Engineered CoS/Ni3S2 Heterointerface Catalysts Grown Directly on Carbon Paper as an Efficient Electrocatalyst for Urea Oxidation
Abstract
1. Introduction
2. Results and Discussion
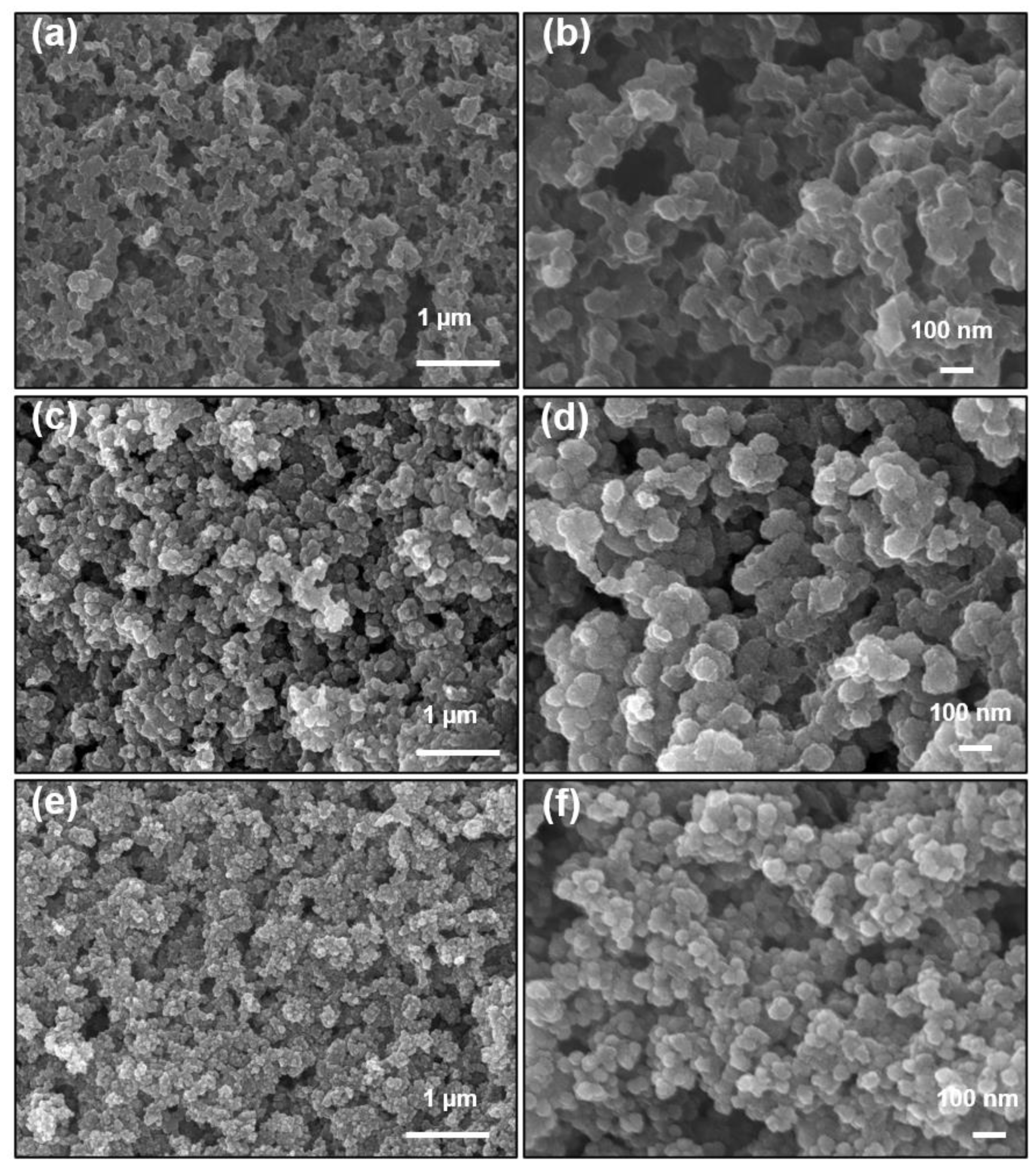
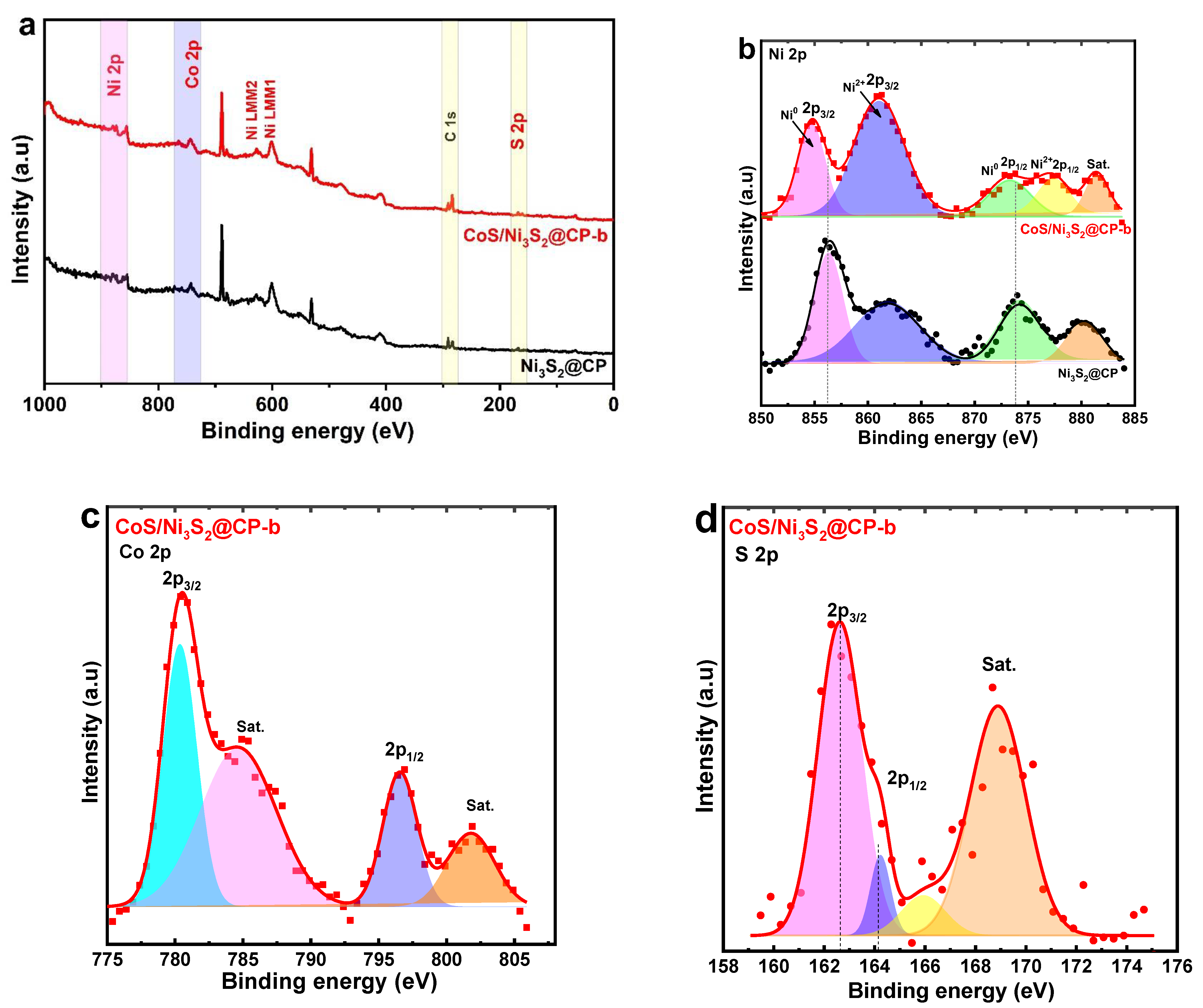
2.1. Catalyst Characterization
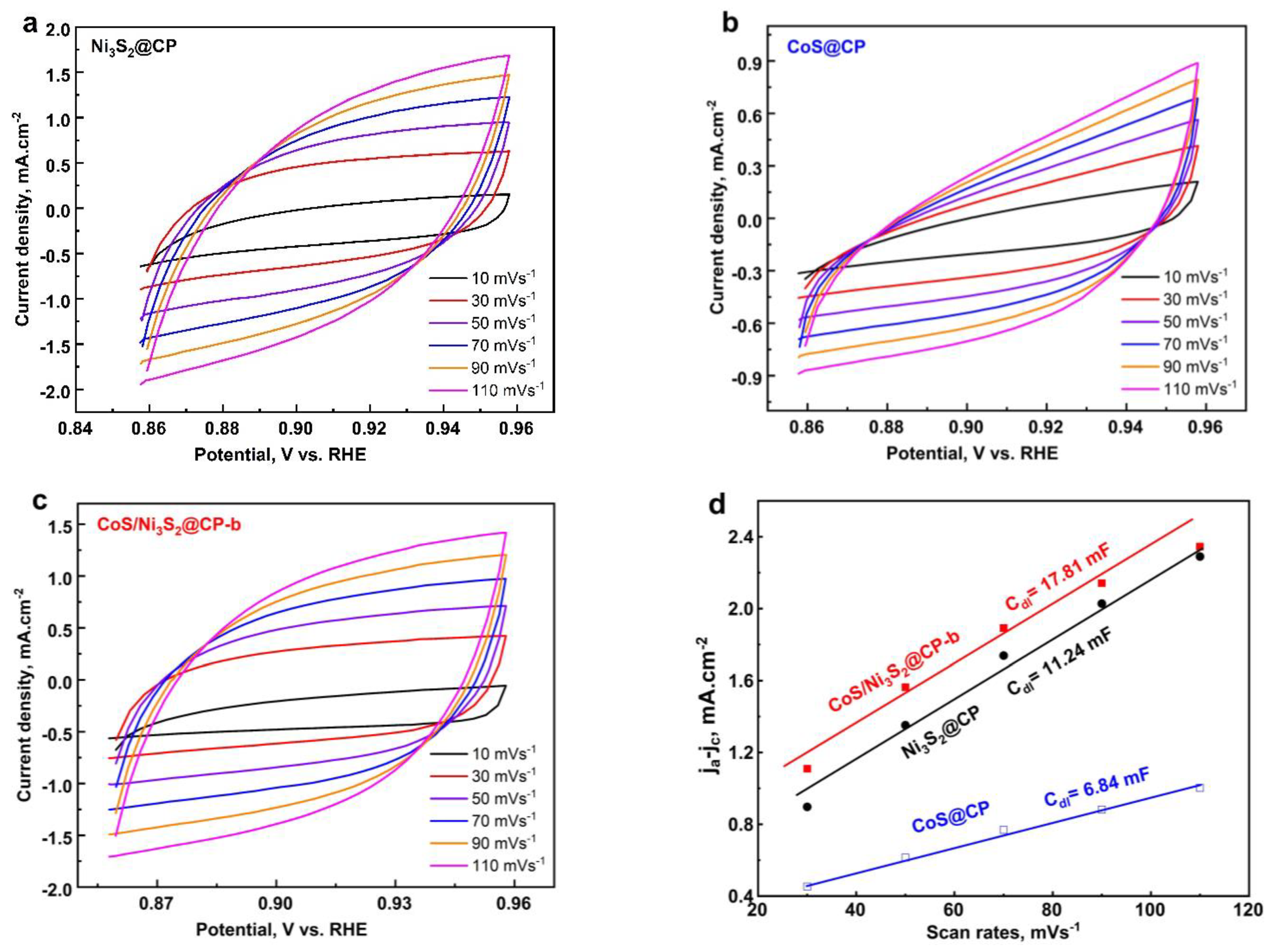
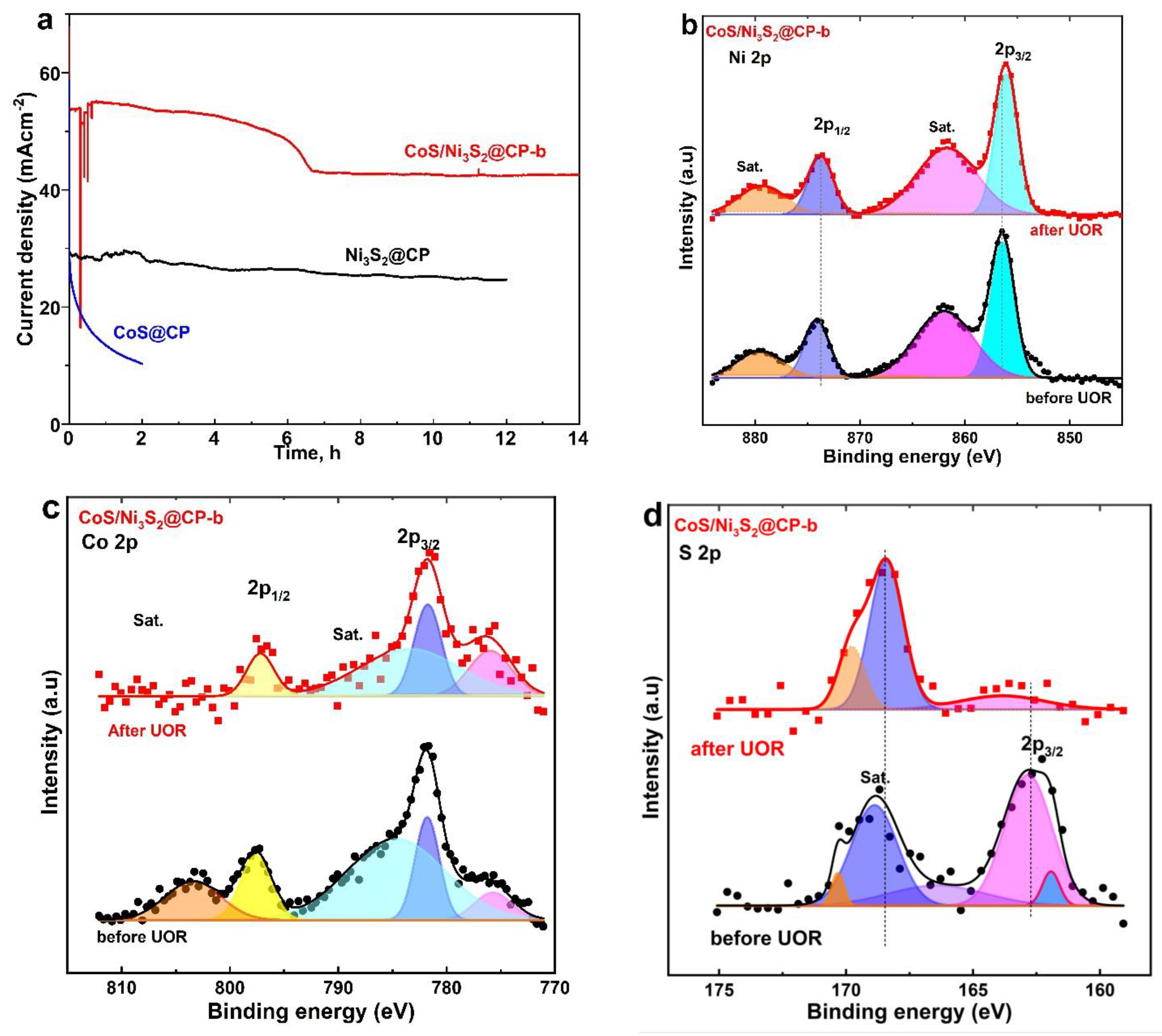
2.2. Electrochemical Features of CoS/Ni3S2@CP Catalysts
3. Experimental
3.1. Chemicals
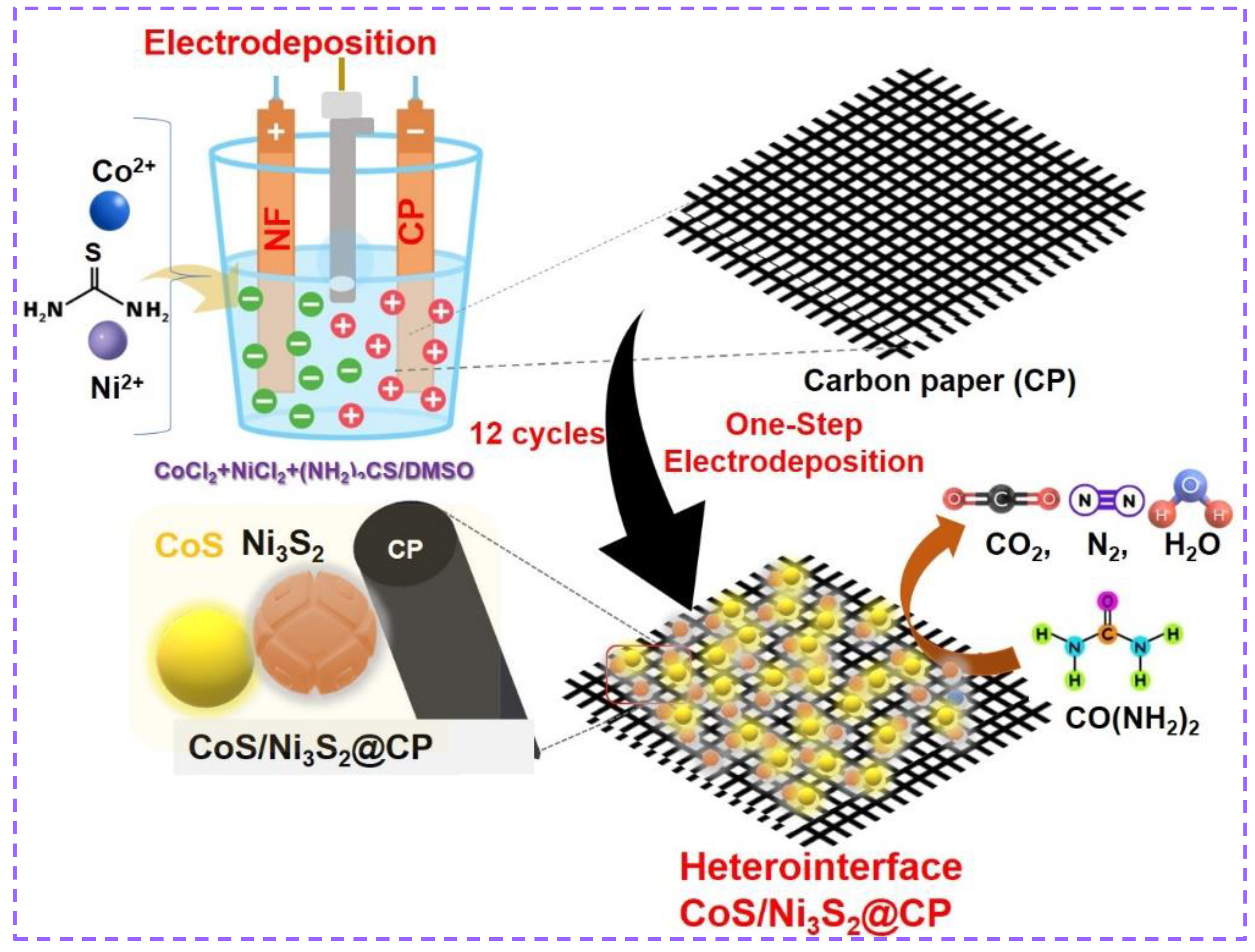
3.2. Electrodeposition of CoS/Ni3S2@CP NP Catalysts
3.3. Characterizations
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Omer, A.M. Renewable Building Energy Systems and Passive Human Comfort Solutions. Renew. Sustain. Energy Rev. 2008, 12, 1562–1587. [Google Scholar] [CrossRef]
- Satyapal, S.; Petrovic, J.; Read, C.; Thomas, G.; Ordaz, G. The U.S. Department of Energy’ s National Hydrogen Storage Project: Progress towards Meeting Hydrogen-Powered Vehicle Requirements. Catal. Today 2007, 120, 246–256. [Google Scholar] [CrossRef]
- Barbir, F. PEM Electrolysis for Production of Hydrogen from Renewable Energy Sources. Sol. Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Christopher, K.; Dimitrios, R. A Review on Exergy Comparison of Hydrogen Production Methods from Renewable Energy Sources. Energy Environ. Sci. 2012, 5, 6640–6651. [Google Scholar] [CrossRef]
- Zhang, S. Design of Electrocatalysts for Oxygen- and Hydrogen-Involving Energy Conversion Reactions. Chem. Soc. Rev. 2023, 44, 2023–2576. [Google Scholar]
- Agyekum, E.B.; Nutakor, C.; Agwa, A.M.; Kamel, S.; Kame, S. A Critical Review of Renewable Hydrogen Production Methods: Factors Affecting Their Scale-up and Its Role in Future Energy Generation. Membranes 2022, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.S.; Arunachalam, P.; Al-Mayouf, A.M.; Alsaleh, A.A.; Alshalwi, M.; Almutairi, Z.A. Effect of Iron and Vanadium Doping on Structural Phase Transition in Cobalt Diselenide Enabling Superior Oxygen/Hydrogen Electrocatalysis. ACS Appl. Energy Mater. 2023, 6, 11718–11731. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, L.; Liu, J. Recent Advances in Cobalt-Based Electrocatalysts for Hydrogen and Oxygen Evolution Reactions. J. Alloys Compd. 2020, 821, 153542. [Google Scholar] [CrossRef]
- Li, H.; Shang, J.; Zhu, H.; Yang, Z.; Ai, Z.; Zhang, L. Oxygen Vacancy Structure Associated Photocatalytic Water Oxidation of BiOCl. ACS Catal. 2016, 6, 8276–8285. [Google Scholar] [CrossRef]
