Methanol Synthesis from CO2 over ZnO-Pd/TiO2 Catalysts: Effect of Pd Precursors on the Formation of ZnPd-ZnO Active Sites
Abstract
1. Introduction
2. Results
2.1. Characterization of Calcined Catalysts
2.2. Characterization of Reduced Catalysts
2.2.1. XRD and HRTEM
2.2.2. CO-DRIFTS
2.3. Activity Tests
2.4. Characterization of Used Catalysts
3. Discussion
4. Materials and Methods
4.1. Catalysts Preparation
4.2. Catalysts Characterization
4.3. Activity Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Centi, G.; Perathoner, S. The chemical engineering aspects of CO2 capture, combined with its utilization. Curr. Opin. Chem. Eng. 2023, 39, 100879. [Google Scholar] [CrossRef]
- Alsarhan, L.M.; Alayyar, A.S.; Alqahtani, N.B.; Khdary, N.H. Circular carbon economy (CCE): A way to invest CO2 and protect the environment, a review. Sustainability 2021, 13, 11625. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Nauman Javed, R.M.; Al-Othman, A.; Almomani, F.; Ajith, S. Unlocking the potential of CO2 hydrogenation into valuable products using noble metal catalysts: A comprehensive review. Environ. Technol. Innov. 2023, 31, 103217. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem. Rev. 2020, 120, 7984–8034. [Google Scholar] [CrossRef] [PubMed]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Methanol synthesis from CO2: A review of the latest developments in heterogeneous catalysis. Materials 2019, 12, 3902. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, B.; Guil-López, R.; Mota, N.; Fierro, J.L.G.; Navarro, R.M. Catalysts for the conversion of CO2 to low weight olefins—A review. Materials 2021, 14, 6952. [Google Scholar] [CrossRef] [PubMed]
- Quilis, C.; Mota, N.; Pawelec, B.; Millán, E.; Navarro Yerga, R.M. Application of intermetallic compounds as catalysts for the selective hydrogenation of CO2 to methanol. ChemCatChem 2024, 16, e202301496. [Google Scholar] [CrossRef]
- Brix, F.; Desbuis, V.; Piccolo, L.; Gaudry, É. Tuning adsorption energies and reaction pathways by alloying: PdZn versus Pd for CO2 hydrogenation to methanol. J. Phys. Chem. Lett. 2020, 11, 7672–7678. [Google Scholar] [CrossRef]
