Enhanced Photocatalytic Degradation of Herbicide 2,4-Dichlorophenoxyacetic Acid Using Sulfated CeO2
Abstract
:1. Introduction
2. Results and Discussion
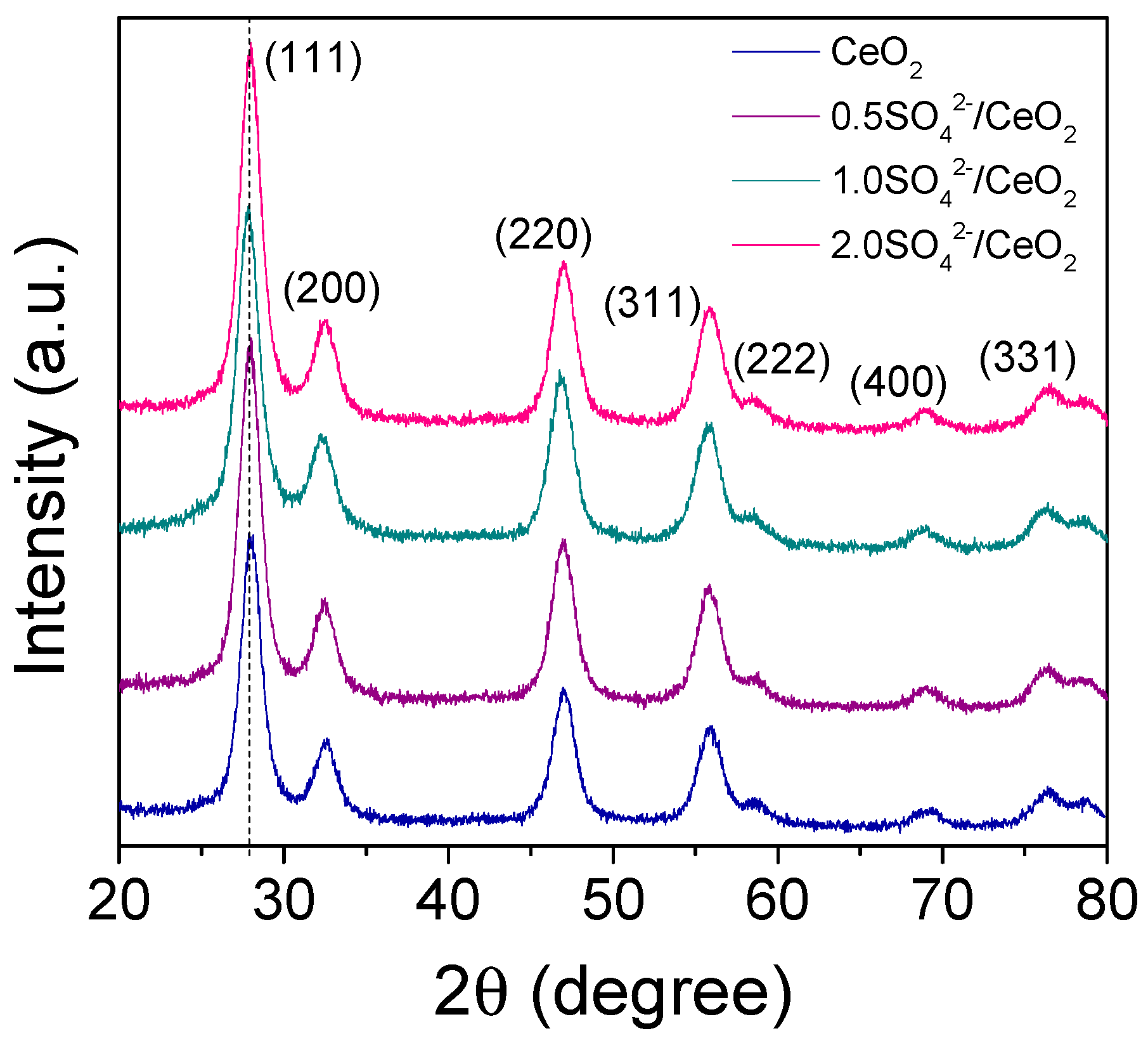
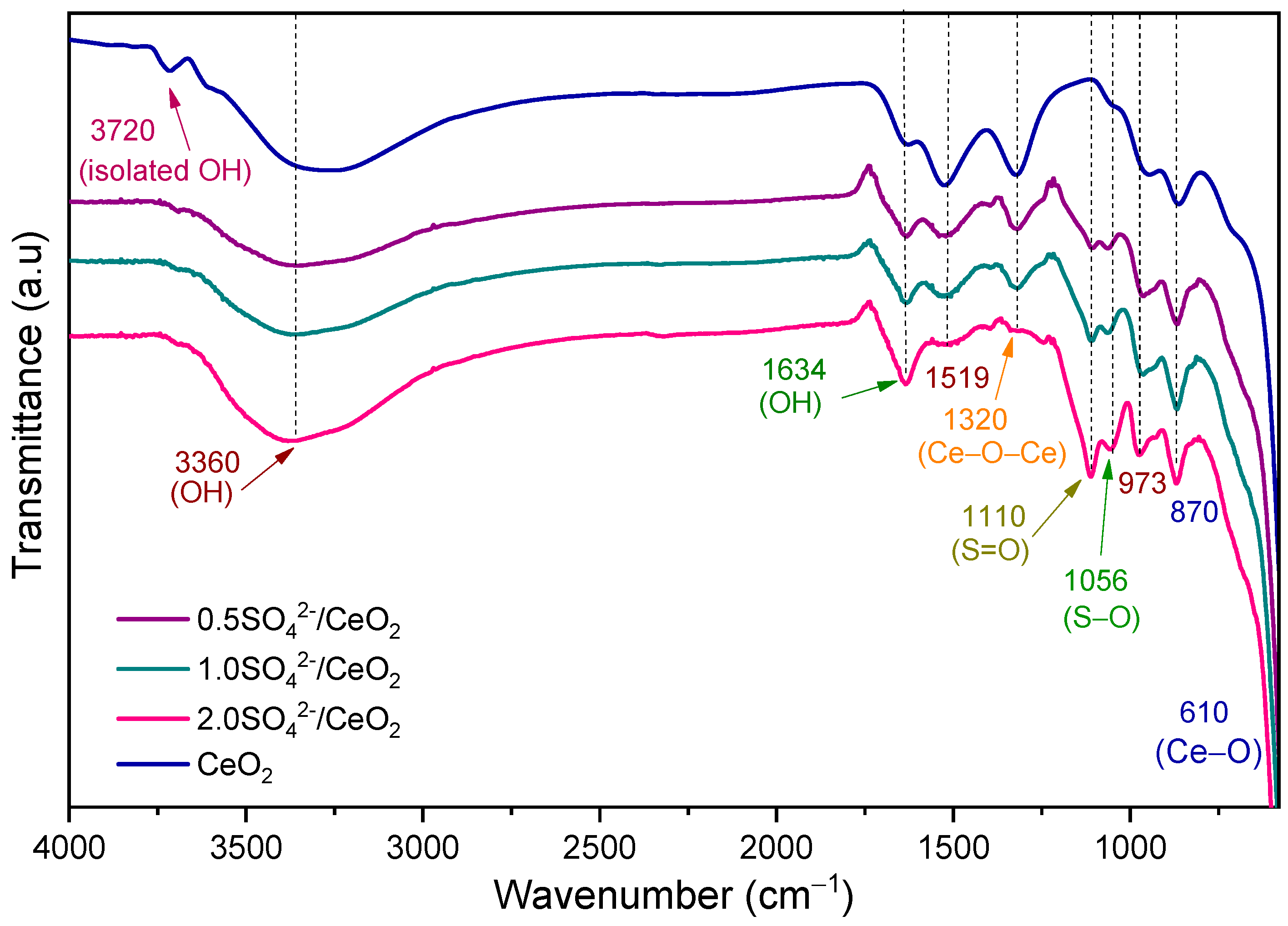
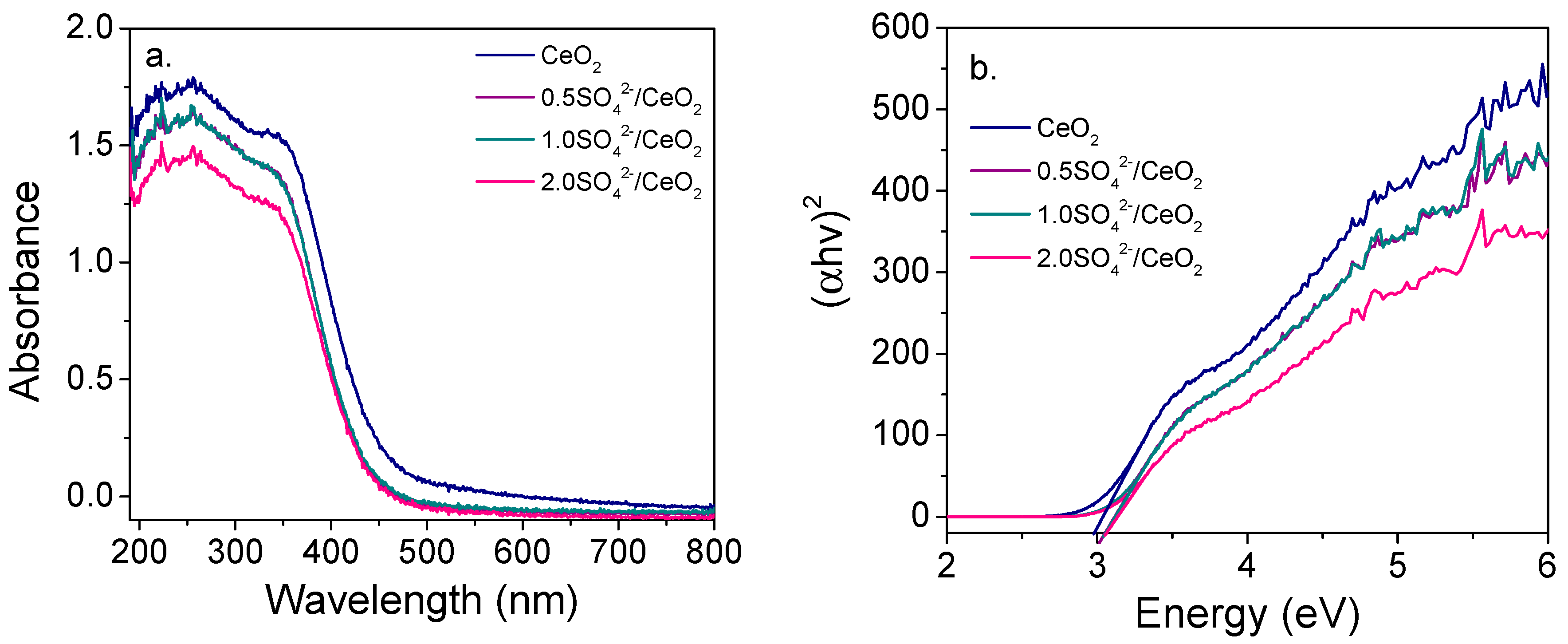
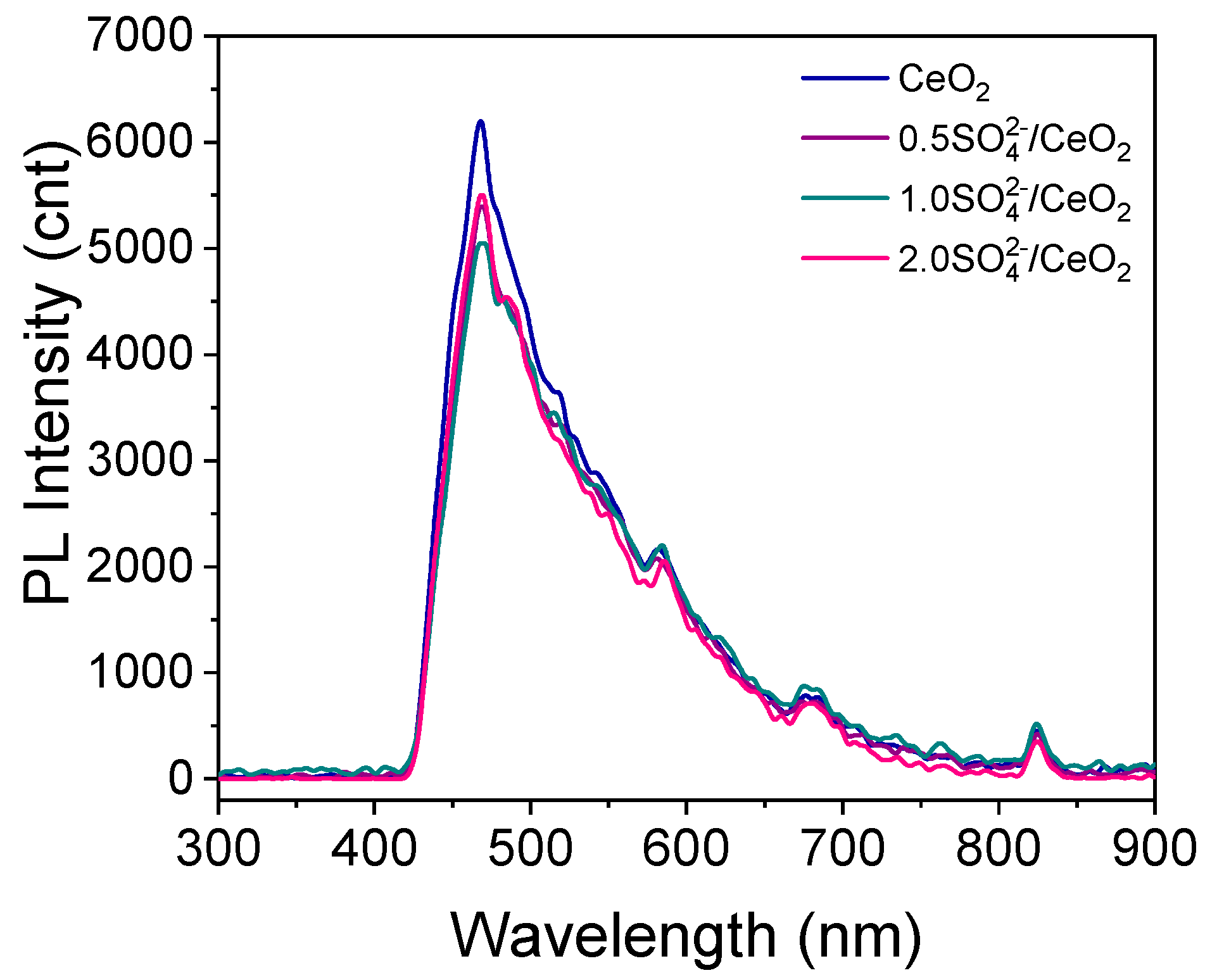
2.1. Characterization of the Photocatalysts
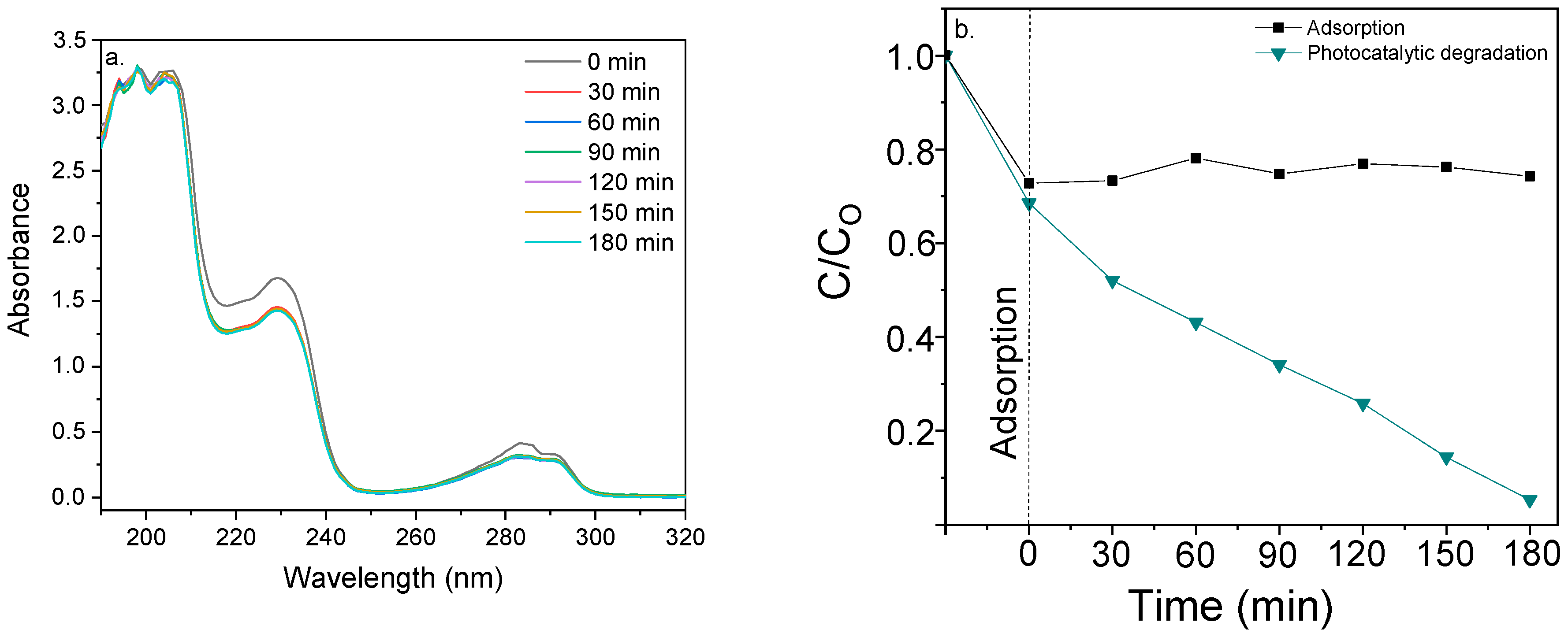
2.2. Evaluation of the Photocatalytic Activity
Photocatalytic Degradation of 2,4-Dichlorophenoxyacetic Acid
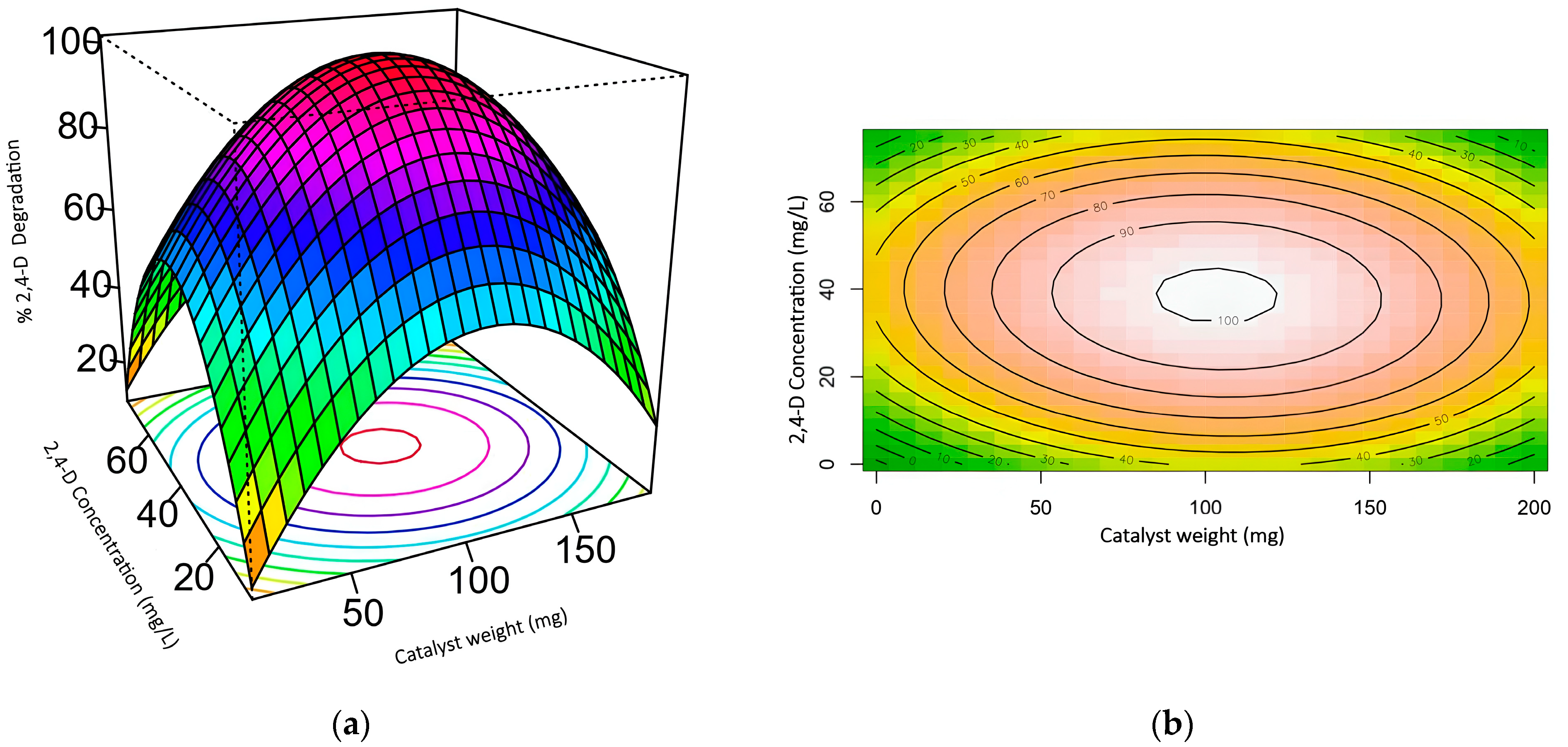
2.3. Response Surface Methodology (RSM)
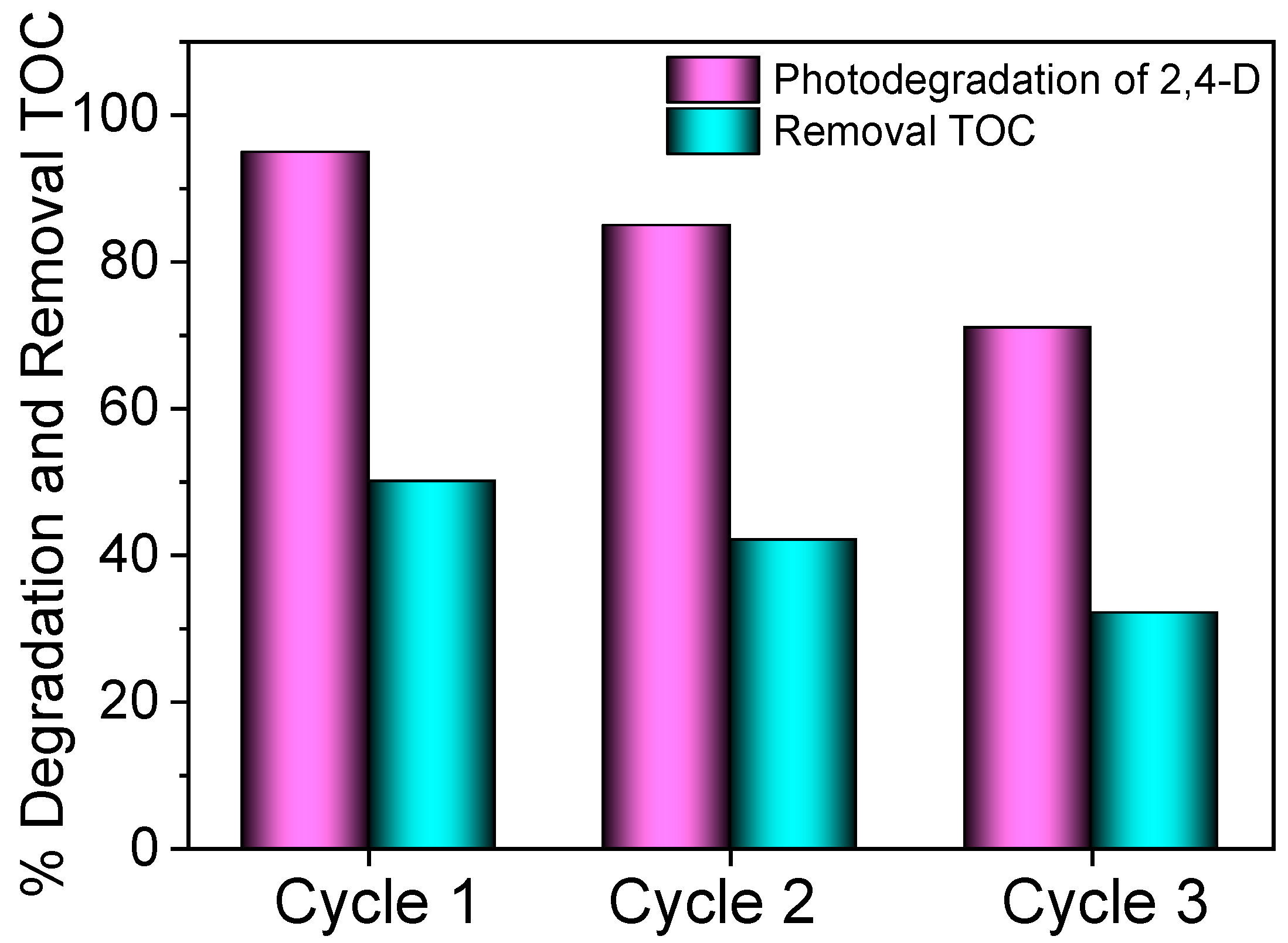
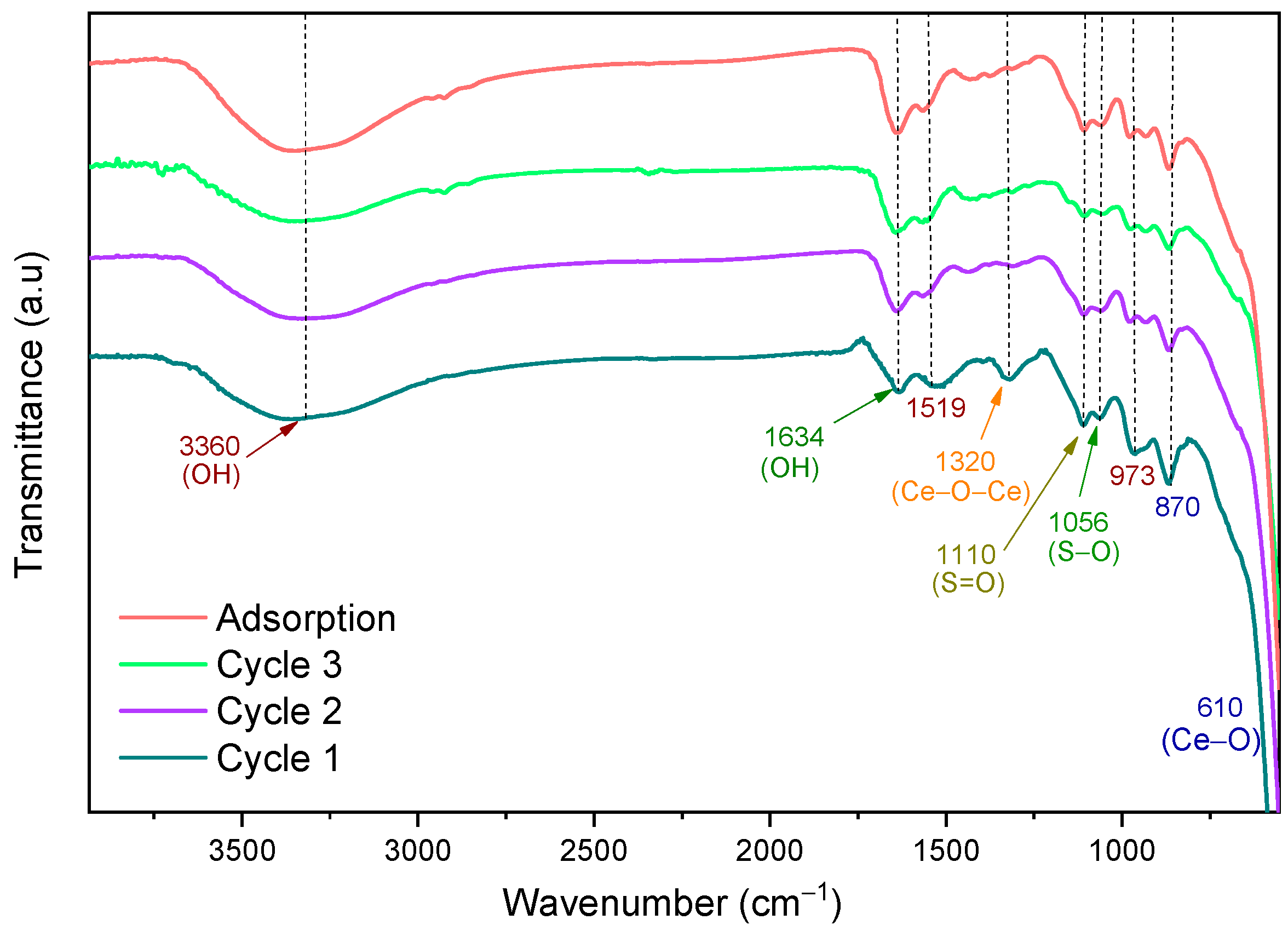
2.4. Reuse of the 1.0SO42−/CeO2 Photocatalyst in the Degradation of 2,4-Dichlorophenoxyacetic Aacid
3. Materials and Methods
3.1. Synthesis of the Photocatalysts
3.2. Charactherization of the Photocatalysts
3.3. Evaluation of the Photocatalytic Activity
3.4. Experimental Design-Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Calculated | Standard Error | t-Value | Pr (>|t|) | |
|---|---|---|---|---|
| (Intercept) | −22.782 | 12.182 | −1.8701 | 0.1106605 |
| Block 2 | 19.766 | 3.5074 | 5.6355 | 0.0013366 |
| Mass | 1.0057 | 1.2655 × 10−1 | 7.9473 | 0.0002111 |
| Concentration | 3.2438 | 3.4174 × 10−1 | 9.4919 | 7.791 × 10−5 |
| Mass:Concentration | −1.3333 × 10−3 | 1.9027 × 10−3 | −0.7008 | 0.5097046 |
| Mass2 | −4.6131 × 10−3 | 4.9778 × 10−4 | −9.2673 | 8.923 × 10−5 |
| Concentration2 | −4.0299 × 10−2 | 3.4945 × 10−3 | −11.5321 | 2.5560 × 10−5 |
| R2 = 0.9726 R2 adjusted = 0.9452 p-value: 0.0001973 F-Statistic: 35.5 The stationary point of the response surface: OPTIMAL CONDITIONS | ||||
| Mass (mg) 103.3840 | Concentration (ppm) 38.53572 | |||
References
- Mehdizadeh, M.; Mushtaq, W.; Siddiqui, S.A.; Ayadi, S.; Kaur, P.; Yeboah, S.; Mazraedoost, S.; K.A.Al Taey, D.; Tampubolon, K. Herbicide Residues in Agroecosystems: Fate, Detection, and Effect on Non-Target Plants. Rev. Agric. Sci. 2021, 9, 157–167. [Google Scholar] [CrossRef] [PubMed]
