Photocatalytic Cement Mortar with Durable Self-Cleaning Performance
Abstract
1. Introduction
2. Results and Discussions
2.1. Monitoring of Hydration-Induced Thermal Process
2.2. Microstructures of Nano-TiO2-Modified Mortars
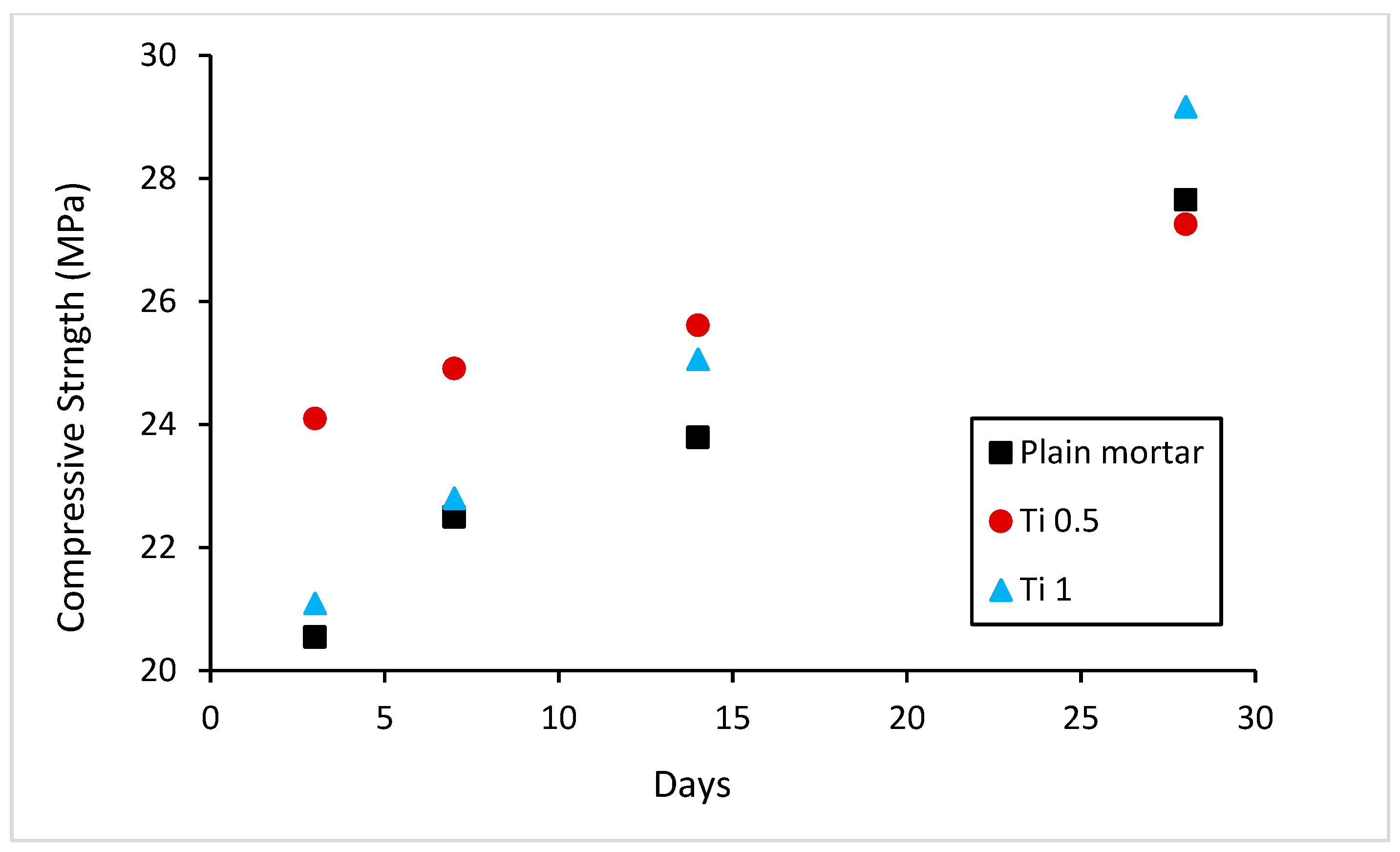
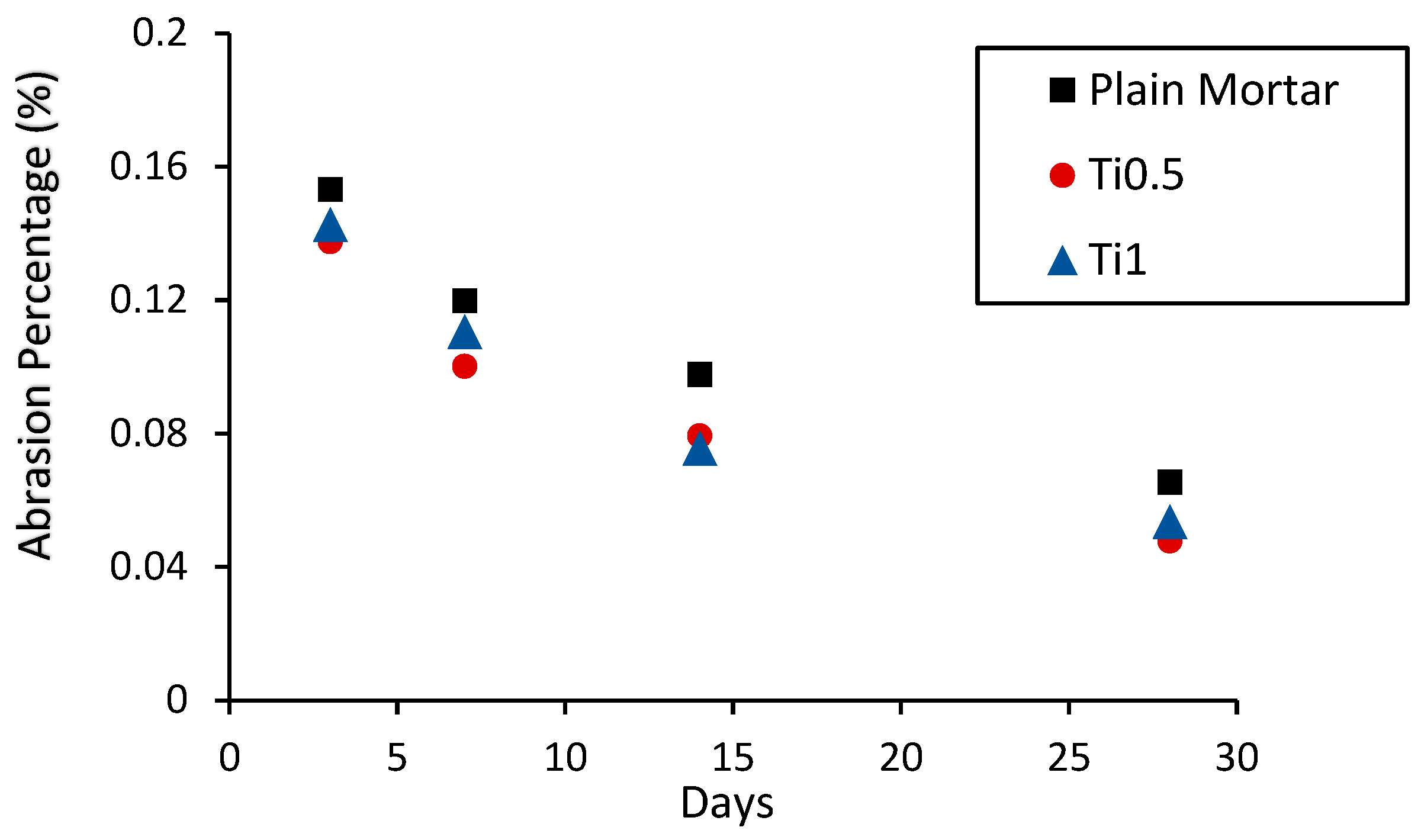
2.3. Mechanical Strength Analysis
2.4. Photocatalytic Performance Measurements
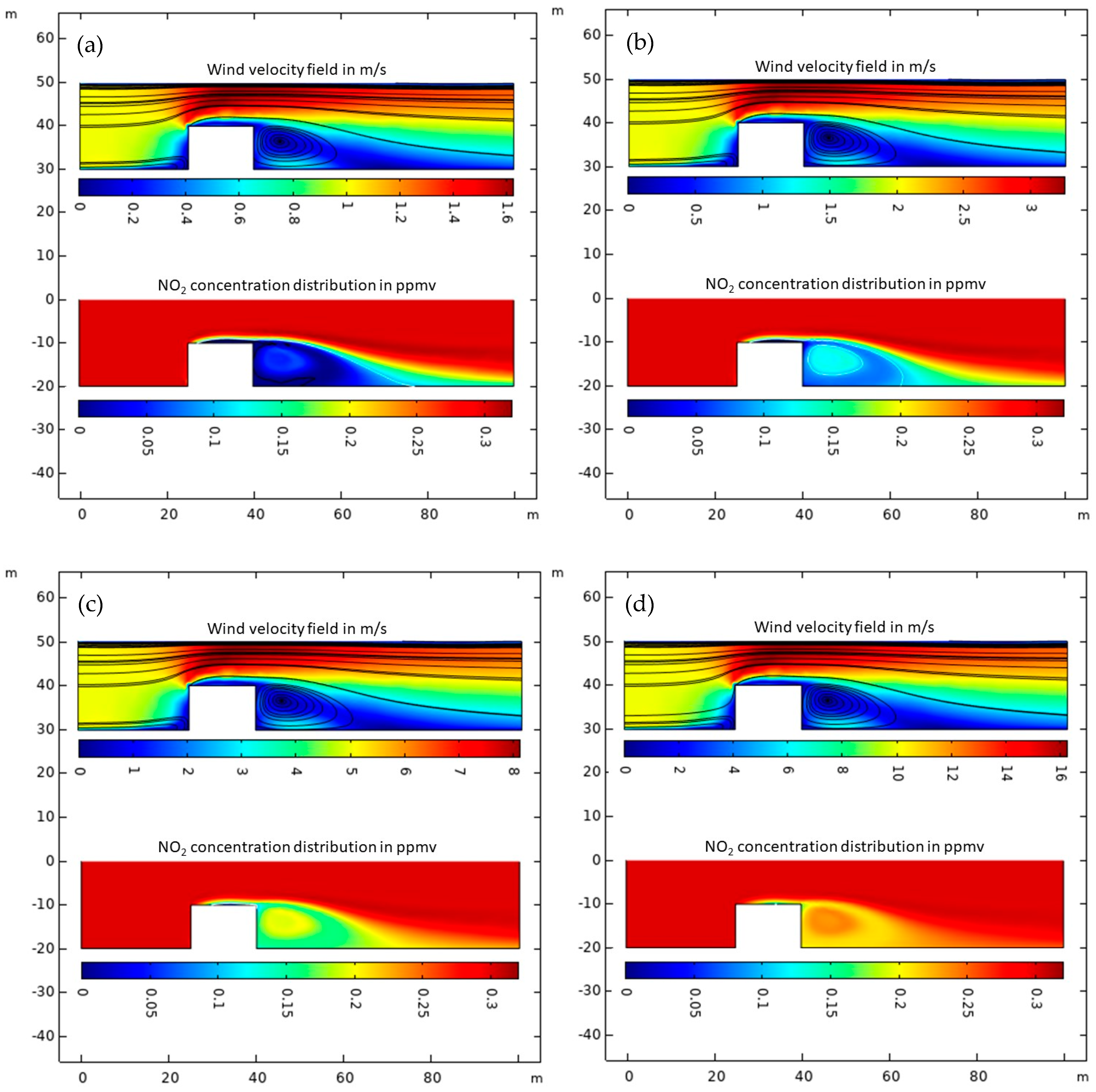
2.5. Air Cleaning Field Test Modeling
3. Materials and Methods
3.1. Specimen Preparations
3.2. Specimen Characterization and Experimental Procedure
3.3. Modeling Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zailan, S.N.; Mahmed, N.; Abdullah, M.M.A.B.; Sandu, A.V. Self-cleaning geopolymer concrete-A review. IOP Conf. Ser. Mater. Sci. 2016, 133, 012026. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Adebanjo, A.U.; Abbas, Y.M.; Shafiq, N.; Khan, M.I.; Farhan, S.A.; Masmoudi, R. Optimizing nano-TiO2 and ZnO integration in silica-based high-performance concrete: Mechanical, durability, and photocatalysis insights for sustainable self-cleaning systems. Constr. Build. Mater. 2024, 446, 138038. [Google Scholar] [CrossRef]
