Strategy in Synthesizing Longer-Chain Levan-Type Fructooligosaccharides by Selective Dextran Macromolecular Cross-Linked Bacillus lehensis G1 Endolevanase Aggregate Immobilization
Abstract
1. Introduction
2. Results and Discussion
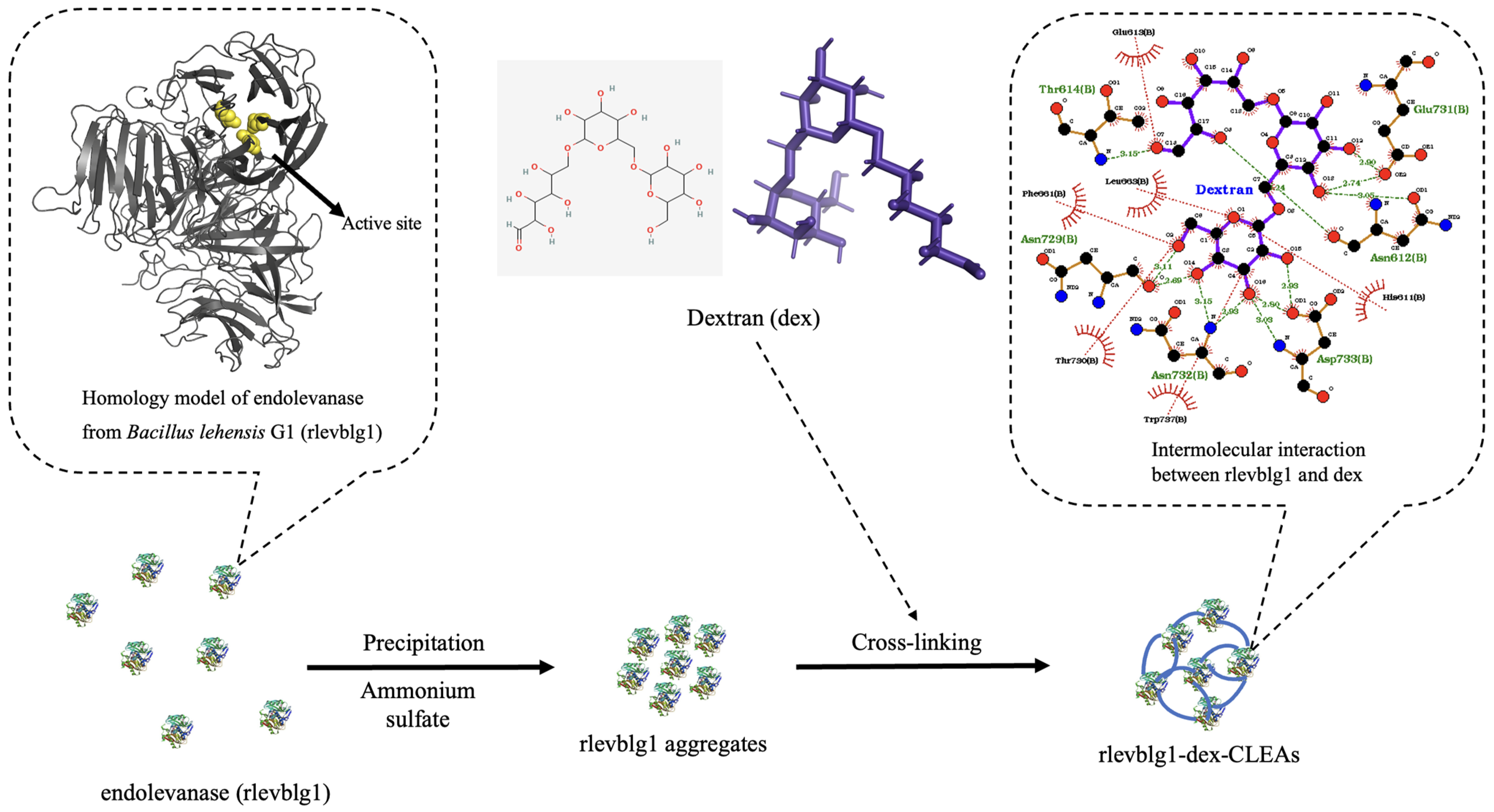
2.1. Optimization and Development of rlevblg1-dex-CLEAs
2.2. Characterizations of rlevblg1-dex-CLEAs
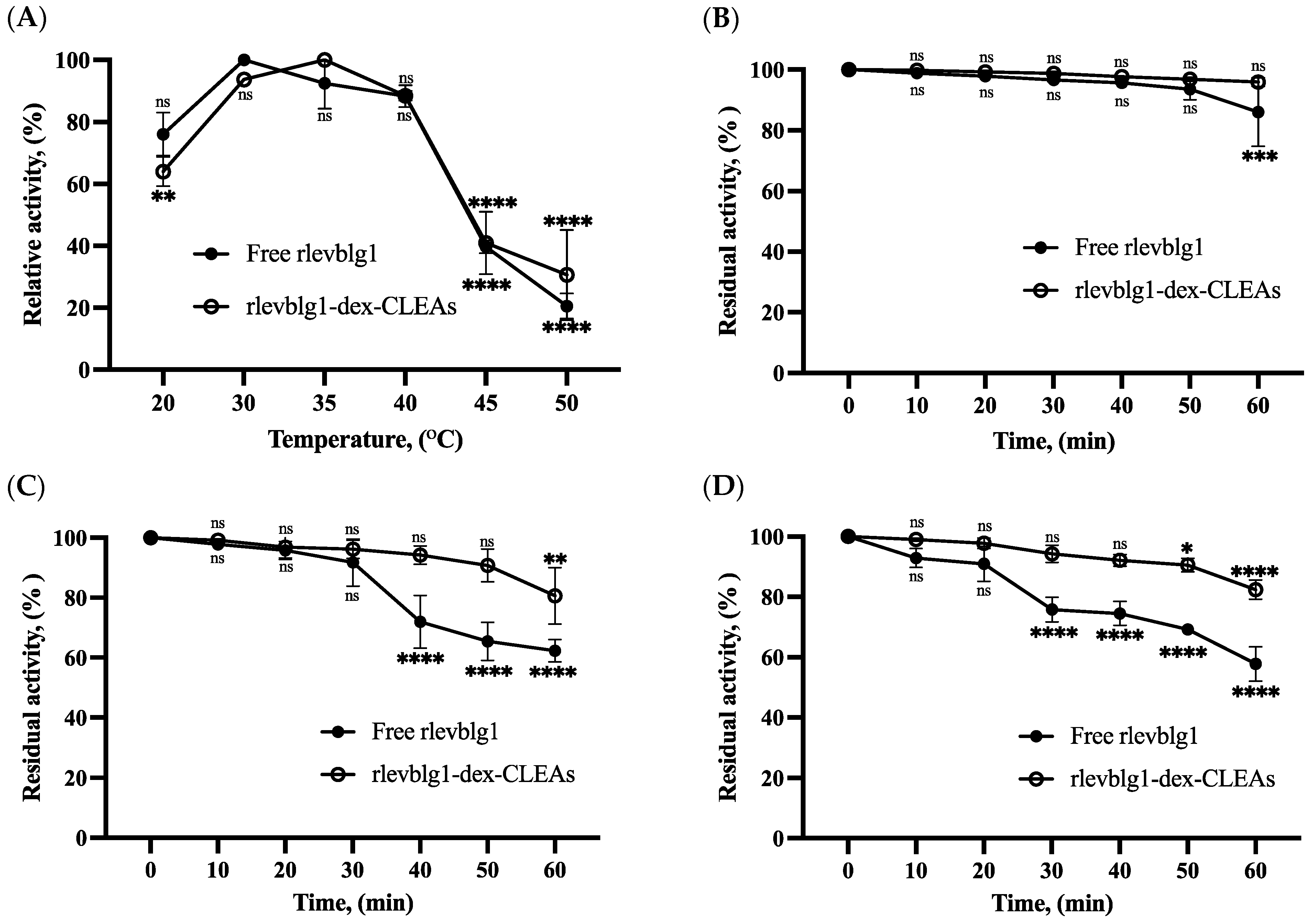
2.2.1. Optimum pH and pH Stability
2.2.2. Optimum Temperature and Thermal Stability
2.2.3. Kinetic Analysis
2.2.4. Effectiveness Factors of rlevblg1-dex-CLEAs
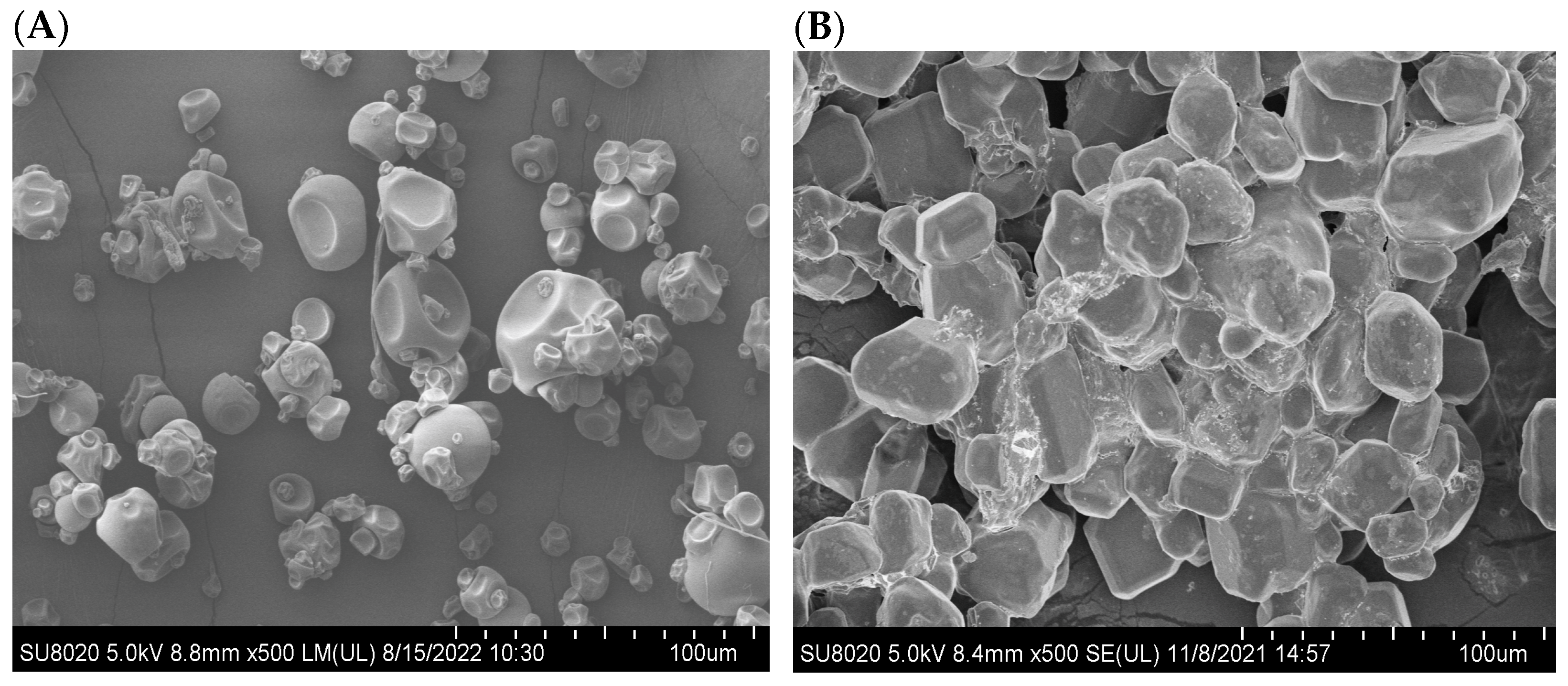
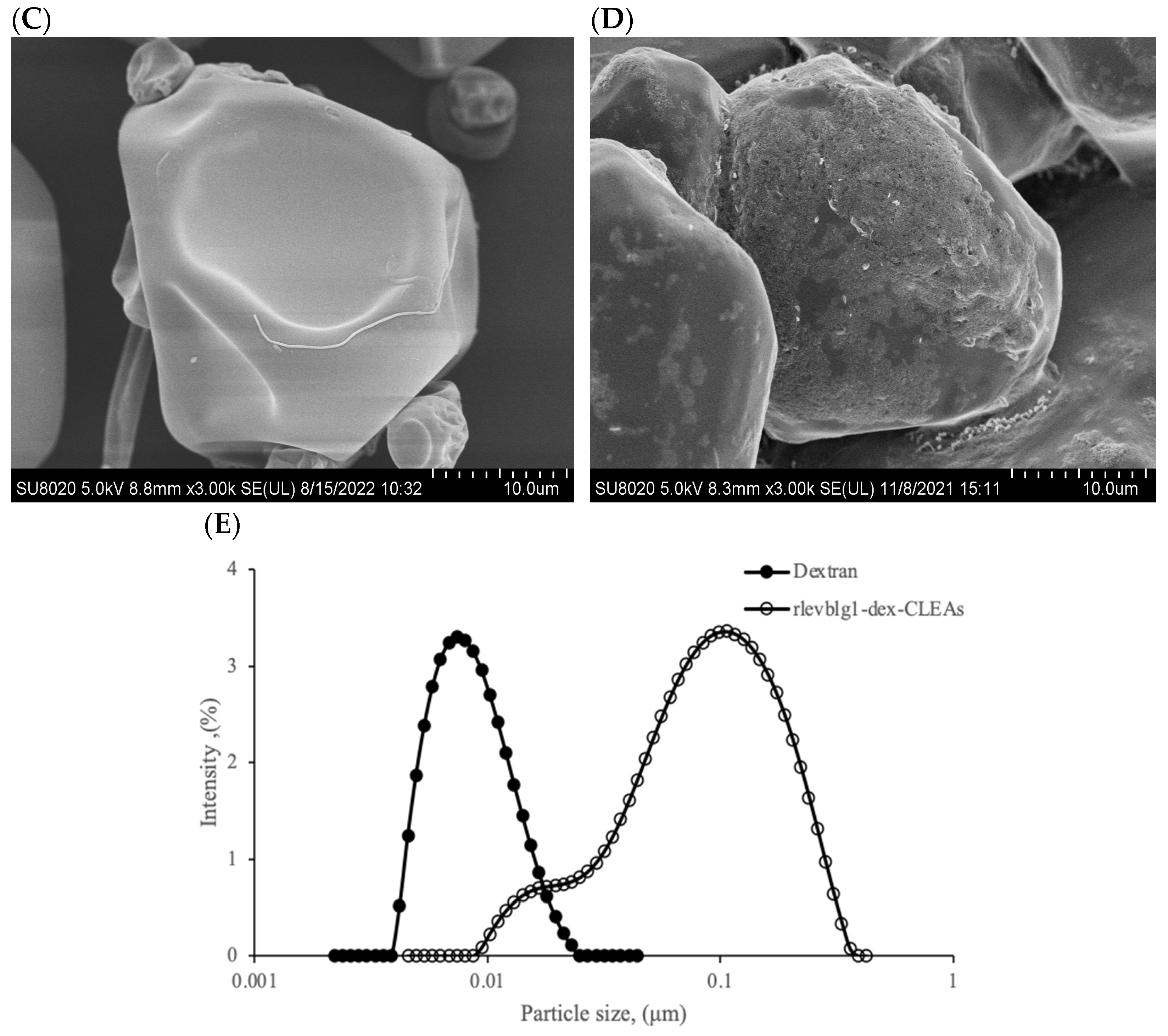
2.2.5. Morphology and Particle Size Distribution
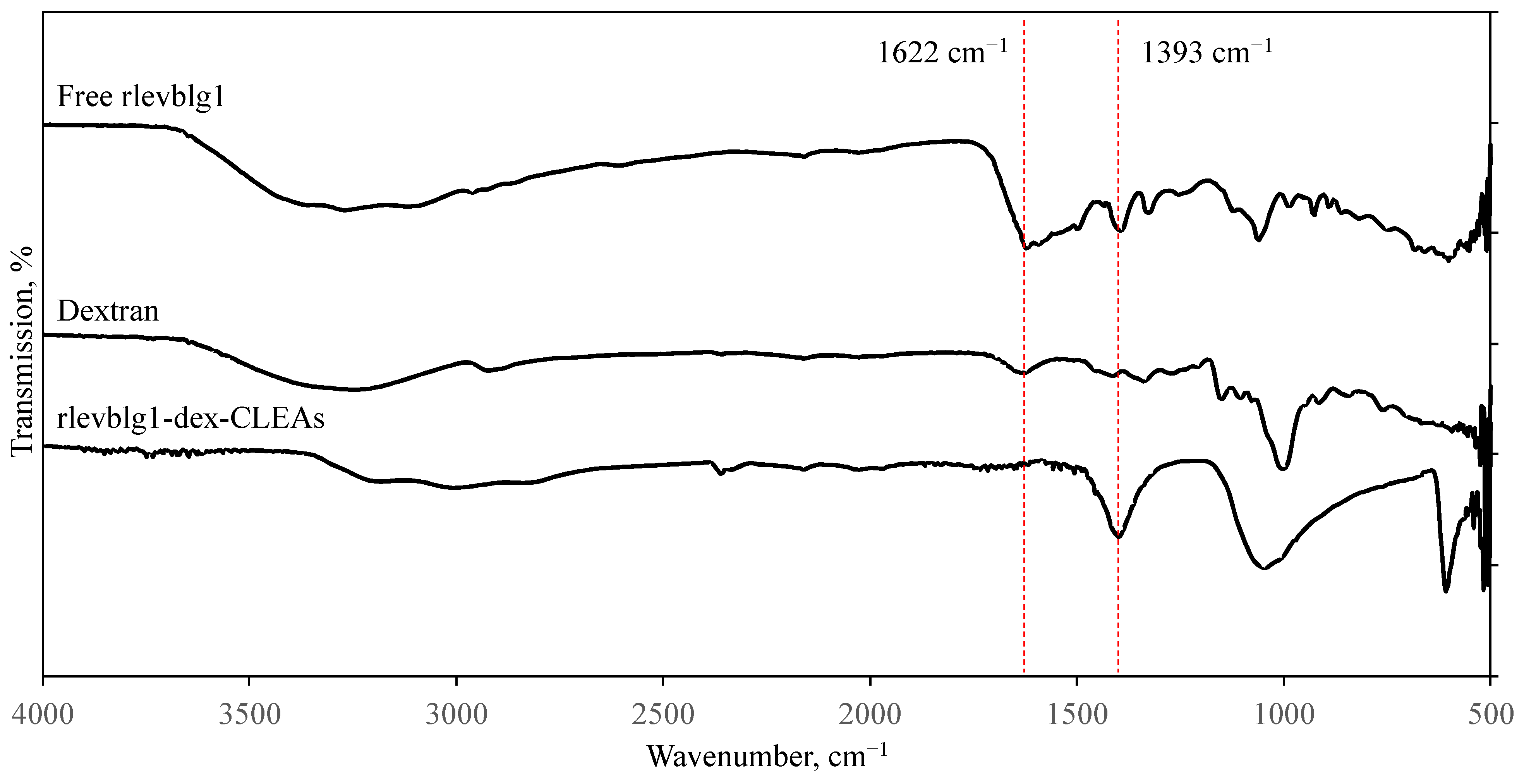
2.2.6. Functional Group Analysis
2.2.7. Reusability and Storage Stability
2.3. L-FOS Production
3. Methodology
3.1. Materials
3.2. Preparation and Optimization of Cross-Linked rlevblg1 Aggregates
3.3. Physiochemical Properties of Free rlevblg1 and rlevblg1-dex-CLEAs
3.4. Characterization of the Biocatalytic Properties and Operational Stability of Free rlevblg1 and rlevblg1-dex-CLEAs
3.4.1. Optimum Temperature and pH
3.4.2. Thermal and pH Stability
3.4.3. Kinetic Analysis of Free and Immobilized rlevblg1
3.4.4. Reusability and Storage Stability of rlevblg1-dex-CLEAs
3.5. Evaluation of the Hydrolysis Reaction Efficiency of rlevblg1-dex-CLEAs
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nawawi, N.N.; Hashim, Z.; Manas, N.H.A.; Azelee, N.I.W.; Illias, R.M. A porous-cross linked enzyme aggregates of maltogenic amylase from Bacillus lehensis G1: Robust biocatalyst with improved stability and substrate diffusion. Int. J. Biol. Macromol. 2020, 148, 1222–1231. [Google Scholar] [CrossRef]
- Matella, N.; Dolan, K.; Lee, Y. Comparison of galactooligosaccharide production in free-enzyme ultrafiltration and in immobilized-enzyme systems. J. Food Sci. 2006, 71, C363–C368. [Google Scholar] [CrossRef]
- Dominguez, A.L.; Rodrigues, L.R.; Lima, N.M.; Teixeira, J.A. An overview of the recent developments on fructooligosaccharide production and applications. Food Bioprocess Technol. 2014, 7, 324–337. [Google Scholar] [CrossRef]