- McGinley, J.; Healy, M.G.; Ryan, P.C.; Mellander, P.E.; Morrison, L.; O’Driscoll, J.H.; Siggins, A. Batch adsorption of herbi-cides from aqueous solution onto diverse reusable materials and granulated activated carbon. J. Environ. Manag. 2022, 323, 116102. [Google Scholar] [CrossRef]
- Shabeer, T.A.; Saha, A.; Gajbhiye, V.; Gupta, S.; Manjaiah, K.; Varghese, E. Simultaneous removal of multiple pesticides from water: Effect of organically modified clays as coagulant aid and adsorbent in coagulation–flocculation process. Environ. Technol. 2014, 35, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Acosta Santoyo, G.; Llanos, J.; Raschitor, A.; Bustos, E.; Cañizares, P.; Rodrigo, M. Performance of ultrafiltration as a pre-concentration stage for the treatment of oxyfluorfen by electrochemical BDD oxidation. Sep. Purif. Technol. 2019, 237, 116366. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, Y.S.; Gao, J.J.; Xu, J.; Li, Z.J.; Fu, X.Y.; Han, H.J.; Wang, L.J.; Zhang, W.H.; Deng, Y.D.; et al. Complete biodegradation of the oldest organic herbicide 2,4-Dichlorophenoxyacetic acid by engineering Escherichia coli. J. Hazard. Mater. 2023, 451, 131099. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, C.; Gutiérrez, K.; Alvarado, I.; Martínez, J.J.; Rojas, H.; Tzompantzi, F.; Gómez, R. Effective phosphated CeO2 materials in the photocatalytic degradation of phenol under UV irradiation. J. Chem. Technol. Biotechnol. 2020, 95, 3213–3220. [Google Scholar] [CrossRef]
- Zhang, X.; Su, L.; Kong, Y.; Ma, D.; Ran, Y.; Peng, S.; Wang, L.; Wang, Y. CeO2 nanoparticles modified by CuO nanoparticles for low-temperature CO oxidation with high catalytic activity. J. Phys. Chem. Solids 2020, 147, 109651. [Google Scholar] [CrossRef]
