Tuning Surface State in CoFe (Oxy)Hydroxide for Improved Oxygen Evolution Electrocatalysis
Abstract
1. Introduction
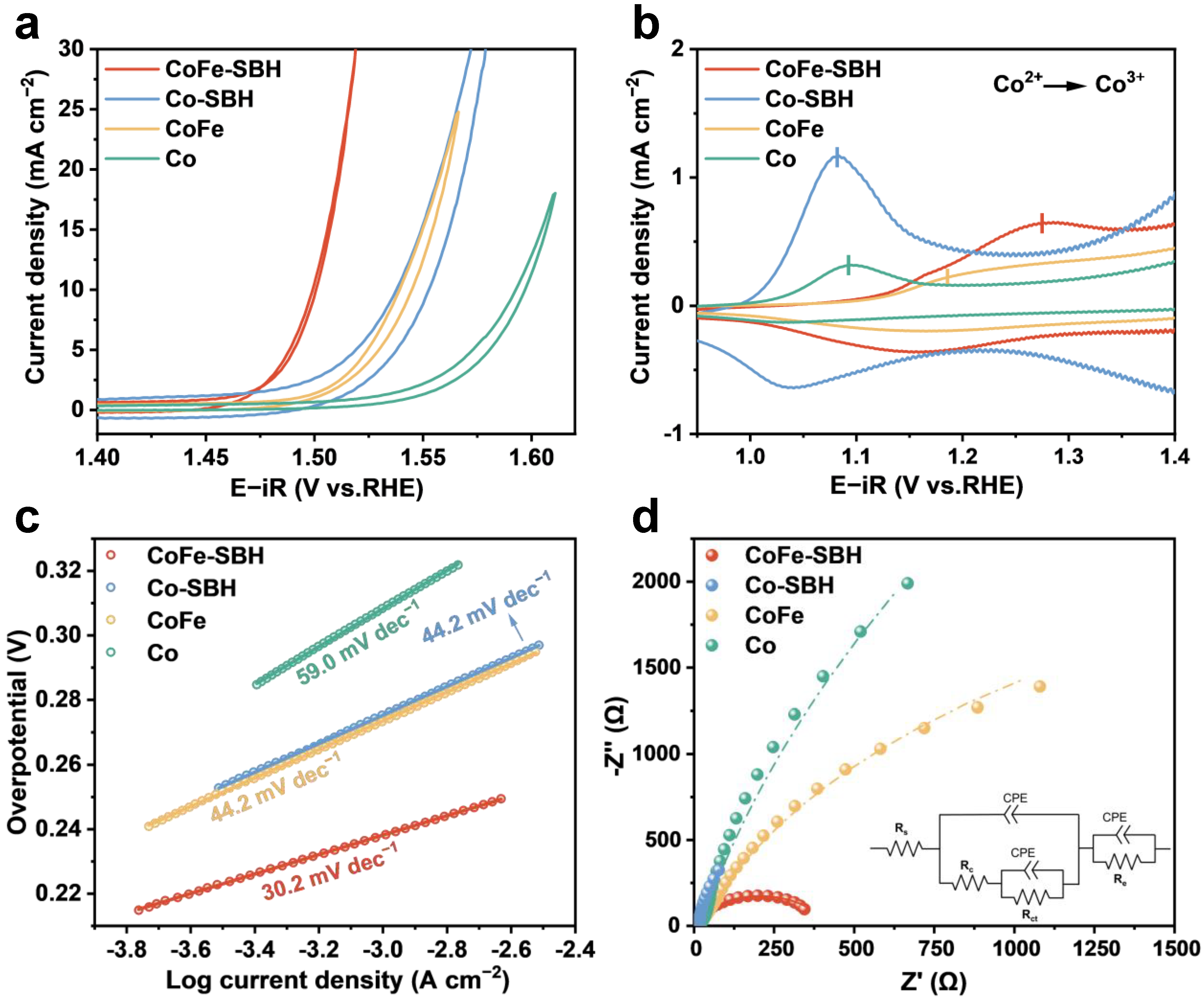
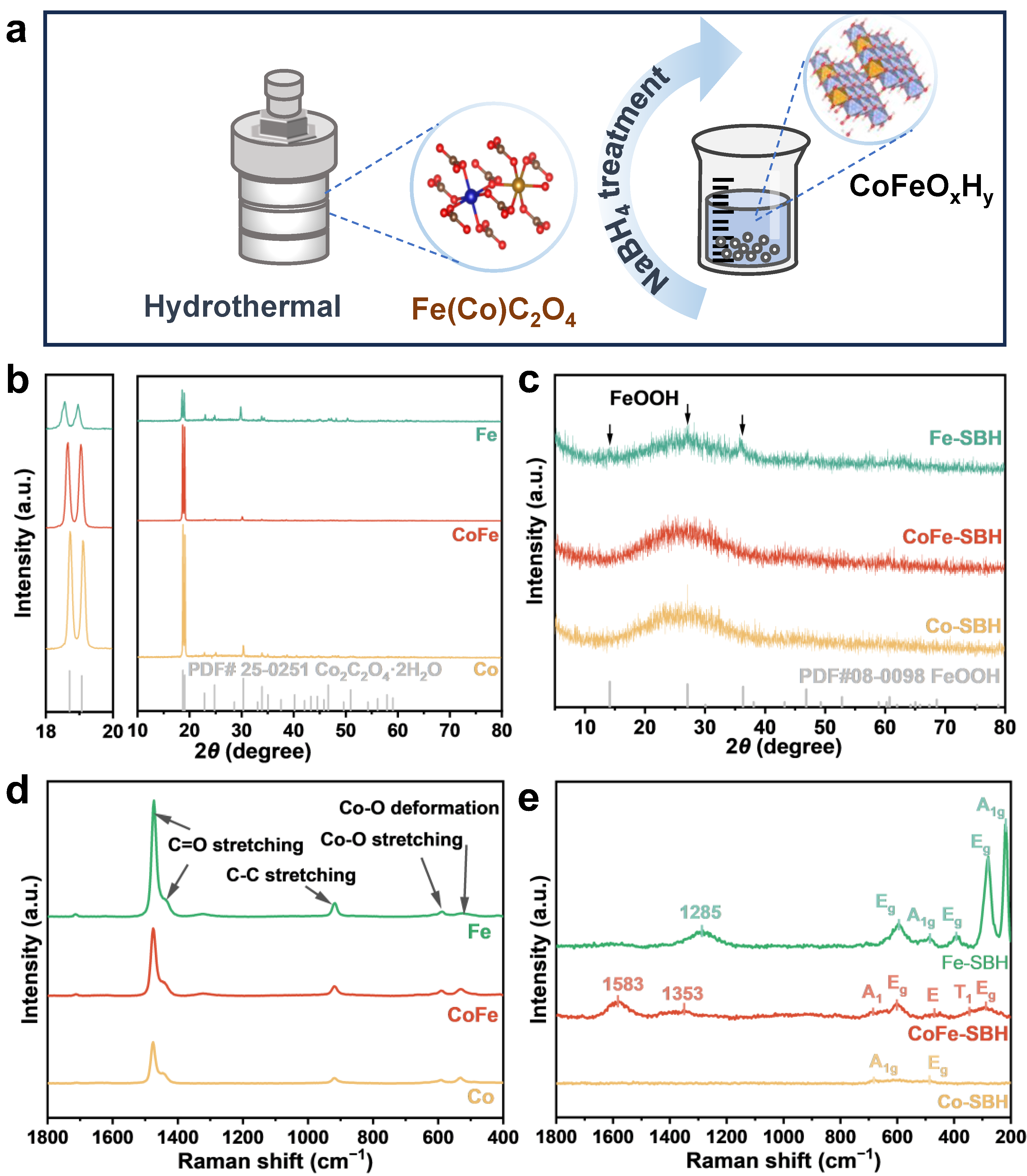
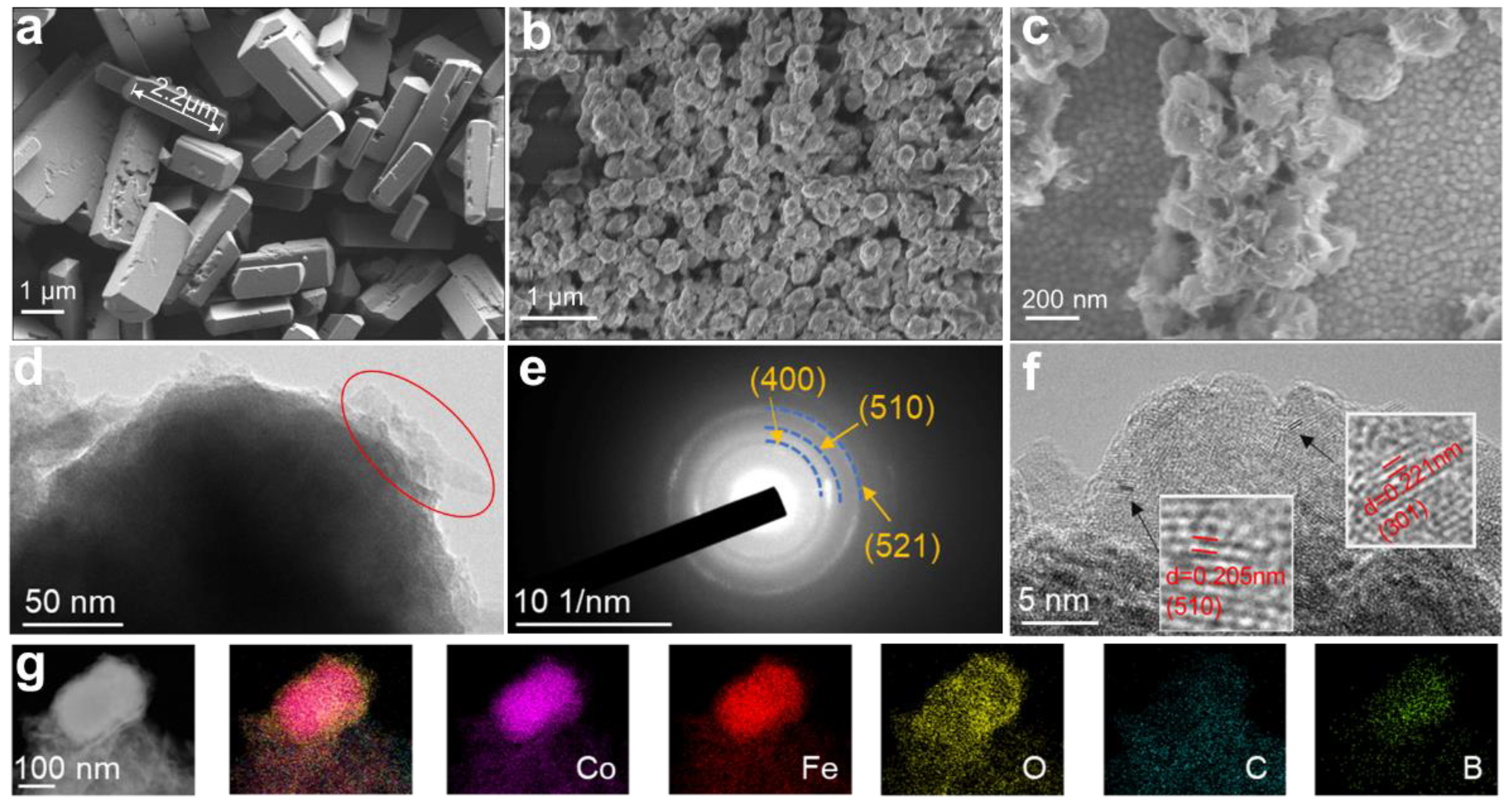
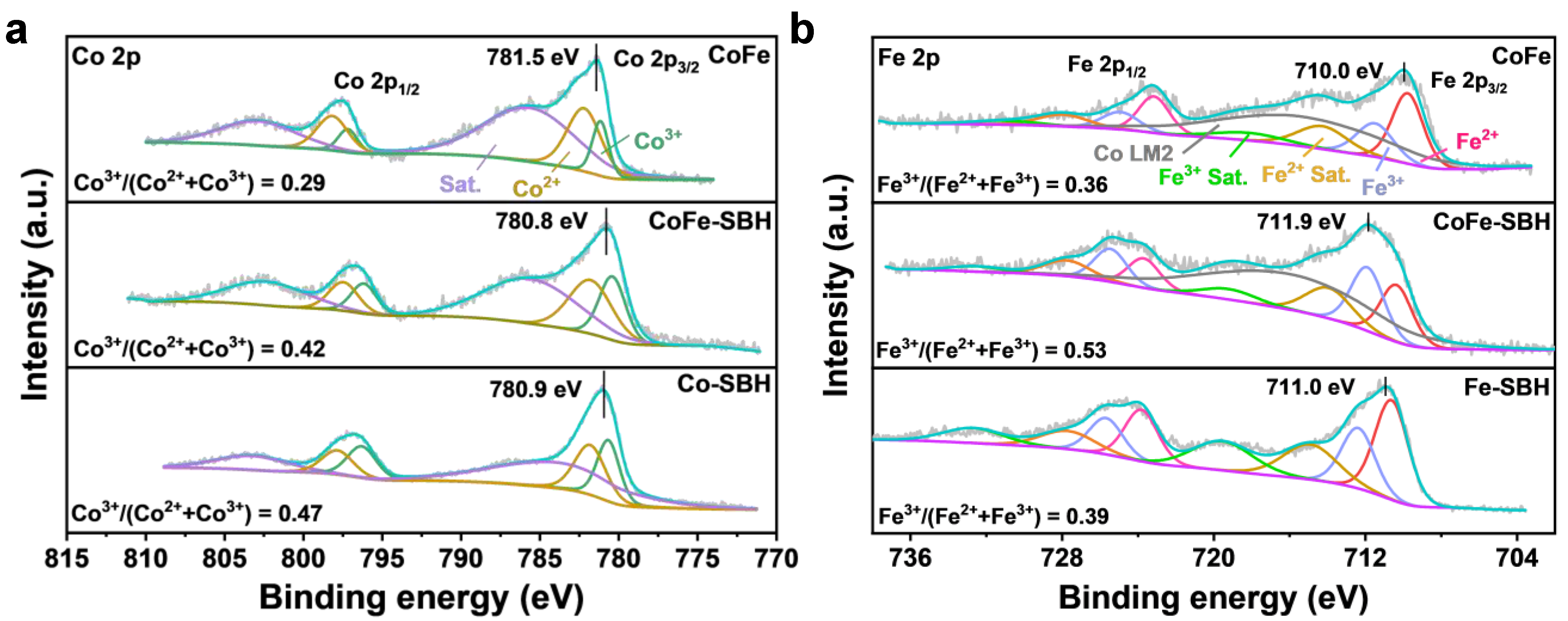
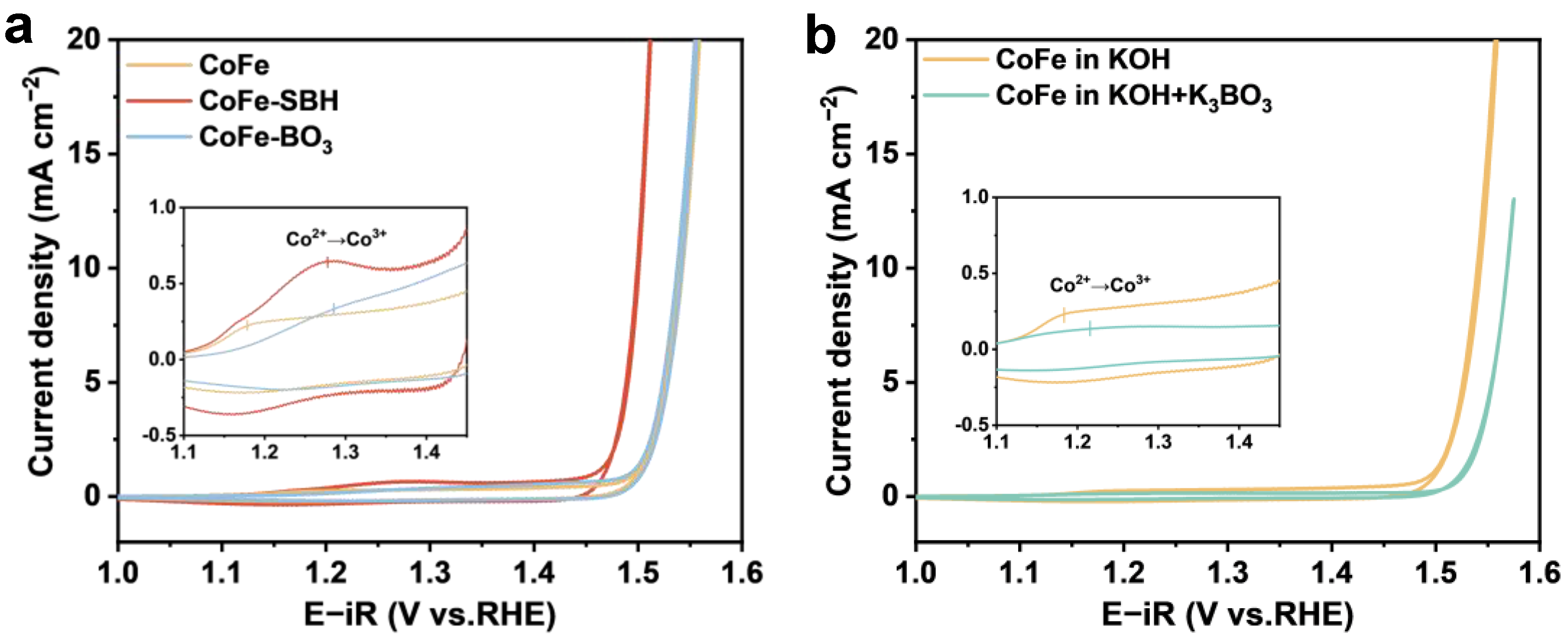
2. Results and Discussion
3. Experimental Section
3.1. Materials and Sample Preparation
3.2. Characterizations
3.3. Electrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Gao, X.; Xiong, D.; Wei, F.; Song, W.G.; Xu, J.; Liu, L. Hydrothermal synthesis of monolithic Co3Se4 nanowire electrodes for oxygen evolution and overall water splitting with high efficiency and extraordinary catalytic stability. Adv. Energy Mater. 2017, 7, 1602579. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. “The Fe Effect”: A review unveiling the critical roles of Fe in enhancing OER activity of Ni and Co based catalysts. Nano Energy 2021, 80, 105514. [Google Scholar] [CrossRef]
- Sun, Y.; Liao, H.; Wang, J.; Chen, B.; Sun, S.; Ong, S.J.H.; Xi, S.; Diao, C.; Du, Y.; Wang, J.O.; et al. Covalency competition dominates the water oxidation structure-activity relationship on spinel oxides. Nat. Catal. 2020, 3, 554–563. [Google Scholar] [CrossRef]
- Wang, J.; Kim, S.J.; Liu, J.; Gao, Y.; Choi, S.; Han, J.; Shin, H.; Jo, S.; Kim, J.; Ciucci, F.; et al. Redirecting dynamic surface restructuring of a layered transition metal oxide catalyst for superior water oxidation. Nat. Catal. 2021, 4, 212–222. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Zhao, Y.; Ge, X.; Lu, Q.; Zhang, T.; Xie, D.; Li, M.; Bu, Y. Design strategies of perovskite nanofibers electrocatalysts for water splitting: A mini review. Chem. Eng. J. 2023, 451, 138710. [Google Scholar] [CrossRef]
- Yao, N.; Wang, G.; Jia, H.; Yin, J.; Cong, H.; Chen, S.; Luo, W. Intermolecular energy gap-induced formation of high-valent cobalt species in CoOOH surface layer on cobalt sulfides for efficient water oxidation. Angew. Chem. Int. Ed. 2022, 61, e202117178. [Google Scholar] [CrossRef]
