Regulating the Structures of Carbon Cloth and Carbon Nanotubes to Boost the Positive Electrode Reaction of Vanadium Redox Flow Batteries
Abstract
1. Introduction
2. Results and Discussion
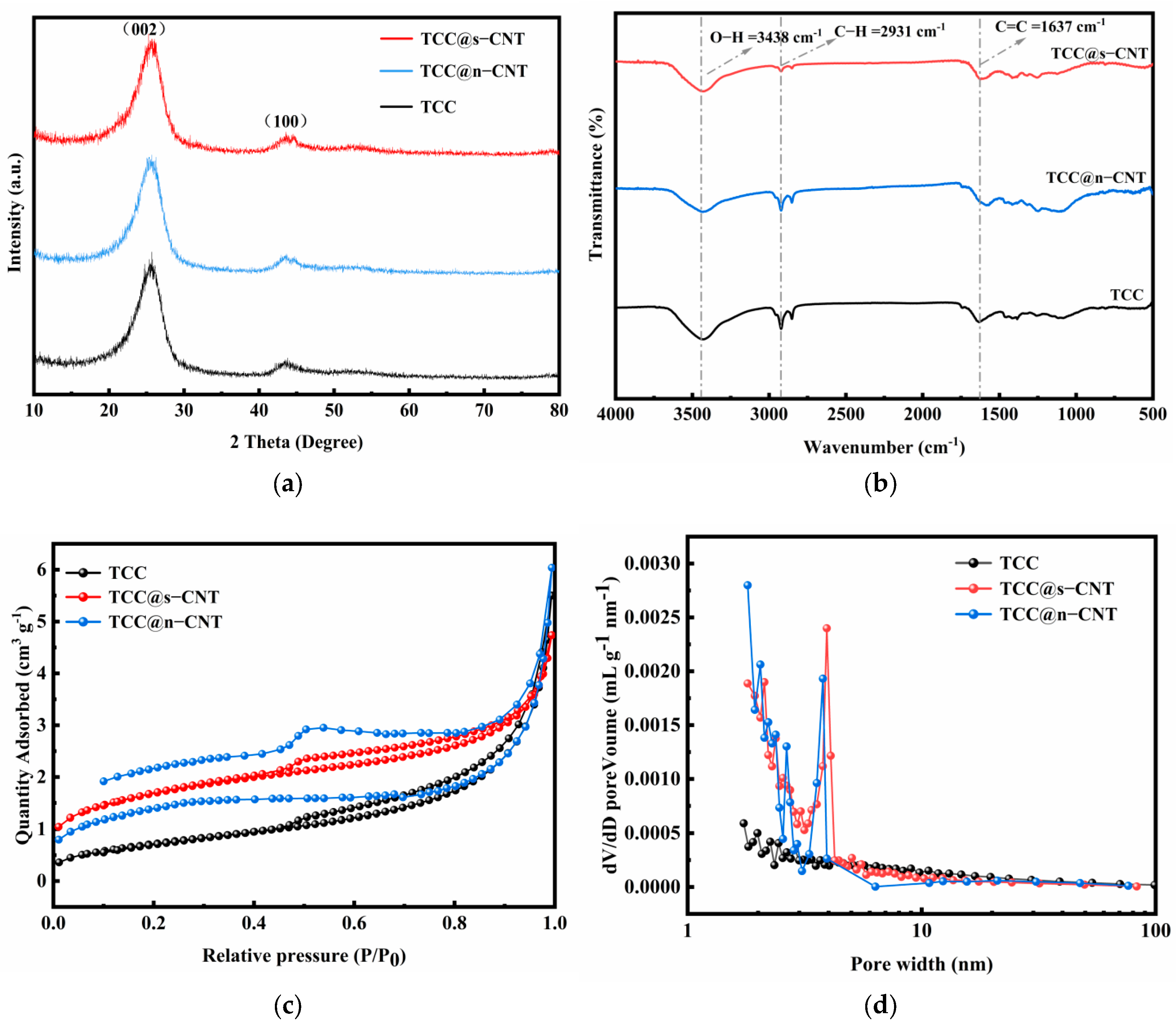
2.1. Morphology and Structure Characterizations
2.2. Electrochemical Properties
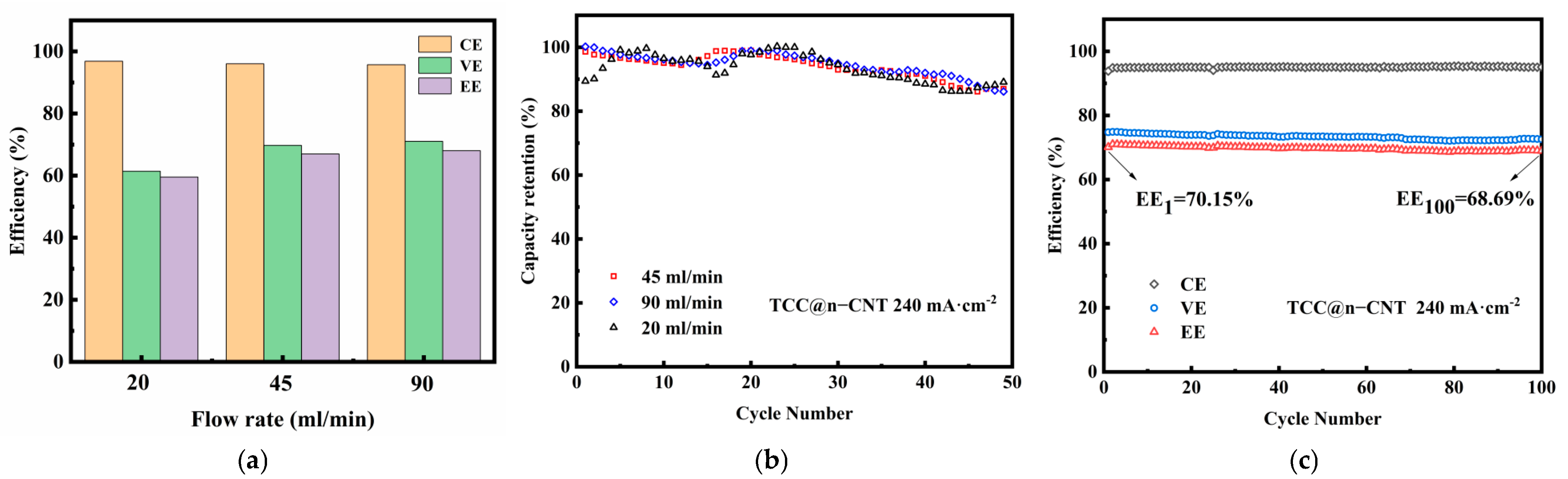
2.3. Single-Cell Cycling Test
3. Materials and Methods
3.1. Electrode Fabrication
3.2. Material Characterization
3.3. Electrochemical Measurement
3.4. Battery Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Sun, C.; Ge, M. Progress in profitable Fe-based flow batteries for broad-scale energy storage. Wiley Interdiscip. Rev. Energy Environ. 2024, 13, e541. [Google Scholar]
- Zhao, Z.; Liu, X.; Zhang, M.; Zhang, L.; Zhang, C.; Li, X.; Yu, G. Development of flow battery technologies using the principles of sustainable chemistry. Chem. Soc. Rev. 2023, 52, 6031–6074. [Google Scholar] [PubMed]
- Chen, S.; Sun, C.; Zhang, H.; Yu, H.; Wang, W.-T. Electrochemical deposition of Bismuth on graphite felt electrodes: Influence on negative half-cell reactions in vanadium redox flow batteries. Appl. Sci. 2024, 14, 3316. [Google Scholar] [CrossRef]
- He, Z.; Lv, Y.; Zhang, T.; Zhu, Y.; Dai, L.; Yao, S.; Zhu, W.; Wang, L. Electrode materials for vanadium redox flow batteries: Intrinsic treatment and introducing catalyst. Chem. Eng. J. 2022, 427, 131680. [Google Scholar]
- Bayeh, A.W.; Kabtamu, D.M.; Chang, Y.-C.; Wondimu, T.H.; Huang, H.-C.; Wang, C.-H. Carbon and metal-based catalysts for vanadium redox flow batteries: A perspective and review of recent progress. Sustain. Energy Fuels 2021, 5, 1668–1707. [Google Scholar]
- Hu, H.; Han, M.; Liu, J.; Zheng, K.; Mu, Y.; Zou, Z.; Yu, F.; Li, W.; Zhao, T. Development status, challenges, and perspectives of key components and systems of all-vanadium redox flow batteries. Future Batter. 2024, 4, 100008. [Google Scholar]