- Li, B.; Zhang, B.; Nie, S.; Shao, L.; Hu, L. Optimization of plasmon-induced photocatalysis in electrospun Au/CeO2 hybrid nanofibers for selective oxidation of benzyl alcohol. J. Catal. 2017, 348, 256–264. [Google Scholar] [CrossRef]
- Chang, J.; Ma, Q.; Ma, J.; Ma, H. Synthesis of Fe3O4 nanowire@CeO2/Ag nanocomposites with enhanced photocatalytic activity under sunlight exposure. Ceram. Int. 2016, 42, 11827–11837. [Google Scholar] [CrossRef]
- Valente, J.S.; Tzompantzi, F.; Prince, J. Highly efficient photocatalytic elimination of phenol and chlorinated phenols by CeO2/MgAl layered double hydroxides. Appl. Catal. B Environ 2011, 102, 276–285. [Google Scholar] [CrossRef]
- Vita, A. Catalytic Applications of CeO2-Based Materials. Catalysts 2020, 10, 576. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Deshmukh, S.P.; More, K.V.; Shevale, V.B.; Mullani, S.B.; Dhodamani, A.G.; Delekar, S.D. Sulfated TiO2/WO3 nanocomposite: An efficient photocatalyst for degradation of Congo red and methyl red dyes under visible light irradiation. Mater. Chem. Phys. 2019, 225, 247–255. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Wang, L.; Hao, Y.; Yang, L.; He, P.; Xue, J. Preparation and Characterization of Sulfated TiO2/Zeolite Composite Catalysts with Enhanced Photocatalytic Activity. Nano 2018, 13, 1850117. [Google Scholar] [CrossRef]
- Svensson, F.G.; Österlund, L. Adsorption and Photo-Degradation of Organophosphates on Sulfate-Terminated Anatase TiO2 Nanoparticles. Catalysts 2023, 13, 526. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Castañeda, C.; Tzompantzi, F.; Gómez, R.; Rojas, H. Enhanced photocatalytic degradation of 4-chlorophenol and 2,4-dichlorophenol on in situ phosphated sol-gel TiO2. J. Chem. Technol. Biotechnol. 2016, 91, 2170–2178. [Google Scholar] [CrossRef]
- Gómez, R.; López, T.; Ortiz-Islas, E.; Navarrete, J.; Sánchez, E.; Tzompanztzi, F.; Bokhimi, X. Effect of sulfation on the photo-activity of TiO2 sol–gel derived catalysts. J. Mol. Catal. A Chem. 2003, 193, 217–226. [Google Scholar] [CrossRef]