- Chen, T.; Qin, S.; Qian, M.; Dai, H.; Fu, Y.; Zhang, Y.; Ye, B.; Lin, Q.; Yang, Q. Defect-Rich Fe-Doped CoP Nanosheets as Efficient Oxygen Evolution Electrocatalysts. Energy Fuels 2021, 35, 10890–10897. [Google Scholar] [CrossRef]
- Zhu, X.; Dou, X.; Dai, J.; An, X.; Guo, Y.; Zhang, L.; Tao, S.; Zhao, J.; Chu, W.; Zeng, X.C.; et al. Metallic Nickel Hydroxide Nanosheets Give Superior Electrocatalytic Oxidation of Urea for Fuel Cells. Angew. Chem. Int. Ed. 2016, 55, 12465–12469. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wang, X.; Wang, J.; Sun, X.; Cui, L.; Yang, W.; Zheng, Y.; Liu, J. CoS 2 Nanoneedle Array on Ti Mesh: A Stable and Efficient Bifunctional Electrocatalyst for Urea-Assisted Electrolytic Hydrogen Production. Electrochim. Acta 2017, 246, 776–782. [Google Scholar] [CrossRef]
- Araujo, R.B.; Martín-Yerga, D.; Santos, E.C.d.; Cornell, A.; Pettersson, L.G.M. Elucidating the Role of Ni to Enhance the Methanol Oxidation Reaction on Pd Electrocatalysts. Electrochim. Acta 2020, 360, 136954. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Moustafa, H.M.; Nassar, M.M.; Abdelkareem, M.A.; Mahmoud, M.S.; Almajid, A.A.; Khalil, K.A. Distinct Influence for Carbon Nano-Morphology on the Activity and Optimum Metal Loading of Ni/C Composite Used for Ethanol Oxidation. Electrochim. Acta 2015, 182, 143–155. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; Al-Mayouf, A.; Amer, M.; Alotaibi, N.; Weller, M.T.; Ghanem, M.A. Structure and Electrochemical Activity of Nickel Aluminium Fl Uoride Nanosheets during Urea Electro-Oxidation in an Alkaline Solution. RSC Adv. 2021, 11, 3190–3201. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.; Chen, P.; Hou, Z. Selective Oxidation of Glycerol in a Base-Free Aqueous Solution: A Short Review. Chin. J. Catal. 2019, 40, 1020–1034. [Google Scholar] [CrossRef]
- Bamuqaddam, A.M.; Aladeemy, S.A.; Ghanem, M.A.; Al-Mayouf, A.M.; Alotaibi, N.H.; Marken, F. Foam Synthesis of Nickel/Nickel (II) Hydroxide Nanoflakes Using Double Templates of Surfactant Liquid Crystal and Hydrogen Bubbles: A High-Performance Catalyst for Methanol Electrooxidation in Alkaline Solution. Nanomaterials 2022, 12, 879. [Google Scholar] [CrossRef]
- Amer, M.S.; Arunachalam, P.; Alsalman, A.M.; Al-Mayouf, A.M.; Almutairi, Z.A.; Aladeemy, S.A.; Hezam, M. Facile Synthesis of Amorphous Nickel Iron Borate Grown on Carbon Paper as Stable Electrode Materials for Promoted Electrocatalytic Urea Oxidation. Catal. Today 2022, 397, 197–205. [Google Scholar] [CrossRef]