- Kim, J.; Park, H. Recent advances in porous electrodes for vanadium redox flow batteries in grid-scale energy storage systems: A mass transfer perspective. J. Power Sources 2022, 545, 231904. [Google Scholar]
- Zhang, H.; Chen, N.; Sun, C.; Luo, X. Investigations on physicochemical properties and electrochemical performance of graphite felt and carbon felt for iron-chromium redox flow battery. Int. J. Energy Res. 2020, 44, 3839–3853. [Google Scholar]
- Park, M.; Jung, A.-J.; Kim, J.; Lee, H.; Cho, J. Synergistic effect of carbon nanofiber/nanotube composite catalyst on carbon felt electrode for high-performance all-vanadium redox flow battery. Nano Lett. 2013, 13, 4833–4839. [Google Scholar]
- Zhang, X.; Ye, X.; Huang, S.; Zhou, X. Promoting pore-level mass transport/reaction in flow batteries: Bi nanodot/vertically standing carbon nanosheet composites on carbon fibers. ACS Appl. Mater. Interfaces 2021, 13, 37111–37112. [Google Scholar]
- Niu, S.; Li, H.; Guo, H.; Liu, Y.; Cheng, Y. Accelerating the reduction kinetics of V4+ to V3+ on atomically Fe-N4 decorated carbon nanotubes for vanadium electrolyte preparation. Small 2024, 20, 2405827. [Google Scholar]
- AlNahyan, M.; Mustafa, I.; Alghaferi, A.; Almarzooqi, F. Porous 3D graphene/multi-walled carbon nanotubes electrodes with improved mass transport and kinetics towards VO2+/VO2+ redox couple. Electrochim. Acta 2021, 385, 138449. [Google Scholar]
- Halada, Š.; Láznička, V.; Němec, T.; Mazúr, P.; Charvát, J.; Slouka, Z. Methodology for fast testing of carbon-based nanostructured 3D electrodes in vanadium redox flow battery. Electrochim. Acta 2024, 498, 144681. [Google Scholar]
- Jelyani, M.Z.; Rashid-Nadimi, S.; Asghari, S. Treated carbon felt as electrode material in vanadium redox flow batteries: A study of the use of carbon nanotubes as electrocatalyst. J. Solid State Chem. 2017, 21, 69–79. [Google Scholar]
