Layered Double Hydroxide (LDH)-Derived Mixed Oxides for Enhanced Light Hydrocarbon Production from CO2 Hydrogenation
Abstract
1. Introduction
2. Results
2.1. Characterization of Catalysts
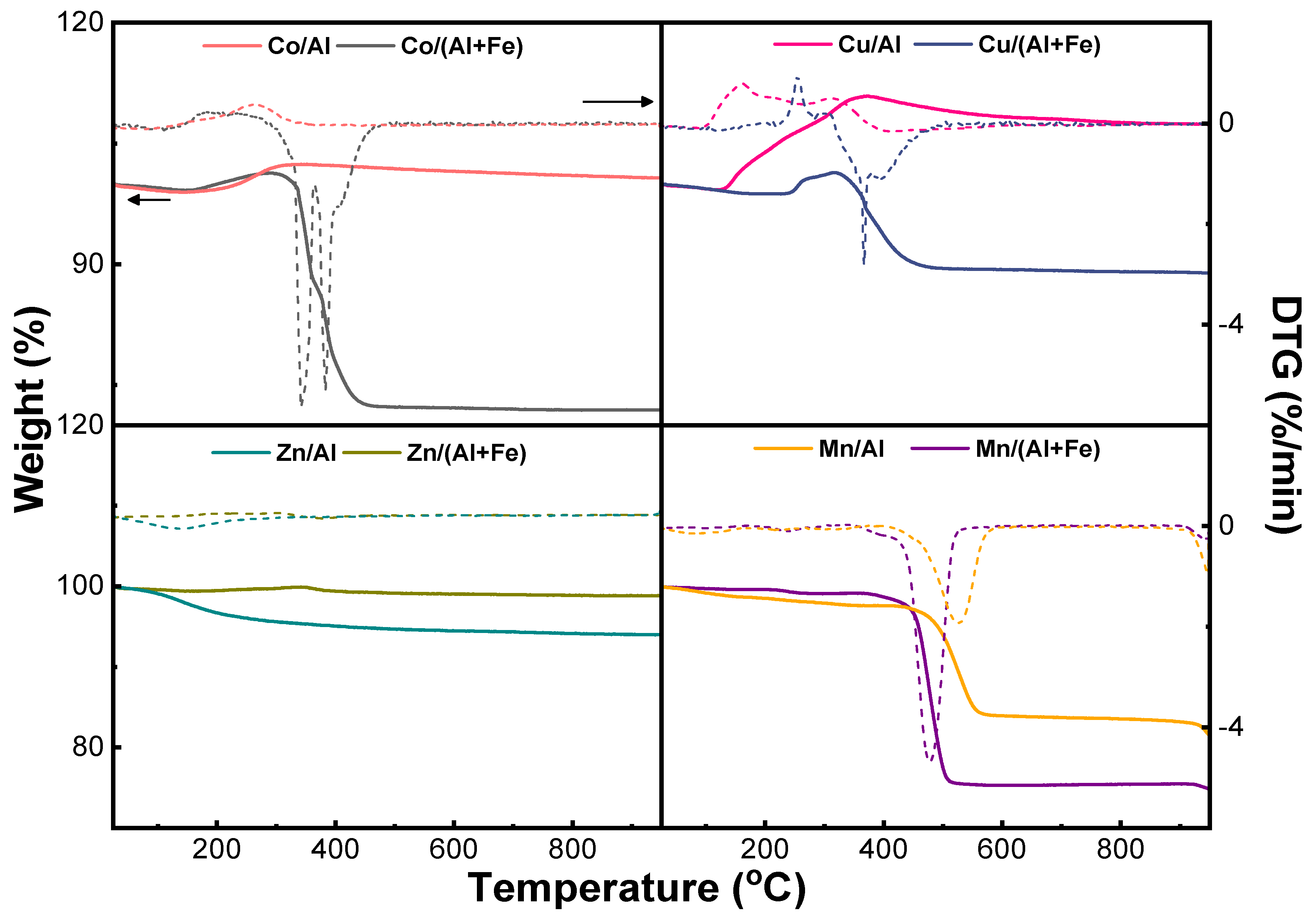
2.1.1. Characterization of As-Prepared Materials
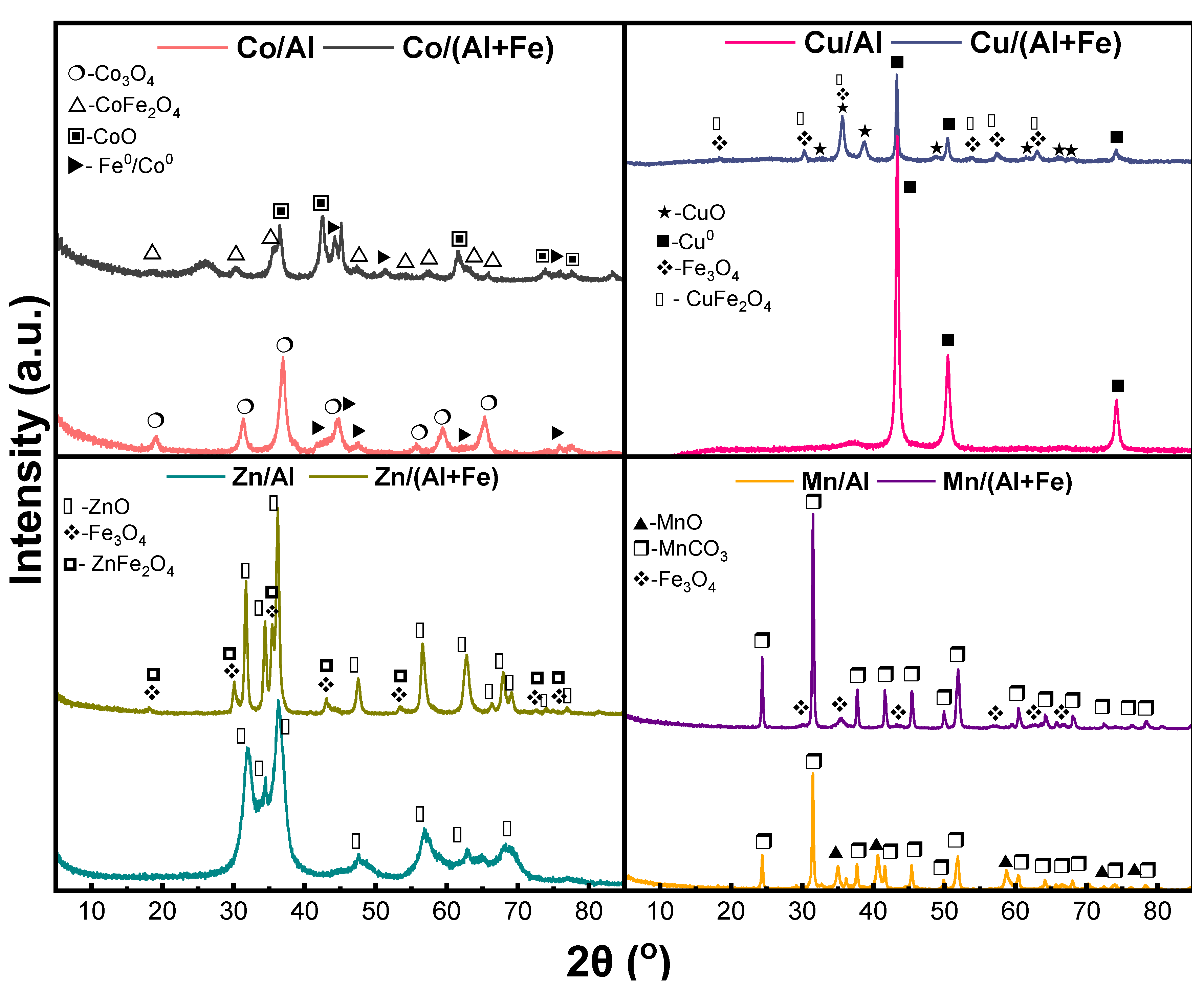
2.1.2. Characterization of Calcined Mixed Oxides
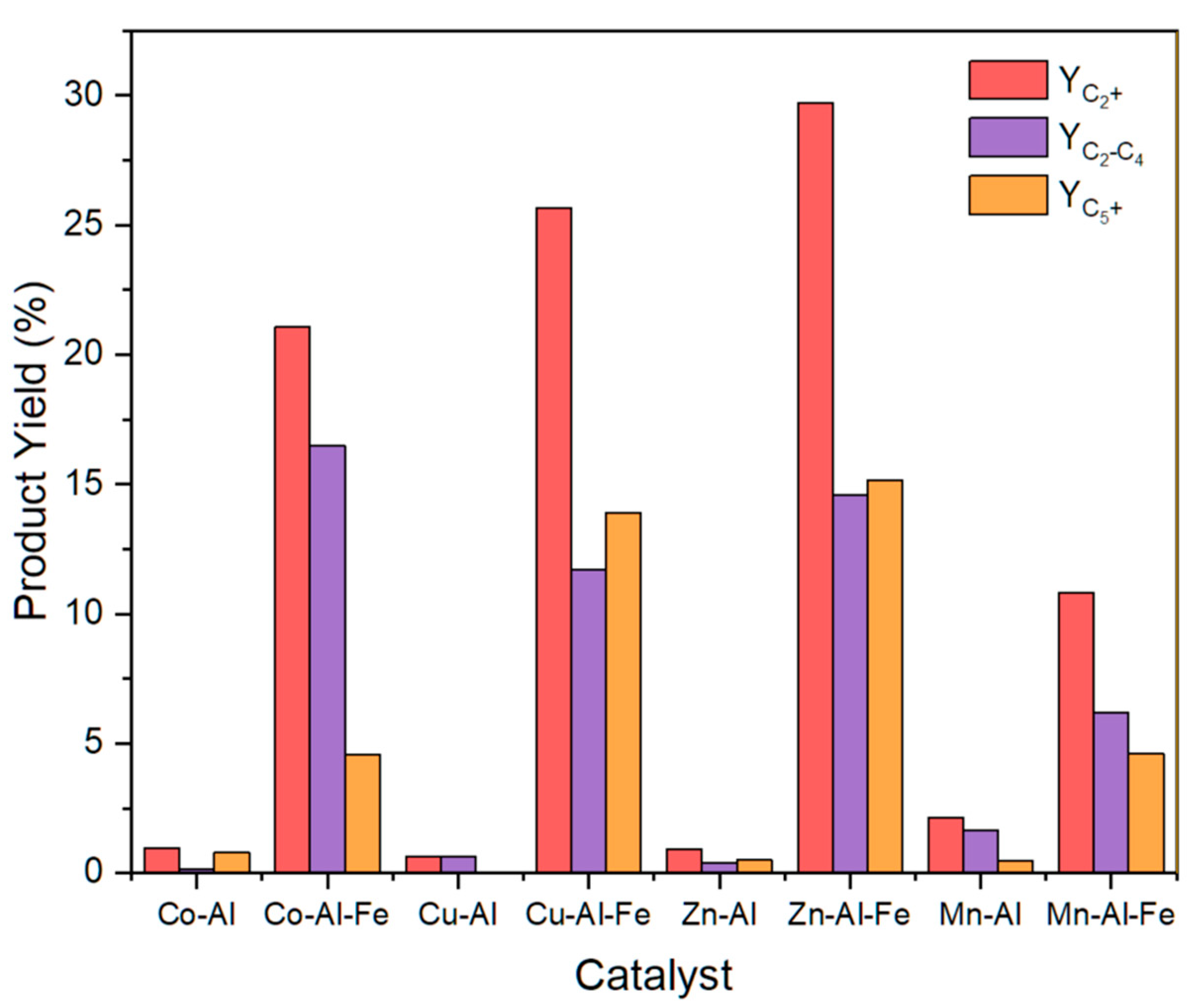
2.2. CO2 Hydrogenation
2.3. Characterization of Spent Catalysts
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Catalysts
4.3. Characterization of Catalysts
4.4. Catalytic Activity Test
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, J.; Liu, J.; Guo, L.; Wang, W.; Wang, C.; Gao, W.; Guo, X.; He, Y.; Yang, G.; Yasuda, S.; et al. CO2 Hydrogenation over Fe-Co Bimetallic Catalysts with Tunable Selectivity through a Graphene Fencing Approach. Nat. Commun. 2024, 15, 512. [Google Scholar] [CrossRef] [PubMed]
- Flores-Granobles, M.; Saeys, M. Quantitative Analysis of CO2 Emissions Reduction Potential of Alternative Light Olefins Production Processes. Green Chem. 2023, 25, 6459–6471. [Google Scholar] [CrossRef]
- Ma, Z.; Porosoff, M.D. Development of Tandem Catalysts for CO2 Hydrogenation to Olefins. ACS Catal. 2019, 9, 2639–2656. [Google Scholar] [CrossRef]
- Jiao, F.; Li, J.; Pan, X.; Xiao, J.; Li, H.; Ma, H.; Wei, M.; Pan, Y.; Zhou, Z.; Li, M.; et al. Selective Conversion of Syngas to Light Olefins. Science 2016, 351, 1065–1068. [Google Scholar] [CrossRef]
- Wang, H.; Nie, X.; Liu, Y.; Janik, M.J.; Han, X.; Deng, Y.; Hu, W.; Song, C.; Guo, X. Mechanistic Insight into Hydrocarbon Synthesis via CO2 Hydrogenation on χ-Fe5C2 Catalysts. ACS Appl. Mater. Interfaces 2022, 14, 37637–37651. [Google Scholar] [CrossRef]
- Ye, D.; Tang, W.; Zhang, T.; Lv, L.; Zou, Z.; Gupta, R.K.; Tang, S. Enhancing the Synergism of Fe3O4 and Fe5C2 to Improve the Process of CO2 Hydrogenation to Olefines. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130145. [Google Scholar] [CrossRef]
- Ronda-Lloret, M.; Rothenberg, G.; Shiju, N.R. A Critical Look at Direct Catalytic Hydrogenation of Carbon Dioxide to Olefins. ChemSusChem 2019, 12, 3896–3914. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, X.; Wang, X.; Song, C. Fe-Cu Bimetallic Catalysts for Selective CO2 Hydrogenation to Olefin-Rich C2+ Hydrocarbons. Ind. Eng. Chem. Res. 2018, 57, 4535–4542. [Google Scholar] [CrossRef]
- Liu, N.; Wei, J.; Xu, J.; Yu, Y.; Yu, J.; Han, Y.; Wang, K.; Orege, J.I.; Ge, Q.; Sun, J. Elucidating the Structural Evolution of Highly Efficient Co–Fe Bimetallic Catalysts for the Hydrogenation of CO2 into Olefins. Appl. Catal. B 2023, 328, 122476. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, X.; Fan, G.; Yang, L.; Li, F. Unveiling the Roles of Fe-Co Interactions over Ternary Spinel-Type ZnCoxFe2−XO4 Catalysts for Highly Efficient CO2 Hydrogenation to Produce Light Olefins. J. Catal. 2021, 400, 355–366. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.; Su, X.; Fan, S.; Ma, Q.; Zhao, T. Selective Formation of Light Olefins from CO2 Hydrogenation over Fe-Zn-K Catalysts. J. CO2 Util. 2015, 12, 95–100. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, H.; Noh, W.Y.; Shin, J.; Han, S.J.; Kim, S.K.; An, K.; Lee, J.S. Cobalt Ferrite Nanoparticles to Form a Catalytic Co-Fe Alloy Carbide Phase for Selective CO2 Hydrogenation to Light Olefins. ACS Catal. 2020, 10, 8660–8671. [Google Scholar] [CrossRef]
