Electrolessly Deposited Cobalt–Phosphorus Coatings for Efficient Hydrogen and Oxygen Evolution Reactions
Abstract
1. Introduction
2. Results and Discussion
2.1. Coatings, Microstructure, and Morphology Studies
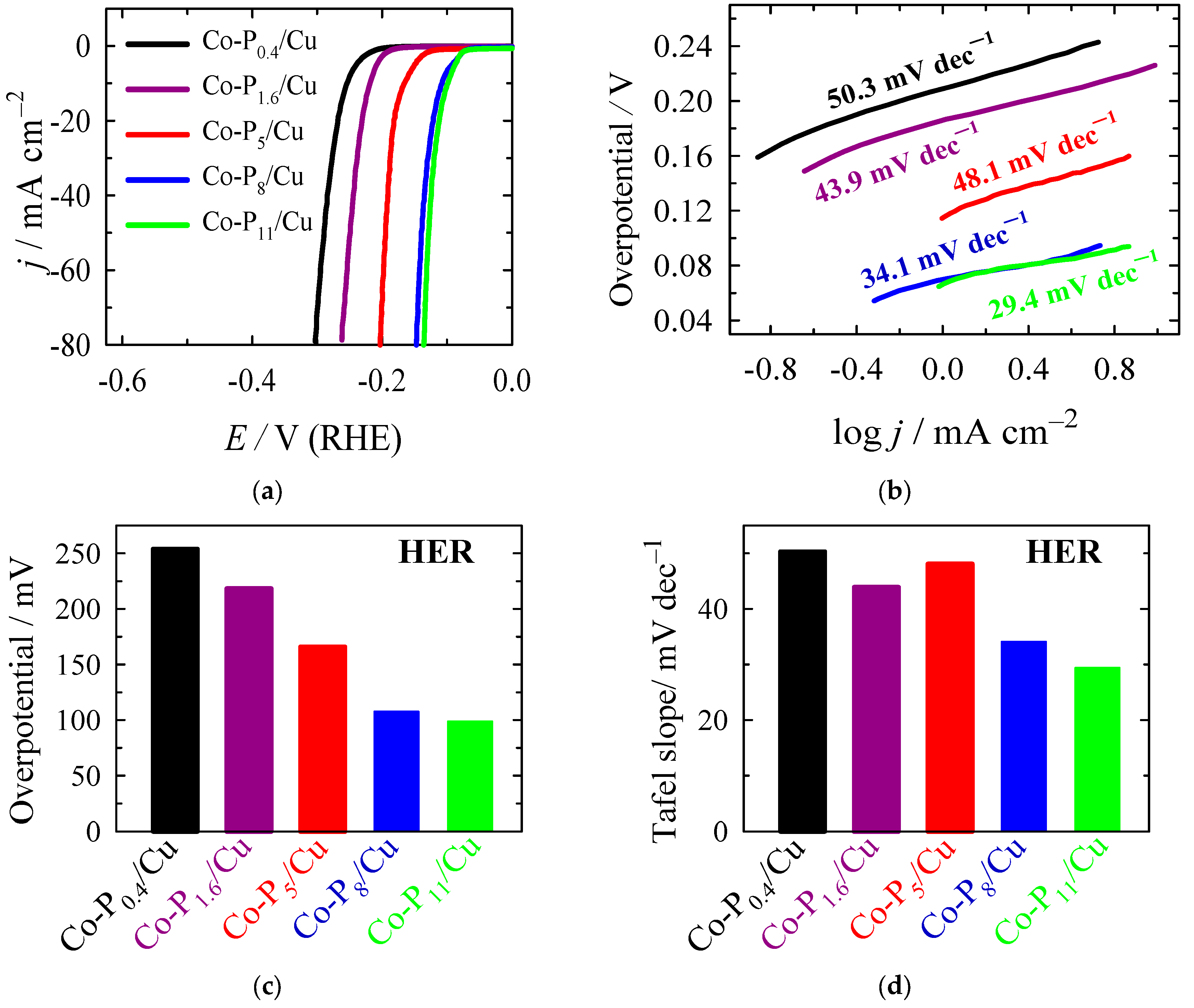
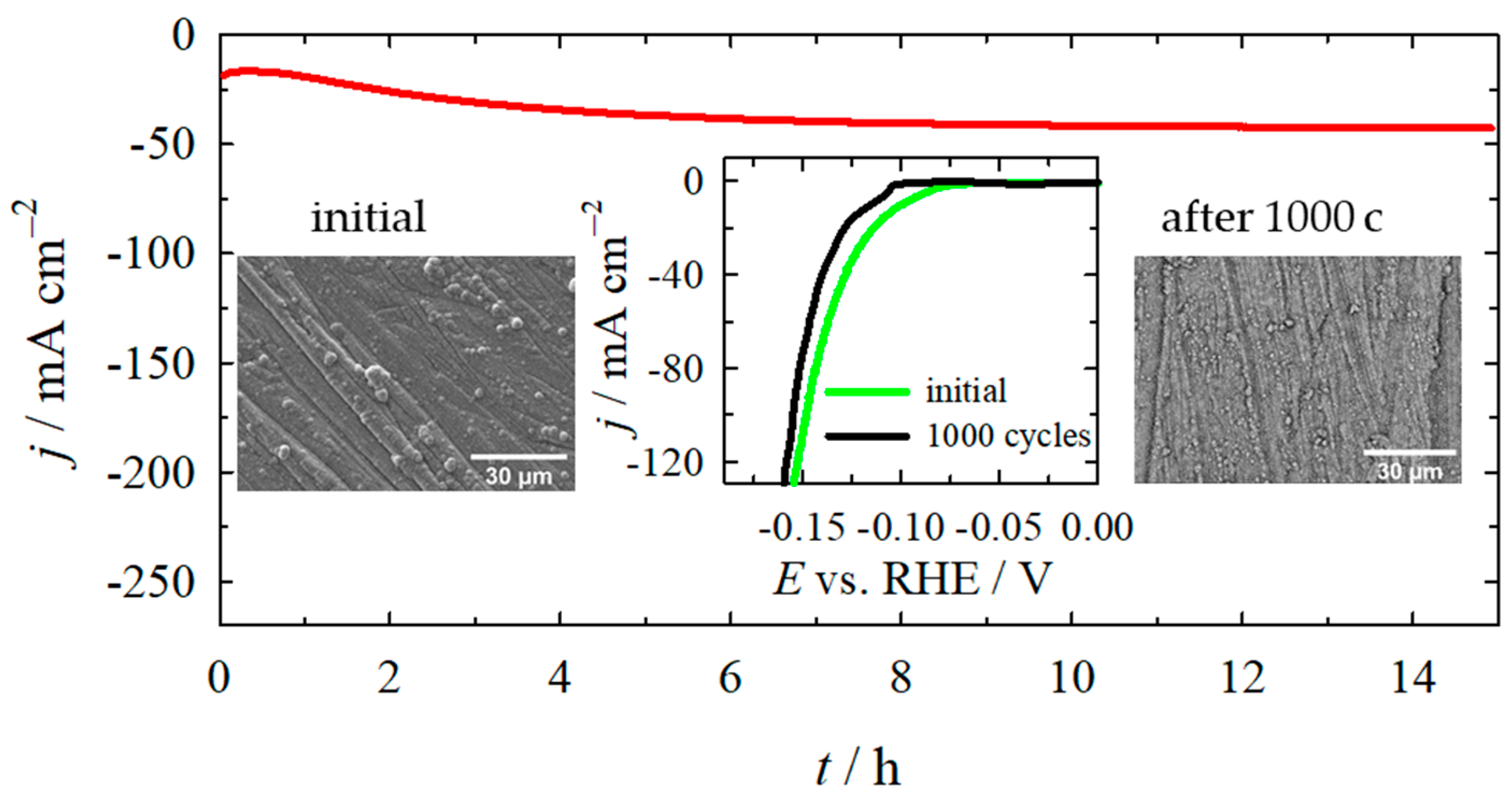
2.2. Electrocatalytic Activity Towards HER
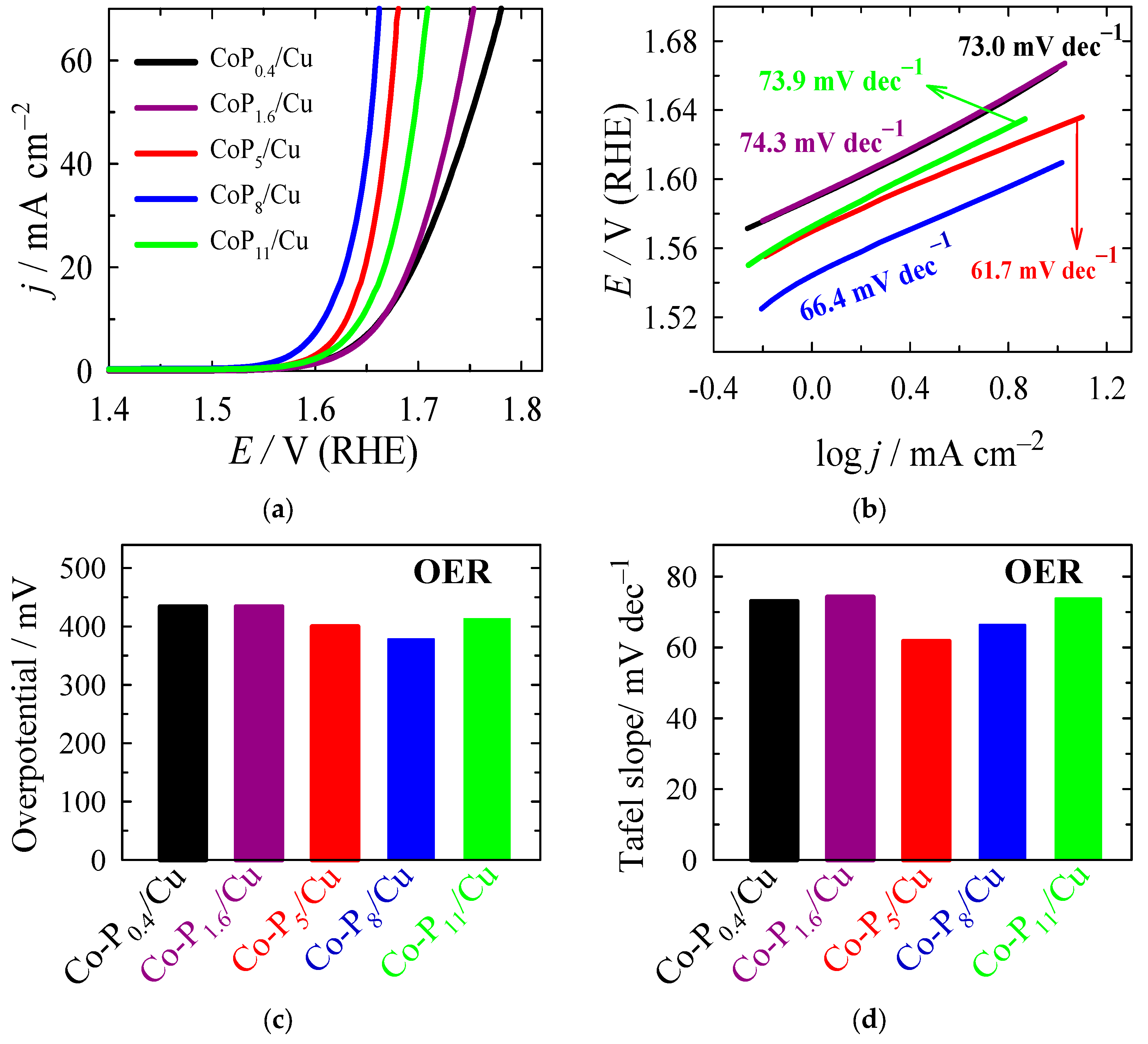
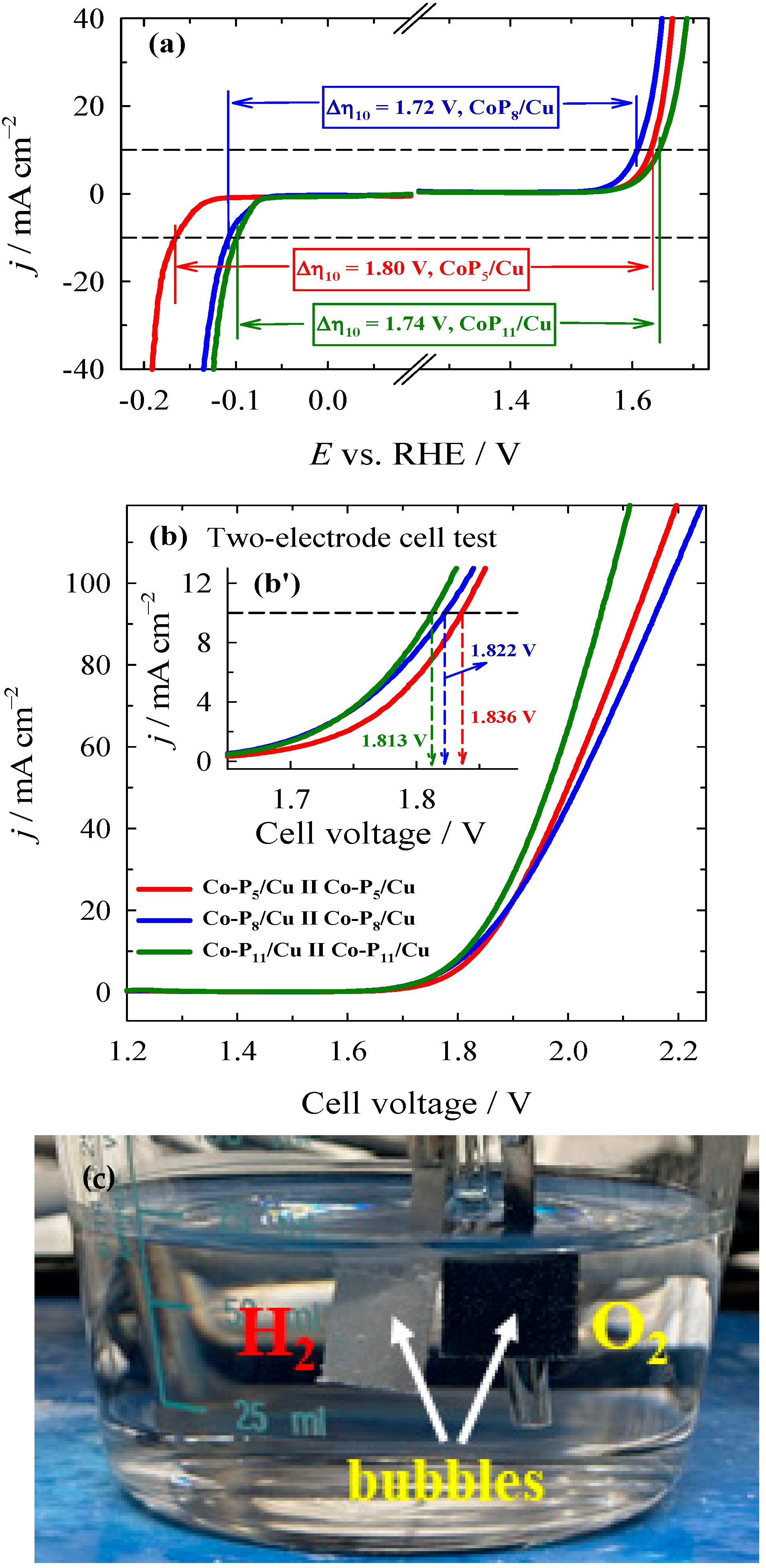
2.3. Electrocatalytic Activity Towards OER
3. Materials and Methods
3.1. Chemical Reagents
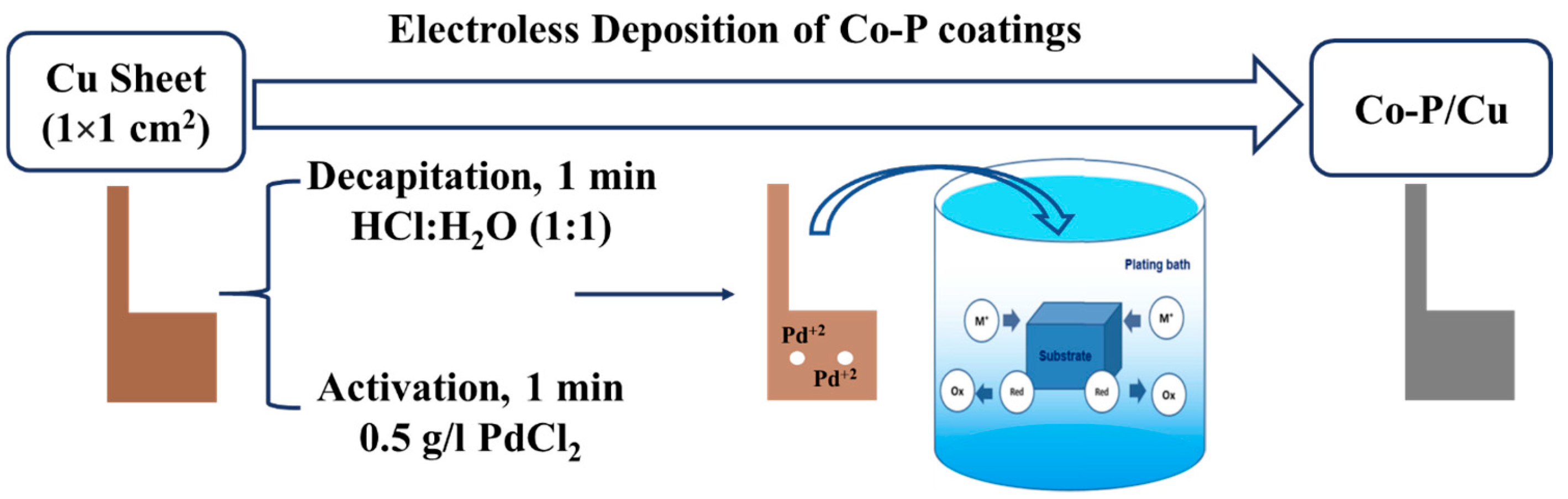
3.2. Preparation of Co-P/Cu Catalysts
3.3. Characterization of Catalysts
3.4. Evaluation of Catalysts Activity for HER and OER
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zai, S.F.; Zhou, Y.T.; Yang, C.C.; Jiang, Q. Al, Fe-codoped CoP nanoparticles anchored on reduced graphene oxide as bifunctional catalysts to enhance overall water splitting. Chem. Eng. J. 2021, 421, 127856. [Google Scholar] [CrossRef]
- Liu, M.; Li, J. Cobalt phosphide hollow polyhedron as efficient bifunctional electrocatalysts for the evolution reaction of hydrogen and oxygen. ACS Appl. Mater. Interfaces 2016, 8, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Pitchai, C.; Vedanarayanan, M.; Gopalakrishnan, S.M. Efficient hydrogen evolution electrocatalysis using nitrogen doped carbon dot decorated palladium copper nanocomposites in acid medium. New J. Chem. 2023, 47, 14355–14363. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An overview of hydrogen production: Current status, potential, and challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, J.; Lim, J.H.; Yoo, B. Cobalt iron-phosphorus synthesized by electrodeposition as highly active and stable bifunctional catalyst for full water splitting. J. Electrochem. Soc. 2018, 165, H271. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, L.; Liu, J. Recent advances in cobalt-based electrocatalysts for hydrogen and oxygen evolution reactions. J. Alloys Compd. 2020, 821, 153542. [Google Scholar] [CrossRef]
- Vilekar, S.A.; Fishtik, I.; Datta, R. Kinetics of the hydrogen electrode reaction. J. Electrochem. Soc. 2010, 157, B1040. [Google Scholar] [CrossRef]
- Li, X.; Hao, X.; Abudula, A.; Guan, G. Nanostructured catalysts for electrochemical water splitting: Current state and prospects. J. Mater. Chem. A 2016, 4, 11973–12000. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.B.; Nørskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Yan, Z.; Chen, X.; Jiao, L.; Cheng, F.; Chen, J. Superhydrophilic amorphous Co–B–P nanosheet electrocatalysts with Pt-like activity and durability for the hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 22062–22069. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, X. Nonprecious metal phosphides as catalysts for hydrogen evolution, oxygen reduction and evolution reactions. Catal. Sci. Technol. 2017, 7, 3676–3691. [Google Scholar] [CrossRef]
- Bose, R.; Jothi, V.R.; Karuppasamy, K.; Alfantazi, A.; Yi, S.C. High performance multicomponent bifunctional catalysts for overall water splitting. J. Mater. Chem. A 2020, 8, 13795–13805. [Google Scholar] [CrossRef]
- Tang, C.; Asiri, A.M.; Luo, Y.; Sun, X. Electrodeposited Ni-P alloy nanoparticle films for efficiently catalyzing hydrogen- and oxygen-evolution reactions. ChemNanoMat 2015, 1, 558–561. [Google Scholar] [CrossRef]
- Jiang, N.; You, B.; Sheng, M.; Sun, Y. Electrodeposited Cobalt-Phosphorous-Derived Films as Competent Bifunctional Catalysts for Overall Water Splitting. Angew. Chem. Int. Ed. 2015, 54, 6251–6254. [Google Scholar] [CrossRef]
- Huang, G.; Liang, W.; Wu, Y.; Li, J.; Jin, Y.Q.; Zeng, H.; Zhang, H.; Xie, F.; Chen, J.; Wang, N.; et al. Co2P/CoP hybrid as a reversible electrocatalyst for hydrogen oxidation/evolution reactions in alkaline medium. J. Catal. 2020, 390, 23–29. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, X.; Yan, H.; Chen, C.; Zhang, D.; Sun, D.; Xu, X. The defect-rich porous CoP4/Co4S3 microcubes as robust electrocatalyst for clean H2 energy production via alkaline overall water splitting. Appl. Catal. A-Gen. 2023, 668, 119461. [Google Scholar] [CrossRef]