- Sabater-Molina, M.; Larqué, E.; Torrella, F.; Zamora, S. Dietary fructooligosaccharides and potential benefits on health. J. Physiol. Biochem. 2009, 65, 315–328. [Google Scholar] [CrossRef]
- Singh, S.P.; Jadaun, J.S.; Narnoliya, L.K.; Pandey, A. Prebiotic oligosaccharides: Special focus on fructooligosaccharides, its biosynthesis and bioactivity. Appl. Biochem. Biotechnol. 2017, 183, 613–635. [Google Scholar] [CrossRef]
- Sangmanee, S.; Nakapong, S.; Pichyangkura, R.; Kuttiyawong, K. Levan-type fructooligosaccharide production using Bacillus licheniformis RN-01 levansucrase Y246S immobilized on chitosan beads. Songklanakarin J. Sci. Technol. 2016, 38, 295–303. [Google Scholar]
- Porras-Domínguez, J.R.; Ávila-Fernández, Á.; Rodríguez-Alegría, M.E.; Miranda-Molina, A.; Escalante, A.; González-Cervantes, R.; Olvera, C.; Munguía, A.L. Levan-type FOS production using a Bacillus licheniformis endolevanase. Process Biochem. 2014, 49, 783–790. [Google Scholar] [CrossRef]
- Marín-Navarro, J.; Talens-Perales, D.; Polaina, J. One-pot production of fructooligosaccharides by a Saccharomyces cerevisiae strain expressing an engineered invertase. Appl. Microbiol. Biotechnol. 2015, 99, 2549–2555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, W.; Ni, D.; Dai, Q.; Guang, C.; Zhang, T.; Mu, W. An overview of levan-degrading enzyme from microbes. Appl. Microbiol. Biotechnol. 2019, 103, 7891–7902. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Aggarwal, S.; Chakravarty, A.; Ikram, S. A comprehensive review on incredible renewable carriers as promising platforms for enzyme immobilization & thereof strategies. Int. J. Biol. Macromol. 2021, 167, 962–986. [Google Scholar] [PubMed]
- Talekar, S.; Joshi, A.; Joshi, G.; Kamat, P.; Haripurkar, R.; Kambale, S. Parameters in preparation and characterization of cross linked enzyme aggregates (CLEAs). Rsc Adv. 2013, 3, 12485–12511. [Google Scholar] [CrossRef]
- Sheldon, R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates as industrial biocatalysts. Org. Process Res. Dev. 2011, 15, 213–223. [Google Scholar] [CrossRef]