- Chaipraditgul, N.; Numpilai, T.; Kui Cheng, C.; Siri-Nguan, N.; Sornchamni, T.; Wattanakit, C.; Limtrakul, J.; Witoon, T. Tuning Interaction of Surface-Adsorbed Species over Fe/K-Al2O3 Modified with Transition Metals (Cu, Mn, V, Zn or Co) on Light Olefins Production from CO2 Hydrogenation. Fuel 2021, 283, 119248. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jang, Y.J.; Park, H.; Kim, W.Y.; Lee, Y.H.; Choi, S.H.; Lee, J.S. Carbon Dioxide Fischer-Tropsch Synthesis: A New Path to Carbon-Neutral Fuels. Appl. Catal. B 2017, 202, 605–610. [Google Scholar] [CrossRef]
- Ojelade, O.A.; Zaman, S.F. A Review on CO2 hydrogenation to Lower Olefins: Understanding the Structure-Property Relationships in Heterogeneous Catalytic Systems. J. CO2 Util. 2021, 47, 101506. [Google Scholar] [CrossRef]
- Li, Z.; Wu, W.; Wang, M.; Wang, Y.; Ma, X.; Luo, L.; Chen, Y.; Fan, K.; Pan, Y.; Li, H.; et al. Ambient-Pressure Hydrogenation of CO2 into Long-Chain Olefins. Nat. Commun. 2022, 13, 2396. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, A.; Jiang, X.; Liu, M.; Sun, Y.; Song, C.; Guo, X. Selective CO2 Hydrogenation to Hydrocarbons on Cu-Promoted Fe-Based Catalysts: Dependence on Cu-Fe Interaction. ACS Sustain. Chem. Eng. 2018, 6, 10182–10190. [Google Scholar] [CrossRef]
- Tang, H.; Qiu, T.; Wang, X.; Zhang, C.; Zhang, Z. A Brief Review of Recent Theoretical Advances in Fe-Based Catalysts for CO2 Hydrogenation. Molecules 2024, 29, 1194. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Tang, Q.; Zuo, Z.; Liu, L.; Dong, J. Effect of MgFe-LDH with Reduction Pretreatment on the Catalytic Performance in Syngas to Light Olefins. Catalysts 2023, 13, 632. [Google Scholar] [CrossRef]
- Luo, M.; Li, M.; Lü, B.; Liu, Q.; Di, Z.; Guo, L. Cobalt Nanoparticle-Decorated LDH/ZIF-Derived Porous Nanoplatelets for Fischer-Tropsch Synthesis. ACS Appl. Nano Mater. 2021, 4, 3734–3741. [Google Scholar] [CrossRef]
- Jayaprakash, S.; Dewangan, N.; Jangam, A.; Das, S.; Kawi, S. LDH-Derived Ni–MgO–Al2O3 Catalysts for Hydrogen-Rich Syngas Production via Steam Reforming of Biomass Tar Model: Effect of Catalyst Synthesis Methods. Int. J. Hydrogen Energy 2021, 46, 18338–18352. [Google Scholar] [CrossRef]
- Dewangan, N.; Hui, W.M.; Jayaprakash, S.; Bawah, A.R.; Poerjoto, A.J.; Jie, T.; Jangam, A.; Hidajat, K.; Kawi, S. Recent Progress on Layered Double Hydroxide (LDH) Derived Metal-Based Catalysts for CO2 Conversion to Valuable Chemicals. Catal. Today 2020, 356, 490–513. [Google Scholar] [CrossRef]
