; Rajalakshmi, N.; Ramaprabhu, S. Novel Platinum-Cobalt Alloy Nanoparticles Dispersed on Nitrogen-Doped Graphene as a Cathode Electrocatalyst for PEMFC Applications. Adv. Funct. Mater. 2012, 22, 3519–3526. [Google Scholar] [CrossRef]
- Yang, H.; Ko, Y.; Lee, W.; Zuttel, A.; Kim, W. Nitrogen-doped carbon black supported Pt-M (M = Pd, Fe, Ni) alloy catalysts for oxygen reduction reaction in proton exchange membrane fuel cell. Mater. Today Energy 2019, 13, 374–381. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Wang, H.N.; Zhang, H.Z.; Ma, X.C.; Zhou, X.M.; Yu, J.M.; Mu, Y.L.; Huang, Y.M.; Huang, T.Z. Hollow carbon nanorods supported platinum-nickel alloy as high efficient catalysts for oxygen reduction reaction. Int. J. Hydrogen Energy 2024, 65, 33–41. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, H.; Qiu, J.; Deng, Y.; Wu, Y.; Wu, J.; Shao, J.; Yan, L. Synergistic catalysis of PtM alloys and nickel hydroxide on highly enhanced electrocatalytic activity and durability for methanol oxidation reaction. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 625, 126942. [Google Scholar] [CrossRef]
- Ahn, C.-Y.; Hwang, W.; Lee, H.; Kim, S.; Park, J.E.; Kim, O.-H.; Her, M.; Cho, Y.-H.; Sung, Y.-E. Effect of N-doped carbon coatings on the durability of highly loaded platinum and alloy catalysts with different carbon supports for polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2018, 43, 10070–10081. [Google Scholar] [CrossRef]
- Huang, K.; Xu, P.; He, X.; Wang, R.; Wang, Y.; Yang, H.; Zhang, R.; Lei, M.; Tang, H. Annealing-Free Platinum-Cobalt Alloy Nanoparticles on Nitrogen-Doped Mesoporous Carbon with Boosted Oxygen Electroreduction Performance. Chemelectrochem 2020, 7, 3341–3346. [Google Scholar] [CrossRef]
- Zhou, S.J.; Yan, R.W.; Zhou, W.C. Synthesis of Cu@Pt-Pd Ternary Metallic Composites for Efficient Electrocatalysis of Methanol. Adv. Mater. Interfaces 2022, 9, 2200761. [Google Scholar] [CrossRef]
- Petkov, V.; Wanjala, B.N.; Loukrakpam, R.; Luo, J.; Yang, L.F.; Zhong, C.J.; Shastri, S. Pt-Au Alloying at the Nanoscale. Nano Lett. 2012, 12, 4289–4299. [Google Scholar] [CrossRef]
- Alayoglu, S.; Zavalij, P.; Eichhorn, B.; Wang, Q.; Frenkel, A.I.; Chupas, P. Structural and Architectural Evaluation of Bimetallic Nanoparticles: A Case Study of Pt-Ru Core-Shell and Alloy Nanoparticles. ACS Nano 2009, 3, 3127–3137. [Google Scholar] [CrossRef]
- Hu, Z.; Allen, F.M.; Wan, C.Z.; Heck, R.M.; Steger, J.J.; Lakis, R.E.; Lyman, C.E. Performance and Structure of Pt–Rh Three-Way Catalysts: Mechanism for Pt/Rh Synergism. J. Catal. 1998, 174, 13–21. [Google Scholar] [CrossRef]
- Zheng, T.; Wu, F.S.; Fu, H.; Zeng, L.; Shang, C.X.; Zhu, L.H.; Guo, Z.X. Rational design of Pt-Pd-Ni trimetallic nanocatalysts for room-temperature benzaldehyde and styrene hydrogenation. Chem.-Asian J. 2021, 16, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, S.; Kim, H. Preparation of CO-tolerant PtRuNi/C ternary electrocatalyst having a composition gradient shell. Chem. Eng. J. 2021, 414, 128792. [Google Scholar] [CrossRef]
- Choi, J.; Cho, J.; Roh, C.W.; Kim, B.S.; Choi, M.S.; Jeong, H.; Ham, H.C.; Lee, H. Au-doped PtCo/C catalyst preventing Co leaching for proton exchange membrane fuel cells. Appl. Catal. B-Environ. 2019, 247, 142–149. [Google Scholar] [CrossRef]
- Li, X.L.; Huang, Y.Q.; Chen, Z.Y.; Hu, S.Q.; Zhu, J.L.; Tsiakaras, P.; Shen, P.K. Novel PtNi nanoflowers regulated by a third element (Rh, Ru, Pd) as efficient multifunctional electrocatalysts for ORR, MOR and HER. Chem. Eng. J. 2023, 454, 140131. [Google Scholar] [CrossRef]
- Nie, M.X.; Xu, Z.Y.; Luo, L.; Wang, Y.; Gan, W.; Yuan, Q.H. One-pot synthesis of ultrafine trimetallic PtPdCu alloy nanoparticles decorated on carbon nanotubes for bifunctional catalysis of ethanol oxidation and oxygen reduction. J. Colloid Interface Sci. 2023, 643, 26–37. [Google Scholar] [CrossRef]
- Fu, C.; Liu, C.; Li, T.; Zhang, X.; Wang, F.; Yang, J.; Jiang, Y.; Cui, P.; Li, H. DFT calculations: A powerful tool for better understanding of electrocatalytic oxygen reduction reactions on Pt-based metallic catalysts. Comput. Mater. Sci. 2019, 170, 109202. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, H.-J.; Feng, X.; Ma, Z.; Ma, Z.-F.; Xue, Y. Progress of Pt and iron-group transition metal alloy catalysts with high ORR activity for PEMFCs. J. Electroanal. Chem. 2024, 959, 118165. [Google Scholar] [CrossRef]
