Abstract
With the intensification of the global energy crisis, hydrogen has attracted significant attention as a high-energy-density and zero-emission clean energy source. Traditional hydrogen production methods are dependent on fossil fuels and simultaneously contribute to environmental pollution. The aqueous phase reforming (APR) of renewable biomass and its derivatives has emerged as a research hotspot in recent years due to its ability to produce green hydrogen in an environmentally friendly manner. This review provides an overview of the advancements in APR of lignocellulosic biomass as a sustainable and environmentally friendly method for hydrogen production. It focuses on the reaction pathways of various biomass feedstocks (such as glucose, cellulose, and lignin), as well as the types and performance of catalysts used in the APR process. Finally, the current challenges and future prospects in this field are briefly discussed.
1. Introduction
Hydrogen, known for its highest energy density and zero carbon footprint (142 kJ/g), is hailed as the “ultimate energy of the 21st century”. As a secondary clean energy source, hydrogen holds tremendous development potential, especially with the global push toward carbon peak and carbon neutrality goals, accelerating its development and utilization [1,2,3]. Hydrogen not only serves as a crucial raw material for producing key commercial chemicals, such as ammonia and methanol, but also as an advanced alternative fuel with wide applications in power generation, heating, and transportation [4,5]. However, conventional methods of hydrogen production, such as natural gas reforming and coal gasification, still depend on non-renewable resources and are accompanied by significant carbon emissions [6]. With the continuous growth of global energy demand, the use of fossil fuels is projected to reach 112.2 million barrels per day by 2035. Specifically, coal and natural gas consumption are forecasted to grow by 38% and 25%, respectively [7,8]. The International Energy Agency’s (IEA) 2024 report projects global hydrogen demand to reach 97 million tons. However, the majority of this demand continues to be met through traditional fossil fuel-based hydrogen production, while the output of low-carbon hydrogen remains limited to less than 1 million tons [9]. Excessive dependence on fossil fuels not only intensifies energy supply instability but also contributes to a range of environmental challenges, such as global warming and air pollution, highlighting the pressing need for the development of more sustainable and environmentally responsible hydrogen production methods [10,11]. Renewable energy sources such as wind, geothermal, solar, and biomass have gained significant attention for their potential to enable large-scale hydrogen production [12,13]. Biomass energy, derived from abundant raw materials, has particularly emerged as a prominent focus in research on hydrogen production from renewable resources.
Lignocellulosic biomass, owing to its renewability and abundance, has been widely acknowledged as a significant raw material for hydrogen production [14,15,16]. Comprising organic compounds, biomass can undergo various thermochemical processes to be converted into hydrogen [17,18]. Biomass-based hydrogen production offers a solution to the depletion of fossil fuels while advancing carbon neutrality by harnessing the carbon dioxide absorbed by plants during their growth, thereby mitigating greenhouse gas emissions. The predominant technologies for biomass hydrogen production, such as steam reforming, partial oxidation, and autothermal reforming, enable efficient conversion of biomass into hydrogen. Nevertheless, these processes are often complex, energy-intensive, and involve multiple reaction stages, which heighten the challenges and costs associated with their industrial implementation [19,20,21,22]. Consequently, researchers are intensively exploring more cost-effective and efficient hydrogen production technologies. APR, in particular, has attracted considerable attention owing to its lower energy consumption and enhanced hydrogen selectivity. APR is a thermochemical catalytic process carried out in the liquid aqueous phase, enabling the production of hydrogen from oxygenated hydrocarbons [23,24]. The APR reaction is composed of two fundamental steps: initially, the reactants undergo endothermic cleavage to yield carbon monoxide (CO) and hydrogen (H2), subsequently, CO participates in an exothermic water-gas shift (WGS) reaction with H2O [25,26]. The use of biomass for hydrogen production through APR offers several distinct advantages:
- (1)
- Biomass is a readily available feedstock, with its derivatives (such as glycerol, ethylene glycol, glucose, etc.) demonstrating considerable potential for APR-based hydrogen production. These raw materials are non-toxic, non-flammable, and exhibit favorable transport safety characteristics.
- (2)
- Biomass naturally contains substantial moisture content, which can be directly harnessed in the APR process for reforming reactions. This eliminates the need for the cumbersome drying pre-treatment stages associated with conventional gasification methods, thereby reducing both energy consumption and process complexity.
- (3)
- Operating under relatively mild conditions, APR enables lower energy usage and cost compared to traditional hydrogen production techniques [27].
- (4)
- The entire reaction process can be carried out in a single reactor, thereby simplifying the equipment configuration and reducing associated costs.
This review provides a comprehensive overview of APR technology for green hydrogen production from lignocellulosic biomass and its derivatives. While previous studies have predominantly focused on the APR of biomass derivatives, limited attention has been given to the specific reaction pathways for biomass feedstocks. Consequently, this work highlights the feasibility and reaction mechanisms of biomass feedstock APR, drawing on both established and recent literature. Additionally, it explores the design of catalysts optimized for the APR process. By synthesizing these recent advancements, this review aims to advance biomass-based APR technologies, thereby making a significant contribution to the development of sustainable energy solutions.
2. Aqueous Phase Reforming of Lignocellulosic Biomass and Derivatives
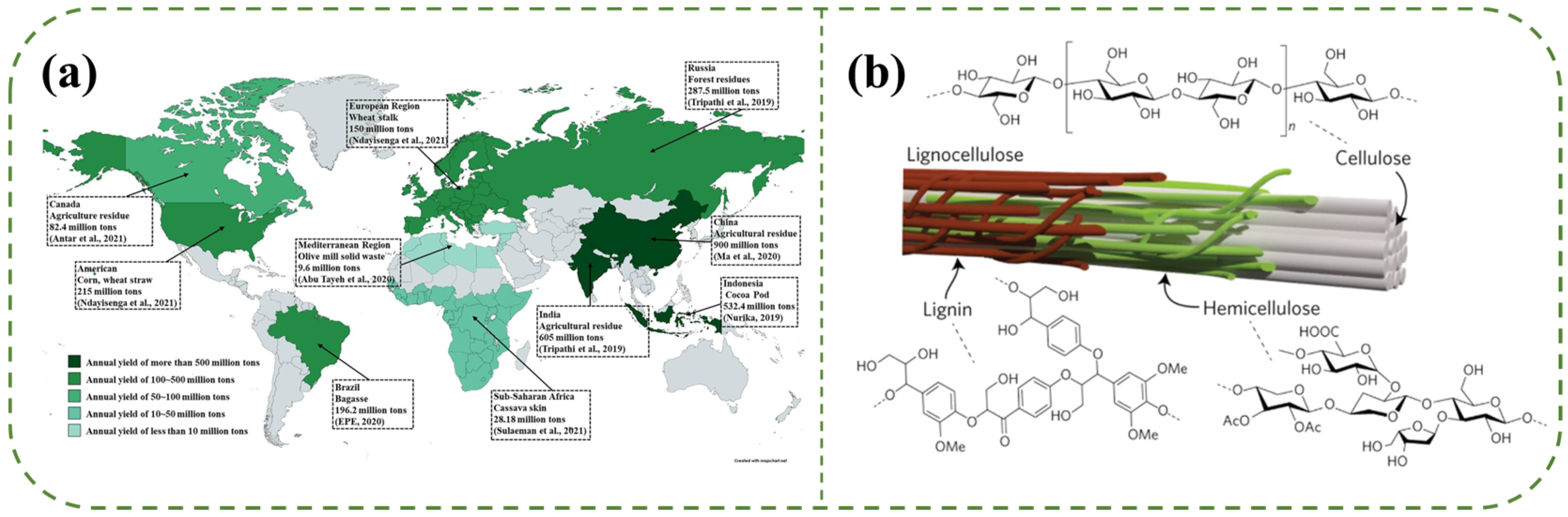
Global annual production of lignocellulosic biomass is approximately 200 billion metric tons, predominantly sourced from agricultural and forestry residues. China stands as the largest producer of agricultural waste, generating over 900 million tons, primarily from rice straw, wheat straw, and corn stover (Figure 1a) [28,29,30]. Lignocellulosic biomass accounts for over 90% of all plant biomass, comprising three polymers with intricate structural configurations: cellulose (35−50 wt%), hemicellulose (20−35 wt%), and lignin (10−25 wt%). Additionally, it contains extractives and inorganic salts [30,31]. As illustrated in Figure 1b, cellulose serves as a reinforcing structural framework within the plant cell wall, enhancing its mechanical strength. Hemicellulose forms hydrogen bonds with cellulose, providing an additional toughening effect. Lignin envelops both cellulose and hemicellulose, functioning as a binding matrix [32]. The interplay among these three polymers results in a rigid and densely cross-linked organic structure [33].
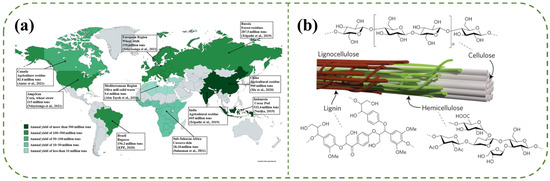
Figure 1.
(a) Global lignocellulose production. Reproduced with permission from [28]; Copyright 2022 Elsevier. (b) Lignocellulose exists as microfibrils in plant cell walls. Reproduced with permission from [34]. Copyright 2017 Springer Nature.
Given the intricate three-component structure of biomass, researchers commonly utilize oxygenated derivative model compounds to conduct preliminary investigations into APR for hydrogen production from lignocellulosic biomass, including methanol, ethanol, ethylene glycol, glycerol, and sorbitol [20,35,36]. Accordingly, this chapter adopts a bottom-up approach, beginning with representative oxygenated hydrocarbons derived from biomass (methanol, ethanol, glycerol), and offers a comprehensive review of the aqueous phase reforming processes for hydrogen production from the three principal constituents of lignocellulosic biomass.
2.1. APR of Methanol for Hydrogen Production
Methanol (CH3OH), the simplest and lightest alcohol with a hydrogen content of 12.6 wt%, serves as an excellent hydrogen carrier and storage material, and can be obtained from biomass waste [37,38]. Owing to the absence of C-C bonds, methanol serves as an ideal model molecule for investigating APR for hydrogen production. Traditional methanol hydrogen production methods include methanol steam reforming (MSR), partial oxidation of methanol, and autothermal steam reforming [39]. To date, MSR is the most widely employed method for hydrogen production. As an endothermic reaction, it necessitates temperatures ranging from 250 to 350 °C to provide the required thermal energy for achieving high hydrogen yields. In contrast, excessive heat in the system can impair catalyst activity [40,41,42]. Partial oxidation of methanol is an exothermic process, where temperature regulation becomes a critical challenge under high heat conditions [43]. Elevated temperatures can enhance the selectivity of by-product CO, consequently diminishing both hydrogen yield and purity [44]. APR of methanol presents an effective solution to address these challenges. Utilizing water as the reaction medium, it operates with relatively low energy consumption. Furthermore, APR achieves efficient hydrogen production at low temperatures (<100 °C), which further enhances energy efficiency [45,46]. In the APR process of methanol, the C−H and/or O−H bonds first break to dehydrogenate and form a CO intermediate. Subsequently, the WGS reaction, facilitated by the dissociated hydroxyl groups adsorbed from water, converts CO into CO2 and H2 [47]. Notably, during aqueous phase reforming, the hydrogen atoms derived from water increase the hydrogen yield, allowing one molecule of methanol to generate three molecules of hydrogen. On the other hand, in the APR reaction, when the C-O bond dissociates, due to the hydrogen-rich environment in the system, side reactions are likely to occur, leading to the formation of hydrocarbons such as methane and some alkenes. The occurrence of these side reactions reduces the amount of methanol available for hydrogen production and may result in catalyst coke formation [48].
2.2. APR of Ethanol for Hydrogen Production
Ethanol (C2H5OH), derived through biomass fermentation or hydrolysis, is regarded as one of the most promising biofuels for the short-term replacement of fossil fuels due to its non-toxic properties and ease of storage [49,50,51]. The conversion of lignocellulosic biomass into bioethanol, followed by its large-scale transformation into hydrogen, not only increases the efficiency of agricultural and forestry waste utilization but also substantially lowers production costs. Currently, steam reforming (SR) and APR are two effective strategies for hydrogen production from ethanol [52,53,54]. The ethanol steam reforming process is typically carried out in two coupled reactors. Nevertheless, the presence of multiple competing reactions and the susceptibility of catalysts to deactivation under high-temperature conditions result in decreased ethanol conversion rates and reduced hydrogen selectivity [55]. In contrast, ethanol aqueous phase reforming can be carried out in a single reactor, and by obviating the need for water evaporation, it significantly mitigates the occurrence of undesirable competing reactions [56]. Ethanol, with its relatively high hydrogen content, is also a by-product in the APR of glycerol and ethylene glycol [57]. Therefore, investigating the APR of ethanol offers valuable insights for overcoming the challenges encountered in the aqueous phase reforming of polyols and sugars. Notably, ethanol molecules have an O/C ratio of less than 1, leading to distinct differences in the APR process compared to methanol and other polyols. In the APR of methanol, the C-H bonds are activated by adjacent OH groups, facilitating their selective conversion to H2 and CO2 [58]. However, the APR of ethanol involves the activation of non-activated C-H bonds, as well as the cleavage of C-C and C-O bonds. Moreover, due to the relatively low bond energy of C-O bonds, their cleavage in the reactant promotes subsequent hydrogenation reactions. Consequently, the products of ethanol APR include not only H2 and CO2 but also CH4 and acetic acid [59,60,61].