- Aladeemy, S.A.; Al-Mayouf, A.M.; Shaddad, M.N.; Amer, M.S.; Almutairi, N.K.; Ghanem, M.A.; Alotaibi, N.H.; Arunachalam, P. Electrooxidation of Urea in Alkaline Solution Using Nickel Hydroxide Activated Carbon Paper Electrodeposited from DMSO Solution. Catalysts 2021, 11, 102. [Google Scholar] [CrossRef]
- Arunachalam, P.; Nagai, K.; Amer, M.S.; Ghanem, M.A.; Ramalingam, R.J.; Al-mayouf, A.M. Recent Developments in the Use of Heterogeneous Semiconductor Photocatalyst Based Materials for A. Catalysts 2021, 11, 160. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, J.; Li, J.; Wu, Q. Urea Electrooxidation: Current Development and Understanding of Ni-Based Catalysts. ChemElectroChem 2020, 7, 3211–3228. [Google Scholar] [CrossRef]
- Singh, R.K.; Schechter, A. Electroactivity of NiCr Catalysts for Urea Oxidation in Alkaline Electrolyte. ChemCatChem 2017, 9, 3374–3379. [Google Scholar] [CrossRef]
- Xie, J.; Qu, H.; Lei, F.; Peng, X.; Liu, W.; Gao, L.; Hao, P.; Cui, G.; Tang, B. Partially Amorphous Nickel-Iron Layered Double Hydroxide Nanosheet Arrays for Robust Bifunctional Electrocatalysis. J. Mater. Chem. A 2018, 6, 16121–16129. [Google Scholar] [CrossRef]
- Ye, K.; Wang, G.; Cao, D.; Wang, G. Recent Advances in the Electro-Oxidation of Urea for Direct Urea Fuel Cell and Urea Electrolysis. Top. Curr. Chem. 2018, 376, 42. [Google Scholar] [CrossRef]
- Urbańczyk, E.; Sowa, M.; Simka, W. Urea Removal from Aqueous Solutions—A Review. J. Appl. Electrochem. 2016, 46, 1011–1029. [Google Scholar] [CrossRef]
- Wang, G.; Wen, Z. Self-Supported Bimetallic Ni–Co Compound Electrodes for Urea- and Neutralization Energy-Assisted Electrolytic Hydrogen Production. Nanoscale 2018, 10, 21087–21095. [Google Scholar] [CrossRef]
- Al-Sulmi, W.A.; Ghanem, M.A.; Aladeemy, S.A.; Al-Mayouf, A.M.; Alotaibi, N.H. Chemical Deposition from a Liquid Crystal Template: A Highly Active Mesoporous Nickel Phosphate Electrocatalyst for Hydrogen Green Production via Urea. Arab. J. Chem. 2023, 16, 105266. [Google Scholar] [CrossRef]