- Zhao, S.; Li, C.; Huang, H.; Liu, Y.; Kang, Z. Carbon n+anodots modified cobalt phosphate as efficient electrocatalyst for water oxidation. J. Mater. 2015, 1, 236–244. [Google Scholar]
- Ashraf, M.A.; Li, C.; Pham, B.T.; Zhang, D. Electrodeposition of Ni–Fe–Mn ternary nanosheets as affordable and efficient electrocatalyst for both hydrogen and oxygen evolution reactions. Int. J. Hydrogen Energy 2020, 45, 24670–24683. [Google Scholar] [CrossRef]
- Verma, J.; Goel, S. Cost-effective electrocatalysts for hydrogen evolution reactions (HER): Challenges and prospects. Int. J. Hydrogen Energy 2022, 47, 38964–38982. [Google Scholar] [CrossRef]
- Xie, L.; Qu, F.; Liu, Z.; Ren, X.; Hao, S.; Ge, R.; Du, G.; Asiri, A.M.; Sun, X.; Chen, L. In situ formation of a 3D core/shell structured Ni3N@ Ni–Bi nanosheet array: An efficient non-noble-metal bifunctional electrocatalyst toward full water splitting under near-neutral conditions. J. Mater. Chem. A 2017, 5, 7806–7810. [Google Scholar] [CrossRef]
- Ma, J.; Cai, A.; Guan, X.; Li, K.; Peng, W.; Fan, X.; Zhang, G.; Zhang, F.; Li, Y. Preparation of ultrathin molybdenum disulfide dispersed on graphene via cobalt doping: A bifunctional catalyst for hydrogen and oxygen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 9583–9591. [Google Scholar] [CrossRef]
- Jiao, L.; Zhou, Y.X.; Jiang, H.L. Metal-organic framework-based CoP/reduced graphene oxide: High-performance bifunctional electrocatalyst for overall water splitting. Chem. Sci. 2016, 7, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dong, J.; You, B.; Sun, Y. Competent overall water-splitting electrocatalysts derived from ZIF-67 grown on carbon cloth. RSC Adv. 2016, 6, 73336–73342. [Google Scholar] [CrossRef]
- Chen, G.F.; Ma, T.Y.; Liu, Z.Q.; Li, N.; Su, Y.Z.; Davey, K.; Qiao, S.Z. Efficient and stable bifunctional electrocatalysts Ni/NixMy (M= P, S) for overall water splitting. Adv. Funct. Mater. 2016, 26, 3314–3323. [Google Scholar] [CrossRef]
- Wang, J.; Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Sun, X. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, D.; Luo, J.; Xu, W.; Li, J.; Li, S.; Cheng, P.; Yuan, D. The urchin-like sphere arrays Co3O4 as a bifunctional catalyst for hydrogen evolution reaction and oxygen evolution reaction. J. Power Sources 2017, 341, 250–256. [Google Scholar] [CrossRef]
- Ji, L.; Wang, J.; Teng, X.; Meyer, T.J.; Chen, Z. CoP nanoframes as bifunctional electrocatalysts for efficient overall water splitting. ACS Catal. 2019, 10, 412–419. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Hu, F.; Wang, Q. Urchin-like CoP nanocrystals as hydrogen evolution reaction and oxygen reduction reaction dual-electrocatalyst with superior stability. Nano Lett. 2015, 15, 7616–7620. [Google Scholar] [CrossRef]
- Hu, G.; Tang, Q.; Jiang, D.E. CoP for hydrogen evolution: Implications from hydrogen adsorption. Phys. Chem. Chem. Phys. 2016, 18, 23864–23871. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, Y.; Lin, J.; Wang, X.; Shen, Z. A hierarchical MoP nanoflake array supported on Ni foam: A bifunctional electrocatalyst for overall water splitting. Small Methods 2018, 2, 1700369. [Google Scholar] [CrossRef]
- Qin, J.F.; Lin, J.H.; Chen, T.S.; Liu, D.P.; Xie, J.Y.; Guo, B.Y.; Wang, L.; Chai, Y.M.; Dong, B. Facile synthesis of V-doped CoP nanoparticles as bifunctional electrocatalyst for efficient water splitting. J. Energy Chem. 2019, 39, 182–187. [Google Scholar] [CrossRef]
- Guan, C.; Xiao, W.; Wu, H.; Liu, X.; Zang, W.; Zhang, H.; Ding, J.; Feng, Y.P.; Pennycook, S.J.; Wang, J. Hollow Mo-doped CoP nanoarrays for efficient overall water splitting. Nano Energy 2018, 48, 73–80. [Google Scholar] [CrossRef]
- Jiang, N.; You, B.; Sheng, M.; Sun, Y. Electrodeposited cobalt-phosphorous-derived films as competent bifunctional catalysts for overall water splitting. Angew. Chem. 2015, 127, 6349–6352. [Google Scholar] [CrossRef]