- Fang, X.; Chen, C.; Jia, H.; Li, Y.; Liu, J.; Wang, Y.; Song, Y.; Du, T.; Liu, L. Progress in Adsorption-Enhanced Hydrogenation of CO2 on Layered Double Hydroxide (LDH) Derived Catalysts. J. Ind. Eng. Chem. 2021, 95, 16–27. [Google Scholar] [CrossRef]
- Vulić, T.; Hadnadjev, M.; Marinković-Nedučin, R. Structure and Morphology of Mg-Al-Fe-Mixed Oxides Derived from Layered Double Hydroxides. J. Microsc. 2008, 232, 634–638. [Google Scholar] [CrossRef]
- Xiang, X.; Hima, H.I.; Wang, H.; Li, F. Facile Synthesis and Catalytic Properties of Nickel-Based Mixed-Metal Oxides with Mesopore Networks from a Novel Hybrid Composite Precursor. Chem. Mater. 2008, 20, 1173–1182. [Google Scholar] [CrossRef]
- Zou, L.; Xiang, X.; Fan, J.; Li, F. Single-Source Precursor to Complex Metal Oxide Monoliths with Tunable Microstructures and Properties: The Case of Mg-Containing Materials. Chem. Mater. 2007, 19, 6518–6527. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-Type Anionic Clays: Preparation, Properties and Applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Yin, W.; Tan, Q.; Liu, L.; Li, X. Synthesis and Characterization of Mg-Al Layered Double Hydroxide. Adv. Mat. Res. 2012, 454, 101–104. [Google Scholar] [CrossRef]
- Boulahbal, A.I.; Santamaría, L.; Azizi, A.; Boutahala, M.; Korili, S.A.; Gil, A. Synthesis of Cu-Al Layered Double Hydroxides from Aluminum Saline Slags. Min. Eng. 2023, 204, 108413. [Google Scholar] [CrossRef]
- Ahmed, A.A.A.; Talib, Z.A.; Hussein, M.Z. bin Thermal, Optical and Dielectric Properties of Zn-Al Layered Double Hydroxide. Appl. Clay Sci. 2012, 56, 68–76. [Google Scholar] [CrossRef]
- Yusoff, N.F.M.; Idris, N.H.; Din, M.F.M.; Majid, S.R.; Harun, N.A.; Rahman, M.M. Investigation on the Electrochemical Performances of Mn2O3 as a Potential Anode for Na-Ion Batteries. Sci. Rep. 2020, 10, 9207. [Google Scholar] [CrossRef] [PubMed]
- Visinescu, D.; Paraschiv, C.; Ianculescu, A.; Jurca, B.; Vasile, B.; Carp, O. The Environmentally Benign Synthesis of Nanosized CoxZn1-XAl2O4 Blue Pigments. Dye. Pigment. 2010, 87, 125–131. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Wu, G.; Wu, W.; Zeng, H.; Jiang, H.; Zhang, W.; Wu, R.; Xue, Q. Effects of Coloration of Spinel CoAl2O4 Cobalt Blue Pigments: Composition, Structure, and Cation Distribution. Inorganics 2023, 11, 368. [Google Scholar] [CrossRef]
- Thommes, M. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Chem. Int. 2016, 38, 25. [Google Scholar] [CrossRef]
- Santamaría, L.; Oliveira García, L.; de Faria, E.H.; Ciuffi, K.J.; Vicente, M.A.; Korili, S.A.; Gil, A. M(II)-Al-Fe Layered Double Hydroxides Synthesized from Aluminum Saline Slag Wastes and Catalytic Performance on Cyclooctene Oxidation. Min. Eng. 2022, 180, 107516. [Google Scholar] [CrossRef]
- Triantafyllidis, K.S.; Peleka, E.N.; Komvokis, V.G.; Mavros, P.P. Iron-Modified Hydrotalcite-like Materials as Highly Efficient Phosphate Sorbents. J. Colloid Interface Sci. 2010, 342, 427–436. [Google Scholar] [CrossRef]
- Ferna, M.; Ulibarri, A.; Labajosb, F.M.; Rives, V. The Effect of Iron on the Crystalline Phases Formed upon Thermal Decomposition of Mg-Al-Fe Hydrotalcites. J. Mater. Chem. 1998, 8, 2507–2514. [Google Scholar]
