Abstract
SBA-15 and organic ionic liquid were incorporated in a post-grafting technique for generating a bifunctional ionic liquid embedded mesoporous SBA-15. The prepared heterogeneous catalyst was employed for the first time to synthesize N-alkylated indoline-2,3-dione at mild conditions to afford excellent yields in a short reaction time. The synthesized DABCOIL@SBA-15 catalyst was meticulously characterized by various techniques, such as FT-IR, solid-state 13C NMR, solid-state 29Si NMR, small-angle X-ray diffraction (XRD), and N2 adsorption–desorption. Further, the morphological behavior of the catalyst was studied by SEM and TEM. The thermal stability and number of active sites were determined by thermogravimetric analysis (TGA). The Hammett equation was used to analyze the synergetic effect of the catalyst and substituent effects on the N-alkylated products of 5-substituted isatin derivatives, which resulted in a negative slope. This negative slope indicates a positive charge in the transition state. Notably, the DABCOIL@SBA-15 catalyst demonstrated its practicality by being reused for seven cycles with consistently high catalytic activity.
1. Introduction
Ionic liquids (ILs) have attracted considerable interest due to their unique qualities and are revolutionizing sustainable technology development [1]. In recent years, ILs have been recognized as convenient and environmentally friendly catalysts for various organic synthetic transformations and fine chemical processes [2,3,4]. The design of ILs with appropriate functional groups is advantageous for various chemical reactions and is conducive to high-yield synthesis processes [3,5,6]. For example, N. Jamasbi et al. recently reported DABCO-based ILs as a catalyst for preparing various organic derivatives [7]. However, unendurable viscosity, poor recyclability, and high cost hindered their application in large-scale preparation [8]. To overcome this problem, immobilizing ILs with a solid support medium is attractive for obtaining heterogenized ILs with high catalytic activity and easy recyclability [9]. Generally, the low cost and easy availability of silica is usually used as an ionic liquid carrier [10,11]. However, only low grafting of ILs was attained due to wide-range pore distribution, uneven pore shape, and low specific surface area, which leads to poor catalytic activities. Therefore, in the recent past, significant efforts have been directed toward developing practical heterogeneous catalysts. In this context, ordered mesoporous materials have been intensively studied to elevate the grafting density of ionic liquids. Particularly, SBA-15 [12,13] has gained increased attention among the other mesoporous materials because of their tunable pore sizes, stability, and high surface area [14,15]. Moreover, SBA-15 has good chemical accessibility and stability in functional group transformation reactions. Further, abundant surface hydroxyl groups could help integrate the active centers of ILs for specific catalytic applications, and the large pore size is also helpful in transporting multifunctional ILs into the pore channels of SBA-15. For example, Caio and others developed a well-defined organo-functionalized mesoporous catalyst by a one-step reaction [16]. Further, Xie and co-workers reported a series of immobilized ionic liquids on SBA-15 heterogeneous catalysts for biodiesel production reactions [17]. Recently, Chen and others synthesized recyclable SBA-15-immobilized Brønsted acidic ionic liquid for a ketalization reaction [18]. In another case, heteropolyanion-based ionic liquid with SBA-15 has been reported for the alkylation of o-xylene with styrene [19]. In this case, the catalyst showed higher catalytic efficiency and reusability, and SBA-15-supported imidazolium-based catalysts were synthesized by Zhiguo et al. for carbon dioxide fixation [20]. Fatemeh Rajabi and others recently synthesized the mesoporous organosilica material for the Knoevenagel condensation reaction, a cross-coupling reaction [21,22]. In another case, Babak and Mojtaba reported the SBA-15-based tungstate heterogeneous catalyst for the selective oxidation of sulfides in water [23]. Xuefeng Bai et al. prepared Ru/SBA-15 catalysts using a simple wet impregnation method for hydrogenation reactions [24]. In another case, mesoporous silica functionalized with imidazole-containing functionalities has been used as a selective adsorbent for the metal ions [25]. These reports revealed that SBA-15 is a potential candidate for acting as a suitable supporting material for incorporating various multifunctional ILs [26].
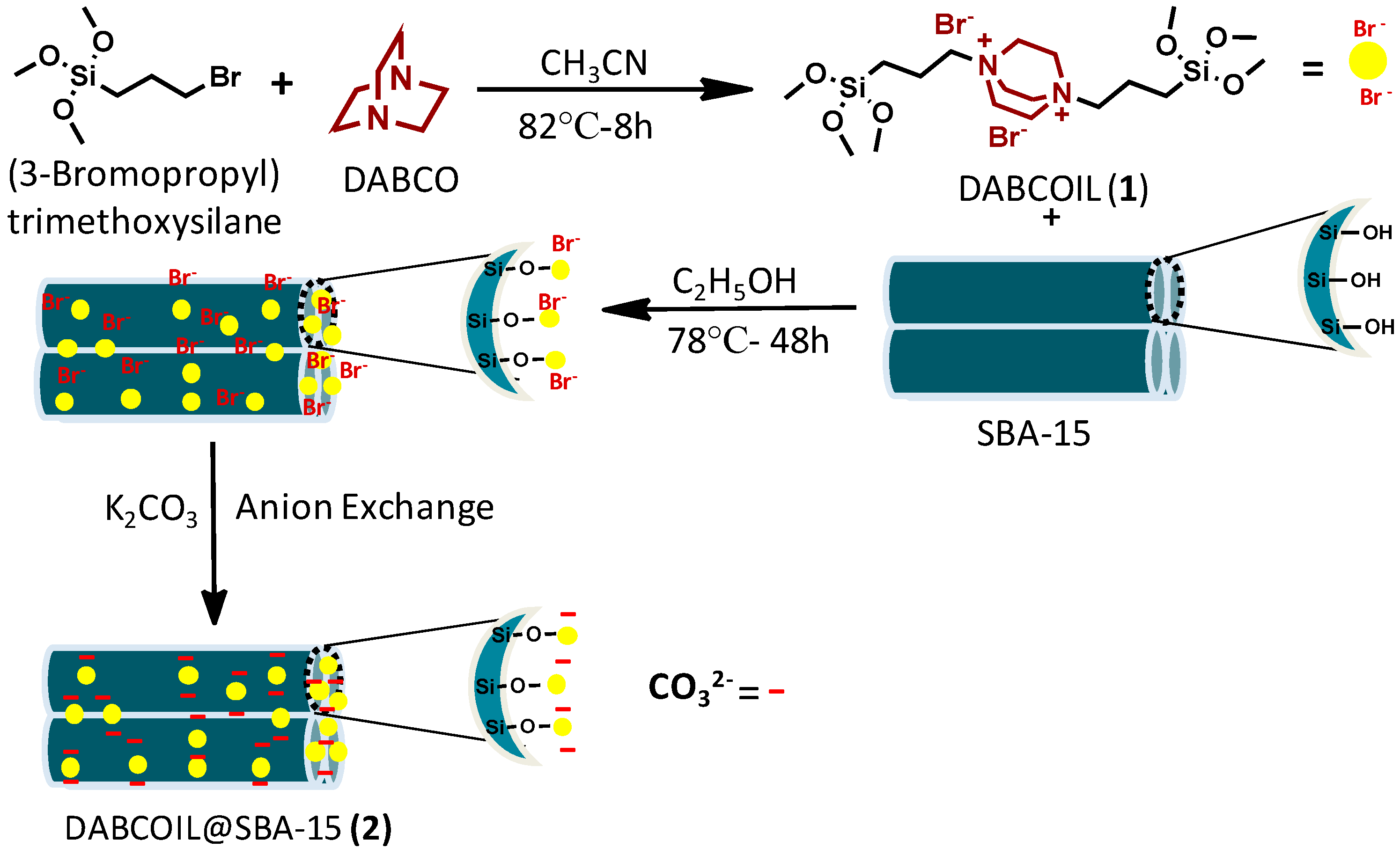
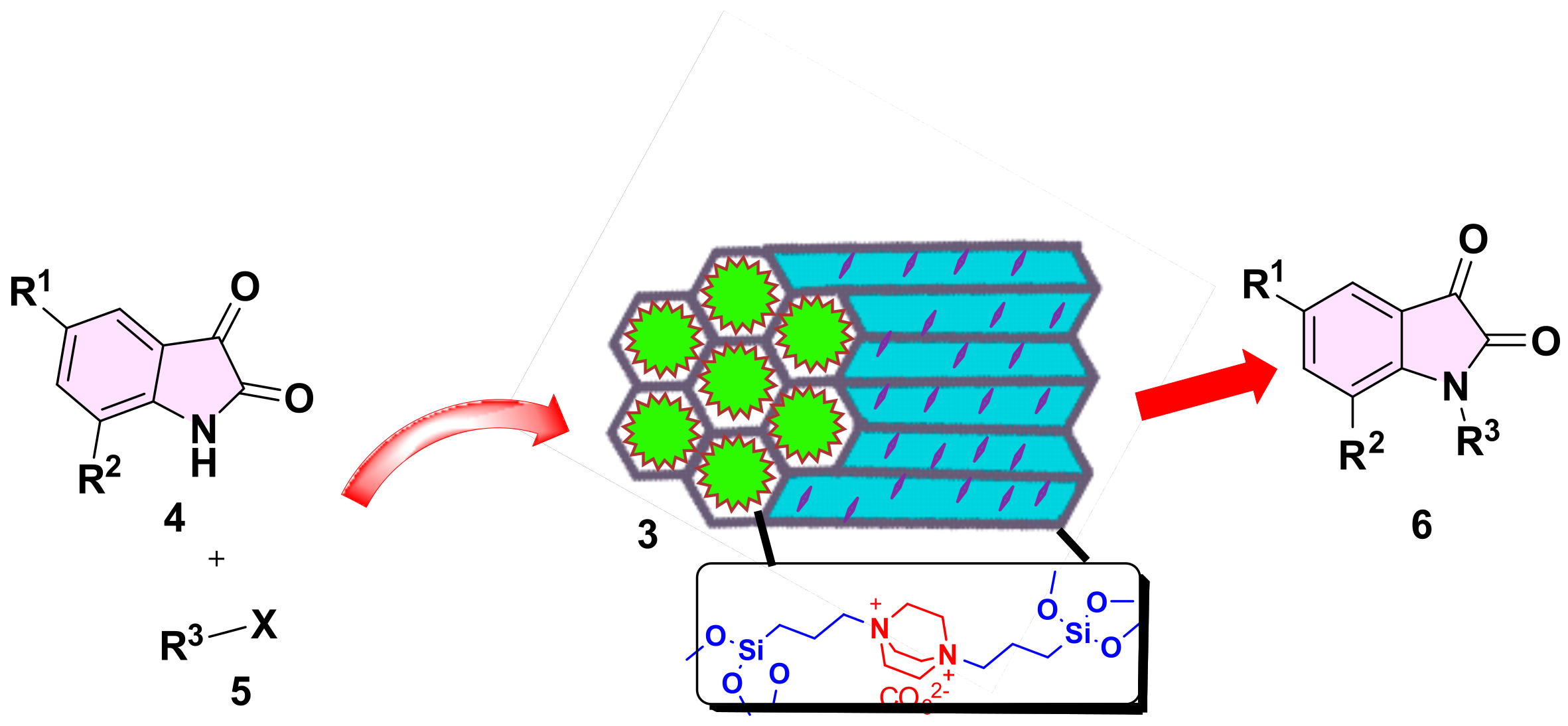
We have previously reported on the use of SBA-15 functionalized organic materials for a variety of applications [27,28]. In this paper, we report the grafting of DBACO-based ILs on SBA-15 support to obtain an efficient DABCOIL@SBA-15 heterogeneous catalyst, which was robustly used as a (pre)catalyst for the synthesis of N-alkylated indoline-2, 3-dione based derivatives. Moreover, the physicochemical properties of SB-15 and functionalized analog are studied in detail. A general synthetic approach is illustrated in Scheme 1, and detailed synthesized DABCO-IL characterized NMR techniques and results are incorporated in the supporting information (Figures S1–S6).
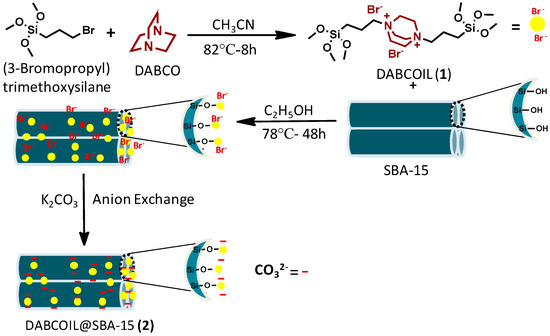
Scheme 1.
