Comparative Studies on Methyl Ester Production from Pretreated Sludge Palm Oil Using Homogeneous and Heterogeneous Base Catalysts
Abstract
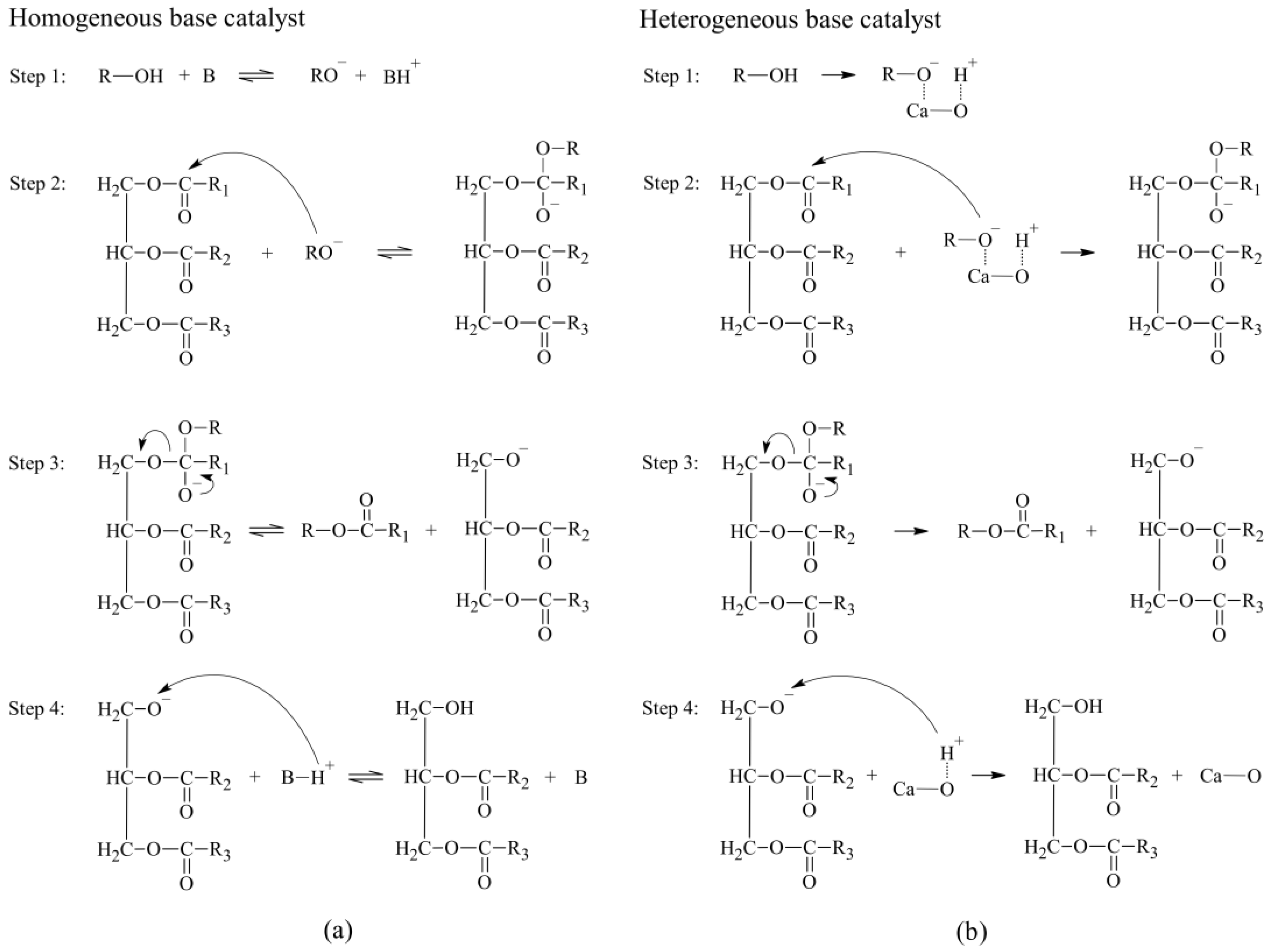
1. Introduction
2. Results and Discussion
2.1. Experimental Result
2.2. Response Surface Methodology and Statistical Analyses
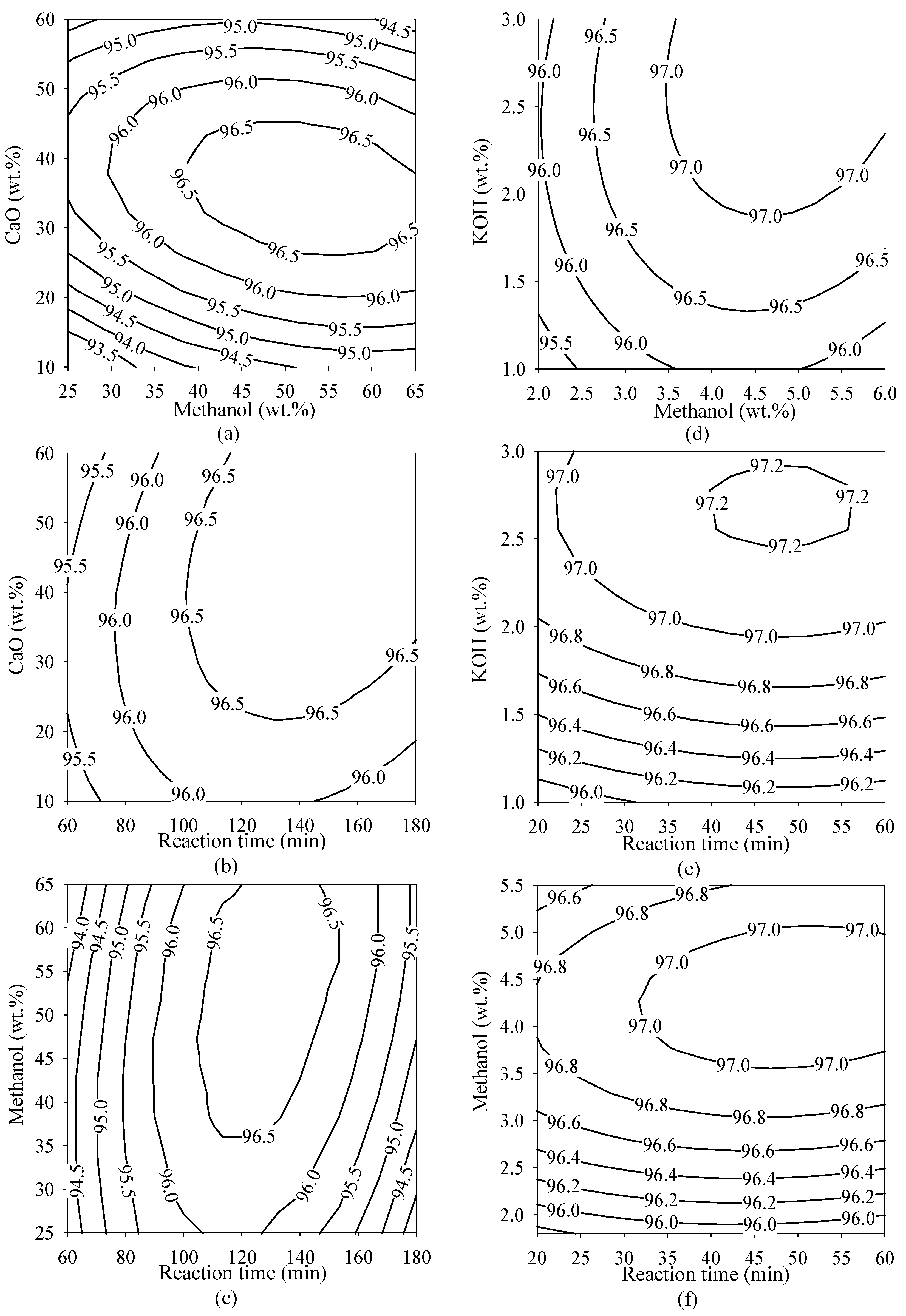
2.3. Response Surface Plots
2.4. Optimal Conditions for Methyl Ester Production
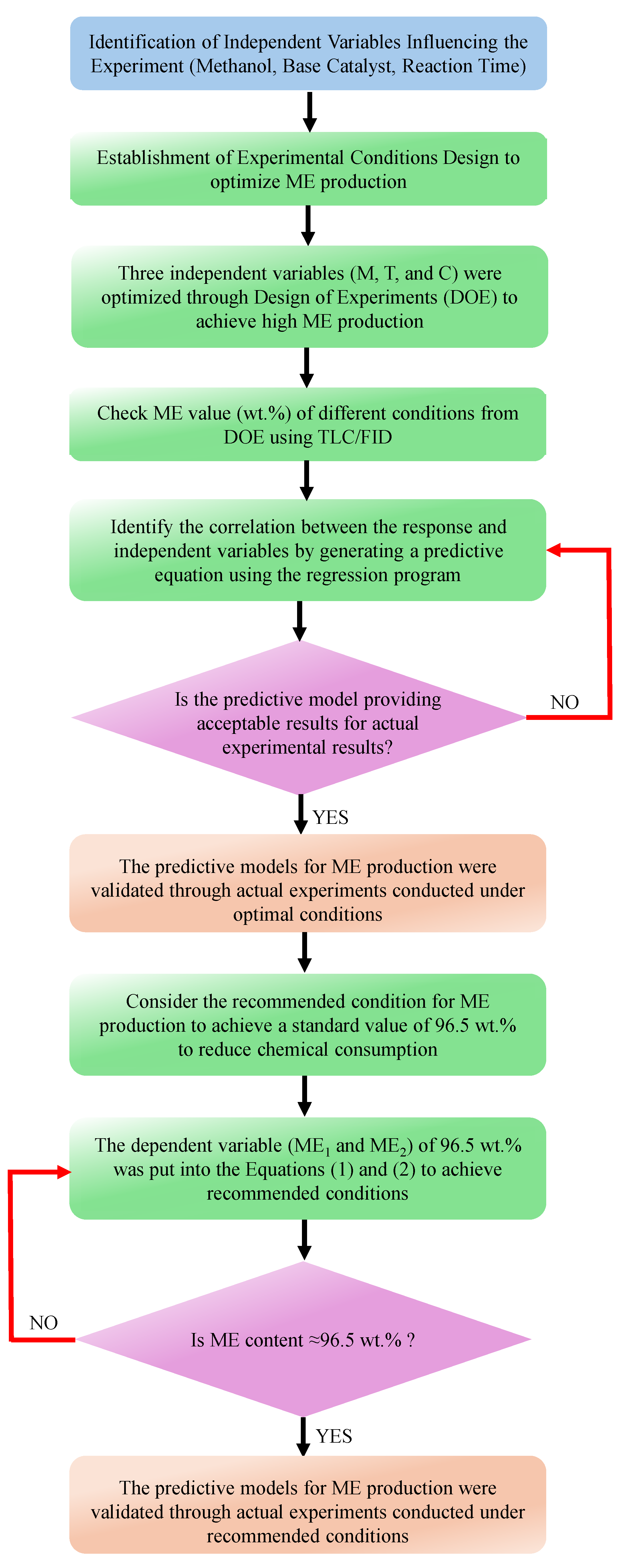
3. Materials and Methods
3.1. Materials
3.2. Procedure
3.2.1. Experimental Setup for the Base-Catalyst Transesterification Reaction
3.2.2. Experimental Design for the Base-Catalyst Transesterification Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chenic, A.; Ștefania; Cretu, A.I.; Burlacu, A.; Moroianu, N.; Vîrjan, D.; Huru, D.; Stanef-Puica, M.R.; Enachescu, V. Logical analysis on the strategy for a sustainable transition of the world to green energy—2050. Smart cities and villages coupled to renewable energy sources with low carbon footprint. Sustainability 2022, 14, 8622. [Google Scholar] [CrossRef]
- Hassan, Q.; Algburi, S.; Sameen, A.Z.; Al-Musawi, T.J.; Al-Jiboory, A.K.; Salman, H.M.; Ali, B.M.; Jaszczur, M. A comprehensive review of international renewable energy growth. Energy Built Environ. 2024, in press. [Google Scholar] [CrossRef]
- Ansari, D.; Holz, F. Between stranded assets and green transformation: Fossil-fuel-producing developing countries towards 2055. World Dev. 2020, 130, 104947. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y. Assessing the interrelationship between fossil fuels resources and the biomass energy market for achieving a sustainable and green economy. Resour. Policy 2024, 88, 104397. [Google Scholar] [CrossRef]
- Maneejuk, P.; Kaewtathip, N.; Yamaka, W. The influence of the Ukraine-Russia conflict on renewable and fossil energy price cycles. Energy Econ. 2024, 129, 107218. [Google Scholar] [CrossRef]
- San-Akca, B.; Sever, S.D.; Yilmaz, S. Does natural gas fuel civil war? Rethinking energy security, international relations, and fossil-fuel conflict. Energy Res. Soc. Sci. 2020, 70, 101690. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Bonifacio, S.; Clowes, J.; Foulds, A.; Holland, R.; Matthews, J.C.; Percival, C.J.; Shallcross, D.E. Investigation of biofuel as a potential renewable energy source. Atmosphere 2021, 12, 1289. [Google Scholar] [CrossRef]
- Mahapatra, S.; Kumar, D.; Singh, B.; Sachan, P.K. Biofuels and their sources of production: A review on cleaner sustainable alternative against conventional fuel, in the framework of the food and energy nexus. Energy Nexus 2021, 4, 100036. [Google Scholar] [CrossRef]
- Temizer, İ.; Eskici, B. Investigation on the combustion characteristics and lubrication of biodiesel and diesel fuel used in a diesel engine. Fuel 2020, 278, 118363. [Google Scholar] [CrossRef]