- Reddy, P.S.; Sudarsanam, P.; Mallesham, B.; Raju, G.; Reddy, B.M. Acetalisation of glycerol with acetone over zirconia and promoted zirconia catalysts under mild reaction conditions. J. Ind. Eng. Chem. 2011, 17, 377–381. [Google Scholar] [CrossRef]
- Khder, A.S.; El Sharkawy, E.A.; El Hakam, S.A.; Ahmed, A.I. Surface characterization and catalytic activity of sulfated tin oxide catalyst. Catal. Commun. 2007, 9, 769–777. [Google Scholar] [CrossRef]
- Marlowe, J.; Acharya, S.; Zuber, A.; Tsilomelekis, G. Characterization of Sulfated SnO2-ZrO2 Catalysts and Their Catalytic Performance on the Tert-Butylation of Phenol. Catalysts 2020, 10, 726. [Google Scholar] [CrossRef]
- Vinothini, R.; Tamizhdurai, P.; Mangesh, V.L.; Vanjinathan, M.; Pazhanisamy, P.; Kumaran, R.; Kumar, N.S.; Al Fatesh, A.S.; Kasim, S. Synthesis of CeO2/SO4/Ni nanoparticle with a greener perspective for the selective oxidation of propylene glycol in continuous reactor. Mol. Catal. 2023, 549, 113475. [Google Scholar] [CrossRef]
- Farisya, M.R.S.; Irmawati, R.; Shafizah, I.N.; Taufiq Yap, Y.H.; Muhamad, E.N.; Lee, S.L.; Salamun, N. Assessment on the effect of sulfuric acid concentration on physicochemical properties of Sulfated-Titania catalyst and glycerol acetylation performance. Catalysts 2021, 11, 1542. [Google Scholar] [CrossRef]
- Ghorai, T.K.; Chakraborty, M.; Pramanik, P. Photocatalytic performance of nano-photocatalyst from TiO2 and Fe2O3 by mechanochemical synthesis. J. Alloys Compd. 2011, 509, 8158–8164. [Google Scholar] [CrossRef]
- Castañeda, C.; Santos, D.; Hernández, J.S.; Álvarez, A.; Rojas, H.; Gómez, R.; Rajabi, F.; Martínez, J.J.; Luque, R. Efficient NiO/F–TiO2 nanocomposites for 4-chlorophenol photodegradation. Chemosphere 2023, 315, 137606. [Google Scholar] [CrossRef]