Synthesis of bi-functionalized ionic liquid grafted-SBA-15.
2. Results
2.1. FT-IR Studies
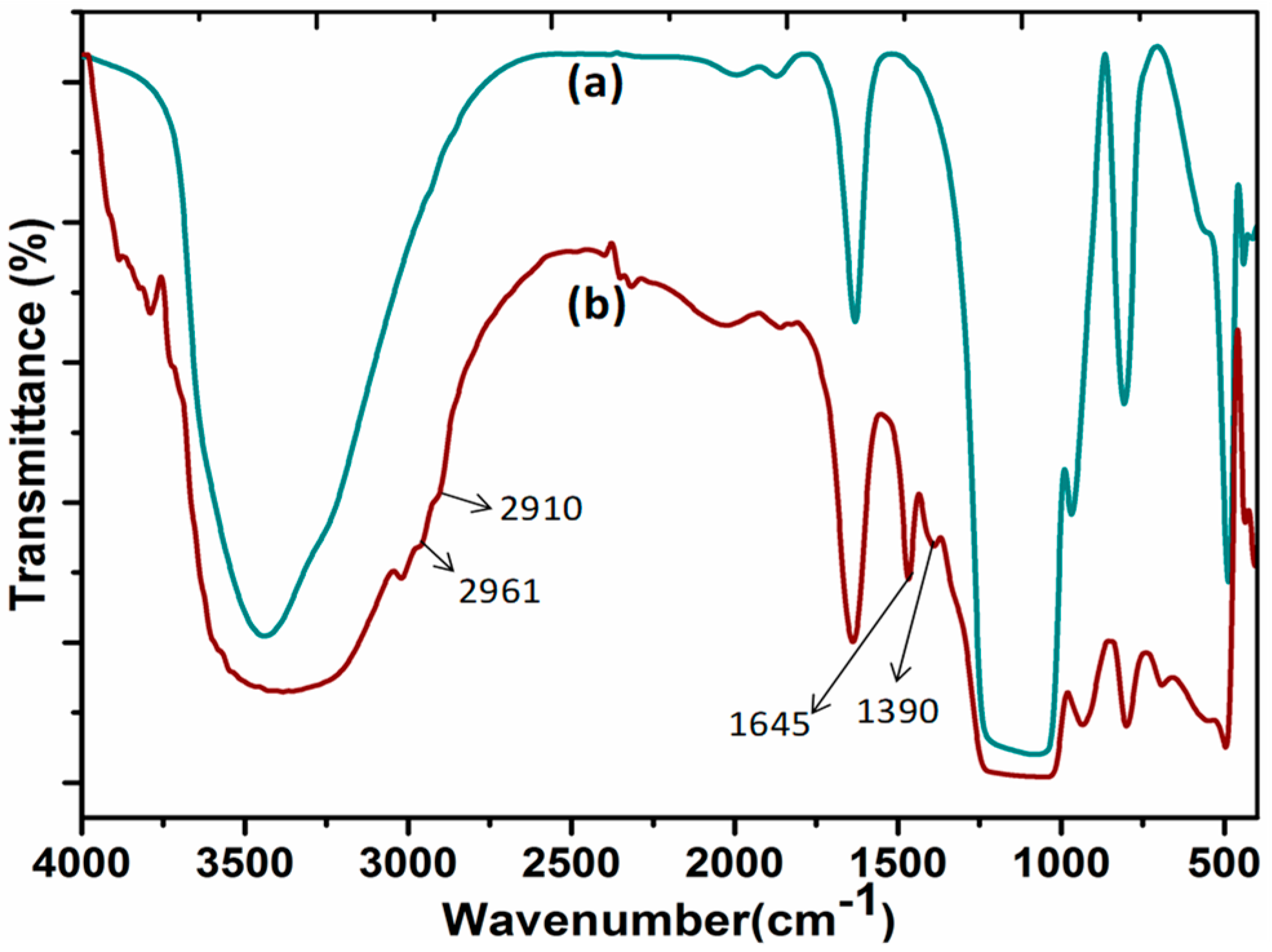
The FT-IR studies confirmed the functionalization of DABCOIL on SBA-15 support. Figure 1 represents the FT-IR spectra of SBA-15 and DABCOIL@SBA-15 catalysts. For SBA-15, the typical peak for the surface –OH group of SBA-15 appeared at 3440 cm−1, and bending vibrations of the –OH peak were also observed at 1631 cm−1. Moreover, asymmetric and symmetric stretching frequencies for Si–O–Si appeared at 1126 and 806 cm−1, which was attributed to the condensed silica network [27]. In the case of the DABCOIL@SBA-15 catalyst, the new peak appeared at 1390 cm−1 due to the C–N vibration of DABCO-IL. Additionally, the peaks appeared at 2961 and 2910 cm−1 for –CH2 symmetric stretching and at 1645 cm−1 for bending vibrations of –CH2 in the silane functionalized DABCOIL [28].
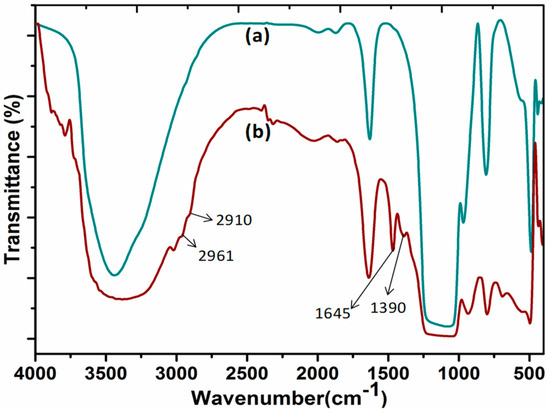
Figure 1.
FT-IR spectra of (a) SBA-15 and (b) DABCOIL@SBA-15.
2.2. Solid-State NMR Studies
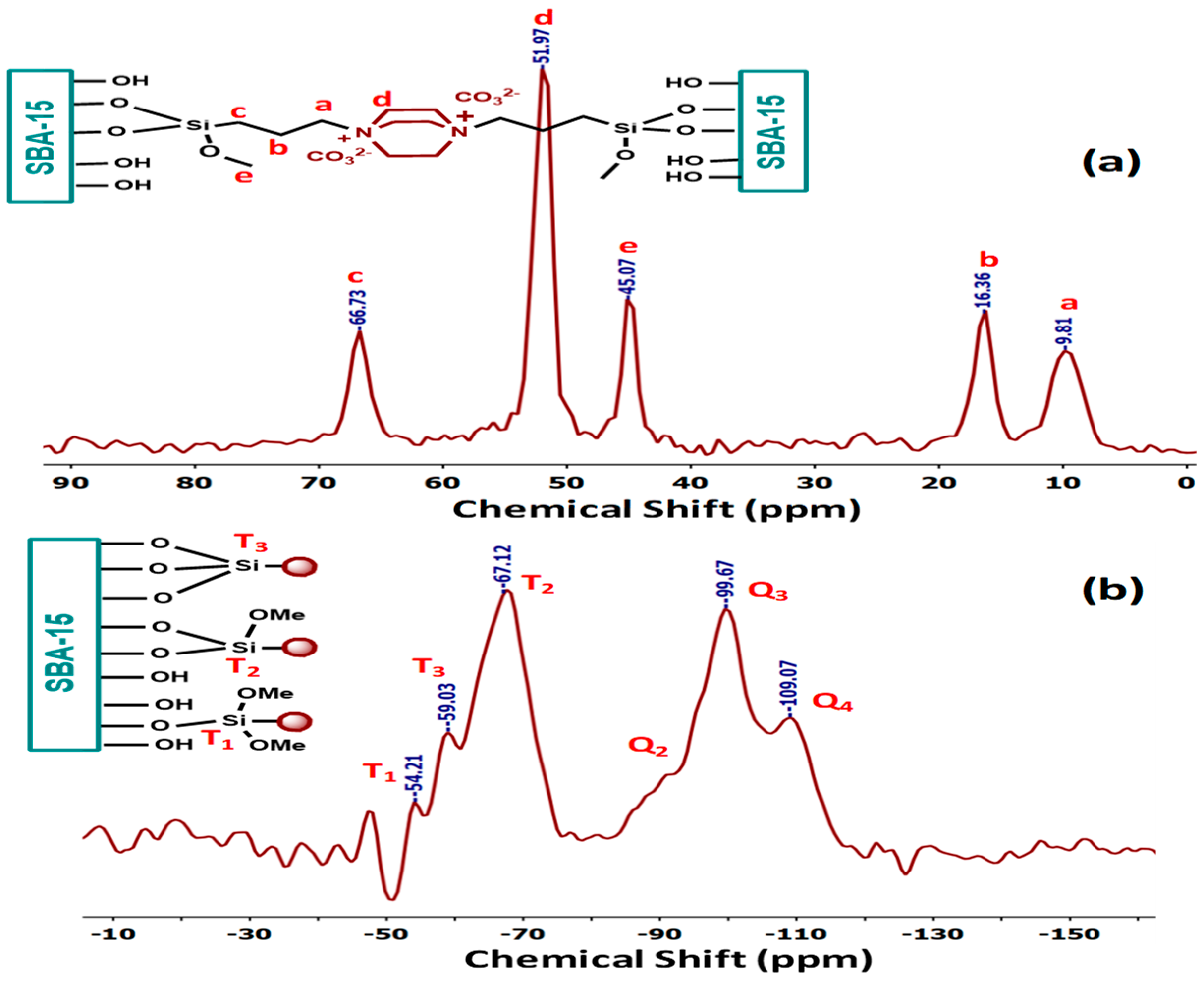
The solid-state 13C CP/MAS-NMR and 29Si CP/MAS-NMR spectra of DABCOIL@SBA-15 are shown in Figure 2. In the 13C CP/MAS-NMR spectrum (Figure 2a), three carbon resonances of the propyl group of the modified SBA-15 peak appeared at 9.81 ppm, 16.36, and 66.73 ppm, respectively.
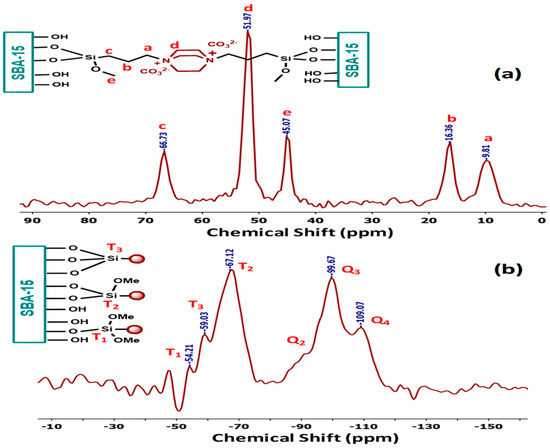
Figure 2.
CP/MAS 13C NMR spectra of (a) DABCOIL@SBA-15 and (b) 29Si NMR of DABCOIL@SBA-15.
The DABCO contains six chemically and magnetically equivalent carbons, showing only one carbon resonance at 51.97 ppm with high peak intensity. A peak appeared at 45.07 ppm for the residual methoxy (O-CH3) [29] group of the siloxane group, as evidenced by 29Si NMR. Further, the covalent bond formation between DABCOIL (1) and SBA-15 was confirmed by the 29Si CP/MAS-NMR spectrum (Figure 2b), which also gives information about the inorganic framework of SBA-15 silica (Q sites) and organic substituted silica (T sites). DABCOIL@SBA-15 showed three peaks in the upfield region for the Q sites, which correspond to Q4 [Si (SiO)4] [30] at −109.07 ppm, Q3 [Si(SiO)3.OH] at −99.67, and Q2 [Si (SiO)2(OH)2] at −90.32 ppm. The spectrum also displayed three peaks in the down-field region for the T sites at −54.21, −59.03, and −67.12 ppm, corresponding to the 29Si atoms of the T1[-CH2-Si(OR)2(OSi)], T2[-CH2-Si(OR)(OSi)2], and T3[-CH2-Si(OSi)3] [29] site. These results revealed the covalent grafting between the organic silane (DABCOIL) and SBA-15. Moreover, the peak intensity of the T sites suggested the dimethoxy condensation was higher than tri methoxy and monomethoxy condensation on SBA-15.