- Lee, S.; Moysiadou, A.; Chu, Y.C.; Chen, H.M.; Hu, X. Tracking high-valent surface iron species in the oxygen evolution reaction on cobalt iron (oxy)hydroxides. Energy Environ. Sci. 2022, 15, 206–214. [Google Scholar] [CrossRef]
- Xu, X.; Song, F.; Hu, X. A nickel iron diselenide-derived efficient oxygen-evolution catalyst. Nat. Commun. 2016, 7, 12324. [Google Scholar] [CrossRef]
- Guo, Y.; Tong, Y.; Chen, P.; Xu, K.; Zhao, J.; Lin, Y.; Chu, W.; Peng, Z.; Wu, C.; Xie, Y. Engineering the electronic state of a perovskite electrocatalyst for synergistically enhanced oxygen evolution reaction. Adv. Mater. 2015, 27, 5989–5994. [Google Scholar] [CrossRef]
- Xu, K.; Cheng, H.; Liu, L.; Lv, H.; Wu, X.; Wu, C.; Xie, Y. Promoting active species generation by electrochemical activation in alkaline media for efficient electrocatalytic oxygen evolution in neutral media. Nano Lett. 2016, 17, 578–583. [Google Scholar] [CrossRef]
- Liu, H.J.; Zhang, S.; Yang, W.Y.; Yu, N.; Liu, C.Y.; Chai, Y.M.; Dong, B. Directional reconstruction of iron oxides to active sites for superior water oxidation. Adv. Funct. Mater. 2023, 33, 2303776. [Google Scholar] [CrossRef]
- Kang-Wen, Q.; Xi, C.; Zhang, Y.; Zhang, R.; Li, Z.; Sheng, G.R.; Liu, H.; Dong, C.K.; Chen, Y.J.; Du, X.W. Laser-induced oxygen vacancies in FeCo2O4 nanoparticles for boosting oxygen evolution and reduction. Chem. Commun. 2019, 55, 8579–8582. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.F.; Hsu, Y.Y.; Chang, C.J.; Hsu, C.S.; Suen, N.T.; Chan, T.S.; Chen, H.M. Unraveling geometrical site confinement in highly efficient iron-doped electrocatalysts toward oxygen evolution reaction. Adv. Energy Mater. 2017, 8, 1701686. [Google Scholar] [CrossRef]
- Enman, L.J.; Stevens, M.B.; Dahan, M.H.; Nellist, M.R.; Toroker, M.C.; Boettcher, S.W. Operando X-Ray absorption spectroscopy shows iron oxidation is concurrent with oxygen evolution in cobalt-iron (oxy)hydroxide electrocatalysts. Angew. Chem. Int. Ed. 2018, 57, 12840–12844. [Google Scholar] [CrossRef]