- Etienne, M.; Vivo-Vilches, J.F.; Vakulko, I.; Genois, C.; Liu, L.; Perdicakis, M.; Hempelmann, R.; Walcarius, A. Layer-by-Layer modification of graphite felt with MWCNT for vanadium redox flow battery. Electrochim. Acta 2019, 313, 131–140. [Google Scholar]
- Lv, Z.; Zhang, J.; Lv, Y.; Cheng, Y.; Jiang, S.P.; Xiang, Y.; Lu, S. The electrocatalytic characterization andmechanism of carbon nanotubes with differentnumbers of walls for the VO2+/VO2+ redoxcouple. Phys. Chem. Chem. Phys. 2018, 20, 7791–7797. [Google Scholar] [CrossRef]
- An, H.; Jeon, S.; Park, J.; Chung, Y. Eco-conscious one-step treatment for oxygen-containing group functionalization on CNTs using TAEA and its use in vanadium redox flow batteries. J. Energy Storage 2024, 103, 114208. [Google Scholar]
- Devi, N.; Singh, P.; Chen, Y.-S. Binder-free CNT-modified excellent electrodes for all-vanadium redox flow batteries. Nanomaterials 2024, 14, 767. [Google Scholar] [CrossRef]
- Yang, H.; Fan, C.; Zhu, Q. Sucrose pyrolysis assembling carbon nanotubes on graphite felt using for vanadium redox flow battery positive electrode. J. Energy Chem. 2018, 27, 451–454. [Google Scholar]
- Kim, M.; Park, J.; Yeo, G.; Ko, M.; Jang, H. Designing CNT-implanted graphite felt as a sustainable electron network for long-cycling of vanadium redox flow batteries. Carbon 2023, 206, 1–6. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Liu, H.; Wang, T.; Liu, C.; Chu, P.; Leung, P. Carbon nanofibers embedded in nanopores decorated graphite felt electrodes for enhanced vanadium redox flow batteries performance. J. Electroanal. Chem. 2024, 971, 118524. [Google Scholar]
- Yang, D.-S.; Lee, J.Y.; Jo, S.-W.; Yoon, S.; Kim, T.-H.; Hong, Y. Electrocatalytic activity of nitrogen-doped CNT graphite felt hybrid for all-vanadium redox flow batteries. Int. J. Hydrogen Energy 2018, 43, 1516–1522. [Google Scholar]