- Sheldon, R. Cross-linked enzyme aggregates (CLEA® s): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.; Schoevaart, R.; Van Langen, L. Cross-linked enzyme aggregates (CLEAs): A novel and versatile method for enzyme immobilization (a review). Biocatal. Biotransform. 2005, 23, 141–147. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. Rsc Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Amaral-Fonseca, M.; Kopp, W.; Giordano, R.d.L.C.; Fernández-Lafuente, R.; Tardioli, P.W. Preparation of magnetic cross-linked amyloglucosidase aggregates: Solving some activity problems. Catalysts 2018, 8, 496. [Google Scholar] [CrossRef]
- Sheldon, R.A. CLEAs, combi-CLEAs and ‘smart’magnetic CLEAs: Biocatalysis in a bio-based economy. Catalysts 2019, 9, 261. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Van Langen, L.M.; Van Rantwijk, F.; Sheldon, R.A. A new, mild cross-linking methodology to prepare cross-linked enzyme aggregates. Biotechnol. Bioeng. 2004, 86, 273–276. [Google Scholar] [CrossRef]
- Arsenault, A.; Cabana, H.; Jones, J.P. Laccase-based CLEAs: Chitosan as a novel cross-linking agent. Enzym. Res. 2011, 1, 376015. [Google Scholar] [CrossRef]
- Nadar, S.S.; Muley, A.B.; Ladole, M.R.; Joshi, P.U. Macromolecular cross-linked enzyme aggregates (M-CLEAs) of α-amylase. Int. J. Biol. Macromol. 2016, 84, 69–78. [Google Scholar] [CrossRef]
- Zhen, Q.; Wang, M.; Qi, W.; Su, R.; He, Z. Preparation of β-mannanase CLEAs using macromolecular cross-linkers. Catal. Sci. Technol. 2013, 3, 1937–1941. [Google Scholar] [CrossRef]
- Jailani, N.; Jaafar, N.R.; Suhaimi, S.; Mackeen, M.M.; Bakar, F.D.A.; Illias, R.M. Cross-linked cyclodextrin glucanotransferase aggregates from Bacillus lehensis G1 for cyclodextrin production: Molecular modeling, developmental, physicochemical, kinetic and thermodynamic properties. Int. J. Biol. Macromol. 2022, 213, 516–533. [Google Scholar] [CrossRef]
- Valdés, E.; Soto, L.; Arcaya, G. Influence of the pH of glutaraldehyde and the use of dextran aldehyde on the preparation of cross-linked enzyme aggregates (CLEAs) of lipase from Burkholderia cepacia. Electron. J. Biotechnol. 2011, 14, 10. [Google Scholar] [CrossRef]