- Amer, M.S.; Arunachalam, P.; Al-Mayouf, A.M.; AlSaleh, A.A.; Almutairi, Z.A. Bifunctional Vanadium Doped Mesoporous Co3O4 on Nickel Foam towards Highly Efficient Overall Urea and Water Splitting in the Alkaline Electrolyte. Environ. Res. 2023, 236, 116818. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, H.; He, H.; Xu, X.; Jin, Y. A High-Performance Binary Ni-Co Hydroxide-Based Water Oxidation Electrode with Three-Dimensional Coaxial Nanotube Array Structure. Adv. Funct. Mater. 2014, 24, 4698–4705. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Liang, Z.; Zou, R.; Guo, S. Earth-Abundant Nanomaterials for Oxygen Reduction. Angew. Chem. Int. Ed. 2016, 55, 2650–2676. [Google Scholar] [CrossRef]
- Wang, J.; Yue, X.; Yang, Y.; Sirisomboonchai, S.; Wang, P.; Ma, X.; Abudula, A.; Guan, G. Earth-Abundant Transition-Metal-Based Bifunctional Catalysts for Overall Electrochemical Water Splitting: A Review. J. Alloys Compd. 2020, 819, 153346. [Google Scholar] [CrossRef]
- Abdel Hameed, R.M.; Medany, S.S. Improved Electrocatalytic Kinetics of Nickel Hydroxide Nanoparticles on Vulcan XC-72R Carbon Black towards Alkaline Urea Oxidation Reaction. Int. J. Hydrogen Energy 2019, 44, 3636–3648. [Google Scholar] [CrossRef]
- Liu, M.; Jiao, Y.; Zhan, S.; Wang, H. Ni3S2 Nanowires Supported on Ni Foam as e Ffi Cient Bifunctional Electrocatalyst for Urea-Assisted Electrolytic Hydrogen Production. Catal. Today 2019, 355, 596–601. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Zhang, Y.; Zeng, L.; Liu, A. Hierarchical Ni 3 S 2 -NiOOH Hetero-Nanocomposite Grown on Nickel Foam as a Noble-Metal-Free Electrocatalyst for Hydrogen Evolution Reaction in Alkaline Electrolyte. Appl. Surf. Sci. 2018, 456, 164–173. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, J.; Guan, J.; Wu, M.; Li, G.R. Ni3S2 in Situ Grown on Ni Foam Coupled with Nitrogen-Doped Carbon Nanotubes as an Efficient Electrocatalyst for the Hydrogen Evolution Reaction in Alkaline Solution. ACS Omega 2019, 4, 20244–20251. [Google Scholar] [CrossRef]
- Sahoo, M.K.; Rao, G.R. Fabrication of NiCo2S4 Nanoball Embedded Nitrogen Doped Mesoporous Carbon on Nickel Foam as an Advanced Charge Storage Material. Electrochim. Acta 2018, 268, 139–149. [Google Scholar] [CrossRef]
- Yang, T.; Li, R.; Li, Z.; Gu, Z.; Wang, G.; Liu, J. Hybrid of NiCo2S4 and Nitrogen and Sulphur-Functionalized Multiple Graphene Aerogel for Application in Supercapacitors and Oxygen Reduction with Significant Electrochemical Synergy. Electrochim. Acta 2016, 211, 59–70. [Google Scholar]
- Ding, R.; Li, X.; Shi, W.; Xu, Q.; Wang, L.; Jiang, H.; Yang, Z.; Liu, E. Mesoporous Ni-P Nanocatalysts for Alkaline Urea Electrooxidation. Electrochim. Acta 2016, 222, 455–462. [Google Scholar] [CrossRef]