- Kim, C.; Dionigi, F.; Beermann, V.; Wang, X.; Möller, T.; Strasser, P. Alloy Nanocatalysts for the Electrochemical Oxygen Reduction (ORR) and the Direct Electrochemical Carbon Dioxide Reduction Reaction (CO2RR). Adv. Mater. 2019, 31, 1805617. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Tao, P.; Song, C.; Shang, W.; Deng, T.; Wu, J. Structural evolution of Pt-based oxygen reduction reaction electrocatalysts. Chin. J. Catal. 2022, 43, 47–58. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Y.; Gao, L.; Zeng, G.; Li, M.; Huang, H. Stabilizing Pt-Based Electrocatalysts for Oxygen Reduction Reaction: Fundamental Understanding and Design Strategies. Adv. Mater. 2021, 33, 2006494. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, P.; Zhao, Y.; Jiang, W.; Zhao, B.; Chen, X.; Li, M. Ultradurable Pt-Based Catalysts for Oxygen Reduction Electrocatalysis. Catalysts 2024, 14, 57. [Google Scholar] [CrossRef]
- Tuaev, X.; Rudi, S.; Petkov, V.; Hoell, A.; Strasser, P. In situ study of atomic structure transformations of Pt–Ni nanoparticle catalysts during electrochemical potential cycling. ACS Nano 2013, 7, 5666–5674. [Google Scholar] [CrossRef]
- Rudi, S.; Teschner, D.; Beermann, V.; Hetaba, W.; Gan, L.; Cui, C.H.; Gliech, M.; Schlögl, R.; Strasser, P. pH-Induced versus Oxygen-Induced Surface Enrichment and Segregation Effects in Pt-Ni Alloy Nanoparticle Fuel Cell Catalysts. ACS Catal. 2017, 7, 6376–6384. [Google Scholar] [CrossRef]
- Kariuki, N.N.; Myers, D.J. Impact of Nickel Ions on the Oxygen Reduction Reaction Kinetics of Pt and on Oxygen Diffusion through Ionomer Thin Films. J. Electrochem. Soc. 2021, 168, 064505. [Google Scholar] [CrossRef]
- Cui, C.; Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 2013, 12, 765–771. [Google Scholar] [CrossRef]
- Asset, T.; Gommes, C.J.; Drnec, J.; Bordet, P.; Chattot, R.; Martens, I.; Nelayah, J.; Job, N.; Maillard, F.; Dubau, L. Disentangling the Degradation Pathways of Highly Defective PtNi/C Nanostructures—An Operando Wide and Small Angle X-ray Scattering Study. ACS Catal. 2019, 9, 160–167. [Google Scholar] [CrossRef]
- Wang, L.; Gao, W.P.; Liu, Z.Y.; Zeng, Z.H.; Liu, Y.F.; Giroux, M.; Chi, M.F.; Wang, G.F.; Greeley, J.; Pan, X.Q.; et al. Core-Shell Nanostructured Cobalt-Platinum Electrocatalysts with Enhanced Durability. ACS Catal. 2018, 8, 35–42. [Google Scholar] [CrossRef]
- Gao, Y.F.; Thakur, N.; Uchiyama, T.; Cao, W.J.; Yamamoto, K.; Watanabe, T.; Kumar, M.; Sato, R.; Teranishi, T.; Imai, H.; et al. Investigating Degradation Mechanisms in PtCo Alloy Catalysts: The Role of Co Content and a Pt-Rich Shell Using Operando High-Energy Resolution Fluorescence Detection X-ray Absorption Spectroscopy. ACS Appl. Mater. Interfaces 2023, 15, 52473–52484. [Google Scholar] [CrossRef]
- Choi, J.; Lee, Y.; Kim, J.; Lee, H. Enhancing stability of octahedral PtNi nanoparticles for oxygen reduction reaction by halide treatment. J. Power Sources 2016, 307, 883–890. [Google Scholar] [CrossRef]
- Chen, G.; Yang, X.; Xie, Z.; Zhao, F.; Zhou, Z.; Yuan, Q. Hollow PtCu octahedral nanoalloys: Efficient bifunctional electrocatalysts towards oxygen reduction reaction and methanol oxidation reaction by regulating near-surface composition. J. Colloid Interface Sci. 2020, 562, 244–251. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, C.; Li, C.; Zeng, Y.; Hwang, S.; Li, B.; Karakalos, S.; Park, J.; Kropf, A.J.; Wegener, E.C.; et al. Atomically dispersed single iron sites for promoting Pt and Pt3Co fuel cell catalysts: Performance and durability improvements. Energy Environ. Sci. 2021, 14, 4948–4960. [Google Scholar] [CrossRef]
- Li, X.; He, Y.H.; Cheng, S.B.; Li, B.Y.; Zeng, Y.C.; Xie, Z.H.; Meng, Q.P.; Ma, L.; Kisslinger, K.; Tong, X.; et al. Atomic Structure Evolution of Pt-Co Binary Catalysts: Single Metal Sites versus Intermetallic Nanocrystals. Adv. Mater. 2021, 33, 2106371. [Google Scholar] [CrossRef]
- Han, X.; Wang, Q.; Zheng, Z.; Nan, Z.; Zhang, X.; Song, Z.; Ma, M.; Zheng, J.; Kuang, Q.; Zheng, L. Size-controlled intermetallic PtZn nanoparticles on N-doped carbon support for enhanced electrocatalytic oxygen reduction. ACS Sustain. Chem. Eng. 2021, 9, 3821–3827. [Google Scholar] [CrossRef]
- Zhou, M.; Li, C.; Fang, J. Noble-Metal Based Random Alloy and Intermetallic Nanocrystals: Syntheses and Applications. Chem. Rev. 2021, 121, 736–795. [Google Scholar] [CrossRef]
- Luo, M.; Sun, Y.; Wang, L.; Guo, S. Tuning Multimetallic Ordered Intermetallic Nanocrystals for Efficient Energy Electrocatalysis. Adv. Energy Mater. 2017, 7, 1602073. [Google Scholar] [CrossRef]
- Li, J.; Sun, S. Intermetallic nanoparticles: Synthetic control and their enhanced electrocatalysis. Acc. Chem. Res. 2019, 52, 2015–2025. [Google Scholar] [CrossRef]
- Hodnik, N.; Jeyabharathi, C.; Meier, J.C.; Kostka, A.; Phani, K.L.; Rečnik, A.; Bele, M.; Hočevar, S.; Gaberšček, M.; Mayrhofer, K.J. Effect of ordering of PtCu 3 nanoparticle structure on the activity and stability for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2014, 16, 13610–13615. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Li, J.; Yuan, T.; Zhou, Y.; Li, X.; Yang, H. Structural transformation of carbon-supported Pt3Cr nanoparticles from a disordered to an ordered phase as a durable oxygen reduction electrocatalyst. Nanoscale 2014, 6, 10686–10692. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Komatsu, T. Intermetallic Compounds: Promising Inorganic Materials for Well-Structured and Electronically Modified Reaction Environments for Efficient Catalysis. ACS Catal. 2017, 7, 735–765. [Google Scholar] [CrossRef]
