Effect of Mn/Cu Molar Ratios on CO Oxidation Activity of Mn-Cu Bimetallic Catalysts
Abstract
:1. Introduction
2. Results and Discussion
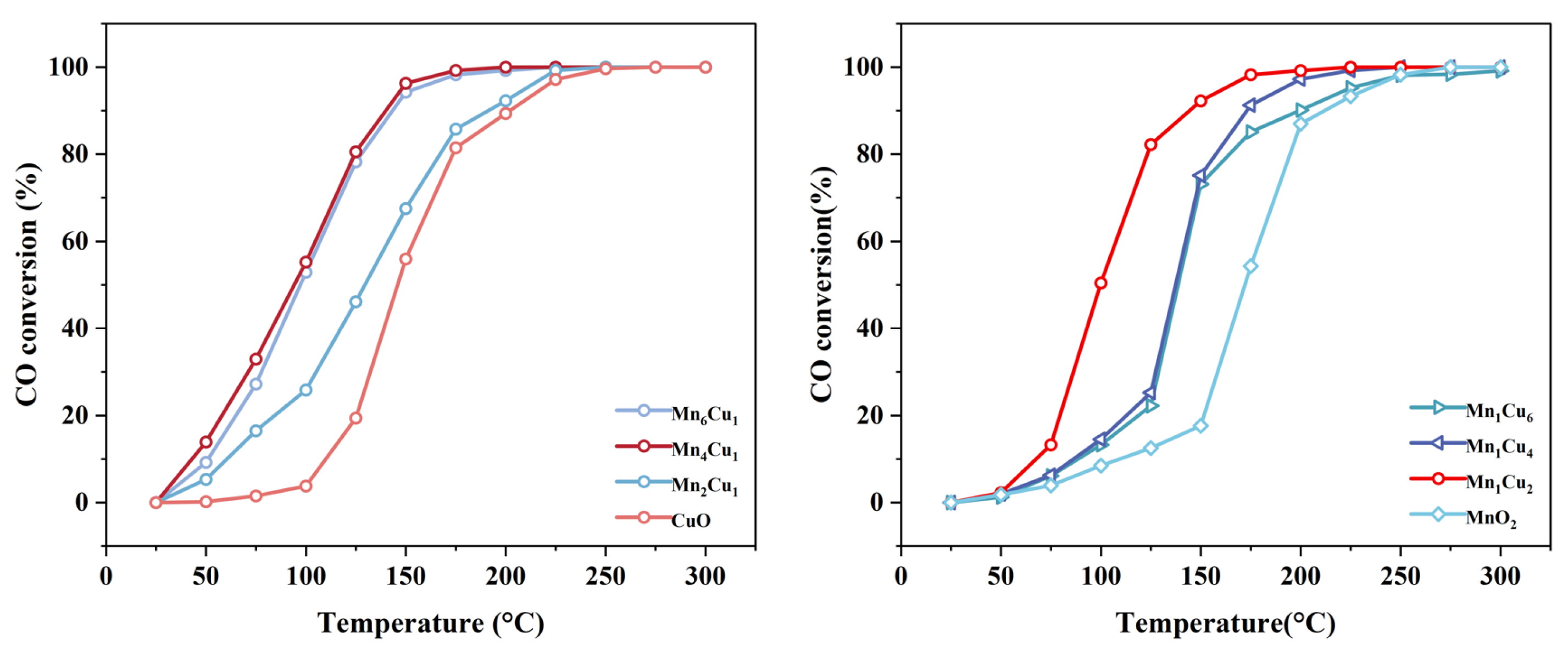
2.1. Effect of Mn/Cu Molar Ratio on Catalytic Activity
2.2. Effect of Mn/Cu Molar Ratio on Microstructure Characteristics
2.3. Effect of Mn/Cu Molar Ratio on Phase Structure
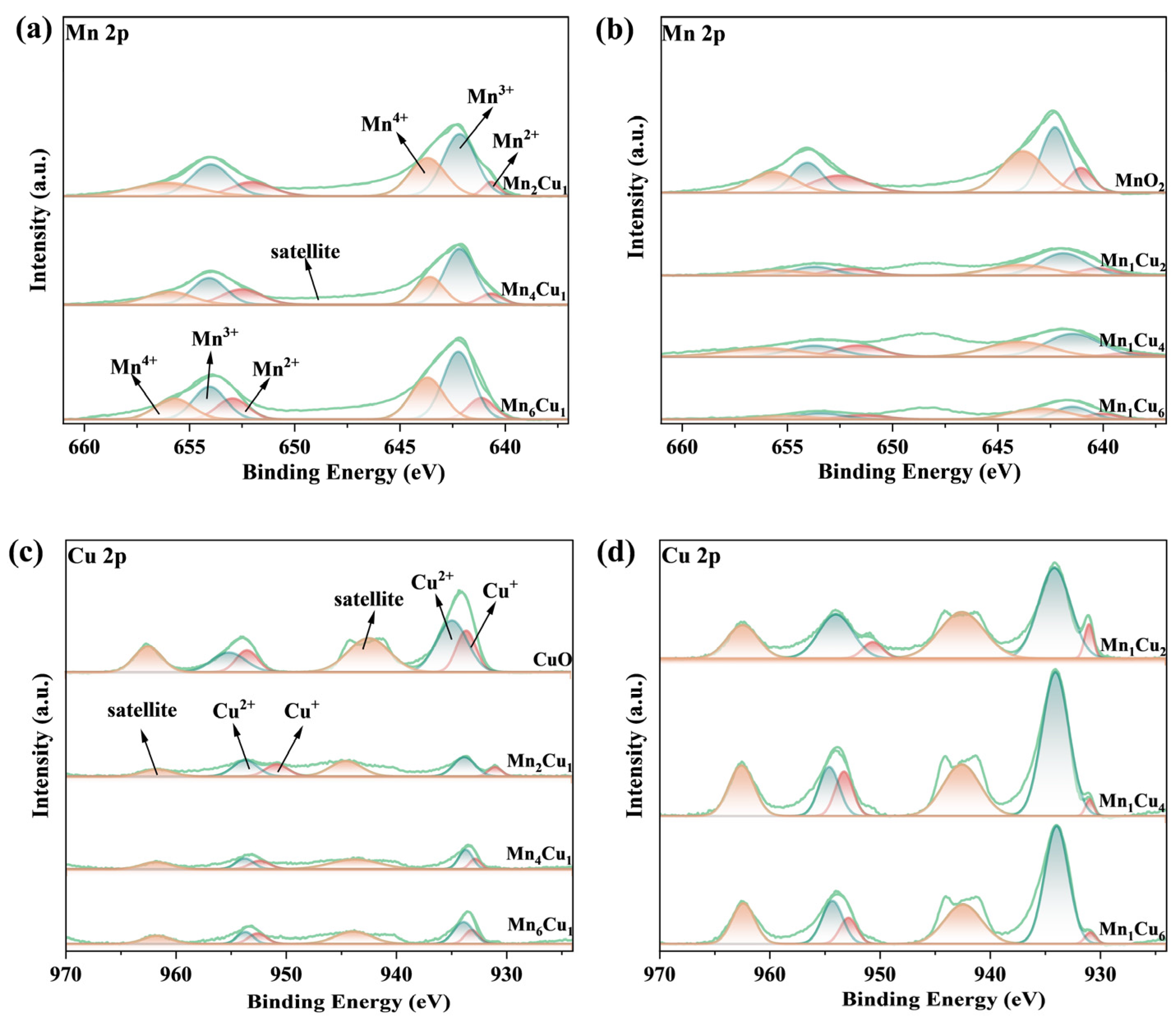
2.4. Effect of Mn/Cu Molar Ratio on Surface Chemical Composition and Valence State Distribution
2.5. Effect of Mn/Cu Molar Ratio on Reducibility
3. Catalyst Synthesis and Characterization
3.1. Catalyst Synthesis of CuO, MnO2 and MnxCuy
3.2. Catalyst Characterization
3.3. Evaluation of Catalyst Activity
4. Conclusions
- (1)
- The Mn4Cu1 catalyst exhibited optimal low-temperature catalytic activity, achieving complete CO conversion (100%) at 175 °C. For the MnxCu1 catalysts, catalytic activity showed a volcano-shaped trend, initially increasing and then decreasing with higher x values. Similarly, the Mn1Cuy catalysts displayed an analogous trend as y decreased.
- (2)
- All synthesized catalysts possessed a well-defined mesoporous structure, with pore diameters distributed between 2 and 50 nm. It should be noted that the Mn4Cu1 catalyst exhibited the highest specific surface area of 45.54 m2/g, significantly surpassing the other catalysts.