- Chebrolu, V.T.; Balakrishnan, B.; Aravindha Raja, S.; Cho, I.; Bak, J.S.; Kim, H.J. The One-Step Electrodeposition of Nickel Phosphide for Enhanced Supercapacitive Performance Using 3-Mercaptopropionic Acid. New J. Chem. 2020, 44, 7690–7697. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Wang, F.; Feng, L. Efficient Catalysis of N Doped NiS/NiS2 Heterogeneous Structure. Chem. Eng. J. 2020, 397, 125507–125516. [Google Scholar] [CrossRef]
- Chen, T.; Wu, Q.; Li, F.; Zhong, R.; Chen, Z. Co2P−Ni3S2 Heterostructured Nanocrystals as Catalysts for Urea Electrooxidation and Urea-Assisted Water Splitting. Appl. Nano Mater. 2023, 6, 18364–18371. [Google Scholar] [CrossRef]
- Shit, S.; Chhetri, S.; Jang, W.; Murmu, N.C.; Koo, H.; Samanta, P.; Kuila, T. Cobalt Sulfide/Nickel Sulfide Heterostructure Directly Grown on Nickel Foam: An Efficient and Durable Electrocatalyst for Overall Water Splitting Application. ACS Appl. Mater. Interfaces 2018, 10, 27712–27722. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, G.Q.; Liu, Y.R.; Dong, B.; Hu, W.H.; Shang, X.; Chai, Y.M.; Liu, C.G. NiSe@NiOOH Core-Shell Hyacinth-like Nanostructures on Nickel Foam Synthesized by in Situ Electrochemical Oxidation as an Efficient Electrocatalyst for the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2016, 8, 20057–20066. [Google Scholar] [CrossRef]
- Li, Q.; Hou, Y.; Yin, J.; Xi, P. The Evolution of Hexagonal Cobalt Nanosheets for CO2 Electrochemical Reduction Reaction. Catalysts 2023, 13, 1384. [Google Scholar] [CrossRef]
- Kuznetsova, I.; Lebedeva, O.; Kultin, D.; Mashkin, M.; Kalmykov, K.; Kustov, L. Enhancing Efficiency of Nitrate Reduction to Ammonia by Fe and Co Nanoparticle-Based Bimetallic Electrocatalyst. Int. J. Mol. Sci. 2024, 25, 7089. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Bao, J.; Zhang, L.; Xin, P.; Yue, C.; Naseri, A.; Wang, H.; Huang, S.; Uvdal, K.; Hu, Z. Interfacial electronic structure modulations of Au@ CuS with defective Ni-doped CoS2 facilitates the electroreduction of N2 into NH3. Chem. Eng. J. 2024, 493, 152456. [Google Scholar] [CrossRef]
- Liu, N.; Guan, J. Core–Shell Co3O4@FeOx Catalysts for Efficient Oxygen Evolution Reaction. Mater. Today Energy 2021, 21, 100715. [Google Scholar] [CrossRef]
- Du, F.; Chen, Z.; Zhang, Y.; He, H.; Zhou, Y.; Li, T.; Zou, Z. Reduced-Graphene-Oxide-Loaded MoS2‡Ni3S2 Nanorod Arrays on Ni Foam as an Efficient and Stable Electrocatalyst for the Hydrogen Evolution Reaction. Electrochem. Commun. 2019, 99, 22–26. [Google Scholar] [CrossRef]