- Yang, Y.; Fei, H.; Ruan, G.; Tour, J.M. Porous cobalt-based thin film as a bifunctional catalyst for hydrogen generation and oxygen generation. Adv. Mater. 2015, 27, 3175–3180. [Google Scholar] [CrossRef] [PubMed]
- Masa, J.; Weide, P.; Peeters, D.; Sinev, I.; Xia, W.; Sun, Z.; Somsen, C.; Muhler, M.; Schuhmann, W. Amorphous cobalt boride (Co2B) as a highly efficient nonprecious catalyst for electrochemical water splitting: Oxygen and hydrogen evolution. Adv. Energy Mater. 2016, 6, 1502313. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Qian, Q.; Wang, G.; Zhang, G. Supramolecular assisted one-pot synthesis of donut-shaped CoP@PNC hybrid nanostructures as multifunctional electrocatalysts for rechargeable Zn-air batteries and self-powered hydrogen production. Energy Storage Mater. 2020, 28, 27–36. [Google Scholar] [CrossRef]
- Chang, J.; Lv, Q.; Li, G.; Ge, J.; Liu, C.; Xing, W. Core-shell structured Ni12P5/Ni3(PO4)2 hollow spheres as difunctional and efficient electrocatalysts for overall water electrolysis. Appl. Catal. B Environ. 2017, 204, 486–496. [Google Scholar] [CrossRef]
- Gong, Y.; Yao, J.; Wang, P.; Li, Z.; Zhou, H.; Xu, C. Perspective of hydrogen energy and recent progress in electrocatalytic water splitting. Chin. J. Chem. Eng. 2022, 43, 282–296. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Jiao, F. Ordered mesoporous cobalt oxide as highly efficient oxygen evolution catalyst. J. Am. Chem. Soc. 2013, 135, 4516–4521. [Google Scholar] [CrossRef]
- Ma, J.; Wei, H.; Liu, Y.; Ren, X.; Li, Y.; Wang, F.; Han, X.; Xu, E.; Cao, X.; Wang, G.; et al. Application of Co3O4-based materials in electrocatalytic hydrogen evolution reaction: A review. Int. J. Hydrogen Energy 2020, 45, 21205–21220. [Google Scholar] [CrossRef]
- Niyitanga, T.; Kim, H. Time-dependent oxidation of graphite and cobalt oxide nanoparticles as electrocatalysts for the oxygen evolution reaction. J. Electroanal. Chem. 2022, 914, 116297. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, T.; Jiang, K.; Da, P.; Peng, Z.; Tang, J.; Kong, B.; Cai, W.B.; Yang, Z.; Zheng, G. Reduced mesoporous Co3O4 nanowires as efficient water oxidation electrocatalysts and supercapacitor electrodes. Adv. Energy Mater. 2014, 4, 1400696. [Google Scholar] [CrossRef]
- Chang, Y.; Shi, N.E.; Zhao, S.; Xu, D.; Liu, C.; Tang, Y.J.; Dai, Z.; Lan, Y.Q.; Han, M.; Bao, J. Coralloid Co2P2O7 nanocrystals encapsulated by thin carbon shells for enhanced electrochemical water oxidation. ACS Appl. Mater. Interfaces 2016, 8, 22534–22544. [Google Scholar] [CrossRef] [PubMed]
- Al-Naggar, A.H.; Shinde, N.M.; Kim, J.S.; Mane, R.S. Water splitting performance of metal and non-metal-doped transition metal oxide electrocatalysts. Coord. Chem. Rev. 2023, 474, 214864. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, Z.; Zhang, P.; Ma, H.; Gao, Z.; Wang, H.; Lu, Y.; He, J.; Zhao, Y. Transition metal ions regulated oxygen evolution reaction performance of Ni-based hydroxides hierarchical nanoarrays. Sci. Rep. 2017, 7, 46154. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Hähnel, A.; Naumann, V.; Lin, C.; Azimi, S.; Schweizer, S.L.; Maijenburg, A.W.; Wehrspohn, R.B. Bifunctional heterostructure assembly of NiFe LDH nanosheets on NiCoP nanowires for highly efficient and stable overall water splitting. Adv. Funct. Mater. 2018, 28, 1706847. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Hu, Y.; Huang, Z.; Guo, L. Co3(OH)2(HPO4)2 as a novel photocatalyst for O2 evolution under visible-light irradiation. Catal. Sci. Technol. 2016, 6, 8080–8088. [Google Scholar] [CrossRef]
- Ding, C.; Yu, Y.; Wang, Y.; Mu, Y.; Dong, X.; Meng, C.; Huang, C.; Zhang, Y. Phosphate-modified cobalt silicate hydroxide with improved oxygen evolution reaction. J. Colloid. Interface Sci. 2023, 648, 251–258. [Google Scholar] [CrossRef]
- Faber, M.S.; Dziedzic, R.; Lukowski, M.A.; Kaiser, N.S.; Ding, Q.; Jin, S. High-performance electrocatalysis using metallic cobalt pyrite (CoS2) micro-and nanostructures. J. Am. Chem. Soc. 2014, 136, 10053–10061. [Google Scholar] [CrossRef]