- (3)
- The MnxCu1 catalysts exhibited mixed crystalline phases of pyrolusite MnO2 and spinel Cu1.4Mn1.6O4, whereas the Mn1Cuy catalysts mainly consisted of monoclinic fluorite CuO.
- (4)
- The Mn4Cu1 catalyst has the highest Mn3+ content, reaching 53.89%, which promotes the generation of activated oxygen species and significantly enhances the catalyst’s redox performance.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duan, W.J.; Li, R.M.; Yang, S.; Han, J.C.; Lv, X.J.; Wang, Z.M.; Yu, Q.B. Theoretical study on coal gasification behavior in CO2 atmosphere driven by slag waste heat. Energy 2024, 305, 132269. [Google Scholar] [CrossRef]
- Duan, W.; Li, R.; Wang, Z.; Ji, J.; Liu, J.; Yu, Q. Optimizing sustainability and profitability: A multi-step approach to the synthesis of X-zeolite from blast furnace slag. Process Saf. Environ. Prot. 2024, 189, 1527–1537. [Google Scholar] [CrossRef]
- Wang, L.; Jia, Y.; Tan, Y.; Ding, B. Numerical Investigation of Heat Transfer Characteristics Between Thermochemical Heat Storage Materials and Compressed Natural Gas in a Moving Bed. Processes 2025, 13, 8. [Google Scholar] [CrossRef]
- Duan, W.; Han, J.; Yang, S.; Wang, Z.; Yu, Q.; Zhan, Y. Understanding CO2 adsorption in layered double oxides synthesized by slag through kinetic and modelling techniques. Energy 2024, 297, 131303. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, X.D.; Jia, M.J.; Ding, B.; Wu, J.J.; Qin, Y.L. Redefining limits: Impact of nozzle diameter on film flow and atomization beyond the synchronizing radius. Chem. Eng. J. 2025, 505, 159816. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, X.D.; Jia, M.J.; He, W.C.; Qin, Y.L.; Ding, B.; Wu, J.J. Evaluating rotating disk vs. cup atomizers: Atomization performance in film fragmentation mode. Chem. Eng. Sci. 2025, 306, 121251. [Google Scholar] [CrossRef]
- Hu, J.; Li, H.; Liu, J.; Du, S. Review of Intelligent Modeling for Sintering Process Under Variable Operating Conditions. Processes 2025, 13, 180. [Google Scholar] [CrossRef]
- Ishida, T.; Murayama, T.; Taketoshi, A.; Haruta, M. Importance of Size and Contact Structure of Gold Nanoparticles for the Genesis of Unique Catalytic Processes. Chem. Rev. 2020, 120, 464–525. [Google Scholar] [CrossRef]
- Lu, R.; He, L.; Wang, Y.; Gao, X.-Q.; Li, W.-C. Promotion effects of nickel-doped Al2O3-nanosheet-supported Au catalysts for CO oxidation. Chin. J. Catal. 2020, 41, 350–356. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Peng, H.; Xu, X.; Liu, W.; Wang, Z.; Zhang, N.; Wang, X. Ag supported on meso-structured SiO2 with different morphologies for CO oxidation: On the inherent factors influencing the activity of Ag catalysts. Microporous Mesoporous Mater. 2017, 242, 90–98. [Google Scholar] [CrossRef]
