Recent Advances in the Synthesis of Substituted Polyacetylenes
Abstract
:1. Introduction
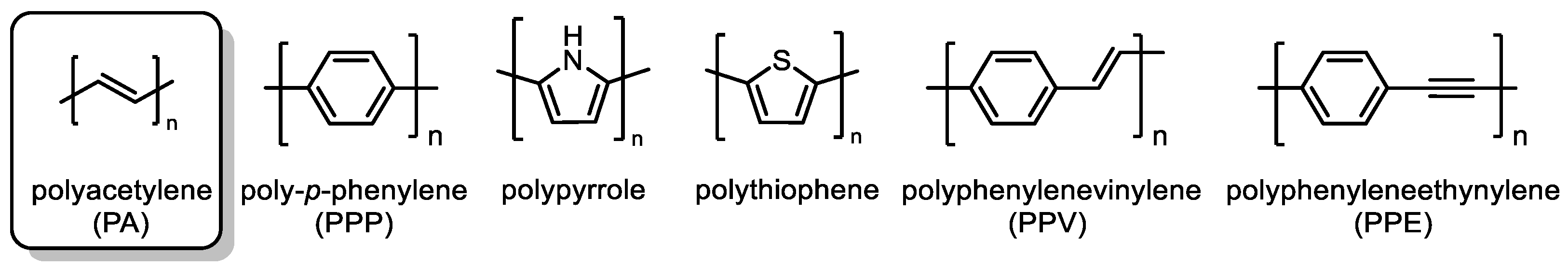
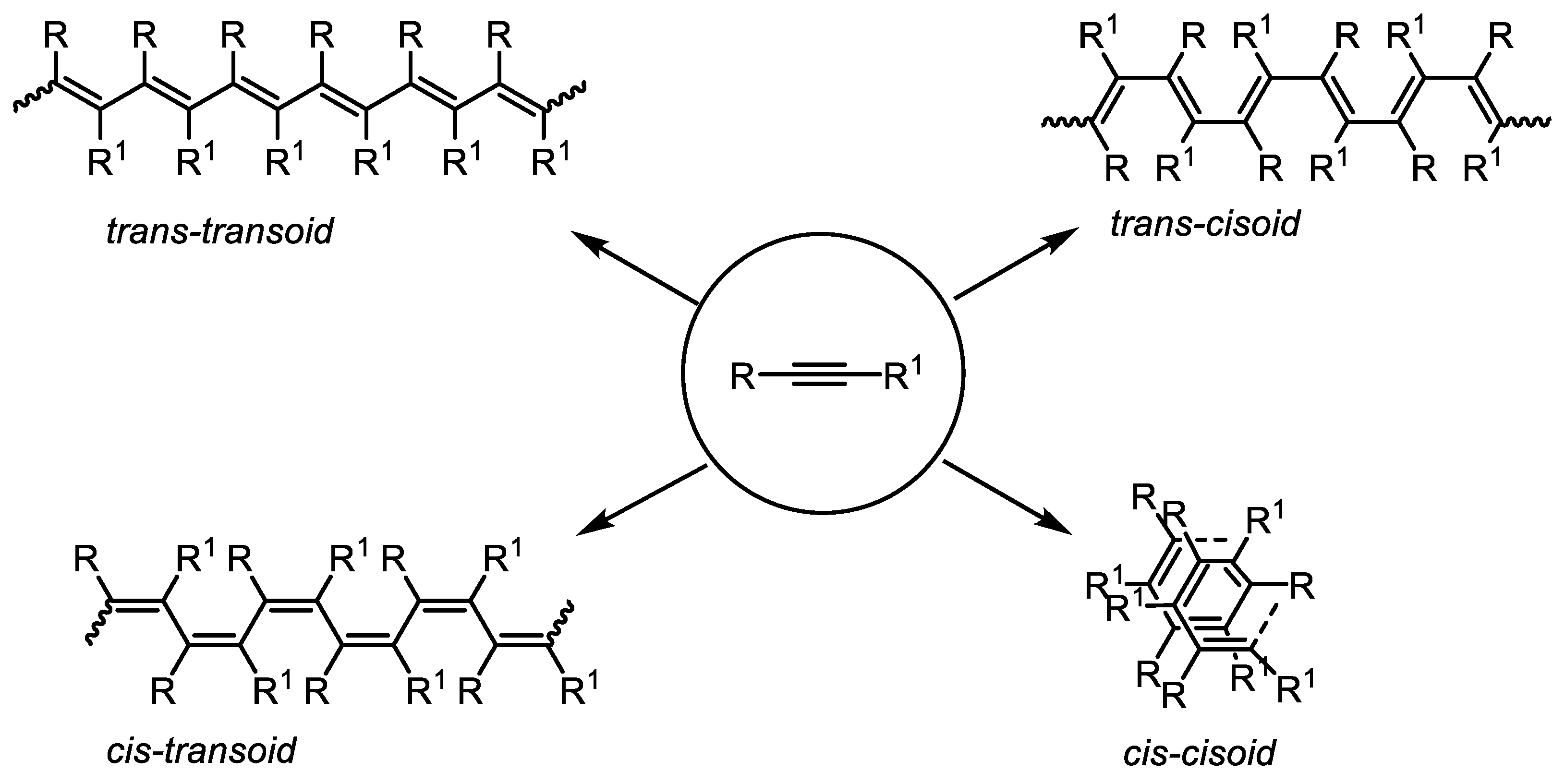
2. Structural Aspects of Substituted Polyacetylenes and Mechanistic Scenarios of Polymerization
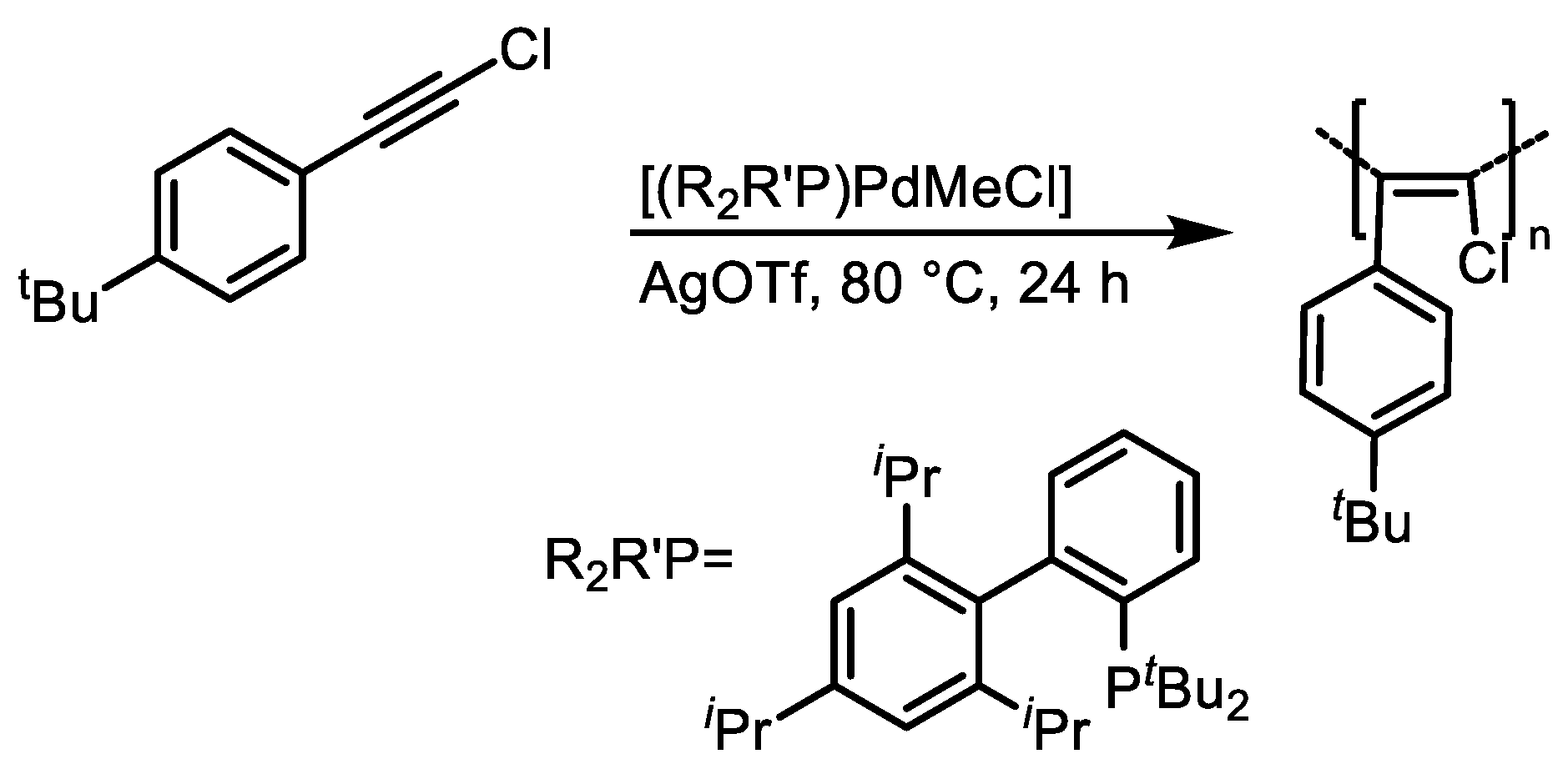
3. Catalyst Systems for the Polymerization of Substituted Acetylenes
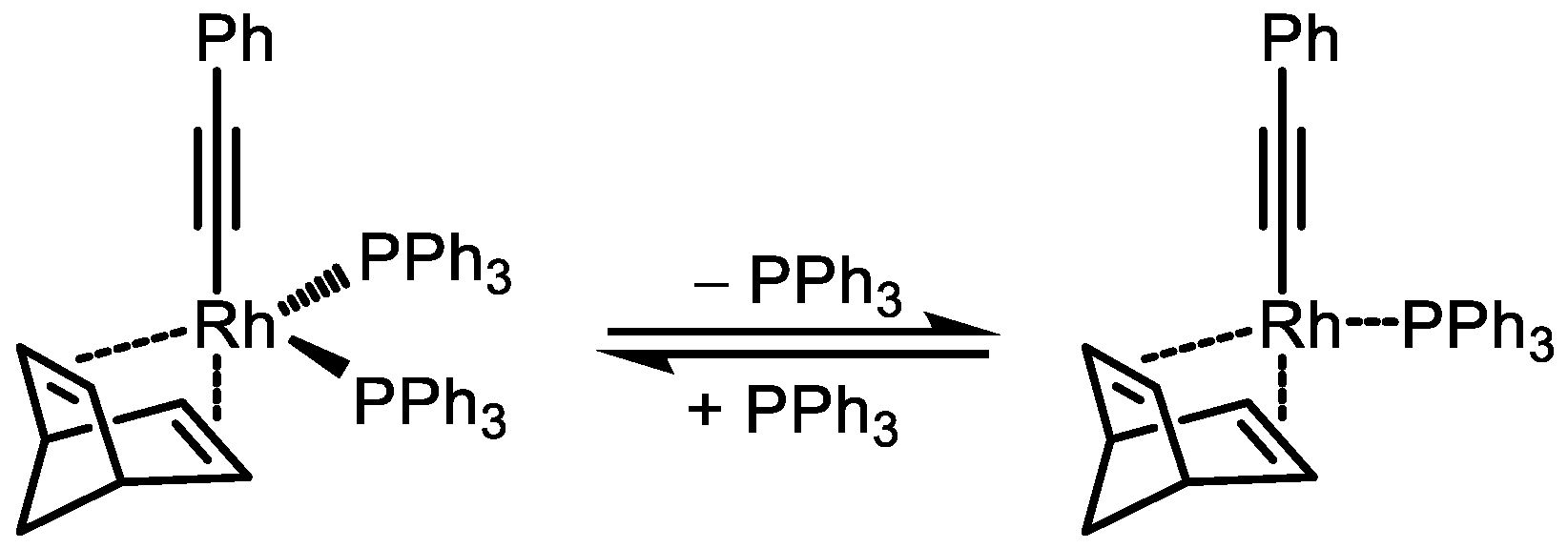
4. Rhodium-Catalyzed Polymerization of Monosubstituted Acetylenes: The Path to Living Polymerization
5. Syntheses and Properties of Functional Monosubstituted Arylacetylenes
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stenger-Smith, J.D. Intrinsically electrically conducting polymers. Synthesis, characterization, and their applications. Progr. Polym. Sci. 1998, 23, 57–79. [Google Scholar] [CrossRef]
- Swager, T.M. 50th Anniversary Perspective: Conducting/Semiconducting Conjugated Polymers; A Personal Perspective on the Macromolecules. Macromolecules 2017, 50, 4867–4886. [Google Scholar] [CrossRef]
- Müllen, K.; Scherf, U. Conjugated Polymers: Where We Come From, Where We Stand, and Where We Might Go. Macromol. Chem. Phys. 2023, 224, 2200337. [Google Scholar] [CrossRef]
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley, D.D.C.; Santos, D.A.D.; Brédas, J.L.; Lögdlund, M.; et al. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Wang, B.-H.; Yin, J.; Xue, M.Z.; Wang, J.l.; Zhong, G.; Ding, X. Dibenzothiophene-5,5-dioxide-containing PPV based copolymer as green–blue electroluminescent material. Synth. Met. 2003, 132, 191–195. [Google Scholar] [CrossRef]
- Law, K.Y. Organic photoconductive materials: Recent trends and developments. Chem. Rev. 1993, 93, 449–486. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, L.J.; Zhang, F.; Andersson, M.; Barrau, S.; Hellstrom, S.; Mammo, W.; Perzon, E.; Inganas, O.; Andersson, M.R. Synthesis, Characterization, and Devices of a Series of Alternating Copolymers for Solar Cells. Chem. Mater. 2009, 21, 3491–3502. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Poddar, A.K.; Patel, S.S.; Patel, H.D. Synthesis, characterization and applications of conductive polymers: A brief review. Polym. Adv. Technol. 2021, 32, 4616–4641. [Google Scholar] [CrossRef]
- Sumdani, M.G.; Islam, M.R.; Yahaya, A.N.A.; Safie, S.I. Recent advancements in synthesis, properties, and applications of conductive polymers for electrochemical energy storage devices: A review. Polym. Eng. Sci. 2022, 62, 269–303. [Google Scholar] [CrossRef]
- Rasmussen, S.C. The Path to Conductive Polyacetylene. Bull. Hist. Chem. 2014, 39, 64–72. [Google Scholar]
