Proton Exchange Membrane Fuel Cell Catalyst Layer Degradation Mechanisms: A Succinct Review
Abstract
:1. Introduction
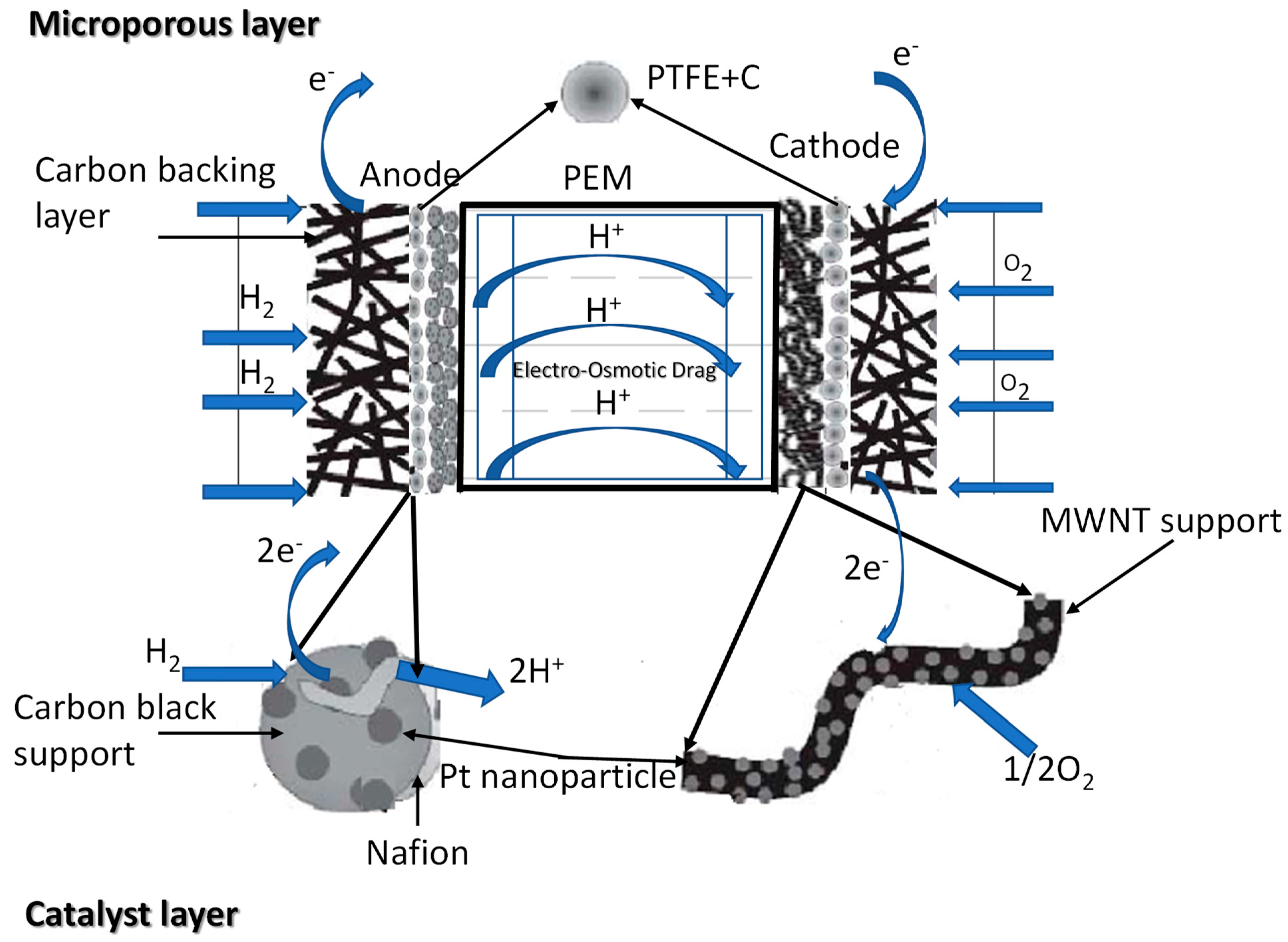
2. PEMFC Design
2.1. Basic Components of the PEMFC
2.1.1. Bipolar Plates
2.1.2. Gas Diffusion Layer
2.1.3. Membrane
2.1.4. Catalyst Layer
Carbon Support
The Ionomer
Pt-Based Catalyst
3. Operation of the PEMFC Process
4. Catalyst Layer Degradation Mechanisms
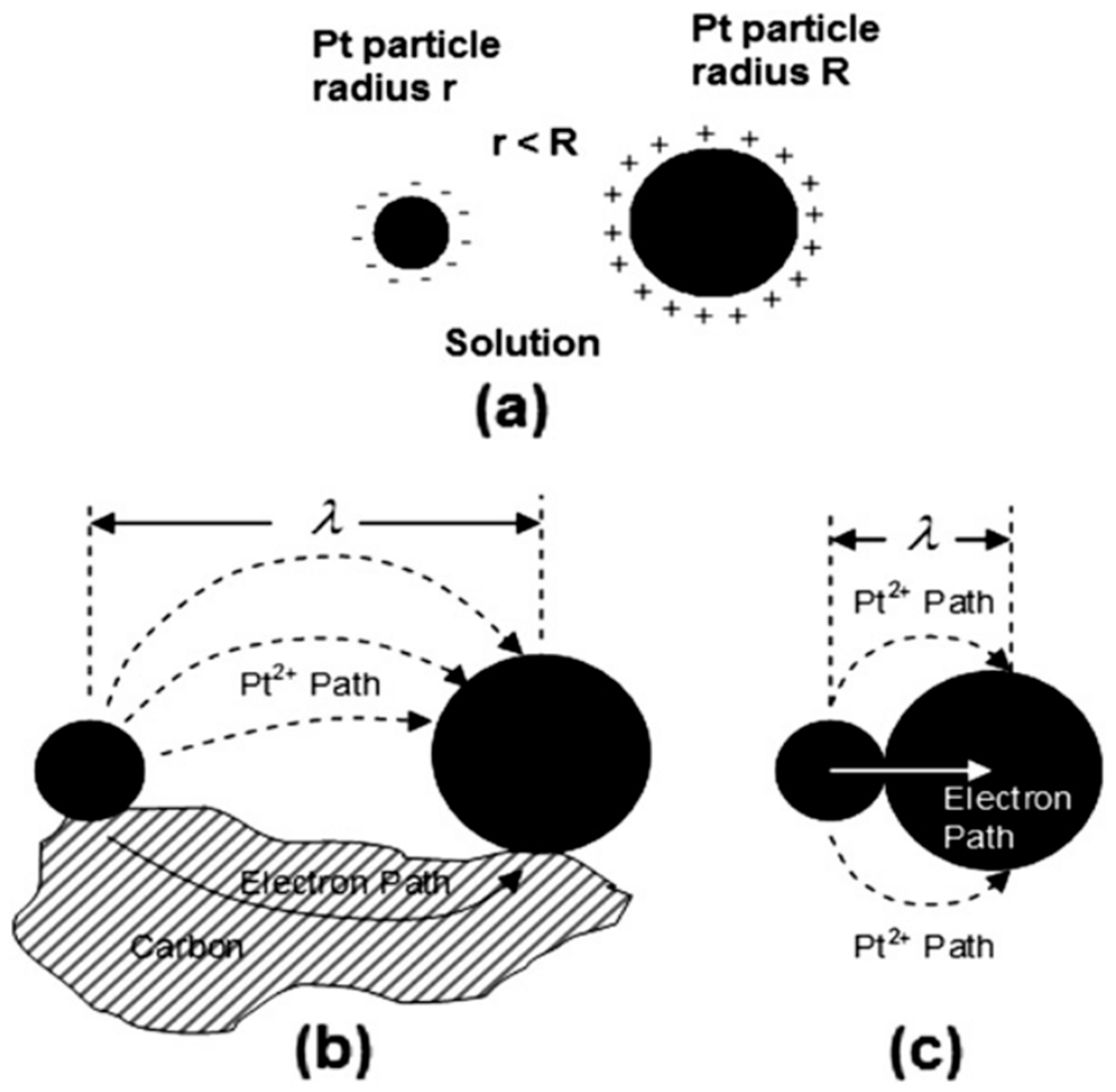
4.1. Platinum Degradation in the PEMFC
Degradation of Bimetallic Nanoparticles in PtM (M = Co, Ni, Cu) Catalysts and Comparison with Pt/C Catalysts
4.2. Carbon Support Degradation
4.3. Nafion Degradation Mechanism
5. The Sources and Effects of Contamination in PEMFC
- Types of Contaminants in PEMFCs
- Carbon monoxide: A primary contaminant in hydrogen fuel, CO can come from impurities in hydrogen during production or storage.