- Ayappan, C.; Xing, R.; Zhang, X.; Luo, D.; Liu, S.; Fujishima, A. TiO2-based photocatalysts for emerging gaseous pollutants removal: From photocatalysts to reactors design. Coord. Chem. Rev. 2024, 515, 215960. [Google Scholar] [CrossRef]
- Liao, G.; Yao, W.; She, A.; Shi, C.; Zuo, J.; Wu, D. Interfacial design of nano-TiO2 modified recycled concrete powder for building self-cleaning. Colloids Surf. A Physicochem. Eng. Asp. 2023, 661, 130925. [Google Scholar] [CrossRef]
- Franzoni, E.; Pizzigatti, C.; Fabris, R. Developing inorganic coatings with nano-TiO2 for heritage concrete and assessing their self-cleaning performance by a new laboratory test. Constr. Build. Mater. 2024, 449, 138282. [Google Scholar] [CrossRef]
- Mostafa, F.E.Z.M.; Smarzewski, P.; El Hafez, G.M.A.; Farghali, A.A.; Morsi, W.M.; Faried, A.S.; Tawfik, T.A. Analyzing the effects of nano-titanium dioxide and nano-zinc oxide nanoparticles on the mechanical and durability properties of self-cleaning concrete. Materials 2023, 16, 6909. [Google Scholar] [CrossRef] [PubMed]
- Neubert, S.; Pliszka, D.; Thavasi, V.; Wintermantel, E.; Ramakrishna, S. Conductive electrospun PANi-PEO/TiO2 fibrous membrane for photo catalysis. Mater. Sci. Eng. B 2011, 176, 640–646. [Google Scholar] [CrossRef]
- Lee, B.Y.; Behler, K.; Kurtoglu, M.E.; Wynosky-Dolfi, M.A.; Rest, R.F.; Gogotsi, Y. Titanium dioxide-coated nanofibers for advanced filters. J. Nanopart. Res. 2010, 12, 2511–2519. [Google Scholar] [CrossRef]