- Liang, X.-L.; Dong, X.; Lin, G.-D.; Zhang, H.-B. Carbon nanotube-supported Pd-ZnO catalyst for hydrogenation of CO2 to methanol. Appl. Catal. B Environ. 2009, 88, 315–322. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kovarik, L.; Szanyi, J. CO2 Reduction on Supported Ru/Al2O3 Catalysts: Cluster Size Dependence of Product Selectivity. ACS Catal. 2013, 3, 2449–2455. [Google Scholar] [CrossRef]
- Conant, T.; Karim, A.M.; Lebarbier, V.; Wang, Y.; Girgsdies, F.; Schlögl, R.; Datye, A. Stability of bimetallic Pd-Zn catalysts for the steam reforming of methanol. J. Catal. 2008, 257, 64–70. [Google Scholar] [CrossRef]
- Fan, X.; Wang, K.; He, X.; Li, S.; Yu, M.; Liang, X. Pd-modified CuO-ZnO-Al2O3 catalysts via mixed-phases-containing precursor for methanol synthesis from CO2 hydrogenation under mild conditions. Carbon Resour. Convers. 2024, 7, 100184. [Google Scholar] [CrossRef]
- Nolen, M.A.; Tacey, S.A.; Kwon, S.; Farberow, C.A. Theoretical assessments of CO2 activation and hydrogenation pathways on transition-metal surfaces. Appl. Surf. Sci. 2023, 637, 157873. [Google Scholar] [CrossRef]
- Lawes, N.; Aggett, K.J.; Smith, L.R.; Slater, T.J.A.; Dearg, M.; Morgan, D.J.; Dummer, N.F.; Taylor, S.H.; Hutchings, G.J.; Bowker, M. Zn loading effects on the selectivity of PdZn catalysts for CO2 hydrogenation to methanol. Catal. Lett. 2024, 54, 1603–1610. [Google Scholar] [CrossRef]
- Tsubaki, N.; Fujimoto, K. Promotional SMSI effect on supported palladium catalysts for methanol synthesis. Top. Catal. 2003, 22, 325–335. [Google Scholar] [CrossRef]
- Ou, Z.; Ran, J.; Niu, J.; Qin, C.; He, W.; Yang, L. A density functional theory study of CO2 hydrogenation to methanol over Pd/TiO2 catalyst: The role of interfacial site. Int. J. Hydrogen Energy 2020, 45, 6328–6340. [Google Scholar] [CrossRef]
- Ren, D.; Ding, J.; Tang, C.; Wang, H.; Huang, W.; Wen, X.; Zhenhua, Z. Titania-crystal-phase-engineered strong metal-support interactions and catalysis in CO2 hydrogenation. Mol. Catal. 2024, 560, 114122. [Google Scholar] [CrossRef]
- Liao, F.; Wu, X.-P.; Zheng, J.; Li, M.; Kroner, A.; Zeng, Z.; Hong, X.; Yuan, Y.; Gong, X.-G.; Tsang, S.C.E. A promising low pressure methanol synthesis route from CO2 hydrogenation over Pd@Zn core-shell catalysts. Green Chem. 2017, 19, 270–280. [Google Scholar] [CrossRef]