- Bu, L.Z.; Liang, J.S.; Ning, F.D.; Huang, J.; Huang, B.L.; Sun, M.Z.; Zhan, C.H.; Ma, Y.H.; Zhou, X.C.; Li, Q.; et al. Low-Coordination Trimetallic PtFeCo Nanosaws for Practical Fuel Cells. Adv. Mater. 2023, 35, 2208672. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Zhao, Z.P.; Cao, L.; Chen, Y.; Zhu, E.B.; Lin, Z.Y.; Li, M.F.; Yan, A.M.; Zettl, A.; Wang, Y.M.; et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234. [Google Scholar] [CrossRef]
- Li, M.G.; Zhao, Z.L.; Xia, Z.H.; Yang, Y.; Luo, M.C.; Huang, Y.R.; Sun, Y.J.; Chao, Y.G.; Yang, W.X.; Yang, W.W.; et al. Lavender-Like Ga-Doped Pt3Co Nanowires for Highly Stable and Active Electrocatalysis. ACS Catal. 2020, 10, 3018–3026. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef]
- Shen, T.; Gong, M.X.; Xiao, D.D.; Shang, T.T.; Zhao, X.; Zhang, J.; Wang, D.L. Engineering Location and Supports of Atomically Ordered L10-PdFe Intermetallics for Ultra-Anticorrosion Electrocatalysis. Adv. Funct. Mater. 2022, 32, 2203921. [Google Scholar] [CrossRef]
- Yang, C.-L.; Wang, L.-N.; Yin, P.; Liu, J.; Chen, M.-X.; Yan, Q.-Q.; Wang, Z.-S.; Xu, S.-L.; Chu, S.-Q.; Cui, C.; et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 2021, 374, 459–464. [Google Scholar] [CrossRef]
- Cheng, Q.; Yang, S.; Fu, C.; Zou, L.; Zou, Z.; Jiang, Z.; Zhang, J.; Yang, H. High-loaded sub-6 nm Pt1Co1 intermetallic compounds with highly efficient performance expression in PEMFCs. Energy Environ. Sci. 2022, 15, 278–286. [Google Scholar] [CrossRef]
- Zhao, X.R.; Cheng, H.; Song, L.; Han, L.L.; Zhang, R.; Kwon, G.H.; Ma, L.; Ehrlich, S.N.; Frenkel, A.I.; Yang, J.; et al. Rhombohedral Ordered Intermetallic Nanocatalyst Boosts the Oxygen Reduction Reaction. ACS Catal. 2020, 11, 184–192. [Google Scholar] [CrossRef]
- Gong, M.; Xiao, D.; Deng, Z.; Zhang, R.; Xia, W.; Zhao, T.; Liu, X.; Shen, T.; Hu, Y.; Lu, Y. Structure evolution of PtCu nanoframes from disordered to ordered for the oxygen reduction reaction. Appl. Catal. B Environ. 2021, 282, 119617. [Google Scholar] [CrossRef]
- Liu, H.J.; Dou, M.L.; Wang, F.; Liu, J.J.; Ji, J.; Li, Z.L. Ordered intermetallic PtFe@Pt core-shell nanoparticles supported on carbon nanotubes with superior activity and durability as oxygen reduction reaction electrocatalysts. RSC Adv. 2015, 5, 66471–66475. [Google Scholar] [CrossRef]
- Jung, C.; Lee, C.; Bang, K.; Lim, J.; Lee, H.; Ryu, H.J.; Cho, E.; Lee, H.M. Synthesis of Chemically Ordered Pt3Fe/C Intermetallic Electrocatalysts for Oxygen Reduction Reaction with Enhanced Activity and Durability via a Removable Carbon Coating. ACS Appl. Mater. Interfaces 2017, 9, 31806–31815. [Google Scholar] [CrossRef]
- Xie, M.; Lyu, Z.; Chen, R.; Shen, M.; Cao, Z.; Xia, Y. Pt–Co@Pt octahedral nanocrystals: Enhancing their activity and durability toward oxygen reduction with an intermetallic core and an ultrathin shell. J. Am. Chem. Soc. 2021, 143, 8509–8518. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Liu, J.; Wang, F. PDA-assisted formation of ordered intermetallic CoPt3 catalysts with enhanced oxygen reduction activity and stability. Nanoscale 2018, 10, 9038–9043. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-T.; Li, D.; Sharma, S.; Wang, C.; Zachman, M.J.; Wegener, E.C.; Kropf, A.J.; Kim, Y.S.; Myers, D.J.; Peterson, A.A.; et al. Ordered CoPt oxygen reduction catalyst with high performance and durability. Chem Catal. 2022, 2, 3559–3572. [Google Scholar] [CrossRef]
- Tong, L.; Fan, L.; Liang, H.-W. Platinum intermetallic nanoparticle cathode catalysts for proton-exchange-membrane fuel cells: Synthesis and ordering effect. Curr. Opin. Electrochem. 2023, 39, 101281. [Google Scholar] [CrossRef]
- Antolini, E. Alloy vs. intermetallic compounds: Effect of the ordering on the electrocatalytic activity for oxygen reduction and the stability of low temperature fuel cell catalysts. Appl. Catal. B Environ. 2017, 217, 201–213. [Google Scholar] [CrossRef]
- Sandbeck, D.J.S.; Secher, N.M.; Speck, F.D.; Sorensen, J.E.; Kibsgaard, J.; Chorkendorff, I.; Cherevko, S. Particle Size Effect on Platinum Dissolution: Considerations for Accelerated Stability Testing of Fuel Cell Catalysts. ACS Catal. 2020, 10, 6281–6290. [Google Scholar] [CrossRef]
- Qin, J.; Zou, P.; Zhang, R.; Wang, C.; Yao, L.; Xin, H.L. Pt–Fe–Cu Ordered Intermetallics Encapsulated with N-Doped Carbon as High-Performance Catalysts for Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2022, 10, 14024–14033. [Google Scholar] [CrossRef]