- Hájek, M.; Vávra, A.; de Paz Carmona, H.; Kocík, J. The catalysed transformation of vegetable oils or animal fats to biofuels and bio-lubricants: A review. Catalysts 2021, 11, 1118. [Google Scholar] [CrossRef]
- Toldrá-Reig, F.; Mora, L.; Toldrá, F. Trends in biodiesel production from animal fat waste. Appl. Sci. 2020, 10, 3644. [Google Scholar] [CrossRef]
- Dey, S.; Reang, N.M.; Das, P.K.; Deb, M. A Comprehensive study on prospects of economy, environment, and efficiency of palm oil biodiesel as a renewable fuel. J. Clean. Prod. 2021, 286, 124981. [Google Scholar] [CrossRef]
- Azad, A.K.; Halder, P.; Wu, Q.; Rasul, M.G.; Hassan, N.M.S.; Karthickeyan, V. Experimental investigation of ternary biodiesel blends combustion in a diesel engine to reduce emissions. Energy Convers. Manag. X 2023, 20, 100499. [Google Scholar] [CrossRef]
- Alahmer, A.; Alahmer, H.; Handam, A.; Rezk, H. Environmental assessment of a diesel engine fueled with various biodiesel blends: Polynomial regression and grey wolf optimization. Sustainability 2022, 14, 1367. [Google Scholar] [CrossRef]
- Kaewbuddee, C.; Sukjit, E.; Srisertpol, J.; Maithomklang, S.; Wathakit, K.; Klinkaew, N.; Liplap, P.; Arjharn, W. Evaluation of waste plastic oil-biodiesel blends as alternative fuels for diesel engines. Energies 2020, 13, 2823. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Kalam, M.A.; Masjuki, H.H.; Gul, M.; Soudagar, M.E.M.; Ong, H.C.; Ahmed, W.; Atabani, A.E.; Razzaq, L.; Yusoff, M. Comparative study of nanoparticles and alcoholic fuel additives-biodiesel-diesel blend for performance and emission improvements. Fuel 2020, 279, 118434. [Google Scholar] [CrossRef]
- Kaniapan, S.; Hassan, S.; Ya, H.; Patma Nesan, K.; Azeem, M. The utilisation of palm oil and oil palm residues and the related challenges as a sustainable alternative in biofuel, bioenergy, and transportation sector: A review. Sustainability 2021, 13, 3110. [Google Scholar] [CrossRef]
- Yusoff, M.N.A.M.; Zulkifli, N.W.M.; Sukiman, N.L.; Chyuan, O.H.; Hassan, M.H.; Hasnul, M.H.; Zulkifli, M.S.A.; Abbas, M.M.; Zakaria, M.Z. Sustainability of palm biodiesel in transportation: A review on biofuel standard, policy and international collaboration between Malaysia and Colombia. Bioenerg. Res. 2021, 14, 43–60. [Google Scholar] [CrossRef]
- Eremeeva, A.M.; Kondrasheva, N.K.; Khasanov, A.F.; Oleynik, I.L. Environmentally friendly diesel fuel obtained from vegetable raw materials and hydrocarbon crude. Energies 2023, 16, 2121. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Iqbal, J.; Bathula, C.; Al-Muhtaseb, A.H. Challenges and perspectives on innovative technologies for biofuel production and sustainable environmental management. Fuel 2022, 325, 124845. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, K.; Sun, S. Simultaneous esterification and transesterification of waste phoenix seed oil with a high free fatty acid content using a free lipase catalyst to prepare biodiesel. Biomass Bioenergy 2021, 144, 105930. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Chong, J.H.; Yuniarto, A.; Hadibarata, T. The current scenario and challenges of biodiesel production in Asian countries: A review. Bioresour. Technol. Rep. 2020, 12, 100608. [Google Scholar] [CrossRef]
- International Monetary Fund. Global Price of Palm Oil. Available online: https://fred.stlouisfed.org/series/PPOILUSDM (accessed on 31 January 2024).
- Jung, S.; Shetti, N.P.; Reddy, K.R.; Nadagouda, M.N.; Park, Y.-K.; Aminabhavi, T.M.; Kwon, E.E. Synthesis of different biofuels from livestock waste materials and their potential as sustainable feedstocks—A review. Energy Convers. Manag. 2021, 236, 114038. [Google Scholar] [CrossRef]
- Bitonto, L.D.; Scelsi, E.; Locaputo, V.; Mustafa, A.; Pastore, C. Enhancing biodiesel production from urban sewage sludge: A novel industrial configuration and optimization model. Sustain. Energy Technol. Assess. 2023, 60, 103567. [Google Scholar] [CrossRef]
- Ng, W.Z.; Obon, A.A.; Lee, C.L.; Ong, Y.H.; Gourich, W.; Maran, K.; Tang, D.B.Y.; Song, C.P.; Chan, E.-S. Techno-economic analysis of enzymatic biodiesel co-produced in palm oil mills from sludge palm oil for improving renewable energy access in rural areas. Energy 2022, 243, 122745. [Google Scholar] [CrossRef]
- Muanruksa, P.; Kaewkannetra, P. Combination of fatty acids extraction and enzymatic esterification for biodiesel production using sludge palm oil as a low-cost substrate. Renew. Energ. 2020, 146, 901–906. [Google Scholar] [CrossRef]
- A Aziz, M.M.; Kassim, K.A.; ElSergany, M.; Anuar, S.; Jorat, M.E.; Yaacob, H.; Ahsan, A.; Imteaz, M.A. Recent advances on palm oil mill effluent (POME) pretreatment and anaerobic reactor for sustainable biogas production. Renew. Sust. Energ. Rev. 2020, 119, 109603. [Google Scholar] [CrossRef]
- Mathew, G.M.; Raina, D.; Narisetty, V.; Kumar, V.; Saran, S.; Pugazhendi, A.; Sindhu, R.; Pandey, A.; Binod, P. Recent advances in biodiesel production: Challenges and solutions. Sci. Total Environ. 2021, 794, 148751. [Google Scholar] [CrossRef]
- Oo, Y.M.; Prateepchaikul, G.; Somnuk, K. Two-stage continuous production process for fatty acid methyl ester from high FFA crude palm oil using rotor-Stator hydrocavitation. Ultrason. Sonochem. 2021, 73, 105529. [Google Scholar] [CrossRef]
- Juera-Ong, P.; Pongraktham, K.; Oo, Y.M.; Somnuk, K. Reduction in free fatty acid concentration in sludge palm oil using heterogeneous and homogeneous catalysis: Process optimization, and reusable heterogeneous catalysts. Catalysts 2022, 12, 1007. [Google Scholar] [CrossRef]