- Manrique, R.; Rodríguez-Pereira, J.; Rincón, S.A.; Bravo Suárez, J.J.; Baldovino-Medrano, V.G.; Jiménez, R.; Karelovic, A. The nature of the active sites of Pd-Ga catalysts in the hydrogenation of CO2 to methanol. Catal. Sci. Technol. 2010, 10, 6644–6658. [Google Scholar] [CrossRef]
- Quilis, C.; Mota, N.; Pawelec, B.; Millán, E.; Navarro Yerga, R.M. Intermetallic ZnPd/TiO2 catalysts for methanol production from CO2 hydrogenation: The effect of ZnO loading on ZnO-PdZn sites and its influence on activity. Appl. Catal. B Environ. 2023, 321, 122064. [Google Scholar] [CrossRef]
- Zabilskiy, M.; Sushkevich, V.L.; Newton, M.A.; Krumeich, F.; Nachtegaal, M.; van Bokhoven, J.A. Mechanistic study of carbon dioxide hydrogenation over ZnPd/ZnO-based catalysts: The role of palladium–zinc alloy in selective methanol synthesis. Angew. Chem. Int. Ed. 2021, 60, 17053–17059. [Google Scholar] [CrossRef] [PubMed]
- Bahruji, H.; Esquius, J.R.; Bowker, M.; Hutchings, G.; Armstrong, R.D.; Jones, W. Solvent free synthesis of ZnPd/TiO2 catalysts for the hydrogenation of CO2 to methanol. Top. Catal. 2018, 61, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Lee, J.S.; Trimm, D.I. Catalytic hydrogenation of CO2 to methanol over Pd/ZnO: Metal-support interaction. Stud. Surf. Sci. Catal. 2004, 153, 61–66. [Google Scholar]
- Lee, D.; Lee, J.-Y.; Lee, J.S. Effects of palladium particle size in hydrogenation of carbon dioxide to methanol over Pd/ZnO catalysts. Stud. Surf. Sci. Catal. 2004, 153, 169–172. [Google Scholar]
- Kim, C.-H.; Lee, J.S.; Trimm, D. The preparation and characterization of Pd-ZnO catalysts for methanol synthesis. Top. Catal. 2003, 22, 319–324. [Google Scholar] [CrossRef]
- Friedrich, M.; Penner, S.; Heggen, M.; Armbrüster, M. High CO2 selectivity in methanol steam reforming through ZnPd/ZnO teamwork. Angew. Chem. Int. Ed. 2013, 52, 4389–4392. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, E.; Althahban, S.M.; Luo, Y.; Kriegel, R.; Shaw, G.; Morgan, D.J.; He, Q.; Watanabe, M.; Armbrüster, M.; Kiely, C.J.; et al. Highly selective PdZn/ZnO catalysts for the methanol steam reforming reaction. Catal. Sci. Technol. 2018, 8, 5848–5857. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Liu, X.; Pan, X.; Pei, G.; Huang, Y.; Wang, X.; Zhang, T.; Geng, H. Methanol synthesis from CO2 and H2 over Pd/ZnO/Al2O3: Catalyst structure dependence of methanol selectivity. Appl. Catal. A Gen. 2016, 514, 51–59. [Google Scholar] [CrossRef]