- Rasmussen, S.C. Acetylene and Its Polymers—150+ Years of History, Springer Briefs in Molecular Science, History of Chemistry; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Rasmussen, S.C. Conjugated and Conducting Organic Polymers: The First 150 Years. ChemPlusChem 2020, 85, 1412–1429. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH). Chem. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Chiang, C.K.; Fincher, C.R.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. Electrical conductivity in doped polyacetylene. Phys. Rev. Lett. 1977, 39, 1098–1101. [Google Scholar] [CrossRef]
- Durrani, M. Awards: Physicist shares chemistry Nobel prize. Phys. World 2000, 13, 6. [Google Scholar] [CrossRef]
- Hudson, B.S. Polyacetylene: Myth and Reality. Materials 2018, 11, 242. [Google Scholar] [CrossRef]
- Novak, B.M.; Risse, W.; Grubbs, R.H. The Development of Well-defined Catalysts for Ring-opening Metathesis Polymerization (ROMP). Adv. Polym. Sci. 1992, 102, 47–72. [Google Scholar] [CrossRef]
- Gibson, V.C. Metathesis Polymerization—Romping towards New Materials. Adv. Mater. 1994, 6, 37–42. [Google Scholar] [CrossRef]
- Bunz, U.H.F.; Mäker, D.; Porz, M. Alkene Metathesis—A Tool for the Synthesis of Conjugated Polymers. Macromol. Rapid Commun. 2012, 33, 886–910. [Google Scholar] [CrossRef]
- Masuda, T. Substituted polyacetylenes. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 165–180. [Google Scholar] [CrossRef]
- Masuda, T. Substituted Polyacetylenes: Synthesis, Properties, and Functions. Polym. Rev. 2017, 57, 1–14. [Google Scholar] [CrossRef]
- Sedlácek, J.; Balcar, H. Substituted Polyacetylenes Prepared with Rh Catalysts: From Linear to Network-Type Conjugated Polymers. Polym. Rev. 2017, 57, 31–51. [Google Scholar] [CrossRef]
- Yashima, E. Chiral and chirality discrimination on helical polyacetylenes. Anal. Sci. 2002, 18, 3–6. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, X.J. Coordination-based circularly polarized luminescence emitters: Design strategy and application in sensing. Coord. Chem. Rev. 2022, 453, 214329. [Google Scholar] [CrossRef]
- Goh, M.; Matsushita, S.; Akagi, K. From helical polyacetylene to helical graphite: Synthesis in the chiral nematic liquid crystal field and morphology-retaining carbonization. Chem. Soc. Rev. 2010, 39, 2466–2476. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.Z.; Chen, H.Z.; Xu, R.S.; Lam, J.W.Y.; Cheuk, K.K.L.; Wong, H.N.C.; Wang, M. Structure−Property Relationships for Photoconduction in Substituted Polyacetylenes. Chem. Mater. 2000, 12, 213–221. [Google Scholar] [CrossRef]
- Liu, J.; Lam, J.W.Y.; Tang, B.Z. Synthesis and Functionality of Substituted Polyacetylenes. In Design and Synthesis of Conjugated Polymers; Mario Leclerc, M., Morin, J.F., Eds.; Wiley-VCH: Weinheim, Germany, 2010; Chapter 1; pp. 1–43. [Google Scholar] [CrossRef]