- Tran, N.N.; Tišma, M.; Budžaki, S.; McMurchie, E.J.; Ngothai, Y.; Morales Gonzalez, O.M.; Hessel, V. Production of biodiesel from recycled grease trap waste: A review. Ind. Eng. Chem. Res. 2021, 60, 16547–16560. [Google Scholar] [CrossRef]
- Loh, J.M.; Gourich, W.; Chew, C.L.; Song, C.P.; Chan, E.S. Improved biodiesel production from sludge palm oil catalyzed by a low-cost liquid lipase under low-input process conditions. Renew. Energy 2021, 177, 348–358. [Google Scholar] [CrossRef]
- Maleki, B.; Ashraf Talesh, S.S.; Mansouri, M. Comparison of catalysts types performance in the generation of sustainable biodiesel via transesterification of various oil sources: A review study. Mater. Today Sustain. 2022, 18, 100157. [Google Scholar] [CrossRef]
- Mandari, V.; Devarai, S.K. Biodiesel production using homogeneous, heterogeneous, and enzyme catalysts via transesterification and esterification reactions: A critical review. Bioenerg. Res. 2022, 15, 935–961. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, A.; Saqib, S.; Lin, H.; Hassan Shah, M.U.; Ullah, S.; Younas, M.; Rezakazemi, M.; Ibrahim, M.; Mahmood, A.; Asif, S.; et al. Current status and challenges in the heterogeneous catalysis for biodiesel production. Renew. Sust. Energ. Rev. 2022, 157, 112012. [Google Scholar] [CrossRef]
- Belkhanchi, H.; Rouan, M.; Hammi, M.; Ziat, Y.; Chigr, M. Synthesis of biodiesel by transesterification of used frying oils (UFO) through basic homogeneous catalysts (NaOH and KOH). Biointerface Res. Appl. Chem. 2021, 11, 12858–12868. [Google Scholar]
- Aworanti, O.; Ao, A.; Se, A. Process parameter estimation of biodiesel production from waste frying oil (vegetable and palm oil) using homogeneous catalyst. J. Food Process Technol. 2019, 10, 811. [Google Scholar]
- Kasirajan, R. Biodiesel production by two step process from an energy source of Chrysophyllum albidum oil using homogeneous catalyst. S. Afr. J. Chem. Eng. 2021, 37, 161–166. [Google Scholar] [CrossRef]
- Mohiddin, M.N.B.; Tan, Y.H.; Seow, Y.X.; Kansedo, J.; Mubarak, N.M.; Abdullah, M.O.; Chan, Y.S.; Khalid, M. Evaluation on feedstock, technologies, catalyst and reactor for sustainable biodiesel production: A review. J. Ind. Eng. Chem. 2021, 98, 60–81. [Google Scholar] [CrossRef]
- Ulukardesler, A.H. Biodiesel production from waste cooking oil using different types of catalysts. Processes 2023, 11, 2035. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef]
- Gupta, V.; Pal Singh, K. The impact of heterogeneous catalyst on biodiesel production; a review. Mater. Today Proc. 2023, 78, 364–371. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Sundararaman, S.; Deivasigamani, P.; Rajasimman, M. Synthesis and characterization of barium doped CaO heterogeneous nanocatalyst for the production of biodiesel from Catharanthus roseus seeds: Kinetics, optimization and performance evaluation. Environ. Res. 2023, 222, 115336. [Google Scholar]