- Zaman, S.F.; Ojelade, O.A.; Alhumade, H.; Mazumder, J.; Mohamed, H.O.; Castaño, P. Elucidating the promoting role of Ca on PdZn/CeO2 catalyst for CO2 valorization to methanol. Fuel 2023, 343, 127927. [Google Scholar] [CrossRef]
- Janković, B.; Mentus, S. Model-fitting and model-free analysis of decomposition of palladium acetylacetonate [Pd(acac)2]. J. Therm. Anal. Calorim. 2008, 94, 395–403. [Google Scholar] [CrossRef]
- Chen, Z.-X.; Neyman, K.; Rösch, N. Theoretical study of segregation of Zn and Pd in Pd–Zn alloys. Surf. Sci. 2004, 548, 291–300. [Google Scholar] [CrossRef]
- Toebes, M.L.; Dillen, A.J.; De Jong, K.P. Synthesis of supported palladium catalyst. J. Mol. Catal. A Chem. 2001, 173, 75–98. [Google Scholar] [CrossRef]
- Van Veen, J.A.R.; Jonkers, G.; Hesselnik, W.H. Interaction of transition-metal acetylacetonates with γ-Al2O3 surfaces. J. Chem. Soc. Faraday Trans. 1 1989, 85, 389–413. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Guillet-Nicolas, R.; García-Martínez, J.; Thommes, M. Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem. Soc. Rev. 2017, 46, 389–414. [Google Scholar] [CrossRef] [PubMed]
- Castellazzi, P.; Groppi, G.; Forzatti, P.; Baylet, A.; Marecot, P.; Duprez, D. Role of Pd loading and dispersion on redox behavior and CH4 combustion activity of Al2O3 supported catalysts. Catal. Today 2010, 155, 18–26. [Google Scholar] [CrossRef]
- Hong, C.-T.; Yeh, C.-T.; Yu, F.-H. Effect of reduction and oxidation treatments on Pd/ZnO catalysts. Appl. Catal. 1989, 48, 385–396. [Google Scholar] [CrossRef]
- Baylet, A.; Marecot, P.; Duprez, D.; Castellazzi, P.; Groppi, G.; Forzatti, P. In situ Raman and in situ XRD analysis of PdO reduction and Pd degrees oxidation supported on gamma-Al2O3 catalyst under different atmospheres. Phys. Chem. Chem. Phys. 2011, 13, 4607–4613. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Carstens, J.N.; Bell, A.T. A study of the dynamics of Pd oxidation and PdO reduction by H2 and CH4. J. Catal. 1998, 176, 125–135. [Google Scholar] [CrossRef]