- Lu, Y.; Khan, Z.A.; Alvarez-Alvarado, M.S.; Zhang, Y.; Huang, Z.; Imran, M. A Critical Review of Sustainable Energy Policies for the Promotion of Renewable Energy Sources. Sustainability 2020, 12, 5078. [Google Scholar] [CrossRef]
- Masuda, T.; Isobe, E.; Higashimura, T.; Takada, K. Poly[1-(trimethylsilyl)-1-propyne]: A new high polymer synthesized with transition-metal catalysts and characterized by extremely high gas permeability. J. Am. Chem. Soc. 1983, 105, 7473–7474. [Google Scholar] [CrossRef]
- Chen, M.; Hu, G.; Shen, T.; Zhang, H.; Sun, J.Z.; Tang, B.Z. Applications of Polyacetylene Derivatives in Gas and Liquid Separation. Molecules 2023, 28, 2748. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lam, J.W.Y.; Tang, B.Z. Acetylenic Polymers: Syntheses, Structures, and Functions. Chem. Rev. 2009, 109, 5799–5867. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, J.Z.; Tang, B.Z. Poly(disubstituted acetylene)s: Advances in polymer preparation and materials application. Prog. Polym. Sci. 2018, 79, 98–120. [Google Scholar] [CrossRef]
- Shiotsuki, M.; Sanda, F.; Masuda, T. Polymerization of substituted acetylenes and features of the formed polymers. Polym. Chem. 2011, 2, 1044–1058. [Google Scholar] [CrossRef]
- Casado, M.A.; Fazal, A.; Oro, L.A. Rhodium-Catalyzed Polymerization of Phenylacetylene and its Derivatives. Arab. J. Sci. Eng. 2013, 38, 1631–1646. [Google Scholar] [CrossRef]
- Tanabe, Y.; Kyotani, H.; Akagi, K.; Shirakawa, H. Mechanism of Cis-Trans Thermal Isomerization of Polyacetylene. Macromolecules 1995, 28, 4173–4178. [Google Scholar] [CrossRef]
- Chien, J.C.W.; Karasz, F.E.; Wnek, G.E. Soliton formation and cis trans isomerization in polyacetylene. Nature 1980, 285, 390–392. [Google Scholar] [CrossRef]
- Mülhaupt, R. Catalytic Polymerization and Post Polymerization Catalysis Fifty Years After the Discovery of Ziegler’s Catalysts. Macromol. Chem. Phys. 2003, 204, 289–327. [Google Scholar] [CrossRef]
- Masuda, T.; Sasaki, N.; Higashimura, T. Polymerization of Phenylacetylenes. III. Structure and Properties of Poly(phenylacetylene)s Obtained by WCl6 or MoCl5. Macromolecules 1975, 8, 717–721. [Google Scholar] [CrossRef]
- Katz, T.J.; Hacker, S.M.; Kendrick, R.D.; Yannoni, C.S. Mechanisms of phenylacetylene polymerization by molybdenum and titanium initiators. J. Am. Chem. Soc. 1985, 107, 2182–2183. [Google Scholar] [CrossRef]
- Jean-Louis Hérisson, P.; Chauvin, Y. Catalyse de transformation des oléfines par les complexes du tungstène. II. Télomérisation des oléfines cycliques en présence d’oléfines acycliques. Macromol. Chem. Phys. 1971, 141, 161–176. [Google Scholar] [CrossRef]