- Sulfur compounds such as hydrogen sulfide: Sulfur is a well-known poison for platinum-based catalysts in PEMFCs. It may be present in low concentrations in hydrogen or in trace amounts in fuel sources.
- Nitrogen compounds: Nitrogen oxides from ambient air can infiltrate the fuel cell system and poison the catalyst.
- Ammonia: Ammonia is another potential contaminant in hydrogen fuel, typically arising from industrial processes.
- Water contaminants: Impurities in water used in the fuel cell, like chlorine or chlorides, can lead to catalyst degradation.
- Catalyst poisoning: Many contaminants, especially CO and sulfur compounds, can directly poison the Pt catalyst.
- b.
- Membrane Degradation: The MEA of the PEMFC can be affected by contaminants such as ammonia and chlorine. In the process, chloride has been particularly reported to chemically degrade the fuel cell membrane, resulting in cracks and a reduction in mechanical strength of the membrane. This process generally affects the performance of the PEMFC.
5.1. Effect of Contamination on PEMFC Performance
- They reduce the power output of the PEMFC.
- They increase the internal resistance, thereby reducing the voltage and efficiency of the fuel cell.
- They increase fuel consumption by blocking the active sites on the catalyst, which necessitates the fuel to require more hydrogen to generate the required same amount of power.
- They increase fuel consumption: When contaminants block active sites on the catalyst, the fuel cell requires more hydrogen to generate the same amount of power, thereby increasing fuel consumption and reducing overall efficiency.
- They cause accelerated catalyst degradation which decreases the fuel cell lifetime.
5.2. Strategies for Mitigating Contamination
6. The Operational Parameters and Circumstances That Affect the Degrading Mechanisms in PEMFC
6.1. High Operating Temperatures
- Increased atomic mobility: Platinum is a noble metal, and while it has high thermal stability, its atoms become more mobile at elevated temperatures. When the temperature exceeds a certain threshold, Pt atoms move more freely, which can lead to migration and agglomeration into larger clusters. These larger clusters have fewer available catalytic sites, which diminishes the catalyst’s overall activity.
- Thermal sintering: At temperatures above 600 °C, Pt tends to undergo sintering, a process where smaller Pt particles combine to form larger ones. This process reduces the number of active sites and thus the efficiency of the catalyst, as larger particles are less effective in catalyzing reactions compared to small ones with a high surface area.
6.2. Reduction–Oxidation Cycles
- Reduction conditions: In catalytic applications, such as in automotive exhaust systems, redox cycling occurs—where the catalyst is exposed to alternating reducing and oxidizing conditions. Under reducing conditions (e.g., exposure to hydrogen), Pt can lose its oxygen atoms and become more mobile, leading to the migration of Pt atoms across the surface or even between the catalyst’s support and platinum particles.
- Oxidation conditions: On the other hand, oxidizing conditions (e.g., exposure to oxygen or high temperatures) can cause platinum to form platinum oxides. The instability of these oxides at high temperatures can drive Pt migration and agglomeration, especially if the oxidation–reduction cycles are frequent or severe.
6.3. High Pressure Conditions
- Pressure-induced migration: Under elevated pressure, platinum atoms may shift positions to minimize their energy state, leading to migration and potentially agglomeration if the Pt atoms encounter favorable conditions for coalescing.
- Phase transition effects: In high-pressure environments, Pt can undergo structural changes, such as shifting from a smaller nanoparticle form to larger agglomerated clusters, particularly at elevated temperatures. This can further diminish the catalyst’s surface area and its effectiveness in driving catalytic reactions.
6.4. The Pt Particle Size and Distribution
- Smaller Pt particles: Initially, platinum is often deposited as nanoparticles to maximize surface area. However, smaller platinum particles are more susceptible to agglomeration and migration under extreme conditions (high temperature, redox cycling). As small Pt particles migrate, they are more likely to combine, forming larger particles that are less active.
7. PEMFC Catalyst and Ionomer Degradation Tests
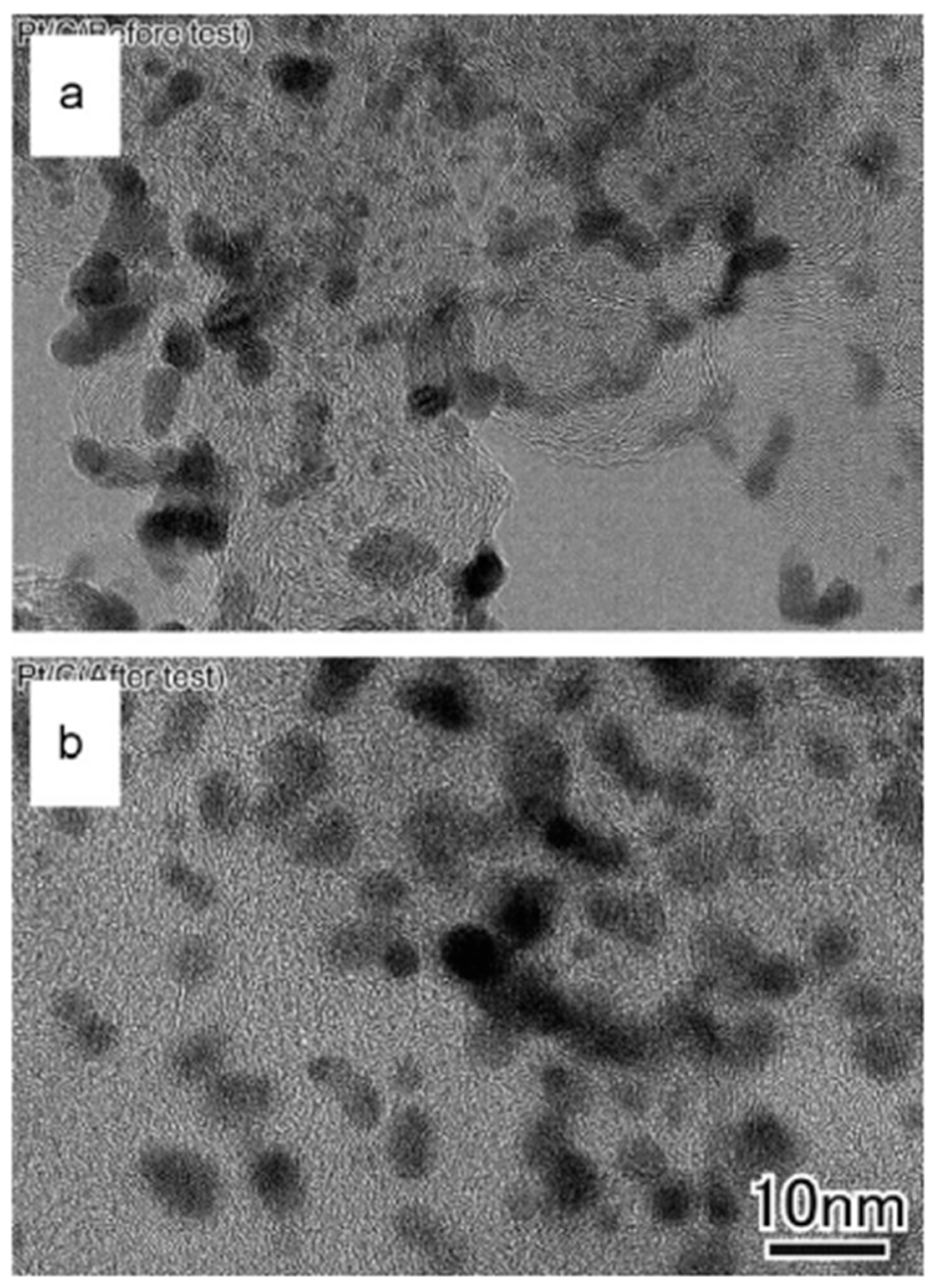
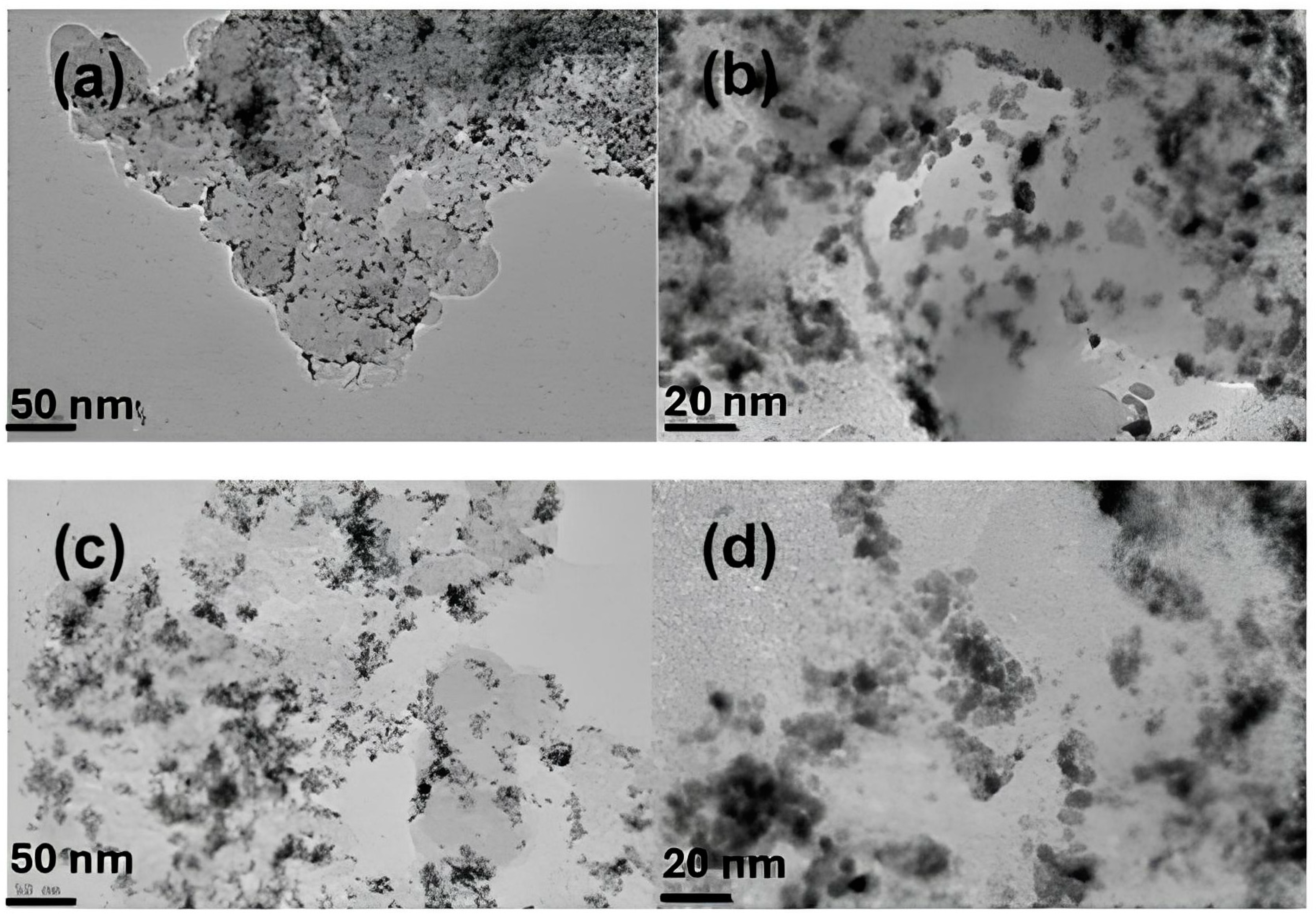
7.1. Microscopic Investigation
7.2. Microstructure of PtM (M = Co, Ni, Cu) Catalysts Before and After Testing
7.2.1. Microstructural Changes Before and After Testing
7.2.2. Structural Size and Composition Before and After Testing
Size of Nanoparticles
Composition of PtM (M = Co, Ni, Cu) Catalysts
7.3. PEMFC Catalyst and Ionomer Degradation Stress Tests for Catalyst, Membrane, and Carbon Support
7.3.1. AST Condition for Catalysts, Ionomers, Membranes, and Carbon Supports Tests
Potential Range
Time
Number of Cycles
Environmental Conditions
7.4. Catalyst and Ionomer Enhancement in the PEMFC
8. Prospect and Concluding Remarks
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Pt | Platinum |
| PEMFC | Proton exchange membrane fuel cells |
| C | Carbon |
| MEA | Membrane electrode assembly |
| CLs | Catalyst layers |
| CCM | Catalyst-coated membrane |
| MPL | Microporous layers |
| GDL | Gas diffusion layer |
| PTFE | Polytetrafluoroethylene |
| BPP | Bipolar plates |
| Vu | Vulcan |
| HSC | High-surface area |
| ROS | Reactive oxygen species |
| ORR | Oxygen reduction reaction |
| PFSA | Perfluorosulfonic acid |
| ACL | Anodic catalyst layer |
| CCL | Cathode catalyst layer |
| HOR | Hydrogen oxidation reaction |
| H2 | Hydrogen |
| O2 | Oxygen (O2) |
| CO | Carbon monoxide |
| CF | Carbon fluoride |
| OH− | Hydro-oxide ion |
| AST | Accelerated stress tests |
| ECSA | Electrochemically active surface area |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| IL-TEM | Identical location-transmission electron microscopy |
| EDX | Energy dispersive X-ray |
| Cr | Chromium |
| Co | Cobalt |
| Ni | Nickel |
| Fe | Iron |
| Pd | Palladium |
References
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Jurasz, J.; Canales, F.; Kies, A.; Guezgouz, M.; Beluco, A. A review on the complementarity of renewable energy sources: Concept, metrics, application and future research directions. Sol. Energy 2020, 195, 703–724. [Google Scholar] [CrossRef]