- Kim, Y.J.; Kim, B.G.; Bae, J.W. CO2 Hydrogenation to Synthetic Natural Gas with Light Hydrocarbons on Mn-Promoted Mesoporous Co3O4-Al2O3 Metal Oxides. J. CO2 Util. 2024, 81, 102724. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, Q.; Liu, S.; Wei, C.; Wang, Y.; Wang, Y.; Huang, S.; Ma, X. Synergistic Effect of Fe-Mn Bimetallic Sites with Close Proximity for Enhanced CO2 Hydrogenation Performance. Front. Chem. Sci. Eng. 2024, 18, 140. [Google Scholar] [CrossRef]
- Lysenko, E.N.; Surzhikov, A.P.; Zhuravkov, S.P.; Vlasov, V.A.; Pustovalov, A.V.; Yavorovsky, N.A. The Oxidation Kinetics Study of Ultrafine Iron Powders by Thermogravimetric Analysis. J. Therm. Anal. Calorim. 2014, 115, 1447–1452. [Google Scholar] [CrossRef]
- Leventis, N.; Donthula, S.; Mandal, C.; Ding, M.S.; Sotiriou-Leventis, C. Explosive versus Thermite Behavior in Iron(0) Aerogels Infiltrated with Perchlorates. Chem. Mater. 2015, 27, 8126–8137. [Google Scholar] [CrossRef]
- Kuhn, C.; Knapp, A.; Deutschmann, M.P.; Spielmann, J.; Tischer, S.; Kramm, U.I.; Nirschl, H.; Deutschmann, O. Iron as Recyclable Metal Fuel: Unraveling Oxidation Behavior and Cyclization Effects Through Thermogravimetric Analysis, Wide-Angle X-Ray Scattering and Mössbauer Spectroscopy. ChemSusChem 2024, 17, e202400351. [Google Scholar] [CrossRef] [PubMed]
- Mahana, D.; Mauraya, A.K.; Singh, P.; Muthusamy, S.K. Evolution of CuO Thin Films through Thermal Oxidation of Cu Films Prepared by Physical Vapour Deposition Techniques. Solid State Commun. 2023, 366–367, 115152. [Google Scholar] [CrossRef]
- Gao, L.; Pang, C.; He, D.; Shen, L.; Gupta, A.; Bao, N. Synthesis of Hierarchical Nanoporous Microstructures via the Kirkendall Effect in Chemical Reduction Process. Sci. Rep. 2015, 5, 16061. [Google Scholar] [CrossRef]
- Burke, M.S.; Kast, M.G.; Trotochaud, L.; Smith, A.M.; Boettcher, S.W. Cobalt-Iron (Oxy)Hydroxide Oxygen Evolution Electrocatalysts: The Role of Structure and Composition on Activity, Stability, and Mechanism. J. Am. Chem. Soc. 2015, 137, 3638–3648. [Google Scholar] [CrossRef]
- Yao, R.; Wei, J.; Ge, Q.; Xu, J.; Han, Y.; Xu, H.; Sun, J. Structure Sensitivity of Iron Oxide Catalyst for CO2 Hydrogenation. Catal. Today 2021, 371, 134–141. [Google Scholar] [CrossRef]
- Pan, Y.; Ding, X.; Zhang, C.; Zhu, M.; Yang, Z.; Han, Y.F. Effects of Different Reductive Agents on Zn-Promoted Iron Oxide Phases in the CO2–Fischer–Tropsch to Linear α-Olefins. Catalysts 2023, 13, 594. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Jacobs, G.; Hamdeh, H.H.; Shafer, W.D.; Liu, F.; Hopps, S.D.; Thomas, G.A.; Davis, B.H. Hydrogenation of Carbon Dioxide over Co-Fe Bimetallic Catalysts. ACS Catal. 2016, 6, 913–927. [Google Scholar] [CrossRef]
- Yang, Y.; Evans, J.; Rodriguez, J.A.; White, M.G.; Liu, P. Fundamental Studies of Methanol Synthesis from CO2 Hydrogenation on Cu(111), Cu Clusters, and Cu/ZnO(0001). Phys. Chem. Chem. Phys. 2010, 12, 9909–9917. [Google Scholar] [CrossRef]