- Czech, T.; Ramakrishna, S.; Sundarrajan, S. Electrospinning and electrospraying techniques for nanocomposite non-woven fabric production. Fibres Text. East. Eur. 2009, 17, 77–81. [Google Scholar]
- Zhang, Y.; Lee, M.W.; An, S.; Sinha-Ray, S.; Khansari, S.; Joshi, B.; Hong, S.; Hong, J.H.; Kim, J.J.; Pourdeyhimi, B.; et al. Antibacterial activity of photocatalytic electrospun titania nanofiber mats and solution-blown soy protein nanofiber mats decorated with silver nanoparticles. Catal. Commun. 2013, 34, 35–40. [Google Scholar] [CrossRef]
- Impellizzeri, G.; Scuderi, V.; Romano, L.; Napolitani, E.; Sanz, R.; Carles, R.; Privitera, V. C ion-implanted TiO2 thin film for photocatalytic applications. J. Appl. Phys. 2015, 117, 105308. [Google Scholar] [CrossRef]
- Li, Z.; Ding, S.; Kong, L.; Wang, X.; Ashour, A.; Han, B.; Ou, J. Nano TiO2-engineered anti-corrosion concrete for sewage system. J. Clean. Prod. 2022, 337, 130508. [Google Scholar] [CrossRef]
- Kajitvichyanukul, P.; Ananpattarachai, J.; Pongpom, S. Sol–gel preparation and properties study of TiO2 thin film for photocatalytic reduction of chromium (VI) in photocatalysis process. Sci. Technol. Adv. Mater. 2005, 6, 352–358. [Google Scholar] [CrossRef]