- Lee, S.; Lin, C.; Kim, S.; Mao, X.; Kim, T.; Kim, S.-J.; Gorte, R.J.; Jung, W. Manganese Oxide Overlayers Promote CO Oxidation on Pt. ACS Catal. 2021, 11, 13935–13946. [Google Scholar]
- Spezzati, G.; Benavidez, A.D.; DeLaRiva, A.T.; Su, Y.; Hofmann, J.P.; Asahina, S.; Olivier, E.J.; Neethling, J.H.; Miller, J.T.; Datye, A.K.; et al. CO oxidation by Pd supported on CeO2(100) and CeO2(111) facets. Appl. Catal. B Environ. 2019, 243, 36–46. [Google Scholar]
- Fang, C.; Jiang, X.; Hu, J.; Song, J.; Sun, N.; Zhang, D.; Kuai, L. Ru Nanoworms Loaded TiO2 for Their Catalytic Performances toward CO Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 5079–5087. [Google Scholar] [PubMed]
- Khan, W.U.; Yu, I.K.M.; Sun, Y.; Polson, M.I.J.; Golovko, V.; Lam, F.L.Y.; Ogino, I.; Tsang, D.C.W.; Yip, A.C.K. Size-activity threshold of titanium dioxide-supported Cu cluster in CO oxidation. Environ. Pollut. 2021, 279, 116899. [Google Scholar] [CrossRef]
- Li, X.; Ren, S.; Chen, Z.; Chen, L.; Wang, M.; Wang, L.; Wang, A. Promoting mechanism of CuO on catalytic performance of CeFe catalyst for simultaneous removal of NOx and CO. Fuel 2023, 347, 128435. [Google Scholar] [CrossRef]
- Liu, C.; Gong, L.; Dai, R.; Lu, M.; Sun, T.; Liu, Q.; Huang, X.; Huang, Z. Mesoporous Mn promoted Co3O4 oxides as an efficient and stable catalyst for low temperature oxidation of CO. Solid State Sci. 2017, 71, 69–74. [Google Scholar]
- Ren, S.; Li, X.; He, C.; Chen, L.; Wang, L.; Li, F. Surface tailoring on bifunctional CuOx/MnO2 catalyst to promote the selective catalytic reduction of NO with NH3 and oxidation of CO with O2. Sep. Purif. Technol. 2024, 346, 127471. [Google Scholar]
- Genty, E.; Brunet, J.; Poupin, C.; Ojala, S.; Siffert, S.; Cousin, R. Influence of CO addition on the toluene total oxidation over Co based mixed oxide catalysts. Appl. Catal. B Environ. 2019, 247, 163–172. [Google Scholar]
- Li, L.; Zhang, C.; Yan, J.; Wang, D.; Peng, Y.; Li, J.; Crittenden, J. Distinctive Bimetallic Oxides for Enhanced Catalytic Toluene Combustion: Insights into the Tunable Fabrication of Mn−Ce Hollow Structure. ChemCatChem 2020, 12, 2872–2879. [Google Scholar] [CrossRef]
- Ma, J.; Jin, G.; Gao, J.; Li, Y.; Dong, L.; Huang, M.; Huang, Q.; Li, B. Catalytic effect of two-phase intergrowth and coexistence CuO–CeO2. J. Mater. Chem. A 2015, 3, 24358–24370. [Google Scholar] [CrossRef]
- Liu, W.; Flytzanistephanopoulos, M. Total Oxidation of Carbon Monoxide and Methane over Transition Metal Fluorite Oxide Composite Catalysts: I. Catalyst Composition and Activity. J. Catal. 1995, 153, 304–316. [Google Scholar]