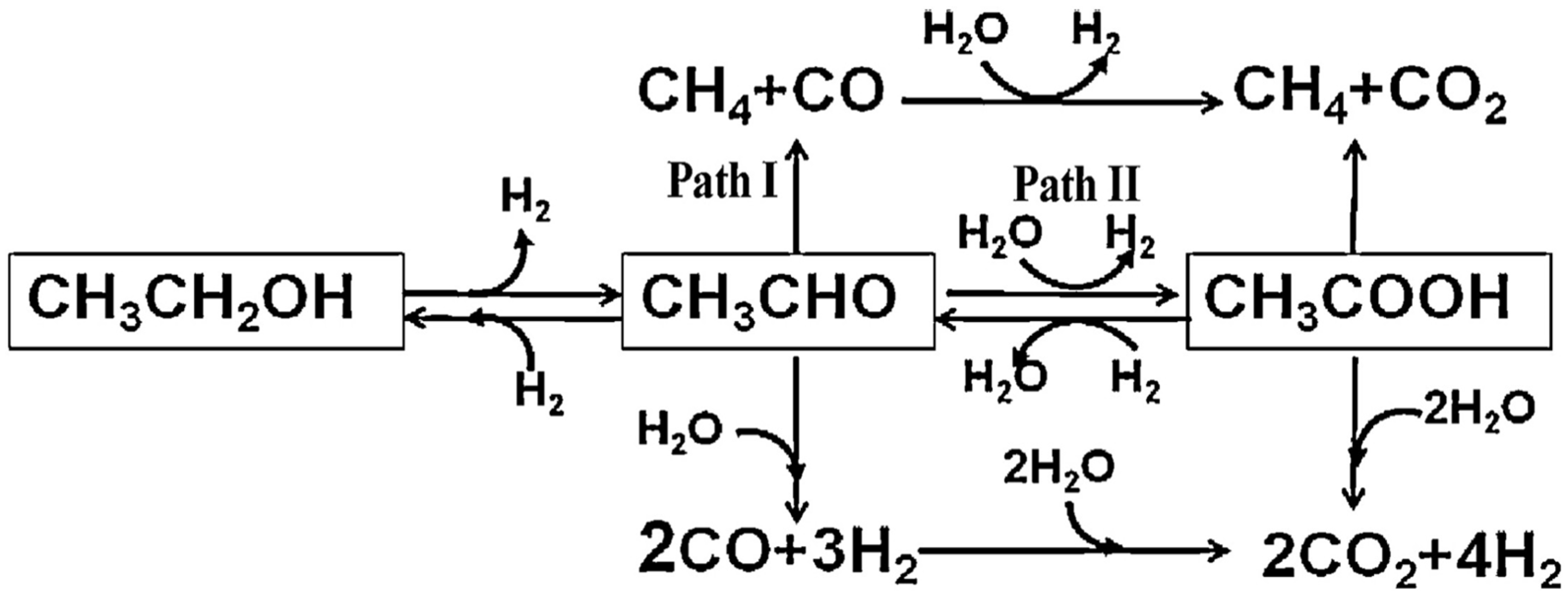
Due to the complexity of ethanol conversion on different catalyst surfaces, extensive research on reaction pathways has led to a widely accepted model for the ethanol APR reaction [62,63]. In this pathway, ethanol first undergoes dehydrogenation to form an acetaldehyde intermediate, which can then be converted via three distinct reaction routes (Figure 2): (1) The C-C bond in acetaldehyde cleaves directly, producing CH4 and CO, with the latter subsequently being converted to CO2 and H2 through the WGS reaction. (2) Acetaldehyde undergoes hydration to form H2 and acetic acid, which then undergoes C-C bond cleavage, yielding CH4 and CO2. Moreover, water may further reform acetic acid to generate CO2 and H2. (3) The acetaldehyde intermediate may react directly with water to form CO and H2, after which CO undergoes reforming with water to produce CO2 and H2. The product distribution in ethanol APR is generally determined by the catalyst’s characteristics and the specific reaction conditions.
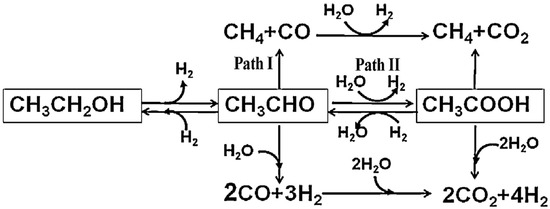
Figure 2.
Reaction network of ethanol APR reaction. Reproduced with permission from [62]. Copyright 2014 Elsevier.
2.3. APR of Glycerol and Oxygenated Hydrocarbons for Hydrogen Production
Glycerol (C3H8O3) serves as a key biomass platform compound and a representative model compound for the catalytic conversion of biomass [64,65]. As a major coproduct of biodiesel production, glycerol constitutes approximately 10 wt% of the total yield, making it an abundant and cost-effective feedstock [66,67]. It is chosen as a model compound for the APR of complex biomass components, such as polyols and sugars, owing to its comparable APR reaction pathway to that of sugar alcohols (e.g., sorbitol and xylitol), while maintaining a relatively simple molecular structure [68,69].
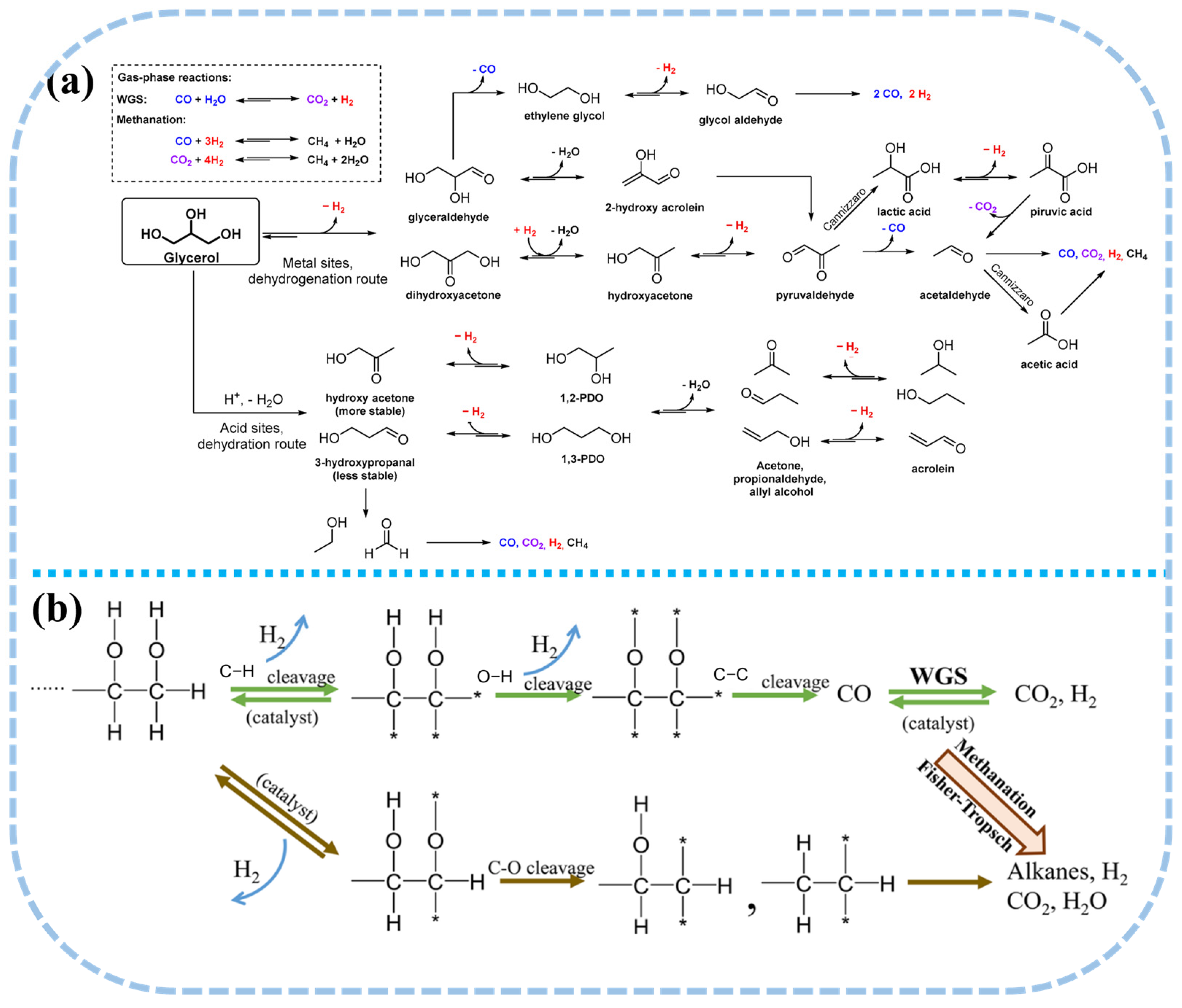
Under optimal conditions, the temperature required for glycerol APR typically falls within the range of 200 to 250 °C. The process primarily involves C-C bond cleavage, leading to the formation of CO and H2, followed by the WGS reaction, which ultimately yields H2 and CO2, with a reforming ratio of 7/3. In practical applications, a single mole of glycerol comprises two moles of C-C bonds, three moles of C-O bonds, and five moles of C-H bonds. Consequently, the aqueous phase reforming process encompasses a series of complex reactions, including hydrogenation, dehydration, dehydrogenation, and C-C bond cleavage, rendering it more intricate than the APR process of methanol [70,71]. Moreover, the liquid phase of glycerol APR also generates oxygenated compounds such as acetic acid, hydroxyacetone, acetaldehyde, and ethanol (Figure 3a). Glycerol readily undergoes C-O bond cleavage through hydrogenation or dehydration reactions, yielding hydroxyacetone and 3-hydroxypropionaldehyde [72,73], which can further be hydrogenated to form propanol or propane [74]. To optimize hydrogen selectivity and minimize the occurrence of side reactions, the catalyst employed in glycerol APR must effectively facilitate the cleavage of C-C and C-H bonds, as well as the WGS reaction, while inhibiting the cleavage of C-O bonds to limit the hydrogenation of CO and CO2. At the low temperatures employed in APR, undesirable reactions are likely to occur. For instance, carbon oxides may undergo further hydrogenation, leading to the formation of methane; glycerol may undergo dehydration or hydrogenation side reactions at acidic sites, resulting in the formation of alcohols; and dehydrogenation reactions on metal surfaces may trigger rearrangement, producing organic acids. These organic acids and alcohols can subsequently be converted into alkanes [75,76].
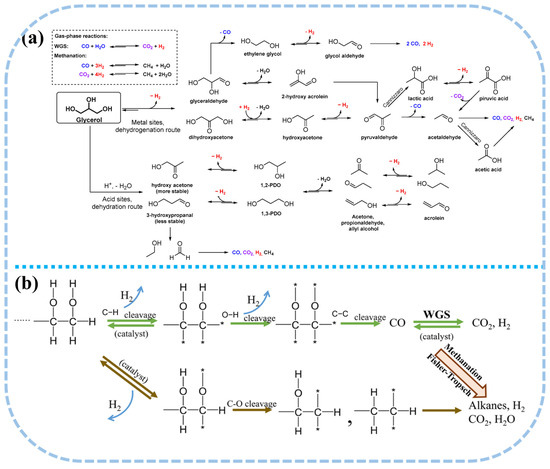
Figure 3.
(a) Possible reaction pathways of glycerol APR reaction. Reproduced with permission from [20]. Copyright 2019 MDPI. (b) Reaction pathways for production of H2 by reactions of oxygenated hydrocarbons APR reaction. (* represents a surface metal site) Reproduced with permission from [77,78]. Copyright 2019 Springer Nature.
Biomass and its derivatives, including methanol, ethanol, glycerol, sugars, sugar alcohols, cellulose, and native biomass, all fall under the category of oxygenated hydrocarbons. By drawing parallels with the APR reaction pathways of methanol and simple polyols, a comparable mechanism for the aqueous reforming of oxygenated hydrocarbons to produce H2 can be inferred (Figure 3b). Upon adsorption onto active sites, reactants undergo C-C and C-H bond cleavage, releasing a portion of H2. Subsequently, the fragmentation of C-C bonds generates smaller molecular intermediates, which, through the WGS reaction, are further reformed to yield additional H2 and CO2. Biomass can generate additional hydrogen from water molecules through the aqueous phase reforming process. However, given that certain C-O bonds possess lower bond dissociation energies than C-C bonds [42], their cleavage leads to the formation of alcohols, alkanes, and other intermediates, which subsequently undergo further conversion into hydrogen. Additionally, side reactions such as methanation and Fischer-Tropsch synthesis may take place, consuming hydrogen and influencing overall reaction efficiency.