- Bai, X.; Yang, S.; Tan, C.; Jia, T.; Guo, L.; Song, W.; Jian, M.; Zhang, X.; Zhang, Z.; Wu, L.; et al. Synthesis of TiO2 based superhydrophobic coatings for efficient anti-corrosion and self-cleaning on stone building surface. J. Clean. Prod. 2022, 380, 134975. [Google Scholar] [CrossRef]
- Folli, A.; Pochard, I.; Nonat, A.; Jakobsen, U.H.; Shepherd, A.M.; Macphee, D.E. Engineering photocatalytic cements: Understanding TiO2 surface chemistry to control and modulate photocatalytic performances. J. Am. Ceram. Soc. 2010, 93, 3360–3369. [Google Scholar] [CrossRef]
- Jennings, H.M. A model for the microstructure of calcium silicate hydrate in cement paste. Cem. Concr. Res. 2000, 30, 101–116. [Google Scholar] [CrossRef]
- Jo, B.W.; Kim, C.H.; Tae, G.H.; Park, J.B. Characteristics of cement mortar with nano-SiO2 particles. Constr. Build. Mater. 2007, 21, 1351–1355. [Google Scholar] [CrossRef]
- Li, H.; Xiao, H.G.; Yuan, J.; Ou, J. Microstructure of cement mortar with nano-particles. Compos. Part B 2004, 35, 185–189. [Google Scholar] [CrossRef]