- Behabtu, H.A.; Messagie, M.; Coosemans, T.; Berecibar, M.; Anlay Fante, K.; Kebede, A.A.; Mierlo, J.V. A review of energy storage technologies’ application potentials in renewable energy sources grid integration. Sustainability 2020, 12, 10511. [Google Scholar] [CrossRef]
- Behl, R.; Chhibar, R.; Jain, S.; Bahl, V.; El Bassam, N. Renewable energy sources and their applications. In Proceedings of the International Conference on Renewable Energy for Institutes and Communities in Urban and Rural Settings, Jevra, India, 27–29 April 2012; pp. 27–29. [Google Scholar]
- Mansir, I.B.; Abubakar, Z.; Ali, A.; Okonkwo, P.C.; Lawal, D.U. A novel geothermal system combined with fuel cell and hydrogen generation to store clean sustainable energy storage. Process Saf. Environ. Prot. 2024, 191, 828–835. [Google Scholar] [CrossRef]
- Zghaibeh, M.; Okonkwo, P.C.; Hasan, N.U.; Farhani, S.; Bacha, F. Energy management system for photovoltaic-battery-fuel cell using arduino board and Matlab Simulink. In Proceedings of the 2022 IEEE Delhi Section Conference (DELCON), New Delhi, India, 11–13 February 2022; pp. 1–6. [Google Scholar]
- Ren, X.; Wang, Y.; Liu, A.; Zhang, Z.; Lv, Q.; Liu, B. Current progress and performance improvement of Pt/C catalysts for fuel cells. J. Mater. Chem. A 2020, 8, 24284–24306. [Google Scholar] [CrossRef]
- Poynton, S.D.; Kizewski, J.P.; Slade, R.C.; Varcoe, J.R. Novel electrolyte membranes and non-Pt catalysts for low temperature fuel cells. Solid State Ion. 2010, 181, 219–222. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, P.C.; Emori, W.; Uzoma, P.C.; Mansir, I.B.; Radwan, A.B.; Ige, O.O.; Abdullah, A.M. A review of bipolar plates materials and graphene coating degradation mechanism in proton exchange membrane fuel cell. Int. J. Energy Res. 2022, 46, 3766–3781. [Google Scholar] [CrossRef]
- Gupta, S.; Fernandes, R.; Patel, R.; Spreitzer, M.; Patel, N. A review of cobalt-based catalysts for sustainable energy and environmental applications. Appl. Catal. A Gen. 2023, 661, 119254. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, X.-Z.; Hin, J.N.C.; Wang, H.; Friedrich, K.A.; Schulze, M. A review of platinum-based catalyst layer degradation in proton exchange membrane fuel cells. J. Power Sources 2009, 194, 588–600. [Google Scholar] [CrossRef]
- Li, B.; Wan, K.; Xie, M.; Chu, T.; Wang, X.; Li, X.; Yang, D.; Ming, P.; Zhang, C. Durability degradation mechanism and consistency analysis for proton exchange membrane fuel cell stack. Appl. Energy 2022, 314, 119020. [Google Scholar] [CrossRef]
- Tarokh, A. Modeling Nafion Ionomer During Catalyst Layer Fabrication Process of Polymer Electrolyte Fuel Cell Using Molecular Dynamics Method. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 2022. [Google Scholar]
- Zhang, Y.; Chen, S.; Wang, Y.; Ding, W.; Wu, R.; Li, L.; Qi, X.; Wei, Z. Study of the degradation mechanisms of carbon-supported platinum fuel cells catalyst via different accelerated stress test. J. Power Sources 2015, 273, 62–69. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Belgacem, I.B.; Emori, W.; Uzoma, P.C. Nafion degradation mechanisms in proton exchange membrane fuel cell (PEMFC) system: A review. Int. J. Hydrogen Energy 2021, 46, 27956–27973. [Google Scholar] [CrossRef]
- Xiao, B.; Zhao, J.; Fan, L.; Liu, Y.; Chan, S.H.; Tu, Z. Effects of moisture dehumidification on the performance and degradation of a proton exchange membrane fuel cell. Energy 2022, 245, 123298. [Google Scholar] [CrossRef]
- Ghassemzadeh, L.; Kreuer, K.-D.; Maier, J.; Müller, K. Chemical degradation of Nafion membranes under mimic fuel cell conditions as investigated by solid-state NMR spectroscopy. J. Phys. Chem. C 2010, 114, 14635–14645. [Google Scholar] [CrossRef]
- Young, A.; Stumper, J.; Gyenge, E. Characterizing the structural degradation in a PEMFC cathode catalyst layer: Carbon corrosion. J. Electrochem. Soc. 2009, 156, B913. [Google Scholar] [CrossRef]
- Mansir, I.B.; Okonkwo, P.C. A focused review of carbon corrosion mechanism in proton exchange membrane fuel cell during start-up and shut-down processes. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 3231–3245. [Google Scholar] [CrossRef]
- Pascone, P. Synthesis, Characterization, and Performance of Graphene Nanoflakes as a Non-Noble Metal Catalyst in Polymer Electrolyte Membrane Fuel Cells. Master’s Thesis, McGill University, Montreal, QB, Canada, 2013. [Google Scholar]
- Zhang, P.; Hong, S.; Song, N.; Han, Z.; Ge, F.; Dai, G.; Dong, H.; Li, C. Alloy as advanced catalysts for electrocatalysis: From materials design to applications. Chin. Chem. Lett. 2024, 35, 109073. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Rangabhashiyam, S.; Singh, A.; Nguyen, V.-H.; Van Le, Q.; Khan, A.A.P.; Hu, C.; Huang, C.-W.; Ahamad, T. Copper sulfides based photocatalysts for degradation of environmental pollution hazards: A review on the recent catalyst design concepts and future perspectives. Surf. Interfaces 2022, 33, 102182. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Balu, A.M.; Muñoz-Batista, M.J.; Luque, R. Environmental catalysis: Present and future. ChemCatChem 2019, 11, 18–38. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Ling, Y.; Zhang, Q.; Yu, X.; Cai, W. Bottom-up Design of High-Performance Pt Electrocatalysts Supported on Carbon Nanotubes with Homogeneous Ionomer Distribution. ChemCatChem 2017, 9, 3307–3313. [Google Scholar] [CrossRef]
- Nam, J.H.; Lee, K.-J.; Hwang, G.-S.; Kim, C.-J.; Kaviany, M. Microporous layer for water morphology control in PEMFC. Int. J. Heat Mass Transf. 2009, 52, 2779–2791. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Dinh, C.-T. Gas diffusion electrode design for electrochemical carbon dioxide reduction. Chem. Soc. Rev. 2020, 49, 7488–7504. [Google Scholar] [CrossRef]
- Lee, F.; Ismail, M.; Ingham, D.; Hughes, K.; Ma, L.; Lyth, S.; Pourkashanian, M. Alternative architectures and materials for PEMFC gas diffusion layers: A review and outlook. Renew. Sustain. Energy Rev. 2022, 166, 112640. [Google Scholar] [CrossRef]
- Jomori, S.; Nonoyama, N.; Yoshida, T. Analysis and modeling of PEMFC degradation: Effect on oxygen transport. J. Power Sources 2012, 215, 18–27. [Google Scholar] [CrossRef]