2.3. Microscopic Studies
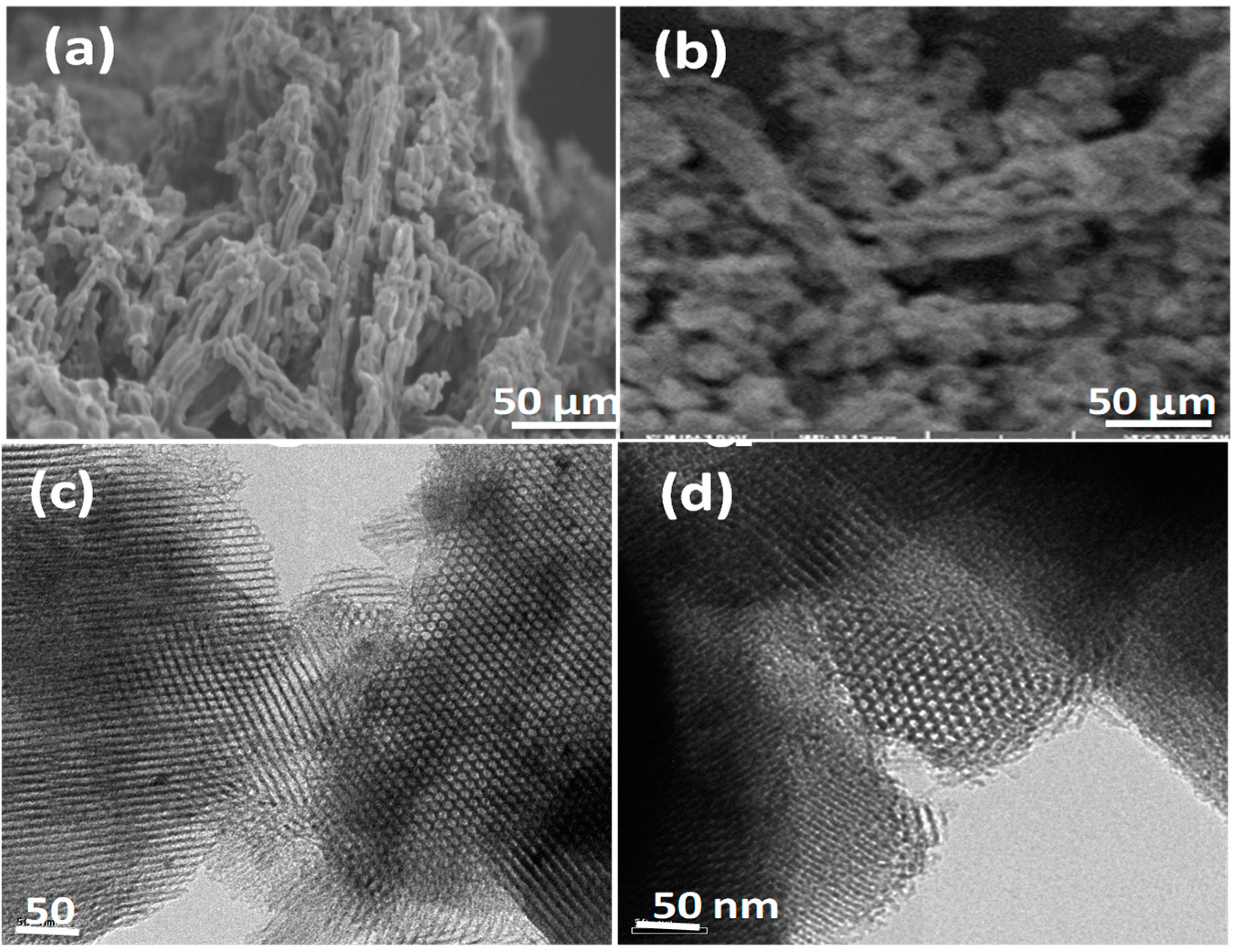
The morphological features of SBA-15 (a) and DABCOIL@SBA-15 (b) were examined by scanning electron microscopy (SEM) and tunneling electron microscopy (TEM) and are shown in Figure 3. The SEM image of SBA-15 (Figure 3a) showed a rodlike aggregated morphology with uniform size and shape [31]. DABCOIL@SBA-15 also showed a rodlike morphology, similar to SBA-15, which had a much-aggregated morphology. This may be attributed to the covalent grafting of DABCOIL on SBA-15 and the subsequent ion exchange process by carbonate ions [32]. The TEM image of SBA-15 (Figure 3c) confirmed the presence of a hexagonal pore and ordered mesoporous arrangement, which was well in agreement with prior reports. In the case of DABCOIL@SBA-15 (Figure 3d), ordered mesoporous were also observed, and this indicated that the morphology of SBA-15 is retained and long-range order and mesoporous structure arrays are not disturbed significantly after DABCOIL (1) grafting [33]. Therefore, the SEM and TEM studies confirmed that the grafting of organic functionalities did not affect the mesoporous structure of parent SBA-15.
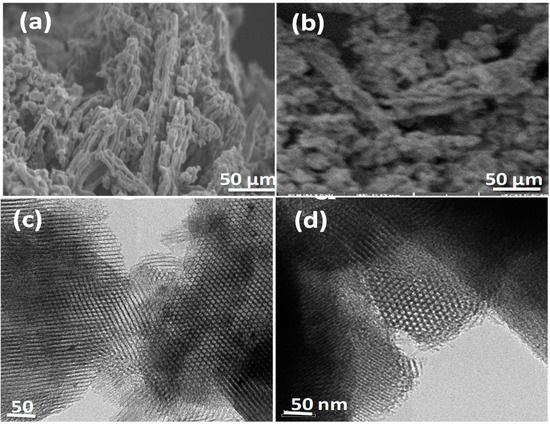
Figure 3.
SEM images of (a) SBA-15 and (b) DABCOIL@SBA-15. TEM images of (c) SBA-15 and (d) DABCOIL@SBA-15.
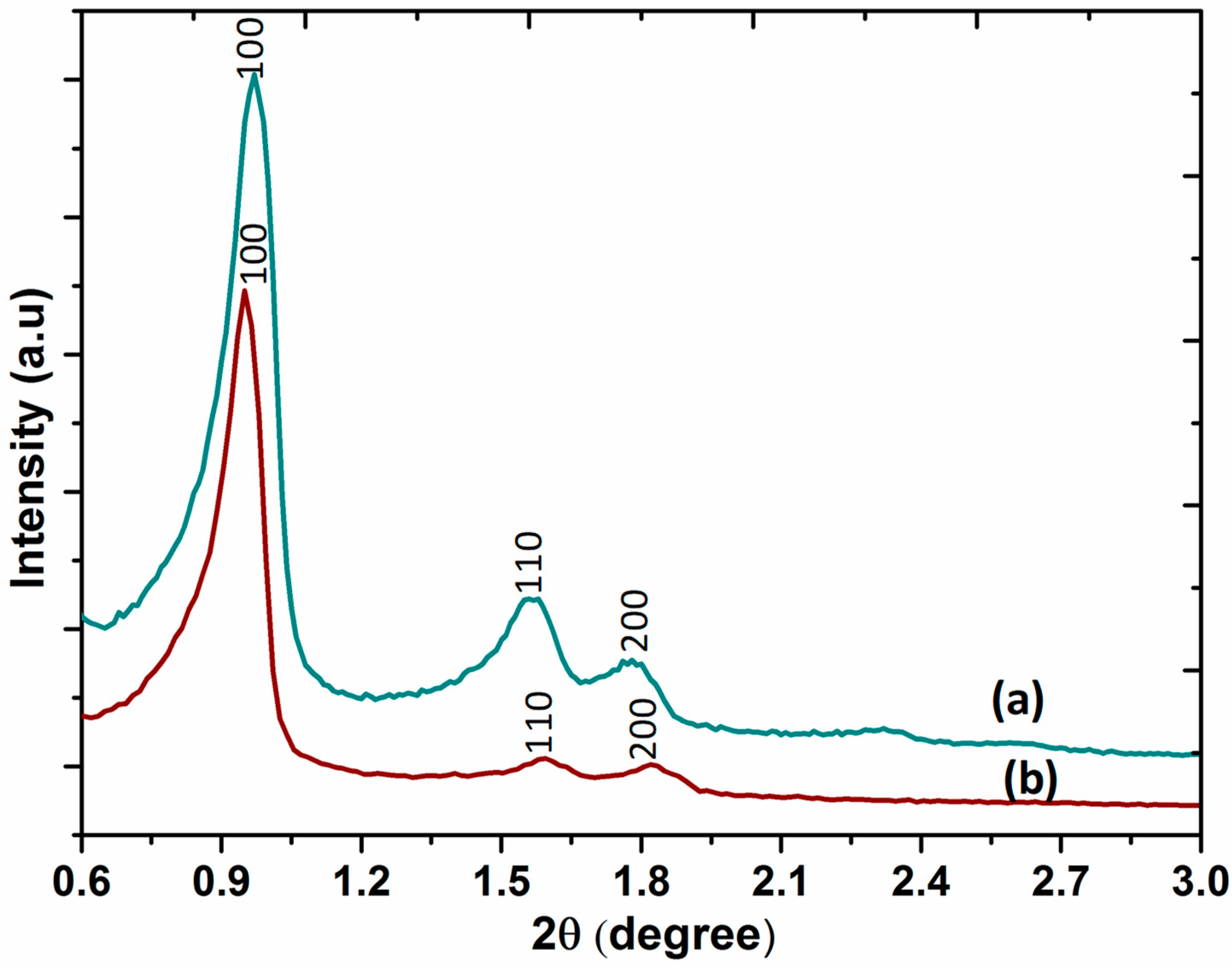
2.4. XRD Analysis
The small-angle X-ray diffraction patterns of SBA-15 and DABCOIL@SBA-15 are shown in Figure 4. The three typical diffraction peaks for SBA-15 appeared at 2θ = 0.97, 1.57, and 1.8 with high peak intensity, which was assigned to 100, 110, and 200 planes of the highly ordered mesoporous structure of SBA-15. These diffraction signals exhibited a long-range ordered mesostructure and uniform mesoporous size distribution with two-dimensional hexagonal space group p6mm symmetry [34]. It also displayed similar diffraction peaks after surface modification (Figure 4b). This indicated that the ordered mesoporous nature of the SBA-15 support was not affected, even after incorporating organic compounds, as confirmed by the TEM images. However, the intensity of the diffraction peaks at 2θ = 0.97 (100), 1.57 (110), and 1.8 (200) was decreased, which is possibly due to the partial blocking of pores of SBA-15 by the silane functionalized ionic liquids [35,36]. Thus, the small-angle XRD results suggested that the ordered mesoporous structure of SBA-15 was not ruined upon functionalization. The particle size distribution and Zeta-potential of the DABCOIL@SBA-15 catalyst were determined by dynamic light scattering (DLS) and are provided in (ESI, Figure S1).
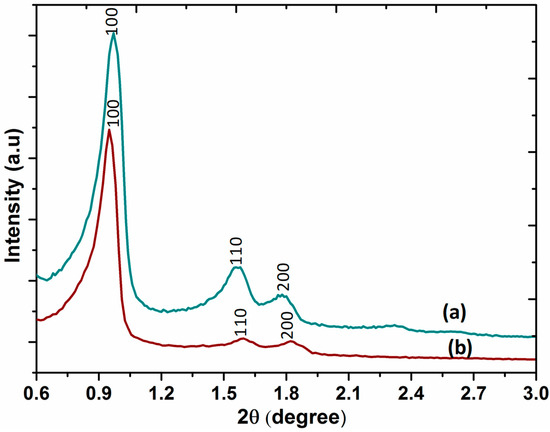
Figure 4.
X-ray diffraction of (a) SBA-15 and (b) DABCOIL@SBA-15.