- Zhang, K.; Wang, H.; Zhang, X.; Liu, L.; Feng, B.; Wang, Y.; Liu, J. Controlled construction of a N-doped carbon nanotube network endows carbon felt with superior performances for high-rate vanadium flow batteries. ACS Sustain. Chem. Eng. 2024, 12, 7318–7328. [Google Scholar]
- Jang, J.; Shin, M.; Kwon, Y.; Jo, C. Carbon cloth modified by direct growth of nitrogen-doped carbon nanofibers and its utilization as electrode for zero gap flow batteries. Chem. Eng. J. 2024, 481, 148644. [Google Scholar]
- Wei, L.; Liu, T.; Zhang, Y.; Liu, H.; Ge, L. Taurine-functionalized carbon nanotubes as electrode catalysts for improvement in the performance of vanadium redox flow battery. Catalysts 2024, 14, 281. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Zhang, D.; Zhang, K.; Hou, S.; Wang, S.; Zhang, Y.; Peng, H.; Liu, J.; Yan, C. Regulating the N/B ratio to construct B, N co-doped carbon nanotubes on carbon felt for high-performance vanadium redox flow batteries. Chem. Eng. J. 2023, 473, 145454. [Google Scholar]
- Jiang, Q.; Ren, Y.; Yang, Y.; Liu, H.; Wang, L.; Li, J.; Dai, L.; He, Z. High-activity and stability graphite felt supported by Fe, N, S co-doped carbon nanofibers derived from bimetal-organic framework for vanadium redox flow battery. Chem. Eng. J. 2023, 460, 141751. [Google Scholar]
- An, H.; Noh, C.; Jeon, S.; Kwon, Y.; Chung, Y. Green functionalization for chelating ligand-like and π-conjugated active sites on carbon nanotubes and its use for vanadium redox flow batteries. J. Energy Storage 2023, 68, 107796. [Google Scholar]
- Mazurenko, I.; Etienne, M.; Francius, G.; Vakulko, I.; Walcarius, A. Macroporous carbon nanotube-carbon composite electrodes. Carbon 2016, 109, 106–116. [Google Scholar]
- Sun, J.; Jiang, H.R.; Wu, M.C.; Fan, X.Z.; Chao, C.Y.H.; Zhao, T.S. Aligned hierarchical electrodes for high-performance aqueous redox flow battery. Appl. Energy 2020, 271, 115235. [Google Scholar]
- Xu, Z.; Jing, M.; Liu, J.; Yan, C.; Fan, X. Advanced dual-gradient carbon nanofibers/graphite felt composite electrode for the next-generation vanadium flow battery. J. Mater. Sci. Technol. 2023, 136, 32–42. [Google Scholar] [CrossRef]