- Aguila, G.; Guerrero, S.; Baeza, P.; Araya, P. Study of the influence of the Cu/Ce loading ratio in the formation of highly active species on ZrO2 supported copper-ceria catalysts. Mater. Chem. Phys. 2019, 223, 666–675. [Google Scholar] [CrossRef]
- Tang, X.; Li, Y.; Huang, X.; Xu, Y.; Zhu, H.; Wang, J.; Shen, W. MnOx–CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: Effect of preparation method and calcination temperature. Appl. Catal. B Environ. 2006, 62, 265–273. [Google Scholar]
- Venkataswamy, P.; Jampaiah, D.; Lin, F.; Alxneit, I.; Reddy, B.M. Structural properties of alumina supported Ce–Mn solid solutions and their markedly enhanced catalytic activity for CO oxidation. Appl. Surf. Sci. 2015, 349, 299–309. [Google Scholar]
- Kacimi, S.; Barbier, J.; Taha, R.; Duprez, D. Oxygen storage capacity of promoted Rh/CeC2 catalysts. Exceptional behavior of RhCu/CeO2. Catal. Lett. 1993, 22, 343–350. [Google Scholar]
- Zhang, M.; Li, W.; Wu, X.; Zhao, F.; Wang, D.; Zha, X.; Li, S.; Liu, H.; Chen, Y. Low-temperature catalytic oxidation of benzene over nanocrystalline Cu-Mn composite oxides by facile sol-gel synthesis. New J. Chem. 2020, 44, 2442–2451. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C.; Mohan, D.; Prasad, R.; Gupta, R.N. Cobalt doped CuMnOx catalysts for the preferential oxidation of carbon monoxide. Appl. Surf. Sci. 2018, 441, 303–316. [Google Scholar]
- Shi, C.; Chang, H.; Wang, C.; Zhang, T.; Peng, Y.; Li, M.; Wang, Y.; Li, J. Improved Activity and H2O Resistance of Cu-Modified MnO2 Catalysts for NO Oxidation. Ind. Eng. Chem. Res. 2018, 57, 920–926. [Google Scholar]
- Li, X.; Ren, S.; Chen, Z.; Wang, M.; Chen, L.; Chen, H.; Yin, X. A Review of Mn-Based Catalysts for Abating NO and CO in Low-Temperature Flue Gas: Performance and Mechanisms. Molecules 2023, 28, 6885. [Google Scholar] [CrossRef]
- Han, M.; Wang, Y.; She, X.; Zhang, Z.; Li, X.; Guo, Z. Recent Progress in CO Oxidation over Non-precious-metal Catalysts. Ind. Eng. Chem. Res. 2024, 64, 36–52. [Google Scholar]
- Ye, Z.; Liu, Y.; Nikiforov, A.; Ji, J.; Zhao, B.; Wang, J. The research on CO oxidation over Ce-Mn oxides: The preparation method effects and oxidation mechanism. Chemosphere 2023, 336, 139130. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Song, J.; Zhang, G.; Sun, X.; Cheng, S.; Zhang, X.; Jiang, Y. Unraveling the promotion for SO2 and H2O resistance of transition metal-doped CeO2-TiO2 catalysts in NH3-SCR reaction: A DFT study. J. Hazard. Mater. 2025, 489, 137563. [Google Scholar] [CrossRef] [PubMed]