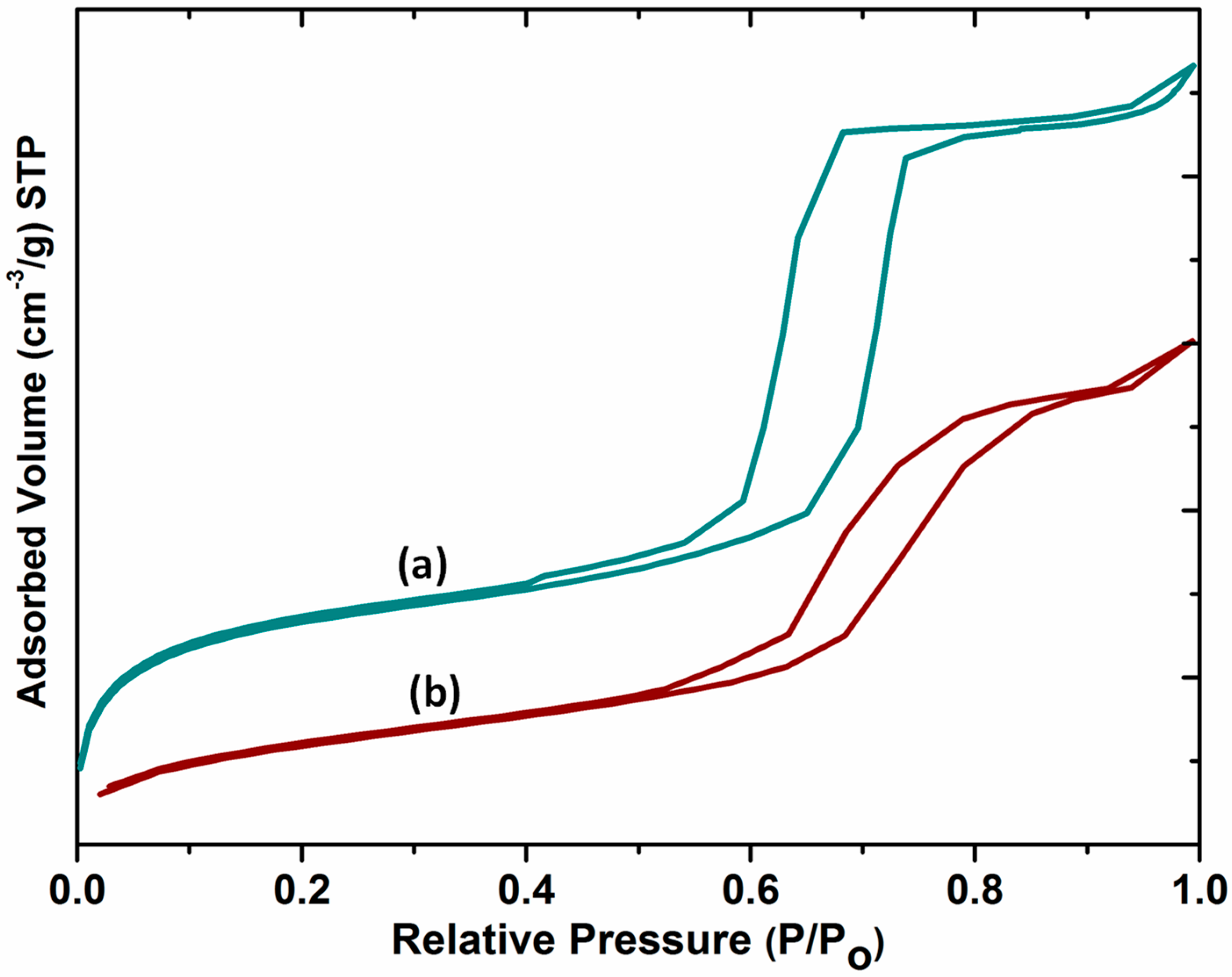
2.5. N2 Adsorption–Desorption
The nitrogen adsorption/desorption isotherms of SBA-15 and DABCOIL@SBA-15 are shown in Figure 5. The samples showed type IV isotherms with an H1 hysteresis loop according to the IUPAC nomenclature. For SBA-15, the sharp increase in adsorption arose at P/Po = 0.6–0.76, which indicates the ordered nature of porous silica materials with narrow pore size distributions, and multilayer adsorption occurred, followed by capillary condensation [30].
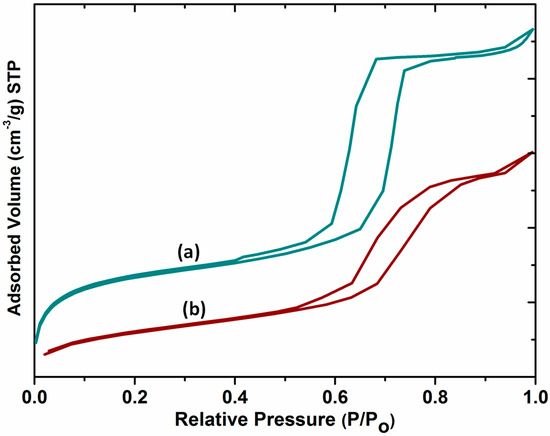
Figure 5.
Nitrogen adsorption/desorption isotherms of (a) SBA-15 and (b) DABCOIL@SBA-15.
After surface modification, it showed a type IV isotherm with a slight decrease in loop intensity due to the partial filling of DABCOIL on the pores [24]. Moreover, the surface area, average pore diameter, and pore volume of the materials also slightly decreased upon modification [19]. The changes are provided in Table 1.

Table 1.
Nitrogen adsorption–desorption isotherm values of SBA-15 and DABCOIL@SBA-15.
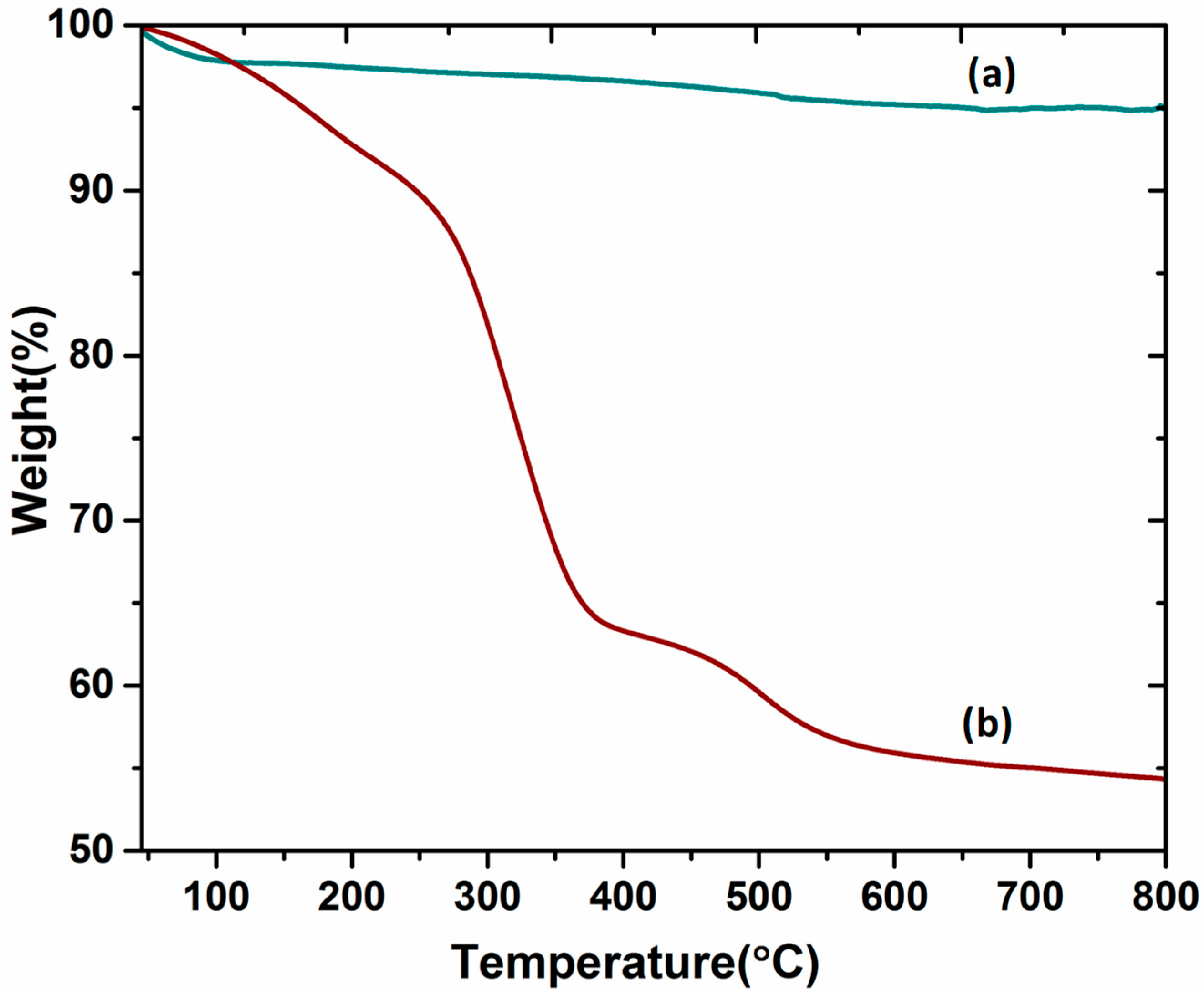
2.6. TGA Analysis
Thermogravimetric analysis (TGA) was performed to understand the thermal stability of the DABCOIL@SBA-15 catalyst. The samples were measured between 25 and 800 °C under a nitrogen atmosphere at a heating rate of 10 °C/min and are represented in Figure 6. The pure SBA-15 (Figure 6a) was stable up to 800 °C with only a trivial mass loss of about 5%. In the case of the DABCOIL@SBA-15 catalyst (Figure 6b), initially, a gradual and minimum weight loss was observed at around 100 °C due to the physically bounded water molecules on the surface of the catalyst [18]. A significant weight loss of about ~25% was observed in the range of 270–380 °C, which may be attributed to the decomposition of organic moieties of modified SBA-15 [35].
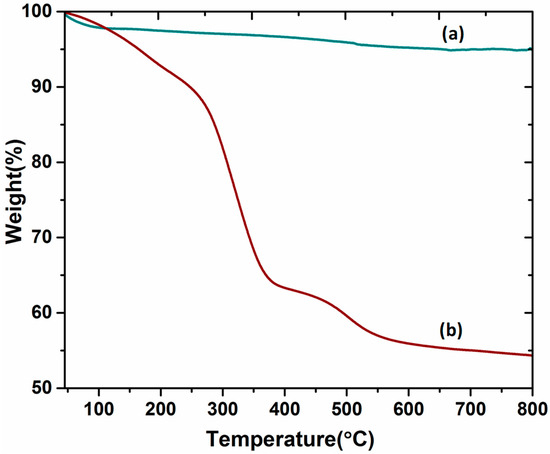
Figure 6.
TGA analysis of (a) SBA-15 and (b) DABCOIL@SBA-15.
Moreover, the silane decomposition profile determined the presence of carbonate ions (CO32−) in the DABCOIL@SBA-15 catalyst, which was found to be 0.15 mol in 0.80 g of the catalyst [36,37]. These results suggested that the DABCOIL@SBA-15 catalyst was stable up to 250 °C with many active sites.
2.7. Activity Test of the Catalyst
Different temperatures, specific concentration regimes, various solvents, and the presence of oxygen/moisture examined the catalytic efficiency of the DABCOIL@SBA-15 heterogeneous catalyst (Scheme 2). This method was impressed by the realistic catalytic strength and provides a viable strategy for product–catalyst discrimination at mild temperatures [38]. Generally, the oxindole compound provides a unique, valuable, and imminent reactivity in biological and pharmacological activities [39]. Therefore, the straightforward N-alkylated approach is practically distinctive [40,41]. Hence, the DABCOIL@SBA-15 catalyst was prepared, and the catalytic effect was examined.
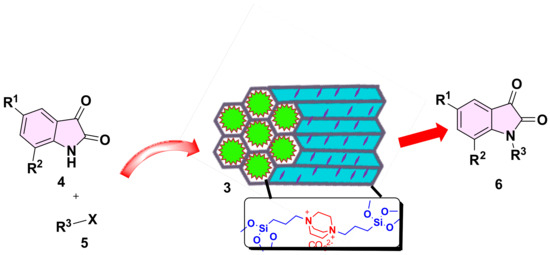
Scheme 2.
Synthesis of N-functionalized isatin derivatives.