- Talekar, S.; Nadar, S.; Joshi, A.; Joshi, G. Pectin cross-linked enzyme aggregates (pectin-CLEAs) of glucoamylase. RSC Adv. 2014, 4, 59444–59453. [Google Scholar] [CrossRef]
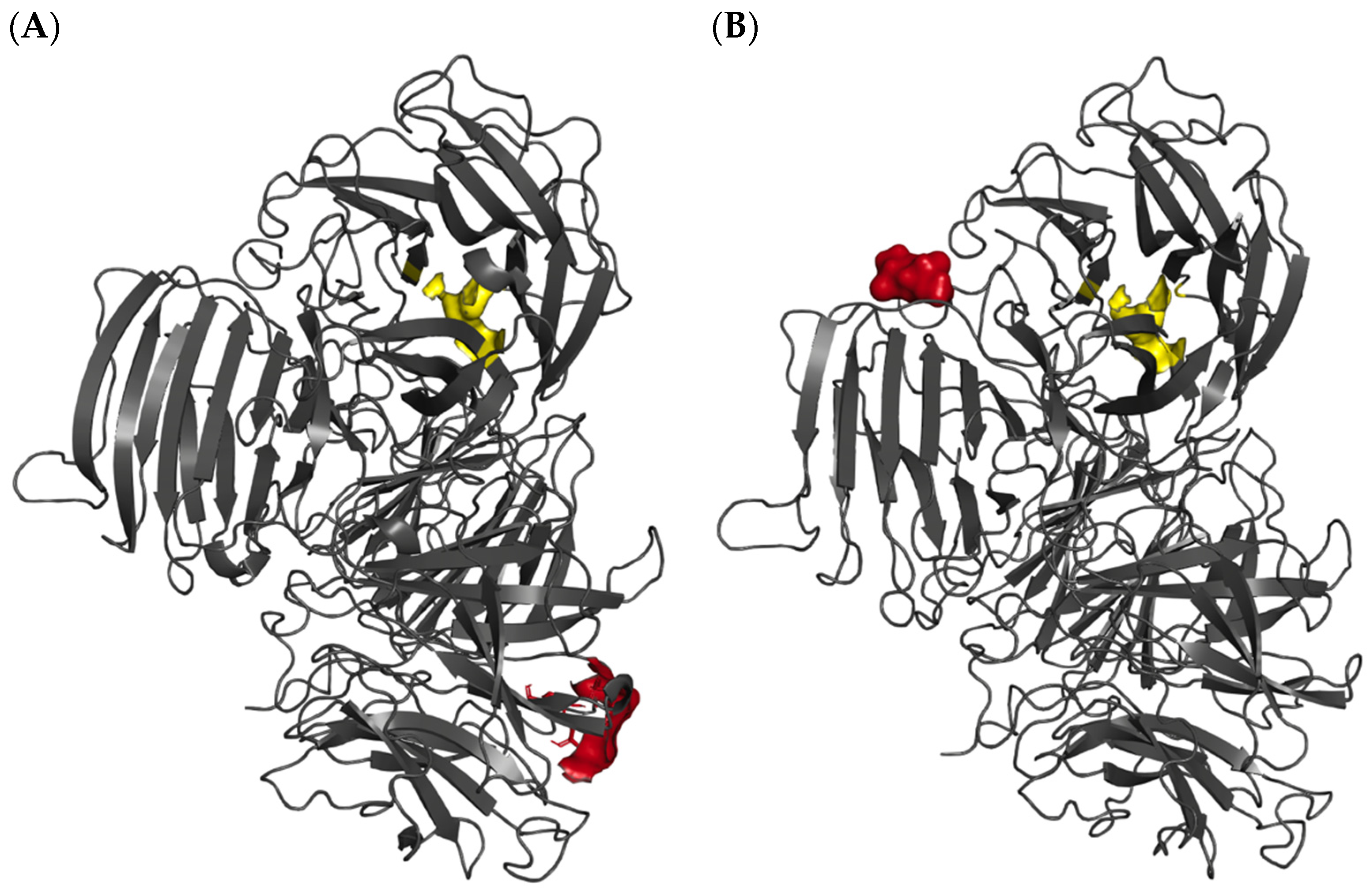
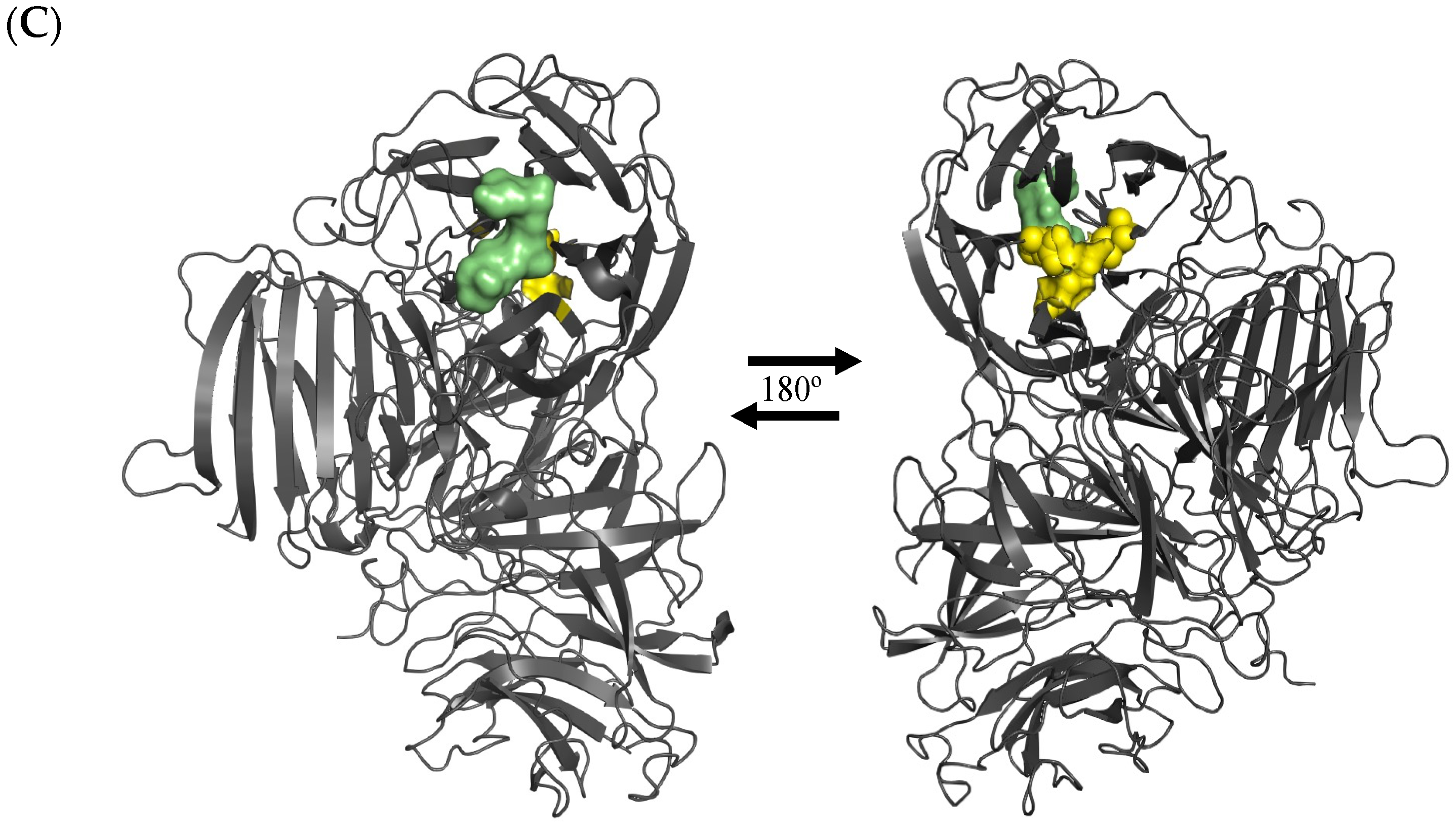
- Makki, H.H.; Jaafar, N.R.; Rahman, R.A.; Rahmat, Z.; Puspaningsih, N.N.T.; Illias, R.M. Protein interaction studies of cross-linked endolevanase aggregates from Bacillus lehensis G1. J. Teknol. (Sci. Eng.) 2024, 86, 159–167. [Google Scholar] [CrossRef]
- Cao, L.; van Langen, L.; Sheldon, R.A. Immobilised enzymes: Carrier-bound or carrier-free? Curr. Opin. Biotechnol. 2003, 14, 387–394. [Google Scholar] [CrossRef]
- Dong, T.; Zhao, L.; Huang, Y.; Tan, X. Preparation of cross-linked aggregates of aminoacylase from Aspergillus melleus by using bovine serum albumin as an inert additive. Bioresour. Technol. 2010, 101, 6569–6571. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Yao, S.; Qian, J.; Guo, H.; Cai, X. Preparation of immobilized lipase on magnetic nanoparticles dialdehyde starch. Carbohydr. Polym. 2019, 218, 324–332. [Google Scholar] [CrossRef]
- Rehman, S.; Bhatti, H.N.; Bilal, M.; Asgher, M. Cross-linked enzyme aggregates (CLEAs) of Pencilluim notatum lipase enzyme with improved activity, stability and reusability characteristics. Int. J. Biol. Macromol. 2016, 91, 1161–1169. [Google Scholar] [CrossRef]
- Talekar, S.; Ghodake, V.; Ghotage, T.; Rathod, P.; Deshmukh, P.; Nadar, S.; Mulla, M.; Ladole, M. Novel magnetic cross-linked enzyme aggregates (magnetic CLEAs) of alpha amylase. Bioresour. Technol. 2012, 123, 542–547. [Google Scholar] [CrossRef]
- Rahman, M.A.; Culsum, U.; Kumar, A.; Gao, H.; Hu, N. Immobilization of a novel cold active esterase onto Fe3O4∼ cellulose nano-composite enhances catalytic properties. Int. J. Biol. Macromol. 2016, 87, 488–497. [Google Scholar] [CrossRef]
- Liao, Q.; Du, X.; Jiang, W.; Tong, Y.; Zhao, Z.; Fang, R.; Feng, J.; Tang, L. Cross-linked enzyme aggregates (CLEAs) of halohydrin dehalogenase from Agrobacterium radiobacter AD1: Preparation, characterization and application as a biocatalyst. J. Biotechnol. 2018, 272, 48–55. [Google Scholar] [CrossRef]