- Li, N.; Hadt, R.G.; Hayes, D.; Chen, L.X.; Nocera, D.G. Detection of high-valent iron species in alloyed oxidic cobaltates for catalysing the oxygen evolution reaction. Nat. Commun. 2021, 12, 4218. [Google Scholar] [CrossRef]
- Burke, M.S.; Kast, M.G.; Trotochaud, L.; Smith, A.M.; Boettcher, S.W. Cobalt-iron (oxy)hydroxide oxygen evolution electrocatalysts: The role of structure and composition on activity, stability, and mechanism. J. Am. Chem. Soc. 2015, 137, 3638–3648. [Google Scholar] [CrossRef]
- Zhang, T.; Nellist, M.R.; Enman, L.J.; Xiang, J.; Boettcher, S.W. Modes of Fe incorporation in Co-Fe (oxy)hydroxide oxygen evolution electrocatalysts. ChemSusChem 2019, 12, 2015–2021. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, T.; Ye, Y.; Dai, W.; Zhu, Y.; Pan, Y. Stabilizing oxygen vacancy in entropy-engineered CoFe2O4-type catalysts for Co-prosperity of efficiency and stability in an oxygen evolution reaction. ACS Appl. Mater. Interfaces 2020, 12, 32548–32555. [Google Scholar] [CrossRef]
- Chen, H.; Song, L.; Ouyang, S.; Wang, J.; Lv, J.; Ye, J. Co and Fe codoped WO2.72 as alkaline-solution-available oxygen evolution reaction catalyst to construct photovoltaic water splitting system with solar-to-hydrogen efficiency of 16.9 %. Adv. Sci. 2019, 6, 1900465. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Liu, H.; Liang, J.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Preparation of hollow cobalt-iron phosphides nanospheres by controllable atom migration for enhanced water oxidation and splitting. Small 2021, 17, 2007858. [Google Scholar] [CrossRef]
- Wang, T.; Wang, C.; Jin, Y.; Sviripa, A.; Liang, J.; Han, J.; Huang, Y.; Li, Q.; Wu, G. Amorphous Co-Fe-P nanospheres for efficient water oxidation. J. Mater. Chem. A 2017, 5, 25378–25384. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, J.; Wang, P.; Chen, D.; Zhang, C.; Xiao, M.; Ma, Q.; Bai, H.; Qin, R.; Ma, J.; et al. Fe-Co-P multi-heterostructure arrays for efficient electrocatalytic water splitting. J. Mater. Chem. A 2021, 9, 24677–24685. [Google Scholar] [CrossRef]