- Santos, S.; Nobre, L.; Gomes, J.; Puna, J.; Quinta-Ferreira, R.; Bordado, J. Soybean oil transesterification for biodiesel production with micro-structured calcium oxide (CaO) from natural waste materials as a heterogeneous catalyst. Energies 2019, 12, 4670. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Bhonsle, A.K.; Bangwal, D.P.; Atray, N. Development of a barium-modified zeolite catalyst for biodiesel production from waste frying oil: Process optimization by design of experiment. Renew. Energ. 2021, 177, 1253–1264. [Google Scholar] [CrossRef]
- Correia, L.M.; Saboya, R.M.A.; de Sousa Campelo, N.; Cecilia, J.A.; Rodríguez-Castellón, E.; Cavalcante, C.L., Jr.; Vieira, R.S. Characterization of calcium oxide catalysts from natural sources and their application in the transesterification of sunflower oil. Bioresour. Technol. 2014, 151, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Akhabue, C.E.; Ogogo, J.A. Modelling and optimization of transesterification of palm kernel oil catalysed by calcium oxide derived from hen eggshell wastes. Ife J.Sci. 2018, 20, 127–138. [Google Scholar] [CrossRef]
- Aziz, H.A.; Abas, N.A.; Ping, B.T.Y.; Idris, Z. Transesterification of palm-based methyl palmitate into esteramine catalyzed by calcium oxide catalyst. J. Surfactants Deterg. 2020, 23, 251–262. [Google Scholar] [CrossRef]
- Malek, M.N.F.A.; Pushparaja, L.; Hussin, N.M.; Embong, N.H.; Bhuyar, P.; Rahim, M.H.A.; Maniam, G.P. Exploration of efficiency of nano calcium oxide (CaO) as catalyst for enhancement of biodiesel production. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e3935. [Google Scholar]
- Badu, M.; Boateng, R.A.; Padevoah, M.M.; Agbemade, B.; Quainoo, T.; Mensah, M.B.; Boadi, N.O. Transesterification of palm kernel oil using calcium oxide as catalyst. Int. J. Chem. Biochem. Sci. 2021, 19, 1–11. [Google Scholar]
- Liu, X.; He, H.; Wang, Y.; Zhu, S.; Piao, X. Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 2008, 87, 216–221. [Google Scholar] [CrossRef]
- Almasi, S.; Najafi, G.; Ghobadian, B.; Jalili, S. Biodiesel production from sour cherry kernel oil as novel feedstock using potassium hydroxide catalyst: Optimization using response surface methodology. Biocatal. Agric. Biotechnol. 2021, 35, 102089. [Google Scholar] [CrossRef]
- Methanol, Puriss. 99.7% (GC), Honeywell Riedel-de Haën, Quantity: 2.5 L|Fisher Scientific. Available online: https://www.fishersci.com/shop/products/methanol-puriss-99-7-gc-honeywell-riedel-de-ha-n-4/60045576#?keyword= (accessed on 14 September 2024).
- Sublimed Sulfur, 99.5–100.5%, Spectrum Chemical, Quantity: 2.5 kg|Fisher Scientific. Available online: https://www.fishersci.com/shop/products/sublimed-sulfur-99-5-100-5-spectrum-chemical/18614127#?keyword=sulfuric (accessed on 14 September 2024).