- Abd Rahman, N.H.; Jaafar, N.R.; Annuar, N.A.S.; Rahman, R.A.; Murad, A.M.A.; El-Enshasy, H.A.; Illias, R.M. Efficient substrate accessibility of cross-linked levanase aggregates using dialdehyde starch as a macromolecular cross-linker. Carbohydr. Polym. 2021, 267, 118159. [Google Scholar] [CrossRef]
- Miao, C.; Li, H.; Zhuang, X.; Wang, Z.; Yang, L.; Lv, P.; Luo, W. Synthesis and properties of porous CLEAs lipase by the calcium carbonate template method and its application in biodiesel production. RSC Adv. 2019, 9, 29665–29675. [Google Scholar] [CrossRef]
- Abd Rahman, N.H.; Jaafar, N.R.; Murad, A.M.A.; Bakar, F.D.A.; Annuar, N.A.S.; Illias, R.M. Novel cross-linked enzyme aggregates of levanase from Bacillus lehensis G1 for short-chain fructooligosaccharides synthesis: Developmental, physicochemical, kinetic and thermodynamic properties. Int. J. Biol. Macromol. 2020, 159, 577–589. [Google Scholar] [CrossRef]
- Yakup Arica, M.; Soydogan, H.; Bayramoglu, G. Reversible immobilization of Candida rugosa lipase on fibrous polymer grafted and sulfonated p (HEMA/EGDMA) beads. Bioprocess Biosyst. Eng. 2010, 33, 227–236. [Google Scholar] [CrossRef]
- Kumar, V.V.; Sivanesan, S.; Cabana, H. Magnetic cross-linked laccase aggregates—Bioremediation tool for decolorization of distinct classes of recalcitrant dyes. Sci. Total Environ. 2014, 487, 830–839. [Google Scholar] [CrossRef]
- Mortazavi, S.; Aghaei, H. Make proper surfaces for immobilization of enzymes: Immobilization of lipase and α-amylase on modified Na-sepiolite. Int. J. Biol. Macromol. 2020, 164, 1–12. [Google Scholar] [CrossRef]
- Sanjay, G.; Sugunan, S. Glucoamylase immobilized on montmorillonite: Synthesis, characterization and starch hydrolysis activity in a fixed bed reactor. Catal. Commun. 2005, 6, 525–530. [Google Scholar] [CrossRef]
- Schoevaart, R.; Wolbers, M.; Golubovic, M.; Ottens, M.; Kieboom, A.; Van Rantwijk, F.; Van Der Wielen, L.; Sheldon, R. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef]
- Torabizadeh, H.; Mikani, M. Nano-magnetic cross-linked enzyme aggregates of naringinase an efficient nanobiocatalyst for naringin hydrolysis. Int. J. Biol. Macromol. 2018, 117, 134–143. [Google Scholar] [CrossRef]