- Choi, Y.H.; Ra, E.C.; Kim, E.H.; Kim, K.Y.; Jang, Y.J.; Kang, K.N.; Choi, S.H.; Jang, J.H.; Lee, J.S. Sodium-Containing Spinel Zinc Ferrite as a Catalyst Precursor for the Selective Synthesis of Liquid Hydrocarbon Fuels. ChemSusChem 2017, 10, 4764–4770. [Google Scholar] [CrossRef]
- Wang, C.; Chen, J.; Zhu, H.; Zhang, W.; Bai, H.; Zhang, J. Highly Effective MFe2O4 (M=Zn, Mg, Cu and Mn) Spinel Catalysts for Fischer-Tropsch Synthesis. J. Fuel Chem. Technol. 2024, 52, 667–676. [Google Scholar] [CrossRef]
- Fallahi, Y.; Burgun, U.; Sarioglan, A.; Atakul, H. Effect of Sodium Incorporation into Fe-Zn Catalyst for Fischer- Tropsch Synthesis to Light Olefins. Mol. Catal. 2023, 535, 112866. [Google Scholar] [CrossRef]
- Mediero-Munoyerro, M.J.; McGregor, J.; McMillan, L.; Al-Yassir, N.; Bingham, P.A.; Forder, S.D.; Gorin, C.; Al-Khattaf, S.; Gladden, L.F.; Midgley, P.A. Structural Changes in FeOx/γ-Al2O3 Catalysts during Ethylbenzene Dehydrogenation. Catal. Struct. React. 2016, 2, 25–32. [Google Scholar] [CrossRef]
- Skrypnik, A.S.; Petrov, S.A.; Kondratenko, V.A.; Yang, Q.; Matvienko, A.A.; Kondratenko, E.V. Spatially Resolved Analysis of CO2 Hydrogenation to Higher Hydrocarbons over Alkali-Metal Promoted Well-Defined FexOyCz. J. Catal. 2023, 425, 286–295. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Hamdeh, H.H.; Jacobs, G.; Qian, D.; Liu, F.; Hopps, S.D.; Thomas, G.A.; Shafer, W.D.; Xiao, Q.; Hu, Y.; et al. Fischer-Tropsch Synthesis: Effect of Cu, Mn and Zn Addition on Activity and Product Selectivity of Cobalt Ferrite. RSC Adv. 2016, 6, 62356–62367. [Google Scholar] [CrossRef]
- Falbo, L.; Martinelli, M.; Visconti, C.G.; Lietti, L.; Forzatti, P.; Bassano, C.; Deiana, P. Effects of Zn and Mn Promotion in Fe-Based Catalysts Used for COx Hydrogenation to Long-Chain Hydrocarbons. Ind. Eng. Chem. Res. 2017, 56, 13146–13156. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Xu, J.; Wang, H.; Ding, M.; Li, Y. Study of Bimetallic Interactions and Promoter Effects of FeZn, FeMn and FeCr Fischer-Tropsch Synthesis Catalysts. J. Mol. Catal. A Chem. 2010, 326, 29–40. [Google Scholar] [CrossRef]
- Wu, D.K.; Wang, X.; Gao, X.H.; Ma, Q.X.; Zhang, J.L.; Fan, S.B.; Zhao, T.S. Preparation of Layered K-Fe-Zn-Ti Catalyst and Its Performance in the Hydrogenation of Carbon Dioxide to Light Olefins. J. Fuel Chem. Technol. 2019, 47, 949–956. [Google Scholar] [CrossRef]
- Song, F.; Han, H.; Zheng, D.; Fan, L.; Cao, Y.; Cai, W. Insight into the Synthesis of Alkali Metal Modified Co-Doped ZnFe2O4 with Faveolate Spinel Structure and Their Enhanced Selectivity of Olefins during CO2 Hydrogenation. Appl. Surf. Sci. 2025, 680, 161482. [Google Scholar] [CrossRef]
- Nam, S.S.; Lee, S.J.; Kim, H.; Jun, K.W.; Choi, M.J.; Lee, K.W. Catalytic Conversion of Carbon Dioxide into Hydrocarbons over Zinc Promoted Iron Catalysts. Energy Convers. Manag. 1997, 38, 397–402. [Google Scholar] [CrossRef]