- Guo, M.; Liu, Y.; Dong, S.; Jiao, X.; Wang, T.; Chen, D. Co9S8-catalyzed growth of thin-walled graphite microtubes for robust, efficient overall water splitting. ChemSusChem 2018, 11, 4150–4155. [Google Scholar] [CrossRef]
- Popczun, E.J.; Read, C.G.; Roske, C.W.; Lewis, N.S.; Schaak, R.E. Highly active electrocatalysis of the hydrogen evolution reaction by cobalt phosphide nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 5427–5430. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, J.; Xiong, D.; Zhang, B.; Liu, Y.; Wu, K.H.; Amorim, I.; Li, W.; Liu, L. Trends in activity for the oxygen evolution reaction on transition metal (M = Fe, Co, Ni) phosphide pre-catalysts. Chem. Sci. 2018, 9, 3470–3476. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, S.; Karthick, K.; Sam Sankar, S.; Karmakar, A.; Madhu, R.; Bera, K.; Kundu, S. Recent advances in engineering of Ni and Co based phosphides for effective electrocatalytic water splitting. ChemElectroChem 2021, 8, 4638–4685. [Google Scholar] [CrossRef]
- Guo, P.; Wu, J.; Li, X.-B.; Luo, J.; Lau, W.-M.; Liu, H.; Sun, X.-L.; Liu, L.-M. A highly stable bifunctional catalyst based on 3D Co(OH)2@NCNTs@NF towards overall water-splitting. Nano Energy 2018, 47, 96–104. [Google Scholar] [CrossRef]
- Ha, D.H.; Han, B.; Risch, M.; Giordano, L.; Yao, K.P.; Karayaylali, P.; Shao-Horn, Y. Activity and stability of cobalt phosphides for hydrogen evolution upon water splitting. Nano Energy 2016, 29, 37–45. [Google Scholar] [CrossRef]
- Liu, T.; Xie, L.; Yang, J.; Kong, R.; Du, G.; Asiri, A.M.; Sun, X.; Chen, L. Self-standing CoP nanosheets array: A three-dimensional bifunctional catalyst electrode for overall water splitting in both neutral and alkaline media. ChemElectroChem 2017, 4, 1840–1845. [Google Scholar] [CrossRef]
- Li, Z.; Sui, J.; Zhang, Q.; Yu, J.; Yu, L.; Dong, L. CoP@NC electrocatalyst promotes hydrogen and oxygen productions for overall water splitting in alkaline media. Int. J. Hydrogen Energy 2021, 46, 2095–2102. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, M.; Cai, M.; Teng, J.; Huang, H.; Fan, Y.; Barboiu, M.; Wang, D.; Su, C.Y. Hollow cobalt phosphide with N-doped carbon skeleton as bifunctional electrocatalyst for overall water splitting. Inorg. Chem. 2019, 58, 14652–14659. [Google Scholar] [CrossRef]
- Beltrán-Suito, R.; Menezes, P.W.; Driess, M. Amorphous outperforms crystalline nanomaterials: Surface modifications of molecularly derived CoP electro (pre) catalysts for efficient water-splitting. J. Mater. Chem. A 2019, 7, 15749–15756. [Google Scholar] [CrossRef]
- Yu, C.; Xu, F.; Luo, L.; Abbo, H.S.; Titinchi, S.J.; Shen, P.K.; Tsiakaras, P.; Yin, S. Bimetallic Ni–Co phosphide nanosheets self-supported on nickel foam as high-performance electrocatalyst for hydrogen evolution reaction. Electrochim. Acta. 2019, 317, 191–198. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Q.; Asiri, A.M.; Sun, X.; Luo, Y. Self-supported FeP nanorod arrays: A cost-effective 3D hydrogen evolution cathode with high catalytic activity. ACS Catal. 2014, 4, 4065–4069. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, R.; Lu, W.; He, L.; Jiang, X.; Asiri, A.M.; Sun, X. Fe-doped CoP nanoarray: A monolithic multifunctional catalyst for highly efficient hydrogen generation. Adv. Mater. 2017, 29, 1602441. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Liang, L.; Li, C.; Wang, M.; Ge, J.; Liu, C.; Xing, W. Ultrathin cobalt phosphide nanosheets as efficient bifunctional catalysts for a water electrolysis cell and the origin for cell performance degradation. Green Chem. 2016, 18, 2287–2295. [Google Scholar] [CrossRef]