- Kast, P.; Friedrich, M.; Girgsdies, F.; Kröhnert, J.; Teschner, D.; Lunkenbein, T.; Behrens, M.; Schlögl, R. Strong metal-support interaction and alloying in Pd/ZnO catalysts for CO oxidation. Catal. Today 2016, 260, 21–31. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kovarik, L.; Szanyi, J. Heterogeneous catalysis on atomically dispersed supported metals: CO2 reduction on multifunctional Pd catalysts. ACS Catal. 2013, 3, 2094–2100. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, H.H.; Hong, S.C. A study on the effect of support reducibility on the reverse water-gas shift reaction over Pt catalysts. Appl. Catal. A 2012, 423–424, 100–107. [Google Scholar]
- Parkinson, G.S.; Novotny, Z.; Argentero, G.; Schmid, M.; Pavelec, J.; Kosak, R.; Blaha, P.; Diebold, U. Carbon monoxide-induced adatom sintering in a Pd-Fe3O4 model catalyst. Nat. Mater. 2013, 12, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, L.M.; Selloni, A.; Lu, G.Q.M.; Smith, S.C. Titania-water interactions: A review of theoretical studies. J. Mater. Chem. 2019, 20, 10319–10334. [Google Scholar] [CrossRef]
- Nelson, N.C.; Chen, L.; Meira, D.; Kovarik, L.; Szanyi, J. In situ dispersion of palladium on TiO2 during reverse water–gas shift reaction: Formation of atomically dispersed palladium. Angew. Chem. Int. Ed. 2020, 59, 17657–17663. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Allec, A.I.; Nguyen, M.-T.; Kovarik, L.; Hoffman, A.S.; Hong, J.; Meira, D.; Shi, H.; Bare, S.R.; Glezakou, V.-A.; et al. Dynamic Evolution of Palladium Single Atoms on Anatase Titania Support Determines the Reverse Water–Gas Shift Activity. J. Am. Chem. Soc. 2023, 145, 10847–10860. [Google Scholar] [CrossRef]
- Li, S.; Xia, Y.; Ou, Y.; Wu, Z.; Jin, Z.; Wang, L.; Meng, X.; Han, Z.-K.; Yuan, W.; Jiang, Y.; et al. Unusual facet-dependent sintering in Pd-TiO2 catalysts revealed by theory and experiment. ACS Catal. 2024, 14, 1608–1619. [Google Scholar] [CrossRef]
- Mclaren, A.; Valdes-Solis, T.; Li, G.; Tsang, S.C. Shape and Size Effects of ZnO Nanocrystals on Photocatalytic Activity. J. Am. Chem. Soc. 2009, 131, 12540–12541. [Google Scholar] [CrossRef] [PubMed]