- Wang, C.H.; Yang, H.C.; Zhang, Y.J.; Wang, Q.B. NiFe Alloy Nanoparticles with hcp Crystal Structure Stimulate Superior Oxygen Evolution Reaction Electrocatalytic Activity. Angew. Chem. Int. Ed. 2019, 58, 6099–6103. [Google Scholar] [CrossRef]
- Wu, J.; Chen, J.; Yu, T.; Zhai, Z.; Zhu, Y.; Wu, X.; Yin, S. Boosting electrochemical kinetics of NiCo2 via MoO2 modification for biomass upgrading assisted hydrogen evolution. ACS Catal. 2023, 13, 13257–13266. [Google Scholar] [CrossRef]
- Wu, J.; Zhai, Z.; Yu, T.; Wu, X.; Huang, S.; Cao, W.; Jiang, Y.; Pei, J.; Yin, S. Tailoring the selective adsorption sites of NiMoO by Ni particles for biomass upgrading assisted hydrogen production. J. Energy Chem. 2023, 86, 480–489. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, K.; Lin, H.; Li, X.; Chan, H.C.; Yang, L.; Gao, Q. MoS2–Ni3S2 heteronanorods as efficient and stable bifunctional electrocatalysts for overall water splitting. ACS Catal. 2017, 7, 2357–2366. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Pohl, D.; Rellinghaus, B.; Dong, R.; Liu, S.; Zhuang, X.; Feng, X. Interface engineering of MoS2/Ni3S2 heterostructures for highly enhanced electrochemical overall-water-splitting activity. Angew. Chem. 2016, 128, 6814–6819. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Lü, S.; Chen, Y.; Fan, H.; Wei, M.; Yang, L.; Yu, W.W.; Meng, X. CoO@CoS/Ni3S2 Hierarchical Nanostructure Arrays for High Performance Asymmetric SupercapacitorCoO@CoS/Ni3S2 Hierarchical Nanostructure Arrays for High Performance Asymmetric Supercapacitor. Appl. Surf. Sci. 2020, 532, 147438. [Google Scholar] [CrossRef]
- Hameed, R.M.A.; Medany, S.S. Influence of Support Material on the Electrocatalytic Activity of Nickel Oxide Nanoparticles for Urea Electro-Oxidation Reaction. J. Colloid Interface Sci. 2018, 513, 536–548. [Google Scholar] [CrossRef]
- Wang, D.; Botte, G.G. In Situ X-Ray Diffraction Study of Urea Electrolysis on Nickel Catalysts. ECS Electrochem. Lett. 2014, 3, 29–32. [Google Scholar] [CrossRef]
- Wang, L.; Saveleva, V.A.; Eslamibidgoli, M.J.; Antipin, D.; Bouillet, C.; Biswas, I.; Gago, A.S.; Hosseiny, S.S.; Gazdzicki, P.; Eikerling, M.H.; et al. Deciphering the Exceptional Performance of NiFe Hydroxide for the Oxygen Evolution Reaction in an Anion Exchange Membrane Electrolyzer. ACS Appl. Energy Mater. 2022, 5, 2221–2230. [Google Scholar] [CrossRef]