Initially, the catalyst (3) (10% w/w) was taken in a 50 mL round-bottom flask, and acetonitrile (5 mL) was added to the catalyst stride 1 h to obtain dispersion. After that, isatin (4a) (0.1 g, 0.00068 mol, 1.0 equiv.) and benzyl bromide (5a) (0.12 g, 0.0068 mol, 1.5 equiv.) were added to the reaction mixture and stirred for 7 h under N2 atm. Finally, the reaction mixture was removed by syringe, and GC-MS confirmed the product with a 90% yield. This method allows the chemoselective N-functionalization of isatin 6j. Moreover, we observed that catalyst (3) demonstrated effective activity and allowed a wide range of electrophiles, such as alkyl halides, acyl halides, anhydrides, and isocyanates, and Michael acceptors for the N-functionalization reaction. Moreover, the effect of the reaction variables on the activity and selectivity was discussed in detail. The optimized reactions were conducted at 50 °C with 6% w/w of DABCOIL@SBA-15 and 1.0 equivalent of isatin (4) and 1.5 equivalent of benzyl bromide in cyclopentyl methyl ether (CPME) [42] for the N-alkylation reaction. The product 6j was obtained with a 98% yield. The reaction optimizations are provided in Table 2. The NMR and mass data of synthesized N-alkyl indoline-2, 3-dione derivatives are provided in (ESI, Table S1).

Table 2.
Optimization of the N-benzylation reaction a.
The Effect of Reaction Temperature
To explore reactivity, we conducted further experiments on reaction temperature in the 30–80 °C range. The relatively high boiling point of the solvent and its polarity maximized the reaction efficiency and shortened the reaction time (Table 2, entries 3, 6, 7, 8, 9). The reaction at higher and lower temperatures considerably lowered the yield of the products (Table 2, entries 1, 3, 6, 7, 8, 9).
The reaction was performed in open-air conditions and achieved a similar conversion yield. Finally, the pure N-alkylated products were obtained by simple recrystallization using methanol. The simple method allowed N-alkylation on diverse functionalized electrophiles ranging from simple to complex species. These preliminary results encouraged us to study the selective C-N bond formation between an aromatic vs. aliphatic alkylation, an ester vs. acid halide, and primary vs. secondary alkylation.
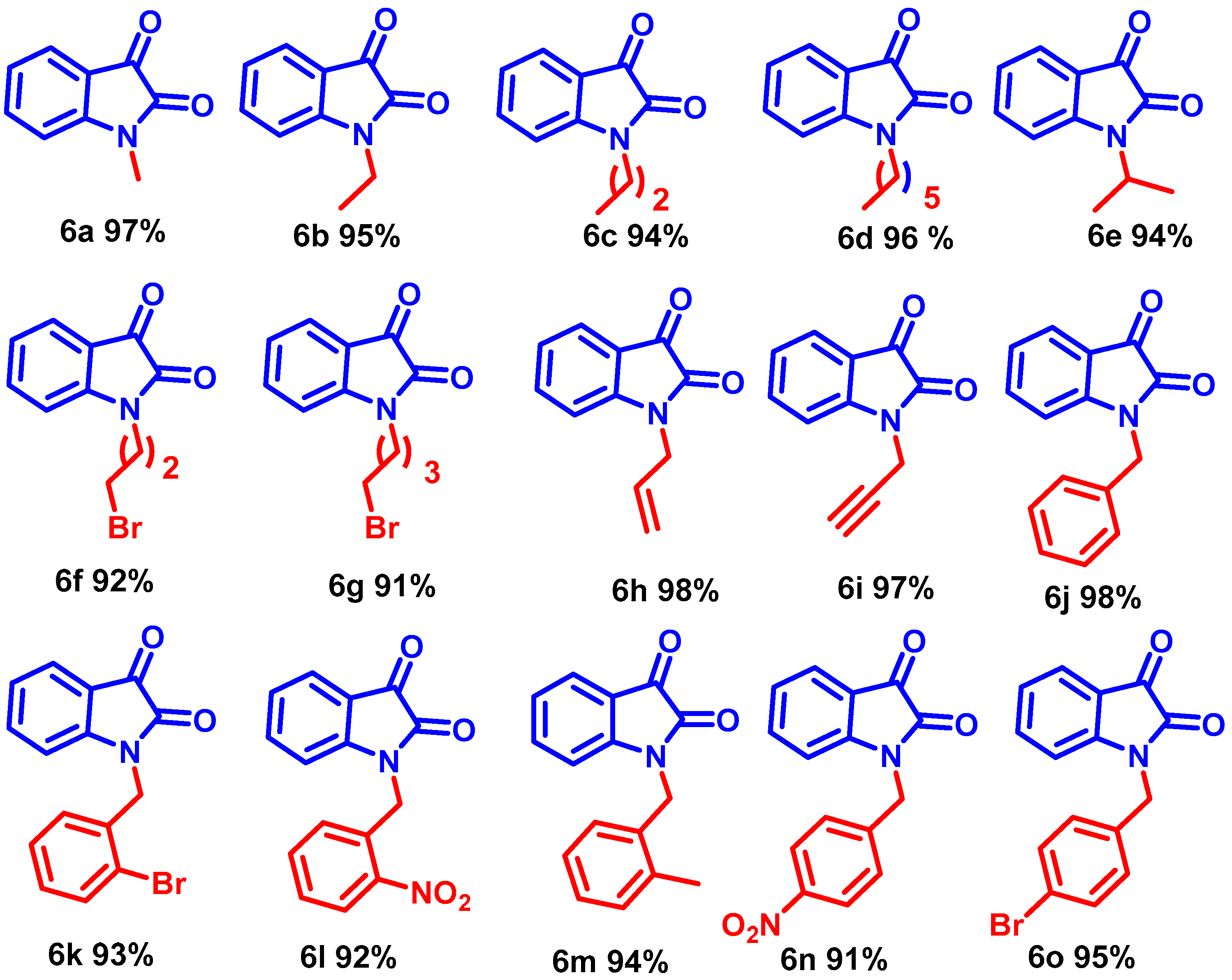
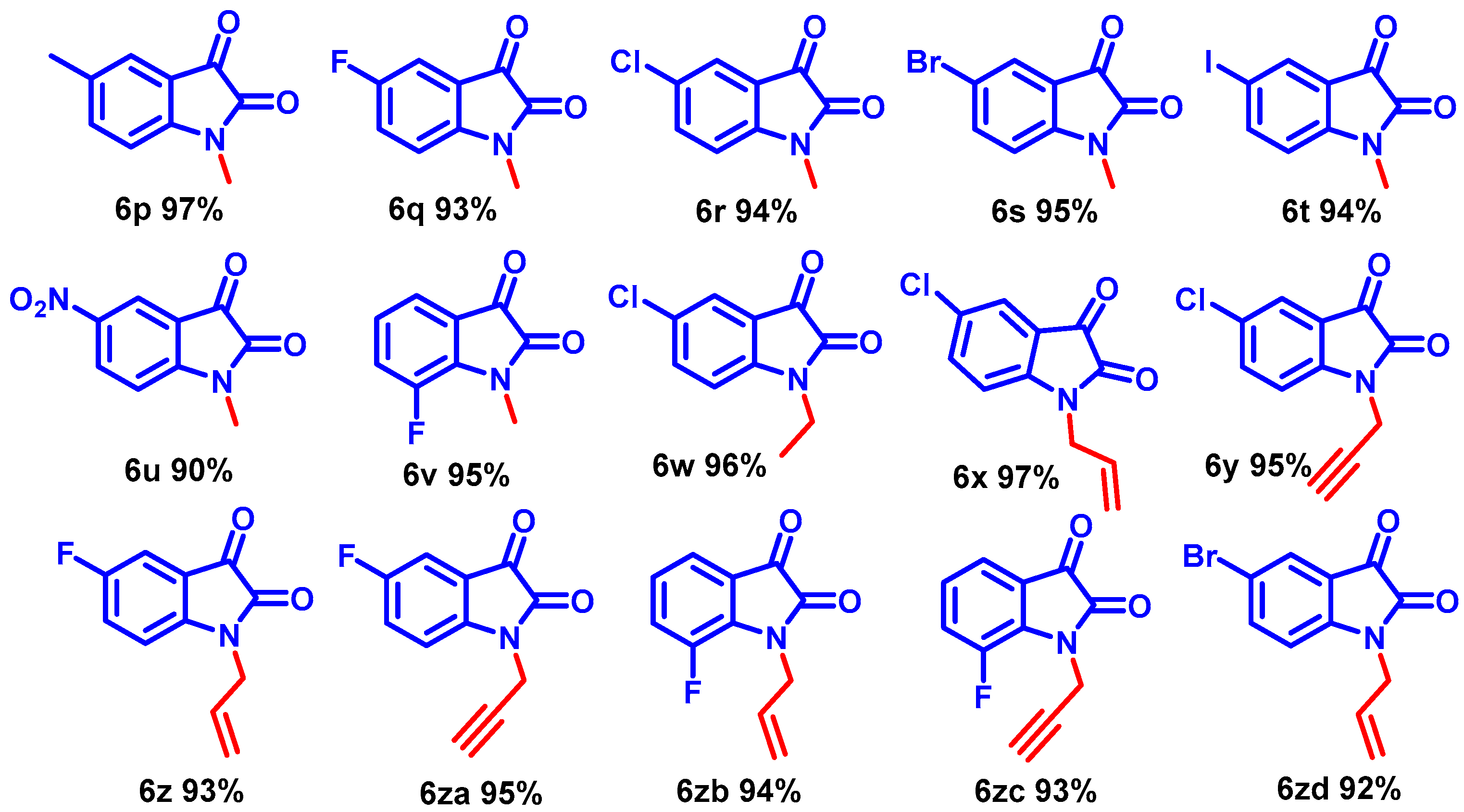
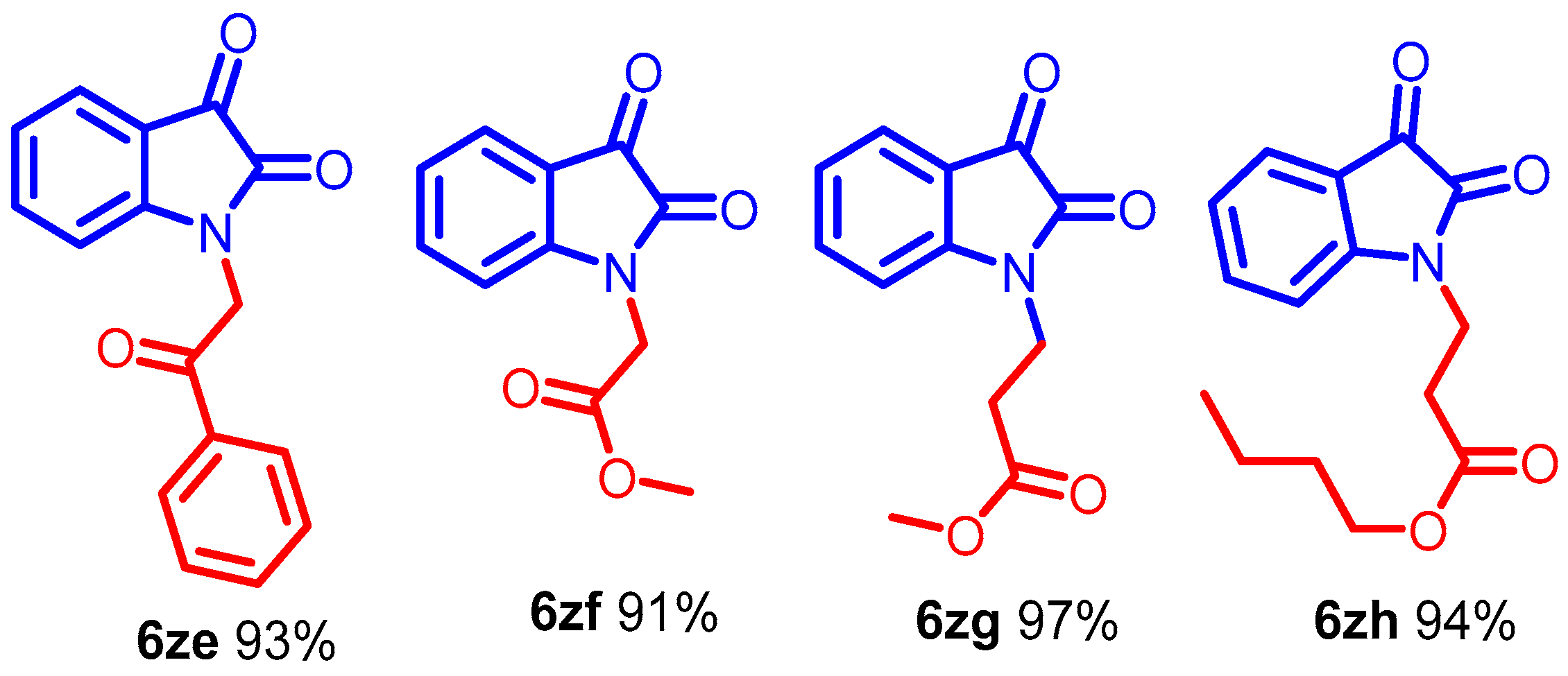
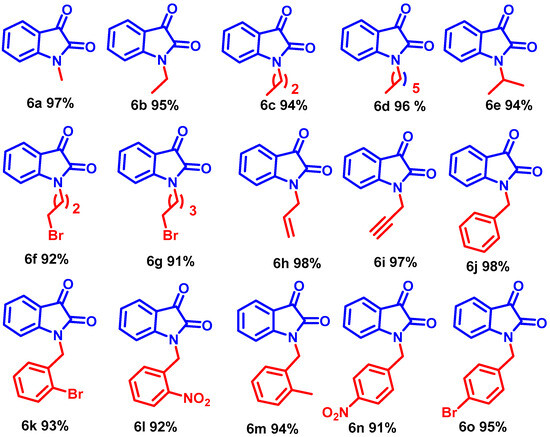
Interestingly, the resulting alkyl iodides reacted faster than the bromides and chlorides; benzyl moieties were less reactive than the alkenyl groups and more reactive than alkyl (Scheme 3, entries 6b–6j). N-alkylation of functionalized secondary halide gave moderate yields compared with the primary analog (Scheme 3, entry 6e) because of the inherent lower reactivity of the secondary alkyl halide. The electronic effects of the substituent on N-phenyl rings were studied under mild reaction conditions (Scheme 3, entries 6j–6o). In this case, incorporating electron-withdrawing functionalities increased the acidity of the N-H protons, dramatically improving the reaction conversion. The NMR and mass spectra of compound 6a-6o are provided in (ESI, Figures S7–S64). For example, para and ortho-substituted benzyl bromide revealed a tiny impact on product yields upon N-alkylation (Scheme 3, entries 6j–6o). Various function-group-substituted benzyl isatins have also been investigated (Scheme 4, entries 6p–6zd). On the other hand, highly reactive acyl bromides, such as phenacyl bromide and methyl bromoacetate, were used for the N-alkylation of isatin under optimized conditions. Moreover, this method was reliable for the conjugate additions with Michael-type acceptors such as α, β-unsaturated esters, and isocyanates to obtain N-functionalized adducts (Scheme 5, 6ze–6zh) in high yields. The NMR and mass spectra of the compound (6p–6zh) are provided in (ESI, Figures S65–S135).