- Tawfik, H.; Hung, Y.; Mahajan, D. Metal bipolar plates for PEM fuel cell—A review. J. Power Sources 2007, 163, 755–767. [Google Scholar] [CrossRef]
- Chun, J.H.; Park, K.T.; Jo, D.H.; Kim, S.G.; Kim, S.H. Numerical modeling and experimental study of the influence of GDL properties on performance in a PEMFC. Int. J. Hydrogen Energy 2011, 36, 1837–1845. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Otor, C. A review of gas diffusion layer properties and water management in proton exchange membrane fuel cell system. Int. J. Energy Res. 2021, 45, 3780–3800. [Google Scholar] [CrossRef]
- Owejan, J.P.; Owejan, J.E.; Gu, W.; Trabold, T.A.; Tighe, T.W.; Mathias, M.F. Water transport mechanisms in PEMFC gas diffusion layers. J. Electrochem. Soc. 2010, 157, B1456. [Google Scholar] [CrossRef]
- Kusoglu, A.; Santare, M.H.; Karlsson, A.M.; Cleghorn, S.; Johnson, W.B. Micromechanics model based on the nanostructure of PFSA membranes. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 2404–2417. [Google Scholar] [CrossRef]
- Luo, Z.; Chang, Z.; Zhang, Y.; Liu, Z.; Li, J. Electro-osmotic drag coefficient and proton conductivity in Nafion® membrane for PEMFC. Int. J. Hydrogen Energy 2010, 35, 3120–3124. [Google Scholar] [CrossRef]
- Hou, M.; Li, Q.; Che, Y. Hydrophilic Modification of Polytetrafluoroethylene (PTFE) Capillary Membranes with Chemical Resistance by Constructing Three-Dimensional Hydrophilic Networks. Polymers 2024, 16, 1154. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Kang, H.; Sohn, Y.-J.; Lee, J.; Shim, S.; Lee, S.G. Molecular dynamics simulation study on the effect of perfluorosulfonic acid side chains on oxygen permeation in hydrated ionomers of PEMFCs. Sci. Rep. 2021, 11, 8702. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Z.; Ran, J.; Zhou, D.; Li, C.; Xu, T. Advances in proton-exchange membranes for fuel cells: An overview on proton conductive channels (PCCs). Phys. Chem. Chem. Phys. 2013, 15, 4870–4887. [Google Scholar] [CrossRef]
- Spendelow, J.; Papageorgopoulos, D. Progress in PEMFC MEA component R&D at the DOE fuel cell technologies program. Fuel Cells 2011, 11, 775–786. [Google Scholar]
- Wang, D.; Cornelius, C.J. Ionomer thermodynamic interrelationships associated with wettability, surface energy, swelling, and water transport. Eur. Polym. J. 2016, 85, 126–138. [Google Scholar] [CrossRef]
- Niu, M.; Gao, Y.; Pan, Q.; Zhang, T. Review on factors of voltage consistency and inconsistent degradation in proton exchange membrane fuel cells. Ionics 2024, 30, 2433–2458. [Google Scholar] [CrossRef]
- Sadeghi, M.A.; Khan, Z.A.; Agnaou, M.; Hu, L.; Litster, S.; Kongkanand, A.; Padgett, E.; Muller, D.A.; Friscic, T.; Gostick, J. Predicting pemfc performance from a volumetric image of catalyst layer structure using pore network modeling. Appl. Energy 2024, 353, 122004. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts—A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, S.; Liao, X.; Jia, Y.; Xu, H.; Wang, C.; He, W.; Zhao, Y. Lattice Boltzmann study of the effect of catalyst layer structure on oxygen reduction reaction within a PEMFC. Int. J. Hydrogen Energy 2024, 52, 1105–1114. [Google Scholar] [CrossRef]
- Ostroverkh, A.; Johánek, V.; Dubau, M.; Kúš, P.; Khalakhan, I.; Šmíd, B.; Fiala, R.; Václavů, M.; Ostroverkh, Y.; Matolín, V. Optimization of ionomer-free ultra-low loading Pt catalyst for anode/cathode of PEMFC via magnetron sputtering. Int. J. Hydrogen Energy 2019, 44, 19344–19356. [Google Scholar] [CrossRef]
- Padgett, E.; Yarlagadda, V.; Holtz, M.E.; Ko, M.; Levin, B.D.; Kukreja, R.S.; Ziegelbauer, J.M.; Andrews, R.N.; Ilavsky, J.; Kongkanand, A. Mitigation of PEM fuel cell catalyst degradation with porous carbon supports. J. Electrochem. Soc. 2019, 166, F198–F207. [Google Scholar] [CrossRef]
- Wang, C.; Krishnan, V.; Wu, D.; Bledsoe, R.; Paddison, S.J.; Duscher, G. Evaluation of the microstructure of dry and hydrated perfluorosulfonic acid ionomers: Microscopy and simulations. J. Mater. Chem. A 2012, 1, 938–944. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pan, F.; Chen, W.; Ding, Z.; Yang, D.; Li, B.; Ming, P.; Zhang, C. The controllable design of catalyst inks to enhance PEMFC performance: A review. Electrochem. Energy Rev. 2021, 4, 67–100. [Google Scholar] [CrossRef]
- Soong, C.-Y.; Yan, W.-M.; Tseng, C.; Liu, H.-C.; Chen, F.; Chu, H.-S. Analysis of reactant gas transport in a PEM fuel cell with partially blocked fuel flow channels. J. Power Sources 2005, 143, 36–47. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Wu, S.; Merida, W. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 2008, 184, 104–119. [Google Scholar] [CrossRef]
- Ehteshami, S.M.M.; Taheri, A.; Chan, S. A review on ions induced contamination of polymer electrolyte membrane fuel cells, poisoning mechanisms and mitigation approaches. J. Ind. Eng. Chem. 2016, 34, 1–8. [Google Scholar] [CrossRef]
- Lohmann-Richters, F.P.; Abel, B.; Varga, Á. In situ determination of the electrochemically active platinum surface area: Key to improvement of solid acid fuel cells. J. Mater. Chem. A 2018, 6, 2700–2707. [Google Scholar] [CrossRef]
- Dijkgraaf, P.; Duisters, H.; Kuster, B.; Van der Wiele, K. Deactivation of platinum catalysts by oxygen: 2. Nature of the catalyst deactivation. J. Catal. 1988, 112, 337–344. [Google Scholar] [CrossRef]
- Merker, J.; Lupton, D.; Topfer, M.; Knake, H. High temperature mechanical properties of the platinum group metals. Platin. Met. Rev. 2001, 45, 74–82. [Google Scholar] [CrossRef]
- Borup, R.L.; Kusoglu, A.; Neyerlin, K.C.; Mukundan, R.; Ahluwalia, R.K.; Cullen, D.A.; More, K.L.; Weber, A.Z.; Myers, D.J. Recent developments in catalyst-related PEM fuel cell durability. Curr. Opin. Electrochem. 2020, 21, 192–200. [Google Scholar] [CrossRef]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Trogadas, P.; Fuller, T.F.; Strasser, P. Carbon as catalyst and support for electrochemical energy conversion. Carbon 2014, 75, 5–42. [Google Scholar] [CrossRef]
- Mayrhofer, K.; Strmcnik, D.; Blizanac, B.; Stamenkovic, V.; Arenz, M.; Markovic, N. Measurement of oxygen reduction activities via the rotating disc electrode method: From Pt model surfaces to carbon-supported high surface area catalysts. Electrochim. Acta 2008, 53, 3181–3188. [Google Scholar] [CrossRef]
- Luo, X.; Ren, C.; Song, J.; Luo, H.; Xiao, K.; Zhang, D.; Hao, J.; Deng, Z.; Dong, C.; Li, X. Design and fabrication of bipolar plates for PEM water electrolyser. J. Mater. Sci. Technol. 2022, 146, 19–41. [Google Scholar] [CrossRef]