- Anjum, M.A.R.; Okyay, M.S.; Kim, M.; Lee, M.H.; Park, N.; Lee, J.S. Bifunctional sulfur-doped cobalt phosphide electrocatalyst outperforms all-noble-metal electrocatalysts in alkaline electrolyzer for overall water splitting. Nano Energy 2018, 53, 286–295. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Liu, Y.P.; Ren, T.Z.; Yuan, Z.Y. Self-supported cobalt phosphide mesoporous nanorod arrays: A flexible and bifunctional electrode for highly active electrocatalytic water reduction and oxidation. Adv. Funct. Mater. 2015, 25, 7337–7347. [Google Scholar] [CrossRef]
- Tabassum, H.; Guo, W.; Meng, W.; Mahmood, A.; Zhao, R.; Wang, Q.; Zou, R. Metal-organic frameworks derived cobalt phosphide architecture encapsulated into B/N Co-doped graphene nanotubes for all pH value electrochemical hydrogen evolution. Adv. Energy Mater. 2017, 7, 601671. [Google Scholar] [CrossRef]
- Vigil, J.A.; Lambert, T.N.; Christensen, B.T. Cobalt phosphide-based nanoparticles as bifunctional electrocatalysts for alkaline water splitting. J. Mater. Chem. A 2016, 4, 7549–7554. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Fan, Q.; Zhang, F.; Xu, S. Hierarchically scaffolded CoP/CoP2 nanoparticles: Controllable synthesis and their application as a well-matched bifunctional electrocatalyst for overall water splitting. Nanoscale 2017, 9, 5677–5685. [Google Scholar] [CrossRef]
- Zhang, M.; Ci, S.; Li, H.; Cai, P.; Xu, H.; Wen, Z. Highly defective porous CoP nanowire as electrocatalyst for full water splitting. Int. J. Hydrogen Energy 2017, 42, 29080–29090. [Google Scholar] [CrossRef]
- Xu, K.; Ding, H.; Zhang, M.; Chen, M.; Hao, Z.; Zhang, L.; Wu, C.; Xie, Y. Regulating water-reduction kinetics in cobalt phosphide for enhancing HER catalytic activity in alkaline solution. Adv. Mater. 2017, 29, 1606980. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, X.Y.; Paik, U. Nickel cobalt phosphides quasi-hollow nanocubes as an efficient electrocatalyst for hydrogen evolution in alkaline solution. Chem. Comm. 2016, 52, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Xiao, Y.; Xiao, M.; Ge, J.; Liu, C.; Han, X.; Zhong, C.; Hu, W.; Xing, W. Surface oxidized cobalt-phosphide nanorods as an advanced oxygen evolution catalyst in alkaline solution. ACS Catal. 2015, 5, 6874–6878. [Google Scholar] [CrossRef]
- Song, M.; He, Y.; Zhang, M.; Zheng, X.; Wang, Y.; Zhang, J.; Han, X.; Zhong, C.; Hu, W.; Deng, Y. Controllable synthesis of Co2P nanorods as high-efficiency bifunctional electrocatalyst for overall water splitting. J. Power Sources 2018, 402, 345–352. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yu, L.; Lou, X.W. General synthesis of multishell mixed-metal oxyphosphide particles with enhanced electrocatalytic activity in the oxygen evolution reaction. Angew. Chem. Int. Ed. 2017, 56, 2386–2389. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yu, X.Y.; Lou, X.W. Carbon-incorporated nickel–cobalt mixed metal phosphide nanoboxes with enhanced electrocatalytic activity for oxygen evolution. Angew. Chem. Int. Ed. 2017, 56, 3897–3900. [Google Scholar] [CrossRef]
- Zhou, G.; Li, M.; Li, Y.; Dong, H.; Sun, D.; Liu, X.; Xu, L.; Tian, Z.; Tang, Y. Regulating the electronic structure of CoP nanosheets by O incorporation for high-efficiency electrochemical overall water splitting. Adv. Funct. Mater. 2020, 30, 1905252. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, N.; Ge, R.; Liu, J.; Li, W.; Chen, Y.; Feng, L.; Che, R. Porous Mn-doped cobalt phosphide nanosheets as highly active electrocatalysts for oxygen evolution reaction. Chem. Eng. J. 2021, 425, 131642. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.; Li, T.; Chen, W.; Chen, G.; Han, L.; Ostrikov, K.K. High-efficiency oxygen evolution catalyzed by Sn–Co–Ni phosphide with oriented crystal phases. J. of Mater. Chem. A. 2022, 10, 13448–13455. [Google Scholar] [CrossRef]
- Yu, X.Y.; Feng, Y.; Guan, B.; Lou, X.W.D.; Paik, U. Carbon coated porous nickel phosphides nanoplates for highly efficient oxygen evolution reaction. Energy Environ. Sci. 2016, 9, 1246–1250. [Google Scholar] [CrossRef]