- Monteiro, P.J.; Kirchheim, A.P.; Chae, S.; Fischer, P.; MacDowell, A.A.; Schaible, E.; Wenk, H.R. Characterizing the nano and micro structure of concrete to improve its durability. Cem. Concr. Compos. 2009, 31, 577–584. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.H.; Ou, J.P. Abrasion resistance of concrete containing nano-particles for pavement. Wear 2006, 260, 1262–1266. [Google Scholar] [CrossRef]
- Qing, Y.; Zenan, Z.; Deyu, K.; Rongshen, C. Influence of nano-SiO2 addition on properties of hardened cement paste as compared with silica fume. Constr. Build. Mater. 2007, 21, 539–545. [Google Scholar] [CrossRef]
- Sanchez, F.; Ince, C. Microstructure and macroscopic properties of hybrid carbon nanofiber/silica fume cement composites. Compos. Sci. Technol. 2009, 69, 1310–1318. [Google Scholar] [CrossRef]
- Senff, L.; Labrincha, J.A.; Ferreira, V.M.; Hotza, D.; Repette, W.L. Effect of nano-silica on rheology and fresh properties of cement pastes and mortars. Constr. Build. Mater. 2009, 23, 2487–2491. [Google Scholar] [CrossRef]
- Li, G.; Lv, L.; Fan, H.; Ma, J.; Li, Y.; Wan, Y.; Zhao, X.S. Effect of the agglomeration of TiO2 nanoparticles on their photocatalytic performance in the aqueous phase. J. Colloid Interface Sci. 2010, 348, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Nanostructured & Amorphous Materials, Inc. (NanoAmor). Available online: http://nanoamor.com (accessed on 9 February 2025).