- Zepeda, T.A.; Aguirre, S.; Galindo-Ortega, Y.I.; Solís-Garcia, A.; Navarro Yerga, R.M.; Pawelec, B.; Fierro-Gonzalez, J.C.; Fuentes, S. Hydrogenation of CO2 to Valuable C2-C5 Hydrocarbons on Mn-Promoted High-Surface-Area Iron Catalysts. Catalysts 2023, 13, 954. [Google Scholar] [CrossRef]
- Yuan, F.; Zhang, G.; Wang, M.; Zhu, J.; Zhang, M.; Ding, F.; Cheng, Z.; Song, C.; Guo, X. Boosting the Production of Light Olefins from CO2 Hydrogenation over Fe-Co Bimetallic Catalysts Derived from Layered Double Hydroxide. Ind. Eng. Chem. Res. 2023, 62, 8210–8221. [Google Scholar] [CrossRef]
- Zhang, L.; Dang, Y.; Zhou, X.; Gao, P.; Petrus van Bavel, A.; Wang, H.; Li, S.; Shi, L.; Yang, Y.; Vovk, E.I.; et al. Direct Conversion of CO2 to a Jet Fuel over CoFe Alloy Catalysts. Innovation 2021, 2, 100170. [Google Scholar] [CrossRef]
- Zhao, Q.; Fan, G.; Li, F. Unique CuOx–FeOx Interfaces in Cu-Decorated Fe-Based Catalysts Facilitating CO2 Hydrogenation to Higher Hydrocarbons. Chem. Eng. J. 2024, 495, 153309. [Google Scholar] [CrossRef]
- Li, Z.; Wang, K.; Xing, Y.; Song, W.; Gao, X.; Ma, Q.; Zhao, T.; Zhang, J. Synthesis of Liquid Hydrocarbon via Direct Hydrogenation of CO2 over FeCu-Based Bifunctional Catalyst Derived from Layered Double Hydroxides. Molecules 2023, 28, 6920. [Google Scholar] [CrossRef]
- Xu, M.; Liu, X.; Cao, C.; Sun, Y.; Zhang, C.; Yang, Z.; Zhu, M.; Ding, X.; Liu, Y.; Tong, Z.; et al. Ternary Fe-Zn-Al Spinel Catalyst for CO2 Hydrogenation to Linear α-Olefins: Synergy Effects between Al and Zn. ACS Sustain. Chem. Eng. 2021, 9, 13818–13830. [Google Scholar] [CrossRef]
- Gong, X.; Liu, Y.; He, R.; Xu, X.; Han, Z.; Chen, J.; Feng, B.; Wang, Z.; Xing, A. Insights into the Structural Evolution Process of Na/ZnFe2O4 Spinel Catalyst in CO2 Hydrogenation. ChemCatChem 2024, 16, e202301341. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, J.; Chen, Z.; Hu, D.; Tian, Y. Unraveling the Role of Reducing Atmospheres on Fe-Zn-Al Catalysts for Highly Selective Carbon Dioxide Hydrogenation to Light Olefins. J. Alloys Compd. 2024, 1009, 176911. [Google Scholar] [CrossRef]
- Satthawong, R.; Koizumi, N.; Song, C.; Prasassarakich, P. Light Olefin Synthesis from CO2 Hydrogenation over K-Promoted Fe-Co Bimetallic Catalysts. Catal. Today 2015, 251, 34–40. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, C.; Jia, L.; Liu, Y.; Sun, C.; Wang, P.; Tu, W. Insights into the Regulation of FeNa Catalysts Modified by Mn Promoter and Their Tuning Effect on the Hydrogenation of CO2 to Light Olefins. J. Catal. 2020, 390, 12–22. [Google Scholar] [CrossRef]
- Liang, B.; Sun, T.; Ma, J.; Duan, H.; Li, L.; Yang, X.; Zhang, Y.; Su, X.; Huang, Y.; Zhang, T. Mn Decorated Na/Fe Catalysts for CO 2 Hydrogenation to Light Olefins. Catal. Sci. Technol. 2019, 9, 456–464. [Google Scholar] [CrossRef]
- Wei, J.; Sun, J.; Wen, Z.; Fang, C.; Ge, Q.; Xu, H. New Insights into the Effect of Sodium on Fe3O4- Based Nanocatalysts for CO2 Hydrogenation to Light Olefins. Catal. Sci. Technol. 2016, 6, 4786–4793. [Google Scholar] [CrossRef]