- Chen, P.; Xu, K.; Fang, Z.; Tong, Y.; Wu, J.; Lu, X.; Peng, X.; Ding, H.; Wu, C.; Xie, Y. Metallic Co4N porous nanowire arrays activated by surface oxidation as electrocatalysts for the oxygen evolution reaction. Angew. Chem. 2015, 127, 14923–14927. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, B.; Xu, J.; Jia, G.; Chen, S.; Rawat, R.S.; Fan, H.J. Rapid synthesis of cobalt nitride nanowires: Highly efficient and low-cost catalysts for oxygen evolution. Angew. Chem. 2016, 128, 8812–8816. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Sci. 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Cha, J.J.; Wang, H.; Lee, H.R.; Cui, Y. First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Bai, C.; Wei, S.; Deng, D.; Lin, X.; Zheng, M.; Dong, Q. A nitrogen-doped nano carbon dodecahedron with Co@ Co3O4 implants as a bi-functional electrocatalyst for efficient overall water splitting. J. Mater. Chem. A 2017, 5, 9533–9536. [Google Scholar] [CrossRef]
- Chen, X.; Zhen, X.; Gong, H.; Li, L.; Xiao, J.; Xu, Z.; Yan, D.; Xiao, G.; Yang, R. Cobalt and nitrogen codoped porous carbon as superior bifunctional electrocatalyst for oxygen reduction and hydrogen evolution reaction in alkaline medium. Chin. Chem. Lett. 2019, 30, 681–685. [Google Scholar] [CrossRef]
- Li, X.; Zhang, R.; Luo, Y.; Liu, Q.; Lu, S.; Chen, G.; Gao, S.; Chen, S.; Sun, X. A cobalt–phosphorus nanoparticle decorated N-doped carbon nanosheet array for efficient and durable hydrogen evolution at alkaline pH. Sustain. Energy Fuels 2020, 4, 3884–3887. [Google Scholar] [CrossRef]
- Liu, M.R.; Hong, Q.L.; Li, Q.H.; Du, Y.; Zhang, H.X.; Chen, S.; Zhou, T.; Zhang, J. Cobalt boron imidazolate framework derived cobalt nanoparticles encapsulated in B/N codoped nanocarbon as efficient bifunctional electrocatalysts for overall water splitting. Adv. Funct. Mater. 2018, 28, 1801136. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, S.; Li, C.; Zhu, C.; Chen, Y.; Gao, P.; Qi, L.; Zhang, X. Hollow CoP nanopaticle/N-doped graphene hybrids as highly active and stable bifunctional catalysts for full water splitting. Nanoscale 2016, 8, 10902–10907. [Google Scholar] [CrossRef]
- Peng, Z.; Yu, Y.; Jiang, D.; Wu, Y.; Xia, B.Y.; Dong, Z. N-doped carbon shell coated CoP nanocrystals encapsulated in porous N-doped carbon substrate as efficient electrocatalyst of water splitting. Carbon 2019, 144, 464–471. [Google Scholar] [CrossRef]
- Ren, A.; Yu, B.; Huang, M.; Liu, Z. Encapsulation of cobalt prussian blue analogue-derived ultra-small CoP nanoparticles in electrospun N-doped porous carbon nanofibers as an efficient bifunctional electrocatalyst for water splitting. Int. J. Hydrogen Energy 2024, 51, 490–502. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Ni, B.J. Cost-effective catalysts for renewable hydrogen production via electrochemical water splitting: Recent advances. Curr. Opin. Green Sustain. Chem. 2021, 27, 100398. [Google Scholar] [CrossRef]
- Liu, C.; Su, F.; Liang, J. Producing cobalt–graphene composite coating by pulse electrodeposition with excellent wear and corrosion resistance. Appl. Surf. Sci. 2015, 351, 889–896. [Google Scholar] [CrossRef]
- Kouotou, P.M.; Tian, Z.Y. CVD synthesis of cobalt spinel for bio-butanol combustion. Surf. Coat. Technol. 2017, 326, 11–17. [Google Scholar] [CrossRef]
- Pandey, N.; Gupta, M.; Gupta, R.; Chakravarty, S.; Shukla, N.; Devishvili, A. Structural and magnetic properties of Co-N thin films deposited using magnetron sputtering at 523 K. J. Alloys Compd. 2017, 694, 1209–1213. [Google Scholar] [CrossRef]
- Cho, J.; Moon, J.; Jeong, K.; Cho, G. Application of PU-sealing into Cu/Ni electroless plated polyester fabrics for e-textiles. Fiber. Polym. 2007, 8, 330–334. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Zhou, Y.; Liang, A.; Zhang, J. Aluminum-induced direct electroless deposition of Co and Co-P coatings on copper and their catalytic performance for electrochemical water splitting. Surf. Coat. Technol. 2018, 352, 42–48. [Google Scholar] [CrossRef]
- Brenner, A.; Riddell, G.E. Deposition of nickel and cobalt by chemical reduction. J. Res. Natl. Bur. Stand. 1947, 39, 385–395. [Google Scholar] [CrossRef]