2.4. APR of Cellulose and Glucose for Hydrogen Production
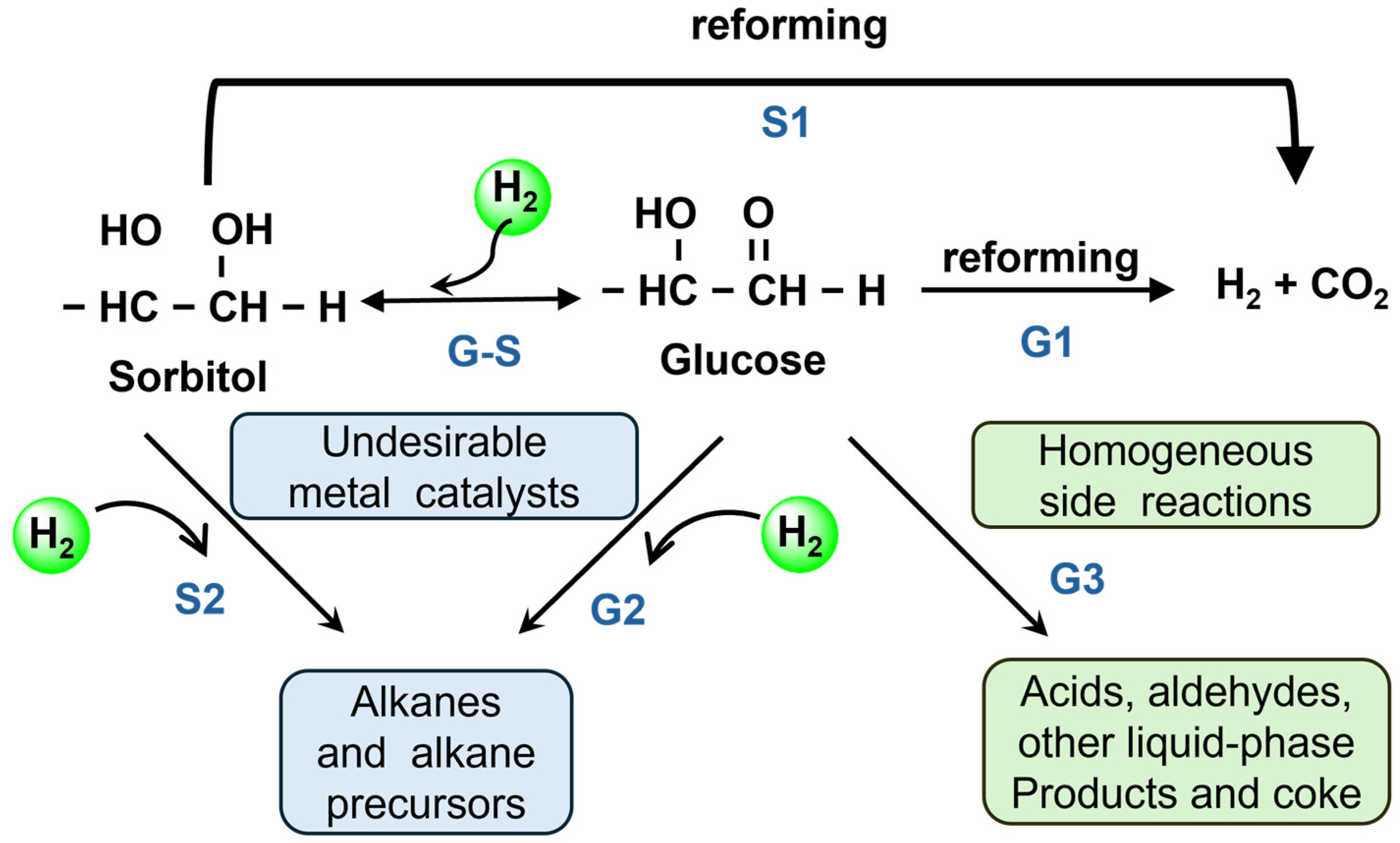
Glucose (C6H12O6) is regarded as an optimal feedstock for hydrogen production via APR among biomass-derived compounds, primarily because it can be directly generated in aqueous solution and readily participate in the reaction without the need for further separation [79]. This inherent characteristic significantly enhances both the reaction efficiency and operational convenience of glucose in aqueous phase reforming. As a polyhydroxy monosaccharide, glucose shares a structurally similar oxygenated framework with simple polyols such as glycerol, leading to a high degree of similarity in their respective reaction pathways during APR. Both processes involve C-H and C-C bond cleavage, the WGS reaction, and the regulation of by-product formation. A key distinction, however, is that glucose APR initially requires degradation and C-C bond cleavage to generate small molecular intermediates, resulting in a more extended reaction pathway and a relatively slower conversion rate compared to glycerol. Extensive studies have delineated the general reaction pathway for glucose APR as follows [80,81,82]: initially, the C-C bonds in glucose undergo dehydrogenation and decarbonylation, generating H2 and CO while progressively shortening the polycarbon backbone. The CO intermediate is then converted into CO2 and additional H2 through the WGS reaction. However, the hydrogen yield from glucose APR is typically lower compared to other feedstocks, primarily due to the isomerization of glucose into fructose, which may subsequently undergo hydrogenation to form sorbitol and other by-products [83]. These competing side reactions often take precedence over the APR process, thereby diminishing its overall efficiency. The potential reactions that may occur during the aqueous phase reforming of glucose are as follows: (1) Glucose (G), readily hydrogenates to form sorbitol (S), both of which undergo C-C bond cleavage, followed by the WGS reaction to complete the aqueous phase reforming (paths G1 and S1). (2) Some C-O bonds also undergo cleavage, resulting in the formation of alkanes, alcohols, and other by-products, which not only consume hydrogen but also generate a significant number of intermediate products, further decreasing the hydrogen yield (paths G2 and S2). (3) In the aqueous phase, acids, aldehydes, and carbonaceous water deposits may form (path G3). Consequently, the occurrence of various side reactions in glucose APR plays a pivotal role in determining both hydrogen yield and selectivity (Figure 4).
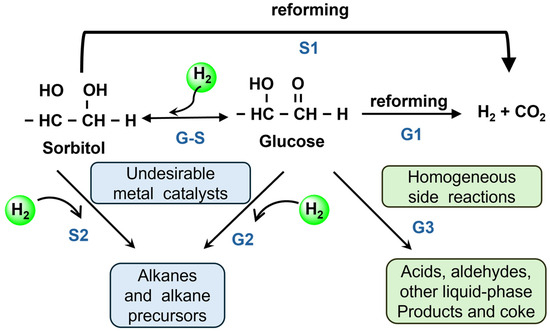
Figure 4.
Reaction pathways involved in glucose and sorbitol reforming. Adapted with permission from [84]. Copyright 2004 Royal Society of Chemistry.
In comparison to the APR of platform molecules for hydrogen production, direct hydrogen production from cellulose offers superior techno-economic advantages. As a key constituent of biomass, cellulose boasts a high carbon content and a robust molecular structure, rendering it an ideal feedstock for hydrogen production. Its inherent stability, coupled with its high thermal resistance and degradation resistance, positions cellulose as a promising material. That said, cellulose’s structure, comprising both crystalline and amorphous regions, makes it inherently challenging to directly engage in APR reactions. On the other hand, glucose, a simpler monosaccharide resulting from cellulose hydrolysis, exhibits enhanced reactivity, enabling it to participate directly in APR reactions. As such, glucose is frequently employed as a model compound to investigate the conversion processes of cellulose [85].
Agrawal and colleagues were the first to successfully achieve the APR of lignocellulose, using a one-pot, two-step hydrogen production reaction in a Parr reactor. At 225 °C, they initially degraded lignocellulose under the influence of sulfuric acid, followed by an APR reaction catalyzed by a metal catalyst to produce hydrogen, achieving a yield of 0.96 mmol/g of H2 [86]. However, at temperatures ranging from 160 to 280 °C, prolonged contact between lignocellulose and acid can lead to the formation of degradation products, such as 5-hydroxymethylfurfural and furfural. This groundbreaking study laid the foundation for advancements in cellulose aqueous phase reforming for hydrogen production. In 2010, the research group led by Zhijian Tian achieved the first direct APR hydrogen production from cellulose, obtaining a hydrogen yield of 27% at 260 °C. The study highlighted that glucose, produced through cellulose hydrolysis, serves as a critical intermediate in the cellulose APR reaction. While the cellulose hydrolysis reaction is relatively slow, once glucose is generated, the reforming reaction proceeds rapidly, ultimately converting it into hydrogen [87]. In the aqueous phase reforming of cellulose, the participation of glucose as a key intermediate streamlines the reaction pathway and circumvents the complexity associated with cellulose degradation.
In the APR reaction pathway of cellulose, the process begins with hydrolysis, converting cellulose into glucose, followed by the cleavage of C-H, O-H, and C-C bonds, along with the WGS reaction [88]. As the carbon chain length of cellulose increases, the ability to cleave C-C bonds in the aqueous phase reforming process becomes more challenging. Concurrently, the selectivity for alkanes rises, resulting in a notable reduction in hydrogen yield compared to polyol APR. This is primarily due to the higher energy requirements for breaking C-C bonds in substrates with longer carbon chains, as well as the increased likelihood of side reactions during the process, which ultimately impacts hydrogen production efficiency. Therefore, a comprehensive understanding of the conversion mechanisms of both cellulose and glucose in APR, along with the development of highly efficient catalysts, is essential for advancing the efficiency of cellulose-based aqueous phase reforming for hydrogen production.
Cellulose (C6H10O5)n is a high-molecular-weight polymer composed of D-glucose units linked by β-1,4-glycosidic bonds, with the number of glucose units ranging from 2000 to 20,000 [89,90]. Cellulose can be depolymerized into glucose through acid hydrolysis or reductive hydrolysis, which can then be further hydrogenated into relevant polyols for APR to produce hydrogen. In contrast to the APR of glucose, direct aqueous phase reforming of cellulose has been less extensively studied, primarily due to the complex molecular structure and reaction pathways of cellulose. Nonetheless, studies on glucose APR provided a crucial foundation for understanding the catalytic mechanisms, reaction intermediates, and product distributions in cellulose APR. These developments offered valuable theoretical insights for improving cellulose conversion efficiency and optimizing hydrogen production processes, thus advancing the sustainable development of biomass-derived hydrogen energy.
2.5. APR of Hemicellulose and Xylose for Hydrogen Production
Giuseppe conducted a systematic investigation into the reaction behavior of xylose during APR reaction, highlighting its substantial potential for H2 [91]. The study revealed a striking resemblance between the reaction pathways of xylose and glucose APR. Both processes involve the cleavage of C-C bonds, the dehydrogenation of C-H bonds, and the subsequent production of hydrogen and carbon dioxide via the WGS. Furthermore, the conversion of xylose and glucose encompasses not only sugar decomposition and dehydrogenation but also the formation of various by-products, such as methane, ethylene, and organic acids. A deeper understanding of the reaction mechanisms underlying xylose APR for hydrogen production provides a crucial theoretical framework for advancing research on the aqueous phase reforming of hemicellulose.
Research on the direct APR of hemicellulose for hydrogen production remains relatively sparse, primarily due to its lower abundance compared to cellulose and its intricate polymeric structure. Hemicellulose is a non-linear polysaccharide, primarily composed of various pentoses (mainly xylose and arabinose), hexose sugars (such as mannose and galactose), and highly branched short chains, with a degree of polymerization ranging from 50 to 200 [92,93]. The extensive array of monosaccharides resulting from the condensation process that forms hemicellulose complicates the systematic study of its APR reaction mechanisms, presenting significant challenges in advancing our understanding of this process [94]. Nonetheless, due to its shorter molecular chains and lower degree of polymerization, hemicellulose is more readily depolymerized than cellulose, facilitating its conversion into usable monosaccharides during hydrolysis. Given these attributes, xylose, the primary monosaccharide in hemicellulose, is commonly employed as a model compound for investigating the APR of hemicellulose.
2.6. APR of Lignin and Phenol for Hydrogen Production
In 2017, Yan reported on the APR of phenol for hydrogen production at relatively low temperatures. The findings revealed that, at a reaction temperature of 240 °C, phenol conversion exceeded 90%, with hydrogen selectivity approaching 80% [95]. The APR mechanism of phenol closely resembles that of polyols, involving primarily dehydrogenation reactions and the WGS reaction. In this process, the C-H bonds in phenol are cleaved, producing hydrogen, while in the WGS reaction, carbon monoxide reacts with water to generate carbon dioxide and additional hydrogen. However, the precise reaction mechanism for phenol aqueous phase reforming remains inadequately understood, particularly regarding the selectivity of different reaction pathways and the influence of catalysts on hydrogen yield. At present, research on the direct APR of lignin for hydrogen production is limited, although considerable foundational work has been established on the APR mechanism of phenol. The insights gained from phenol aqueous phase reforming have provided novel approaches and methodologies for the application of lignin in hydrogen production via APR.
Lignin is a highly intricate, aromatic, three-dimensional cross-linked phenolic polymer, composed of phenylpropanoid units substituted with “hydroxyl” and “methoxy” groups [96,97]. Its primary chemical composition predominantly consists of carbon (C), hydrogen (H), and oxygen (O), with trace amounts of nitrogen. The structure of lignin varies across plant species, but its complex 3D configuration is primarily formed by C-C and C-O bonds [98]. As a vital component of biomass, lignin’s molecular structure is exceptionally intricate and highly cross-linked, making it one of the most challenging biomass constituents to depolymerize. This inherent difficulty in depolymerization renders the conversion and depolymerization of lignin in the aqueous phase particularly crucial, as its efficient breakdown directly influences the overall efficiency of biomass conversion, especially in aqueous phase reforming reactions. During lignin hydrolysis, its complex molecular structure generates various bioactive compounds, including phenolic compounds and aromatic ring-containing polymers. These hydrolysis products play a significant role in subsequent conversion and utilization, which is why phenol is commonly investigated as a monomer derived from lignin hydrolysis. Currently, research on the direct APR of lignin for hydrogen production is relatively limited, despite a solid foundation in the understanding of the APR mechanisms for phenol. The findings from phenol aqueous-phase reforming offer new perspectives and methodologies for the aqueous-phase reforming of lignin in hydrogen production.
The structures of the compounds discussed in this section, along with the corresponding reaction equations for aqueous phase reforming, are presented in Table 1.

Table 1.
Structures of various biomass-derived compounds and aqueous phase reforming reaction equations.
3. Efficient Catalysts for Aqueous Phase Reforming Hydrogen Production
The selection of catalysts plays a crucial role in the efficient APR of biomass for hydrogen production. Based on the APR reaction pathways of oxygenated hydrocarbons, the cleavage of C-O bonds significantly influences hydrogen selectivity. Therefore, an ideal APR catalyst should exhibit high activity for C-C bond cleavage while maintaining low activity for C-O bond cleavage. Such a catalyst would facilitate the WGS reaction while suppressing methane formation and Fischer-Tropsch synthesis reactions. Moreover, the suitability of a catalyst depends not only on the specific requirements of the aqueous reforming process but also on the reaction conditions and the intrinsic properties of the biomass feedstock [99] (Table 2).

Table 2.
Aqueous phase reforming of different biomass derivatives.