- Lin, C.; Huang, Z.; Zhang, Z.; Zeng, T.; Chen, R.; Tan, Y.; Wu, W.; Mu, S.; Cheng, N. Structurally Ordered Pt3Co Nanoparticles Anchored on N-Doped Graphene for Highly Efficient Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2020, 8, 16938–16945. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, G.; Li, Y.; Liang, L.; Grundish, N.S.; Tang, Y.; Goodenough, J.B.; Cui, Z. General Strategy for Synthesis of Ordered Pt3M Intermetallics with Ultrasmall Particle Size. Angew. Chem. Int. Ed. 2020, 59, 7857–7863. [Google Scholar] [CrossRef]
- Lin, F.; Li, M.; Zeng, L.; Luo, M.; Guo, S. Intermetallic Nanocrystals for Fuel-Cells-Based Electrocatalysis. Chem. Rev. 2023, 123, 12507–12593. [Google Scholar] [CrossRef] [PubMed]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Miracle, D.B.; Miller, J.D.; Senkov, O.N.; Woodward, C.; Uchic, M.D.; Tiley, J. Exploration and Development of High Entropy Alloys for Structural Applications. Entropy 2014, 16, 494–525. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Xin, Y.; Li, S.; Qian, Y.; Zhu, W.; Yuan, H.; Jiang, P.; Guo, R.; Wang, L. High-Entropy Alloys as a Platform for Catalysis: Progress, Challenges, and Opportunities. ACS Catal. 2020, 10, 11280–11306. [Google Scholar] [CrossRef]
- Huo, X.; Yu, H.; Xing, B.; Zuo, X.; Zhang, N. Review of High Entropy Alloys Electrocatalysts for Hydrogen Evolution, Oxygen Evolution, and Oxygen Reduction Reaction. Chem. Rec. 2022, 22, e202200175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, X.; Wu, Y.; Wang, H.; Jiang, S.; Wang, S.; Hui, X.; Wu, Y.; Gault, B.; Kontis, P.; et al. Enhanced strength and ductility in a high-entropy alloy via ordered oxygen complexes. Nature 2018, 563, 546–550. [Google Scholar] [CrossRef]
- Liu, F.; Liaw, P.K.; Zhang, Y. Recent Progress with BCC-Structured High-Entropy Alloys. Metals 2022, 12, 501. [Google Scholar] [CrossRef]
- Wu, D.S.; Kusada, K.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Gueye, I.; Seo, O.; Kim, J.; Hiroi, S.; Sakata, O.; et al. On the electronic structure and hydrogen evolution reaction activity of platinum group metal-based high-entropy-alloy nanoparticles. Chem. Sci. 2020, 11, 12731–12736. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Makineni, S.K.; Lu, W.; Shang, Y.; Lu, Z.; Li, Z.; Gault, B. On the formation of hierarchical microstructure in a Mo-doped NiCoCr medium-entropy alloy with enhanced strength-ductility synergy. Scr. Mater. 2020, 175, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Fu, Y. High-entropy alloys: Emerging materials for advanced functional applications. J. Mater. Chem. A 2021, 9, 663–701. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Y.S.; Pan, H.; Wang, J.R.; Sun, K.J.; Gao, F.M. A fine 3d transition metal regulation strategy toward high-entropy alloy mesoporous nanotubes as efficient electrocatalysts. Chem. Eng. J. 2023, 477, 147099. [Google Scholar] [CrossRef]
- Xie, C.; Yan, D.; Li, H.; Du, S.; Chen, W.; Wang, Y.; Zou, Y.; Chen, R.; Wang, S. Defect Chemistry in Heterogeneous Catalysis: Recognition, Understanding, and Utilization. ACS Catal. 2020, 10, 11082–11098. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W.; Gan, J.-Y. Diffusion barrier properties of AlMoNbSiTaTiVZr high-entropy alloy layer between copper and silicon. Thin Solid Film 2008, 516, 5527–5530. [Google Scholar] [CrossRef]
- Tsai, K.Y.; Tsai, M.H.; Yeh, J.W. Sluggish diffusion in Co–Cr–Fe–Mn–Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Amiri, A.; Shahbazian-Yassar, R. Recent progress of high-entropy materials for energy storage and conversion. J. Mater. Chem. A 2021, 9, 782–823. [Google Scholar] [CrossRef]
- Li, H.; Lai, J.; Li, Z.; Wang, L. Multi-sites electrocatalysis in high-entropy alloys. Adv. Funct. Mater. 2021, 31, 2106715. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Z.; Chen, K.; Chen, C.; Zhu, Y.; Li, C. Platinum based high entropy alloy oxygen reduction electrocatalysts for proton exchange membrane fuel cells. Mater. Today Nano 2023, 21, 100282. [Google Scholar] [CrossRef]
- Ranganathan, S. Alloyed pleasures: Multimetallic cocktails. Curr. Sci. 2003, 85, 1404–1406. [Google Scholar]
- Chen, Z.Q.; Wen, J.B.; Wang, C.H.; Kang, X.W. Convex Cube-Shaped Pt34Fe5Ni20Cu31Mo9Ru High Entropy Alloy Catalysts toward High-Performance Multifunctional Electrocatalysis. Small 2022, 18, 2204255. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Dong, Q.; Brozena, A.; Luo, J.; Miao, J.; Chi, M.; Wang, C.; Kevrekidis, I.G.; Ren, Z.J.; Greeley, J.; et al. High-entropy nanoparticles: Synthesis-structure-property relationships and data-driven discovery. Science 2022, 376, eabn3103. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Yan, R.; Zhao, L.; Long, Y.; Shi, L.; Cheng, Q.; Liu, D.; Hu, C. A hollow PdCuMoNiCo high-entropy alloy as an efficient bi-functional electrocatalyst for oxygen reduction and formic acid oxidation. J. Mater. Chem. A 2022, 10, 14857–14865. [Google Scholar] [CrossRef]