- Lam, J.W.Y.; Tang, B.Z. Functional Polyacetylenes. Acc. Chem. Res. 2005, 38, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Luppi, B.T.; Muralidharan, A.V.; Ostermann, N.; Cheong, I.T.; Ferguson, M.J.; Siewert, I.; Rivard, E. Redox-Active Heteroatom-Functionalized Polyacetylenes. Angew. Chem. Int. Ed. 2022, 61, e202114586. [Google Scholar] [CrossRef] [PubMed]
- Gorman, C.B.; Ginsburg, E.J.; Sailor, M.J.; Moore, J.S.; Jozefiak, T.H.; Lewis, N.S.; Grubbs, R.H.; Marder, S.R.; Perry, J.W. Substituted polyacetylenes through the ring-opening metathesis polymerization (ROMP) of substituted cyclooctatetraenes: A route into soluble polyacetylene. Synth. Met. 1991, 41, 1033–1038. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, S.Y.; Canlier, A.; Hwang, T.S. Controlled Dehydrochlorination of Poly(vinyl chloride) for Fabrication of Membranes with Polyacetylene-Like Structure: XPS Analysis and Ion Exchange Membrane Discussion. Macromol. Res. 2019, 27, 33–47. [Google Scholar] [CrossRef]
- O’Rourke, G.; Hennebel, T.; Stalpaert, M.; Skorynina, A.; Bugaev, A.; Janssens, K.; Van Emelen, L.; Lemmens, V.; De Oliveira Silva, R.; Colemonts, C.; et al. Catalytic tandem dehydrochlorination–hydrogenation of PVC towards valorisation of chlorinated plastic waste. Chem. Sci. 2023, 14, 4401–4412. [Google Scholar] [CrossRef] [PubMed]
- Aldissi, M. Review of the synthesis of polyacetylene and its stabilization to ambient atmosphere. Synth. Met. 1984, 9, 131–141. [Google Scholar] [CrossRef]
- Taniguchi, T.; Yoshida, T.; Echizen, K.; Takayama, K.; Nishimura, T.; Maeda, K. Facile and Versatile Synthesis of End-Functionalized Poly(phenylacetylene)s: A Multicomponent Catalytic System for Well-Controlled Living Polymerization of Phenylacetylenes. Angew. Chem. Int. Ed. 2020, 59, 8670–8680. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Taniguchi, T.; Sakata, Y.; Akine, S.; Nishimura, T.; Maeda, K. Rhodium(I) Complexes Bearing an Aryl-Substituted 1,3,5-Hexatriene Chain: Catalysts for Living Polymerization of Phenylacetylene and Potential Helical Chirality of 1,3,5-Hexatrienes. Angew. Chem. Int. Ed. 2021, 60, 22201–22206. [Google Scholar] [CrossRef] [PubMed]
- Misumi, Y.; Masuda, T. Living Polymerization of Phenylacetylene by Novel Rhodium Catalysts. Quantitative Initiation and Introduction of Functional Groups at the Initiating Chain End. Macromolecules 1998, 31, 7572–7573. [Google Scholar] [CrossRef]
- Miyairi, M.; Taniguchi, T.; Nishimura, T.; Maeda, K. Facile Synthesis of Linear and Cyclic Poly(diphenylacetylene)s by Molybdenum and Tungsten Catalysis. Angew. Chem. Int. Ed. 2023, 62, e202302332. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Shimada, H.; Hashimoto, T. Metathesis polymerization of monomers containing two diphenylacetylene units: Synthesis and properties of poly(diphenylacetylene)s bearing diphenylacetylene units on the side chain. Polym. Chem. 2020, 11, 6471–6478. [Google Scholar] [CrossRef]