- Schossler, R.T.; Ojo, S.; Jiang, Z.; Hu, J.; Yu, X. A novel interpretable machine learning model approach for the prediction of TiO2 photocatalytic degradation of air contaminants. Sci. Rep. 2024, 14, 13070. [Google Scholar] [CrossRef]
- Yu, X.; Drnevich, V.P. Soil Water Content and Dry Density by Time Domain Reflectometry. ASCE 2004, 130, 922–934. [Google Scholar] [CrossRef]
- Yu, X.; Drnevich, V.P. Density Compensation of TDR Calibration for Geotechnical Applications. J. ASTM Int. 2005, 2, 16. [Google Scholar] [CrossRef]
- Mills, A. An overview of the methylene blue ISO test for assessing the activities of photocatalytic films. Appl. Catal. B 2012, 128, 144–149. [Google Scholar] [CrossRef]
- Hamidi, F.; Aslani, F. TiO2-based photocatalytic cementitious composites: Materials, properties, influential parameters, and assessment techniques. Nanomaterials 2019, 9, 1444. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Air Quality Guide for Nitrogen Dioxide; EPA-456/F-11-003; U.S. Environmental Protection Agency: Washington, DC, USA, 2011. Available online: https://document.airnow.gov/air-quality-guide-for-nitrogen-dioxide.pdf (accessed on 9 February 2025).
- Rochetto, U.L.; Tomaz, E. Degradation of volatile organic compounds in the gas phase by heterogeneous photocatalysis with titanium dioxide/ultraviolet light. J. Air Waste Manag. Assoc. 2015, 65, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Dhada, I.; Nagar, P.K.; Sharma, M. Challenges of TiO2-based photooxidation of volatile organic compounds: Designing, coating, and regenerating catalyst. Ind. Eng. Chem. Res. 2015, 54, 5381–5387. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, J.; Zhang, X.; Zhao, Y.; Fan, X.; Zhong, S.; Zhang, H.; Yu, X. A generalized predictive model for TiO2–Catalyzed photo-degradation rate constants of water contaminants through artificial neural network. Environ. Res. 2020, 187, 109697. [Google Scholar] [CrossRef] [PubMed]









| Purity | APS | SSA | Color | Morphology | Bulk Density | True Density |
|---|---|---|---|---|---|---|
| >99% | 30–40 nm | 30 m2 g−1 | white | spherical | 0.4 g cm−3 | 3.94 g cm−3 |
| Specimen | Water (g) | Cement (g) | Sand (g) | Nano-TiO2 (g) | Water-Reducer (g) | Total (g) |
|---|---|---|---|---|---|---|
| PM | 65 | 130 | 320 | 0 | 0.39 | 515.39 |
| Ti 0.5 | 65 | 130 | 320 | 0.65 | 0.52 | 516.17 |
| Ti 1 | 65 | 130 | 320 | 1.3 | 0.65 | 516.95 |
| TiO2 Coverage (g/m2) | Rate Constant k (m/s) | UV Intensity (W/m2) | Source |
|---|---|---|---|
| 50 | 4.02 × 10−3 | 10 (corresponds to the sunlight UV intensity on partial cloudy days in the Middle Europe) | [35] |
| 5 | 4.02 × 10−4 | Extrapolate from [35] | |
| 10 | 8.04 × 10−4 | ||
| 20 | 1.61 × 10−3 | ||
| 40 | 3.22 × 10−3 | ||
| 100 | 8.04 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Zhang, B.; Yu, X. Photocatalytic Cement Mortar with Durable Self-Cleaning Performance. Catalysts 2025, 15, 249. https://doi.org/10.3390/catal15030249
Jiang Z, Zhang B, Yu X. Photocatalytic Cement Mortar with Durable Self-Cleaning Performance. Catalysts. 2025; 15(3):249. https://doi.org/10.3390/catal15030249
Chicago/Turabian StyleJiang, Zhuoying, Bin Zhang, and Xiong Yu. 2025. "Photocatalytic Cement Mortar with Durable Self-Cleaning Performance" Catalysts 15, no. 3: 249. https://doi.org/10.3390/catal15030249
APA StyleJiang, Z., Zhang, B., & Yu, X. (2025). Photocatalytic Cement Mortar with Durable Self-Cleaning Performance. Catalysts, 15(3), 249. https://doi.org/10.3390/catal15030249