- Liu, H.J.; Zhang, S.; Zhou, Y.N.; Yu, W.L.; Ma, Y.; Wang, S.T.; Chai, Y.M.; Dong, B. Dynamically stabilized electronic regulation and electrochemical reconstruction in Co and S atomic pair doped Fe3O4 for water oxidation. Small 2023, 19, 2301255. [Google Scholar] [CrossRef]
- Li, X.; Duan, F.; Deng, M.; Zheng, W.; Lin, Y.; Dan, Y.; Cheng, X.; Chen, L. Effect of Fe doping on Co-S/carbon cloth as bifunctional electrocatalyst for enhanced water splitting. J. Electroanal. Chem. 2022, 922, 116723. [Google Scholar] [CrossRef]
- Suryawanshi, U.P.; Suryawanshi, M.P.; Ghorpade, U.V.; Shin, S.W.; Kim, J.; Kim, J.H. An earth-abundant, amorphous cobalt-iron-borate (Co-Fe-Bi) prepared on Ni foam as highly efficient and durable electrocatalysts for oxygen evolution. Appl. Surf. Sci. 2019, 495, 143462. [Google Scholar] [CrossRef]
- Tian, L.; Zhong, D.; Zhao, T.; Liu, Y.; Hao, L.; Fang, Q.; Lang, X.; Zhao, X.; Hao, G.; Liu, G.; et al. Oxygen-vacancy-rich Co3O4@Fe-B-O heterostructure for efficient oxygen evolution reaction in alkaline and neutral media. J. Colloid Interface Sci. 2023, 646, 452–460. [Google Scholar] [CrossRef]
- Sun, J.; Guo, N.; Shao, Z.; Huang, K.; Li, Y.; He, F.; Wang, Q. A facile strategy to construct amorphous spinel-based electrocatalysts with massive oxygen vacancies using ionic liquid dopant. Adv. Energy Mater. 2018, 8, 1800980. [Google Scholar] [CrossRef]
- Yang, W.; Guo, J.; Ma, J.; Wu, N.; Xiao, J.; Wu, M. FeCo nanoalloys encapsulated in N-doped carbon nanofibers as a trifunctional catalyst for rechargeable Zn-air batteries and overall water electrolysis. J. Alloys Compd. 2022, 926, 166937. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, Y.; Zhou, X.; Ye, Y.; Nie, K.; Wang, J.; Xie, M.; Zhang, Z.; Liu, Z.; Cheng, T.; et al. Promoting nickel oxidation state transitions in single-layer NiFeB hydroxide nanosheets for efficient oxygen evolution. Nat. Commun. 