- Kibis, L.S.; Titkov, A.I.; Stadnichenko, A.; Koscheev, S.V.; Boronin, A.I. X-ray photoelectron spectroscopy study of Pd oxidation by RF discharge in oxygen. Appl. Surf. Sci. 2009, 255, 9248–9254. [Google Scholar] [CrossRef]
- Pawlonka, J.; Gac, W.; Greluk, M.; Słowik, G. Applcation of microemulsion method for development of methanol steam reforming Pd/ZnO catalysts. J. Therm. Anal. Calorim. 2016, 125, 1265–1272. [Google Scholar] [CrossRef]
- Wang, X.; Shi, H.; Kwak, J.H.; Szanyi, J. Mechanism of CO2 hydrogenation on Pd/Al2O3 catalysts, Kinetics and transient DRIFT-MS studies. ACS Catal. 2015, 5, 6337–6349. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowker, M.; Hutchings, G.; Dimitratos, N.; Wells, P.; Gibson, E.; Jones, W.; Brookes, C.; Morgan, D.; Lalev, G. ZnPd/ZnO catalysts for direct CO2 hydrogenation to methanol. J. Catal. 2016, 343, 133–146. [Google Scholar] [CrossRef]
- Mathew, T.; Saju, S.; Raveendran, S.N. Survey of heterogeneous catalysts for the CO2 reduction to CO via reverse water gas shift. In Engineering Solutions for CO2 Conversion; Reina, T.R., Arellano-Garcia, H., Odriozola, J.A., Eds.; Wiley: Hoboken, NJ, USA, 2021; Chapter 12; pp. 281–316. [Google Scholar]
- Chen, S.; Abdel-Mageed, A.M.; Li, D.; Bansmann, J.; Cisneros, S.; Biskupek, J.; Huang, W.; Behm, R.J. Morphology-engineered highly active and stable Ru/TiO2 catalysts for selective CO methanation. Angew. Chem. Int. Ed. 2019, 58, 10732–10736. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Sano, T.; Kobayashi, H.; Yamashita, H. Surface engineering of a supported PdAg catalyst for hydrogenation of CO2 to formic acid: Elucidating the active Pd atoms in alloy nanoparticles. J. Am. Chem. Soc. 2018, 140, 8902–8909. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, N.; Pax, R.A.; Gray, E.M. Review of functional titanium oxides. I: TiO2 and its modifications. Prog. Solid State Chem. 2016, 44, 86–105. [Google Scholar] [CrossRef]
- Ali, I.; Suhail, M.; Alothman, Z.A.; Alwarthan, A. Recent advances in syntheses, properties and applications of TiO2 nanostructures. RSC Adv. 2018, 8, 30125–30147. [Google Scholar] [CrossRef] [PubMed]
- González, I.D.; Navarro, R.M.; Álvarez-Galván, M.C.; Rosa, F.; Fierro, J.L.G. Performance enhancement in the water-gas shift reaction of platinum deposited over a cerium-modified TiO2 support. Catal. Commun. 2008, 9, 1759–1765. [Google Scholar] [CrossRef]