- Bu, L.; Guo, S.; Zhang, X.; Shen, X.; Su, D.; Lu, G.; Zhu, X.; Yao, J.; Guo, J.; Huang, X. Surface engineering of hierarchical platinum-cobalt nanowires for efficient electrocatalysis. Nat. Commun. 2016, 7, 11850. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Jiang, Y.; Yang, H.; Wang, H.; Fang, Y.; Wang, L.; Xie, M.; Qiu, P.; Luo, W. Constructing Structurally Ordered High-Entropy Alloy Nanoparticles on Nitrogen-Rich Mesoporous Carbon Nanosheets for High-Performance Oxygen Reduction. Adv. Mater. 2022, 34, 2110128. [Google Scholar] [CrossRef]
- Chen, T.; Ning, F.H.; Qi, J.Z.; Feng, G.; Wang, Y.C.; Song, J.; Yang, T.H.; Liu, X.; Chen, L.W.; Xia, D.G. PtFeCoNiCu high-entropy solid solution alloy as highly efficient electrocatalyst for the oxygen reduction reaction. iScience 2023, 26, 105890. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, Z.; Xie, P.; Huang, Z.; Li, T.; Morris, D.; Finfrock, Z.; Zhou, J.; Jiao, M.; Gao, J.; et al. Computationally aided, entropy-driven synthesis of highly efficient and durable multi-elemental alloy catalysts. Sci. Adv. 2020, 6, eaaz0510. [Google Scholar] [CrossRef]
- Kong, K.; Hyun, J.; Kim, Y.; Kim, W.; Kim, D. Nanoporous structure synthesized by selective phase dissolution of AlCoCrFeNi high entropy alloy and its electrochemical properties as supercapacitor electrode. J. Power Sources 2019, 437, 226927. [Google Scholar] [CrossRef]
- Li, S.Y.; Tang, X.W.; Jia, H.L.; Li, H.L.; Xie, G.Q.; Liu, X.J.; Lin, X.; Qiu, H.J. Nanoporous high-entropy alloys with low Pt loadings for high-performance electrochemical oxygen reduction. J. Catal. 2020, 383, 164–171. [Google Scholar] [CrossRef]
- Batchelor, T.A.A.; Pedersen, J.K.; Winther, S.H.; Castelli, I.E.; Jacobsen, K.W.; Rossmeisl, J. High-Entropy Alloys as a Discovery Platform for Electrocatalysis. Joule 2019, 3, 834–845. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, N.; Liu, H.F.; Ma, W.; Hippalgaonkar, K.; Liu, Z.; Huang, Y.Z. Ordering-Dependent Hydrogen Evolution and Oxygen Reduction Electrocatalysis of High-Entropy Intermetallic Pt4FeCoCuNi. Adv. Mater. 2023, 35, 2302067. [Google Scholar] [CrossRef]
- Chen, J.; Ren, L.; Chen, X.; Wang, Q.; Chen, C.; Fan, J.; Wang, S.; Binas, V.; Shen, S. Well-defined nanostructures of high entropy alloys for electrocatalysis. Exploration 2024, 20230036. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, Z.; Yu, W.; Niu, H.; Wang, X.; Guo, Y. Machine-learning-assisted discovery of highly efficient high-entropy alloy catalysts for the oxygen reduction reaction. Patterns 2022, 3, 100553. [Google Scholar] [CrossRef]
- Xu, X.; Shao, Z.; Jiang, S.P. High-Entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, X.; Su, Y.-Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E.J.M.; Lou, X.W.; et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856. [Google Scholar] [CrossRef]
- Ren, J.-T.; Chen, L.; Wang, H.-Y.; Yuan, Z.-Y. High-entropy alloys in electrocatalysis: From fundamentals to applications. Chem. Soc. Rev. 2023, 52, 8319–8373. [Google Scholar] [CrossRef]
- Liu, B.; Feng, R.; Busch, M.; Wang, S.; Wu, H.; Liu, P.; Gu, J.; Bahadoran, A.; Matsumura, D.; Tsuji, T.; et al. Synergistic Hybrid Electrocatalysts of Platinum Alloy and Single-Atom Platinum for an Efficient and Durable Oxygen Reduction Reaction. ACS Nano 2022, 16, 14121–14133. [Google Scholar] [CrossRef]
- Kongkanand, A.; Mathias, M.F. The Priority and Challenge of High-Power Performance of Low-Platinum Proton-Exchange Membrane Fuel Cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Gasteiger, H.A.; Shao-Horn, Y. Hydrogen Oxidation and Evolution Reaction Kinetics on Platinum: Acid vs Alkaline Electrolytes. J. Electrochem. Soc. 2010, 157, B1529–B1536. [Google Scholar] [CrossRef]
- Wang, J.; Ding, W.; Wei, Z.D. Performance of Polymer Electrolyte Membrane Fuel Cells at Ultra-Low Platinum Loadings. Acta Phys.-Chim. Sin. 2021, 37, 2009094. [Google Scholar] [CrossRef]
- Du, L.; Prabhakaran, V.; Xie, X.; Park, S.; Wang, Y.; Shao, Y. Low-PGM and PGM-Free Catalysts for Proton Exchange Membrane Fuel Cells: Stability Challenges and Material Solutions. Adv. Mater. 2021, 33, 1908232. [Google Scholar] [CrossRef] [PubMed]
- He, Y.H.; Tan, Q.; Lu, L.L.; Sokolowski, J.; Wu, G. Metal-Nitrogen-Carbon Catalysts for Oxygen Reduction in PEM Fuel Cells: Self-Template Synthesis Approach to Enhancing Catalytic Activity and Stability. Electrochem. Energy Rev. 2019, 2, 231–251. [Google Scholar] [CrossRef]
- Ye, C.W.; Xu, L. Recent advances in the design of a high performance metal-nitrogen-carbon catalyst for the oxygen reduction reaction. J. Mater. Chem. A 2021, 9, 22218–22247. [Google Scholar] [CrossRef]