- Humelnicu, A.C.; Samoila, P.; Asandulesa, M.; Cojocaru, C.; Bele, A.; Marinoiu, A.T.; Sacca, A.; Harabagiu, V. Chitosan-Sulfated Titania Composite Membranes with Potential Applications in Fuel Cell: Influence of Cross-Linker Nature. Polymers 2020, 12, 1125. [Google Scholar] [CrossRef]
- Lefèvre, G. In situ Fourier-transform infrared spectroscopy studies of inorganic ions adsorption on metal oxides and hydroxides. Adv. Colloid Interface Sci. 2004, 107, 109–123. [Google Scholar] [CrossRef]
- Sumalatha, V.; Anujya, C.; Balchander, V.; Dhanalaxmi, B.; Kumar, M.P.; Ayodhya, D. Hydrothermal fabrication of n-CeO2/p-CuS heterojunction nanocomposite for enhanced photodegradation of pharmaceutical drugs in wastewater under visible-light and fluorometric sensor for detection of uric acid. Inorg. Chem. Commun. 2023, 155, 110962. [Google Scholar] [CrossRef]
- Chen, P. Crystal Sizes and Energy Gaps of Cerium Oxide Using Co-Precipitation Method. Mater. Sci. Appl. 2022, 13, 213–231. [Google Scholar] [CrossRef]
- Arora, N.; Joshi, D.P. Band gap dependence of semiconducting nano-wires on cross-sectional shape and size. Indian J. Phys. 2017, 91, 1493–1501. [Google Scholar] [CrossRef]
- Chen, J.; Feng, W.; Zhao, W. Anion-doped CeO2 for high-performance lithium-sulfur batteries. Appl. Surf. Sci. 2022, 584, 152613. [Google Scholar] [CrossRef]
- Bourja, L.; Bakiz, B.; Benlhachemi, A.; Ezahri, M.; Villain, S.; Favotto, C.; Valmalette, J.C.; Gavarri, J.R. Structural modifications of nanostructured ceria CeO2, xH2O during dehydration process. Powder Technol. 2012, 215–216, 66–71. [Google Scholar] [CrossRef]
- Heering, C.; Nateghi, B.; Janiak, C. Charge-Assisted Hydrogen-Bonded Networks of NH4+ and [Co(NH3)6]3+ with the New Linker Anion of 4-Phosphono-Biphenyl-4′-Carboxylic Acid. Crystals 2016, 6, 22. [Google Scholar] [CrossRef]