Scheme 3.
Synthesis of N-alkyl isatin derivatives in optimized condition.
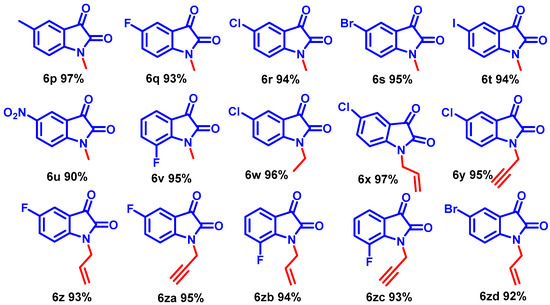
Scheme 4.
N-alkylation on substituted isatins in optimized condition.
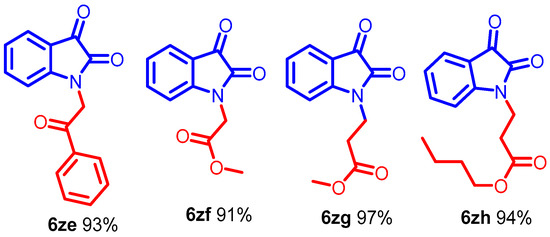
Scheme 5.
Synthesis of N-alkyl isatin with acyl bromide and Michael-type acceptors.
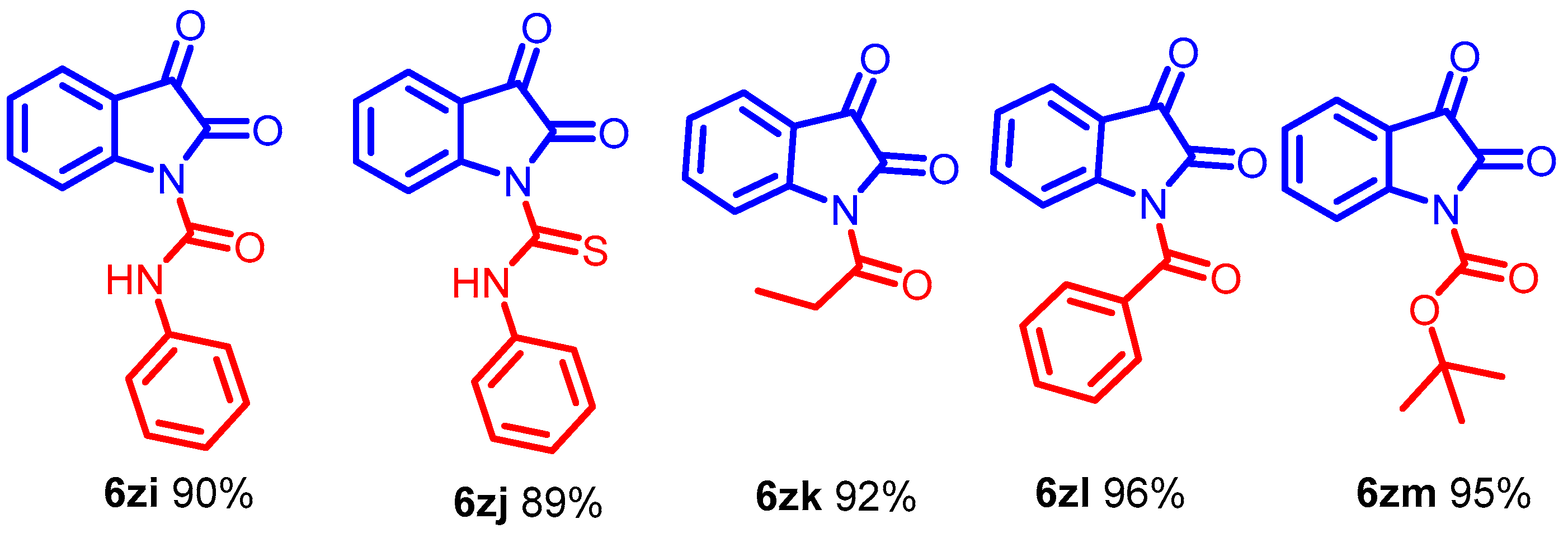
The DABCOIL@SBA-15 catalyst synthesized urea-type adducts (Scheme 6, 6zi–6zm) by adding isatin anions to isocyanates. Interestingly, phenyl isocyanate is suitable for transforming generous compounds (Scheme 6, 6zi–6zj) in high yields. Moreover, the procedure can functionalize α-oxoimide using propanoyl chloride and benzoic anhydride to obtain the acylated products (Scheme 6, 6zk–6zl) [43]. The use of the DABCOIL@SBA-15 heterogeneous catalyst further enabled the synthesis of the Boc anhydride compound (Scheme 6, 6zm). The formation of a solid residue indicated the completion of the reaction.
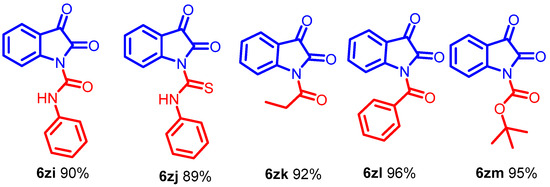
Scheme 6.
Synthesis of N-alkyl isatin with different acyl donors for α-oxoimide, Optimized condition.
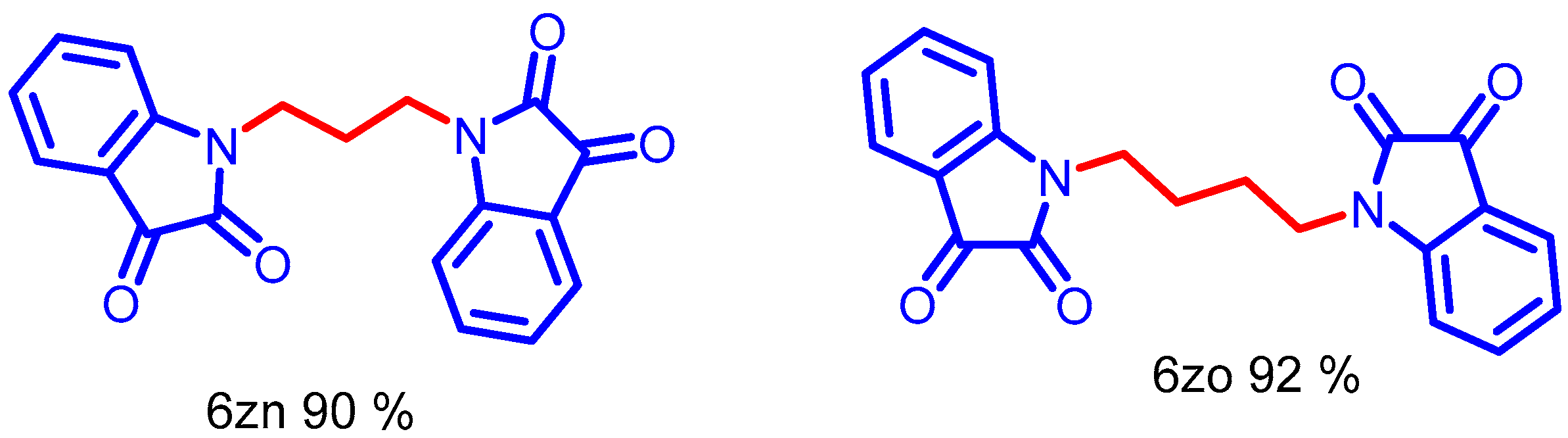
A bis-electrophilic reagent such as 1,4-dibromobutane and 1, 3 propane reacted with isatin to generate bis-isatin-based compounds in high yields (Scheme 7, 6zn–6zo). The NMR and mass spectra of compound 6zi–6zo are provided in (ESI, Figures S136–S161).
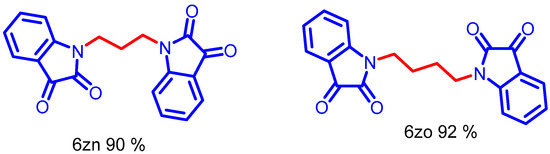
Scheme 7.
Synthesis of N-alkyl bis-isatin with di-halo carbon tether, Optimized condition.