2022, 13, 6094. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, S.; Lin, H.; Han, S.; Zhong, W.; Xie, Y.; Hu, J.; Yang, S. NaBH4 induces a high ratio of Ni3+/Ni2+ boosting OER activity of the NiFe LDH electrocatalyst. RSC Adv. 2020, 10, 33475–33482. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Long, X.; Chen, L.; Cao, Q.; Wang, J.; Qiu, C.; Lim, J.; Yang, S. Formation of FeOOH nanosheets induces substitutional doping of CeO2−x with high-valence Ni for efficient water oxidation. Adv. Energy Mater. 2020, 11, 2002731. [Google Scholar] [CrossRef]
- Su, L.; Du, H.; Tang, C.; Nan, K.; Wu, J.; Li, C.M. Borate-ion intercalated NiFe layered double hydroxide to simultaneously boost mass transport and charge transfer for catalysis of water oxidation. J. Colloid Interface Sci. 2018, 528, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Han, M.H.; Ko, Y.J.; Min, B.K.; Chae, K.H.; Oh, H.S. Electrode reconstruction strategy for oxygen evolution reaction: Maintaining Fe-CoOOH phase with intermediate-spin state during electrolysis. Nat. Commun. 2022, 13, 605. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Sun, Y.; Ren, X.; Wang, J.; Song, J.; Pan, Y.; Mu, Y.; Zhang, J.; Cheng, Q.; Xian, G.; et al. Reconstruction of thiospinel to active sites and spin channels for water oxidation. Adv. Mater. 2022, 35, 2207041. [Google Scholar] [CrossRef]
- Reith, L.; Hausmann, J.N.; Mebs, S.; Mondal, I.; Dau, H.; Driess, M.; Menezes, P.W. In situ detection of iron in oxidation states ≥ IV in cobalt-iron oxyhydroxide reconstructed during oxygen evolution reaction. Adv. Energy Mater. 2023, 13, 2203886. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16745–17236. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015, 137, 4275–4592. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; Liu, H.; Zhang, Q.; Liu, B.; Yang, M.; Dai, H.; He, D.; Feng, X.; Xiao, X. Unraveling the modulation essence of p bands in Co-based oxide stability on acidic oxygen evolution reaction. Nano Res. 2024, 17, 5922–5929. [Google Scholar] [CrossRef]