- Amberlyst 15 Strongly Acidic, Cation Exchanger, Dry 39389-20-3. Available online: http://www.sigmaaldrich.com/ (accessed on 14 September 2024).
- Potassium Hydroxide, ca. 85%, Extra Pure, Flakes, Thermo Scientific Chemicals, Quantity: 1 kg|Fisher Scientific. Available online: https://www.fishersci.com/shop/products/potassium-hydroxide-ca-85-extra-pure-flakes-thermo-scientific/AC232550250#?keyword= (accessed on 14 September 2024).
- Calcium Oxide Powder 90.0%, 500 M&P IMPEX. Available online: https://www.mpimpex.co.th/product/calcium-oxide-powder-90-0/11000339797002185 (accessed on 14 September 2024).

| Author | Process | Reaction | Type of Reactor | Raw Material | Molar Ratio of Methanol to Oil | Type of Catalyst wt.% | Temperature (°C) | Time (h) | Yield (%) | Ester (wt.%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Correia et al. [47] | Batch | Transesterification | Round bottom flask | Sunflower oil | 9:1 | CaO (3) | 60 | 4 | - | 97.75 |
| Akhabue and Ohogo [48] | Batch | Transesterification | Round bottom flask | Palm kernel oil | 9.02:1 | CaO (3.106) | 51.4 | 2.26 | - | 94.63 |
| Aziz et al. [49] | Batch | Transesterification | Round bottom flask | Palm-based methyl palmitate | 1.8:1 | CaO (0.1) | 170 | 6 | - | 94.5 |
| Malek et al. [50] | Batch | Transesterification | Round bottom flask | Refined Palm oil | 15:9 | CaO (5) | 65 | 3 | - | 88.7 |
| Badu et al. [51] | Batch | Transesterification | Round bottom flask | Palm kernel oil | 6:1 | CaO (1) | 60 | 3.5 | - | 94.84 |
| In this study | Batch | Transesterification | Five-neck round bottom flask | Pretreated sludge palm | 5.83:1 | CaO (31.1) | 60 | 1.98 | 72.6 | 96.51 |
| Heterogeneous Catalytic Reaction | Homogeneous Catalytic Reaction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | M1 (wt.%) | T1 (min) | C1 (wt.%) | ME1 (wt.%) | Error | M2 (wt.%) | T2 (min) | C2 (wt.%) | ME2 (wt.%) | Error | ||
| Actual | Predicted | Actual | Predicted | |||||||||
| 1 | 25 | 60 | 10 | 92.8 | 92.8 | −0.02 | 1.8 | 20 | 1 | 95.02 | 95.03 | −0.01 |
| 2 | 25 | 180 | 10 | 91.2 | 91.4 | −0.23 | 1.8 | 60 | 1 | 95.12 | 95.09 | 0.03 |
| 3 | 25 | 120 | 35 | 94.6 | 94.2 | 0.40 | 1.8 | 40 | 2 | 95.85 | 95.91 | −0.06 |
| 4 | 25 | 60 | 60 | 93.8 | 94.2 | −0.39 | 1.8 | 20 | 3 | 95.7 | 95.67 | 0.03 |
| 5 | 25 | 180 | 60 | 94.4 | 94.2 | 0.23 | 1.8 | 60 | 3 | 95.74 | 95.73 | 0.01 |
| 6 | 45 | 120 | 10 | 95.7 | 95.6 | 0.12 | 3.7 | 40 | 1 | 96.05 | 96.07 | −0.02 |
| 7 | 45 | 60 | 35 | 96.0 | 96.1 | −0.09 | 3.7 | 20 | 2 | 96.76 | 96.78 | −0.02 |
| 8 | 45 | 120 | 35 | 96.9 | 96.7 | 0.17 | 3.7 | 40 | 2 | 96.92 | 97.02 | −0.10 |
| 9 | 45 | 120 | 35 | 96.8 | 96.7 | 0.06 | 3.7 | 40 | 2 | 97.1 | 97.02 | 0.08 |