3.1. Noble Metal Catalysts
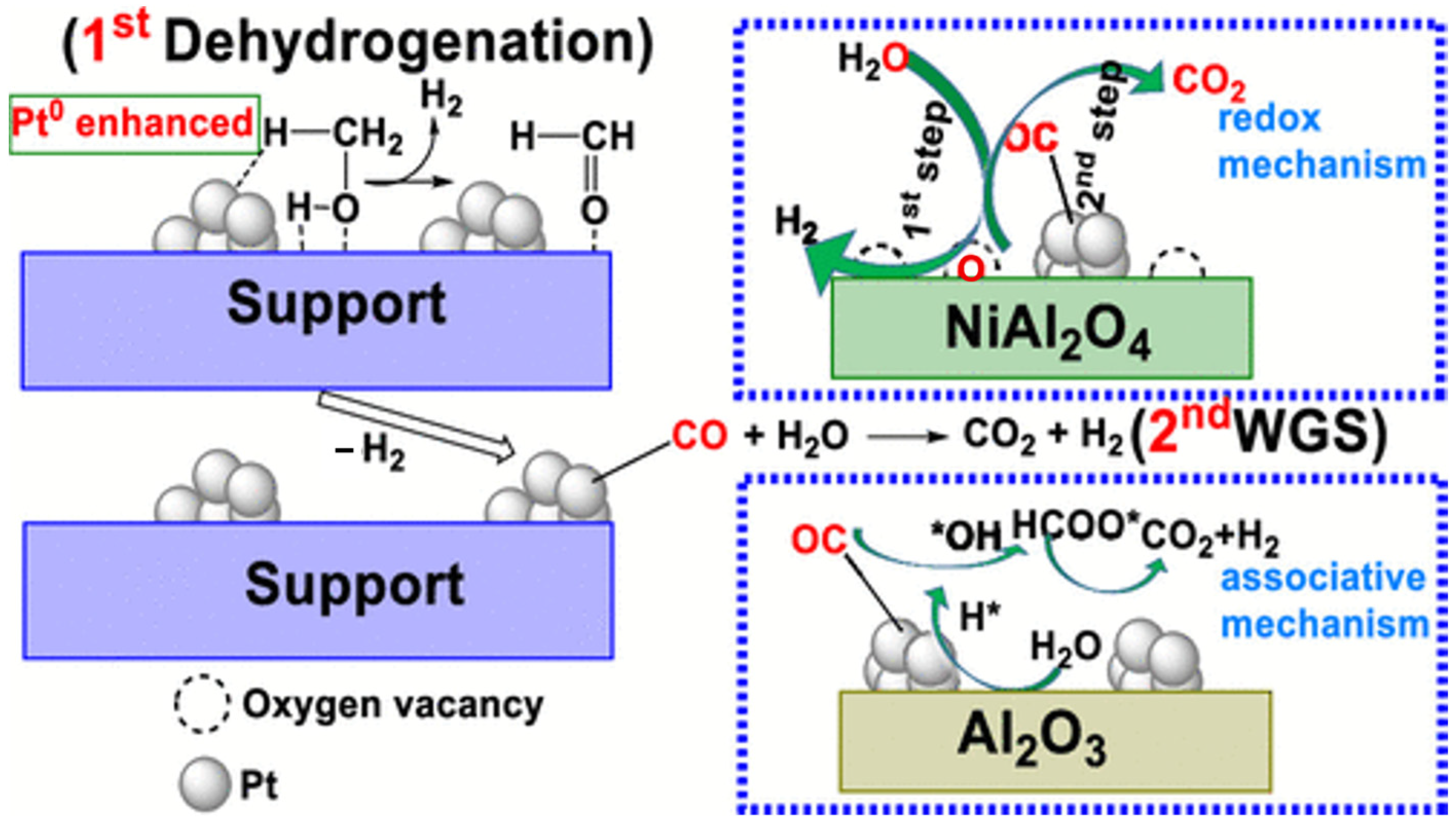
Platinum is widely recognized as a highly versatile metal, renowned for its exceptional activity and selectivity, and has been extensively utilized in various catalytic processes. Dumesic was the first to propose the use of Pt/Al2O3 as a catalyst for the APR of biomass-derived hydrocarbons to produce hydrogen. Research has demonstrated that Pt-based catalysts not only efficiently convert diverse feedstocks—such as glycerol, glucose, and sorbitol—into hydrogen but also exhibit remarkable long-term stability and resistance to catalyst deactivation [71]. Shabaker et al. also investigated the APR of ethylene glycol and methanol using the Pt/Al2O3 catalyst, revealing that both compounds achieved nearly 100% H2 selectivity on Pt/Al2O3. Their study further suggested that C-C bond cleavage is not the rate-limiting step in the aqueous phase reforming of oxygenated hydrocarbons [47]. The choice of Al2O3 crystalline phase as the catalyst support was found to significantly influence hydrogen production in APR by modulating the electronic density of Pt nanoparticles. At 30 °C, the Pt/α-Al2O3 catalyst exhibited a turnover frequency (TOF) of 69.8 h−1, approximately five times higher than that of Pt/γ-Al2O3 under identical conditions. Mechanistic analysis indicated that the α-Al2O3 phase, characterized by a larger lattice spacing, enhances the electronic density of Pt nanoparticles, altering their electronic structure. This modification further regulates the d-band center of Pt, effectively promoting the dissociation of H2O and enhancing the overall hydrogen production efficiency [106]. Researchers have emphasized the crucial role of the WGS reaction in the APR process. By comparing the APR activity of Pt-based catalysts on various supports, they found that catalysts with higher WGS capability exhibited better APR performance, with the activity order being: Pt/MgO > Pt/Al2O3 > Pt/CeO2 > Pt/TiO2 > Pt/SiO2. This suggested that the basic properties of the support significantly influence the APR performance of Pt-based catalysts [73,107]. As a result, modifying the support led to higher hydrogen yields. For instance, combining the active metal oxide support NiO with Al2O3 to form the spinel NiAl2O4 produced a Pt/NiAl2O4 catalyst for methanol APR, which achieved a hydrogen yield of 95.7%, four times higher than that of Pt/γ-Al2O3 [100]. This improvement was attributed to the generation of oxygen vacancies on the Pt/NiAl2O4 catalyst due to the incorporation of NiAl2O4 spinel, which facilitated the reduction of PtOx to metallic Pt, thereby enhancing catalytic performance in methanol dehydrogenation. Additionally, the Pt/NiAl2O4 catalyst modified the WGS reaction pathway, favoring a redox-based mechanism, whereas Pt/γ-Al2O3 followed an associative pathway, resulting in a slower WGS reaction (Figure 5).
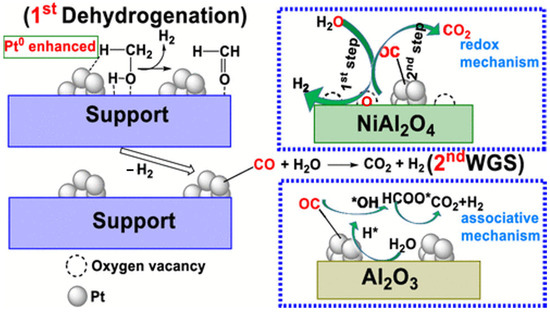
Figure 5.
Schematic illustrations of the mechanism of methanol APR over Pt/γ-Al2O3 and Pt/NiAl2O4. Reproduced with permission from [100]. Copyright 2019 ACS Publications.
Recently, carbides have emerged as promising catalytic supports for hydrogen production due to their significant effect on hydrogen adsorption activity [108]. Studies have shown that the 0.2% Pt/α-MoC catalyst exhibits the highest hydrogen TOF for methanol APR among existing catalysts [109]. This catalyst enables hydrogen generation without the presence of alkalis at low temperatures (150–190 °C), demonstrating exceptionally high conversion rates. Pt is atomically dispersed on the surface of α-MoC, with strong interactions between the Pt1 sites and α-MoC. Detailed reaction pathway calculations indicate that the Pt1/α-MoC catalyst promotes the cleavage of C-H bonds and water dissociation in methanol molecules by reducing the dissociation energy of both methanol and water, thereby accelerating hydrogen production.
Several noble metals, including palladium (Pd), rhodium (Rh), and ruthenium (Ru), have proven effective in the APR of oxygenated compounds, with Rh and Ru being particularly efficient in facilitating C-C bond cleavage. However, their hydrogen production activity remains inferior to that of Pt-based catalysts. Davda et al. investigated the APR activity of Pt, Ru, Rh, Pd, and Ir catalysts supported on silica for ethylene glycol reforming. Kinetic studies revealed that Pt exhibited the highest hydrogen production capacity, while Pd demonstrated the lowest activity but the highest selectivity. Additionally, Pd exhibited reduced selectivity for C-O bond cleavage [110]. Liu et al. prepared Pd/Fe3O4 catalysts via co-precipitation, which displayed outstanding performance in the APR of ethylene glycol, achieving a TOF of 109 min−1, the highest reported to date. This exceptional activity was attributed to the strong metal-support interaction between small Pd nanoparticles and magnetite (Fe3O4), which significantly facilitated the WGS reaction, a key step in APR [111]. Pd-based catalysts also show great potential in the APR of biomass feedstocks. At 250 °C, Pd/C catalysts achieved hydrogen yields of 35.48% for pinewood and 29.97% for poplar, demonstrating high stability. Compared to other noble metal catalysts, Pd/C offers a cost-effective alternative while maintaining superior catalytic performance [112]. Furthermore, gold-based catalysts, such as Au/α-MoC, have shown remarkable efficacy in promoting low-temperature WGS reactions, offering high hydrogen selectivity and conversion efficiency. Its breakthrough opens new avenues for designing advanced catalysts for biomass APR [113].
3.2. Non-Noble Metal Catalysts
Noble metal catalysts have exhibited exceptional activity in biomass APR for hydrogen production. But the high cost and limited reserves of these metals present significant challenges to the industrial scalability of APR processes. Consequently, the development of cost-effective catalysts that offer high activity, selectivity, and stability has become essential. Among these, transition metal nickel (Ni) has garnered significant attention in recent research due to its abundant availability, low cost, and excellent capability for C-C bond cleavage.
The Ni/CeO2 catalyst synthesized via the combustion method achieved a maximum glycerol conversion rate of 30% at 543 K, with a hydrogen molar fraction exceeding 70%, highlighting its significant potential for APR applications [114]. Compared to pure metal oxide supports, composite metal oxide supports exhibit superior catalytic performance and enhanced resistance to carbon deposition. Iriondo et al. investigated glycerol APR using Ni-based catalysts supported on various modified oxides, including ceria, zirconia, and lanthanum-modified alumina. The initial catalytic activity followed the trend: Ni/Al2O3–La2O3 > Ni/Al2O3–CeO2 > Ni/Al2O3–ZrO2 > Ni/Al2O3 [115]. The incorporation of Ce and La facilitated the dispersion and surface modification of metallic Ni, thereby enhancing hydrogen yield and catalyst stability during the APR process. Although nickel-based catalysts exhibit high initial APR activity, they tend to undergo rapid deactivation under reaction conditions, primarily due to changes in the oxidation state of Ni and the formation of surface NiAl2O4 species.
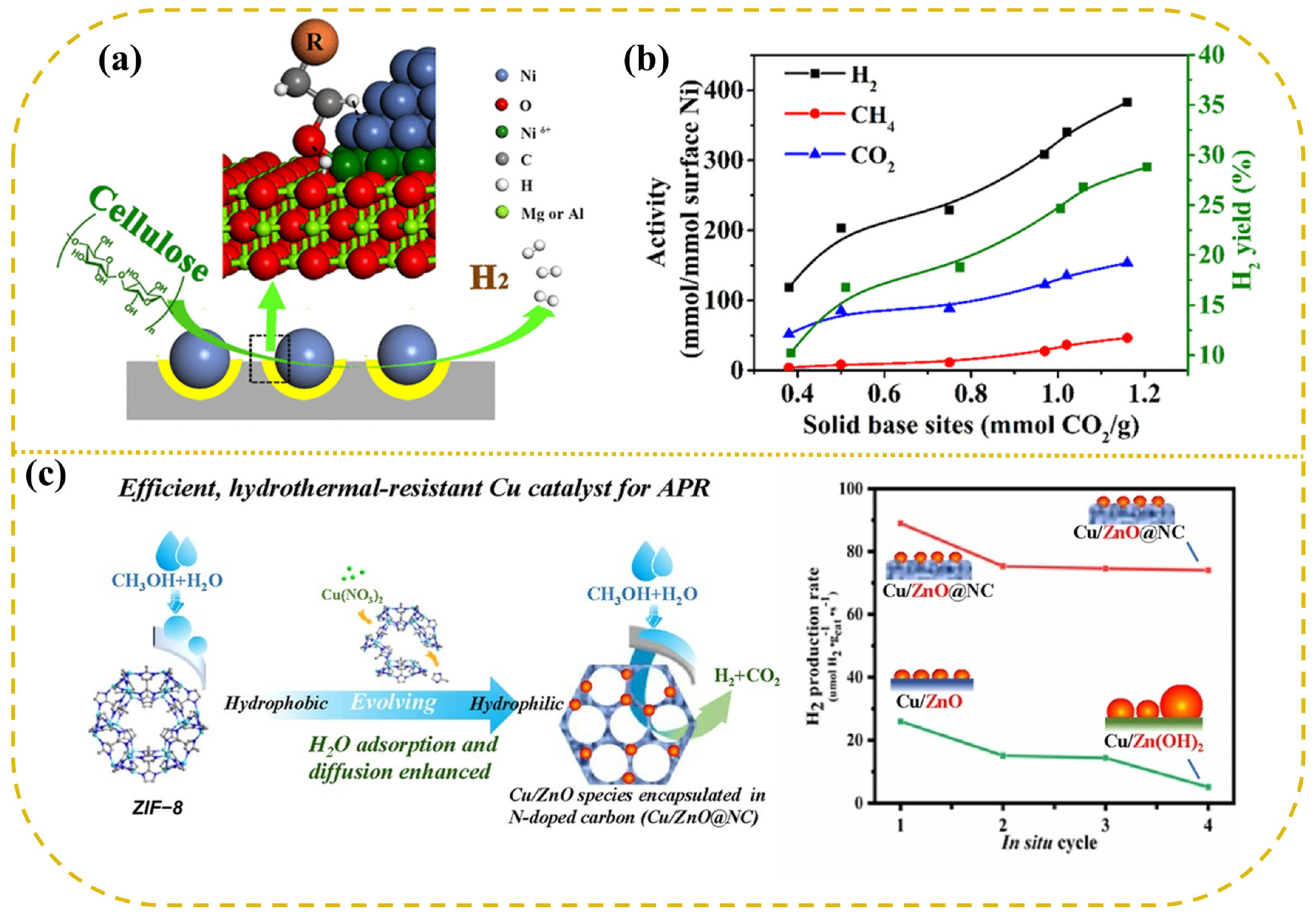
Ni-based catalysts supported on traditional metal oxides typically exhibit hydrogen production activities slightly lower than those of Pt. As a result, researchers have increasingly focused on carbide-based supports. Lin developed a novel Ni/α-MoC catalyst, which demonstrated an impressive hydrogen generation rate of 171 μmol g−1 s−1 at 240 °C with 2% Ni/α-MoC, showing six times the catalytic activity of the conventional 2% Pt/Al2O3 catalyst and establishing the highest activity ever reported for a non-precious metal catalyst [116]. On the surface of α-MoC, Ni was nearly entirely atomically dispersed, and at low Ni loadings (≤2%), Ni atoms were linked to α-MoC via carbon bridges, forming a Ni1−Cx structure. DFT calculations indicated that the Ni1−Cx structure exhibits a low energy barrier for methanol dissociation and demonstrates exceptional catalytic activity in the processes of water dissociation, methanol activation, and subsequent reforming reactions. Compared to conventional Ni and α-MoC catalysts, Ni/α-MoC catalysts exhibited lower reaction energy barriers and superior catalytic efficiency. Moreover, catalysts prepared by loading Ni particles onto layered double metal oxide (LDO) supports, and by in situ reduction of Ni-containing magnesium-aluminum layered double hydroxides (LDHs), have shown remarkable performance in cellulose aqueous phase reforming, achieving a hydrogen yield of 30.9%, far exceeding the hydrogen yields reported for other catalysts in the literature [117]. The LDO support facilitates the cleavage of O-H bonds, thereby promoting the dissociation of C-H and C-C bonds, while the Niδ+ sites at the Ni-LDO interface enhance the oxidation of CO intermediates, thereby boosting the WGS reaction and mitigating CO formation (Figure 6a,b). In comparative studies involving Raney Ni and several noble metal catalysts (Pt, Pd, Ru) for the APR of wheat straw hydrolysate, Raney Ni exhibited superior hydrogen production performance, generating fewer by-products and offering notable advantages in terms of cost-effectiveness and stability [118].