- Afshari, B.B.; Jamshidi, M.; Rostami, M.; Ghamarpoor, R. Improving the Mechanical/Anticorrosive Properties of a Nitrile Rubber-Based Adhesive Filled with Cerium Oxide Nanoparticles Using a Two-Step Surface Modification Method. ACS Omega 2022, 7, 44912–44927. [Google Scholar] [CrossRef] [PubMed]
- Poornaprakash, B.; Subramanyam, K.; Kumar, M.; Kim, Y.; Reddy, M.S.P. Enhanced photoluminescence characteristics and intrinsic ferromagnetism in Co-substituted CeO2 nanoparticles. Mater. Sci. Semicond. Process. 2020, 123, 105566. [Google Scholar] [CrossRef]
- Malleshappa, J.; Nagabhushana, H.; Prasad, B.D.; Sharma, S.C.; Vidya, Y.S.; Anantharaju, K.S. Structural, photoluminescence and thermoluminescence properties of CeO2 nanoparticles. Optik 2016, 127, 855–861. [Google Scholar] [CrossRef]
- Tzompantzi Flores, C.; Castillo Rodríguez, J.C.; Gómez, R.; Hernández, R.P.; Santolalla Vargas, C.E.; Tzompantzi, F. Photocatalytic evaluation of the ZrO2:Zn5(OH)6(CO3)2 composite for the H2 production via water splitting. Top. Catal. 2020, 63, 575–585. [Google Scholar] [CrossRef]
- Castañeda Martínez, C.P.; Alvarado Ortega, I.A.; Rojas Sarmiento, H.A.; Tzompantzi Morales, F.J.; Gómez Romero, J.R. Photocatalytic degradation of the 2,4-dichlorophenoxyacetic acid herbicide using supported iridium materials. Ciencia Desarrollo 2021, 12, 125–134. [Google Scholar] [CrossRef]
- Carvalho, L.M.; Soares Filho, A.F.; Lima, M.S.; Cruz Filho, J.F.; Dantas, T.C.M.; Luz, G.E. 2,4-Dichlorophenoxyacetic acid (2,4-D) photodegradation on WO3-TiO2-SBA-15 nanostructured composite. Environ. Sci. Pollut. Res. 2020, 28, 7774–7785. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, C.; Martínez, J.J.; Santos, L.; Rojas, H.; Osman, S.M.; Gómez, R.; Luque, R. Caffeine photocatalytic degradation using composites of NiO/TiO2–F and CuO/TiO2–F under UV irradiation. Chemosphere 2021, 288, 132506. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, T.; Sengupta, S. Chapter 4-Contaminants of Emerging Concern: Occurrence, fate, and remediation. In Advances in Water Purification Techniques; Elsevier: Amsterdam, The Netherlands, 2019; pp. 67–114. [Google Scholar] [CrossRef]