- Boggs, B.K.; King, R.L.; Botte, G.G. Urea Electrolysis: Direct Hydrogen Production from Urine. Chem. Commun. 2009, 2009, 4859–4861. [Google Scholar] [CrossRef] [PubMed]
- Vedharathinam, V.; Botte, G.G. Understanding the Electro-Catalytic Oxidation Mechanism of Urea on Nickel Electrodes in Alkaline Medium. Electrochim. Acta 2012, 81, 292–300. [Google Scholar] [CrossRef]
- Yi, P.; Song, Y.; Liu, Z.; Xie, P.; Liang, G.; Liu, R.; Chen, L.; Sun, J. Boosting Alkaline Urea Oxidation with a Nickel Sulfide/Cobalt Oxide Heterojunction Catalyst via Interface Engineering. Adv. Compos. Hybird Mater. 2023, 6, 228. [Google Scholar] [CrossRef]
- Li, F.; Cheng, J.; Zhang, D.; Fu, W.-F.; Chen, Y.; Wen, Z.; Lv, X.-J. Heteroporous MoS2/Ni3S2 towards Superior Electrocatalytic 491 Overall Urea Splitting. Chem. Commun. 2018, 54, 5181–5184. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Chen, J.; Luo, L.; Zhang, H.; Chen, W.; Gao, Z.; Yin, S.; Tsiakaras, P. Novel Bifunctional V2O3Nanosheets Coupled with N-Doped-Carbon Encapsulated Ni Heterostructure for Enhanced Electrocatalytic Oxidation of Urea-Rich Wastewater. ACS Appl. Mater. Interfaces 2020, 12, 38061–38069. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guan, J.; Chen, W.; Wu, Y.; Li, S.; Du, X.; Zhang, M. Nickel-Cobalt Derived Nanowires/Nanosheets as Electrocatalyst for Efficient H2 Generation via Urea Oxidation Reaction. J. Alloys Compd. 2021, 891, 161790. [Google Scholar] [CrossRef]
- Wang, Y.-T.; He, X.-F.; Chen, X.-M.; Zhang, Y.; Li, F.-T.; Zhou, Y.; Meng, C. Laser Synthesis of Cobalt-Doped Ni3S4-NiS/Ni as High-Efficiency Supercapacitor Electrode and Urea Oxidation Electrocatalyst. Appl. Surf. Sci. 2022, 596, 153600. [Google Scholar] [CrossRef]



| Electrodes | Overpotential | Stability | Current Density | Electrolyte | Tafel Slope | Ref. |
|---|---|---|---|---|---|---|
| Ni3S2/Co3O4-NF | 1.288 V at 10 mA.cm−2 | 100 h | - | - | [60] | |
| Co2P−Ni3S2-2 | 1.338 V at 10 mA.cm−2 | 100 h | 100 mA.cm−2 at 1.5 V | 1.0 M KOH + 0.5 M urea | 54.52 mV/dm | [41] |
| MoS2/Ni3S2/Ni/NF | 1.33 V at 50 mA.cm−2 | 20 h | 200 mA.cm−2 at 1.35 V | 1.0 M KOH + 0.33 M urea | 42 mV/dm | [61] |
| Ni3S2/NF | 1.30 V at 10 mA.cm−2 | - | 120 mA.cm−2 at 1.7 V | 1.0 M NaOH + 0.33 M urea | 46 mV/dm | [33] |
| N-NiS/NiS2 | 1.38 V at 10 mA.cm−2 | 2 h | 120 mA.cm−2 at 1.5V | 1.0 M KOH + 0.33 M urea | 28.34 mV/dm | [40] |
| Ni@C−V2O3/NF | 1.32 V at 10 mA.cm−2 | 50 h | ~500 mA.cm−2 at 1.4V | 1.0 M KOH + 0.5 M urea | 41.22 mV/dm | [62] |