- Castanon, J.R.; Sano, N.; Shiotsuki, M.; Sanda, F. New Approach to the Polymerization of Disubstituted Acetylenes by Bulky Monophosphine-Ligated Palladium Catalysts. ACS Macro Lett. 2014, 3, 51–54. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Daugulis, O.; Brookhart, M. Polymerization of Terminal Acetylenes by a Bulky Monophosphine-Palladium Catalyst. Organometallics 2023, 42, 235–239. [Google Scholar] [CrossRef]
- Ojwach, S.O.; Guzei, I.A.; Darkwa, J.; Mapolie, S.F. Palladium complexes of multidentate pyrazolylmethyl pyridine ligands: Synthesis, structures and phenylacetylene polymerization. Polyhedron 2007, 26, 851–861. [Google Scholar] [CrossRef]
- Katsumata, T.; Shiotsuki, M.; Sanda, F.; Sauvage, X.; Delaude, L.; Masuda, T. Polymerization of ortho-Substituted Phenylacetylenes with Well-Defined Ruthenium-Alkylidene Catalysts and Related Metathesis Initiators. Macromol. Chem. Phys. 2009, 210, 1891–1902. [Google Scholar] [CrossRef]
- Buchowicz, W.; Wojtczak, W.; Pietrzykowski, A.; Lupa, A.; Jerzykiewicz, L.B.; Makal, A.; Woźniak, K. Synthesis, Structure, and Polymerization Activity of Cyclopentadienylnickel(II) N-Heterocyclic Carbene Complexes: Selective Cross-Metathesis in Metal Coordination Spheres. Eur. J. Inorg. Chem. 2010, 2010, 648–656. [Google Scholar] [CrossRef]
- Echizen, K.; Taniguchi, T.; Nishimura, T.; Maeda, K. Well-Controlled Living Polymerization of Phenylacetylenes in Water: Synthesis of Water-Soluble Stereoregular Telechelic Poly(phenylacetylene)s. Angew. Chem. Int. Ed. 2022, 61, e202202676. [Google Scholar] [CrossRef] [PubMed]
- Nikishkin, N.I.; Huskens, J.; Verboom, W. Highly active and robust rhodium(I) catalyst for the polymerization of arylacetylenes in polar and aqueous medium under air atmosphere. Polymer 2013, 54, 3175–3181. [Google Scholar] [CrossRef]
- Kern, R.J. Preparation and properties of isomeric polyphenylacetylenes. J. Polym. Sci. 1969, 7, 621–631. [Google Scholar] [CrossRef]
- Mayershofer, M.G.; Nuyken, O. Living polymerization of substituted acetylenes. J. Polym. Sci. 2005, 43, 5723–5747. [Google Scholar] [CrossRef]
- Tabata, M.; Yang, W.; Yokota, K. Polymerization of m-Chlorophenylacetylene Initiated by [Rh(norbornadiene)Cl]2-Triethylamine Catalyst Containing Long-Lived Propagation Species. Polym. J. 1990, 22, 1105–1107. [Google Scholar] [CrossRef]
- Saeed, I.; Shiotsuki, M.; Masuda, T. Effect of Diene Ligands in the Rhodium-Catalyzed Polymerization of Phenylacetylene. Macromolecules 2006, 39, 8977–8981. [Google Scholar] [CrossRef]