- Yang, K.; Kas, R.; Smith, W.A.; Burdyny, T. Role of the carbon-based gas diffusion layer on flooding in a gas diffusion electrode cell for electrochemical CO2 reduction. ACS Energy Lett. 2020, 6, 33–40. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Z.; Kou, S.; Lu, G.; Chen, D.; Liu, Z. Carbon-supported catalysts with atomically dispersed metal sites for oxygen electroreduction: Present and future perspectives. J. Mater. Chem. A 2021, 9, 15919–15936. [Google Scholar] [CrossRef]
- Motupally, S.; Becker, A.J.; Weidner, J.W. Diffusion of water in Nafion 115 membranes. J. Electrochem. Soc. 2000, 147, 3171. [Google Scholar] [CrossRef]
- Kundu, S.; Simon, L.C.; Fowler, M.; Grot, S. Mechanical properties of Nafion™ electrolyte membranes under hydrated conditions. Polymer 2005, 46, 11707–11715. [Google Scholar] [CrossRef]
- Lu, G.; Liu, F.; Wang, C.-Y. Water transport through Nafion 112 membrane in DMFCs. Electrochem. Solid State Lett. 2004, 8, A1. [Google Scholar] [CrossRef]
- Sasikumar, G.; Ihm, J.; Ryu, H. Optimum Nafion content in PEM fuel cell electrodes. Electrochim. Acta 2004, 50, 601–605. [Google Scholar] [CrossRef]
- Kusoglu, A.; Cho, K.T.; Prato, R.A.; Weber, A.Z. Structural and transport properties of Nafion in hydrobromic-acid solutions. Solid State Ion. 2013, 252, 68–74. [Google Scholar] [CrossRef]
- Kudo, K.; Morimoto, Y. Analysis of Oxygen Transport Resistance of Nafion thin film on Pt electrode. ECS Trans. 2013, 50, 1487. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Ige, O.O.; Uzoma, P.C.; Emori, W.; Benamor, A.; Abdullah, A.M. Platinum degradation mechanisms in proton exchange membrane fuel cell (PEMFC) system: A review. Int. J. Hydrogen Energy 2021, 46, 15850–15865. [Google Scholar] [CrossRef]
- Antolini, E. The use of silicon in the membrane electrode assembly of fuel cells. ChemCatChem 2024, 16, e202301443. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Gao, Y. Understanding and approaches for the durability issues of Pt-based catalysts for PEM fuel cell. J. Power Sources 2007, 171, 558–566. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S.; Pei, K.; Ozaki, J.-i.; Kishimoto, T.; Imashiro, Y. A review of the stability and durability of non-precious metal catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2015, 285, 334–348. [Google Scholar] [CrossRef]
- Ali-Löytty, H.; Hannula, M.; Valden, M.; Eilert, A.; Ogasawara, H.; Nilsson, A. Chemical dissolution of Pt (111) during potential cycling under negative pH conditions studied by operando X-ray photoelectron spectroscopy. J. Phys. Chem. C 2019, 123, 25128–25134. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Y.; Gao, L.; Zeng, G.; Li, M.; Huang, H. Stabilizing Pt-based electrocatalysts for oxygen reduction reaction: Fundamental understanding and design strategies. Adv. Mater. 2021, 33, 2006494. [Google Scholar] [CrossRef]
- Fuchs, T.; Briega-Martos, V.; Drnec, J.; Stubb, N.; Martens, I.; Calle-Vallejo, F.; Harrington, D.A.; Cherevko, S.; Magnussen, O.M. Anodic and cathodic platinum dissolution processes involve different oxide species. Angew. Chem. 2023, 135, e202304293. [Google Scholar] [CrossRef]
- Guilminot, E.; Corcella, A.; Charlot, F.; Maillard, F.; Chatenet, M. Detection of Pt z+ ions and Pt nanoparticles inside the membrane of a used PEMFC. J. Electrochem. Soc. 2006, 154, B96. [Google Scholar] [CrossRef]
- Shao-Horn, Y.; Sheng, W.; Chen, S.; Ferreira, P.J.; Holby, E.; Morgan, D. Instability of supported platinum nanoparticles in low-temperature fuel cells. Top. Catal. 2007, 46, 285–305. [Google Scholar] [CrossRef]
- Chowdury, M.S.K.; Park, Y.; Park, S.B.; Park, Y.-i. Degradation Mechanisms, Long-Term durability Challenges, and mitigation methods for proton exchange membranes and membrane electrode assemblies with Pt/C electrocatalysts in Low-Temperature and High-Temperature fuel Cells: A comprehensive review. J. Electroanal. Chem. 2024, 975, 118712. [Google Scholar] [CrossRef]
- Helmly, S.; Hiesgen, R.; Morawietz, T.; Yuan, X.-Z.; Wang, H.; Friedrich, K.A. Microscopic investigation of platinum deposition in PEMFC cross-sections using AFM and SEM. J. Electrochem. Soc. 2013, 160, F687. [Google Scholar] [CrossRef]
- Dubau, L.; Castanheira, L.; Maillard, F.; Chatenet, M.; Lottin, O.; Maranzana, G.; Dillet, J.; Lamibrac, A.; Perrin, J.C.; Moukheiber, E. A review of PEM fuel cell durability: Materials degradation, local heterogeneities of aging and possible mitigation strategies. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 540–560. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Virkar, A.V. Electrochemical Ostwald ripening of Pt and Ag catalysts supported on carbon. J. Power Sources 2013, 234, 82–90. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, X.; Lu, J.; Du, A.; Lin, R. Pt Active Site Density Optimization of the Catalyst Layer for an Ultralow-Pt-Loading PEMFC. Energy Fuels 2024, 38, 21423–21431. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; ALOthman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Progress in modified carbon support materials for Pt and Pt-alloy cathode catalysts in polymer electrolyte membrane fuel cells. Prog. Mater. Sci. 2016, 82, 445–498. [Google Scholar] [CrossRef]
- Du, L.; Prabhakaran, V.; Xie, X.; Park, S.; Wang, Y.; Shao, Y. Low-PGM and PGM-free catalysts for proton exchange membrane fuel cells: Stability challenges and material solutions. Adv. Mater. 2021, 33, 1908232. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Chen, S.; Wang, Q.; Gao, X.; Li, J.; Li, J.; Li, L.; Ding, W.; Wei, Z. Leaching-and sintering-resistant hollow or structurally ordered intermetallic PtFe alloy catalysts for oxygen reduction reactions. Nanoscale 2019, 11, 20115–20122. [Google Scholar] [CrossRef] [PubMed]
- Litkohi, H.R.; Bahari, A.; Gatabi, M.P. Improved oxygen reduction reaction in PEMFCs by functionalized CNTs supported Pt–M (M= Fe, Ni, Fe–Ni) bi-and tri-metallic nanoparticles as efficient electrocatalyst. Int. J. Hydrogen Energy 2020, 45, 23543–23556. [Google Scholar] [CrossRef]
- Cheng, D.; Yuan, S.; Ferrando, R. Structure, chemical ordering and thermal stability of Pt–Ni alloy nanoclusters. J. Phys. Condens. Matter 2013, 25, 355008. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.; Coskun, S.; Pyun, K.R.; Doganay, D.; Tunca, S.; Koylan, S.; Kim, D.; Unalan, H.E.; Ko, S.H. Advances in protective layer-coating on metal nanowires with enhanced stability and their applications. Appl. Mater. Today 2021, 22, 100909. [Google Scholar] [CrossRef]