2.8. Hammett Analysis for the Synergetic Effect of Catalyst
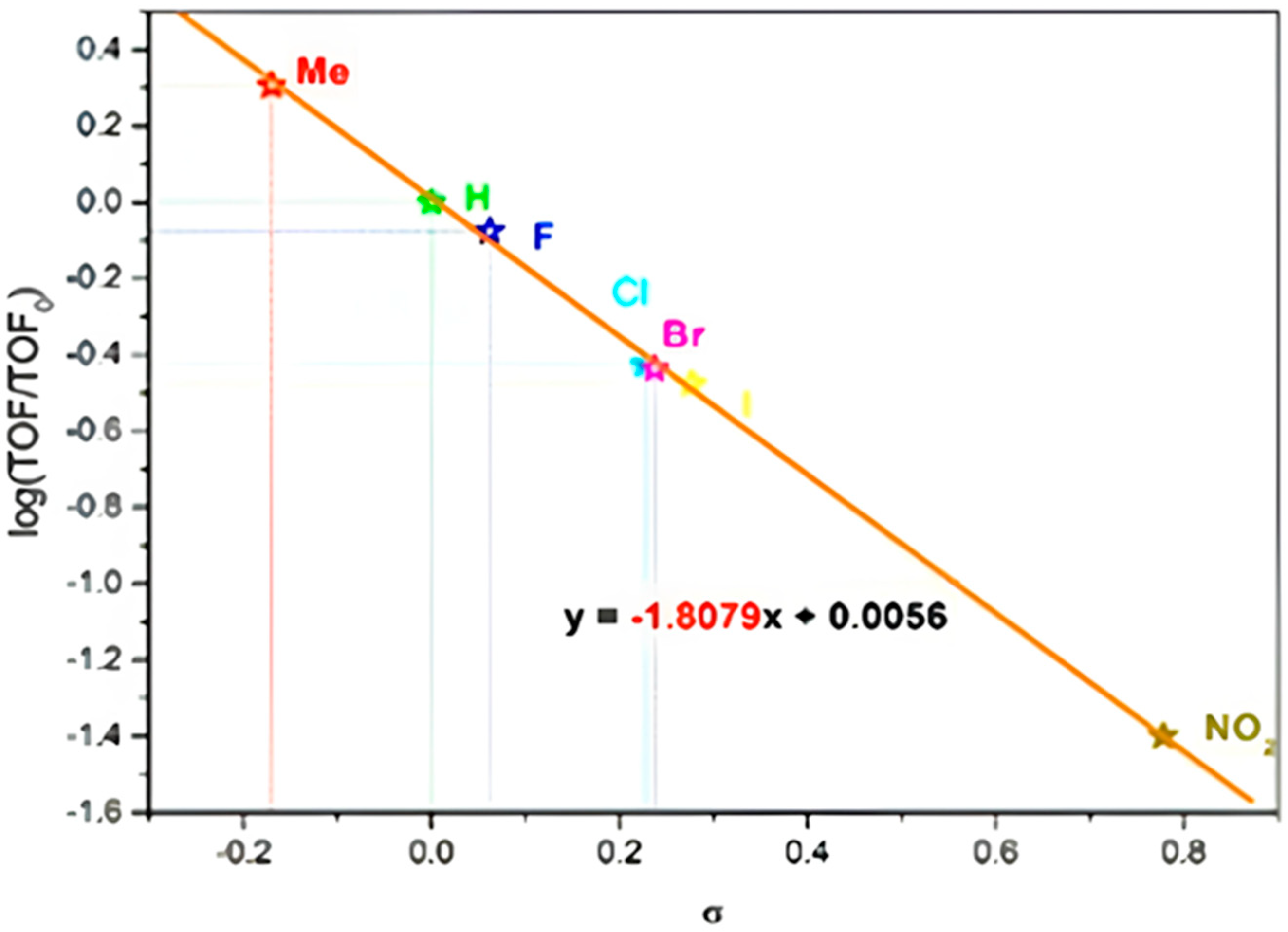
Mechanistic studies of N-alkyl isatin were conducted using Hammett analysis. First, the isatin and their six derivatives, such as 5-nitro-isatin, 5-chloro-isatin, 5-bromo-isatin, 5-iodo-isatin, 5-fluoro-isatin, and 4-methyl-isatin, were used for the study. The reactions were studied in an ideal optimized condition. Samples were collected in 20 min intervals; each reaction was repeated four times, and mean turnover frequencies (TOFs) were recorded. The Hammett constants were taken from previously reported values [44]. The crude N-methyl and alkyl isatin derivatives were examined by GC-mass spectroscopy to determine the selectivity of products [45].
Turnover frequency (TOF) values of seven isatin derivatives were plotted by the standard σ values of the substituent constants (Figure 7), and a slope of −1.8079 showed the reasonable substituent effect on the activity of the reaction [40]. The negative slope value revealed that groups at a para position on the isatin triggered the rate of N-alkylation; the decreased slope suggested the significant substituent effects on the kinetics of N-methyl isatin, but closer inspection revealed a subtle stabilization effect based on the electronic nature of the substituent. Mechanistically, the electron donating group increased the electron density at the nitrogen atom of isatin through resonance or inductive donating effect, which increased the reactivity and made the nucleophiles stronger.
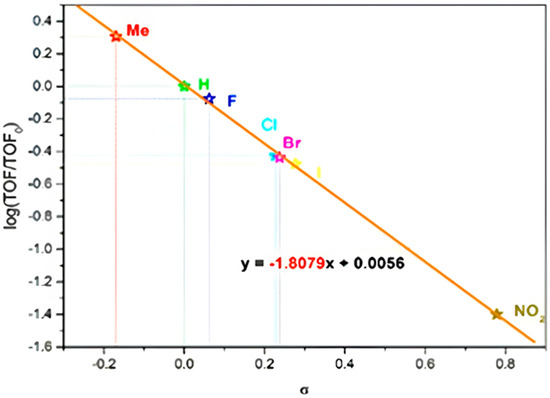
Figure 7.
Hammett plot for competitive reaction.
2.9. Reusability Test of the Catalyst
The synthesis of recoverable catalysts is essential from an economic and environmental viewpoint. Compound 6j was taken as the standard for recyclability studies. The DABCOIL@SBA-15 catalyst was recovered after the complete conversion of the first cycle by simple sintered glass crucible filtration, washed with DCM solvent, and reused for the next cycle. The catalytic performance was slightly decreased after testing seven cycles. However, the performance of DABCOIL@SBA-15 was comparable or even superior to that of the mineral-based catalysts. The results are summarized in Table 3.

Table 3.
Recyclability of DABCOIL@SBA-15 catalyst.
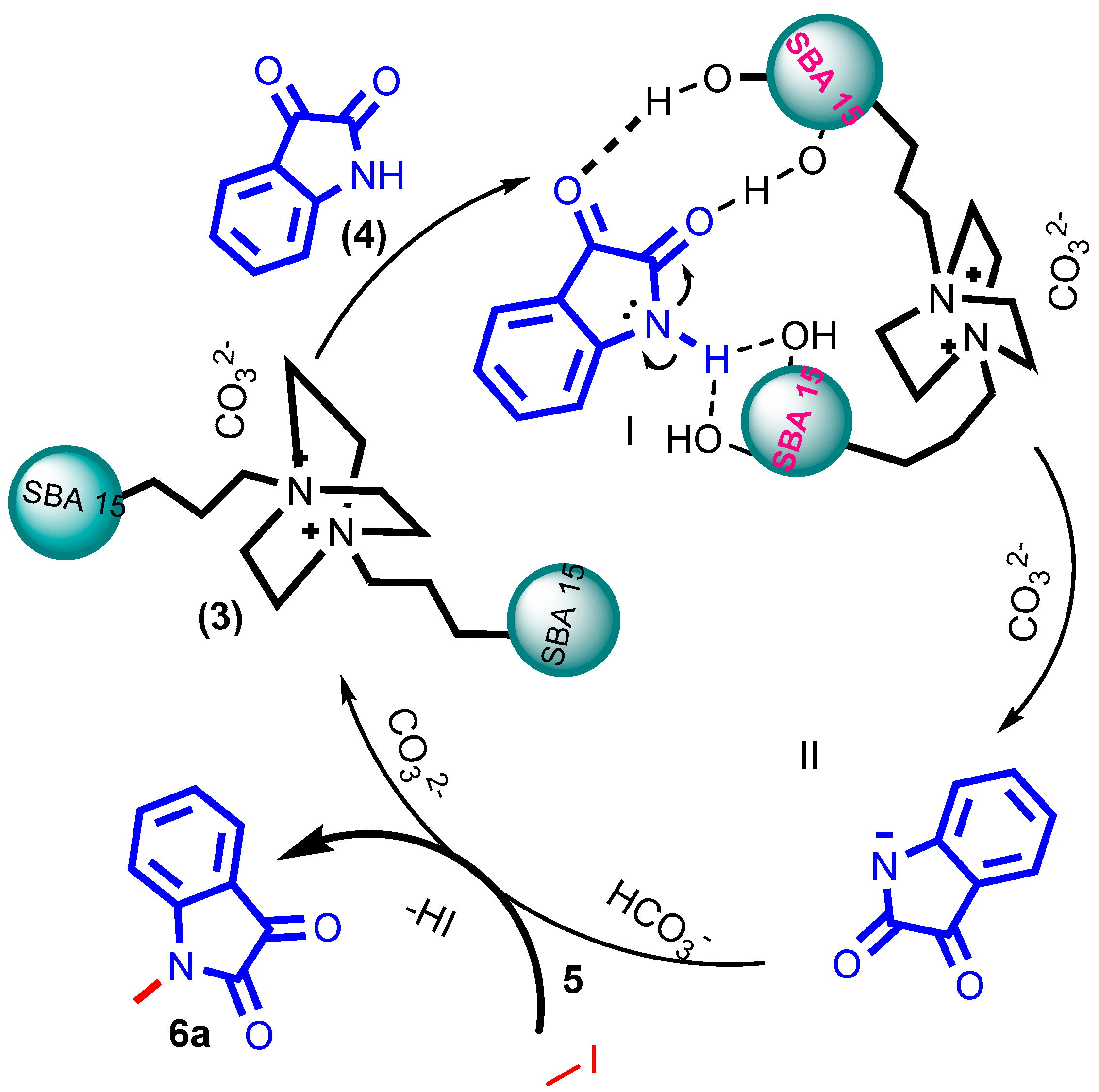
2.10. Plausible Mechanism
A plausible mechanism for synthesizing N-alkylated isatin 6a has been proposed in Scheme 8. Initially, the inherent acidic nature of the surface acidic hydroxyl groups of silanol interacts with the carbonyl group of isatins, making the N-substitution feasible [46]. Then, the silanol dragged the amide lone pair electrons [47] and the hydrogen. This served as hydrogen and facilitated the proton transfer reactions because of the weak acidic nature of the amide proton [48]. Next, the nucleophilic attack of the isatin was generated by activating the carbonate, which begins to abstract the amide proton, and negative nitrogen ions were stabilized by the surface silanol, which allows for simultaneous activation of the reactants to afford N-nucleophile step II. Finally, these nucleophilic attacks the alkyl group to form stable product 6a via the SN2 substitution reaction.
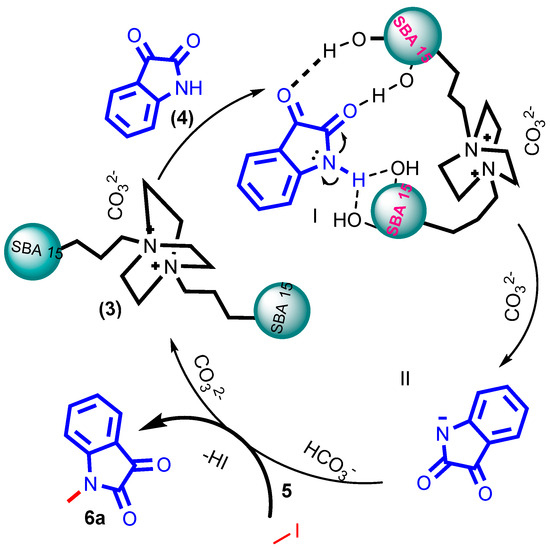
Scheme 8.
The proposed plausible mechanism for N-alkylated reaction.
3. Conclusions
In conclusion, we successfully prepared a DABCOIL@SBA-15 (3) heterogeneous catalyst by grafting DABCOIL (1) onto the SBA-15 surface. Various spectroscopic and microscopic techniques characterized the catalyst. Further, the catalytic efficiency of heterogeneous catalysts was investigated in detail. Moreover, the catalyst was utilized for the N-alkylation reaction of various isatin derivatives and demonstrated good isolated yields of 41 derivatives. This method offers operational simplicity, large-scale synthesis, and quick product isolation. The substituent effect and electronic nature of the derivatives were studied using the Hammett equation. We found that the catalyst was highly efficient for the N-alkylation reaction of isatin-based compounds and could be reused for multiple cycles without trailing their catalytic activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14090629/s1, The detailed synthesis, 1H, 13C NMR data, and additional data can be found in the supporting information [12,27,28,33,34,35,38,49,50,51,52].
Author Contributions
G.S.: Conceptualization, methodology, investigation, data curation, formal analysis, writing original draft, review, and editing. V.R.S.: funding, resource, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding authors upon request.