- Kanki, K.; Misumi, Y.; Masuda, T. Remarkable Cocatalytic Effect of Organometallics and Rate Control by Triphenylphosphine in the Rh-Catalyzed Polymerization of Phenylacetylene. Macromolecules 1999, 32, 2384–2386. [Google Scholar] [CrossRef]
- Goldberg, Y.; Alper, H. Polymerisation of phenylacetylene catalysed by a zwitterionic rhodium(I) complex under hydrosilyation conditions. J. Chem. Soc. Chem. Commun. 1994, 10, 1209–1210. [Google Scholar] [CrossRef]
- Szwarc, M.; Levy, M.; Milkovich, R. Polymerization inititiated by electron transfer to monomer: A new method of formation of block polymers. J. Am. Chem. Soc. 1956, 78, 2656–2657. [Google Scholar] [CrossRef]
- Webster, O.W. Living Polymerization Methods. Science 1991, 251, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Eckerle, P.; Miyatake, T.; Ikariya, T.; Noyori, R. Living Polymerization of Phenylacetylenes Initiated by Rh(C≡CC6H5)(2,5-norbornadiene)[P(C6H5)3]2. J. Am. Chem. Soc. 1994, 116, 12131–12132. [Google Scholar] [CrossRef]
- Misumi, Y.; Kanki, K.; Miyake, M.; Masuda, T. Living polymerization of phenylacetylene by rhodium-based ternary catalysts, (diene)Rh(I) complex/vinyllithium/phosphorus ligand. Effects of catalyst components. Macromol. Chem. Phys. 2000, 201, 2239–2244. [Google Scholar] [CrossRef]
- Echizen, K.; Taniguchi, T.; Nishimura, T.; Maeda, K. Synthesis of Stereoregular Telechelic Poly(phenylacetylene)s: Facile Terminal Chain-End Functionalization of Poly(phenylacetylene)s by Terminative Coupling with Acrylates and Acrylamides in Rhodium-Catalyzed Living Polymerization of Phenylacetylenes. J. Am. Chem. Soc. 2021, 143, 3604–3612. [Google Scholar] [CrossRef]
- Ito, K.; Taniguchi, T.; Nishimura, T.; Maeda, K. Well-Controlled Living Polymerization of N-Propargylamides and Their Derivatives by Rhodium Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202117234. [Google Scholar] [CrossRef]
- Freire, F.; Quiñoá, E.; Riguera, R. Supramolecular Assemblies from Poly(phenylacetylene)s. Chem. Rev. 2016, 116, 1242–1271. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.; Quiñoá, E.; Riguera, R.; Freire, F. Architecture of Chiral Poly(phenylacetylene)s: From Compressed/Highly Dynamic to Stretched/Quasi-Static Helices. J. Am. Chem. Soc. 2016, 138, 9620–9628. [Google Scholar] [CrossRef]
- Freire, F.; Quiñoá, E.; Riguera, R. Chiral nanostructure in polymers under different deposition conditions observed using atomic force microscopy of monolayers: Poly(phenylacetylene)s as a case study. Chem. Commun. 2017, 53, 481–492. [Google Scholar] [CrossRef]
- Reggelin, M.; Doerr, S.; Klussmann, M.; Schultz, M.; Holbach, M. Helically chiral polymers: A class of ligands for asymmetric catalysis. Proc. Natl. Acad. Sci. USA 2004, 101, 5461–5466. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Yashima, E. Helical Polyacetylenes Induced via Noncovalent Chiral Interactions and Their Applications as Chiral Materials. Top. Curr. Chem. 2017, 375, 72. [Google Scholar] [CrossRef] [PubMed]