- Yu, X.; Ye, S. Recent advances in activity and durability enhancement of Pt/C catalytic cathode in PEMFC: Part II: Degradation mechanism and durability enhancement of carbon supported platinum catalyst. J. Power Sources 2007, 172, 145–154. [Google Scholar] [CrossRef]
- Yang, W.; Vogler, B.; Lei, Y.; Wu, T. Metallic ion leaching from heterogeneous catalysts: An overlooked effect in the study of catalytic ozonation processes. Environ. Sci. Water Res. Technol. 2017, 3, 1143–1151. [Google Scholar] [CrossRef]
- Ranganthan, O. The Leaching of Cobalt in Platinum-Cobalt Fuel Cell Catalysts. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2022. [Google Scholar]
- Li, X.; Zhao, J.; Su, D. Structural changes of intermetallic catalysts under reaction conditions. Small Struct. 2021, 2, 2100011. [Google Scholar] [CrossRef]
- Wu, D.; Shen, X.; Pan, Y.; Yao, L.; Peng, Z. Platinum alloy catalysts for oxygen reduction reaction: Advances, challenges and perspectives. ChemNanoMat 2020, 6, 32–41. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, L.; Gan, M.; Fu, S.; Zhao, Y. TiN@ nitrogen-doped carbon supported Pt nanoparticles as high-performance anode catalyst for methanol electrooxidation. J. Power Sources 2016, 324, 199–207. [Google Scholar] [CrossRef]
- Zhao, J.; Tu, Z.; Chan, S.H. Carbon corrosion mechanism and mitigation strategies in a proton exchange membrane fuel cell (PEMFC): A review. J. Power Sources 2021, 488, 229434. [Google Scholar] [CrossRef]
- Peron, J.; Mani, A.; Zhao, X.; Edwards, D.; Adachi, M.; Soboleva, T.; Shi, Z.; Xie, Z.; Navessin, T.; Holdcroft, S. Properties of Nafion® NR-211 membranes for PEMFCs. J. Membr. Sci. 2010, 356, 44–51. [Google Scholar] [CrossRef]
- Chen, C.; Fuller, T.F. The effect of humidity on the degradation of Nafion® membrane. Polym. Degrad. Stab. 2009, 94, 1436–1447. [Google Scholar] [CrossRef]
- S Kushwaha, O.; V Avadhani, C.; P Singh, R. Effect of UV rays on degradation and stability of high performance polymer membranes. Adv. Mater. Lett. 2014, 5, 272–279. [Google Scholar] [CrossRef]
- Collier, A.; Wang, H.; Yuan, X.Z.; Zhang, J.; Wilkinson, D.P. Degradation of polymer electrolyte membranes. Int. J. Hydrogen Energy 2006, 31, 1838–1854. [Google Scholar] [CrossRef]
- Madhav, D.; Wang, J.; Keloth, R.; Mus, J.; Buysschaert, F.; Vandeginste, V. A Review of Proton Exchange Membrane Degradation Pathways, Mechanisms, and Mitigation Strategies in a Fuel Cell. Energies 2024, 17, 998. [Google Scholar] [CrossRef]
- Patil, V.; Reshmi, P.; Prajna, S.; Haleshappa, D.; Jayarama, A.; Pinto, R. Degradation mechanisms in PEM fuel cells: A brief review. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Frühwirt, P.; Kregar, A.; Törring, J.T.; Katrašnik, T.; Gescheidt, G. Holistic approach to chemical degradation of Nafion membranes in fuel cells: Modelling and predictions. Phys. Chem. Chem. Phys. 2020, 22, 5647–5666. [Google Scholar] [CrossRef]
- Xie, J.; Ban, S.; Liu, B.; Zhou, H. A molecular simulation study of chemical degradation and mechanical deformation of hydrated Nafion membranes. Appl. Surf. Sci. 2016, 362, 441–447. [Google Scholar] [CrossRef]
- Danilczuk, M.; Schlick, S.; Coms, F.D. Degradation mechanism of perfluorinated membranes. In The Chemistry of Membranes Used in Fuel Cells: Degradation and Stabilization; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 19–53. [Google Scholar]
- Lee, J.-S.; Hwang, I.-T.; Jung, C.-H.; Choi, J.-H. Surface modification of Nafion membranes by ion implantation to reduce methanol crossover in direct methanol fuel cells. RSC Adv. 2016, 6, 62467–62470. [Google Scholar] [CrossRef]
- Rodgers, M.P.; Bonville, L.J.; Kunz, H.R.; Slattery, D.K.; Fenton, J.M. Fuel cell perfluorinated sulfonic acid membrane degradation correlating accelerated stress testing and lifetime. Chem. Rev. 2012, 112, 6075–6103. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.H.; Sha, Y.; Liu, W.-G.; Merinov, B.V.; Shirvanian, P.; Goddard III, W.A. Mechanism for degradation of Nafion in PEM fuel cells from quantum mechanics calculations. J. Am. Chem. Soc. 2011, 133, 19857–19863. [Google Scholar] [CrossRef] [PubMed]
- Wallnöfer-Ogris, E.; Poimer, F.; Köll, R.; Macherhammer, M.-G.; Trattner, A. Main degradation mechanisms of polymer electrolyte membrane fuel cell stacks–Mechanisms, influencing factors, consequences, and mitigation strategies. Int. J. Hydrogen Energy 2024, 50, 1159–1182. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.-A.; Qi, R. Review of ionomers in catalyst layers of proton exchange membrane (PEM) modules: Key parameters, characterization and manipulation methods. Int. J. Green Energy 2024, 21, 2872–2897. [Google Scholar] [CrossRef]
- Ted, H.Y.; Liu, W.-G.; Sha, Y.; Merinov, B.V.; Shirvanian, P.; Goddard, W.A., III. The effect of different environments on Nafion degradation: Quantum mechanics study. J. Membr. Sci. 2013, 437, 276–285. [Google Scholar]
- Fernandes, A.C.; Ticianelli, E.A. A performance and degradation study of Nafion 212 membrane for proton exchange membrane fuel cells. J. Power Sources 2009, 193, 547–554. [Google Scholar] [CrossRef]
- Benziger, J.; Chia, E.; Karnas, E.; Moxley, J.; Teuscher, C.; Kevrekidis, I. The stirred tank reactor polymer electrolyte membrane fuel cell. AIChE J. 2004, 50, 1889–1900. [Google Scholar] [CrossRef]
- Lehr, A.B. The Temperature-Dependent Deformation of Nafion for Fuel Cell Applications. Bachelor’s Thesis, Princeton University, Princeton, NJ, USA, 2005. [Google Scholar]
- Majsztrik, P.; Bocarsly, A.; Benziger, J. Water permeation through Nafion membranes: The role of water activity. J. Phys. Chem. B 2008, 112, 16280–16289. [Google Scholar] [CrossRef] [PubMed]
- Pedapati, P.R.; Dhanushkodi, S.R.; Chidambaram, R.K.; Taler, D.; Sobota, T.; Taler, J. Design and Manufacturing Challenges in PEMFC Flow Fields—A Review. Energies 2024, 17, 3499. [Google Scholar] [CrossRef]
- Pourrahmani, H.; Siavashi, M.; Yavarinasab, A.; Matian, M.; Chitgar, N.; Wang, L.; Van Herle, J. A review on the long-term performance of proton exchange membrane fuel cells: From degradation modeling to the effects of bipolar plates, sealings, and contaminants. Energies 2022, 15, 5081. [Google Scholar] [CrossRef]