- Ramirez, L.M.F.; Rihouey, C.; Chaubet, F.; Le Cerf, D.; Picton, L. Characterization of dextran particle size: How frit-inlet asymmetrical flow field-flow fractionation (FI-AF4) coupled online with dynamic light scattering (DLS) leads to enhanced size distribution. J. Chromatogr. A 2021, 1653, 462404. [Google Scholar] [CrossRef]
- Kamal, M.A.; Brizioli, M.; Zinn, T.; Narayanan, T.; Cerbino, R.; Giavazzi, F.; Pal, A. Dynamics of anisotropic colloidal systems: What to choose, DLS, DDM or XPCS? J. Colloid Interface Sci. 2024, 660, 314–320. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Ghiaci, M.; Aghaei, H.; Soleimanian, S.; Sedaghat, M. Enzyme immobilization: Part 2: Immobilization of alkaline phosphatase on Na-bentonite and modified bentonite. Appl. Clay Sci. 2009, 43, 308–316. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef]
- D’Souza, L.; Devi, P.; Divya Shridhar, M.; Naik, C.G. Use of Fourier Transform Infrared (FTIR) spectroscopy to study cadmium-induced changes in Padina tetrastromatica (Hauck). Anal. Chem. Insights 2008, 3, 117739010800300001. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. Cellulose fibers hydrophobization via a hybrid chemical modification. Polymers 2019, 11, 1174. [Google Scholar] [CrossRef]
- Lv, B.-H.; Tan, W.; Zhu, C.-C.; Shang, X.; Zhang, L. Properties of a stable and sustained-release formulation of recombinant human parathyroid hormone (rhPTH) with chitosan and silk fibroin microparticles. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 7532. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Qian, J.; Zhao, C.; Yang, H.; Zhao, X.; Guo, H. Study on the relationship between crosslinking degree and properties of TPP crosslinked chitosan nanoparticles. Carbohydr. Polym. 2020, 241, 116349. [Google Scholar] [CrossRef]
- Ellerbrock, R.H.; Gerke, H.H. FTIR spectral band shifts explained by OM–cation interactions. J. Plant Nutr. Soil Sci. 2021, 184, 388–397. [Google Scholar] [CrossRef]
- Steen Redeker, E.; Ta, D.T.; Cortens, D.; Billen, B.; Guedens, W.; Adriaensens, P. Protein engineering for directed immobilization. Bioconjugate Chem. 2013, 24, 1761–1777. [Google Scholar] [CrossRef]