- Wei, Z.; Qu, G.; Huang, Z.; Wang, Y.; Li, D.; Yang, X.; Zhang, S.; Chen, A.; Wang, Y.; Hong, H.; et al. Gradient distribution of zincophilic sites for stable aqueous zinc-based flow batteries with high capacity. Adv. Mater. 2024, 36, 2414388. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Fu, W.; Shi, H.; Chen, Z.; Su, H.; Xu, Q. An electrospinning carbon nanofiber composite electrode with gradient porous structure for deep eutectic solvent electrolyte-based iron-vanadium redox flow battery. J. Energy Storage 2023, 74 Pt A, 109392. [Google Scholar] [CrossRef]
- Li, J.; Lu, M.; Yang, W.; Zhang, R.; Su, J.; Xu, Q. Development of high-performance and ultra-stability hierarchical nested-network-pore carbon electrode for vanadium redox flow batteries. J. Energy Storage 2024, 97, 112987. [Google Scholar] [CrossRef]
- Song, F.; Wang, Z.; Sun, G.; Ma, T.; Wu, D.; Chen, L.; Li, H.; Wu, F. In-situ CNT-loaded organic cathodes for sulfide all-solid-state Li metal batteries. eTransportation 2023, 18, 100261. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, K.; Ren, Z.; Li, L.; Chang, L.; Zhang, C.; Wang, R.; Li, B.; Wu, G.; Ying, H. High-performance MXene/carbon nanotube electrochemical actuators for biomimetic soft robotic applications. Adv. Funct. Mater. 2024, 34, 2408496. [Google Scholar] [CrossRef]
- Park, S.H.; Ha, J.; Kim, D.W.; Hwang, C.; Choi, J.-I.; Park, H.S.; Kim, Y. Mesoporous graphite felt electrode prepared via thermal oxidative etching on all-vanadium redox flow batteries. Chem. Eng. J. 2024, 500, 157328. [Google Scholar] [CrossRef]
- Yang, D.; Tian, D.; Xue, C.; Gao, F.; Liu, Y.; Li, H.; Bao, O.; Liang, J.; Zhao, Z.; Qiu, J. Tuned fabrication of the aligned and opened CNT membrane with exceptionally high permeability and selectivity for bioalcohol recovery. Nano Lett. 2018, 18, 6150–6156. [Google Scholar] [CrossRef]