- Mo, R.; Zhang, X.; Chen, Z.; Huang, S.; Li, Y.; Liang, L.; Tian, Z.Q.; Shen, P.K. Highly efficient PtCo nanoparticles on Co–N–C nanorods with hierarchical pore structure for oxygen reduction reaction. Int. J. Hydrogen Energy 2021, 46, 15991–16002. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Dodelet, J.P.; Wu, G.; Zelenay, P. PGM-Free Cathode Catalysts for PEM Fuel Cells: A Mini-Review on Stability Challenges. Adv. Mater. 2019, 31, e1807615. [Google Scholar] [CrossRef]
- Zhang, H.G.; Osgood, H.; Xie, X.H.; Shao, Y.Y.; Wu, G. Engineering nanostructures of PGM-free oxygen-reduction catalysts using metal-organic frameworks. Nano Energy 2017, 31, 331–350. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Xu, M.; Tian, C.; Dong, Y.; Wang, C.-A. Pt3Co/Co Composite Catalysts on Porous N-Doped Carbon Support Derived from ZIF-67 with Enhanced HER and ORR Activities. Inorg. Chem. 2022, 61, 19309–19318. [Google Scholar] [CrossRef]
- Gong, M.; Mehmood, A.; Ali, B.; Nam, K.-W.; Kucernak, A. Oxygen Reduction Reaction Activity in Non-Precious Single-Atom (M–N/C) Catalysts─Contribution of Metal and Carbon/Nitrogen Framework-Based Sites. ACS Catal. 2023, 13, 6661–6674. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wen, Y.; Jiang, H.; Yu, S.; Dong, C.; Fan, Y.; Liu, B.; Li, C. Identifying Activity Trends for the Electrochemical Production of H2O2 on M–N–C Single-Atom Catalysts Using Theoretical Kinetic Computations. J. Phys. Chem. C 2022, 126, 10388–10398. [Google Scholar] [CrossRef]
- Sun, Y.; Silvioli, L.; Sahraie, N.R.; Ju, W.; Li, J.; Zitolo, A.; Li, S.; Bagger, A.; Arnarson, L.; Wang, X.; et al. Activity–Selectivity Trends in the Electrochemical Production of Hydrogen Peroxide over Single-Site Metal–Nitrogen–Carbon Catalysts. J. Am. Chem. Soc. 2019, 141, 12372–12381. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Zhang, L.; Shen, Y. Activity Origin and Catalytic Mechanism of the M–N–C Catalysts for the Oxygen Reduction Reaction. ACS Mater. Lett. 2024, 6, 2858–2887. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Jiang, H.; Li, Y.; Zhu, H.; Zheng, L.; Gu, L.; Liang, H.-P. In Situ Alloying with Hybrid Mesoporous Fe–N–C to Accelerate the Catalysis Efficiency of Pt for the Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2023, 11, 10051–10060. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, Y.; DiSalvo, F.J.; Abruña, H.D. Synergistic Bimetallic Metallic Organic Framework-Derived Pt-Co Oxygen Reduction Electrocatalysts. ACS Nano 2020, 14, 13069–13080. [Google Scholar] [CrossRef]
- Yang, K.; Jiang, P.; Chen, J.T.; Chen, Q.W. Nanoporous PtFe Nanoparticles Supported on N-Doped Porous Carbon Sheets Derived from Metal-Organic Frameworks as Highly Efficient and Durable Oxygen Reduction Reaction Catalysts. ACS Appl. Mater. Interfaces 2017, 9, 32106–32113. [Google Scholar] [CrossRef]
- Yin, S.H.; Yan, Y.N.; Chen, L.; Cheng, N.Y.; Cheng, X.Y.; Huang, R.; Huang, H.; Zhang, B.W.; Jiang, Y.X.; Sun, S.G. FeN4 Active Sites Electronically Coupled with PtFe Alloys for Ultralow Pt Loading Hybrid Electrocatalysts in Proton Exchange Membrane Fuel Cells. ACS Nano 2023, 18, 551–559. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Q.; Xu, G.L.; Qin, X.P.; Hwang, I.H.; Sun, C.J.; Liu, M.; Hua, W.; Wu, H.W.; Zhu, S.Q.; et al. Atomically dispersed Pt and Fe sites and Pt-Fe nanoparticles for durable proton exchange membrane fuel cells. Nat. Catal. 2022, 5, 503–512. [Google Scholar] [CrossRef]
- Lai, J.; Chen, S.; Liu, X.; Yan, X.; Qin, Z.; Xie, L.; Lin, Z.; Cai, Z.; Zhao, Y.; Wang, H.-L.; et al. Synergy between Intermetallic Pt Alloy and Porous Co–N4 Carbon Nanofibers Enables Durable Fuel Cells with Low Mass Transport Resistance. ACS Catal. 2023, 13, 11996–12006. [Google Scholar] [CrossRef]
- Guo, P.; Liu, B.; Dai, Y.K.; Gong, X.F.; Xia, Y.F.; Zhang, Y.L.; Liu, B.; Zhao, L.; Sui, X.L.; Wang, Z.B. Coupling fine Pt nanoparticles and Co-Nx moiety as a synergistic bi-active site catalyst for oxygen reduction reaction in acid media. J. Colloid Interface Sci. 2022, 613, 276–284. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, G.; Du, L.; Zhang, J.; Chiang, F.-K.; Wen, Y.; Wang, X.; Wu, Y.; Chen, N.; Sun, S. PGM-Free Fe/N/C and Ultralow Loading Pt/C Hybrid Cathode Catalysts with Enhanced Stability and Activity in PEM Fuel Cells. ACS Appl. Mater. Interfaces 2020, 12, 13739–13749. [Google Scholar] [CrossRef]
- Du, N.N.; Wang, C.M.; Long, R.; Xiong, Y.J. N-doped carbon-stabilized PtCo nanoparticles derived from Pt@ZIF-67: Highly active and durable catalysts for oxygen reduction reaction. Nano Res. 2017, 10, 3228–3237. [Google Scholar] [CrossRef]
- Liang, L.H.; Jin, H.H.; Zhou, H.; Liu, B.S.; Hu, C.X.; Chen, D.; Wang, Z.; Hu, Z.Y.; Zhao, Y.F.; Li, H.W.; et al. Cobalt single atom site isolated Pt nanoparticles for efficient ORR and HER in acid media. Nano Energy 2021, 88, 106221. [Google Scholar] [CrossRef]