- Ottone, C.; Romero, O.; Urrutia, P.; Bernal, C.; Illanes, A.; Wilson, L. Enzyme biocatalysis and sustainability. In Nanostructured Catalysts for Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 383–413. [Google Scholar]
- Ernits, K.; Eek, P.; Lukk, T.; Visnapuu, T.; Alamäe, T. First crystal structure of an endo-levanase–the BT1760 from a human gut commensal Bacteroides thetaiotaomicron. Sci. Rep. 2019, 9, 8443. [Google Scholar]
- Ávila-Fernández, Á.; Montiel, S.; Rodríguez-Alegría, M.E.; Caspeta, L.; López Munguía, A. Simultaneous enzyme production, Levan-type FOS synthesis and sugar by-products elimination using a recombinant Pichia pastoris strain expressing a levansucrase-endolevanase fusion enzyme. Microb. Cell Factories 2023, 22, 18. [Google Scholar] [CrossRef]
- Miasnikov, A.N. Characterization of a novel endo-levanase and its gene from Bacillus sp. L7. FEMS Microbiol. Lett. 1997, 154, 23–28. [Google Scholar] [CrossRef]
- Murakami, H.; Kuramoto, T.; Mizutani, K.; Nakano, H.; Kitahata, S. Purification and some properties of a new levanase from Bacillus sp. No. 71. Biosci. Biotechnol. Biochem. 1992, 56, 608–613. [Google Scholar] [CrossRef][Green Version]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Kumar, V.V.; Kumar, M.P.; Thiruvenkadaravi, K.; Baskaralingam, P.; Kumar, P.S.; Sivanesan, S. Preparation and characterization of porous cross linked laccase aggregates for the decolorization of triphenyl methane and reactive dyes. Bioresour. Technol. 2012, 119, 28–34. [Google Scholar] [CrossRef] [PubMed]



| Kinetic Parameter | Vmax (mM·min−1) | Km (mM) | kcat (s−1) | Catalytic Efficiency (kcat/Km) (mM−1 s−1) |
|---|---|---|---|---|
| Free rlevblg1 | 10.26 ± 0.1 | 14.80 ± 1.6 | 11.88 ± 0.1 | 0.81 ± 0.0 |
| rlevblg1-dex-CLEAs | 8.38± 0.9 | 13.79 ± 2.7 | 9.70 ± 1.0 | 0.71 ± 0.2 |
| Levan mg/mL | Free rlevblg1 Velocity (μmole/mg·min) | ƞ | rlevblg1-dex-CLEAs Velocity (μmole/mg·min) | ƞ |
|---|---|---|---|---|
| 1 | 0.48 ± 0.12 | 1.00 | 0.27 ± 0.03 | 0.56 |
| 2 | 1.42 ± 0.41 | 1.00 | 1.14 ± 0.14 | 0.80 |
| 4 | 2.82 ± 0.24 | 1.00 | 2.05 ± 0.03 | 0.72 |
| 6 | 2.97 ± 0.06 | 1.00 | 2.47 ± 0.00 | 0.83 |