- Rao, R.; Sharma, R.; Abild-Pedersen, F.; Nørskov, J.K.; Harutyunyan, A.R. Insights into carbon nanotube nucleation: Cap formation governed by catalyst interfacial step flow. Sci. Rep. 2014, 4, 6510. [Google Scholar] [CrossRef]
- He, K.; Chen, N.; Wang, C.; Wei, L.; Chen, J. Method for determining crystal grain size by X-ray diffraction. Cryst. Res. Technol. 2018, 53, 1700157. [Google Scholar] [CrossRef]
- Wang, W.; Shang, L.; Chang, G.; Yan, C.; Shi, R.; Zhao, Y.; Waterhouse, G.I.N.; Yang, D.; Zhang, T. Intrinsic carbon-defect-driven electrocatalytic reduction of carbon dioxide. Adv. Mater. 2018, 31, 1808276. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Han, Z.; Jia, G.; Zhu, S.; Xu, Y.; Zhang, X.; Yao, Y.; Li, Y. Covalently bonded graphene sheets on carbon nanotubes: Direct growth and outstanding properties. Adv. Funct. Mater. 2023, 33, 2306785. [Google Scholar] [CrossRef]
- Luob, H.; Lub, H.; Qiu, J. Carbon fibers surface-grown with helical carbon nanotubes and polyaniline for high-performance electrode materials and flexible supercapacitors. J. Electroanal. Chem. 2018, 828, 24–32. [Google Scholar]
- Wu, R.; Wang, X.; Ge, L.; Zheng, Z.; Zhu, Y.; Zhou, C.; Yuan, J.; Zhu, S.; Gu, Y.; Zhou, W.; et al. N, S co-doped carbon with embedment of FeNi alloy as bifunctional oxygen electrocatalysts for rechargeable Zinc-air batteries. Carbon 2023, 202, 141–149. [Google Scholar] [CrossRef]
- Sun, C.; Zlotorowicz, A.; Nawn, G.; Negro, E.; Bertasi, F.; Pagot, G.; Vezzù, K.; Pace, G.; Guarnieri, M.; Vito, D. [Nafion/(WO3)x] hybrid membranes for vanadium redox flow batteries. Solid State Ion. 2018, 319, 110–116. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Vito, D. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Zhai, L.; Zhu, Y.-L.; Wang, G.; He, H.; Wang, F.; Jiang, F.; Chai, S.; Li, X.; Guo, H.; Wu, L.; et al. Ionic-nanophase hybridization of Nafion by supramolecular patching for enhanced proton selectivity in redox flow batteries. Nano Lett. 2023, 23, 3887–3896. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery. Int. J. Energy Res. 2019, 43, 8739–8752. [Google Scholar] [CrossRef]