- Liang, L.H.; Jin, H.H.; Zhou, H.; Liu, B.S.; Hu, C.X.; Chen, D.; Zhu, J.W.; Wang, Z.; Li, H.W.; Liu, S.L.; et al. Ultra-small platinum nanoparticles segregated by nickle sites for efficient ORR and HER processes. J. Energy Chem. 2022, 65, 48–54. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Y.; Xu, G.-L.; Yang, F.; Zhu, S.; Sun, C.-J.; Cui, Y.; Xu, Z.; Zhao, Q.; Jang, J.; et al. Fe–N–C Boosts the Stability of Supported Platinum Nanoparticles for Fuel Cells. J. Am. Chem. Soc. 2022, 144, 20372–20384. [Google Scholar] [CrossRef]
- Jha, S.; Hassan, N.; Bhat, M.A.; Ingole, P.P. Enhanced Multifunctional Electrocatalytic Activity of Pt-Co Nanoalloy-Decorated Graphene Oxide Sheets through Strong Metal–Support Interaction. Energy Fuels 2022, 36, 15055–15065. [Google Scholar] [CrossRef]
- Ao, X.; Zhang, W.; Zhao, B.T.; Ding, Y.; Nam, G.; Soule, L.; Abdelhafiz, A.; Wang, C.D.; Liu, M.L. Atomically dispersed Fe-N-C decorated with Pt-alloy core-shell nanoparticles for improved activity and durability towards oxygen reduction. Energy Environ. Sci. 2020, 13, 3032–3040. [Google Scholar] [CrossRef]
- Wang, X.X.; Hwang, S.; Pan, Y.-T.; Chen, K.; He, Y.; Karakalos, S.; Zhang, H.; Spendelow, J.S.; Su, D.; Wu, G. Ordered Pt3Co Intermetallic Nanoparticles Derived from Metal–Organic Frameworks for Oxygen Reduction. Nano Lett. 2018, 18, 4163–4171. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Zhou, X.Y.; Wang, Y.; Chen, Y.M.; Xu, Z.Y.; Qiu, Y.J.; Yuan, Q.H.; Lin, X.; Qiu, H.J. Exploiting the Synergistic Electronic Interaction between Pt-Skin Wrapped Intermetallic PtCo Nanoparticles and Co-N-C Support for Efficient ORR/EOR Electrocatalysis in a Direct Ethanol Fuel Cell. Small 2022, 18, e2202071. [Google Scholar] [CrossRef]
- Guo, P.; Xia, Y.; Liu, B.; Ma, M.; Shen, L.; Dai, Y.; Zhang, Z.; Zhao, Z.; Zhang, Y.; Zhao, L.; et al. Low-Loading Sub-3 nm PtCo Nanoparticles Supported on Co–N–C with Dual Effect for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. ACS Appl. Mater. Interfaces 2022, 14, 53819–53827. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.J.; Wang, J.; Huang, Z.F.; Zhang, R.R.; Wang, W.; Pan, L.; Zhang, J.F.; Zhu, W.K.; Zhang, X.W.; Shi, C.X.; et al. Pt/Fe2O3 with Pt-Fe pair sites as a catalyst for oxygen reduction with ultralow Pt loading. Nat. Energy 2021, 6, 614–623. [Google Scholar] [CrossRef]
- Bae, G.; Kim, H.; Choi, H.; Jeong, P.; Kim, D.H.; Kwon, H.C.; Lee, K.S.; Choi, M.; Oh, H.S.; Jaouen, F.; et al. Quantification of Active Site Density and Turnover Frequency: From Single-Atom Metal to Nanoparticle Electrocatalysts. JACS Au 2021, 1, 586–597. [Google Scholar] [CrossRef]
- Primbs, M.; Sun, Y.Y.; Roy, A.; Malko, D.; Mehmood, A.; Sougrati, M.T.; Blanchard, P.Y.; Granozzi, G.; Kosmala, T.; Daniel, G.; et al. Establishing reactivity descriptors for platinum group metal (PGM)-free Fe-N-C catalysts for PEM fuel cells. Energy Environ. Sci. 2020, 13, 2480–2500. [Google Scholar] [CrossRef]
- Sahraie, N.R.; Kramm, U.I.; Steinberg, J.; Zhang, Y.J.; Thomas, A.; Reier, T.; Paraknowitsch, J.P.; Strasser, P. Quantifying the density and utilization of active sites in non-precious metal oxygen electroreduction catalysts. Nat. Commun. 2015, 6, 8618. [Google Scholar] [CrossRef] [PubMed]
- Snitkoff-Sol, R.Z.; Friedman, A.; Honig, H.C.; Yurko, Y.; Kozhushner, A.; Zachman, M.J.; Zelenay, P.; Bond, A.M.; Elbaz, L. Quantifying the electrochemical active site density of precious metal-free catalysts in situ in fuel cells. Nat. Catal. 2022, 5, 163–170. [Google Scholar] [CrossRef]
- Jiao, L.; Jiang, H.L. Metal-Organic-Framework-Based Single-Atom Catalysts for Energy Applications. Chem 2019, 5, 786–804. [Google Scholar] [CrossRef]
- Gara, M.; Laborda, E.; Holdway, P.; Crossley, A.; Jones, C.J.V.; Compton, R.G. Oxygen reduction at sparse arrays of platinum nanoparticles in aqueous acid: Hydrogen peroxide as a liberated two electron intermediate. Phys. Chem. Chem. Phys. 2013, 15, 19487–19495. [Google Scholar] [CrossRef] [PubMed]
- von Weber, A.; Baxter, E.T.; Proch, S.; Kane, M.D.; Rosenfelder, M.; White, H.S.; Anderson, S.L. Size-dependent electronic structure controls activity for ethanol electro-oxidation at Ptn/indium tin oxide (n = 1 to 14). Phys. Chem. Chem. Phys. 2015, 17, 17601–17610. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Raistrick, I.D.; Srinivasan, S. Oxygen Reduction Kinetics on a Platinum RDE Coated with a Recast Nafion Film. J. Electrochem. Soc. 1987, 134, 1455. [Google Scholar] [CrossRef]
- Busari, F.K.; Babar, Z.U.D.; Raza, A.; Li, G. Unlocking new frontiers: Boosting up electrochemical catalysis with metal clusters and single-atoms. Sustain. Mater. Technol. 2024, 40, e00958. [Google Scholar] [CrossRef]
- Dong, Y.N.; Li, H.; Mei, X.H.; Han, C.; Gong, X.; Song, P.; Xu, W.L. Synergetic Effect between Platinum Nanoparticles and Single Atoms Revealed in the Catalytic Mechanism of Traditional Pt/C for the Oxygen Reduction Reaction. ACS Appl. Energy Mater. 2024, 7, 5352–5358. [Google Scholar] [CrossRef]