- Elishav, O.; Shener, Y.; Beilin, V.; Landau, M.V.; Herskowitz, M.; Shter, G.E.; Grader, G.S.; Grader, G.S. Electrospun Fe-Al-O Nanobelts for Selective CO2 Hydrogenation to Light Olefins. ACS Appl. Mater. Interfaces 2020, 12, 24855–24867. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, X.; Song, G.; Cai, Y.; Shi, B.; Liu, Y.; Ding, X.; Yang, Z.; Tian, P.; Cao, C.; et al. Regulating Iron Species Compositions by Fe-Al Interaction in CO2 Hydrogenation. J. Catal. 2022, 413, 331–341. [Google Scholar] [CrossRef]
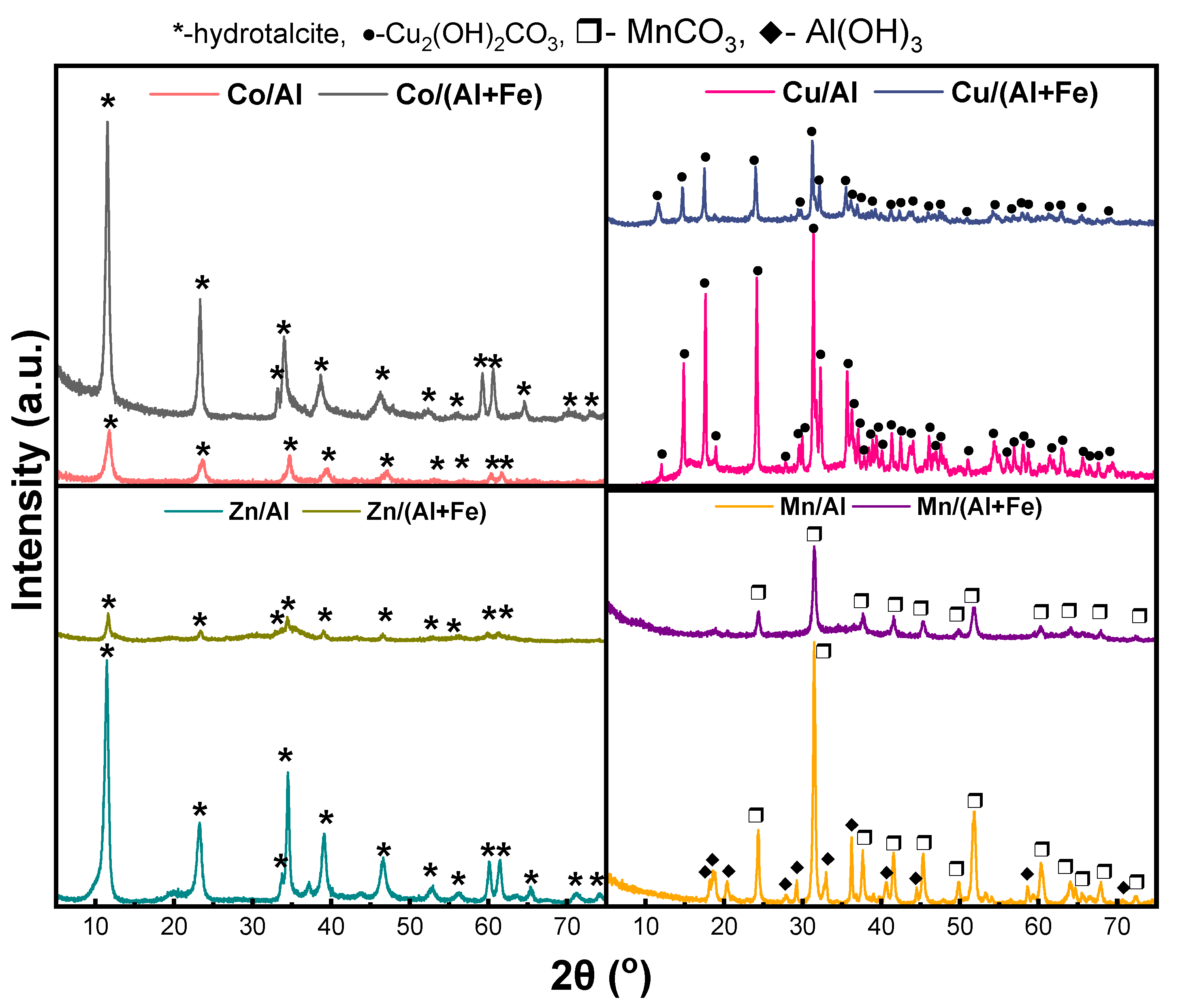

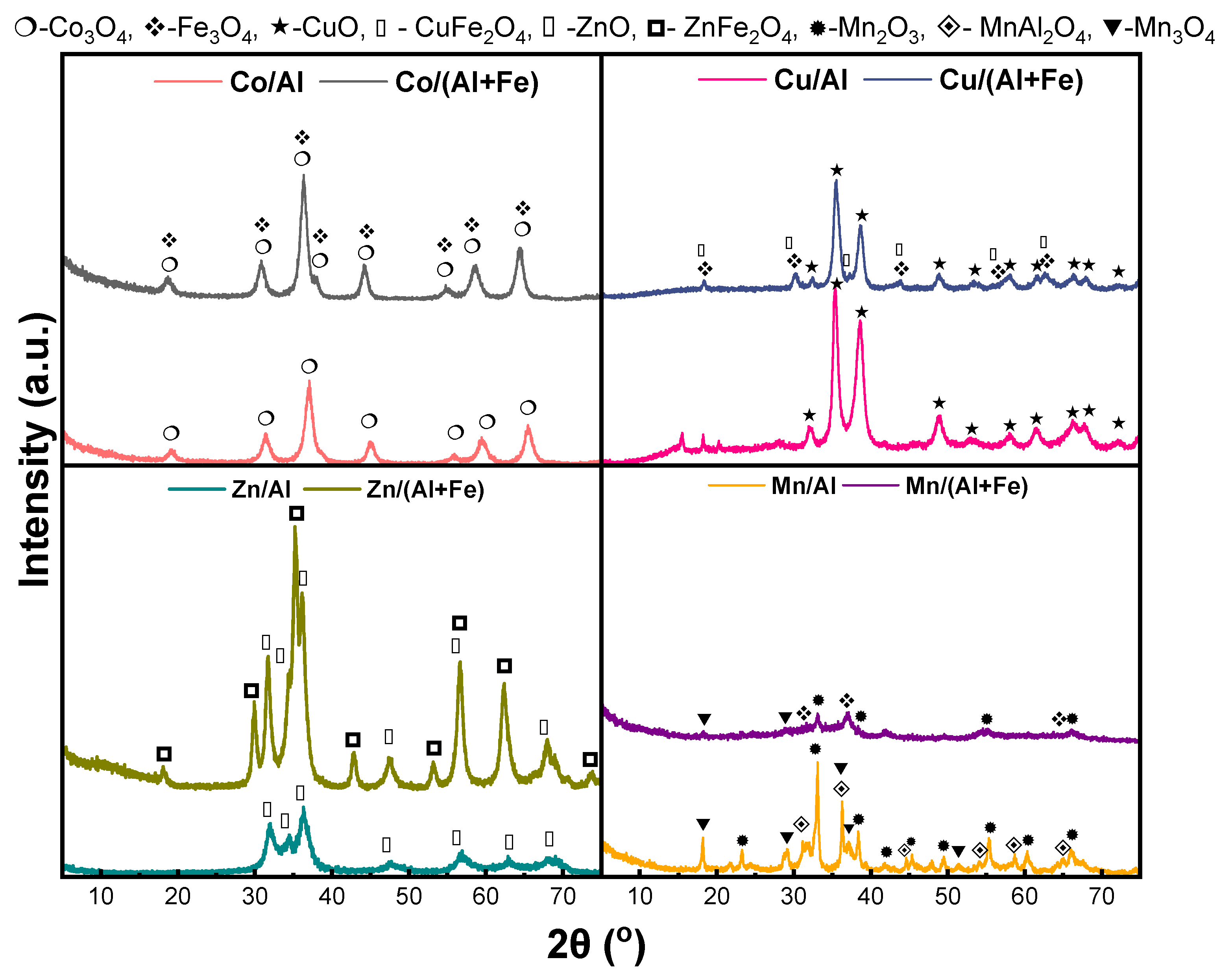

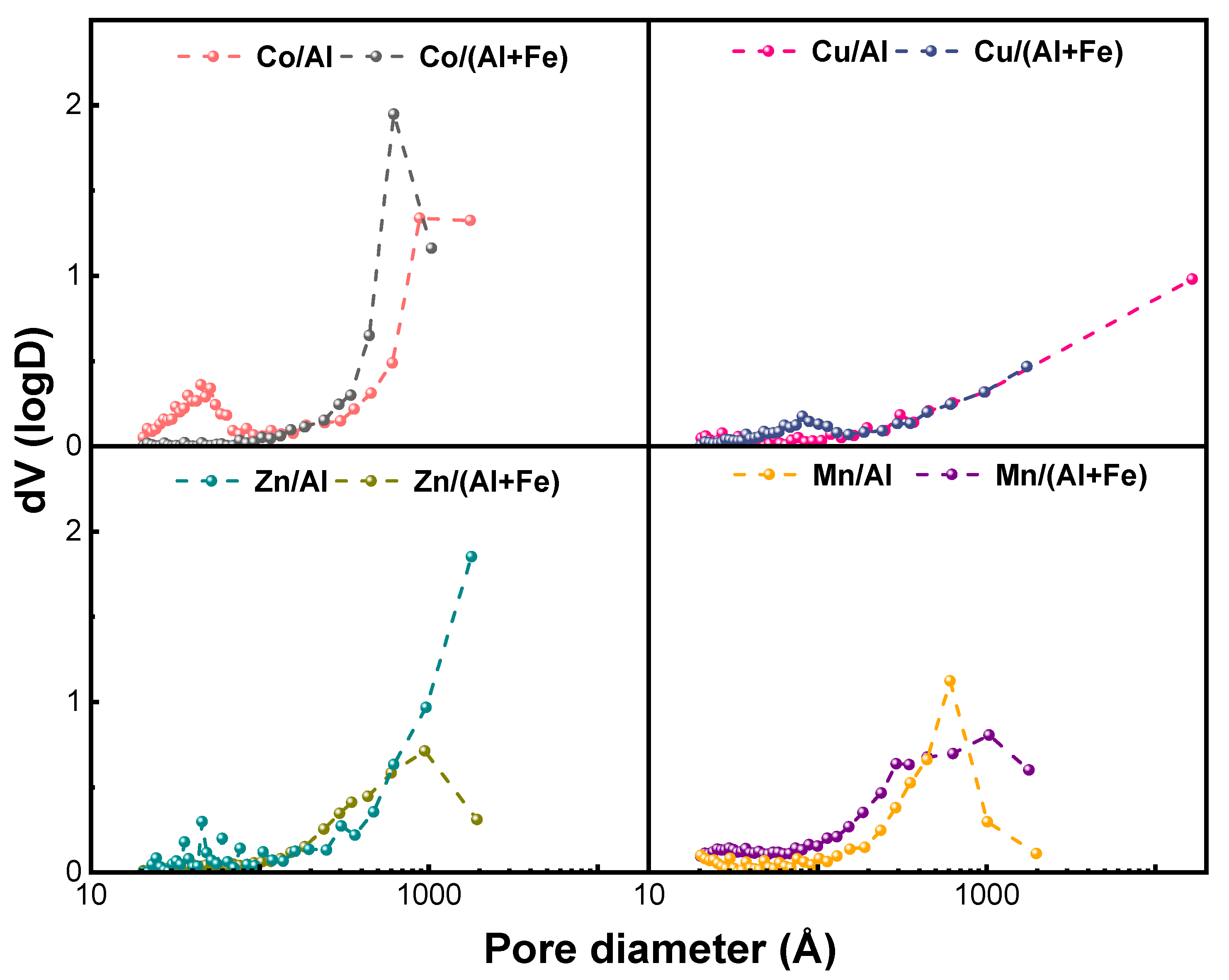

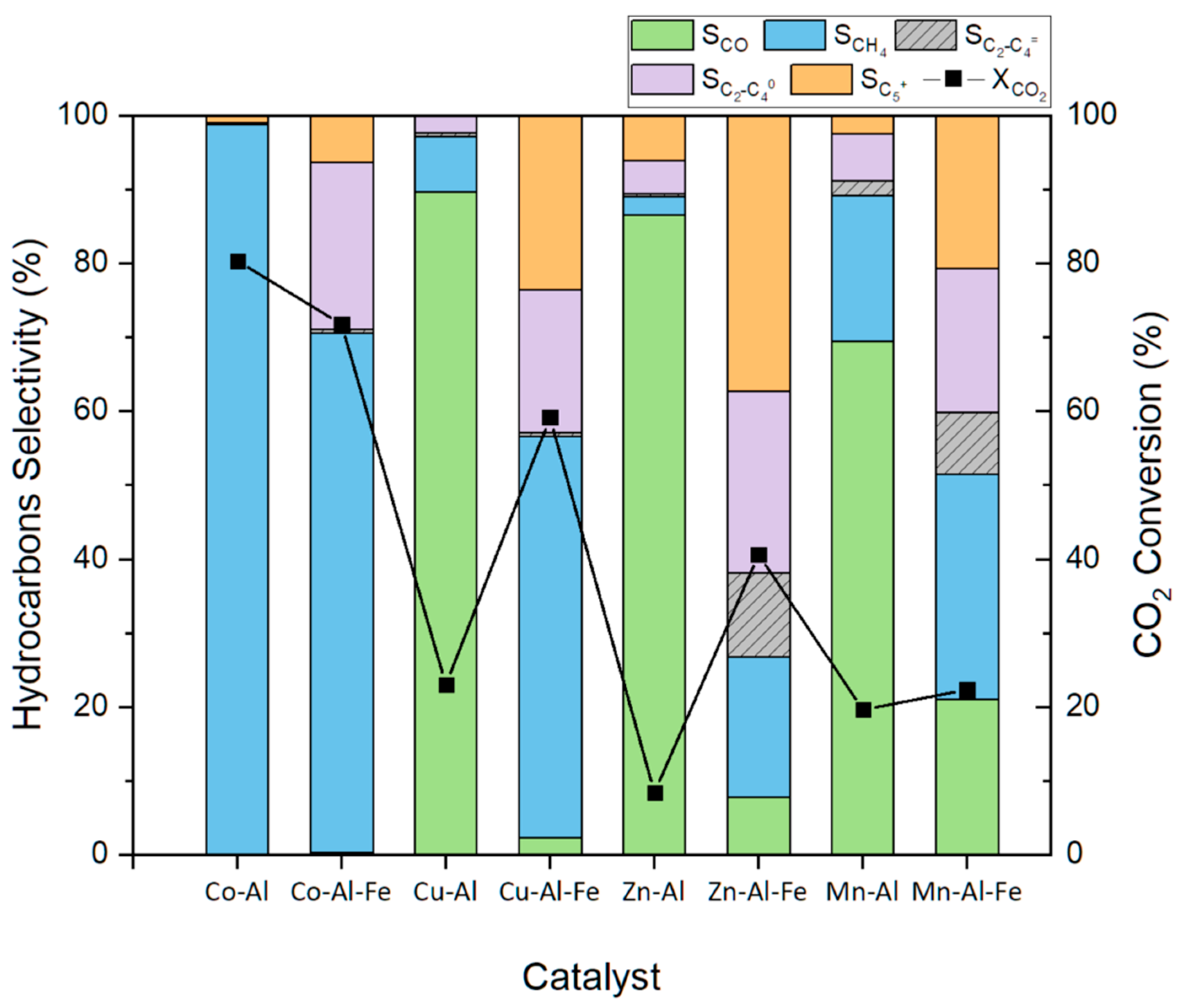
| Materials | Theoretical Molar Ratios | Experimental Molar Ratios | Co (wt.%) | Cu (wt.%) | Zn (wt.%) | Mn (wt.%) | Al (wt.%) | Fe (wt.%) |
|---|---|---|---|---|---|---|---|---|
| Co-Al | Co/Al = 2 | Co/Al = 2.4 | 61.1 | - | - | - | 11.6 | - |
| Co-Al-Fe | Co/(Al+Fe) = 2, Fe/Al = 4 | Co/(Al+Fe) = 2.3, Fe/Al = 4.0 | 44.7 | - | - | - | 1.8 | 14.9 |
| Cu-Al | Cu/Al = 2 | Cu/Al = 2.3 | - | 39.9 | - | - | 7.3 | - |
| Cu-Al-Fe | Cu/(Al+Fe) = 2, Fe/Al = 4 | Cu/(Al+Fe) = 2.3, Fe/Al = 4.1 | - | 50.5 | - | - | 1.8 | 15.3 |
| Zn-Al | Zn/Al = 2 | Zn/Al = 2.6 | - | - | 69.5 | - | 11.1 | - |
| Zn-Al-Fe | Zn/(Al+Fe) = 2.2, Fe/Al = 4 | Zn/(Al+Fe) = 2.2, Fe/Al = 4.3 | - | - | 48.0 | - | 1.7 | 15.1 |
| Mn-Al | Mn/Al = 2 | Mn/Al = 3.1 | - | - | - | 53.7 | 8.5 | - |
| Mn-Al-Fe | Mn/(Al+Fe) = 2, Fe/Al = 4 | Mn/(Al+Fe) = 2.8, Fe/Al = 4.6 | - | - | - | 50.1 | 1.6 | 15.1 |
| Materials | Theoretical Molar Ratio | Dcryst a (nm) | SBET (m2/g) b | Vtotal (cm3/g) c | dparticle (nm) d |
|---|---|---|---|---|---|
| Co-Al | Co/Al = 2 | 6.7 | 139 | 1.014 | 200 |
| Co-Al-Fe | Co/(Al+Fe) = 2, Fe/Al = 4 | 7.5 | 65 | 0.888 | 260 |
| Cu-Al | Cu/Al = 2 | 9.8 | 55 | 1.608 | 480 |
| Cu-Al-Fe | Cu/(Al+Fe) = 2, Fe/Al = 4 | 9.2 | 53 | 0.367 | 330 |
| Zn-Al | Zn/Al = 2 | 4.9 | 95 | 1.102 | 390 |
| Zn-Al-Fe | Zn/(Al+Fe) = 2.2, Fe/Al = 4 | 12.7 | 61 | 0.556 | 430 |
| Mn-Al | Mn/Al = 2 | 22.3 | 134 | 0.557 | 710 |
| Mn-Al-Fe | Mn/(Al+Fe) = 2, Fe/Al = 4 | - | 157 | 0.863 | 330 |
| Materials | XCO2 (%) | Product Selectivity (%) | |||
|---|---|---|---|---|---|
| CO | CH4 | C2−C4 | C5+ | ||
| Co-Al | 80.3 | 0.0 | 98.7 | 0.2 | 1.0 |
| Co-Al-Fe | 71.7 | 0.3 | 70.3 | 23.0 | 6.4 |
| Cu-Al | 23.0 | 89.7 | 7.5 | 2.8 | 0.0 |
| Cu-Al-Fe | 59.2 | 2.3 | 54.3 | 19.8 | 23.5 |
| Zn-Al | 8.4 | 86.5 | 2.6 | 4.8 | 6.1 |
| Zn-Al-Fe | 40.6 | 7.8 | 18.9 | 35.9 | 37.3 |
| Mn-Al | 19.6 | 69.4 | 19.8 | 8.4 | 2.5 |
| Mn-Al-Fe | 22.3 | 21.1 | 30.4 | 27.8 | 20.7 |
| Materials | Dcryst (nm) a | C (wt.%) b | Weight Gain (wt.%) c | DTGmax (°C) c | Weight Loss (wt.%) c | DTGmax (°C) c |
|---|---|---|---|---|---|---|
| Co-Al | 7.6 | 0.0 | 3.3 | 264 | - | - |
| Co-Al-Fe | 10.3 | 32.8 | 1.0 | 250 | 27.0 | 342–383 |
| Cu-Al | 17.1 | 0.2 | 9.8 | 314 | - | - |
| Cu-Al-Fe | 24.4 | 8.3 | 2.7 | 254 | 12.0 | 367 |
| Zn-Al | 7.6 | 1.2 | - | - | 4.7 | 136 |
| Zn-Al-Fe | 17.0 | 1.7 | - | - | - | - |
| Mn-Al | 30.2 | 4.4 | - | - | 14.0 | 525 |
