Large-Scale Preparation of Granular Sludge@MOF-Derived Hierarchical Porous Carbon Catalysts for Advanced Oxidation Process: Preparation Process and Intrinsic Degradation Mechanism
Abstract
:1. Introduction
2. Results and Discussion
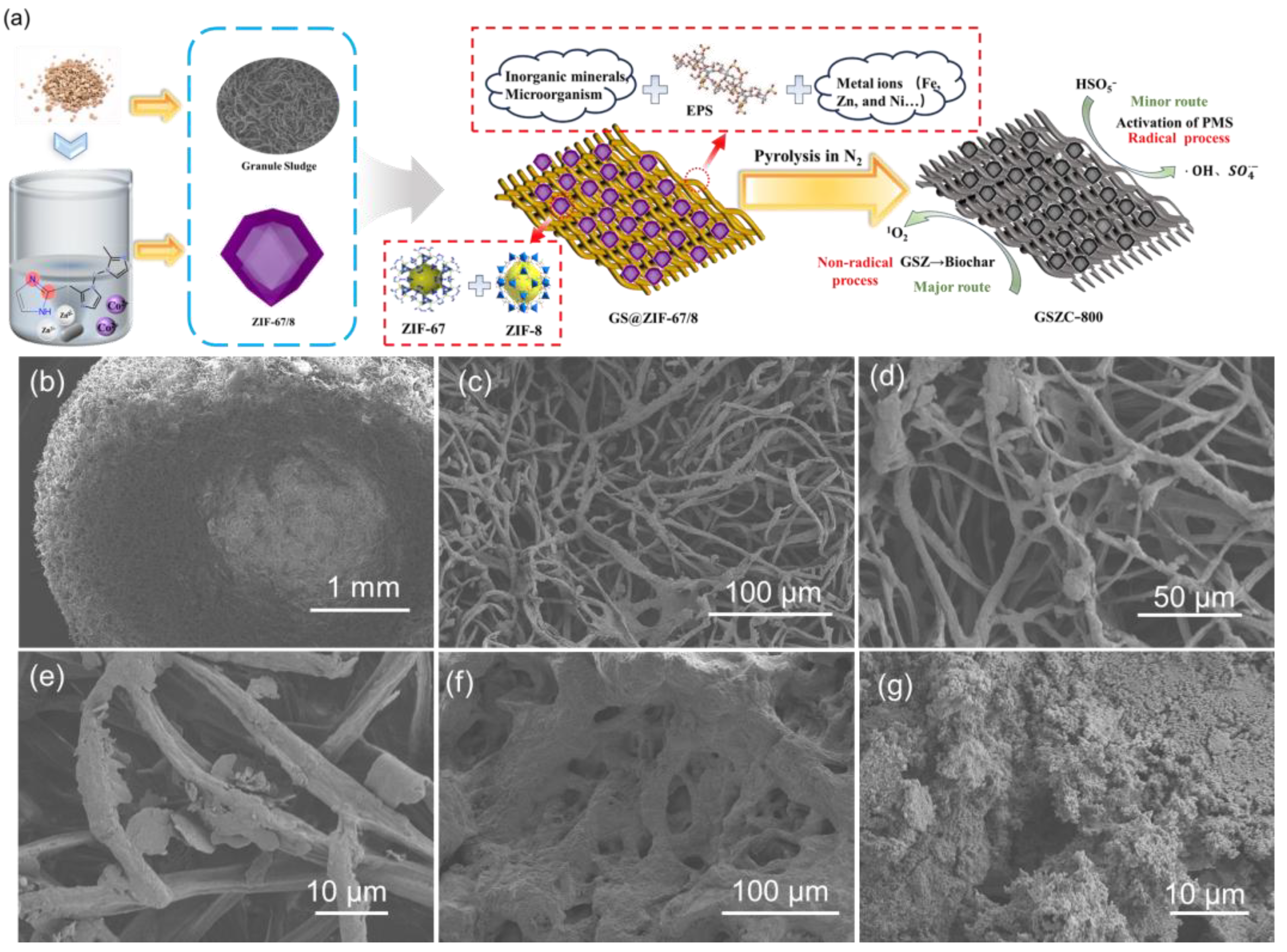
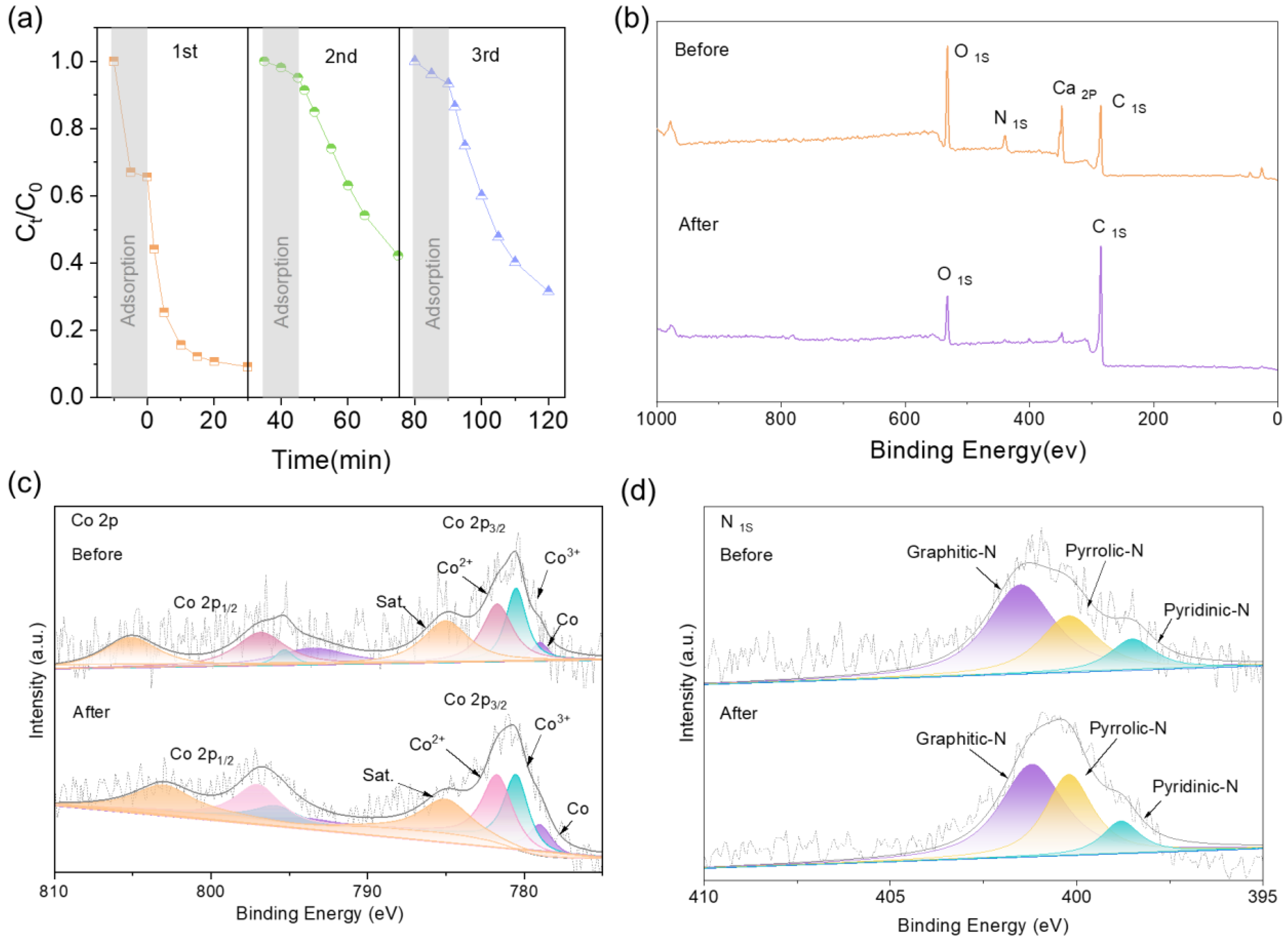
2.1. Catalyst Characterization
2.2. Catalytic Degradation Experiments
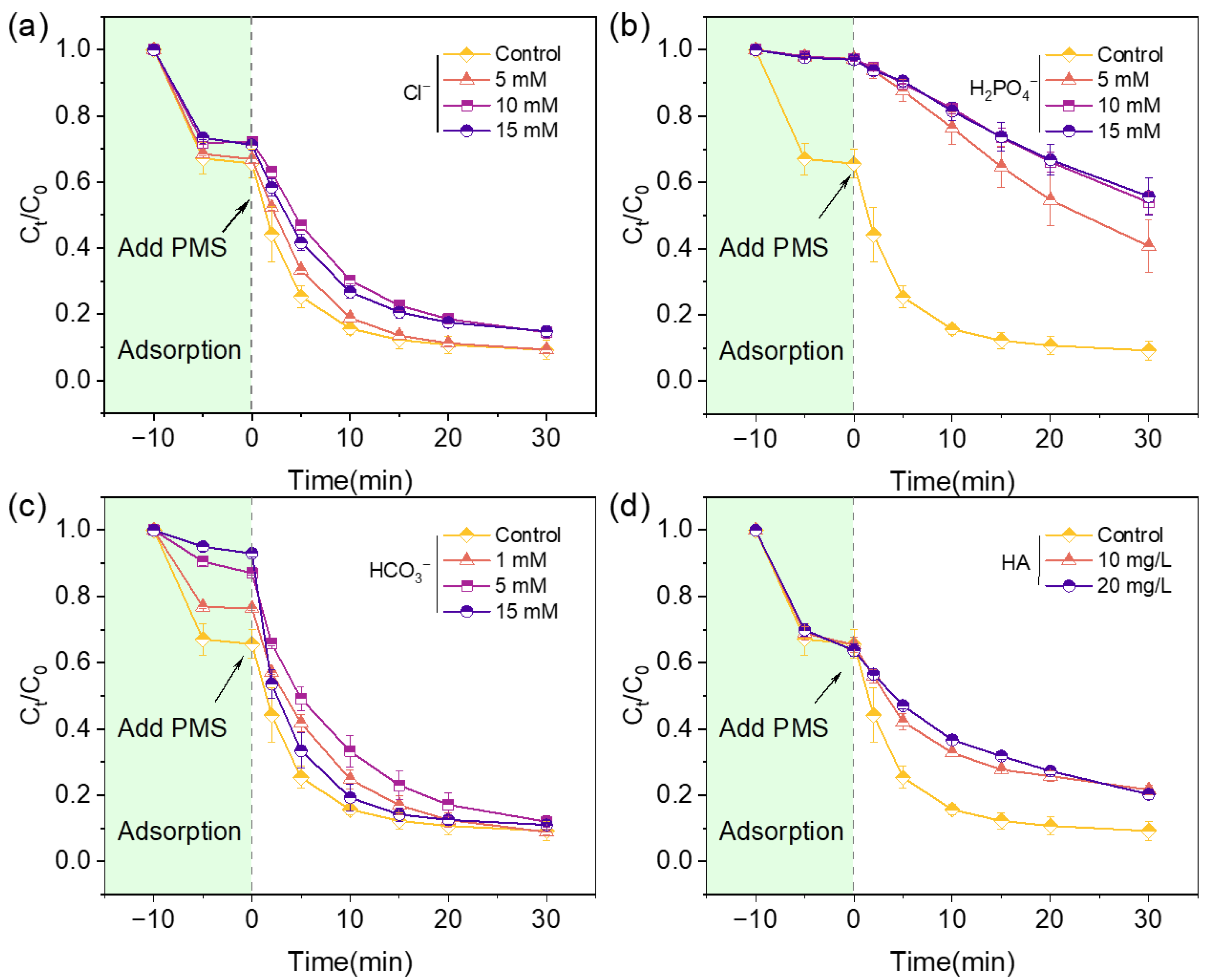
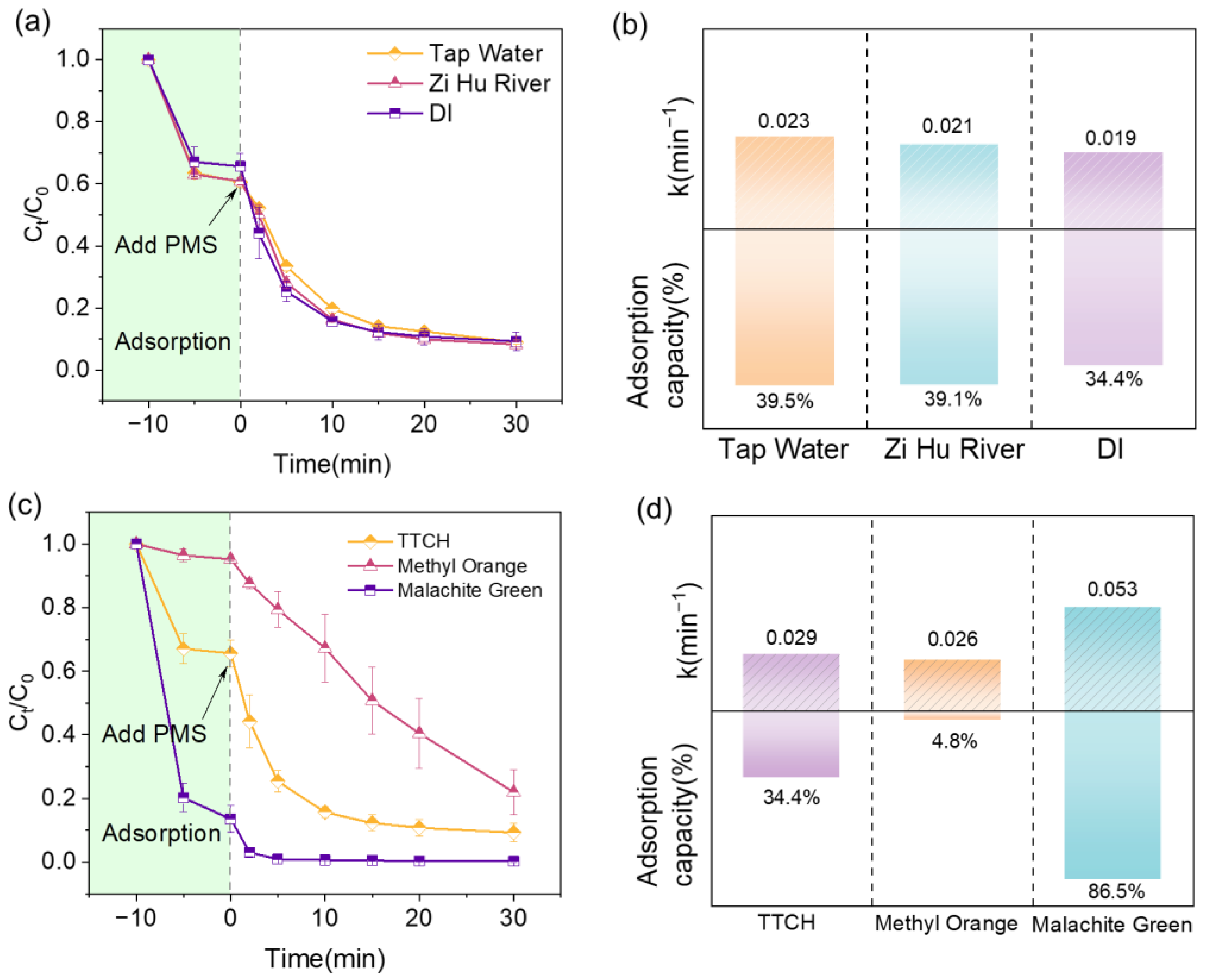
2.3. The Anions and Natural Organic Matter Effects
2.4. Degradation Mechanism
2.4.1. Radical Quenching Experiment Results
2.4.2. Results of Premixing Experiments
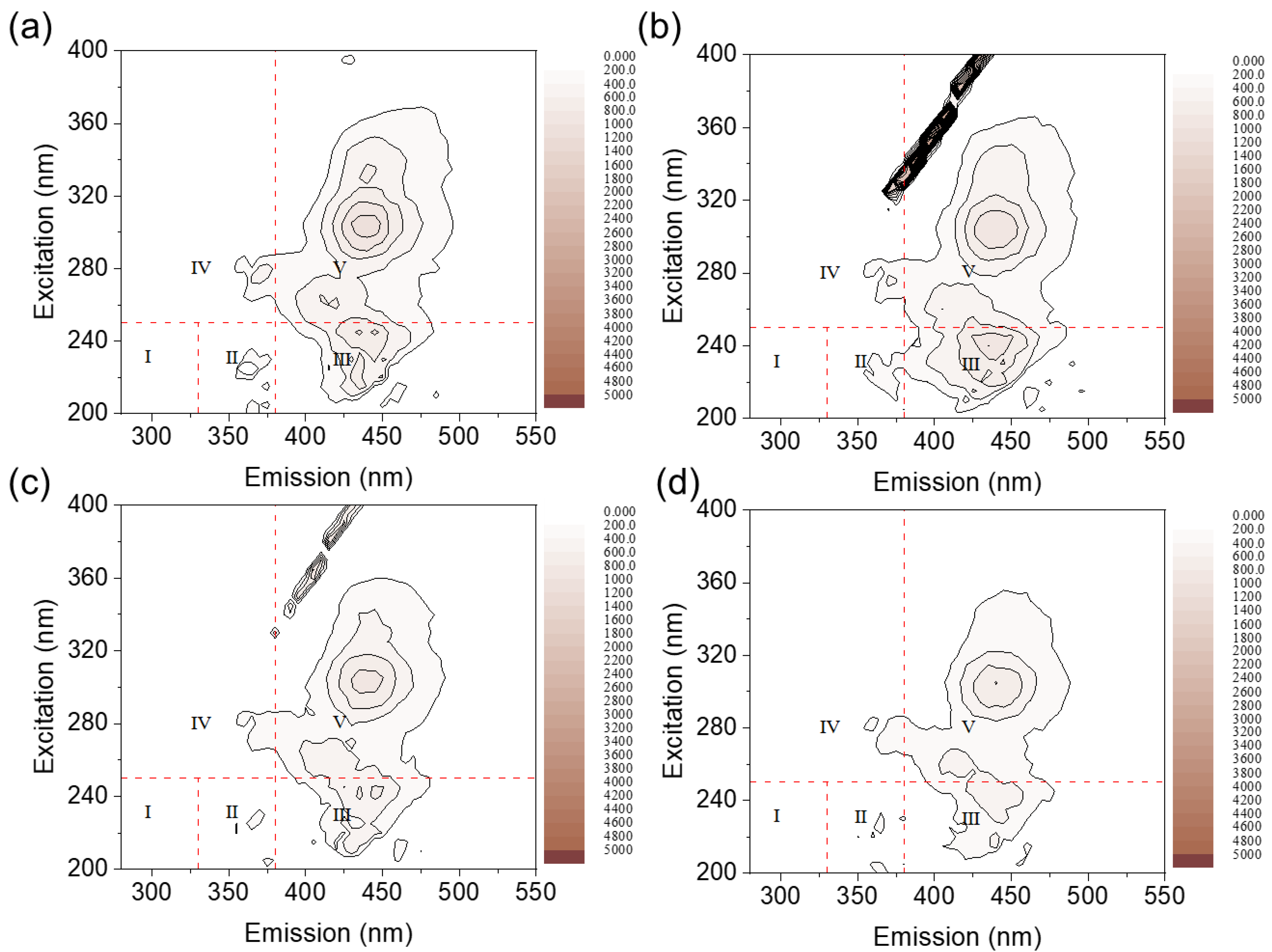
2.4.3. Two-Dimensional Fluorescence Analysis
2.4.4. Results of Three-Dimensional Fluorescence Experiments
2.5. Regeneration Performance and Actual Water Application Results
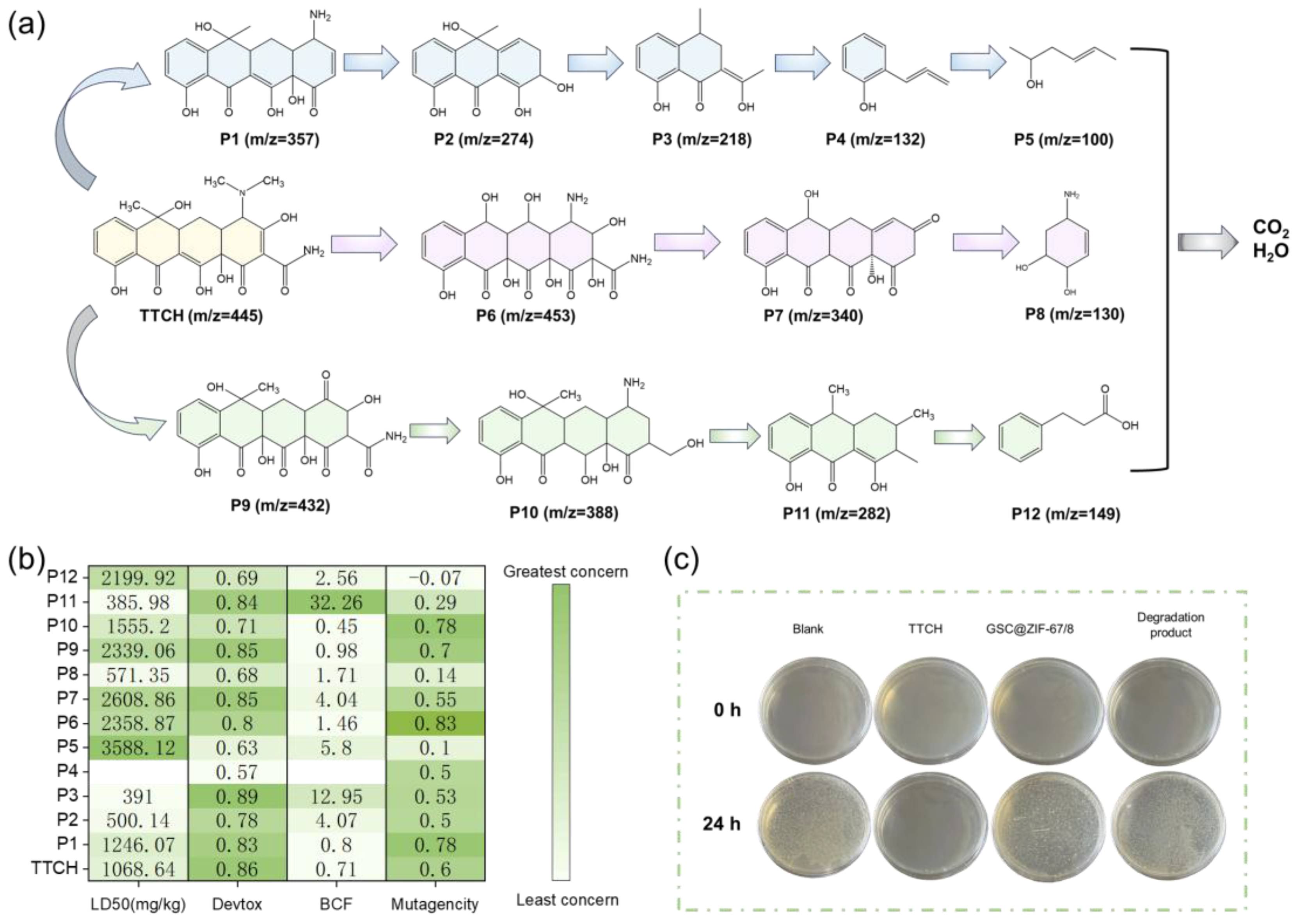
2.6. Possible Degradation Pathways and Toxicology Research
3. Experiment
3.1. Materials
3.1.1. Synthesis of Granular Sludge Loading ZIF-67/8 (GSZ)
3.1.2. Synthesis of Granular Sludge Loading ZIF-67/8 Biomass Porous Carbon Materials (GSZC-800)
3.2. Experiment Procedures
3.2.1. Catalytic Performance Experiment
3.2.2. Anion and Natural Organic Matter Interference Experiments
3.2.3. Radical Quenching Experiment
3.2.4. Fluorescence Spectral Analysis
3.2.5. Experiments of Regeneration Performance and Application in Real Water Bodies
3.2.6. Biological Toxicity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Kiran Kumar Reddy, G. Aerobic granular sludge technology: Mechanisms of granulation and biotechnological applications. Bioresour. Technol. 2018, 247, 1128–1143. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, G.; Wei, D.; Yan, T.; Xue, X.; Shi, S.; Wei, Q. Preparation and utilization of anaerobic granular sludge-based biochar for the adsorption of methylene blue from aqueous solutions. J. Mol. Liq. 2014, 198, 334–340. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Lins, P.V.d.S.; Oliveira, L.M.T.d.M.; Sepulveda, P.; Ighalo, J.O.; Rajapaksha, A.U.; Meili, L. Sewage sludge-derived biochar for the adsorptive removal of wastewater pollutants: A critical review. Environ. Pollut. 2022, 293, 118581. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.; Fu, B. Conversion of sewage sludge into environmental catalyst and microbial fuel cell electrode material: A review. Sci. Total Environ. 2019, 666, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Fowler, G.D.; Pullket, S.; Graham, N.J.D. The production of attrition resistant, sewage–sludge derived, granular activated carbon. Sep. Purif. Technol. 2012, 98, 240–248. [Google Scholar] [CrossRef]
- Zhang, A.; Li, X.; Xing, J.; Xu, G. Adsorption of potentially toxic elements in water by modified biochar: A review. J. Environ. Chem. Eng. 2020, 8, 104196. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater. Chem. Eng. J. 2019, 356, 350–358. [Google Scholar] [CrossRef]
- Wen, G.; Pan, Z.-H.; Ma, J.; Liu, Z.-Q.; Zhao, L.; Li, J.-J. Reuse of sewage sludge as a catalyst in ozonation—Efficiency for the removal of oxalic acid and the control of bromate formation. J. Hazard. Mater. 2012, 239–240, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chi, Q.; Liu, X.; Wang, Y. Influence of pyrolysis temperature on characteristics and environmental risk of heavy metals in pyrolyzed biochar made from hydrothermally treated sewage sludge. Chemosphere 2019, 216, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, C.; Na, J.; Hossain, M.S.A.; Yan, X.; Zhang, H.; Amin, M.A.; Qi, J.; Yamauchi, Y.; Li, J. Macroscopic MOF Architectures: Effective Strategies for Practical Application in Water Treatment. Small 2022, 18, 2104387. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.; Tang, J.; Kim, M.; Lim, H.; Malgras, V.; You, J.; Xu, Q.; Li, J.; Yamauchi, Y. New Strategies for Novel MOF-Derived Carbon Materials Based on Nanoarchitectures. Chem 2020, 6, 19–40. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Li, C.; Zhang, Q.; Sun, J.; Wang, X.; Guo, J.; Xie, L.; Xie, J.; He, B.; et al. MOF for template-directed growth of well-oriented nanowire hybrid arrays on carbon nanotube fibers for wearable electronics integrated with triboelectric nanogenerators. Nano Energy 2018, 45, 420–431. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Li, Y.; Yao, L.; Zhang, H. Accelerated photocatalytic degradation of organic pollutant over metal-organic framework MIL-53(Fe) under visible LED light mediated by persulfate. Appl. Catal. B Environ. 2017, 202, 165–174. [Google Scholar] [CrossRef]
- Dai, R.; Wang, X.; Tang, C.Y.; Wang, Z. Dually Charged MOF-Based Thin-Film Nanocomposite Nanofiltration Membrane for Enhanced Removal of Charged Pharmaceutically Active Compounds. Environ. Sci. Technol. 2020, 54, 7619–7628. [Google Scholar] [CrossRef]
- Zhang, S.; Du, M.; Shao, P.; Wang, L.; Ye, J.; Chen, J.; Chen, J. Carbonic Anhydrase Enzyme-MOFs Composite with a Superior Catalytic Performance to Promote CO2 Absorption into Tertiary Amine Solution. Environ. Sci. Technol. 2018, 52, 12708–12716. [Google Scholar] [CrossRef]
- Li, H.; Tian, J.; Zhu, Z.; Cui, F.; Zhu, Y.-A.; Duan, X.; Wang, S. Magnetic nitrogen-doped nanocarbons for enhanced metal-free catalytic oxidation: Integrated experimental and theoretical investigations for mechanism and application. Chem. Eng. J. 2018, 354, 507–516. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Jhung, S.H. Adsorptive removal of wide range of pharmaceuticals and personal care products from water using bio-MOF-1 derived porous carbon. Microporous Mesoporous Mater. 2018, 270, 102–108. [Google Scholar] [CrossRef]