- Stankevičienė, I.; Jagminiene, A.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Electroless Co-B deposition using dimethylamine borane as reducing agent in the presence of different amines. ECS Trans. 2015, 64, 17. [Google Scholar] [CrossRef]
- Hao, W.; Huang, H.; Chen, Z.; Wang, L.; Ma, X.; Huang, M.; Ou, X.; Guo, Y. Electroless plating-induced morphology self-assembly of free-standing Co–P–B enabling efficient overall water splitting. Electrochim. Acta. 2020, 354, 136645. [Google Scholar] [CrossRef]
- Yu, Y.; Song, Z.; Ge, H.; Wei, G. Preparation of CoP films by ultrasonic electroless deposition at low initial temperature. Prog. Nat. Sci. Mater. Int. 2014, 24, 232–238. [Google Scholar] [CrossRef]
- Vitry, V.; Bonin, L. Increase of boron content in electroless nickel-boron coating by modification of plating conditions. Surf. Coat. Technol. 2017, 311, 164–171. [Google Scholar] [CrossRef]
- Liang, M.W.; Yen, H.T.; Hsieh, T.E. Investigation of electroless cobalt-phosphorous layer and its diffusion barrier properties of Pb-Sn solder. J. Electron. Mater. 2006, 35, 1593–1599. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Cheng, C. Ru-based electrocatalysts for hydrogen evolution reaction: Recent research advances and perspectives. Mater. Today Phys. 2020, 15, 100274. [Google Scholar] [CrossRef]
- Cossar, E.; Houache, M.S.E.; Zhang, Z.; Baranova, E.A. Comparison of electrochemical active surface area methods for various nickel nanostructures. J. Electroanal. Chem. 2020, 870, 114246. [Google Scholar] [CrossRef]
- Katkar, P.K.; Marje, S.J.; Kale, S.B.; Lokhande, A.C.; Lokhande, C.D.; Patil, U.M. Synthesis of hydrous cobalt phosphate electro-catalysts by a facile hydrothermal method for enhanced oxygen evolution reaction: Effect of urea variation. CrystEngComm. 2019, 21, 884–893. [Google Scholar] [CrossRef]
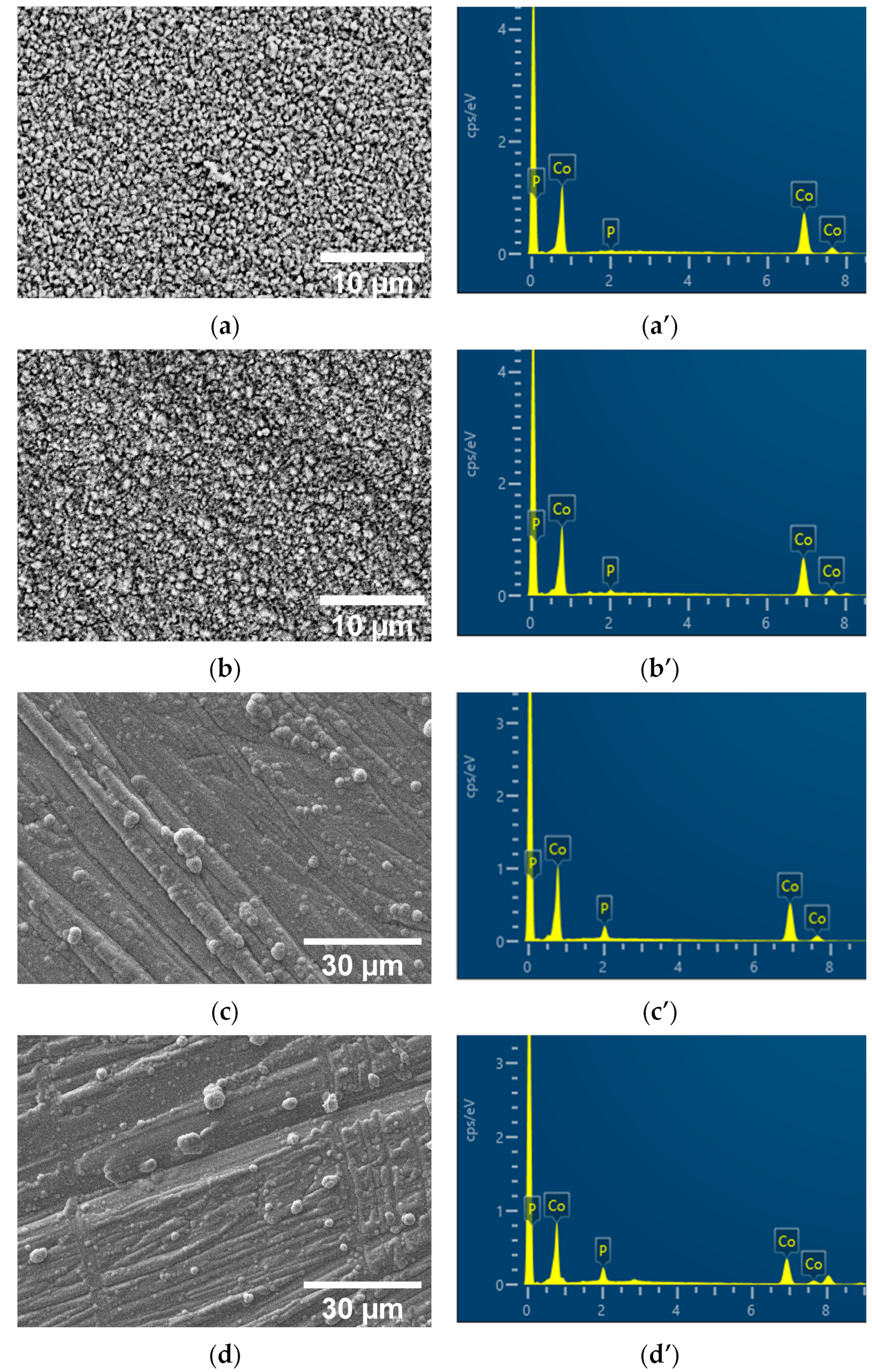



| Sample | Element, wt% | |
|---|---|---|
| Co | P | |
| CoP0.4/Cu | 99.62 | 0.38 |
| CoP1.6/Cu | 98.45 | 1.55 |
| CoP5/Cu | 95.13 | 4.87 |
| CoP8/Cu | 92.02 | 7.98 |
| CoP11/Cu | 88.87 | 11.13 |
| Sample | Eonset, V at j = −1 mA cm−2 | η10 *, mV | Tafel Slope, mV dec−1 |
|---|---|---|---|
| Co-P0.4/Cu | −0.210 | 253.9 | 50.3 |
| Co-P1.6/Cu | −0.186 | 218.2 | 43.9 |
| Co-P5/Cu | −0.114 | 165.9 | 48.1 |
| Co-P8/Cu | −0.071 | 107.6 | 34.1 |
| Co-P11/Cu | −0.067 | 98.9 | 29.4 |
| Catalyst | η10, mV at j = −10 mA cm−2 | Tafel Slope, mV dec−1 | Electrolyte | Ref. |
|---|---|---|---|---|
| Co-P11/Cu | 98.9 | 29.4 | 1 M KOH | This work |
| Co-P8/Cu | 107.6 | 34.1 | 1 M KOH | This work |
| Ni-Co-P/NF | 85 | 46 | 1 M KOH | [60] |
| FeP NAs/CC | 218 | 146 | 1 M KOH | [61] |
| CoP Nanoparticle | 170 | 66 | 1 M KOH | [55] |
| Ni/iP | 130 | 58.5 | 1 M KOH | [29] |
| Fe–CoP/Ti | 78 | 75 | 1 M KOH | [62] |
| Co-P-B/CC MPs | 87 | 69.2 | 1 M KOH | [99] |
| MoP/NF | 114 | 54.6 | 1 M KOH | [30] |
| Co–B/NF | 103 | 98.3 | 1 M KOH | [10] |
| Co–P/NF | 65 | 59.7 | 1 M KOH | [10] |
| NiFeLDH@NiCoP/NF | 120 | 88.2 | 1 M KOH | [46] |
| NiCoP/NF | 185 | 124.4 | 1 M KOH | [46] |
| Co/NBC | 117 | 146 | 1 M KOH | [87] |
| CoP NS | 207 | 124.5 | 1 M KOH | [63] |
| CoP NS/C | 111 | 70.9 | 1 M KOH | [63] |
| Fe-codoped CoP/RGO | 145 | 60 | 1 M KOH | [1] |
| CoP/RGO | 272 | 134 | 1 M KOH | [1] |
| Al-doped CoP/RGO | 206 | 106 | 1 M KOH | [1] |
| Fe-doped CoP/RGO | 208 | 107 | 1 M KOH | [1] |
| S:CoP NPs | 175 | 71 | 1 M KOH | [64] |
| CoP NPs | 216 | 90 | 1 M KOH | [64] |
| CoP-MNA/Ni Foam | 150 | 51 | 1 M KOH | [65] |
| CoP@BCN | 215 | 52 | 1 M KOH | [66] |
| (CoxNi1-x)2P | 180 | 63 | 1 M KOH | [67] |
| f-CoP/CoP2/Al2O3 | 138 | 73 | 1 M KOH | [68] |
| Co-P/N-doped carbon matrices | 154 | 51 | 1 M KOH | [88] |
| Co-P-2 | 188 | – | 1 M KOH | [96] |
| CoP nanowires | 147 | 69 | 1 M KOH | [69] |
| Co2P | 247 | 86.3 | 1 M KOH | [70] |
| CoP hollow polyhedron | 159 | 59 | 1 M KOH | [2] |
| Ni-Co-P | 150 | 60.6 | 1 M KOH | [71] |
| V-doped CoP | 235 | 86.1 | 1 M KOH | [31] |
| CoP/PNC | 165 | 70 | 1 M KOH | [89] |