- Wei, X.; Wang, R.-Z.; Zhao, W.; Chen, G.; Chai, M.-R.; Zhang, L.; Zhang, J. Recent research progress in PEM fuel cell electrocatalyst degradation and mitigation strategies. EnergyChem 2021, 3, 100061. [Google Scholar] [CrossRef]
- Bilondi, A.M.; Abdollahzadeh, M.; Kermani, M.; Heidary, H.; Havaej, P. Numerical study of anode side CO contamination effects on PEM fuel cell performance; and mitigation methods. Energy Convers. Manag. 2018, 177, 519–534. [Google Scholar] [CrossRef]
- Haque, M.; Kawawaki, T.; Negishi, Y. Navigating challenges and possibilities for improving polymer electrolyte membrane fuel cells via Pt electrocatalyst, support and ionomer advancements. Int. J. Hydrogen Energy 2024, 85, 30–47. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S. Current status and future development of catalyst materials and catalyst layers for proton exchange membrane fuel cells: An industrial perspective. ACS Energy Lett. 2017, 2, 629–638. [Google Scholar] [CrossRef]
- Zhao, X.; Sasaki, K. Advanced Pt-based core–shell electrocatalysts for fuel cell cathodes. Acc. Chem. Res. 2022, 55, 1226–1236. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, Z.; Chan, S.H. Performance evaluation and degradation mechanism for proton exchange membrane fuel cell with dual exhaust gas recirculation. Adv. Energy Sustain. Res. 2023, 4, 2200180. [Google Scholar] [CrossRef]
- Mittal, V.O.; Kunz, H.R.; Fenton, J.M. Membrane degradation mechanisms in PEMFCs. J. Electrochem. Soc. 2007, 154, B652. [Google Scholar] [CrossRef]
- Chen, S.; Hao, M.; Hu, Y.; Liu, K.; Li, Y. Insight into the evolution of membrane chemical degradation in proton exchange membrane fuel cells: From theoretical analysis to model developing. J. Power Sources 2024, 599, 234238. [Google Scholar] [CrossRef]
- He, W.; Tang, F.; Li, X.; Zhang, C.; Ming, P. Quantification and evolution on degradation mechanisms of proton exchange membrane fuel cell catalyst layer under dynamic testing conditions. Int. J. Hydrogen Energy 2023, 48, 18032–18040. [Google Scholar] [CrossRef]
- Healy, J.; Hayden, C.; Xie, T.; Olson, K.; Waldo, R.; Brundage, M.; Gasteiger, H.; Abbott, J. Aspects of the chemical degradation of PFSA ionomers used in PEM fuel cells. Fuel Cells 2005, 5, 302–308. [Google Scholar] [CrossRef]
- Vielstich, W.; Kuver, A.; Krausa, M.; Ferreira, A.C.; Petrov, K.; Srinivasan, S. Proton exchange membrane fuel cells using gas-fed methanol. In Proceedings of the Symposium on Batteries and Fuel Cells for Stationary and Electric Vehicle Applications, Honolulu, HI, USA, 16–21 May 1993; Electrochemical Society Inc.: Pennington, NJ, USA, 1993; pp. 269–280. [Google Scholar]
- Shi, S.; Sun, X.; Lin, Q.; Chen, J.; Fu, Y.; Hong, X.; Li, C.; Guo, X.; Chen, G.; Chen, X. Fatigue crack propagation behavior of fuel cell membranes after chemical degradation. Int. J. Hydrogen Energy 2020, 45, 27653–27664. [Google Scholar] [CrossRef]
- Taniguchi, A.; Akita, T.; Yasuda, K.; Miyazaki, Y. Analysis of degradation in PEMFC caused by cell reversal during air starvation. Int. J. Hydrogen Energy 2008, 33, 2323–2329. [Google Scholar] [CrossRef]
- Sandbeck, D.J.; Secher, N.M.; Speck, F.D.; Sørensen, J.E.; Kibsgaard, J.; Chorkendorff, I.; Cherevko, S. Particle size effect on platinum dissolution: Considerations for accelerated stability testing of fuel cell catalysts. ACS Catal. 2020, 10, 6281–6290. [Google Scholar] [CrossRef]
- Yu, K.; Groom, D.J.; Wang, X.; Yang, Z.; Gummalla, M.; Ball, S.C.; Myers, D.J.; Ferreira, P.J. Degradation mechanisms of platinum nanoparticle catalysts in proton exchange membrane fuel cells: The role of particle size. Chem. Mater. 2014, 26, 5540–5548. [Google Scholar] [CrossRef]
- Artyushkova, K.; Serov, A.; Doan, H.; Danilovic, N.; Capuano, C.; Sakamoto, T.; Kishi, H.; Yamaguchi, S.; Mukerjee, S.; Atanassov, P. Application of X-ray photoelectron spectroscopy to studies of electrodes in fuel cells and electrolyzers. J. Electron Spectrosc. Relat. Phenom. 2019, 231, 127–139. [Google Scholar] [CrossRef]
- Yu, H.; Bonville, L.; Jankovic, J.; Maric, R. Microscopic insights on the degradation of a PEM water electrolyzer with ultra-low catalyst loading. Appl. Catal. B Environ. 2020, 260, 118194. [Google Scholar] [CrossRef]
- Tang, H.; Wan, Z.; Pan, M. Self-assembled Nafion–silica nanoparticles for elevated-high temperature polymer electrolyte membrane fuel cells. Electrochem. Commun. 2007, 9, 2003–2008. [Google Scholar] [CrossRef]
- Hartl, K.; Hanzlik, M.; Arenz, M. IL-TEM investigations on the degradation mechanism of Pt/C electrocatalysts with different carbon supports. Energy Environ. Sci. 2011, 4, 234–238. [Google Scholar] [CrossRef]
- Guilminot, E.; Corcella, A.; Chatenet, M.; Maillard, F.; Charlot, F.; Berthomé, G.; Iojoiu, C.; Sanchez, J.-Y.; Rossinot, E.; Claude, E. Membrane and active layer degradation upon PEMFC steady-state operation: I. platinum dissolution and redistribution within the MEA. J. Electrochem. Soc. 2007, 154, B1106. [Google Scholar] [CrossRef]
- Chatenet, M.; Guetaz, L.; Maillard, F. Electron microscopy to study membrane electrode assembly (MEA) materials and structure degradation. In Handbook of Fuel Cells; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Lopez-Haro, M.; Dubau, L.; Guétaz, L.; Bayle-Guillemaud, P.; Chatenet, M.; André, J.; Caqué, N.; Rossinot, E.; Maillard, F. Atomic-scale structure and composition of Pt3Co/C nanocrystallites during real PEMFC operation: A STEM–EELS study. Appl. Catal. B Environ. 2014, 152, 300–308. [Google Scholar] [CrossRef]
- Zhu, E.; Wu, M.; Xu, H.; Peng, B.; Liu, Z.; Huang, Y.; Li, Y. Stability of Platinum-Group-Metal-Based Electrocatalysts in Proton Exchange Membrane Fuel Cells. Adv. Funct. Mater. 2022, 32, 2203883. [Google Scholar] [CrossRef]
- Park, J.-H.; Yim, S.-D.; Kim, T.; Park, S.-H.; Yoon, Y.-G.; Park, G.-G.; Yang, T.-H.; Park, E.-D. Understanding the mechanism of membrane electrode assembly degradation by carbon corrosion by analyzing the microstructural changes in the cathode catalyst layers and polarization losses in proton exchange membrane fuel cell. Electrochim. Acta 2012, 83, 294–304. [Google Scholar] [CrossRef]
- Rao, C.V.; Viswanathan, B. Monodispersed platinum nanoparticle supported carbon electrodes for hydrogen oxidation and oxygen reduction in proton exchange membrane fuel cells. J. Phys. Chem. C 2010, 114, 8661–8667. [Google Scholar] [CrossRef]