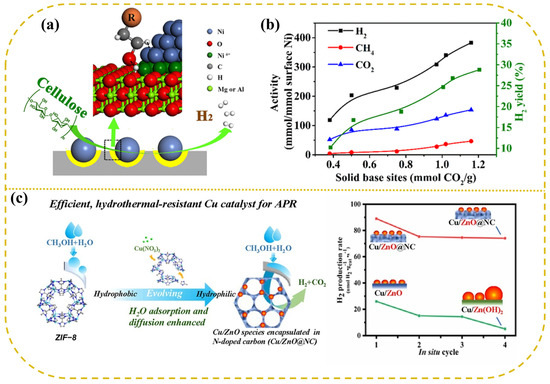
Figure 6.
The Ni/LDO catalyst for APR of cellulose of (a) structure and (b) gas production activity. Reproduced with permission from [117]. Copyright 2018 ACS Publications. (c) The sketch map of APR of methanol over Cu catalyst. Reproduced with permission from [119]. Copyright 2022 Elsevier.
Other transition metals, such as copper (Cu) and tungsten (W), demonstrated significant potential for hydrogen production via APR. In the methanol APR process, a Cu/ZnO@NC catalyst synthesized using ZIF-8 as a precursor exhibited a hydrogen release rate four times higher than that of the Cu/ZnO catalyst, with performance comparable to that of the commercial 5% Pt/C catalyst [119]. Stability tests showed that the hydrogen production rate of the 27% Cu/ZnO@NC catalyst remained virtually unchanged, indicating exceptional resistance to hydrothermal stability (Figure 6c). Additionally, tungsten-based catalysts prepared by magnetron sputtering showed impressive performance in cellulose aqueous phase reforming at 260 °C, achieving a hydrogen TOF of 18.1 min−1, significantly higher than that of the conventional 5% Pt/C catalyst (TOF = 1.6 min−1), while maintaining high hydrogen selectivity. Under identical reaction conditions, the tungsten catalyst exhibited a higher hydrogen production rate and better resistance to poisoning compared to the platinum catalyst [88].
3.3. Bimetallic Catalysts
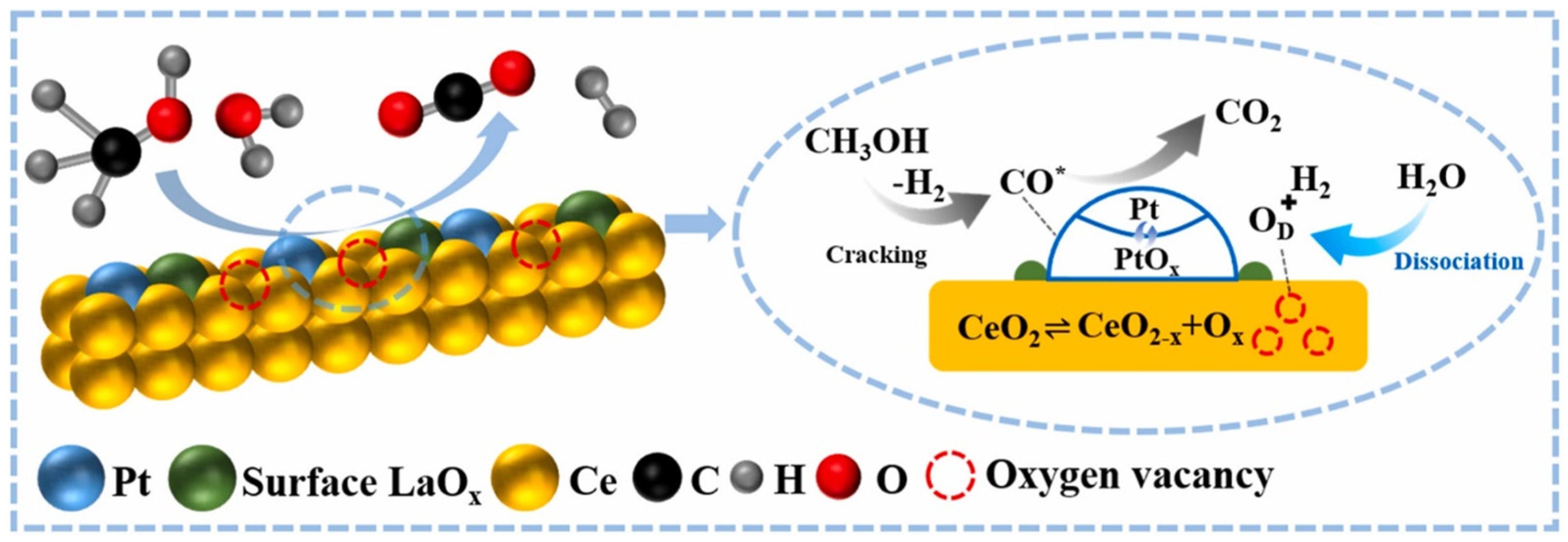
Monometallic catalysts have certain limitations in terms of activity, selectivity, and resistance to deactivation. Bimetallic catalysts, due to their unique electronic effects and synergistic interactions, offer superior catalytic performance in APR reactions. For instance, the incorporation of non-precious metals (M = Fe, Co, Ni, Cu) into Pt/γ-Al2O3 catalysts to form Pt-M/γ-Al2O3 bimetallic systems has been shown to significantly improve performance. Among these, the Pt1Fe1/γ-Al2O3 catalyst exhibited the highest hydrogen production rate, carbon conversion efficiency to gas, and hydrogen selectivity. The PtFe alloy catalyst notably increased hydrogen selectivity by intensifying the water-gas shift reaction and promoting the conversion of CO [120]. The Pt-Fe (1:3)/CMK-9 catalyst has demonstrated remarkable catalytic activity for a range of biomass-derived compounds, including glycerol, xylitol, and sorbitol. The incorporation of Fe into the Pt/CMK-9 catalyst significantly enhanced its catalytic performance [121]. Similar synergistic effects have also been observed with the addition of other noble metals, such as Ru and Pd, to Pt-based catalysts [122,123]. Lu employed a photochemical reduction method to introduce La into Pt/CeO2 catalysts, resulting in PtLa/CeO2 catalysts that exhibited substantially higher hydrogen production rates and methanol conversion compared to Pt/CeO2. This improvement is attributed to La doping, which enhances the metal-support interaction and markedly increases the number of oxygen vacancies on the catalyst surface. The vacancies were continuously replenished by free oxygen from H2O molecules, facilitating a cyclic process that accelerated the redox pathway of the water-gas shift (WGS) reaction, ultimately resulting in a significant increase in hydrogen production (Figure 7) [124]. On the other hand, the addition of Ce to the non-precious metal catalyst Ni/γ-Al2O3 significantly enhanced the hydrogen production rate in methanol APR, increasing it to 85 mmol·min−1·g−1. Doping with Cu also provided a notable improvement in the catalyst’s performance [125,126]. Similar synergistic effects were observed when Co and Fe were incorporated into Ni-based catalysts [127]. Additionally, Si et al. utilized Raney Ni-Mo catalysts for the APR of biomass feedstocks, such as agricultural and forestry waste [128]. At lower operating temperatures, the biomass feedstocks were almost completely converted, with hydrogen production reaching 58.6 .
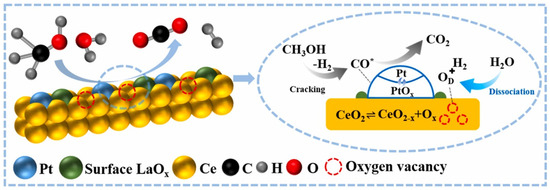
Figure 7.
Schematic illustrations of the mechanism of methanol APR over PtLa/CeO2. (CO* stands intermediate CO*). Reproduced with permission from [124]. Copyright 2024 Elsevier.
4. Feasibility and Lifecycle Assessment of APR for Hydrogen
Compared to traditional technologies, APR for hydrogen production operates at a lower reaction temperature and exhibits relatively higher technical efficiency (Table 3). For instance, ethanol steam reforming, while having the advantages of low cost and a wide range of feedstock sources, faces challenges such as catalyst cost and durability under high temperature and pressure conditions. On the other hand, gasification technology can process various forms of biomass, producing syngas at high temperatures, which can then be further converted into hydrogen. However, its purification process is complex, thermal efficiency is low, and environmental emissions are a prominent issue. In contrast to pyrolysis, which requires drying the feedstock, APR uses subcritical water (100–250 °C) as the reaction medium, resulting in lower energy consumption. Although the hydrogen yield from APR is relatively lower, the significantly lower reaction temperature helps make the process more energy-efficient. For example, the hydrogen production cost from glycerol in APR is lower than SR ($3.55 kg−1 vs. $3.65 kg−1).

Table 3.
Comparison of the current technologies of feedstock for H2 production.
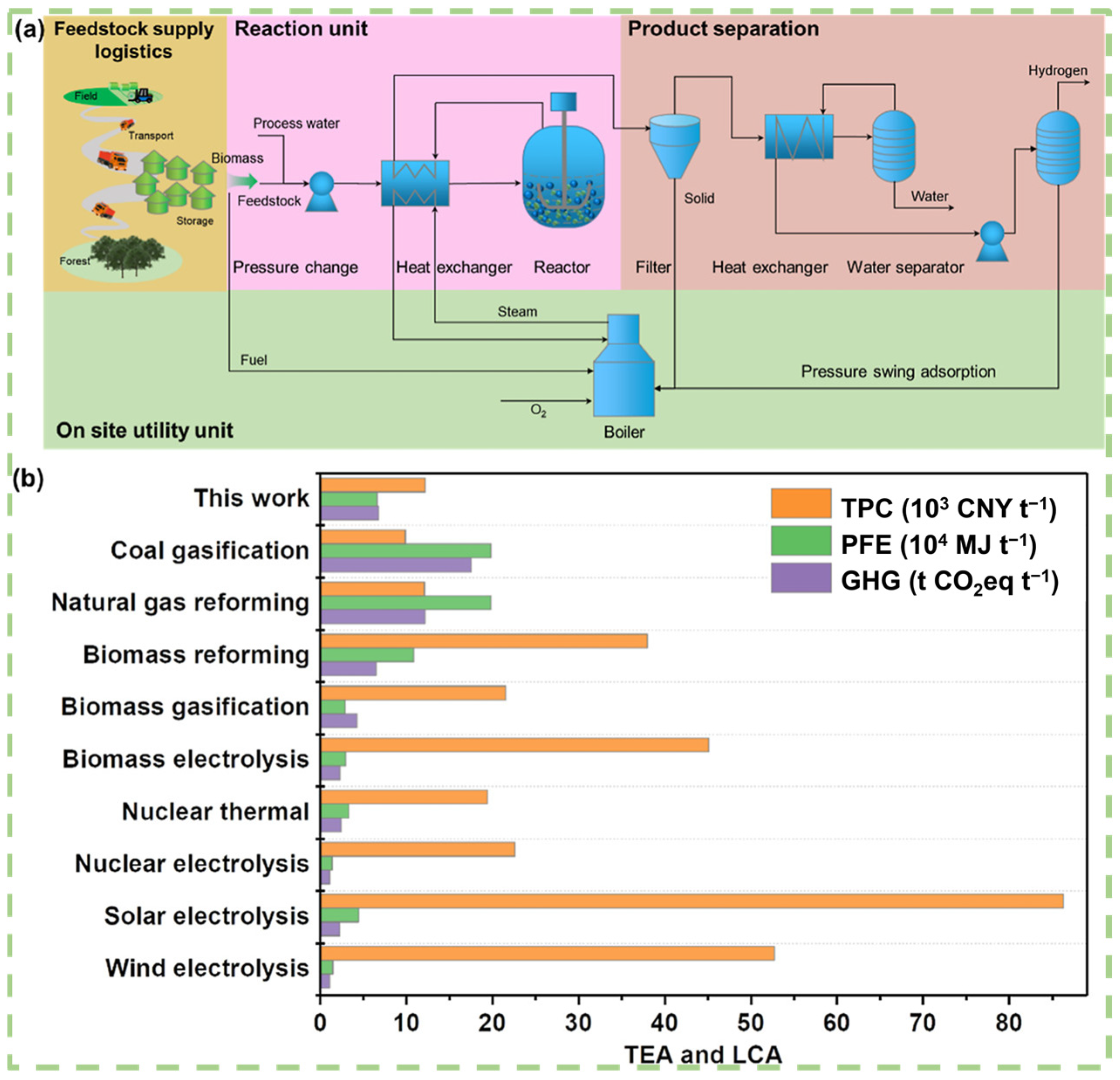
From a lifecycle assessment (LCA) perspective, aqueous phase reforming for hydrogen production demonstrates advantages in terms of lower greenhouse gas emissions and energy consumption across various stages, including raw material acquisition, production processes, and product applications. This advantage is especially notable as catalyst recovery and waste treatment technologies continue to improve, resulting in more significant overall environmental benefits. Si et al. conducted a techno-economic analysis (TEA) and LCA evaluation (Figure 8) for lignocellulosic biomass feedstocks. The results show that the total production cost (TPC) of hydrogen derived from biomass is approximately 1699.58 $ t−1, slightly higher than that of mature fossil hydrogen (coal gasification: 1381.8 $ t−1, natural gas reforming: 1689.2 $ t−1), but still much lower than emerging non-fossil hydrogen. Additionally, its greenhouse gas emissions and lifecycle primary energy consumption are only 38.8% and 33.6%, respectively, of those from coal-based hydrogen production (Figure 8b). These results strongly demonstrate the environmental advantages of biomass-derived hydrogen from aqueous phase reforming, highlighting its potential for reducing environmental burdens [128].