- El-Shobaky, G.A.; El-Shobaky, H.G.; Badawy, A.A.; Mohamed, G.M. Effect of Li2O—Doping on physicochemical, surface and catalytic properties of nanosized CuO–Mn2O3/cordierite system. Mater. Chem. Phys. 2012, 136, 1143–1147. [Google Scholar] [CrossRef]
- Kupková, K.; Topka, P.; Balabánová, J.; Koštejn, M.; Jirátová, K.; Giraudon, J.-M.; Lamonier, J.-F.; Maixner, J.; Kovanda, F. Cobalt-Copper Oxide Catalysts for VOC Abatement: Effect of Co:Cu Ratio on Performance in Ethanol Oxidation. Catalysts 2023, 13, 107. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, Y.; Xu, X.; Zhu, T. Study of Cu/Mn Catalysts for Coreactions of NH3-SCR and CO Oxidation. Catal. Lett. 2022, 152, 1752–1759. [Google Scholar] [CrossRef]
- White, R.J.; Budarin, V.; Luque, R.; Clark, J.H.; Macquarrie, D.J. Tuneable porous carbonaceous materials from renewable resources. Chem. Soc. Rev. 2009, 38, 3401–3418. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, Y.; Zhao, W.; Wu, P.; Zhang, J.; Zheng, L.; Ding, T.; Li, X. Hydroxyl promoted preferential and total oxidation of CO over ε-MnO2 catalyst. Catal. Today 2020, 355, 214–221. [Google Scholar] [CrossRef]
- Yuan, X.; Qing, M.; Meng, L.; Zhao, H. One-Step Synthesis of Nanostructured Cu–Mn/TiO2 via Flame Spray Pyrolysis: Application to Catalytic Combustion of CO and CH4. Energy Fuels 2020, 34, 14447–14457. [Google Scholar] [CrossRef]
- Wu, G.; Guan, N.; Li, L. Low temperature CO oxidation on Cu-Cu2O/TiO2 catalyst prepared by photodeposition. Catal. Sci. Technol. 2011, 1, 601–608. [Google Scholar] [CrossRef]
- Yan, Z.D.; Sun, Y.C.; Li, P.U.; Zhan, S.; Sun, X.; Chen, S.U.; Jiang, Y. High-performance MnOx-CuO catalysts for low-temperature CO oxidation: Metal interaction and reaction mechanism. Mol. Catal. 2025, 576, 114945. [Google Scholar]
- Liu, T.; Wei, L.; Yao, Y.; Dong, L.; Li, B. La promoted CuO-MnOx catalysts for optimizing SCR performance of NO with CO. Appl. Surf. Sci. 2021, 546, 148971. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Centeno, M.A.; Ivanova, S.; Eloy, P.; Gaigneaux, E.M.; Odriozola, J.A. Cu-modified cryptomelane oxide as active catalyst for CO oxidation reactions. Appl. Catal. B Environ. 2012, 123–124, 27–35. [Google Scholar] [CrossRef]
- Jiang, Y.; Cheng, S.; Sun, X.; Zhang, G.; Su, C.; Lin, R.; Yang, Z. Integrating Research and Teaching: Designing an Experiment on the Catalytic Oxidation of VOCs over Mn-Cu Catalysts. J. Chem. Educ. 2024, 101, 3466–3472. [Google Scholar] [CrossRef]