- Neumann, M.; Teschner, D.; Knop-Gericke, A.; Reschetilowski, W.; Armbrüster, M. Controlled synthesis and catalytic properties of supported In–Pd intermetallic compounds. J. Catal. 2016, 340, 49–59. [Google Scholar] [CrossRef]
- Tamura, H.; Katayama, N.; Furuichi, R. Modeling of ion-exchange reactions on metal oxides with the Frumkin isotherm. 1. Acid−base and charge characteristics of MnO2, TiO2, Fe3O4, and Al2O3 surfaces and adsorption affinity of alkali metal ions. Environ. Sci. Technol. 1996, 30, 1198–1204. [Google Scholar] [CrossRef]
- McNamee, C.E.; Tsujii, Y.; Matsumoto, M. Physicochemical Characterization of an anatase TiO2 surface and the adsorption of a nonionic surfactant: An atomic force microscopy study. Langmuir 2005, 1, 11283–11288. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of 677 gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics: Eden Prairie, MN, USA, 2000. [Google Scholar]
- Obeso–Estrella, R.; Pawelec, B.; Mota, N.; Flores–Sanchez, L.A.; Quintana-Melgoza, J.M.; Yocupicio-Gaxiola, R.I.; Zepeda, T.A. Method for analysing HR-TEM micrographs to propose and/or describe structures and their interaction in crystalline materials. MethodsX 2022, 9, 101855. [Google Scholar] [CrossRef] [PubMed]
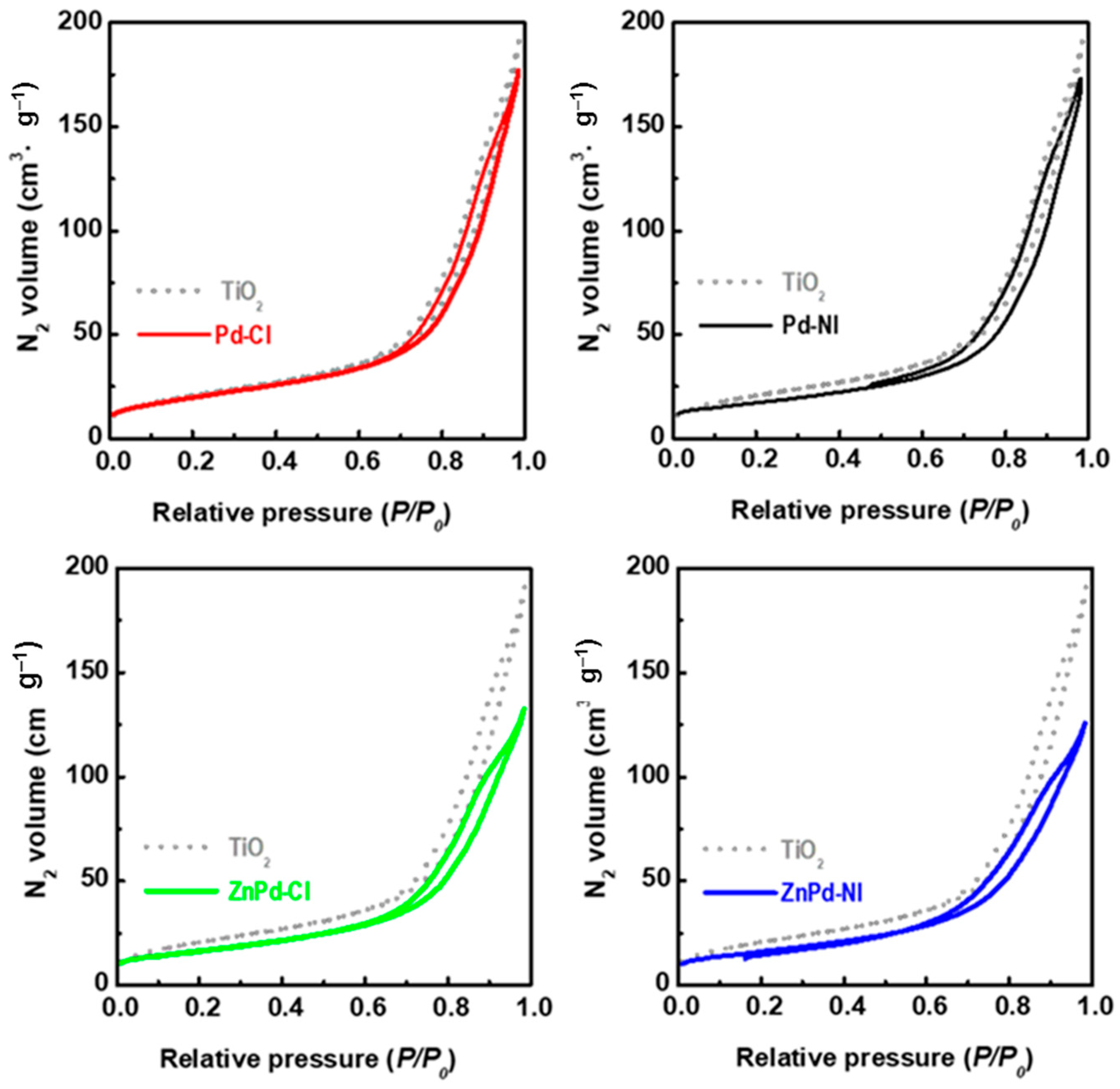
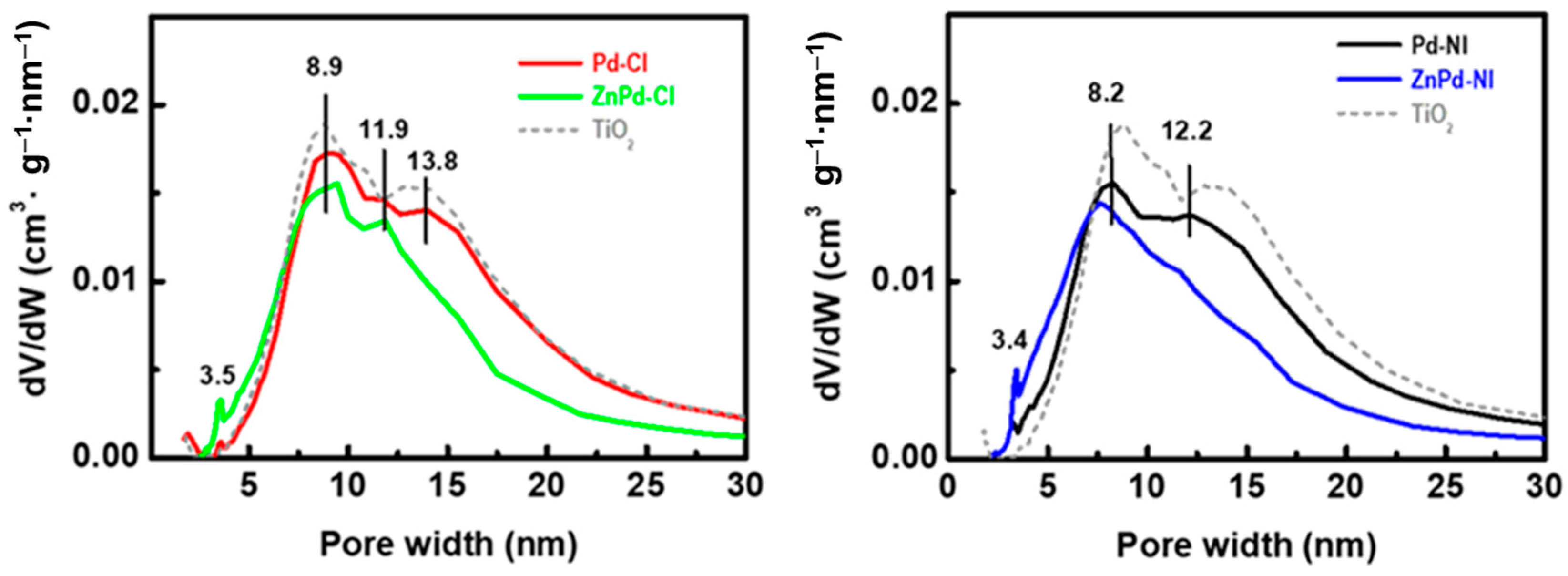
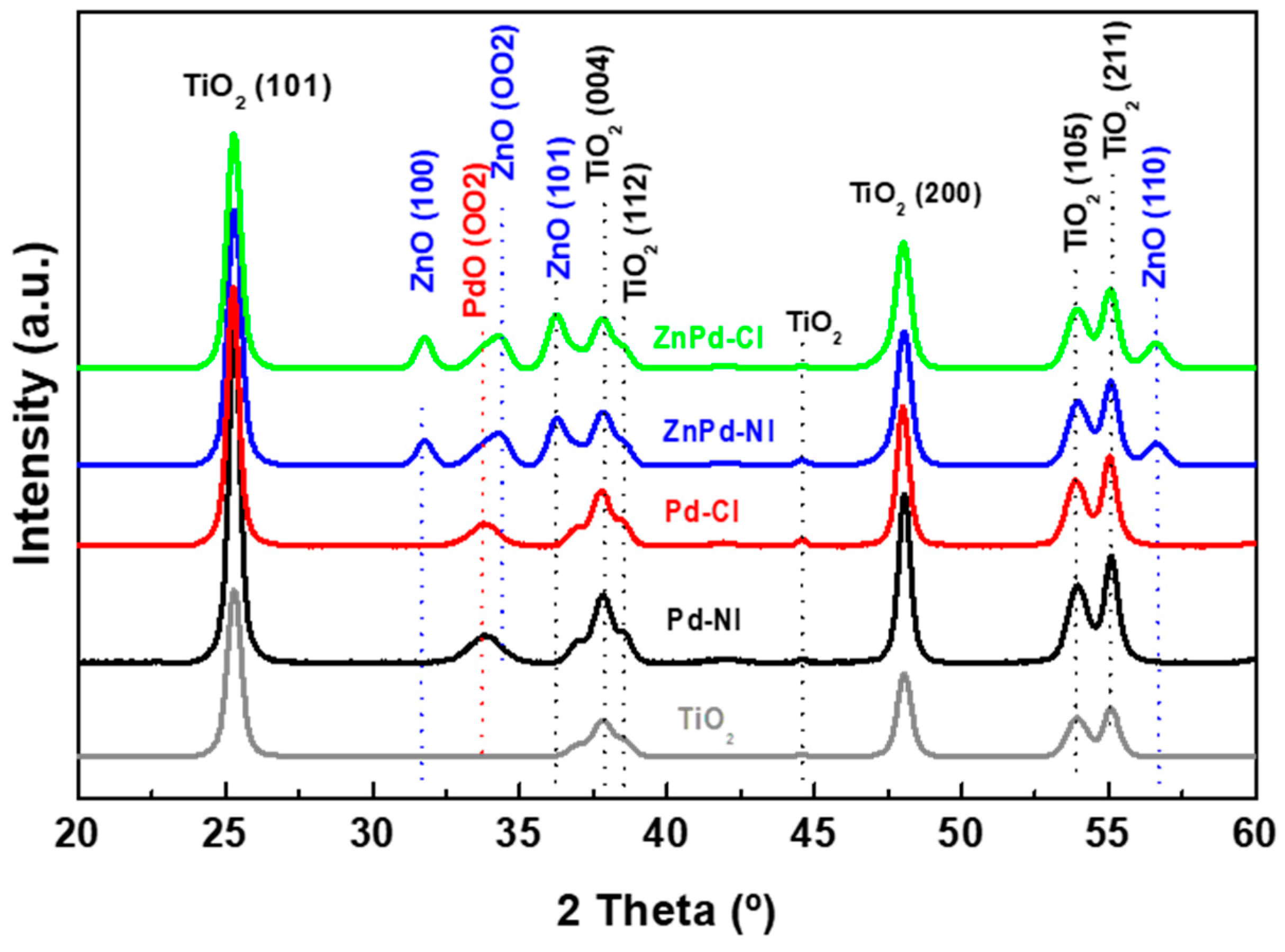
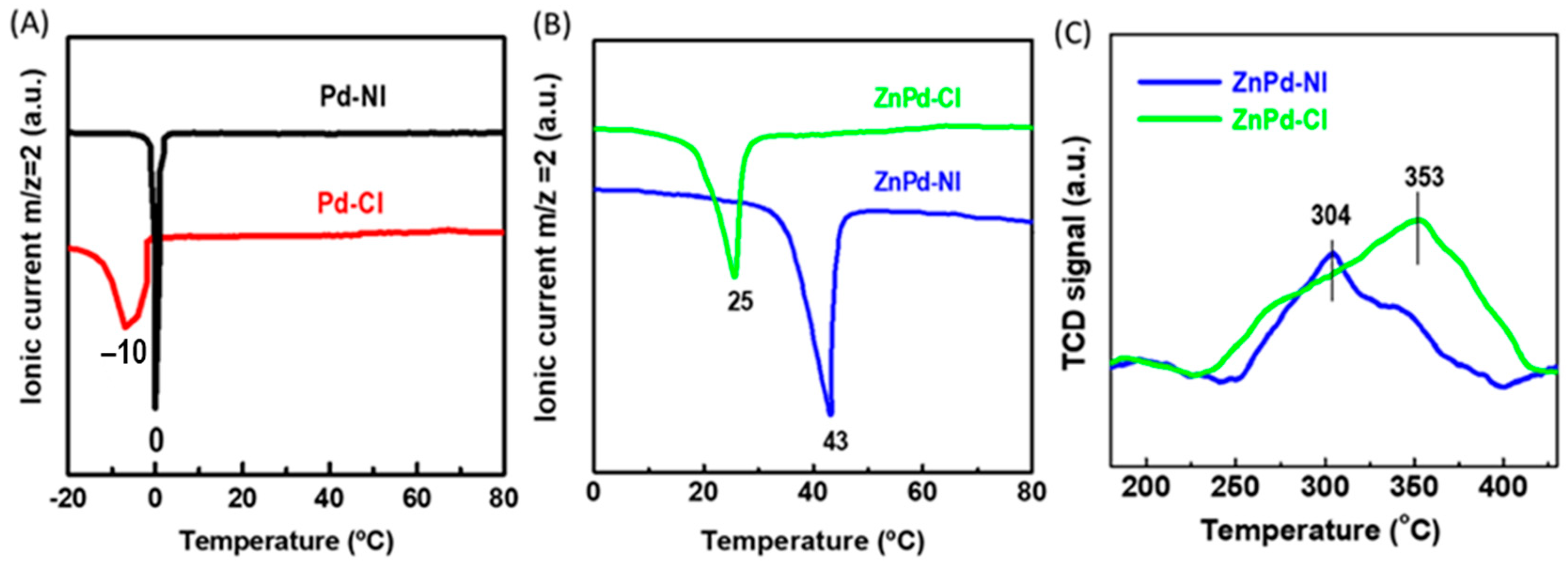
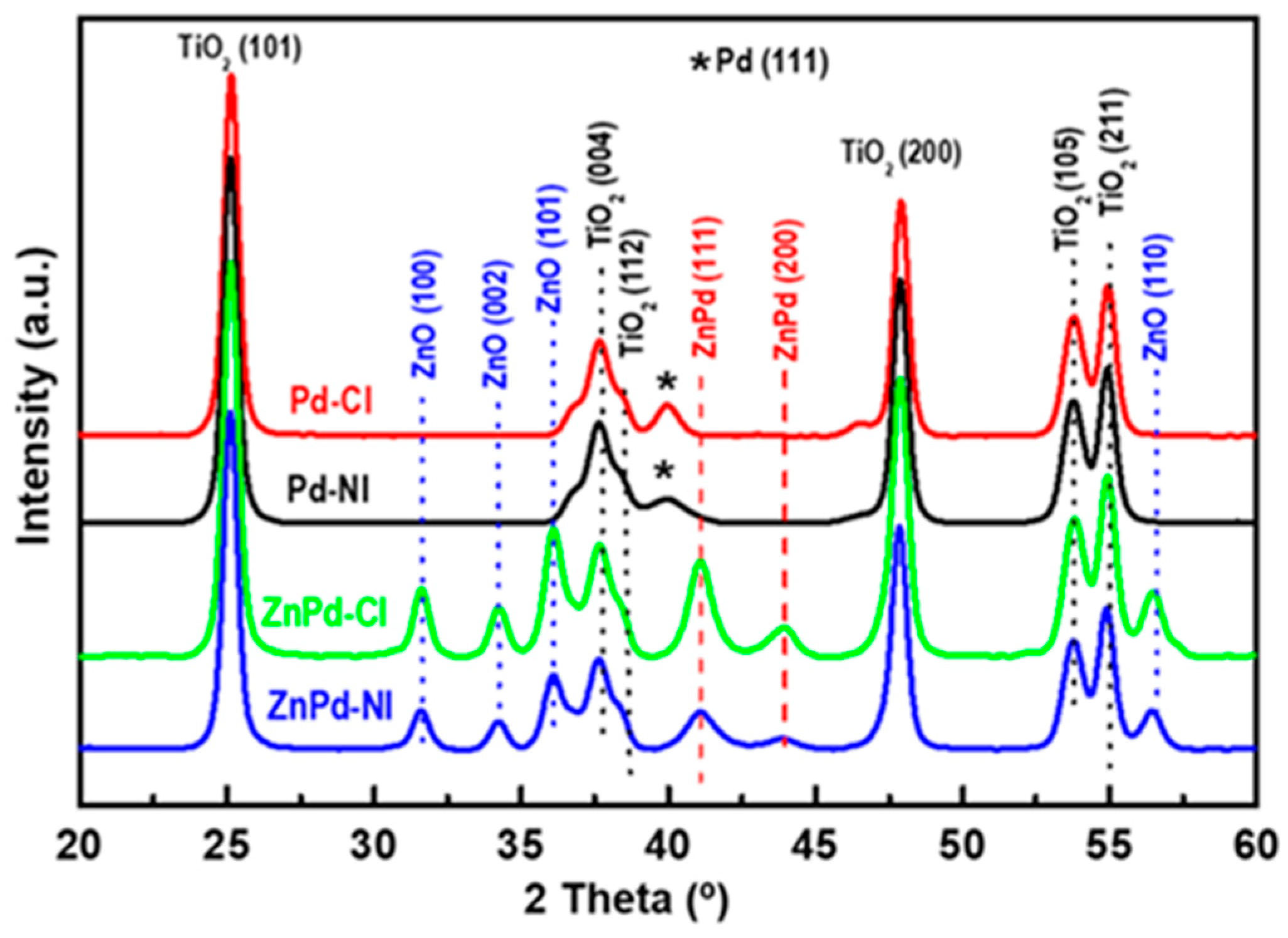
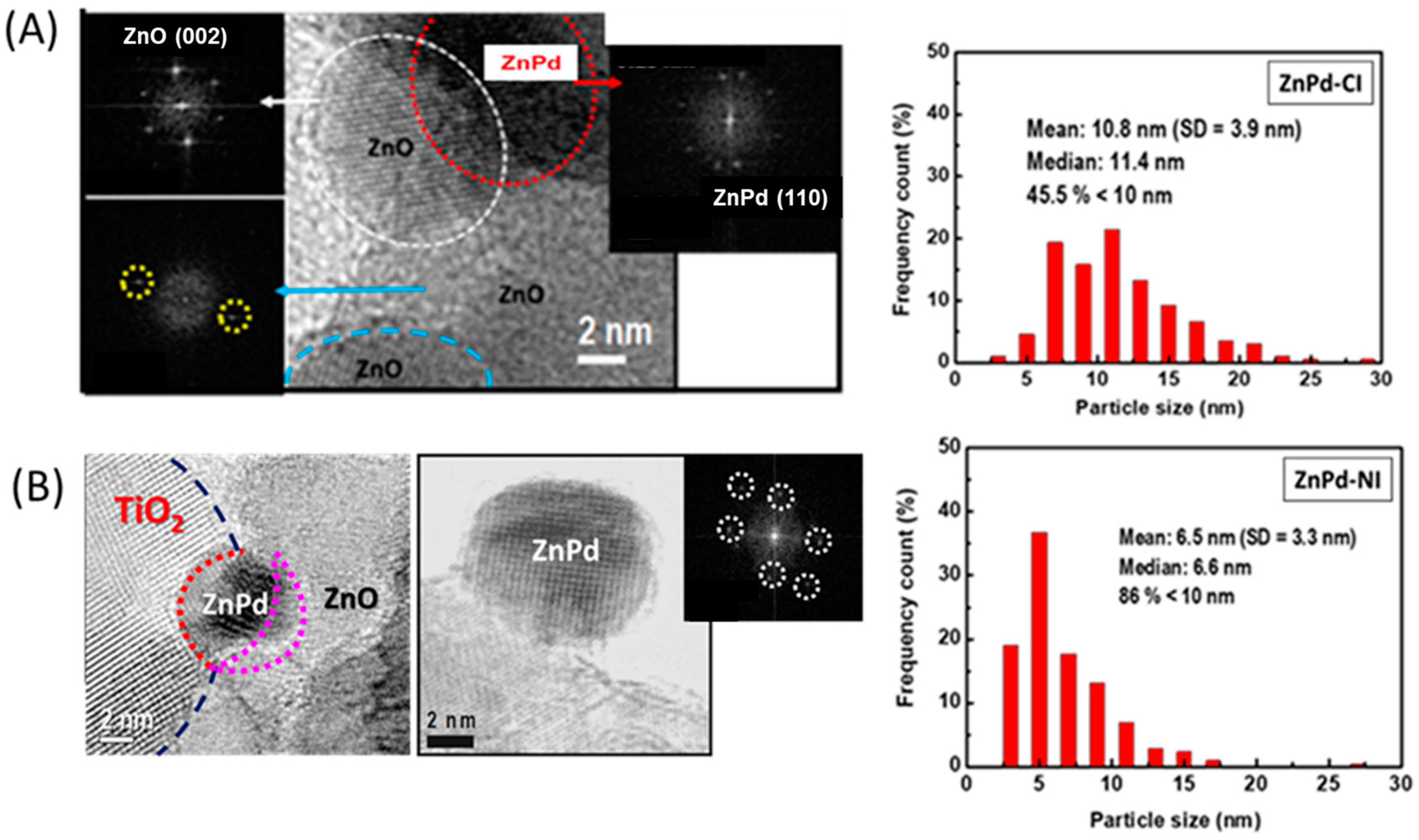
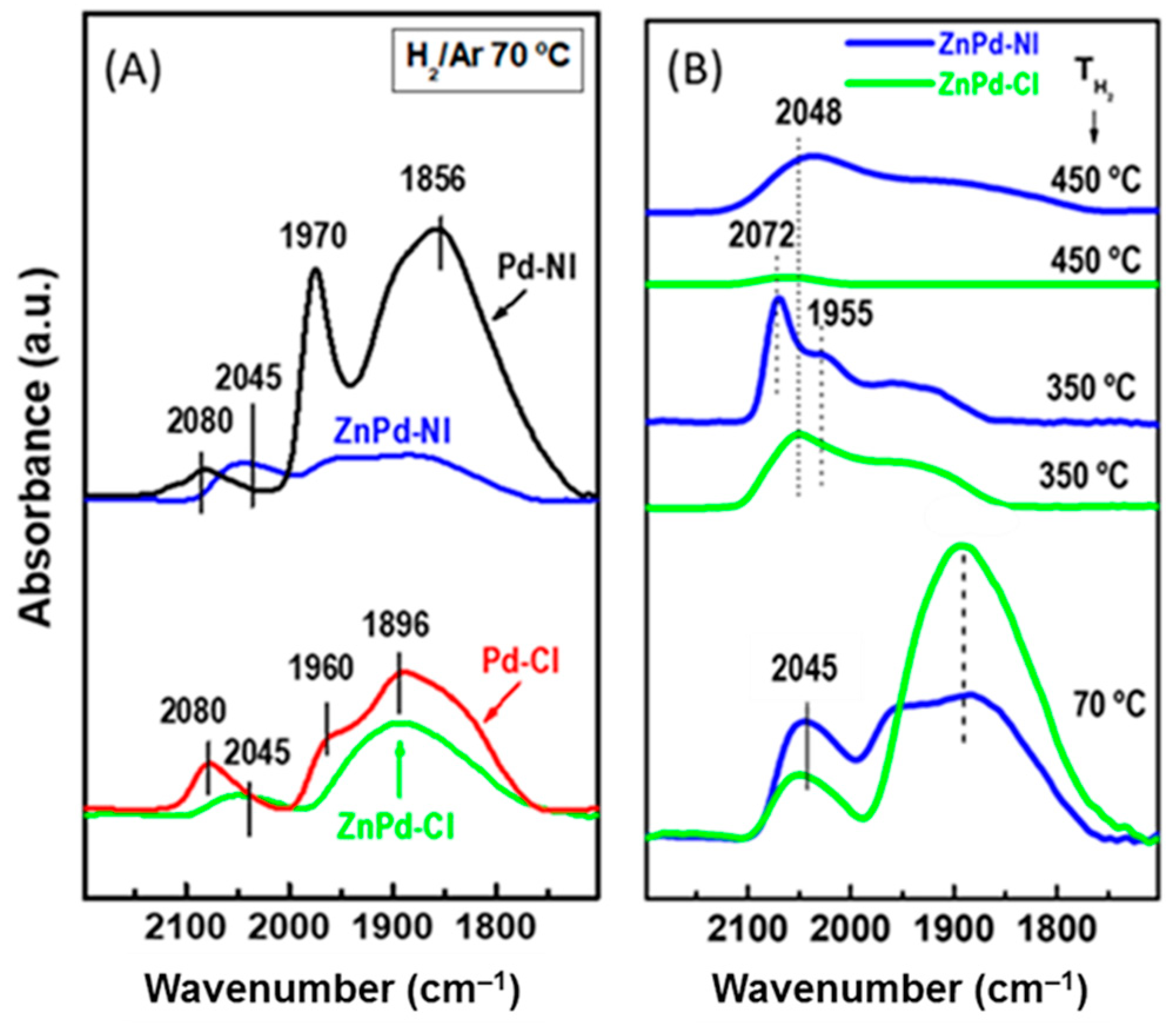
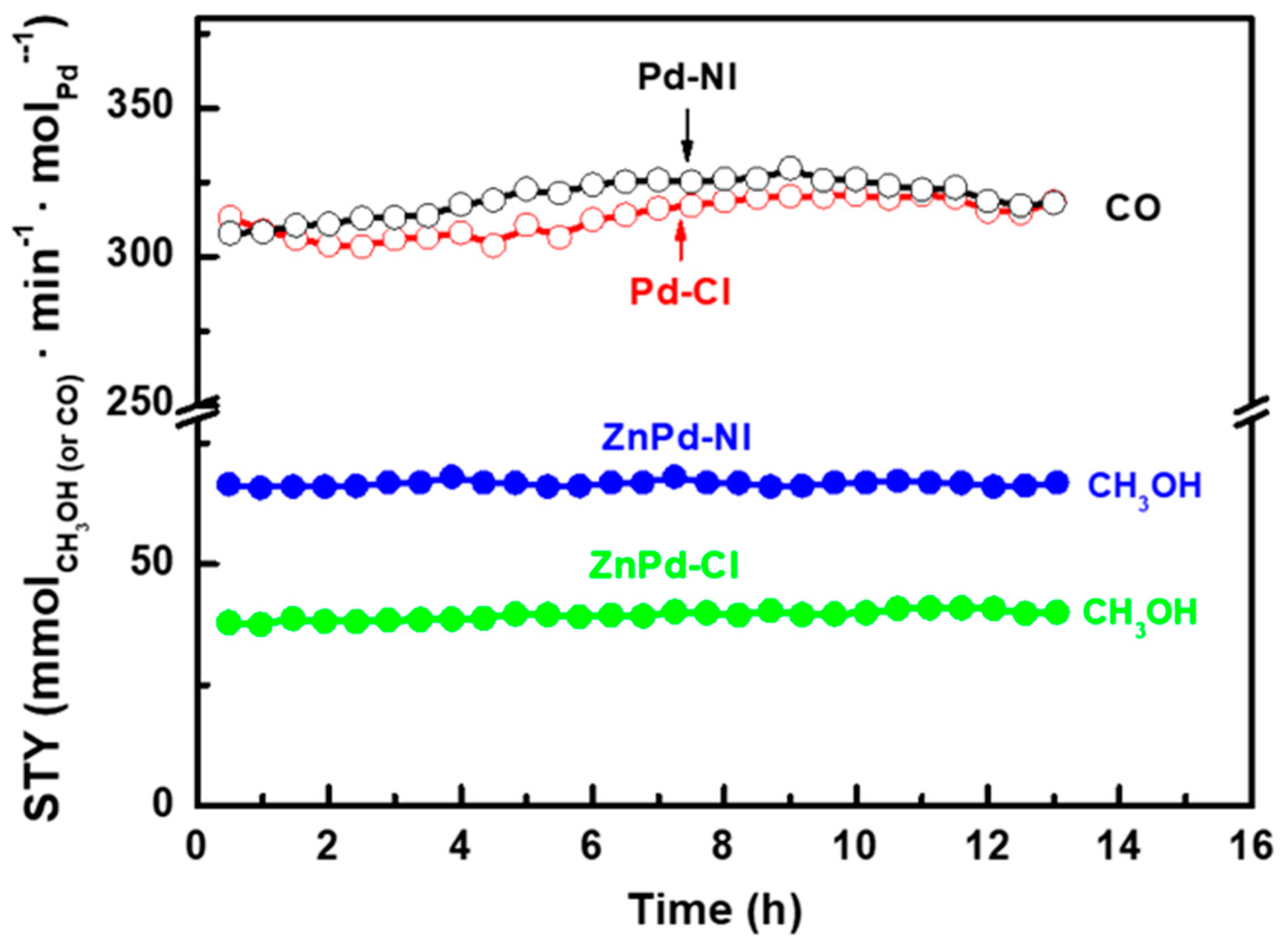
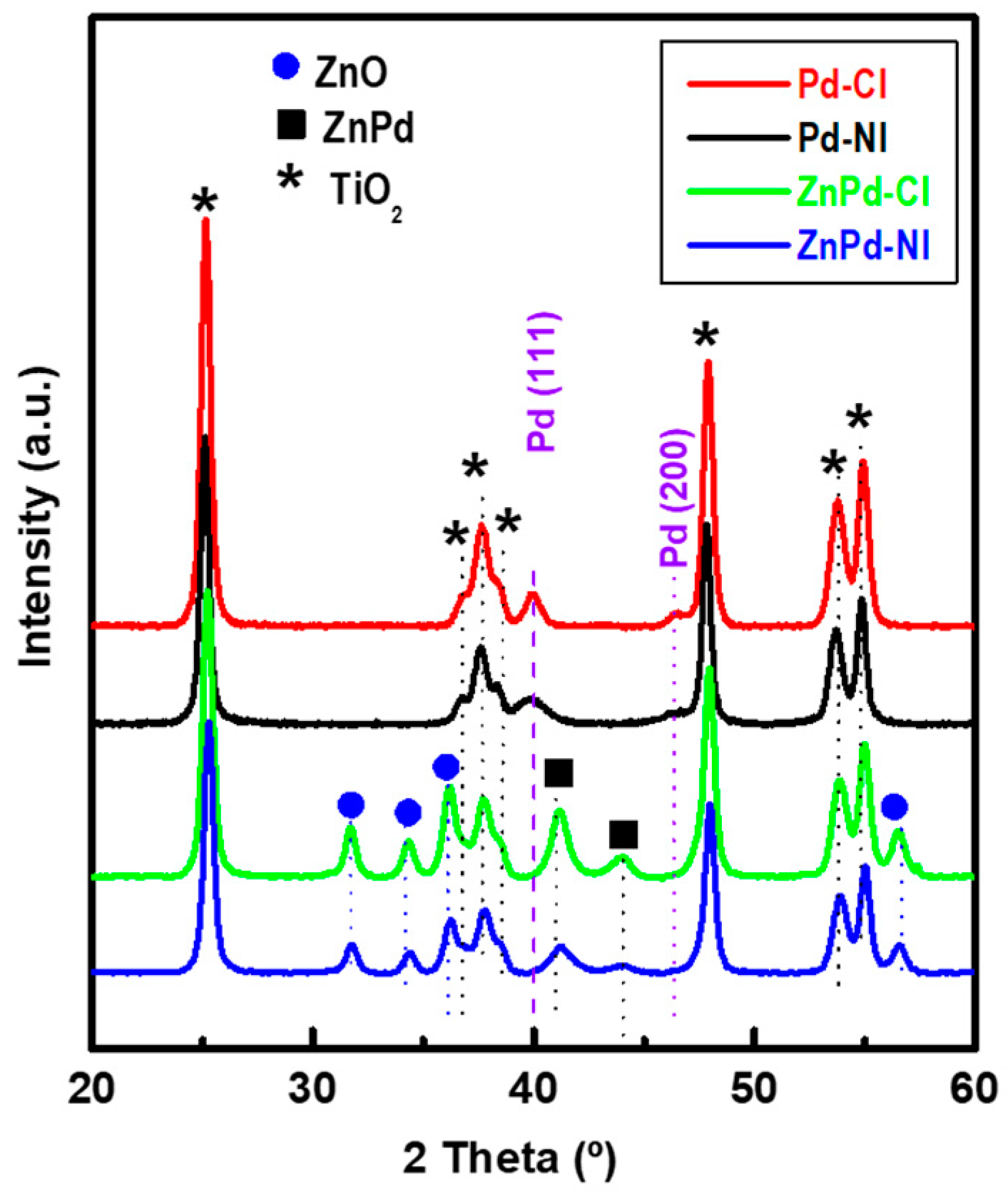
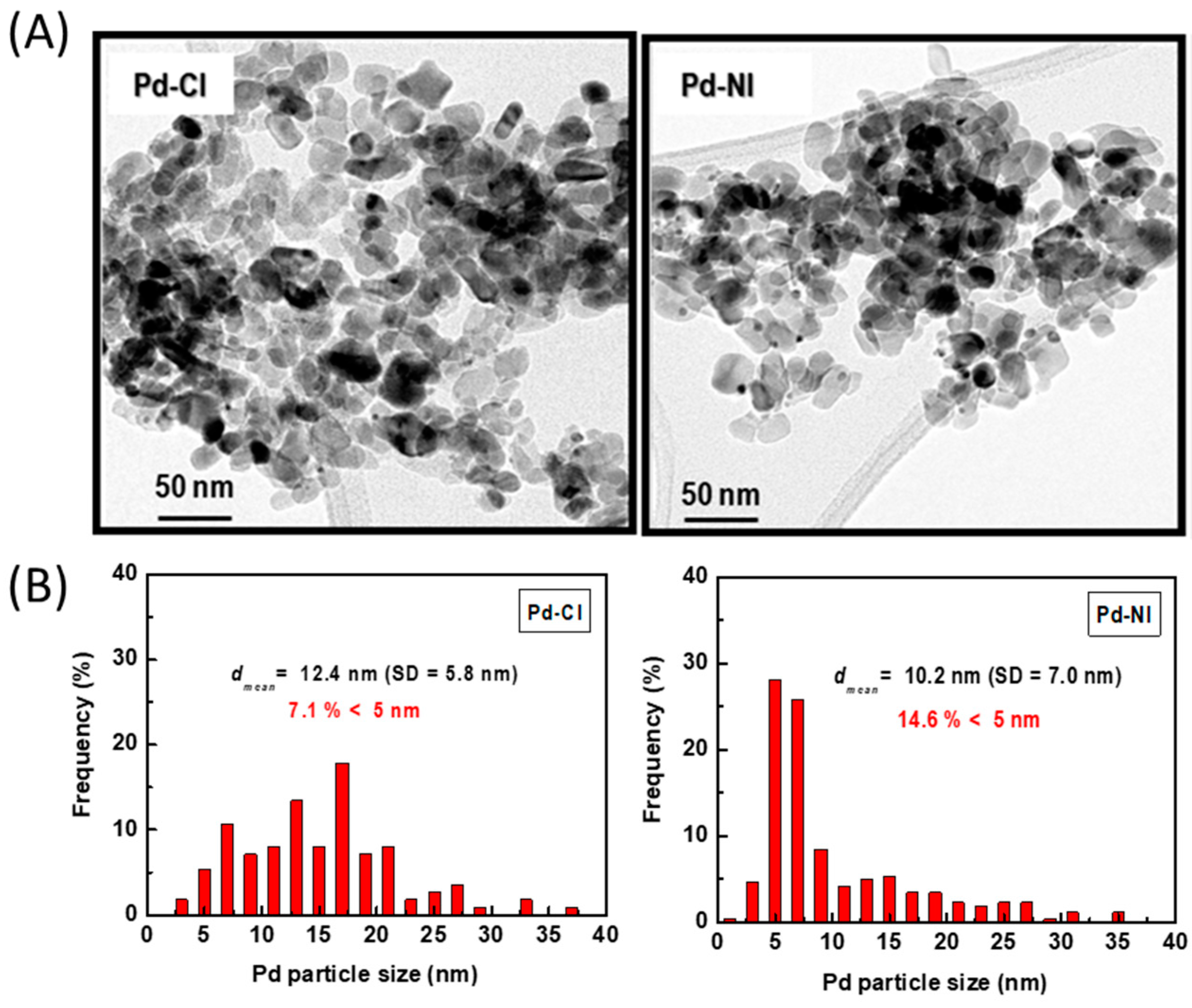
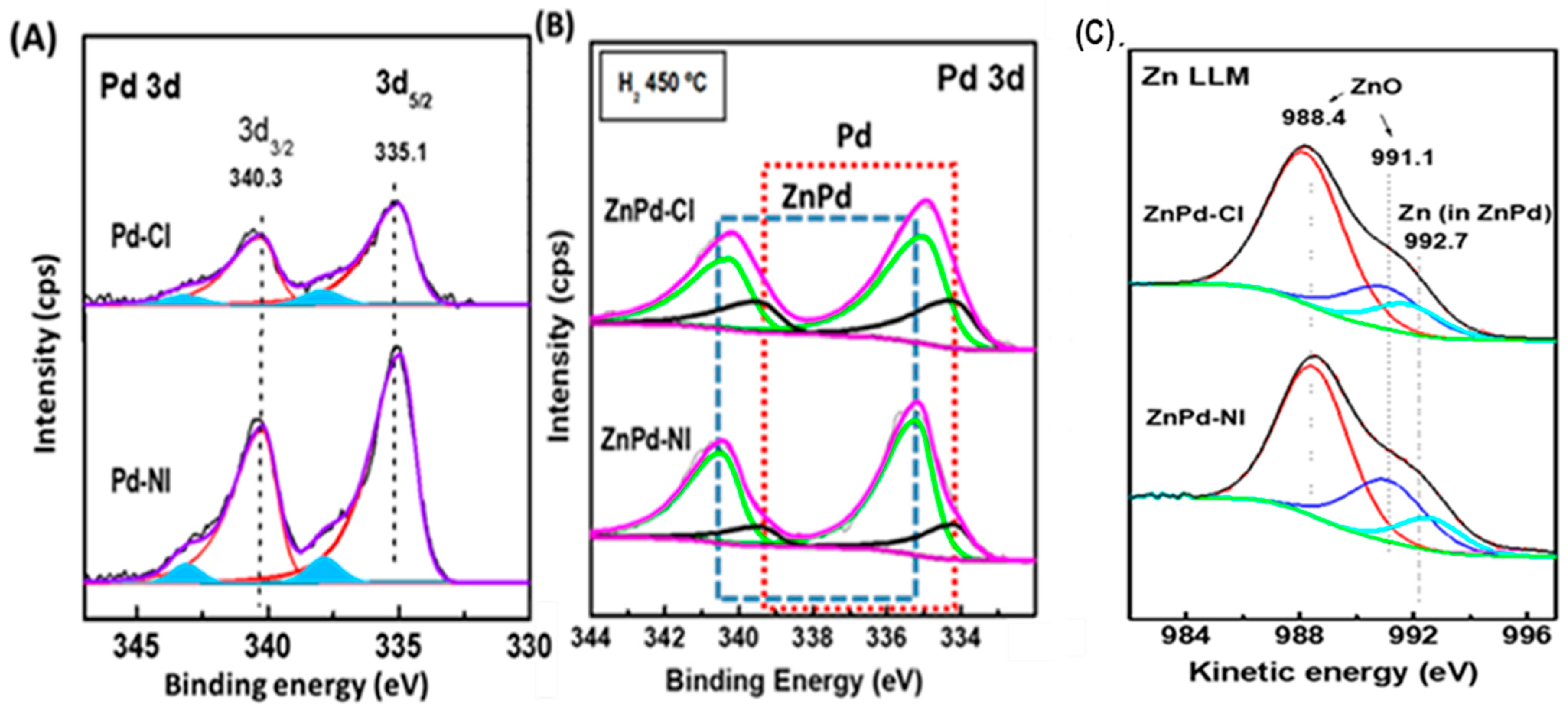


| Sample | Pd b (wt.%) | ZnO b (wt.%) | SBET a (m2/gTiO2) | Vpore c (cm3/gTiO2) | dd (nm) |
|---|---|---|---|---|---|
| TiO2 a | - | - | 76 | 0.30 | 13.9 |
| Pd-CI | 3.6 | - | 76 | 0.29 | 13.9 |
| Pd-NI | 3.9 | - | 75 | 0.28 | 13.5 |
| ZnPd-CI | 4.2 | 17.8 | 88 | 0.31 | 12.1 |
| ZnPd-NI | 4.3 | 19.2 | 86 | 0.29 | 11.9 |
| Sample | TiO2 (101) (nm) | PdO (002) (nm) | ZnO (100) (nm) | PdO (002)/ ZnO (100) |
|---|---|---|---|---|
| TiO2 | 17.4 | - | - | - |
| Pd-CI | 17.6 | 9.2 | - | - |