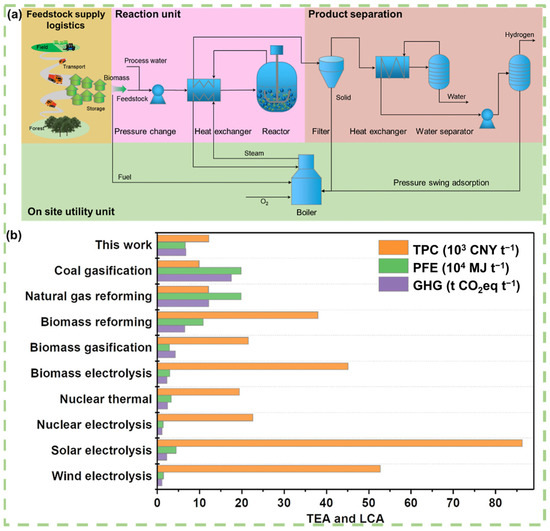
Figure 8.
TEA and LCA of H2 production. (a) Conceptual model process for hydrogen production from raw biomass. (b) The TPC, life cycle PFE depletion, and GHG emissions of H2 production. Reproduced with permission from [128]. Copyright 2022 ACS Publications.
5. Conclusions and Perspectives
Convert renewable biomass and its derivatives into high-value green hydrogen to generate significant optimism for the future sustainability of energy. In recent years, with the growing demand for renewable energy, aqueous phase reforming technology has become a focal point of research in the biomass energy sector, due to its ability to efficiently convert biomass into hydrogen under mild conditions. APR-based hydrogen production offers significant advantages, including mild reaction conditions, renewable feedstocks, high hydrogen yield, environmental sustainability, and strong economic feasibility, positioning it as a green and sustainable technology for hydrogen production. This paper comprehensively reviews the latest advancements in the APR process, focusing on lignocellulosic biomass and its model compounds.
The key factors influencing the APR reaction encompass catalyst selection, biomass feedstock type, and the optimization of reaction conditions. This paper provides a comprehensive examination of the application of noble metals, non-precious metals, and bimetallic catalysts in the APR process. The findings highlight that multiple factors, such as the choice of active metal, support material, additives, and preparation methods, play a critical role in determining catalytic performance for hydrogen production. Noble metal catalysts, including platinum, palladium, and ruthenium-based systems, demonstrate high hydrogen selectivity but exhibit relatively low conversion rates. Non-precious metal nickel-based catalysts, while cost-effective, yield lower hydrogen outputs compared to platinum catalysts, suffer from inferior stability, and are susceptible to rapid deactivation. In contrast, bimetallic catalysts effectively mitigate these limitations by leveraging the synergistic effects of two metals to enhance catalytic activity and optimize C-C bond cleavage and the WGS reaction, thereby improving hydrogen yields. Moreover, they reduce by-product formation, increase reaction selectivity, and enhance catalyst stability and resistance to poisoning. By fine-tuning metal ratios and selecting appropriate supports, bimetallic catalysts can further improve durability and maintain high catalytic efficiency over extended reaction times.
Despite the significant potential of biomass APR technology for hydrogen production, it still faces several challenges. Therefore, future research could focus on the following key areas: (1) the development of efficient, low-cost catalysts, particularly transition metal catalysts and their composite forms, to reduce catalytic costs and enhance long-term catalyst stability; (2) the optimization of reaction conditions and feedstock concentrations to improve hydrogen selectivity and yield; and (3) an in-depth exploration of efficient conversion pathways for biomass feedstocks (such as hemicellulose, lignin, etc.), thereby broadening the range of feedstocks available for biomass aqueous phase reforming.
Biomass aqueous phase reforming for hydrogen production is poised to emerge as a crucial method for the future generation of hydrogen energy, offering substantial potential to contribute to the advancement of sustainable energy solutions. It is hoped that forthcoming research will accelerate the commercialization of APR technology, positioning it as a viable, sustainable alternative to traditional fossil fuels, thereby mitigating current environmental challenges and meeting the increasingly pressing global demand for energy.
Author Contributions
M.L.: Conceptualization, investigation, and writing—review and editing; W.J. and C.H.: Investigation, visualization, and resources; X.S.: Conceptualization, project administration, supervision, and writing—review and editing; Q.L.: Investigation, and methodology; R.L. and T.L.: Conceptualization, project administration, supervision, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the State Key Laboratory of Catalysis (2024SKL-A-007), and the National Natural Science Foundation of China (22208339 and 22379131).
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors thank the financial support from the funding sources mentioned above.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, J.; Yin, Y. Fermentative hydrogen production using various biomass-based materials as feedstock. Renew. Sustain. Energy Rev. 2018, 92, 284–306. [Google Scholar] [CrossRef]
- Saha, S.; Mondal, A.; Kurade, M.B.; Ahn, Y.; Banerjee, P.; Park, H.-K.; Pandey, A.; Kim, T.H.; Jeon, B.-H. Cutting-edge technological advancements in biomass-derived hydrogen production. Rev. Environ. Sci. Bio/Technol. 2023, 22, 397–426. [Google Scholar] [CrossRef]
- Ahmad, K.; Jaoude, M.A.; Bankole, A.A.; Polychronopoulou, K. Hydrogen production towards carbon-free economy: A comprehensive thermodynamic analysis. Energy Convers. Manag. 2025, 326, 119492–119502. [Google Scholar] [CrossRef]
- Marbán, G.; Valdés-Solís, T. Towards the hydrogen economy? Int. J. Hydrog. Energy 2007, 32, 1625–1637. [Google Scholar] [CrossRef]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Valizadeh, S.; Hakimian, H.; Farooq, A.; Jeon, B.-H.; Chen, W.-H.; Hoon Lee, S.; Jung, S.-C.; Won Seo, M.; Park, Y.-K. Valorization of biomass through gasification for green hydrogen generation: A comprehensive review. Bioresour. Technol. 2022, 365, 128143–128155. [Google Scholar] [CrossRef]
- Taipabu, M.I.; Viswanathan, K.; Wu, W.; Hattu, N.; Atabani, A.E. A critical review of the hydrogen production from biomass-based feedstocks: Challenge, solution, and future prospect. Process Saf. Environ. Prot. 2022, 164, 384–407. [Google Scholar] [CrossRef]
- Jeon, S.; Farooq, A.; Lee, I.H.; Lee, D.; Seo, M.W.; Jung, S.-C.; Hussain, M.; Khan, M.A.; Jeon, B.-H.; Jang, S.-H.; et al. Green conversion of wood plastic composites: A study on gasification with an activated bio-char catalyst. Int. J. Hydrog. Energy 2024, 54, 96–106. [Google Scholar] [CrossRef]
- Global Hydrogen Review 2024; IEA: Paris, France, 2024; Available online: https://www.iea.org/reports/global-hydrogen-review-2024 (accessed on 3 October 2024).
- Mohan, S.V.; Pandey, A. Biohydrogen Production; CRC Press: Boca Raton, FL, USA, 2013; pp. 1–24. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Lee, Y.-J.; Lee, D.-W. Biohydrogen production: Strategies to improve process efficiency through microbial routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef]
- Si, X.; Lu, R.; Zhao, Z.; Yang, X.; Wang, F.; Jiang, H.; Luo, X.; Wang, A.; Feng, Z.; Xu, J.; et al. Catalytic production of low-carbon footprint sustainable natural gas. Nat. Commun. 2022, 13, 258. [Google Scholar] [CrossRef]
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in energy transition: A review. Int. J. Hydrog. Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Huber, G.W.; Shabaker, J.W.; Dumesic, J.A. Raney Ni-Sn catalyst for H2 production from biomass-derived hydrocarbons. Science 2003, 300, 2075–2077. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Si, X.; Chen, J.; Li, X.; Lu, F. Catalytic complete cleavage of C–O and C–C bonds in biomass to natural gas over Ru(0). ACS Catal. 2022, 12, 5549–5558. [Google Scholar] [CrossRef]
- Xia, Q.; Chen, Z.; Shao, Y.; Gong, X.; Wang, H.; Liu, X.; Parker, S.F.; Han, X.; Yang, S.; Wang, Y. Direct hydrodeoxygenation of raw woody biomass into liquid alkanes. Nat. Commun. 2016, 7, 11162. [Google Scholar] [CrossRef]
- El-Emam, R.S.; Özcan, H. Comprehensive review on the techno-economics of sustainable large-scale clean hydrogen production. J. Clean. Prod. 2019, 220, 593–609. [Google Scholar] [CrossRef]
- González Martínez, M.; Elsaddik, M.; Nzihou, A. Monitoring, analysis, and quantification of hydrogen from biomass and biowaste: A review. Int. J. Hydrog. Energy 2023, 48, 22113–22131. [Google Scholar] [CrossRef]
- Cao, A.N.T.; Ng, K.H.; Ahmed, S.F.; Nguyen, H.T.; Kumar, P.S.; Tran, H.-T.; Rajamohan, N.; Yusuf, M.; Show, P.L.; Balakrishnan, A.; et al. Hydrogen generation by heterogeneous catalytic steam reforming of short-chain alcohols: A review. Environ. Chem. Lett. 2023, 22, 561–583. [Google Scholar] [CrossRef]
- Fasolini, A.; Cespi, D.; Tabanelli, T.; Cucciniello, R.; Cavani, F. Hydrogen from renewables: A case study of glycerol reforming. Catalysts 2019, 9, 722. [Google Scholar] [CrossRef]
- Wang, K.; Dou, B.; Jiang, B.; Song, Y.; Zhang, C.; Zhang, Q.; Chen, H.; Xu, Y. Renewable hydrogen production from chemical looping steam reforming of ethanol using xCeNi/SBA-15 oxygen carriers in a fixed-bed reactor. Int. J. Hydrog. Energy 2016, 41, 12899–12909. [Google Scholar] [CrossRef]
- Silva, J.M.; Soria, M.A.; Madeira, L.M. Challenges and strategies for optimization of glycerol steam reforming process. Renew. Sustain. Energy Rev. 2015, 42, 1187–1213. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Baeza, J.A.; Calvo, L.; Gilarranz, M.A. Aqueous phase reforming of starch wastewater over Pt and Pt-based bimetallic catalysts for green hydrogen production. Chem. Eng. J. 2023, 460, 141770–141781. [Google Scholar] [CrossRef]
- Rosmini, C.; Urrea, M.P.; Tusini, E.; Indris, S.; Kovacheva, D.; Karashanova, D.; Kolev, H.; Zimina, A.; Grunwaldt, J.-D.; Rønning, M.; et al. Unveiling the synergistic effects of pH and Sn content for tuning the catalytic performance of Ni0/NixSny intermetallic compounds dispersed on Ce-Zr mixed oxides in the aqueous phase reforming of ethylene glycol. Appl. Catal. B Environ. Energy 2024, 350, 123904–123920. [Google Scholar] [CrossRef]
- Wheeler, C. The water–gas-shift reaction at short contact times. J. Catal. 2004, 223, 191–199. [Google Scholar] [CrossRef]
- Huber, G.W.; Shabaker, J.W.; Evans, S.T.; Dumesic, J.A. Aqueous-phase reforming of ethylene glycol over supported Pt and Pd bimetallic catalysts. Appl. Catal. B Environ. 2006, 62, 226–235. [Google Scholar] [CrossRef]
- Hanika, J.; Lederer, J.; Tukač, V.; Veselý, V.; Kováč, D. Hydrogen production via synthetic gas by biomass/oil partial oxidation. Chem. Eng. J. 2011, 176–177, 286–290. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.F.; Zhang, C.C.; Nan, J.; Ren, N.Q.; Lee, D.J.; Chen, C. Advances in pretreatment of lignocellulosic biomass for bioenergy production: Challenges and perspectives. Bioresour. Technol. 2022, 343, 126123–126134. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Qiu, X.; Xu, C. Hydrothermal treatment of lignocellulosic biomass towards low-carbon development: Production of high-value-added bioproducts. EnergyChem 2024, 6, 100133–100170. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, Y.; Liu, Y. State of the art of straw treatment technology: Challenges and solutions forward. Bioresour. Technol. 2020, 313, 123656–123664. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef]
- Li, H.; Fang, Z.; Smith, R.L.; Yang, S. Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials. Prog. Energy Combust. Sci. 2016, 55, 98–194. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Wakerley, D.W.; Kuehnel, M.F.; Orchard, K.L.; Ly, K.H.; Rosser, T.E.; Reisner, E. Solar-driven reforming of lignocellulose to H2 with a CdS/CdOx photocatalyst. Nat. Energy 2017, 2, 17021. [Google Scholar] [CrossRef]
- Jamil, F.; Inayat, A.; Hussain, M.; Ghenai, C.; Shanableh, A.; Sarwer, A.; Shah, N.S.; Park, Y.-K. Green hydrogen production through a facile aqueous-phase reforming technique from waste biomass: A comprehensive review. Int. J. Hydrog. Energy 2024, 96, 126–146. [Google Scholar] [CrossRef]