- Inaba, A.; Nishimura, T.; Yamamoto, M.; Das, S.; Yurtsever, A.; Miyata, K.; Fukuma, T.; Kawaguchi, S.; Kikuchi, M.; Taniguchi, T.; et al. Synthesis of optically active star polymers consisting of helical poly(phenylacetylene) chains by the living polymerization of phenylacetylenes and their chiroptical properties. RSC Adv. 2023, 13, 30978–30984. [Google Scholar] [CrossRef] [PubMed]
- Mino, S.; Matsui, K.; Goto, M.; Ryoki, A.; Suzuki, T.; Fujimoto, K.; Sogawa, H.; Kudo, H.; Sanda, F. Star-Shaped Polymers with Helical Polyacetylene Arms. Comparison of Solution- and Solid-State Properties with Linear Helical Polyacetylenes. Macromolecules 2024, 57, 10824–10834. [Google Scholar] [CrossRef]
- Lu, X.; Ren, L.; Zhang, X.; Whittaker, A.K.; Li, W.; Zhang, A. Thermo/Light Dual-Responsive Helical Dendronized Poly(phenylacetylene)s. Macromolecules 2024, 57, 5915–5928. [Google Scholar] [CrossRef]
- Lam, J.W.Y.; Dong, Y.; Kwok, H.S.; Tang, B.Z. Light-Emitting Polyacetylenes: Synthesis and Electrooptical Properties of Poly(1-phenyl-1-alkyne)s Bearing Naphthyl Pendants. Macromolecules 2006, 39, 6997–7003. [Google Scholar] [CrossRef]
- Wang, S.; Hu, D.; Guan, X.; Cai, S.; Shi, G.; Shuai, Z.; Zhang, J.; Peng, Q.; Wan, X. Brightening up Circularly Polarized Luminescence of Monosubstituted Polyacetylene by Conformation Control: Mechanism, Switching, and Sensing. Angew. Chem. Int. Ed. 2021, 60, 21918–21926. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.Z.; Zhao, H.; Shen, X.Y.; Mahtab, F.; Lam, J.W.Y.; Sun, J.Z.; Tang, B.Z. Luminogenic Polyacetylenes and Conjugated Polyelectrolytes: Synthesis, Hybridization with Carbon Nanotubes, Aggregation-Induced Emission, Superamplification in Emission Quenching by Explosives, and Fluorescent Assay for Protein Quantitation. Macromolecules 2009, 42, 9400–9411. [Google Scholar] [CrossRef]
- Sun, J.Z.; Qin, A.J.; Tang, B.Z. Functional polyacetylenes: Hybrids with carbon nanotubes. Polym. Chem. 2013, 4, 211–223. [Google Scholar] [CrossRef]
- Tang, B.Z.; Kotera, N. Synthesis of optically active polyacetylene containing an asymmetric silicon by using organotransition-metal complexes as catalysts. Macromolecules 1989, 22, 4388–4390. [Google Scholar] [CrossRef]
- Gangadhar, P.S.; Reddy, G.; Prasanthkumar, S.; Giribabu, L. Phenothiazine functional materials for organic optoelectronic applications. Phys. Chem. Chem. Phys. 2021, 23, 14969–14996. [Google Scholar] [CrossRef]
- Otteny, F.; Desmaizieres, G.; Esser, B. Phenothiazine-based Redox Polymers for Energy Storage. In Redox Polymers for Energy and Nanomedicine; Casado, N., Mecerreyes, D., Eds.; The Royal Society of Chemistry: London, UK, 2020; Chapter 5; pp. 166–197. [Google Scholar] [CrossRef]