- Yan, X.M.; Mei, P.; Gao, L. Photocatalytic Degradation of Rhodamine B over Sulfated SiO2-TiO2 Composite by Sulphuric Acid Impregnation Method. J. Adv. Oxid. Technol. 2016, 19, 52–58. [Google Scholar] [CrossRef]
- Hernández, J.S.; Guevara, D.; Shamshurin, M.; Benassi, E.; Sokolov, M.N.; Feliz, M. Octahedral Tantalum Bromide Clusters as Catalysts for Light-Driven Hydrogen Evolution. Inorg. Chem. 2023, 62, 19060–19069. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, M.; Konstantinou, I. Photocatalytic degradation of pentachlorophenol by visible light Ν–F–TiO2 in the presence of oxalate ions: Optimization, modeling, and scavenging studies. Environ. Sci. Pollut. Res. 2015, 22, 9438–9448. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, C.; Tzompantzi, F.; Gómez, R. Photocatalytic reduction of 4-nitrophenol on in situ fluorinated sol–gel TiO2 under UV irradiation using Na2SO3 as reducing agent. J. Sol-Gel Sci. Technol. 2016, 80, 426–435. [Google Scholar] [CrossRef]
- Elghniji, K.; Hentati, O.; Mlaik, N.; Mahfoudh, A.; Ksibi, M. Photocatalytic degradation of 4-chlorophenol under P-modified TiO2/UV system: Kinetics, intermediates, phytotoxicity and acute toxicity. J. Environ. Sci. 2012, 24, 479–487. [Google Scholar] [CrossRef]




| Photocatalyst | Crystallite Size (nm) | Lattice Parameter a (nm) | SBET (m2/g) | Pore Size (Å) | Eg (eV) |
|---|---|---|---|---|---|
| CeO2 | 6.2 | 0.552 | 146 | 46 | 3.00 |
| 0.5SO42−/CeO2 | 5.6 | 0.554 | 149 | 46 | 3.08 |
| 1.0SO42−/CeO2 | 5.2 | 0.552 | 158 | 43 | 3.08 |
| 2.0SO42−/CeO2 | 5.0 | 0.552 | 161 | 45 | 3.08 |
| Photocatalyst | 2,4-Dichlorophenoxyacetic Acid | ||
|---|---|---|---|
| min−1) | t1/2 (min) | R2 | |
| CeO2 | 5.5 | 126 | 0.972 |
| 0.5SO42−/CeO2 | 5.8 | 119 | 0.970 |
| 1.0SO42−/CeO2 | 8.6 | 80 | 0.989 |
| 2.0SO42−/CeO2 | 6.9 | 100 | 0.982 |