- Ma, S.; Goenaga, G.A.; Call, A.V.; Liu, D.-J. Cobalt Imidazolate Framework as Precursor for Oxygen Reduction Reaction Electrocatalysts. Chem. Eur. J. 2011, 17, 2063–2067. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Cai, X.; Jiang, H.-L. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef]
- Shi, K.-X.; Qiu, F.; Wang, J.-W.; Wang, P.; Li, H.-Y.; Wang, C.-C. Sulfamethoxazole degradation via peroxydisulfate activation over WO3/MIL-100(Fe) under low power LED visible light. Sep. Purif. Technol. 2023, 309, 122991. [Google Scholar] [CrossRef]
- Wang, F.-X.; Zhang, Z.-C.; Wang, C.-C. Selective oxidation of aqueous organic pollutants over MOFs-based catalysts: A mini review. Chem. Eng. J. 2023, 459, 141538. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.-T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Ahnfeldt, T.; Gunzelmann, D.; Wack, J.S.; Senker, J.; Stock, N. Controlled modification of the inorganic and organic bricks in an Al-based MOF by direct and post-synthetic synthesis routes. CrystEngComm 2012, 14, 4126–4136. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Zhao, J.; Cong, Z.; Hu, J.; Lu, H.; Wang, L.; Wang, H.; Malyi, O.I.; Pu, X.; Zhang, Y.; Shao, H.; et al. Regulating zinc electroplating chemistry to achieve high energy coaxial fiber Zn ion supercapacitor for self-powered textile-based monitoring system. Nano Energy 2022, 93, 106893. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Z.; Shao, W.; Tian, T.; Wang, X.; Chen, Z.; Qiao, W.; Gu, C. A confined expansion pore-making strategy to transform Zn-MOF to porous carbon nanofiber for water treatment: Insight into formation and degradation mechanism. J. Colloid Interface Sci. 2023, 652, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xiao, C.; Zhang, C.; Qi, J.; Wang, C.; Sun, X.; Wang, L.; Xu, Q.; Li, J. Large-Scale Synthesis of Biomass@MOF-Derived Porous Carbon/Cobalt Nanofiber for Environmental Remediation by Advanced Oxidation Processes. ACS EST Eng. 2021, 1, 249–260. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Xue, H.; Pang, H.; Xu, Q. Metal–organic frameworks as a platform for clean energy applications. EnergyChem 2020, 2, 100027. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, H.; Miao, Y.; Tian, C.; Li, W.; Song, Y.; Zhang, Y. Influence of the Conductivity of Polymer Matrix on the Photocatalytic Activity of PAN–PANI–ZIF8 Electrospun Fiber Membranes. Fibers Polym. 2023, 24, 1253–1264. [Google Scholar] [CrossRef]
- Dong, X.; Ma, L.Q.; Li, Y. Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J. Hazard. Mater. 2011, 190, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef]
- Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y.; Song, Y. In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem. Commun. 2011, 47 47, 12694–12696. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical Generation by the Interaction of Transition Metals with Common Oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Z.; Ruan, J.; Shao, W.; Wei, W.; Zhang, W.; Qiao, W.; Li, J. Functional polymers-assisted confined pyrolysis strategy to transform MOF into hierarchical Co/N-doped carbon for peroxymonosulfate advanced oxidation processes. Sep. Purif. Technol. 2023, 305, 122407. [Google Scholar] [CrossRef]
- Huang, G.-X.; Si, J.-Y.; Qian, C.; Wang, W.-K.; Mei, S.-C.; Wang, C.-Y.; Yu, H.-Q. Ultrasensitive Fluorescence Detection of Peroxymonosulfate Based on a Sulfate Radical-Mediated Aromatic Hydroxylation. Anal. Chem. 2018, 90, 14439–14446. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Molavi, H.; Salimi, M.S. Green Synthesis of Cerium-Based Metal–Organic Framework (Ce-UiO-66 MOF) for Wastewater Treatment. Langmuir 2023, 39, 17798–17807. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jafarpour, E.; Mirzaei, K.; Shojaei, A.; Jafarpour, P.; Beikmohammadi Eyni, M.; Mirzaei, S.; Molavi, H. Novel ZIF-8/CNC Nanohybrid with an Interconnected Structure: Toward a Sustainable Adsorbent for Efficient Removal of Cd(II) Ions. ACS Appl. Mater. Interfaces 2024, 16, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ruan, J.; Wang, L.; Zhao, Z.; Shao, W.; Li, J.; Chen, Z.; Gu, C.; Qiao, W. MXene-like carbon sheet/carbon nanotubes derived from metal-organic frameworks for efficient removal of tetracycline by non-radical dominated advanced oxidation processes. Sep. Purif. Technol. 2022, 300, 121851. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, M.; Yuan, D.; Li, X.; Wang, Z.; Zhang, X.; Jiao, T.; Ke, J. Fe3O4 nanoparticles three-dimensional electro-peroxydisulfate for improving tetracycline degradation. Chemosphere 2021, 268, 129315. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Wan, D.; Huang, J.; Liu, Y. Peroxymonosulfate-enhanced photocatalysis by carbonyl-modified g-C3N4 for effective degradation of the tetracycline hydrochloride. Sci. Total Environ. 2020, 749, 142313. [Google Scholar] [CrossRef]
- Yang, D.; Hong, P.; Hu, Y.; Li, Y.; Wang, C.; He, J.; Sun, B.; Zhu, S.; Kong, L.; Liu, J. Carbon framework-encapsulated copper oxide particles to activate peroxymonosulfate for the efficient degradation of tetracycline. Appl. Surf. Sci. 2021, 552, 149424. [Google Scholar] [CrossRef]
- Tian, T.; Shen, X.; Zhang, M.; Chen, Z.; Shao, W.; Liu, C.; Li, W.; Gu, C.; Qiao, W. A hydrothermal-assisted strategy to transform Fe-doped MOF into 3D interwoven network structure of CNTs boosting the degradation of antibiotics via peroxymonosulfate activation. J. Environ. Chem. Eng. 2024, 12, 113644. [Google Scholar] [CrossRef]
- Su, X.; Guo, Y.; Yan, L.; Wang, Q.; Zhang, W.; Li, X.; Song, W.; Li, Y.; Liu, G. MoS2 nanosheets vertically aligned on biochar as a robust peroxymonosulfate activator for removal of tetracycline. Sep. Purif. Technol. 2022, 282, 120118. [Google Scholar] [CrossRef]