- Huang, M.; Cao, C.; Liu, L.; Wei, W.; Zhu, Q.-L.; Huang, Z. Controlled synthesis of MOF-derived hollow and yolk–shell nanocages for improved water oxidation and selective ethylene glycol reformation. eScience 2023, 3, 2300321. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Y.; Sun, S.; Liang, J.; Hong, S.; Zhang, H.; Yang, C.; Zhang, X.; Cai, Z.; Li, J.; et al. Carbon oxyanion self-transformation on NiFe oxalates enables long-term ampere-level current density seawater oxidation. Angew. Chem. Int. Ed. 2024, 63, e202316522. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, H.; Tan, X.; Lin, S.; Yu, K.; Mu, X.; Tao, Z.; Ji, P.; Mu, S. Fe-S dually modulated adsorbate evolution and lattice oxygen compatible mechanism for water oxidation. Nat. Commun. 2024, 15, 8293. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; An, B.; Liu, W.; Su, H.; Li, N.; Gao, Y.; Ge, L. Cation-induced interface electric field redistribution and molecular orbital coupling in Co-FeS/MoS2 for boosting electrocatalytic overall water splitting. Chem. Eng. J. 2024, 498, 120122. [Google Scholar] [CrossRef]
- Kuang, Z.; Liu, S.; Li, X.; Wang, M.; Ren, X.; Ding, J.; Ge, R.; Zhou, W.; Rykov, A.I.; Sougrati, M.T.; et al. Topotactically constructed nickel–iron (oxy)hydroxide with abundant in-situ produced high-valent iron species for efficient water oxidation. J. Energy Chem. 2021, 57, 212–218. [Google Scholar] [CrossRef]
- Hunter, B.M.; Thompson, N.B.; Müller, A.M.; Rossman, G.R.; Hill, M.G.; Winkler, J.R.; Gray, H.B. Trapping an iron (VI) water-splitting intermediate in nonaqueous media. Joule 2018, 2, 747–763. [Google Scholar] [CrossRef]
- Chenakin, S.; Kruse, N. XPS characterization of transition metal oxalates. Appl. Surf. Sci. 2020, 515, 146041. [Google Scholar] [CrossRef]