Acknowledgments
The first author (G.S.) acknowledges CSIR, Government of India, for the financial support of a Senior Research Fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2019, 297, 112038. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic liquids—New “solutions” for transition metal catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar]
- Ni, B.; Headley, A.D. Ionic-Liquid-Supported (ILS) catalysts for asymmetric organic synthesis. Chem. Eur. J. 2010, 16, 4426–4436. [Google Scholar] [PubMed]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Qureshi, Z.S.; Deshmukh, K.M.; Bhanage, B.M. Applications of ionic liquids in organic synthesis and catalysis. Clean Technol. Environ. Policy 2013, 16, 1487–1513. [Google Scholar] [CrossRef]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Jamasbi, N.; Irankhah-Khanghah, M.; Shirini, F.; Tajik, H.; Langarudi, M.S. DABCO-based ionic liquids: Introduction of two met-al-free catalysts for one-pot synthesis of 1, 2, 4-triazolo [4, 3-a] pyrimidines and pyrido [2, 3-d] pyrimidines. New J. Chem. 2018, 42, 9016–9027. [Google Scholar]
- Sahoo, S.; Kumar, P.; Lefebvre, F.; Halligudi, S. Oxidative kinetic resolution of alcohols using chiral Mn–salen complex immobilized onto ionic liquid modified silica. Appl. Catal. A Gen. 2008, 354, 17–25. [Google Scholar] [CrossRef]
- Sharma, P.; Gupta, M. Silica functionalized sulphonic acid coated with ionic liquid: An efficient and recyclable heterogeneous catalyst for the one-pot synthesis of 1,4-dihydropyridines under solvent-free conditions. Green Chem. 2014, 17, 1100–1106. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Green chemistry by nano-catalysis. Green Chem. 2010, 12, 743–754. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Luque, R.; Fihri, A.; Zhu, H.; Bouhrara, M.; Basset, J.-M. Magnetically Recoverable Nanocatalysts. Chem. Rev. 2011, 111, 3036–3075. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, J.; Kiasat, A.R. Covalently anchored n-propyl-4-aza-1-azoniabicyclo [2.2.2] octane chloride on SBA-15 as a basic nanocatalyst for the synthesis of pyran heterocyclic compounds. RSC Adv. 2014, 4, 4403–4412. [Google Scholar]
- Li, J.; Qi, T.; Wang, L.; Liu, C.; Zhang, Y. Synthesis and characterization of imidazole-functionalized SBA-15 as an adsorbent of hexavalent chromium. Mater. Lett. 2007, 61, 3197–3200. [Google Scholar] [CrossRef]
- Badamali, S.K.; Luque, R.; Clark, J.H.; Breeden, S.W. Unprecedented oxidative properties of mesoporous silica materials: Towards microwave-assisted oxidation of lignin model compounds. Catal. Commun. 2012, 31, 1–4. [Google Scholar] [CrossRef]
- Wang, Y.; Caruso, F. Mesoporous Silica Spheres as Supports for Enzyme Immobilization and Encapsulation. Chem. Mater. 2005, 17, 953–961. [Google Scholar] [CrossRef]
- Costa, J.A.S.; de Jesus, R.A.; Santos, D.O.; Neris, J.B.; Figueiredo, R.T.; Paranhos, C.M. Synthesis, functionalization, and environmental application of silica-based mesoporous materials of the M41S and SBA-n families: A review. J. Environ. Chem. Eng. 2021, 9, 105259. [Google Scholar] [CrossRef]
- Xie, W.; Hu, L.; Yang, X. Basic ionic liquid supported on mesoporous SBA-15 silica as an efficient heterogeneous catalyst for bio-diesel production. Ind. Eng. Chem. Res. 2015, 54, 1505–1512. [Google Scholar]
- Li, R.; Song, H.; Wang, G.; Chen, J. Efficient and reusable SBA-15-immobilized Bronsted acidic ionic liquid for the ketalization of cyclohexanone with glycol. RSC Adv. 2018, 8, 7179–7185. [Google Scholar]
- Sheng, X.; Zhou, Y.; Yang, Y.; Zhang, Y.; Zhang, Z.; Zhou, S.; Fu, X.; Zhao, S. Synthesis of immobilized heteropolyanion-based ionic liquids on mesoporous silica SBA-15 as a heterogeneous catalyst for alkylation. RSC Adv. 2014, 4, 30697–30703. [Google Scholar] [CrossRef]
- Tao, Y.; Ma, M.; Li, W.; Zhou, L.; Li, L.; Guo, Z.; Zhang, C.; Lv, Z. Palladium covalently anchored imidazolium functionalized SBA-15: An efficient recoverable heterogeneous catalyst for the hydrocarboxylation of styrene. J. Solid State Chem. 2023, 330, 124471. [Google Scholar] [CrossRef]
- Rajabi, F.; Ebrahimi, A.Z.; Rabiee, A.; Pineda, A.; Luque, R. Synthesis and Characterization of Novel Pyridine Periodic Mesoporous Organosilicas and Its Catalytic Activity in the Knoevenagel Condensation Reaction. Materials 2020, 13, 1097. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, F.; Burange, A.S.; Voskressensky, L.G.; Luque, R. Supported phosphine free bis-NHC palladium pincer complex: An efficient reusable nanocatalyst for Suzuki-Miyaura coupling reaction. Mol. Catal. 2021, 515, 111928. [Google Scholar] [CrossRef]
- Karimi, B.; Khorasani, M. Selectivity Adjustment of SBA-15 Based Tungstate Catalyst in Oxidation of Sulfides by Incorporating a Hydrophobic Organic Group inside the Mesochannels. ACS Catal. 2013, 3, 1657–1664. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X.; Bai, X. Preparation of SBA-15 supported Ru nanocatalysts by electrostatic adsorption–ultrasonic in situ reduction method and its catalytic performance for hydrogen storage of N-ethylcarbazole. Environ. Sci. Pollut. Res. 2023, 30, 98034–98047. [Google Scholar] [CrossRef]
- Navik, R.; Wang, E.; Ding, X.; Qiu, K.; Li, J. Atmospheric carbon dioxide capture by adsorption on amine-functionalized silica composites: A review. Environ. Chem. Lett. 2024, 22, 1791–1830. [Google Scholar] [CrossRef]
- Ekeoma, B.C.; Yusuf, M.; Johari, K.; Abdullah, B. Mesoporous silica supported Ni-based catalysts for methane dry reforming: A review of recent studies. Int. J. Hydrogen Energy 2022, 47, 41596–41620. [Google Scholar] [CrossRef]
- Elumalai, V.; Dharmalingam, S. Synthesis characterization and performance evaluation of ionic liquid immobilized SBA-15/quaternised polysulfone composite membrane for alkaline fuel cell. Microporous Mesoporous Mater. 2016, 236, 260–268. [Google Scholar]
- Vijayakumar, E.; Sangeetha, D. A quaternized mesoporous silica/polysulfone composite membrane for an efficient alkaline fuel cell application. RSC Adv. 2015, 5, 42828–42835. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.; Wan, Y.; Chai, W.; Zhang, F.; Lu, Y. Aqueous medium Ullmann reaction over a novel Pd/Ph–Al-MCM-41 as a new route of clean organic synthesis. Green Chem. 2006, 9, 273–280. [Google Scholar] [CrossRef]
- Reichhardt, N.V.; Guillet-Nicolas, R.; Thommes, M.; Klösgen, B.; Nylander, T.; Kleitz, F.; Alfredsson, V. Mapping the location of grafted PNIPAAM in mesoporous SBA-15 silica using gas adsorption analysis. Phys. Chem. Chem. Phys. 2012, 14, 5651–5661. [Google Scholar] [CrossRef]
- Kingchok, S.; Pornsuwan, S. Comparison of spherical and rod-like morphologies of SBA-15 for enzyme immobilization. J. Porous Mater. 2020, 27, 1547–1557. [Google Scholar]
- Batail, N.; Bendjeriou, A.; Djakovitch, L.; Dufaud, V. Larock indole synthesis using palladium complexes immobilized onto mesoporous silica. Appl. Catal. A Gen. 2010, 388, 179–187. [Google Scholar]
- Chen, S.Y.; Huang, C.Y.; Yokoi, T.; Tang, C.Y.; Huang, S.J.; Lee, J.J.; Chan, J.C.C.; Tatsumi, T.; Cheng, S. Synthesis and catalytic activity of amino-functionalized SBA-15 materials with controllable channel lengths and amino loadings. J. Mater. Chem. 2011, 22, 2233–2243. [Google Scholar] [CrossRef]
- Rostamnia, S.; Doustkhah, E.; Zeynizadeh, B. Cationic modification of SBA-15 pore walls for Pd supporting: Pd@ SBA-15/ILDABCO as a catalyst for Suzuki coupling in water medium. Microporous Mesoporous Mater. 2016, 222, 87–93. [Google Scholar]
- Li, Q.-F.; Yue, D.; Lu, W.; Zhang, X.; Li, C.; Wang, Z. Hybrid luminescence materials assembled by [Ln(DPA)3]3− and mesoporous host through ion-pairing interactions with high quantum efficiencies and long lifetimes. Sci. Rep. 2015, 5, 8385. [Google Scholar] [CrossRef]
- Yue, C.; Zhang, P.; Wu, H.; Fan, M.; Jiang, P. Novel Bronsted-Lewis Acid Heterogeneous Catalyst: Functionalized Imidazolium Ferric Salts@SBA-15 for Efficient Production of Biodiesel. ChemistrySelect 2019, 4, 11275–11281. [Google Scholar]
- Finn, M.; An, N.; Voutchkova-Kostal, A. Immobilization of imidazolium ionic liquids on hydrotalcites using silane linkers: Retardation of memory effect. RSC Adv. 2015, 5, 13016–13020. [Google Scholar]
- Sun, L.-B.; Liu, X.-Q.; Zhou, H.-C. Design and fabrication of mesoporous heterogeneous basic catalysts. Chem. Soc. Rev. 2015, 44, 5092–5147. [Google Scholar] [CrossRef]
- Jiang, X.; Cao, Y.; Wang, Y.; Liu, L.; Shen, F.; Wang, R. A unique approach to the concise synthesis of highly optically active spi-rooxazolines and the discovery of a more potent oxindole-type phytoalexin analog. J. Am. Chem. Soc. 2010, 13, 15328–15333. [Google Scholar]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2008, 38, 606–631. [Google Scholar] [CrossRef]
- Humphrey, J.M.; Chamberlin, A.R. Chemical Synthesis of Natural Product Peptides: Coupling Methods for the Incorporation of Noncoded Amino Acids into Peptides. Chem. Rev. 1997, 97, 2243–2266. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Yamagiwa, N.; Torisawa, Y. Cyclopentyl Methyl Ether as a New and Alternative Process Solvent. Org. Process. Res. Dev. 2007, 11, 251–258. [Google Scholar] [CrossRef]
- Barrett, M.J.; Davies, P.W.; Grainger, R.S. Regioselective functionalization of dibenzothiophenes through gold-catalyzed intermo-lecular alkyne oxyarylation. Org. Biomol. Chem. 2015, 13, 8676–8686. [Google Scholar] [PubMed]
- Song, H.; Rioux, R.M.; Hoefelmeyer, J.D.; Komor, R.; Niesz, K.; Grass, M.; Yang, P.; Somorjai, G.A. Hydrothermal Growth of Mesoporous SBA-15 Silica in the Presence of PVP-Stabilized Pt Nanoparticles: Synthesis, Characterization, and Catalytic Properties. J. Am. Chem. Soc. 2006, 128, 3027–3037. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, R.K.; Hwang, S.; Davis, M.E. Multifunctional Heterogeneous Catalysts: SBA-15-Containing Primary Amines and Sulfonic Acids. Angew. Chem. 2006, 118, 6480–6483. [Google Scholar] [CrossRef]
- Huh, S.; Chen, H.; Wiench, J.W.; Pruski, M.; Lin, V.S. Cooperative Catalysis by General Acid and Base Bifunctionalized Mesoporous Silica Nanospheres. Angew. Chem. 2005, 117, 1860–1864. [Google Scholar] [CrossRef]
- Margelefsky, E.L.; Zeidan, R.K.; Davis, M.E. Cooperative catalysis by silica-supported organic functional groups. Chem. Soc. Rev. 2008, 37, 1118–1126. [Google Scholar] [CrossRef]
- Sulpizi, M.; Gaigeot, M.-P.; Sprik, M. The Silica–Water Interface: How the Silanols Determine the Surface Acidity and Modulate the Water Properties. J. Chem. Theory Comput. 2012, 8, 1037–1047. [Google Scholar] [CrossRef]
- Castejon, H.; Wiberg, K.B. Solvent Effects on Methyl Transfer Reactions. 1. The Menshutkin Reaction. J. Am. Chem. Soc. 1999, 121, 2139–2146. [Google Scholar] [CrossRef]
- De Vos, D.; Vankelecom, I.F.; Jacobs, P.A. (Eds.) Chiral Catalyst Immobilization and Recycling; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Zhang, W.; Wang, Q.; Wu, H.; Wu, P.; He, M. A highly ordered mesoporous polymer supported imidazolium-based ionic liquid: An efficient catalyst for cycloaddition of CO2 with epoxides to produce cyclic carbonates. Green Chem. 2014, 16, 4767–4774. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.T.; Lin, V.S. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol-gel process and applications in controlled release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).