| 10 | 45 | 120 | 35 | 96.8 | 96.7 | 0.06 | 3.7 | 40 | 2 | 97.15 | 97.02 | 0.13 |
| 11 | 45 | 120 | 35 | 96.9 | 96.7 | 0.18 | 3.7 | 40 | 2 | 97.04 | 97.02 | 0.02 |
| 12 | 45 | 180 | 35 | 96.1 | 96.6 | −0.43 | 3.7 | 60 | 2 | 96.94 | 96.99 | −0.05 |
| 13 | 45 | 120 | 60 | 96.5 | 96.6 | −0.05 | 3.7 | 40 | 3 | 97.12 | 97.17 | −0.05 |
| 14 | 65 | 60 | 10 | 93.2 | 93.4 | −0.22 | 5.5 | 20 | 1 | 95.25 | 95.30 | −0.05 |
| 15 | 65 | 180 | 10 | 94.8 | 94.4 | 0.34 | 5.5 | 60 | 1 | 95.71 | 95.66 | 0.05 |
| 16 | 65 | 120 | 35 | 94.6 | 94.9 | −0.33 | 5.5 | 40 | 2 | 96.77 | 96.78 | −0.01 |
| 17 | 65 | 60 | 60 | 93.0 | 92.6 | 0.35 | 5.5 | 20 | 3 | 96.89 | 96.84 | 0.05 |
| 18 | 65 | 180 | 60 | 94.9 | 95.0 | −0.14 | 5.5 | 60 | 3 | 97.17 | 97.21 | −0.04 |
| Coefficient | Heterogeneous Catalytic Reaction | Homogeneous Catalytic Reaction | ||
|---|---|---|---|---|
| Value | p-Value | Value | p-Value | |
| β0 | 83.0389 | 4.9 × 10−14 | 91.2652 | 1.0 × 10−17 |
| β1 | 0.4748 | 4.8 × 10−6 | 1.3581 | 7.0 × 10−7 |
| β2 | 0.1151 | 2.6 × 10−3 | 1.6853 | 1.3 × 10−5 |
| β3 | –0.0053 | 2.2 × 10−6 | 0.0242 | 3.7 × 10−2 |
| β4 | –0.0011 | 1.8 × 10−3 | –0.1972 | 1.9 × 10−7 |
| β5 | 0.0005 | 5.9 × 10−4 | 0.1231 | 1.8 × 10−5 |
| β6 | –0.0011 | 9.1 × 10−3 | 0.0021 | 2.3 × 10−2 |
| β7 | 0.0002 | 1.8 × 10−2 | –0.3971 | 1.4 × 10−5 |
| β8 | –0.0001 | 6.9 × 10−4 | –0.0003 | 2.1 × 10−2 |
| R2 R2adjusted | 0.976 0.956 | - | 0.995 0.990 | - |
| Heterogeneous Catalytic Reaction | |||||
| Source | SS | MS | F0 | Fcritical | DOF |
| Regression | 46.16 | 5.770 | 46.73 | 3.230 (F0.05,8,9) | 8 |
| Residual | 1.111 | 0.123 | - | - | 9 |
| LOF Error | 1.098 | 0.183 | 41.3554 | 0.00563 | 6 |
| Pure Error | 0.01328 | 0.00443 | - | - | 3 |
| Total | 47.27 | - | - | - | 17 |
| Homogeneous catalytic reaction | |||||
| Source | SS | MS | F0 | Fcritical | DOF |
| Regression | 10.23 | 1.279 | 212.13 | 3.230 (F0.05,8,9) | 8 |
| Residual | 0.05428 | 0.00603 | - | - | 9 |
| LOF Error | 0.02480 | 0.00413 | 0.4207 | 0.831 | 6 |
| Pure Error | 0.02948 | 0.00982 | - | - | 3 |
| Total | 10.29 | - | - | - | 17 |
| Conditions, Compositions, Density, and Yield | PSPO | Using CaO Catalyst | Using KOH Catalyst | ||
|---|---|---|---|---|---|
| Optimum | Recommended | Optimum | Recommended | ||
| Conditions | |||||
| Methanol (wt.%) | - | 47.08 | 41.61 | 4.58 | 3.45 |
| Reaction time (min) | - | 156.0 | 119.0 | 50.84 | 40.0 |
| Base catalyst (wt.%) | - | 47.21 | 31.3 | 2.83 | 1.39 |
| Predicted ME (wt.%) | - | 96.94 | 96.50 | 97.37 | 96.5 |
| Compositions | |||||
| FFA (wt.%) | 1.26 | 0.98 | 1.08 | 0.23 | 0.34 |
| Actual ME (wt.%) | 88.20 | 96.88 | 96.51 | 97.2 | 96.59 |
| TG (wt.%) | 6.62 | 1.00 | 1.00 | 1.03 | 1.39 |
| DG (wt.%) | 3.33 | 0.72 | 1.03 | 1.40 | 1.54 |
| MG (wt.%) | 0.58 | 0.44 | 0.38 | 0.14 | 0.13 |
| Properties | |||||
| Density at 60 °C (kg/L) | 0.851 | 0.858 | 0.854 | 0.852 | 0.856 |