- Wang, G.; Chen, S.; Quan, X.; Yu, H.; Zhang, Y. Enhanced activation of peroxymonosulfate by nitrogen doped porous carbon for effective removal of organic pollutants. Carbon 2017, 115, 730–739. [Google Scholar] [CrossRef]
- Huong, P.T.; Jitae, K.; Al Tahtamouni, T.M.; Le Minh Tri, N.; Kim, H.-H.; Cho, K.H.; Lee, C. Novel activation of peroxymonosulfate by biochar derived from rice husk toward oxidation of organic contaminants in wastewater. J. Water Process Eng. 2020, 33, 101037. [Google Scholar] [CrossRef]
- Han, S.; Xiao, P. Catalytic degradation of tetracycline using peroxymonosulfate activated by cobalt and iron co-loaded pomelo peel biochar nanocomposite: Characterization, performance and reaction mechanism. Sep. Purif. Technol. 2022, 287, 120533. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, Y.; Chen, Z.; Yu, R.; Ye, Y.; Ma, R.; Li, K.; Wang, L.; Wang, D.; Ni, B.-J. Sulfur-decorated Fe/C composite synthesized from MIL-88A(Fe) for peroxymonosulfate activation towards tetracycline degradation: Multiple active sites and non-radical pathway dominated mechanism. J. Environ. Manag. 2023, 344, 118440. [Google Scholar] [CrossRef]
- Hu, F.; Ning, S.; Li, Z.; Zhu, H.; Fujita, T.; Yin, X.; Chen, L.; Zeng, D.; Hamza, M.F.; Wei, Y.; et al. A new strategy to construct MOF-on-MOF derivatives for the removal of tetracycline hydrochloride from water by activation of peroxymonosulfate. Chemosphere 2024, 362, 142676. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, D.; Zhang, R.; Ding, Y.; Ren, Z.; Fu, M.; Cao, X.; Zeng, G. Singlet oxygen-dominated activation of peroxymonosulfate by passion fruit shell derived biochar for catalytic degradation of tetracycline through a non-radical oxidation pathway. J. Hazard. Mater. 2021, 419, 126495. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, L.; Zhang, X.; Liu, Z.; Li, T.; Wang, H. Green synthesis of FeCu@biochar nanocomposites through a mechanochemical method for enhanced tetracycline degradation via peroxymonosulfate activation. Sep. Purif. Technol. 2024, 328, 125077. [Google Scholar] [CrossRef]