| Mn-Al-Fe | 30.0 | 8.4 | - | - | 23.8 | 477 |
| Catalyst | Reaction Conditions T (°C), P (bar), WHSV (mL·gcat−1.h−1) | XCO2 (%) | SCH4 (%) | SCO (%) | SC2–C4 (%) | SC2+ (%) | Ref. |
|---|---|---|---|---|---|---|---|
| ZnFe2O4 a | 320, 25, 4800 | 42.1 | 10.1 | 10.7 | 28.8 | 79.0 | [10] |
| Fe6Zn1Al1 | 330, 15, 15,000 | 39.1 | 12.4 | 22.5 | 21.1 c | 62.6 | [66] |
| 10Fe3Zn1K/Al2O3 | 400, 30, 3600 | 38.6 | 35.8 | 33.8 | 24.1 | 30.9 | [17] |
| FeZn | 300, 10, 5000 | 40.6 | 27.3 | 10.7 | - | 46.9 | [67] |
| Fe-Zn-Al | 320, 10, 13,000 | 31.6 | 11.9 | 20.7 | 36.2 | 68.08 | [68] |
| ZnCo0.5Fe1.5O4 a | 320, 25, 4800 | 49.6 | 17.8 | 5.8 | 39.8 | 76.2 | [10] |
| FeCo-1:2-LDH a | 320, 30, 7200 | 48.2 | 43.9 | 4.2 | 36.7 | 51.9 | [62] |
| Co-Fe a | 240, 30, 5500 | 19.6 | 68.1 | 2.9 | 2.2 | 28.8 | [63] |
| ZnCo0.5Fe1.5O4 a | 320, 25, 4800 | 49.6 | 17.8 | 5.8 | 39.8 | 76.2 | [10] |
| Fe-Co/Al2O3 | 300, 11, 3600 | 25.0 | 44.0 | 13.0 | - | 43.0 | [69] |
| Co4Fe1 | 320, 20, 8000 | 43.7 | 34.1 | 7.6 | 31.7 | 53.5 | [9] |
| Na-AlFeCu a | 320, 50, 35 b | 44.5 | 13.6 | 9.9 | 23.8 | 76.3 | [65] |
| Fe–Cu(0.75)/Al2O3 | 300, 11, 3600 | 22.8 | 18.0 | 45.0 | - | 37.0 | [8] |
| 10Fe3Cu1K/Al2O3 | 400, 30, 3600 | 41.7 | 27.8 | 26.5 | 31.9 | 45.7 | [17] |
| 0.3-CuFe/ZrO2 | 320, 20, 4800 | 35.4 | 12.3 | 11.9 | 38.0 | 77.1 | [64] |
| 10Fe3Mn1K/Al2O3 | 400, 30, 3600 | 42.0 | 36.1 | 23.0 | 29.8 | 40.9 | [17] |
| 3MnNaFe | 290, 15, 20,000 | 30.1 | 30.9 | 24.2 | 33.3 | 43.9 | [70] |
| 10Mn-Na/Fe | 320, 30, 2040 | 37.7 | 14.0 | 12.9 | 34.1 | 73.8 | [71] |
| Fe3O4 | 320, 30, 2000 | 29.3 | 50.2 | 16.6 | 30.4 | 33.02 | [72] |
| Fe-Al-O Nanobelts | 300, 10, 1 d | 48.0 | 10.0 | 16.0 | 57.0 e | 69.0 | [73] |
| FeAl350 | 330, 15, 9000 | 48.2 | 17.3 | 10.1 | 38.1 | 72.6 | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandela, E.; Margellou, A.G.; Kotsaridou, A.; Marnellos, G.E.; Konsolakis, M.; Triantafyllidis, K.S. Layered Double Hydroxide (LDH)-Derived Mixed Oxides for Enhanced Light Hydrocarbon Production from CO2 Hydrogenation. Catalysts 2025, 15, 323. https://doi.org/10.3390/catal15040323
Mandela E, Margellou AG, Kotsaridou A, Marnellos GE, Konsolakis M, Triantafyllidis KS. Layered Double Hydroxide (LDH)-Derived Mixed Oxides for Enhanced Light Hydrocarbon Production from CO2 Hydrogenation. Catalysts. 2025; 15(4):323. https://doi.org/10.3390/catal15040323
Chicago/Turabian StyleMandela, Evridiki, Antigoni G. Margellou, Athanasia Kotsaridou, George E. Marnellos, Michalis Konsolakis, and Konstantinos S. Triantafyllidis. 2025. "Layered Double Hydroxide (LDH)-Derived Mixed Oxides for Enhanced Light Hydrocarbon Production from CO2 Hydrogenation" Catalysts 15, no. 4: 323. https://doi.org/10.3390/catal15040323
APA StyleMandela, E., Margellou, A. G., Kotsaridou, A., Marnellos, G. E., Konsolakis, M., & Triantafyllidis, K. S. (2025). Layered Double Hydroxide (LDH)-Derived Mixed Oxides for Enhanced Light Hydrocarbon Production from CO2 Hydrogenation. Catalysts, 15(4), 323. https://doi.org/10.3390/catal15040323