- Chenakin, S.P.; Szukiewicz, R.; Barbosa, R.; Kruse, N. Surface analysis of transition metal oxalates: Damage aspects. J. Electron Spectrosc. Relat. Phenom. 2016, 209, 66–77. [Google Scholar] [CrossRef]
- Zhao, X.; Pattengale, B.; Fan, D.; Zou, Z.; Zhao, Y.; Du, J.; Huang, J.; Xu, C. Mixed-node metal–organic frameworks as efficient electrocatalysts for oxygen evolution reaction. ACS Energy Lett. 2018, 3, 2520–2526. [Google Scholar] [CrossRef]
- Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv. Mater. 2017, 29, 1606793. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Zhong, C.; Li, S.; Shen, Z.; Pu, J.; Liu, J.; Zhou, Y.; Zhang, H.; Ma, H. (Co/Fe)4O4 Cubane-containing nanorings fabricated by phosphorylating cobalt ferrite for highly efficient oxygen evolution reaction. ACS Catal. 2019, 9, 3878–3887. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, S.; Song, J.; Xi, S.; Chen, B.; Du, Y.; Fisher, A.C.; Chen, F.; Wang, X.; Zhang, H.; et al. Enlarged Co-O Covalency in octahedral sites leading to highly efficient spinel oxides for oxygen evolution reaction. Adv. Mater. 2018, 30, 1802912. [Google Scholar] [CrossRef]
- Bai, L.; Hsu, C.S.; Alexander, D.T.; Chen, H.M.; Hu, X. Double-atom catalysts as a molecular platform for heterogeneous oxygen evolution electrocatalysis. Nat. Energy 2021, 6, 1054–1066. [Google Scholar] [CrossRef]
- Hai, G.; Jia, X.; Zhang, K.; Liu, X.; Wu, Z.; Wang, G. High-performance oxygen evolution catalyst using two-dimensional ultrathin metal-organic frameworks nanosheets. Nano Energy 2018, 44, 345–352. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Tan, P.; Dos Santos, E.C.; Holmes, S.M.; Li, H.; Pan, J.; D’Agostino, C. A Doping-induced SrCo(0.4)Fe(0.6)O3/CoFe2O4 nanocomposite for efficient oxygen evolution in alkaline media. Small 2024, 20, 2308948. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, D.; Luo, G.; Lu, Y.; Chen, C.; Zou, Y.; Dong, C.; Li, Y.; Wang, S. Zirconium-regulation-induced bifunctionality in 3D cobalt-iron oxide nanosheets for overall water splitting. Adv. Mater. 2019, 31, 1901439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guan, B.; Lu, X.; Xi, S.; Du, Y.; Lou, X. Metal atom-doped Co3O4 hierarchical nanoplates for electrocatalytic oxygen evolution. Adv. Mater. 2020, 32, 2002235. [Google Scholar] [CrossRef]