- Mayer, L.; Müller, T.J.J. 3,10-Diaryl Phenothiazines—One-pot Synthesis and Conformational Tuning of Ground and Excited State Electronics. Eur. J. Org. Chem. 2021, 2021, 3516–3527. [Google Scholar] [CrossRef]
- Meyer, T.; Müller, T.J.J. Consecutive Three-Component Synthesis of Donor-Substituted Merocyanines by One-pot Suzuki-Knoevenagel Condensation (SuKnoCon) Sequence. Org. Mater. 2020, 2, 64–70. [Google Scholar] [CrossRef]
- Stephan, M.; Stute, B.; von Lieres, E.; Müller, T.J.J. Consecutive Three-component Synthesis of Phenothiazine Based Merocyanines—Bayesian Optimization, Electronic properties, and DSSC Characteristics. Eur. J. Org. Chem. 2022, 2022, e202200163. [Google Scholar] [CrossRef]
- Pisetsky, W.; Budny, P.; Müller, T.J.J. Synthesis and Photophysical Properties of Luminescent Phenothiazinyl Merocyanine Substituted Polyacetylenes. Angew. Chem. Int. Ed. 2024, 63, e202316246. [Google Scholar] [CrossRef] [PubMed]
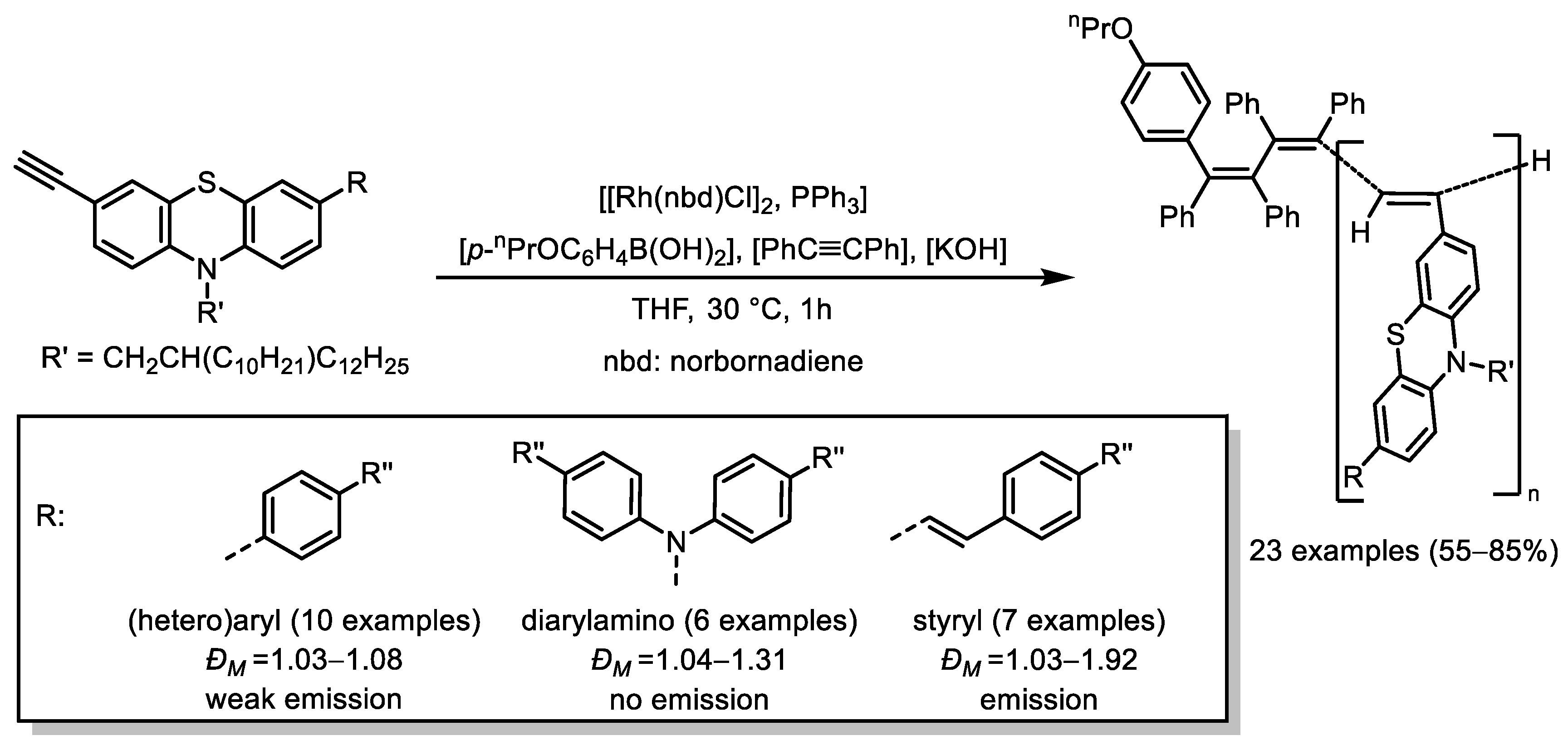
- Pisetsky, W.; Müller, T.J.J. Polyacetylenes with (Hetero)Aryl-, Styryl-, and Amino-Phenothiazinyl Sidechains—Synthesis and Photophysics. RSC Adv. 2024, 14, 10638–10643. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Nishimura, T.; Maeda, K. Development of Methods for Controlled Polymerization of Monosubstituted Acetylenes Enabling Versatile Design of Polymer End Structures. J. Synth. Org. Chem. Jpn. 2023, 81, 594–606. [Google Scholar] [CrossRef]
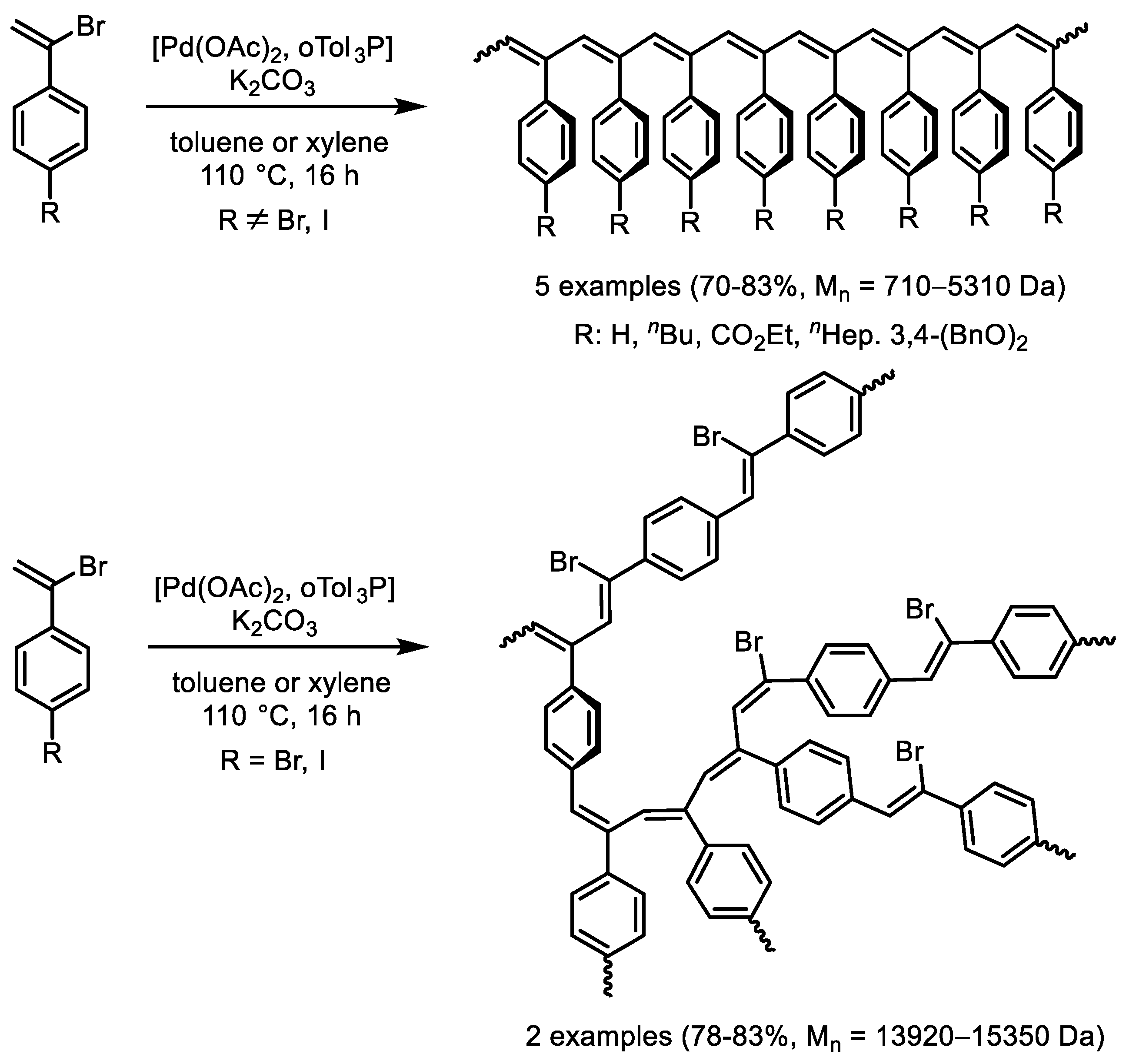
- He, L.; Yu, Y.; Liu, F.; He, J. Synthesis of linear and branched poly(phenylacetylene)s through Mizoroki-Heck coupling reaction of vinyl bromides. Eur. Polym. J. 2023, 195, 112233. [Google Scholar] [CrossRef]





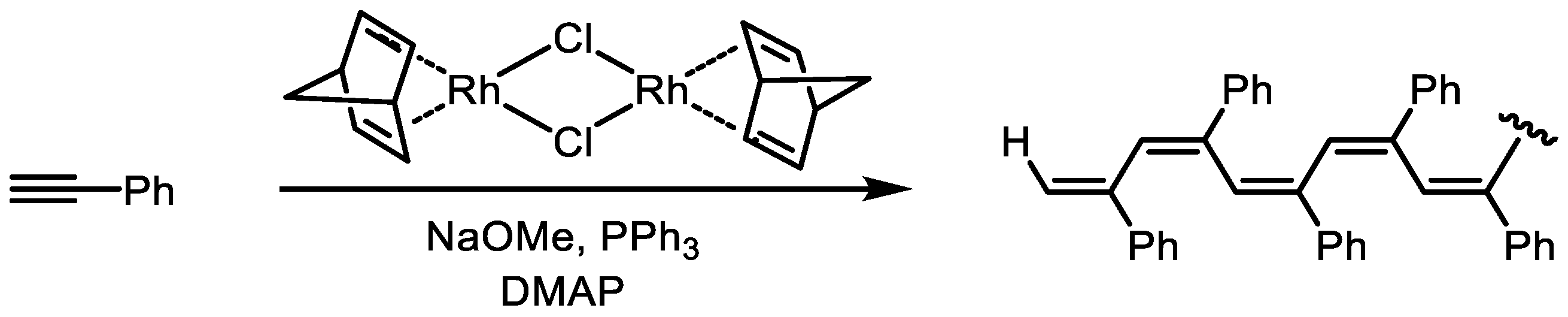
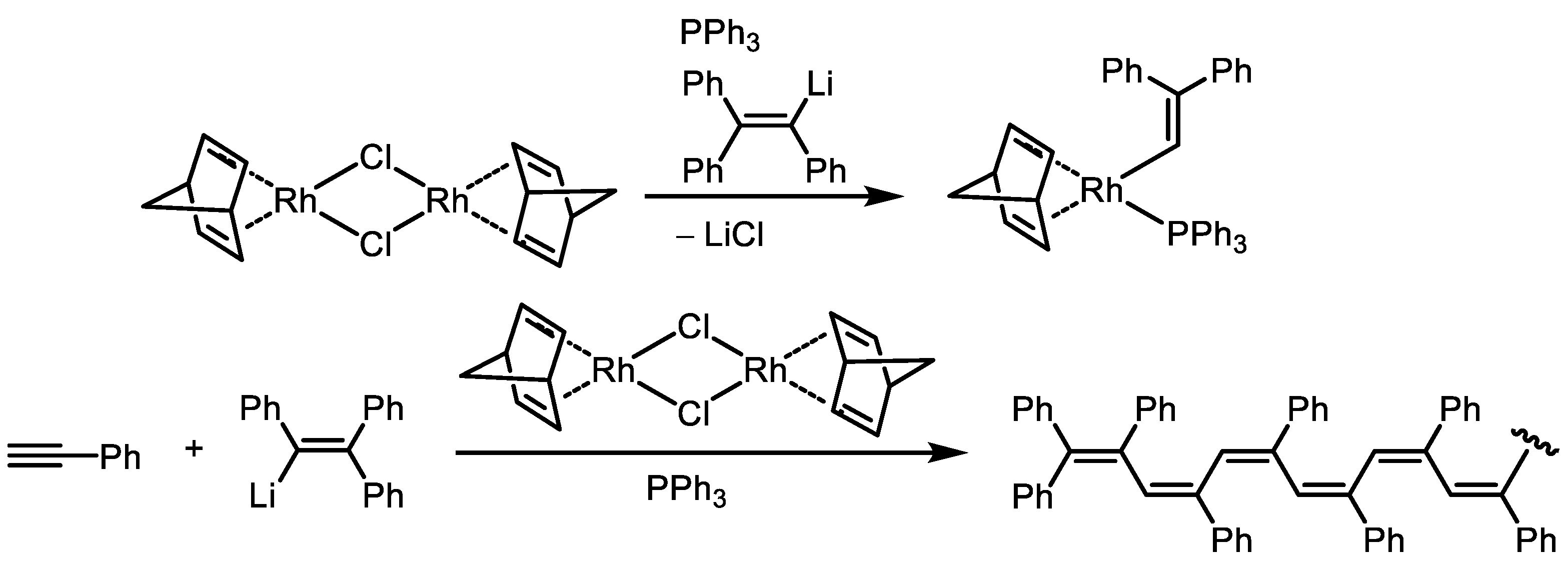


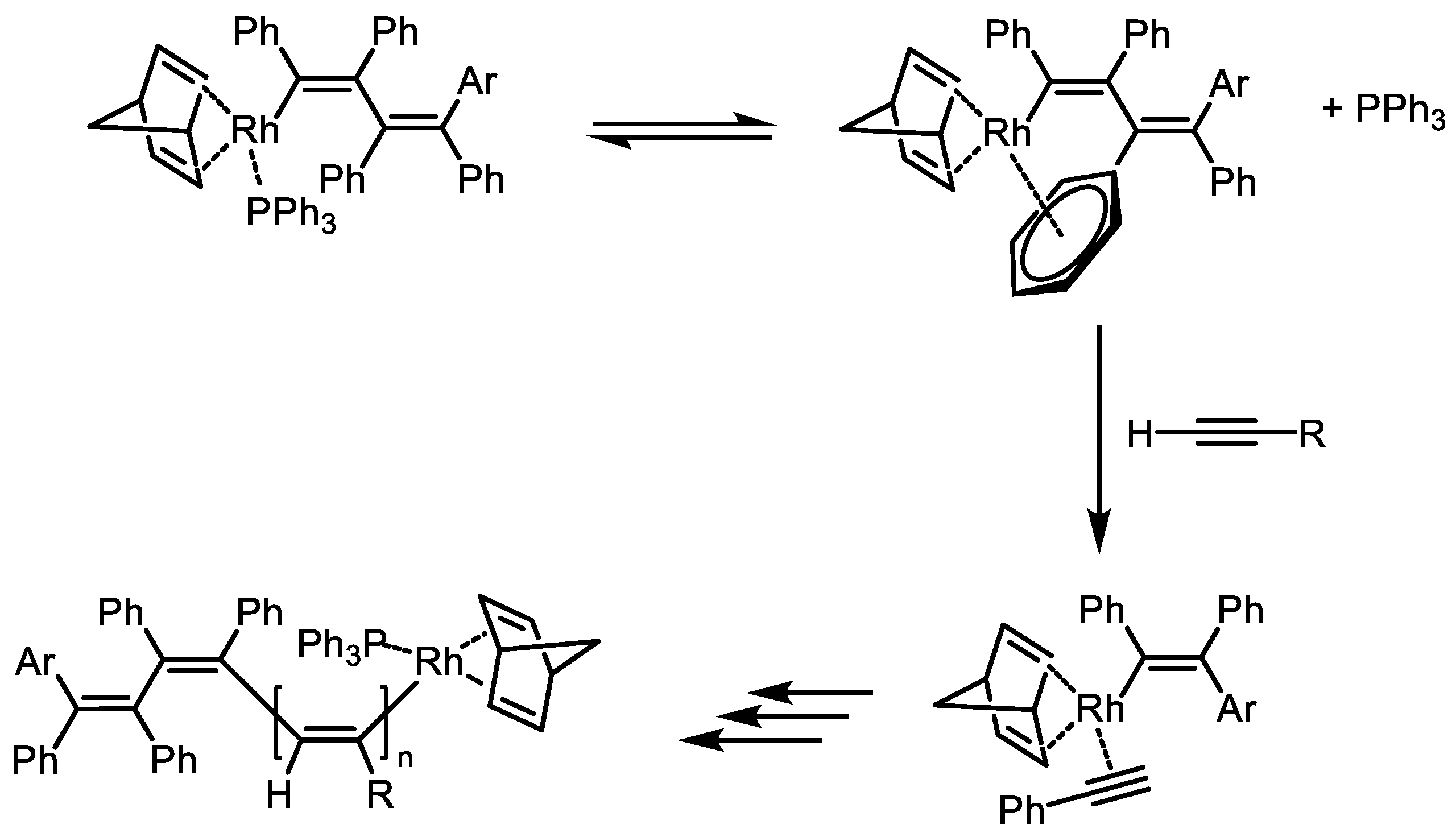
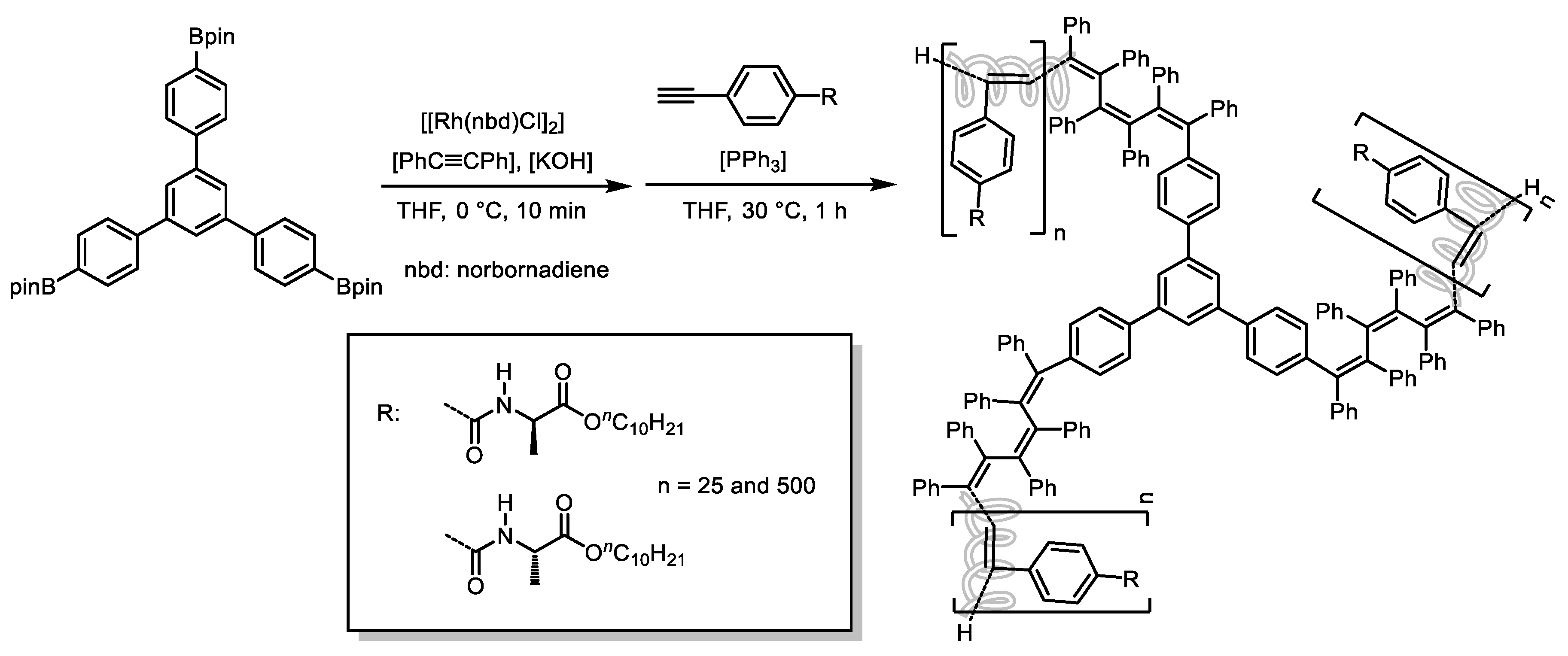



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisetsky, W.; Müller, T.J.J. Recent Advances in the Synthesis of Substituted Polyacetylenes. Catalysts 2025, 15, 50. https://doi.org/10.3390/catal15010050
Pisetsky W, Müller TJJ. Recent Advances in the Synthesis of Substituted Polyacetylenes. Catalysts. 2025; 15(1):50. https://doi.org/10.3390/catal15010050
Chicago/Turabian StylePisetsky, Wladislaw, and Thomas J. J. Müller. 2025. "Recent Advances in the Synthesis of Substituted Polyacetylenes" Catalysts 15, no. 1: 50. https://doi.org/10.3390/catal15010050
APA StylePisetsky, W., & Müller, T. J. J. (2025). Recent Advances in the Synthesis of Substituted Polyacetylenes. Catalysts, 15(1), 50. https://doi.org/10.3390/catal15010050