| Viscosity at 40 °C (cSt) | 5.71 | 5.04 | 5.29 | 5.36 | 5.88 |
| Cloud point (°C) | 13 | 14.0 | 14.0 | 12 | 12 |
| Pour point (°C) | 10 | 13.0 | 13.0 | 10 | 10 |
| Yield a | - | 75.9 | 72.6 | 76.0 | 70.8 |
| Reaction | Raw Material | Catalyst | Chemical | Condition | Weight (kg/batch) | Chemical Price (USD/kg) | Production Cost (USD/batch) |
|---|---|---|---|---|---|---|---|
| First-step esterification [31] | SPO | Homogeneous acid catalyst (H2SO4) | Methanol | 58.35 wt.% | 0.175 | 12.18 a | 2.13 |
| H2SO4 | 16.81 wt.% | 0.050 | 48.53 b | 2.45 | |||
| Total | 4.58 | ||||||
| First-step esterification [31] | SPO | Heterogeneous acid catalyst (Amberlyst-15) | Methanol | 44.66 wt.% | 0.134 | 12.18 a | 1.63 |
| Amberlyst-15 | 38.57 wt.% | 0.116 | 424.00 c | 49.06 | |||
| Total | 50.69 | ||||||
| Second-step transesterification (this study) | PSPO | Homogeneous base catalyst (KOH) | Methanol | 3.45 wt.% | 0.010 | 12.18 a | 0.13 |
| KOH | 1.39 wt.% | 0.005 | 21.15 d | 0.10 | |||
| Total | 0.23 | ||||||
| Second-step transesterification (this study) | PSPO | Heterogeneous base catalyst (CaO) | Methanol | 41.61 wt.% | 0.125 | 12.18 a | 1.52 |
| CaO | 31.30 wt.% | 0.094 | 47.24 e | 4.44 | |||
| Total | - | - | - | 5.96 |
| Independent Variable | Symbol | Levels of Independent Variable | ||
|---|---|---|---|---|
| –1 | 0 | 1 | ||
| Heterogeneous catalytic reaction | ||||
| Methanol (wt.%) | M1 | 25 | 45 | 65 |
| Reaction time (min) | T1 | 60 | 120 | 180 |
| CaO loading (wt.%) | C1 | 10 | 35 | 60 |
| Homogeneous catalytic reaction | ||||
| Methanol (wt.%) | M2 | 1.8 | 3.7 | 5.5 |
| Reaction time (min) | T2 | 20 | 40 | 60 |
| KOH loading (wt.%) | C2 | 1 | 2 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oo, Y.M.; Juera-Ong, P.; Pongraktham, K.; Somnuk, K. Comparative Studies on Methyl Ester Production from Pretreated Sludge Palm Oil Using Homogeneous and Heterogeneous Base Catalysts. Catalysts 2024, 14, 647. https://doi.org/10.3390/catal14090647
Oo YM, Juera-Ong P, Pongraktham K, Somnuk K. Comparative Studies on Methyl Ester Production from Pretreated Sludge Palm Oil Using Homogeneous and Heterogeneous Base Catalysts. Catalysts. 2024; 14(9):647. https://doi.org/10.3390/catal14090647
Chicago/Turabian StyleOo, Ye Min, Panupong Juera-Ong, Kritsakon Pongraktham, and Krit Somnuk. 2024. "Comparative Studies on Methyl Ester Production from Pretreated Sludge Palm Oil Using Homogeneous and Heterogeneous Base Catalysts" Catalysts 14, no. 9: 647. https://doi.org/10.3390/catal14090647
APA StyleOo, Y. M., Juera-Ong, P., Pongraktham, K., & Somnuk, K. (2024). Comparative Studies on Methyl Ester Production from Pretreated Sludge Palm Oil Using Homogeneous and Heterogeneous Base Catalysts. Catalysts, 14(9), 647. https://doi.org/10.3390/catal14090647