- Tian, Z.; Lu, Y.; Wang, J.; Shu, R.; Wang, C.; Chen, Y. Advances in hydrogen production by aqueous phase reforming of biomass oxygenated derivatives. Fuel 2024, 357, 129691–129713. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Liu, Q.; Du, S.; Liu, T.; Gong, L.; Wu, Y.; Lin, J.; Yang, P.; Huang, G.; Li, M.; Wu, Y.; et al. Efficient low-temperature hydrogen production by electrochemical-assisted methanol steam reforming. Angew. Chem. Int. Ed. 2024, 63, e202315157. [Google Scholar] [CrossRef]
- Lee, J.K.; Ko, J.B.; Kim, D.H. Methanol steam reforming over Cu/ZnO/Al2O3 catalyst: Kinetics and effectiveness factor. Appl. Catal. A Gen. 2004, 278, 25–35. [Google Scholar] [CrossRef]
- Wang, C.; Boucher, M.; Yang, M.; Saltsburg, H.; Flytzani-Stephanopoulos, M. ZnO-modified zirconia as gold catalyst support for the low-temperature methanol steam reforming reaction. Appl. Catal. B Environ. 2014, 154–155, 142–152. [Google Scholar] [CrossRef]
- Herdem, M.S.; Sinaki, M.Y.; Farhad, S.; Hamdullahpur, F. An overview of the methanol reforming process: Comparison of fuels, catalysts, reformers, and systems. Int. J. Energy Res. 2019, 43, 5076–5105. [Google Scholar] [CrossRef]
- Mei, D.; Qiu, X.; Liu, H.; Wu, Q.; Yu, S.; Xu, L.; Zuo, T.; Wang, Y. Progress on methanol reforming technologies for highly efficient hydrogen production and applications. Int. J. Hydrog. Energy 2022, 47, 35757–35777. [Google Scholar] [CrossRef]
- Kulprathipanja, A.; Falconer, J.L. Partial oxidation of methanol for hydrogen production using ITO/Al2O3 nanoparticle catalysts. Appl. Catal. A Gen. 2004, 261, 77–86. [Google Scholar] [CrossRef]
- Nielsen, M.; Alberico, E.; Baumann, W.; Drexler, H.-J.; Junge, H.; Gladiali, S.; Beller, M. Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide. Nature 2013, 495, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lugo, R.E.; Trincado, M.; Vogt, M.; Tewes, F.; Santiso-Quinones, G.; Grützmacher, H. A homogeneous transition metal complex for clean hydrogen production from methanol–water mixtures. Nat. Chem. 2013, 5, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Shabaker, J. Aqueous-phase reforming of methanol and ethylene glycol over alumina-supported platinum catalysts. J. Catal. 2003, 215, 344–352. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, W.; Liu, T.; Liu, J.; Wang, C.; Lei, L.; Liao, M.; Wang, C.; Chen, Y. Study on the promotion of FeOx species on water-gas shift reaction in methanol aqueous phase reforming over PtFe/Al2O3 catalyst. Int. J. Hydrog. Energy 2022, 47, 41468–41479. [Google Scholar] [CrossRef]
- Wong, S.S.; Shu, R.; Zhang, J.; Liu, H.; Yan, N. Downstream processing of lignin derived feedstock into end products. Chem. Soc. Rev. 2020, 49, 5510–5560. [Google Scholar] [CrossRef]
- Barroso, M.N.; Gomez, M.F.; Arrúa, L.A.; Abello, M.C. Co catalysts modified by rare earths (La, Ce or Pr) for hydrogen production from ethanol. Int. J. Hydrog. Energy 2014, 39, 8712–8719. [Google Scholar] [CrossRef]
- Augusto, B.L.; Costa, L.O.O.; Noronha, F.B.; Colman, R.C.; Mattos, L.V. Ethanol reforming over Ni/CeGd catalysts with low Ni content. Int. J. Hydrog. Energy 2012, 37, 12258–12270. [Google Scholar] [CrossRef]
- Jacobs, G.; Keogh, R.; Davis, B. Steam reforming of ethanol over Pt/ceria with co-fed hydrogen. J. Catal. 2007, 245, 326–337. [Google Scholar] [CrossRef]
- Zhong, Z.; Ang, H.; Choong, C.; Chen, L.; Huang, L.; Lin, J. The role of acidic sites and the catalytic reaction pathways on the Rh/ZrO2 catalysts for ethanol steam reforming. Phys. Chem. Chem. Phys. 2009, 11, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Loganathan, K.; Pham, H.N.; Datye, A.K.; Leclerc, C.A. Surface modification of solution combustion synthesized Ni/Al2O3 catalyst for aqueous-phase reforming of ethanol. Int. J. Hydrog. Energy 2010, 35, 11700–11708. [Google Scholar] [CrossRef]
- Tang, Z.; Monroe, J.; Dong, J.; Nenoff, T.; Weinkauf, D. Platinum-loaded NaY zeolite for aqueous-phase reforming of methanol and ethanol to hydrogen. Ind. Eng. Chem. Res. 2009, 48, 2728–2733. [Google Scholar] [CrossRef]
- Cruz, I.O.; Ribeiro, N.F.P.; Aranda, D.A.G.; Souza, M.M.V.M. Hydrogen production by aqueous-phase reforming of ethanol over nickel catalysts prepared from hydrotalcite precursors. Catal. Commun. 2008, 9, 2606–2611. [Google Scholar] [CrossRef]
- González-Arias, J.; Zhang, Z.; Reina, T.R.; Odriozola, J.A. Hydrogen production by catalytic aqueous-phase reforming of waste biomass: A review. Environ. Chem. Lett. 2023, 21, 3089–3104. [Google Scholar] [CrossRef]
- Trane, R.; Dahl, S.; Skjøth-Rasmussen, M.S.; Jensen, A.D. Catalytic steam reforming of bio-oil. Int. J. Hydrog. Energy 2012, 37, 6447–6472. [Google Scholar] [CrossRef]
- Wan, H.; Chaudhari, R.V.; Subramaniam, B. Aqueous phase hydrogenation of acetic acid and its promotional effect on p-cresol hydrodeoxygenation. Energy Fuels 2012, 27, 487–493. [Google Scholar] [CrossRef]
- Roy, B.; Artyushkova, K.; Pham, H.N.; Li, L.; Datye, A.K.; Leclerc, C.A. Effect of preparation method on the performance of the Ni/Al2O3 catalysts for aqueous-phase reforming of ethanol: Part II-characterization. Int. J. Hydrog. Energy 2012, 37, 18815–18826. [Google Scholar] [CrossRef]
- Roy, B.; Martinez, U.; Loganathan, K.; Datye, A.K.; Leclerc, C.A. Effect of preparation methods on the performance of Ni/Al2O3 catalysts for aqueous-phase reforming of ethanol: Part I-catalytic activity. Int. J. Hydrog. Energy 2012, 37, 8143–8153. [Google Scholar] [CrossRef]
- Nozawa, T.; Mizukoshi, Y.; Yoshida, A.; Naito, S. Aqueous phase reforming of ethanol and acetic acid over TiO2 supported Ru catalysts. Appl. Catal. B Environ. 2014, 146, 221–226. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, S.; Chen, Y.; Wang, D.; Cai, W. Hydrogen production from ethanol reforming: Catalysts and reaction mechanism. Renew. Sustain. Energy Rev. 2015, 44, 132–148. [Google Scholar] [CrossRef]
- Manochio, C.; Andrade, B.R.; Rodriguez, R.P.; Moraes, B.S. Ethanol from biomass: A comparative overview. Renew. Sustain. Energy Rev. 2017, 80, 743–755. [Google Scholar] [CrossRef]
- Wei, Z.; Karim, A.; Li, Y.; Wang, Y. Elucidation of the roles of Re in aqueous-phase reforming of glycerol over Pt–Re/C catalysts. ACS Catal. 2015, 5, 7312–7320. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energy Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Armando, D.-A.; Carlos, C.-H.J.; Israel, F.-B.M.; Jazmín, H.-D.; Orlando, P.-V.L.; Javier, E.-M.F.; Antonio, S.-M.G.; Gilver, R.-C. Life cycle analysis of hydrogen production from aqueous phase reforming of glycerol. Int. J. Hydrog. Energy 2024, 1, 196–206. [Google Scholar] [CrossRef]
- King, D.L.; Zhang, L.; Xia, G.; Karim, A.M.; Heldebrant, D.J.; Wang, X.; Peterson, T.; Wang, Y. Aqueous phase reforming of glycerol for hydrogen production over Pt–Re supported on carbon. Appl. Catal. B Environ. 2010, 99, 206–213. [Google Scholar] [CrossRef]
- Ciftci, A.; Ligthart, D.A.J.M.; Hensen, E.J.M. Influence of Pt particle size and Re addition by catalytic reduction on aqueous phase reforming of glycerol for carbon-supported Pt (Re) catalysts. Appl. Catal. B Environ. 2015, 174–175, 126–135. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts. Appl. Catal. B Environ. 2005, 56, 171–186. [Google Scholar] [CrossRef]
- Guo, Y.; Azmat, M.U.; Liu, X.; Wang, Y.; Lu, G. Effect of support’s basic properties on hydrogen production in aqueous-phase reforming of glycerol and correlation between WGS and APR. Appl. Energy 2012, 92, 218–223. [Google Scholar] [CrossRef]
- Vikla, A.K.K.; Koichumanova, K.; He, S.; Seshan, K. Aqueous-phase reforming of hydroxyacetone solution to bio-based H2 over supported Pt catalysts. Green Energy Environ. 2024, 9, 777–788. [Google Scholar] [CrossRef]
- Koichumanova, K.; Vikla, A.K.K.; Cortese, R.; Ferrante, F.; Seshan, K.; Duca, D.; Lefferts, L. In situ ATR-IR studies in aqueous phase reforming of hydroxyacetone on Pt/ZrO2 and Pt/AlO(OH) catalysts: The role of aldol condensation. Appl. Catal. B Environ. 2018, 232, 454–463. [Google Scholar] [CrossRef]
- Tokarev, A.V.; Kirilin, A.V.; Murzina, E.V.; Eränen, K.; Kustov, L.M.; Murzin, D.Y.; Mikkola, J.P. The role of bio-ethanol in aqueous phase reforming to sustainable hydrogen. Int. J. Hydrog. Energy 2010, 35, 12642–12649. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Lopez-Sanchez, J.A. Review of hydrogen production by catalytic aqueous-phase reforming. ChemistrySelect 2017, 2, 6563–6576. [Google Scholar] [CrossRef]
- Zambare, R.S.; Vaidya, P.D. Platinum catalyst supported on hydrotalcite for hydrogen production by aqueous-phase reforming of glycerol, glucose, glycine and humic acid. Int. J. Hydrog. Energy 2024, 87, 310–320. [Google Scholar] [CrossRef]
- Leng, S.; Barghi, S.; Xu, C. A short review on green H2 production by aqueous phase reforming of biomass derivatives. Npj Mater. Sustain. 2024, 2, 19. [Google Scholar] [CrossRef]
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef]
- Melero, J.A.; Iglesias, J.; Garcia, A. Biomass as renewable feedstock in standard refinery units. Feasibility, opportunities and challenges. Energy Environ. Sci. 2012, 5, 7393–7420. [Google Scholar] [CrossRef]
- Wen, G.; Xu, Y.; Xu, Z.; Tian, Z. Characterization and catalytic properties of the Ni/Al2O3 catalysts for aqueous-phase reforming of glucose. Catal. Lett. 2008, 129, 250–257. [Google Scholar] [CrossRef]
- Tanksale, A.; Beltramini, J.N.; Lu, G.Q. Reaction mechanisms for renewable hydrogen from liquid phase reforming of sugar compounds. Dev. Chem. Eng. Miner. Process. 2008, 14, 9–18. [Google Scholar] [CrossRef]
- Koklin, A.E.; Klimenko, T.A.; Kondratyuk, A.V.; Lunin, V.V.; Bogdan, V.I. Transformation of aqueous solutions of glucose over the Pt/C catalyst. Kinet. Catal. 2015, 56, 84–88. [Google Scholar] [CrossRef]
- Tanksale, A.; Beltramini, J.N.; Lu, G.M. A review of catalytic hydrogen production processes from biomass. Renew. Sustain. Energy Rev. 2010, 14, 166–182. [Google Scholar] [CrossRef]
- Davda, R.R.; Dumesic, J.A. Renewable hydrogen by aqueous-phase reforming of glucose. Chem. Commun. 2004, 1, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Susanti, R.F.; Dianningrum, L.W.; Yum, T.; Kim, Y.; Lee, Y.-W.; Kim, J. High-yield hydrogen production by supercritical water gasification of various feedstocks: Alcohols, glucose, glycerol and long-chain alkanes. Chem. Eng. Res. Des. 2014, 92, 1834–1844. [Google Scholar] [CrossRef]
- Valenzuela, M.B.; Jones, C.W.; Agrawal, P.K. Batch aqueous-phase reforming of woody biomass. Energy Fuels 2006, 20, 1744–1752. [Google Scholar] [CrossRef]
- Wen, G.; Xu, Y.; Xu, Z.; Tian, Z. Direct conversion of cellulose into hydrogen by aqueous-phase reforming process. Catal. Commun. 2010, 11, 522–526. [Google Scholar] [CrossRef]