| Pd-NI | 17.6 | 7.2 | - | - |
| ZnPd-CI | 17.6 | 9.9 | 16.8 | 0.09/0.13 |
| ZnPd-NI | 17.6 | 9.0 | 16.9 | 0.08/0.10 |
| Catalyst | χCO2 (%) | Selectivity (%) | STY (mmol·min−·molPd−1) | |||
|---|---|---|---|---|---|---|
| MeOH | CO | CH4 | CH3OH a | CO | ||
| Pd-CI | 5.5 ± 0.1 | - | 94.8 ± 0.2 | 5.2 ± 0.2 | - | 313.2 ± 6.2 |
| Pd-NI | 5.9 ± 0.1 | - | 95.0 ± 0.6 | 5.0 ± 0.6 | - | 319.8 ± 6.3 |
| ZnPd-CI | 2.3 ± 0.02 | 35.5 ± 0.01 | 64.5 ± 0.01 | - | 41.6 ± 1.0 | 71.3 ± 1.4 |
| ZnPd-NI | 2.2 ± 0.02 | 64.7 ± 0.2 | 35.3 ± 0.2 | - | 67.8 ± 0.6 | 37.4 ± 0.3 |
| Catalysts | Pd0 (111) | ZnPd (111) | ZnO (100) | IZnO(100)/IZnO(002) |
|---|---|---|---|---|
| Freshly red./used | Freshly red./used | Freshly red./used | Freshly red./used | |
| Pd-CI | 9.1/9.8 | - | - | - |
| Pd-NI | 7.7/7.0 | - | - | - |
| ZnPd-CI | - | 10.7/10.1 | 15.5/15.0 | 1.44/1.39 |
| ZnPd-NI | - | 8.5/9.4 | 14.1/13.8 | 1.31/1.41 |
| Atomic Ratios | Pd-CI | Pd-NI | ZnPd-CI | ZnPd-NI |
|---|---|---|---|---|
| Pd/Titotal | 0.12 | 0.27 | 0.20 | 0.33 |
| Zn/Ti | - | - | 15.88 | 13.7 |
| ZnPd/Pdtotal | - | - | 0.48 | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quilis Romero, C.; Mota Toledo, N.; Pawelec, B.; Navarro Yerga, R.M. Methanol Synthesis from CO2 over ZnO-Pd/TiO2 Catalysts: Effect of Pd Precursors on the Formation of ZnPd-ZnO Active Sites. Catalysts 2025, 15, 55. https://doi.org/10.3390/catal15010055
Quilis Romero C, Mota Toledo N, Pawelec B, Navarro Yerga RM. Methanol Synthesis from CO2 over ZnO-Pd/TiO2 Catalysts: Effect of Pd Precursors on the Formation of ZnPd-ZnO Active Sites. Catalysts. 2025; 15(1):55. https://doi.org/10.3390/catal15010055
Chicago/Turabian StyleQuilis Romero, Carlos, Noelia Mota Toledo, Barbara Pawelec, and Rufino M. Navarro Yerga. 2025. "Methanol Synthesis from CO2 over ZnO-Pd/TiO2 Catalysts: Effect of Pd Precursors on the Formation of ZnPd-ZnO Active Sites" Catalysts 15, no. 1: 55. https://doi.org/10.3390/catal15010055
APA StyleQuilis Romero, C., Mota Toledo, N., Pawelec, B., & Navarro Yerga, R. M. (2025). Methanol Synthesis from CO2 over ZnO-Pd/TiO2 Catalysts: Effect of Pd Precursors on the Formation of ZnPd-ZnO Active Sites. Catalysts, 15(1), 55. https://doi.org/10.3390/catal15010055