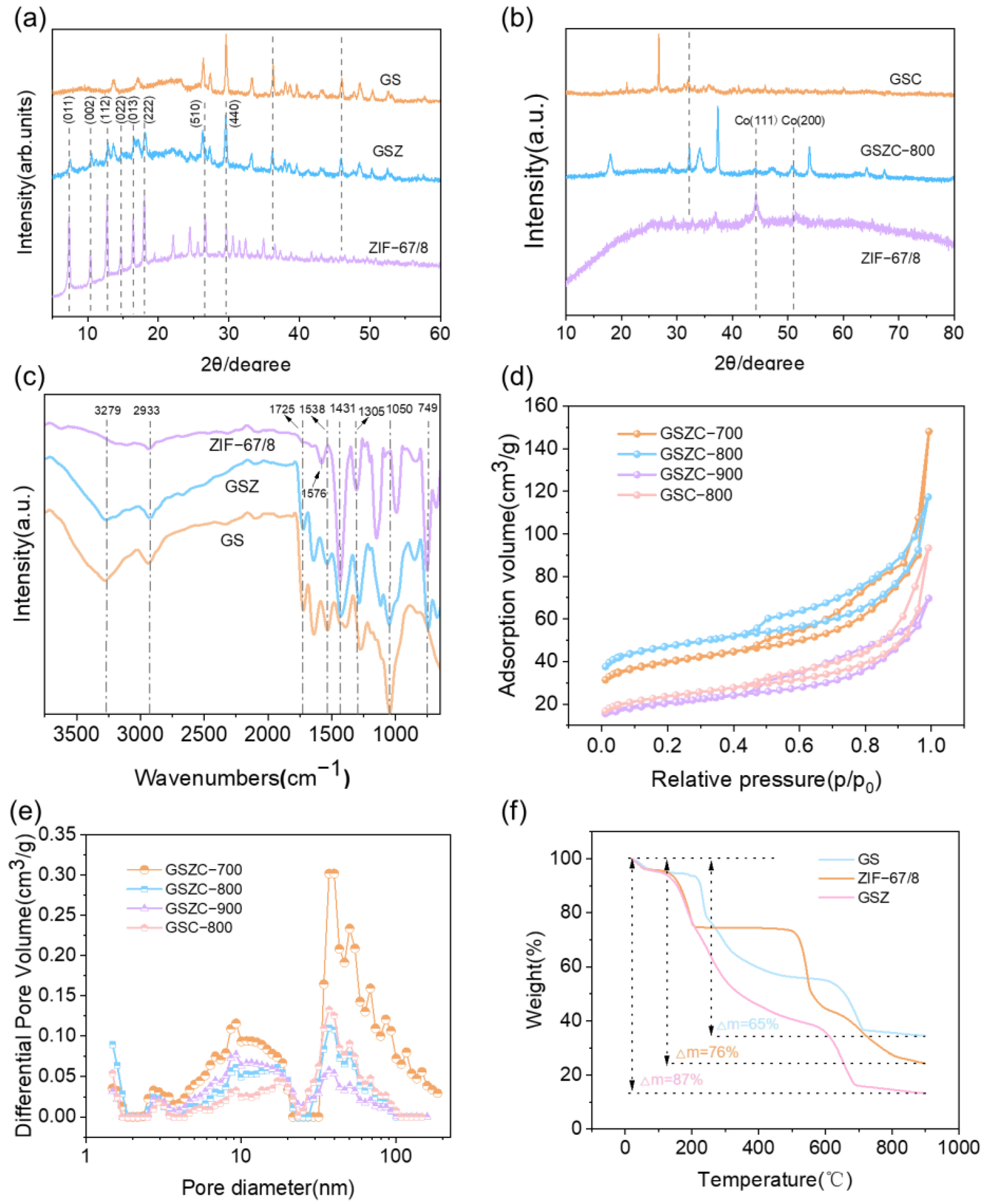



| Sample | SBET (m2/g) | Vpore (cm3g−1) | Pore Size (nm) |
|---|---|---|---|
| GSZC-700 | 129.43 | 0.20 | 7.08 |
| GSZC-800 | 150.61 | 0.14 | 4.82 |
| GSZC-900 | 69.50 | 0.10 | 6.20 |
| GSC-800 | 79.10 | 0.13 | 7.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, Z.; Liu, C.; Li, W.; Yao, X.; Tian, T.; Zhao, C.; Tao, S.; Qiao, W.; Zhang, M. Large-Scale Preparation of Granular Sludge@MOF-Derived Hierarchical Porous Carbon Catalysts for Advanced Oxidation Process: Preparation Process and Intrinsic Degradation Mechanism. Catalysts 2025, 15, 264. https://doi.org/10.3390/catal15030264
Liu Y, Chen Z, Liu C, Li W, Yao X, Tian T, Zhao C, Tao S, Qiao W, Zhang M. Large-Scale Preparation of Granular Sludge@MOF-Derived Hierarchical Porous Carbon Catalysts for Advanced Oxidation Process: Preparation Process and Intrinsic Degradation Mechanism. Catalysts. 2025; 15(3):264. https://doi.org/10.3390/catal15030264
Chicago/Turabian StyleLiu, Yu, Zhonglin Chen, Chenyong Liu, Wenhui Li, Xiyue Yao, Tian Tian, Chenyu Zhao, Shaoqun Tao, Weichuan Qiao, and Ming Zhang. 2025. "Large-Scale Preparation of Granular Sludge@MOF-Derived Hierarchical Porous Carbon Catalysts for Advanced Oxidation Process: Preparation Process and Intrinsic Degradation Mechanism" Catalysts 15, no. 3: 264. https://doi.org/10.3390/catal15030264
APA StyleLiu, Y., Chen, Z., Liu, C., Li, W., Yao, X., Tian, T., Zhao, C., Tao, S., Qiao, W., & Zhang, M. (2025). Large-Scale Preparation of Granular Sludge@MOF-Derived Hierarchical Porous Carbon Catalysts for Advanced Oxidation Process: Preparation Process and Intrinsic Degradation Mechanism. Catalysts, 15(3), 264. https://doi.org/10.3390/catal15030264