- Cai, X.; Xie, J.; Guo, X.; Zheng, X.; Liu, Y.; Cao, F.; Dong, X.; Kong, Q.; Zhang, J. Suppressing polysulfide shuttle in lithium-sulfur batteries using CNTs/C3N4/S cathodes. Mater. Today Commun. 2023, 35, 106138. [Google Scholar] [CrossRef]

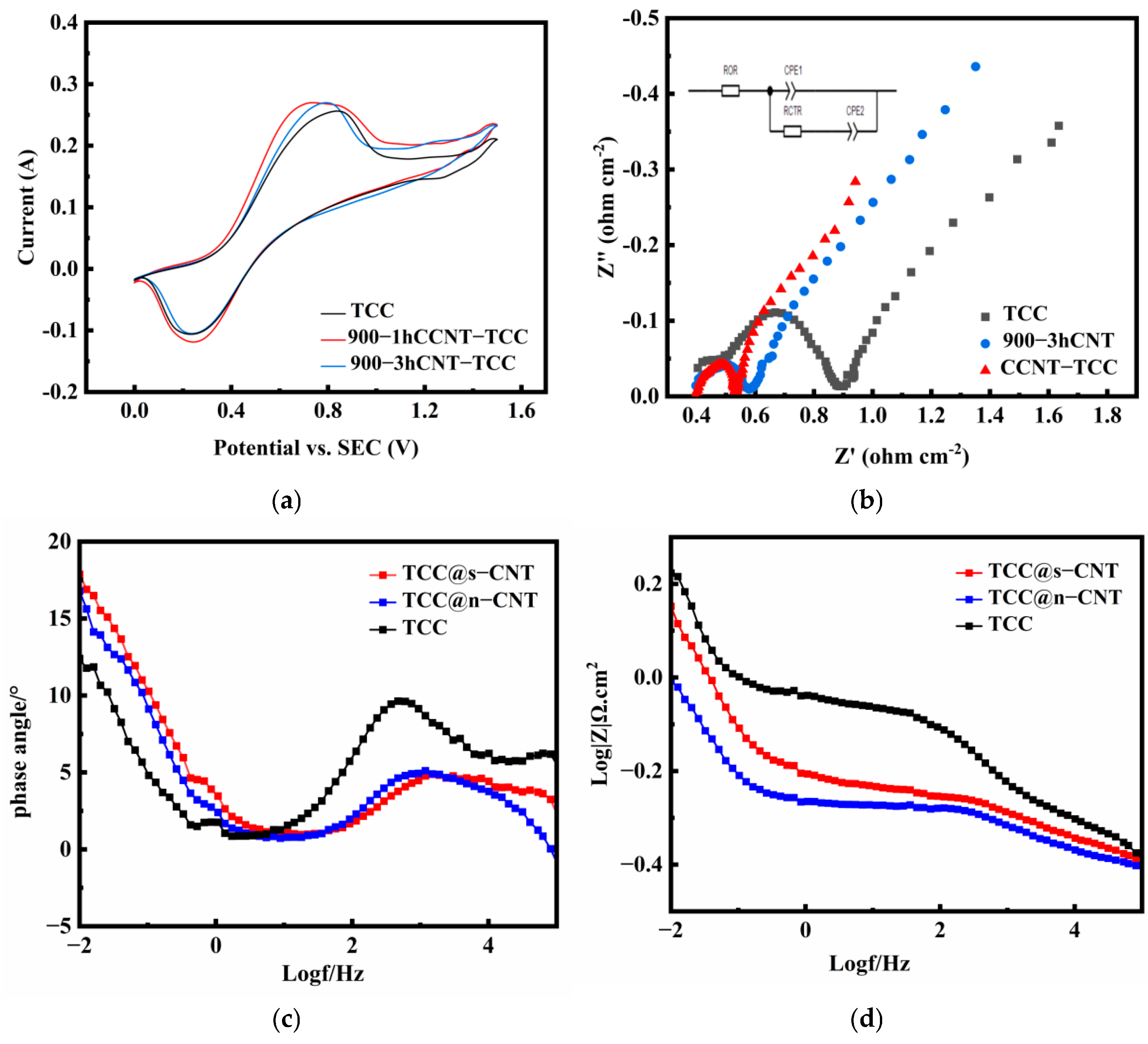

| Samples | ΔE (V) | Ipa (A) | Ipc(A) | Ipa/Ipc | RS (Ω·cm2) | RCTR (Ω·cm2) |
|---|---|---|---|---|---|---|
| TCC | 0.619 | 0.2562 | 0.1094 | 2.341 | 0.409 | 0.479 |
| TCC@s-CNT | 0.471 | 0.2699 | 0.1187 | 2.273 | 0.390 | 0.125 |
| TCC@n-CNT | 0.554 | 0.2698 | 0.1060 | 2.545 | 0.390 | 0.170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Sun, C.; Liu, S.; Zhao, B.; Ge, M.; Zhang, H. Regulating the Structures of Carbon Cloth and Carbon Nanotubes to Boost the Positive Electrode Reaction of Vanadium Redox Flow Batteries. Catalysts 2025, 15, 345. https://doi.org/10.3390/catal15040345
Huang X, Sun C, Liu S, Zhao B, Ge M, Zhang H. Regulating the Structures of Carbon Cloth and Carbon Nanotubes to Boost the Positive Electrode Reaction of Vanadium Redox Flow Batteries. Catalysts. 2025; 15(4):345. https://doi.org/10.3390/catal15040345
Chicago/Turabian StyleHuang, Xinyu, Chuanyu Sun, Shuqi Liu, Bangsen Zhao, Mingming Ge, and Huan Zhang. 2025. "Regulating the Structures of Carbon Cloth and Carbon Nanotubes to Boost the Positive Electrode Reaction of Vanadium Redox Flow Batteries" Catalysts 15, no. 4: 345. https://doi.org/10.3390/catal15040345
APA StyleHuang, X., Sun, C., Liu, S., Zhao, B., Ge, M., & Zhang, H. (2025). Regulating the Structures of Carbon Cloth and Carbon Nanotubes to Boost the Positive Electrode Reaction of Vanadium Redox Flow Batteries. Catalysts, 15(4), 345. https://doi.org/10.3390/catal15040345