- Sun, J.; Xue, H.; Zhang, Y.; Zhang, X.; Guo, N.; Song, T.; Dong, H.; Kong, Y.; Zhang, J.; Wang, Q. Unraveling the synergistic effect of heteroatomic substitution and vacancy engineering in CoFe2O4 for superior electrocatalysis performance. Nano Lett. 2022, 22, 3503–3511. [Google Scholar] [CrossRef]
- Tsai, F.; Deng, Y.; Pao, C.; Chen, J.; Lee, J.; Lai, K.; Liaw, W. The HER/OER mechanistic study of an FeCoNi-based electrocatalyst for alkaline water splitting. J. Mater. Chem. A 2020, 8, 9939–9950. [Google Scholar] [CrossRef]
- Xin, S.; Tang, Y.; Jia, B.; Zhang, Z.; Li, C.; Bao, R.; Li, C.; Yi, J.; Wang, J.; Ma, T. Coupling adsorbed evolution and lattice oxygen mechanism in Fe-Co(OH)2/Fe2O3 heterostructure for enhanced electrochemical water oxidation. Adv. Funct. Mater. 2023, 33, 2305243. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Wang, C.; Qiu, L.; Liu, F.; Chen, S.; Chang, H. Tuning Surface State in CoFe (Oxy)Hydroxide for Improved Oxygen Evolution Electrocatalysis. Catalysts 2025, 15, 11. https://doi.org/10.3390/catal15010011
Guo W, Wang C, Qiu L, Liu F, Chen S, Chang H. Tuning Surface State in CoFe (Oxy)Hydroxide for Improved Oxygen Evolution Electrocatalysis. Catalysts. 2025; 15(1):11. https://doi.org/10.3390/catal15010011
Chicago/Turabian StyleGuo, Wen, Chizhong Wang, Lei Qiu, Fanghua Liu, Sizhe Chen, and Huazhen Chang. 2025. "Tuning Surface State in CoFe (Oxy)Hydroxide for Improved Oxygen Evolution Electrocatalysis" Catalysts 15, no. 1: 11. https://doi.org/10.3390/catal15010011
APA StyleGuo, W., Wang, C., Qiu, L., Liu, F., Chen, S., & Chang, H. (2025). Tuning Surface State in CoFe (Oxy)Hydroxide for Improved Oxygen Evolution Electrocatalysis. Catalysts, 15(1), 11. https://doi.org/10.3390/catal15010011