- Jayasayee, K.; Van Veen, J.R.; Manivasagam, T.G.; Celebi, S.; Hensen, E.J.; De Bruijn, F.A. Oxygen reduction reaction (ORR) activity and durability of carbon supported PtM (Co, Ni, Cu) alloys: Influence of particle size and non-noble metals. Appl. Catal. B Environ. 2012, 111, 515–526. [Google Scholar] [CrossRef]
- Rasouli, S. Degradation Mechanisms of Pt and Pt Alloy Nanocatalysts in Proton Exchange Membrane Fuel Cells. Ph.D. Thesis, The University of Texas, Austin, TX, USA, 2017. [Google Scholar]
- Tagliazucca, V.; Schlichte, K.; Schüth, F.; Weidenthaler, C. Molybdenum-based catalysts for the decomposition of ammonia: In situ X-ray diffraction studies, microstructure, and catalytic properties. J. Catal. 2013, 305, 277–289. [Google Scholar] [CrossRef]
- Sharma, R.; Andersen, S.M. An opinion on catalyst degradation mechanisms during catalyst support focused accelerated stress test (AST) for proton exchange membrane fuel cells (PEMFCs). Appl. Catal. B Environ. 2018, 239, 636–643. [Google Scholar] [CrossRef]
- Schmittinger, W.; Vahidi, A. A review of the main parameters influencing long-term performance and durability of PEM fuel cells. J. Power Sources 2008, 180, 1–14. [Google Scholar] [CrossRef]
- Wang, C.; Ricketts, M.; Soleymani, A.P.; Jankovic, J.; Waldecker, J.; Chen, J. Effect of carbon support characteristics on fuel cell durability in accelerated stress testing. J. Electrochem. Soc. 2021, 168, 044507. [Google Scholar] [CrossRef]
- Paperzh, K.; Alekseenko, A.; Pankov, I.; Guterman, V. Accelerated stress tests for Pt/C electrocatalysts: An approach to understanding the degradation mechanisms. J. Electroanal. Chem. 2024, 952, 117972. [Google Scholar] [CrossRef]
- Baldizzone, C.; Gan, L.; Hodnik, N.; Keeley, G.P.; Kostka, A.; Heggen, M.; Strasser, P.; Mayrhofer, K.J. Stability of dealloyed porous Pt/Ni nanoparticles. ACS Catal. 2015, 5, 5000–5007. [Google Scholar] [CrossRef]
- Belenov, S.; Alekseenko, A.; Pavlets, A.; Nevelskaya, A.; Danilenko, M. Architecture evolution of different nanoparticles types: Relationship between the structure and functional properties of catalysts for PEMFC. Catalysts 2022, 12, 638. [Google Scholar] [CrossRef]
- Hartnig, C. Catalyst and membrane technology for low temperature fuel cells. In Polymer Electrolyte Membrane and Direct Methanol Fuel Cell Technology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 56–80. [Google Scholar]
- Sinniah, J.D.; Wong, W.Y.; Loh, K.S.; Yunus, R.M.; Timmiati, S.N. Perspectives on carbon-alternative materials as Pt catalyst supports for a durable oxygen reduction reaction in proton exchange membrane fuel cells. J. Power Sources 2022, 534, 231422. [Google Scholar] [CrossRef]
- Serov, A.; Kwak, C. Review of non-platinum anode catalysts for DMFC and PEMFC application. Appl. Catal. B Environ. 2009, 90, 313–320. [Google Scholar] [CrossRef]
- Tackett, B.M.; Sheng, W.; Chen, J.G. Opportunities and challenges in utilizing metal-modified transition metal carbides as low-cost electrocatalysts. Joule 2017, 1, 253–263. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.; Bondarenko, A.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Jung, W.S.; Xie, T.; Kriston, A.; Ganesan, P.; Gamliel, D.; Murphy, B.; Popov, B.N. Development of Hybrid Cathode Catalyst for PEM Fuel Cells. ECS Trans. 2013, 50, 1875. [Google Scholar] [CrossRef]
- Zhu, F.; Luo, L.; Wu, A.; Wang, C.; Cheng, X.; Shen, S.; Ke, C.; Yang, H.; Zhang, J. Improving the high-current-density performance of PEMFC through much enhanced utilization of platinum electrocatalysts on carbon. ACS Appl. Mater. Interfaces 2020, 12, 26076–26083. [Google Scholar] [CrossRef] [PubMed]
- Brouzgou, A.; Song, S.; Tsiakaras, P. Low and non-platinum electrocatalysts for PEMFCs: Current status, challenges and prospects. Appl. Catal. B Environ. 2012, 127, 371–388. [Google Scholar] [CrossRef]
- Seifitokaldani, A.; Savadogo, O. Electrochemically stable titanium oxy-nitride support for platinum electro-catalyst for PEM fuel cell applications. Electrochim. Acta 2015, 167, 237–245. [Google Scholar] [CrossRef]


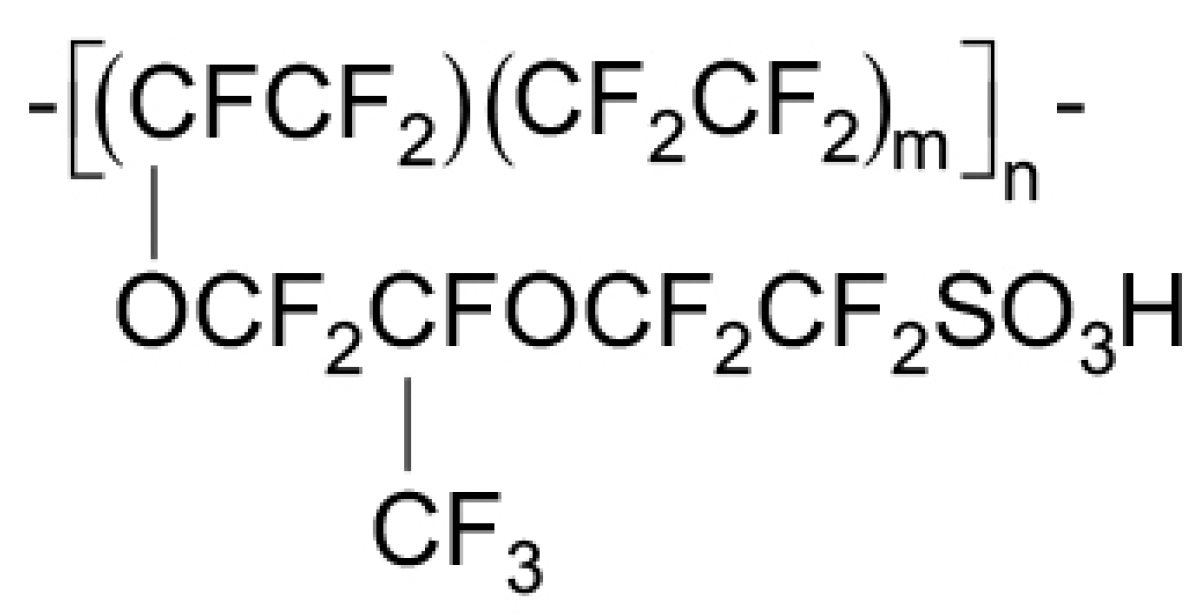


| Catalytic Layer Component | Function | Ref. |
|---|---|---|
| Platinum | Resistance to the stresses imposed by PEMFC operation | [51] |
| Protect the PEMFC from contamination and contamination-induced poisoning | [52] | |
| Large gas–electrode contact area | [53] | |
| Removal of byproducts and oxygen, as well as an efficient mechanism for transporting protons | [54] | |
| Provides superior electronic and mechanical characteristics | [55] | |
| Resistance to the stresses imposed by PEMFC operation | [56] | |
| Carbon support | Provides structural stability to the catalyst | [57] |
| Creates a partway for electrons to access the catalyst particles | [58] | |
| Used as catalyst support material due to its high surface area | [59] | |
| Employed as support for the active metal in the catalyst layer | [58] | |
| Used in bipolar plate fabrication | [60] | |
| Employed in the gas diffusion layer design | [61] | |
| Enhances the ORR process | [62] | |
| Nafion | Acts as a barrier to the self-diffusion of water | [63] |
| In terms of temperature and mechanical stress, PEMFC-compatible designs must be robust enough for use in actual applications | [64] | |
| Carries out the fuel cell’s water management and electrical conductivity | [65] | |
| Carries out the role of binder within the catalyst layer | [66] | |
| Helps the electrolyte’s proton transport properties | [67] | |
| Offers resistance at the interface to oxygen diffusion and helps regulate cellular humidity | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okonkwo, P.C. Proton Exchange Membrane Fuel Cell Catalyst Layer Degradation Mechanisms: A Succinct Review. Catalysts 2025, 15, 97. https://doi.org/10.3390/catal15010097
Okonkwo PC. Proton Exchange Membrane Fuel Cell Catalyst Layer Degradation Mechanisms: A Succinct Review. Catalysts. 2025; 15(1):97. https://doi.org/10.3390/catal15010097
Chicago/Turabian StyleOkonkwo, Paul C. 2025. "Proton Exchange Membrane Fuel Cell Catalyst Layer Degradation Mechanisms: A Succinct Review" Catalysts 15, no. 1: 97. https://doi.org/10.3390/catal15010097
APA StyleOkonkwo, P. C. (2025). Proton Exchange Membrane Fuel Cell Catalyst Layer Degradation Mechanisms: A Succinct Review. Catalysts, 15(1), 97. https://doi.org/10.3390/catal15010097