| Ni1.5Co1.5-O/CC | 1.36 V at 10 mA.cm−2 | ~100 mA.cm−2 at 1.5V | 1.0 M KOH + 0.33 M urea | 59.52 mV/dm | [63] | |
| Co-doped Ni3S4-NiS/Ni | 1.350 V at 10 mA.cm−2 | 1000 cycle | - | 1.0 M KOH + 0.33 M urea | 15.04 mV/dm | [64] |
| CoS/Ni3S2@CP-b | 1.32 V at 10 mA.cm−2 | 14 h | 103.3 mA.cm−2 at 1.7 V | 1 M KOH/0.33 M urea | 206 mV/dm | This work |
| Anodic Materials | EIS Oarameters: R1 + Q2/R2 + Q3/R3 | ||||
|---|---|---|---|---|---|
| R1, ohm | Q2, F.S(α − 1) | R2, ohm | Q3, F.S(α − 1) | R3, ohm | |
| CoS/Ni3S2@CP-b at 1.60 V | 3.99 | 83.81 × 10−6 | 2.502 | 0.1965 | 0.372 |
| CoS/Ni3S2@CP-b at 1.50 V | 3.75 | 859.07 × 10−6 | 2.369 | 0.3097 | 1.246 |
| CoS/Ni3S2@CP-b at 1.45 V | 3.77 | 0.2635 | 1.54 | 62.02 × 10−6 | 2.42 |
| CoS/Ni3S2@CP-b at 1.35 V | 3.37 | 0.1442 × 10−3 | 2.78 | 0.2001 | 4.081 |
| Ni3S2@CP at 1.45 V | 3.97 | 0.2988 | 2.795 | 0.3725 | 3.452 |
| CoS@CP at 1.45 V | 4.86 | 0.1836 | 1.148 | 0.1279 | 3.572 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aladeemy, S.A.; Arunachalam, P.; Al-Mayouf, A.M.; Sudha, P.N.; Rekha, A.; Vidhya, A.; Hemapriya, J.; Latha, S.; Prasad, P.S.; Pavithra, S.; et al. Engineered CoS/Ni3S2 Heterointerface Catalysts Grown Directly on Carbon Paper as an Efficient Electrocatalyst for Urea Oxidation. Catalysts 2024, 14, 570. https://doi.org/10.3390/catal14090570
Aladeemy SA, Arunachalam P, Al-Mayouf AM, Sudha PN, Rekha A, Vidhya A, Hemapriya J, Latha S, Prasad PS, Pavithra S, et al. Engineered CoS/Ni3S2 Heterointerface Catalysts Grown Directly on Carbon Paper as an Efficient Electrocatalyst for Urea Oxidation. Catalysts. 2024; 14(9):570. https://doi.org/10.3390/catal14090570
Chicago/Turabian StyleAladeemy, Saba A., Prabhakarn Arunachalam, Abdullah M. Al-Mayouf, P. N. Sudha, A. Rekha, A. Vidhya, J. Hemapriya, Srinivasan Latha, P. Supriya Prasad, S. Pavithra, and et al. 2024. "Engineered CoS/Ni3S2 Heterointerface Catalysts Grown Directly on Carbon Paper as an Efficient Electrocatalyst for Urea Oxidation" Catalysts 14, no. 9: 570. https://doi.org/10.3390/catal14090570
APA StyleAladeemy, S. A., Arunachalam, P., Al-Mayouf, A. M., Sudha, P. N., Rekha, A., Vidhya, A., Hemapriya, J., Latha, S., Prasad, P. S., Pavithra, S., Arunadevi, R., & Hameed, S. T. (2024). Engineered CoS/Ni3S2 Heterointerface Catalysts Grown Directly on Carbon Paper as an Efficient Electrocatalyst for Urea Oxidation. Catalysts, 14(9), 570. https://doi.org/10.3390/catal14090570