- Zeng, Y.C.; Liang, J.S.; Li, C.Z.; Qiao, Z.; Li, B.Y.; Hwang, S.; Kariuki, N.N.; Chang, C.W.; Wang, M.Y.; Lyons, M.; et al. Regulating Catalytic Properties and Thermal Stability of Pt and PtCo Intermetallic Fuel-Cell Catalysts via Strong Coupling Effects between Single-Metal Site-Rich Carbon and Pt. J. Am. Chem. Soc. 2023, 145, 17643–17655. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, X.; Jiang, B.; Zhuo, H.; Chen, W.; Chen, Y.; Li, X. Low-loading Pt nanoparticles combined with the atomically dispersed FeN4 sites supported by FeSA-N-C for improved activity and stability towards oxygen reduction reaction/hydrogen evolution reaction in acid and alkaline media. J. Colloid Interface Sci. 2023, 635, 514–523. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Liu, B. Coordination Engineering of Single-Atom Catalysts for the Oxygen Reduction Reaction: A Review. Adv. Energy Mater. 2021, 11, 2002473. [Google Scholar] [CrossRef]
- Qi, Z.; Zhou, Y.; Guan, R.; Fu, Y.; Baek, J.-B. Tuning the Coordination Environment of Carbon-Based Single-Atom Catalysts via Doping with Multiple Heteroatoms and Their Applications in Electrocatalysis. Adv. Mater. 2023, 35, 2210575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Ge, R.; Zhang, J.; Cairney, J.M.; Li, Y.; Zhu, M.; Li, S.; Li, W. The effect of coordination environment on the activity and selectivity of single-atom catalysts. Coord. Chem. Rev. 2022, 461, 214493. [Google Scholar] [CrossRef]
- He, Y.; Shi, Q.; Shan, W.; Li, X.; Kropf, A.J.; Wegener, E.C.; Wright, J.; Karakalos, S.; Su, D.; Cullen, D.A.; et al. Dynamically Unveiling Metal–Nitrogen Coordination during Thermal Activation to Design High-Efficient Atomically Dispersed CoN4 Active Sites. Angew. Chem. Int. Ed. 2021, 60, 9516–9526. [Google Scholar] [CrossRef]
- Chen, Y.P.; Zheng, X.S.; Cai, J.Y.; Zhao, G.Q.; Zhang, B.X.; Luo, Z.X.; Wang, G.M.; Pan, H.G.; Sun, W.P. Sulfur Doping Triggering Enhanced Pt-N Coordination in Graphitic Carbon Nitride-Supported Pt Electrocatalysts toward Efficient Oxygen Reduction Reaction. ACS Catal. 2022, 12, 7406–7414. [Google Scholar] [CrossRef]
- Lang, R.; Du, X.R.; Huang, Y.K.; Jiang, X.Z.; Zhang, Q.; Guo, Y.L.; Liu, K.P.; Qiao, B.T.; Wang, A.Q.; Zhang, T. Single-Atom Catalysts Based on the Metal-Oxide Interaction. Chem. Rev. 2020, 120, 11986–12043. [Google Scholar] [CrossRef]
- Chen, L.; He, C.Z.; Wang, R.; Li, Q.; Zeng, J.; Liu, W.; Wang, Y.C.; Wang, Q.C.; Ye, T.; Tang, Y.G.; et al. Potential active sites of Mo single atoms for electrocatalytic reduction of N2. Chin. Chem. Lett. 2021, 32, 53–56. [Google Scholar] [CrossRef]
- Poh, C.K.; Lim, S.H.; Lin, J.Y.; Feng, Y.P. Tungsten Carbide Supports for Single-Atom Platinum-Based Fuel-Cell Catalysts: First-Principles Study on the Metal-Support Interactions and O2 Dissociation on WxC Low-Index Surfaces. J. Phys. Chem. C 2014, 118, 13525–13538. [Google Scholar] [CrossRef]
- Kong, Z.J.; Zhang, D.C.; Lu, Y.X.; Yang, C.M.; Du, S.Q.; Li, W.; Tao, L.; Wang, S.Y. Advanced Cathode Electrocatalysts for Fuel Cells: Understanding, Construction, and Application of Carbon-Based and Platinum-Based Nanomaterials. ACS Mater. Lett. 2021, 3, 1610–1634. [Google Scholar] [CrossRef]
- Humayun, M.; Israr, M.; Khan, A.; Bououdina, M. State-of-the-art single-atom catalysts in electrocatalysis: From fundamentals to applications. Nano Energy 2023, 113, 108570. [Google Scholar] [CrossRef]
- Ou, Z.Q.; An, Z.; Ma, Z.; Li, N.; Han, Y.N.; Yang, G.J.; Jiang, Q.K.; Chen, Q.; Chu, W.L.; Wang, S.L.; et al. 3D Porous Graphene-like Carbons Encaged Single-Atom-Based Pt for Ultralow Loading and High-Performance Fuel Cells. ACS Catal. 2023, 13, 1856–1862. [Google Scholar] [CrossRef]
- Iqbal, R.; Ali, S.; Saleem, A.; Majeed, M.K.; Hussain, A.; Rauf, S.; Akbar, A.R.; Xu, H.; Qiao, L.; Zhao, W. Electrically conductive Pt-MOFs for acidic oxygen reduction: Optimized performance via altering conjugated ligands. Chem. Eng. J. 2023, 455, 140799. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Huang, Z.; Yu, J.; Wang, H.; Qin, Y.; Xing, L.; Du, L. Research Progress of Pt-Based Catalysts toward Cathodic Oxygen Reduction Reactions for Proton Exchange Membrane Fuel Cells. Catalysts 2024, 14, 569. https://doi.org/10.3390/catal14090569
Chen Y, Huang Z, Yu J, Wang H, Qin Y, Xing L, Du L. Research Progress of Pt-Based Catalysts toward Cathodic Oxygen Reduction Reactions for Proton Exchange Membrane Fuel Cells. Catalysts. 2024; 14(9):569. https://doi.org/10.3390/catal14090569
Chicago/Turabian StyleChen, Yue, Zhiyin Huang, Jiefen Yu, Haiyi Wang, Yukuan Qin, Lixin Xing, and Lei Du. 2024. "Research Progress of Pt-Based Catalysts toward Cathodic Oxygen Reduction Reactions for Proton Exchange Membrane Fuel Cells" Catalysts 14, no. 9: 569. https://doi.org/10.3390/catal14090569