- Soták, T.; Hronec, M.; Vávra, I.; Dobročka, E. Sputtering processed tungsten catalysts for aqueous phase reforming of cellulose. Int. J. Hydrog. Energy 2016, 41, 21936–21944. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. Pretreatment: The key to efficient utilization of lignocellulosic materials. Biomass Bioenergy 2012, 46, 70–78. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Pipitone, G.; Zoppi, G.; Frattini, A.; Bocchini, S.; Pirone, R.; Bensaid, S. Aqueous phase reforming of sugar-based biorefinery streams: From the simplicity of model compounds to the complexity of real feeds. Catal. Today 2020, 345, 267–279. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Lundqvist, J.; Teleman, A.; Junel, L.; Zacchi, G.; Dahlman, O.; Tjerneld, F.; Stålbrand, H. Isolation and characterization of galactoglucomannan from spruce (Picea abies). Carbohydr. Polym. 2002, 48, 29–39. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Yan, B.; Li, W.; Tao, J.; Xu, N.; Li, X.; Chen, G. Hydrogen production by aqueous phase reforming of phenol over Ni/ZSM-5 catalysts. Int. J. Hydrog. Energy 2017, 42, 6674–6682. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A Critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Wu, M.Y.; Lin, J.T.; Xu, Z.Q.; Hua, T.C.; Lv, Y.C.; Liu, Y.F.; Pei, R.H.; Wu, Q.; Liu, M.H. Selective catalytic degradation of a lignin model compound into phenol over transition metal sulfates. RSC Adv. 2020, 10, 3013–3019. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Azmat, M.U.; Xu, W.; Ren, J.; Wang, Y.; Lu, G. Hydrogen production by aqueous-phase reforming of glycerol over Ni-B catalysts. Int. J. Hydrog. Energy 2012, 37, 227–234. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Liu, X.; Guo, Y.; Pao, C.-W.; Chen, J.-L.; Hu, Y.; Wang, Y. NiAl2O4 spinel supported Pt catalyst: High performance and origin in aqueousphase reforming of methanol. ACS Catal. 2019, 9, 9671–9682. [Google Scholar] [CrossRef]
- Luo, N.; Fu, X.; Cao, F.; Xiao, T.; Edwards, P.P. Glycerol aqueous phase reforming for hydrogen generation over Pt catalyst—Effect of catalyst composition and reaction conditions. Fuel 2008, 87, 3483–3489. [Google Scholar] [CrossRef]
- Rahman, M.M.; Church, T.L.; Minett, A.I.; Harris, A.T. Effect of CeO2 addition to Al2O3 supports for Pt catalysts on the aqueous-phase reforming of glycerol. ChemSusChem 2013, 6, 1006–1013. [Google Scholar] [CrossRef]
- Reynoso, A.J.; Iriarte-Velasco, U.; Gutiérrez-Ortiz, M.A.; Ayastuy, J.L. Highly stable Pt/CoAl2O4 catalysts in aqueous-phase reforming of glycerol. Catal. Today 2021, 367, 278–289. [Google Scholar] [CrossRef]
- Irmak, S.; Öztürk, İ. Hydrogen rich gas production by thermocatalytic decomposition of kenaf biomass. Int. J. Hydrog. Energy 2010, 35, 5312–5317. [Google Scholar] [CrossRef]
- Alvear, M.; Aho, A.; Simakova, I.L.; Grénman, H.; Salmi, T.; Murzin, D.Y. Aqueous phase reforming of xylitol and xylose in the presence of formic acid. Catal. Sci. Technol. 2020, 10, 5245–5255. [Google Scholar] [CrossRef]
- Liao, Q.; Wang, Y.; Chen, C.; Zhang, S. Electronic regulation of Pt for low-temperature hydrogen generation from methanol and water. ACS Sustain. Chem. Eng. 2024, 12, 18486–18492. [Google Scholar] [CrossRef]
- Menezes, A.O.; Rodrigues, M.T.; Zimmaro, A.; Borges, L.E.P.; Fraga, M.A. Production of renewable hydrogen from aqueous-phase reforming of glycerol over Pt catalysts supported on different oxides. Renew. Energy 2011, 36, 595–599. [Google Scholar] [CrossRef]
- Czaplicka, N.; Rogala, A.; Wysocka, I. Metal (Mo, W, Ti) Carbide catalysts: Synthesis and application as alternative catalysts for dry reforming of hydrocarbons—A review. Int. J. Mol. Sci. 2021, 22, 12337. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Gao, R.; Yao, S.; Zhang, X.; Xu, W.; Zheng, S.; Jiang, Z.; Yu, Q.; Li, Y.W.; et al. Low-temperature hydrogen production from water and methanol using Pt/alpha-MoC catalysts. Nature 2017, 544, 80–83. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. Aqueous-phase reforming of ethylene glycol on silica-supported metal catalysts. Appl. Catal. B Environ. 2003, 43, 13–26. [Google Scholar] [CrossRef]
- Liu, J.; Sun, B.; Hu, J.; Pei, Y.; Li, H.; Qiao, M. Aqueous-phase reforming of ethylene glycol to hydrogen on Pd/Fe3O4 catalyst prepared by co-precipitation: Metal–support interaction and excellent intrinsic activity. J. Catal. 2010, 274, 287–295. [Google Scholar] [CrossRef]
- Meryemoglu, B.; Kaya Ozsel, B.; Irmak, S. Evaluation of hardwood or softwood bark biomass as feed materials for aqueous-phase reforming gasification process. Int. J. Hydrog. Energy 2024, 53, 1044–1051. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, X.; Zhou, W.; Gao, R.; Xu, W.; Ye, Y.; Lin, L.; Wen, X.; Liu, P.; Chen, B.; et al. Atomic-layered Au clusters on α-MoC as catalysts for the low-temperature water-gas shift reaction. Science 2017, 357, 389–393. [Google Scholar] [CrossRef]
- Manfro, R.L.; da Costa, A.F.; Ribeiro, N.F.P.; Souza, M.M.V.M. Hydrogen production by aqueous-phase reforming of glycerol over nickel catalysts supported on CeO2. Fuel Process. Technol. 2011, 92, 330–335. [Google Scholar] [CrossRef]
- Iriondo, A.; Barrio, V.L.; Cambra, J.F.; Arias, P.L.; Güemez, M.B.; Navarro, R.M.; Sánchez-Sánchez, M.C.; Fierro, J.L.G. Hydrogen production from glycerol over nickel catalysts supported on Al2O3 modified by Mg, Zr, Ce or La. Top. Catal. 2008, 49, 46–58. [Google Scholar] [CrossRef]
- Lin, L.; Yu, Q.; Peng, M.; Li, A.; Yao, S.; Tian, S.; Liu, X.; Li, A.; Jiang, Z.; Gao, R.; et al. Atomically dispersed Ni/alpha-MoC catalyst for hydrogen production from methanol/water. J. Am. Chem. Soc. 2021, 143, 309–317. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, W.; An, Z.; Song, H.; He, J. Interface–promoted dehydrogenation and water–gas shift toward high-efficient H2 production from aqueous phase reforming of cellulose. ACS Sustain. Chem. Eng. 2018, 6, 7313–7324. [Google Scholar] [CrossRef]
- Meryemoglu, B.; Hesenov, A.; Irmak, S.; Atanur, O.M.; Erbatur, O. Aqueous-phase reforming of biomass using various types of supported precious metal and raney-nickel catalysts for hydrogen production. Int. J. Hydrog. Energy 2010, 35, 12580–12587. [Google Scholar] [CrossRef]
- Zheng, Z.; Fang, Y.; Yang, J.; Ma, L.; Meng, Q.; Lin, X.; Liu, Y.; Zhang, Q.; Wang, T. A highly active and hydrothermal-resistant Cu/ZnO@NC catalyst for aqueous phase reforming of methanol to hydrogen. Int. J. Hydrog. Energy 2022, 47, 950–961. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Wang, Y. Catalytic and DRIFTS studies of Pt-based bimetallic alloy catalysts in aqueous-phase reforming of glycerol. Ind. Eng. Chem. Res. 2019, 58, 2749–2758. [Google Scholar] [CrossRef]
- Kim, M.-C.; Kim, T.-W.; Kim, H.J.; Kim, C.-U.; Bae, J.W. Aqueous phase reforming of polyols for hydrogen production using supported Pt Fe bimetallic catalysts. Renew. Energy 2016, 95, 396–403. [Google Scholar] [CrossRef]
- Chang, A.C.C.; Louh, R.F.; Wong, D.; Tseng, J.; Lee, Y.S. Hydrogen production by aqueous-phase biomass reforming over carbon textile supported Pt–Ru bimetallic catalysts. Int. J. Hydrog. Energy 2011, 36, 8794–8799. [Google Scholar] [CrossRef]
- Alvear, M.; Aho, A.; Simakova, I.L.; Grénman, H.; Salmi, T.; Murzin, D.Y. Aqueous phase reforming of alcohols over a bimetallic Pt-Pd catalyst in the presence of formic acid. Chem. Eng. J. 2020, 398, 125541–125550. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, C.; Luo, X.; Shu, R.; Lei, L.; Liu, J.; Tian, Z.; Liao, Y.; Chen, Y. Aqueous phase reforming of methanol for hydrogen production over highly-dispersed PtLa/CeO2 catalyst prepared by photochemical reduction method. Int. J. Hydrog. Energy 2024, 62, 1054–1066. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Wu, X.; Cao, X.; Geng, H.; Zhang, C.; Liu, S. Improving the hydrothermal stability and hydrogen selectivity of Ni-Cu based catalysts for the aqueous-phase reforming of methanol. Int. J. Hydrog. Energy 2023, 48, 12699–12711. [Google Scholar] [CrossRef]
- Coronado, I.; Stekrova, M.; García Moreno, L.; Reinikainen, M.; Simell, P.; Karinen, R.; Lehtonen, J. Aqueous-phase reforming of methanol over nickel-based catalysts for hydrogen production. Biomass Bioenergy 2017, 106, 29–37. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, N. Hydrogen production from ethylene glycol aqueous phase reforming over Ni–Al layered hydrotalcite-derived catalysts. Catalysts 2020, 10, 54. [Google Scholar] [CrossRef]
- Si, X.; Zhao, Z.; Chen, J.; Lu, R.; Lu, F. Low-temperature efficient hydrogen production from raw biomass on the Ni–Mo catalyst. ACS Catal. 2022, 12, 10629–10637. [Google Scholar] [CrossRef]
- Jazani, O.; Bennett, J.; Liguori, S. Carbon-low, renewable hydrogen production from methanol steam reforming in membrane reactors—A review. Chem. Eng. Process.-Process Intensif. 2023, 189, 109382. [Google Scholar] [CrossRef]
- Gasparetto, H.; Salau, N.P.G. A review on ethanol steam reforming focusing on yttria-stabilized zirconia catalysts: A look into hydrogen production for fuel cells. Fuel 2024, 371, 132–140. [Google Scholar] [CrossRef]
- Compagnoni, M.; Mostafavi, E.; Tripodi, A.; Mahinpey, N.; Rossetti, I. Techno-economic analysis of a bioethanol to hydrogen centralized plant. Energy Fuels 2017, 31, 12988–12996. [Google Scholar] [CrossRef]
- Lee, B.; Heo, J.; Kim, S.; Kim, C.-H.; Ryi, S.-K.; Lim, H. Integrated techno-economic analysis under uncertainty of glycerol steam reforming for H2 production at distributed H2 refueling stations. Energy Convers. Manag. 2019, 180, 250–257. [Google Scholar] [CrossRef]
- Cormos, A.-M.; Cormos, C.-C. Techno-economic and environmental performances of glycerol reforming for hydrogen and power production with low carbon dioxide emissions. Int. J. Hydrog. Energy 2017, 42, 7798–7810. [Google Scholar] [CrossRef]
- Khodabandehloo, M.; Larimi, A.; Khorasheh, F. Comparative process modeling and techno-economic evaluation of renewable hydrogen production by glycerol reforming in aqueous and gaseous phases. Energy Convers. Manag. 2020, 225, 113483. [Google Scholar] [CrossRef]
- Ganeshan, P.; Vigneswaran, V.S.; Gowd, S.C.; Kondusamy, D.; Sanjay Kumar, C.; Krishnamoorthy, N.; Kumar, D.; Juneja, A.; Paramasivan, B.; Raju, N.N.; et al. How does techno-economic analysis and lifecycle assessment help in commercializing the biohydrogen supply chain? Fuel 2023, 341, 127601. [Google Scholar] [CrossRef]
- Bahari, M.B.; Mamat, C.R.; Jalil, A.A.; Siang, T.J.; Hassan, N.S.; Khusnun, N.F.; Nabgan, W.; Roslan, N.A.; Abidin, S.Z.; Setiabudi, H.D.; et al. Cobalt-based catalysts for hydrogen production by thermochemical valorization of glycerol: A review. Environ. Chem. Lett. 2022, 20, 2361–2384. [Google Scholar] [CrossRef]
- Valliyappan, T.; Bakhshi, N.N.; Dalai, A.K. Pyrolysis of glycerol for the production of hydrogen or syn gas. Bioresour. Technol. 2008, 99, 4476–4483. [Google Scholar] [CrossRef] [PubMed]
- Yukesh Kannah, R.; Kavitha, S.; Preethi; Parthiba Karthikeyan, O.; Kumar, G.; Dai-Viet, N.V.; Rajesh Banu, J. Techno-economic assessment of various hydrogen production methods—A review. Bioresour. Technol. 2021, 319, 124175. [Google Scholar] [CrossRef]
- Sorunmu, Y.; Billen, P.; Spatari, S. A review of thermochemical upgrading of pyrolysis bio-oil: Techno-economic analysis, life cycle assessment, and technology readiness. GCB Bioenergy 2020, 12, 4–18. [Google Scholar] [CrossRef]
- Lopez, G.; Santamaria, L.; Lemonidou, A.; Zhang, S.; Wu, C.; Sipra, A.T.; Gao, N. Hydrogen generation from biomass by pyrolysis. Nat. Rev. Methods Primers 2022, 2, 20. [Google Scholar] [CrossRef]
- Samad, T.E.; Adeyemi, I.; Ghenai, C.; Janajreh, I. Assessment of glycerol gasification: Devolatilization kinetics and parametric analysis. Chem. Pap. 2023, 77, 3277–3291. [Google Scholar] [CrossRef]
- Cerone, N.; Zimbardi, F.; Contuzzi, L.; Striūgas, N.; Eimontas, J.; Zito, G.D. Production of hydrogen-rich syngas from biomass gasification by double step steam catalytic tar reforming. Int. J. Hydrog. Energy 2024, 95, 1215–1221. [Google Scholar] [CrossRef]
- Sara, H.R.; Enrico, B.; Mauro, V.; Andrea, D.C.; Vincenzo, N. Techno-economic analysis of hydrogen production using biomass gasification -A small scale power plant study. Energy Procedia 2016, 101, 806–813. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen production technologies: Current state and future developments. Conf. Pap. Sci. 2013, 2013, 690627. [Google Scholar] [CrossRef]
- Levin, D.B.; Chahine, R. Challenges for renewable hydrogen production from biomass. Int. J. Hydrog. Energy 2010, 35, 4962–4969. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).