| Pt/C (20 wt% Pt/XC-72) on NF | 30 | 49 | 1 M KOH | [14] |
| Pt/C on GCE | 50 | 108 | 1 M KOH | [13] |
| Pt/C | – | 108 | 1 M KOH | [14] |
| Ni-Co-P | 44 | 52 | 1 M KOH | [15] |
| 20 wt% Pt/C | 13.5 | 58.9 | 1 M KOH | [16] |
| Sample | Eonset, V at j = 1 mA cm−2 | ηonset, mV | E, V at j = 10 mA cm−2 | η10 *, mV | Tafel Slope, mV dec−1 |
|---|---|---|---|---|---|
| Co-P0.4/Cu | 1.590 | 360 | 1.664 | 434 | 73.0 |
| Co-P1.6/Cu | 1.589 | 359 | 1.664 | 434 | 74.3 |
| Co-P5/Cu | 1.569 | 339 | 1.630 | 400 | 61.7 |
| Co-P8/Cu | 1.544 | 314 | 1.608 | 378 | 66.4 |
| Co-P11/Cu | 1.574 | 344 | 1.643 | 413 | 73.9 |
| Catalyst | η10, mV at j = 10 mA cm−2 | Tafel Slope, mV dec−1 | Electrolyte | Ref. |
|---|---|---|---|---|
| Co-P8/Cu | 378 | 66.4 | 1 M KOH | This work |
| Co-P5/Cu | 400 | 61.7 | 1 M KOH | This work |
| CoP nanoparticles | 340 | 99 | 1 M KOH | [72] |
| Co2P nanorods | 310 | 61.0 | 1 M KOH | [73] |
| CoP nanowires | 326 | 80 | 1 M KOH | [69] |
| CoP nanosheet | 361 | 69.5 | 1 M KOH | [63] |
| CoP NPs@NF | 320 | 102 | 1 M KOH | [64] |
| CoP-MNA/Ni Foam | 390 | 65 | 1 M KOH | [65] |
| CoP hollow polyhedron | 400 | 57 | 1 M KOH | [2] |
| Co-Mn oxyphosphide | 370 | 52 | 1 M KOH | [74] |
| NiCoP Nanoboxes | 370 | 115 | 1 M KOH | [75] |
| Reduced mesoporous Co3O4 nanowires | 400 | 72 | 1 M KOH | [42] |
| O-CoP/GCE | 310 | 59.9 | 1 M KOH | [76] |
| S:CoP/NF | 300 | 82 | 1 M KOH | [64] |
| Mo-CoP/CC | 305 | 56 | 1 M KOH | [32] |
| V-doped CoP/GCE | 340 | 95.7 | 1 M KOH | [31] |
| Co-P film | 350 | 47 | 1 M KOH | [33] |
| CoPi/PCDs | 350 | - | 1 M KOH | [17] |
| CL-Co2P2O7@C nanohybrids | 397 | 70 | 1 M KOH | [43] |
| Co2P2O7 nanostructure | 490 | 86 | 1 M KOH | [43] |
| Co(PO3)2 nanosheets | 574 | 106 | 1 M KOH | [43] |
| Hydrous cobalt phosphate thin films | 292 | 98 | 1 M KOH | [105] |
| Co–Fe–P–O | 267 | 30 | 1 M KOH | [47] |
| Co phosphide/Co phosphate thin film (PCPTF) | 300 | 65 | 1 M KOH | [34] |
| Ni-P | 300 | 64 | 1 M KOH | [79] |
| Co2B | 360 | 45 | 1 M KOH | [35] |
| Mn-CoP | 288 | 77.2 | 1 M KOH | [77] |
| SnPi@CoP–Ni5P4/NCF | 364 | 52 | 1 M KOH | [78] |
| CoSi-P | 309 | 121 | 1 M KOH | [48] |
| RuO2 on NF | 290 | 81 | 1 M KOH | [13] |
| RuO2/CF | 360 | 164 | 1 M KOH | [15] |
| IrO2 commercial | 339 | 94.5 | 1 M KOH | [16] |
| Ir/C | 254 | 71.9 | 1 M KOH | [17] |
| Catalyst | Cell Voltage, V | Electrolyte | Ref. |
|---|---|---|---|
| Co-P11/Cu | 1.81 | 1 M KOH | This work |
| CoP/rGO-400 | 1.70 | 1 M KOH | [22] |
| Co-P/NC/CC | 1.77 | 1 M KOH | [23] |
| Co-P/NC-CC | 1.95 | 1 M KOH | [23] |
| Co(OH)2@NCNTs@NF | 1.72 | 1 M KOH | [54] |
| Pt/C‖IrO2 | 1.71 | 1 M KOH | [56] |
| Pt/C‖Pt/C | 1.83 | 1 M KOH | [81] |
| Hydrous cobalt phosphate thin films | 1.80 | 1 M KOH | [105] |
| CoP@PNC-DoS | 1.74 | 1 M KOH | [36] |
| S:CoP NPs | 1.72 | 1 M KOH | [64] |
| Co phosphide/Co phosphate thin film (PCPTF) | 1.92 | 1 M KOH | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amber, H.; Balčiūnaitė, A.; Sukackienė, Z.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. Electrolessly Deposited Cobalt–Phosphorus Coatings for Efficient Hydrogen and Oxygen Evolution Reactions. Catalysts 2025, 15, 8. https://doi.org/10.3390/catal15010008
Amber H, Balčiūnaitė A, Sukackienė Z, Tamašauskaitė-Tamašiūnaitė L, Norkus E. Electrolessly Deposited Cobalt–Phosphorus Coatings for Efficient Hydrogen and Oxygen Evolution Reactions. Catalysts. 2025; 15(1):8. https://doi.org/10.3390/catal15010008
Chicago/Turabian StyleAmber, Huma, Aldona Balčiūnaitė, Zita Sukackienė, Loreta Tamašauskaitė-Tamašiūnaitė, and Eugenijus Norkus. 2025. "Electrolessly Deposited Cobalt–Phosphorus Coatings for Efficient Hydrogen and Oxygen Evolution Reactions" Catalysts 15, no. 1: 8. https://doi.org/10.3390/catal15010008
APA StyleAmber, H., Balčiūnaitė, A., Sukackienė, Z., Tamašauskaitė-Tamašiūnaitė, L., & Norkus, E. (2025). Electrolessly Deposited Cobalt–Phosphorus Coatings for Efficient Hydrogen and Oxygen Evolution Reactions. Catalysts, 15(1), 8. https://doi.org/10.3390/catal15010008