| 8 | 3.38 ± 0.37 | 1.00 | 2.98 ± 0.21 | 0.88 |
| 10 | 4.39 ± 0.32 | 1.00 | 3.85 ± 0.46 | 0.87 |
| 12 | 4.62 ± 0.18 | 1.00 | 4.44 ± 0.66 | 0.96 |
| 14 | 4.80 ± 0.19 | 1.00 | 4.17 ± 0.00 | 0.86 |
| 20 | 4.94± 0.36 | 1.00 | 4.29 ± 0.01 | 0.86 |
| 25 | 3.86 ± 1.02 | 1.00 | 3.18 ± 0.42 | 0.82 |
| Time (h) | Fructose (mg/g) | DP2 (mg/g) | DP3 (mg/g) | DP5 (mg/g) | Total Reducing Sugar (mg/g) | Total L-FOS (mg/g) | Product Ratio |
|---|---|---|---|---|---|---|---|
| 1 | 0.0 ± 0.0 | 61.7 ± 26.2 | 21.9 ± 12.1 | 1284.2 ± 1.3 | 1367.9 | 1367.9 | 1.00 |
| 2 | 14.6 ± 8.6 | 249.1 ± 28.9 | 40.5 ± 15.0 | 1425.8 ± 15.3 | 1730.1 | 1715.5 | 0.99 |
| 3 | 16.9 ± 0.5 | 208.6 ± 1.2 | 167.8 ± 5.2 | 1519.7 ± 22.0 | 1913.1 | 1896.2 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makki, H.H.; Jaafar, N.R.; Jailani, N.; Alqasem, A.A.; Rahmat, Z.; Illias, R.M. Strategy in Synthesizing Longer-Chain Levan-Type Fructooligosaccharides by Selective Dextran Macromolecular Cross-Linked Bacillus lehensis G1 Endolevanase Aggregate Immobilization. Catalysts 2024, 14, 584. https://doi.org/10.3390/catal14090584
Makki HH, Jaafar NR, Jailani N, Alqasem AA, Rahmat Z, Illias RM. Strategy in Synthesizing Longer-Chain Levan-Type Fructooligosaccharides by Selective Dextran Macromolecular Cross-Linked Bacillus lehensis G1 Endolevanase Aggregate Immobilization. Catalysts. 2024; 14(9):584. https://doi.org/10.3390/catal14090584
Chicago/Turabian StyleMakki, Hotaf Hassan, Nardiah Rizwana Jaafar, Nashriq Jailani, Abdullah A. Alqasem, Zaidah Rahmat, and Rosli Md. Illias. 2024. "Strategy in Synthesizing Longer-Chain Levan-Type Fructooligosaccharides by Selective Dextran Macromolecular Cross-Linked Bacillus lehensis G1 Endolevanase Aggregate Immobilization" Catalysts 14, no. 9: 584. https://doi.org/10.3390/catal14090584
APA StyleMakki, H. H., Jaafar, N. R., Jailani, N., Alqasem, A. A., Rahmat, Z., & Illias, R. M. (2024). Strategy in Synthesizing Longer-Chain Levan-Type Fructooligosaccharides by Selective Dextran Macromolecular Cross-Linked Bacillus lehensis G1 Endolevanase Aggregate Immobilization. Catalysts, 14(9), 584. https://doi.org/10.3390/catal14090584