- Jiang, Y.; Ji, Z.; Sun, X.; Ge, H.; Jiang, Y.; Xu, Y.; Yang, Z. 3DOM Cu-Ce binary composite oxides for selective catalytic reduction of NO with CO. J. Environ. Chem. Eng. 2024, 12, 113684. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Y.; Zhang, G.; Sun, X.; Song, J.; Cheng, S.; Yang, Z. Mass transfer characteristics of multi-pollutants in nano-pores of CeO2-TiO2 based SCR catalyst: A molecular dynamics study. Process Saf. Environ. Prot. 2024, 191, 1591–1599. [Google Scholar] [CrossRef]


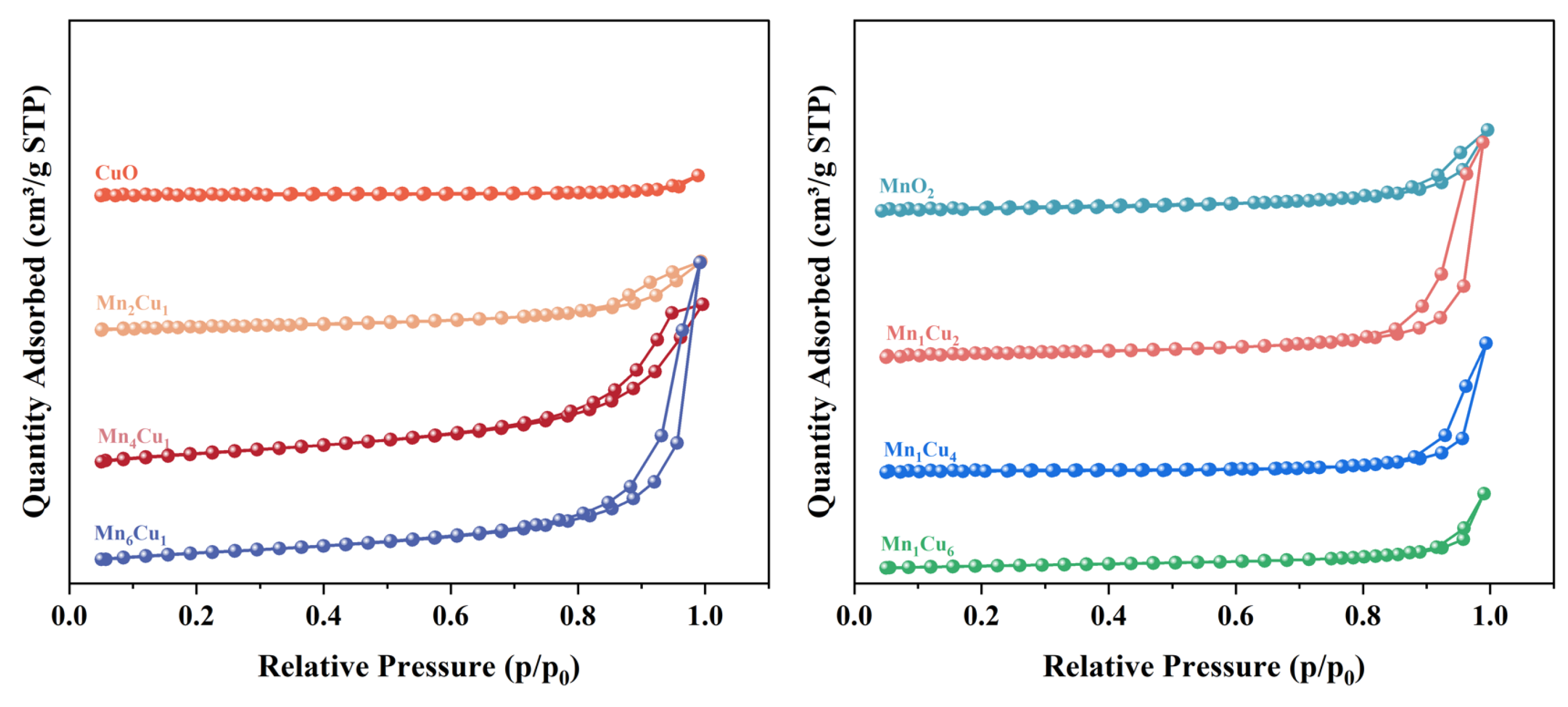




| Catalyst | Specific Surface Areas (m2·g−1) | Pore Volume (cm3·g−1) | Pore Size (nm) |
|---|---|---|---|
| CuO | 4.58 | 0.01 | 24.52 |
| Mn2Cu1 | 18.38 | 0.04 | 14.28 |
| Mn4Cu1 | 45.54 | 0.09 | 11.14 |
| Mn6Cu1 | 37.78 | 0.18 | 23.15 |
| MnO2 | 8.99 | 0.03 | 15.39 |
| Mn1Cu2 | 14.62 | 0.10 | 26.60 |
| Mn1Cu4 | 9.70 | 0.06 | 31.53 |
| Mn1Cu6 | 8.61 | 0.03 | 22.37 |
| Catalysts | Surface Chemical Composition (%) | Mn3+/Mn (%) | Oβ/O (%) | Cu+/Cu (%) | ||
|---|---|---|---|---|---|---|
| Mn | O | Cu | ||||
| MnO2 | 30.15 | 69.85 | — | 36.28 | 27.03 | / |
| Mn1Cu2 | 27.01 | 67.19 | 5.80 | 49.01 | 30.98 | 22.14 |
| Mn1Cu4 | 22.97 | 68.00 | 5.81 | 44.21 | 29.87 | 10.25 |
| Mn1Cu6 | 26.73 | 65.02 | 8.25 | 39.49 | 28.12 | 8.48 |
| CuO | 27.26 | 63.67 | 9.07 | / | 25.30 | 22.44 |
| Mn2Cu1 | 11.27 | 65.45 | 23.28 | 52.15 | 29.98 | 20.91 |
| Mn4Cu1 | 8.12 | 60.86 | 31.02 | 53.89 | 32.26 | 19.81 |
| Mn6Cu1 | 7.01 | 56.19 | 36.8 | 48.84 | 2 | 23.01 |
| Catalysts | Characteristic Temperatures | H2 Consumption (mmol/g) | |||
|---|---|---|---|---|---|
| α (°C) | β (°C) | λ (°C) | η (°C) | ||
| MnO2 | / | / | 356 | 402 | 10.75 |
| Mn1Cu2 | 234 | 278 | 329 | 366 | 10.80 |
| Mn1Cu4 | 235 | 282 | 336 | 369 | 10.66 |
| Mn1Cu6 | 277 | 324 | / | / | 10.51 |
| CuO | 260 | 304 | / | / | 10.22 |
| Mn2Cu1 | 220 | 274 | 324 | 363 | 10.55 |
| Mn4Cu1 | 209 | 265 | 303 | 336 | 10.82 |
| Mn6Cu1 | 227 | 279 | 332 | 371 | 10.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.; Sun, Y.; Li, P.; Jiang, Y.; Sun, X.; Yang, Z. Effect of Mn/Cu Molar Ratios on CO Oxidation Activity of Mn-Cu Bimetallic Catalysts. Catalysts 2025, 15, 353. https://doi.org/10.3390/catal15040353
Liang C, Sun Y, Li P, Jiang Y, Sun X, Yang Z. Effect of Mn/Cu Molar Ratios on CO Oxidation Activity of Mn-Cu Bimetallic Catalysts. Catalysts. 2025; 15(4):353. https://doi.org/10.3390/catal15040353
Chicago/Turabian StyleLiang, Cong, Yingchun Sun, Peiyuan Li, Ye Jiang, Xin Sun, and Zhengda Yang. 2025. "Effect of Mn/Cu Molar Ratios on CO Oxidation Activity of Mn-Cu Bimetallic Catalysts" Catalysts 15, no. 4: 353. https://doi.org/10.3390/catal15040353
APA StyleLiang, C., Sun, Y., Li, P., Jiang, Y., Sun, X., & Yang, Z. (2025). Effect of Mn/Cu Molar Ratios on CO Oxidation Activity of Mn-Cu Bimetallic Catalysts. Catalysts, 15(4), 353. https://doi.org/10.3390/catal15040353