| Test N° | Catalyst Mass (x1) | Contaminant Concentration (x2) | %Y Calculated | %Y Experimental | % Error |
|---|---|---|---|---|---|
| 1 | (−1) 50 | (−1) 25 | 71.9 | 71 | 1.22 |
| 2 | (1) 150 | (−1) 25 | 80.2 | 79 | 1.48 |
| 3 | (−1) 50 | (1) 55 | 72.5 | 65 | 10.3 |
| 4 | (1) 150 | (1) 55 | 80.8 | 69 | 14.6 |
| 5 | (−0.5) 10 | (0) 40 | 52.1 | 73 | 40.2 |
| 6 | (0.5) 190 | (0) 40 | 67.0 | 75 | 11.9 |
| 7 | (0) 100 | (−0.5) 5 | 46.9 | 64 | 36.6 |
| 8 | (0) 100 | (0.5) 75 | 48.3 | 60 | 24.3 |
| 9 | (0) 100 | (0) 40 | 96.9 | 92 | 5.09 |
| 10 | (0) 100 | (0) 40 | 96.9 | 91 | 6.12 |
| 11 | (0) 100 | (0) 40 | 96.9 | 93 | 4.06 |
| 12 | (0) 100 | (0) 40 | 96.9 | 92 | 5.09 |
| 13 | (0) 100 | (0) 40 | 96.9 | 90 | 7.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, C.; Castañeda, C.; Sosa, E.; Martínez, J.J.; Mancipe, S.; Rojas, H.; Tzompantzi, F.; Gómez, R. Enhanced Photocatalytic Degradation of Herbicide 2,4-Dichlorophenoxyacetic Acid Using Sulfated CeO2. Catalysts 2024, 14, 594. https://doi.org/10.3390/catal14090594
Rodríguez C, Castañeda C, Sosa E, Martínez JJ, Mancipe S, Rojas H, Tzompantzi F, Gómez R. Enhanced Photocatalytic Degradation of Herbicide 2,4-Dichlorophenoxyacetic Acid Using Sulfated CeO2. Catalysts. 2024; 14(9):594. https://doi.org/10.3390/catal14090594
Chicago/Turabian StyleRodríguez, Carlos, Claudia Castañeda, Edwin Sosa, José J. Martínez, Sonia Mancipe, Hugo Rojas, Francisco Tzompantzi, and Ricardo Gómez. 2024. "Enhanced Photocatalytic Degradation of Herbicide 2,4-Dichlorophenoxyacetic Acid Using Sulfated CeO2" Catalysts 14, no. 9: 594. https://doi.org/10.3390/catal14090594
APA StyleRodríguez, C., Castañeda, C., Sosa, E., Martínez, J. J., Mancipe, S., Rojas, H., Tzompantzi, F., & Gómez, R. (2024). Enhanced Photocatalytic Degradation of Herbicide 2,4-Dichlorophenoxyacetic Acid Using Sulfated CeO2. Catalysts, 14(9), 594. https://doi.org/10.3390/catal14090594







