Abstract
Over time, nanoparticles’ chemistry has shown exceptional ability to solve a wide range of problems in various fields, including the control of microbiological air quality in buildings. Herein, magnesium ferrite (MgFe2O4) was synthesized using coprecipitation, then characterized using X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), Brunauer–Emmett–Teller (BET), scanning electron microscopy (SEM), Transmission Electron Microscopy (TEM) and photoelectron spectroscopy (XPS). MgFe2O4 nanoparticles were then assessed for their ability to inhibit Escherichia coli ATCC 8739 growth and airborne bacterial viability in a laboratory atmosphere through a direct air filtration system. The material showed strong inhibitory activity against E. coli by eliminating practically all viable cells in the tested suspensions after 1 h contact time in the presence of light. Finally, the prepared air filtration setup revealed that passing air bacteria through non-woven fabric filters impregnated with MgFe2O4 effectively eliminates them. Thus, only 1 colony-forming unit (CFU) was obtained from 36 L of filtered air, while a control filter (without MgFe2O4) allowed the passage of 2.6 × 105 CFU to the liquid medium. The obtained results initiate potential applications of MgFe2O4 nanoparticles in controlling microbiological indoor air quality (IAQ), especially in healthcare facilities where microbial resistance to antibiotics is the most notable, individuals are the most exposed, and contamination risks are the highest.
1. Introduction
Since the beginning of the 21st century, concerns about environmental pollution have acquired a central place in scientific and political agendas [1,2]. The accelerated growth of the human population, together with the industrial and technological development, has led to a significant increase in environmental contaminants [3,4]. Moreover, the rapid development of medicinal, chemical, pharmaceutical, agricultural and textile industries has released a multitude of toxic substances, including heavy metals, phosphate and nitrogen, solvents, organic dyes, drugs such as antibiotics and various harmful chemicals such as pesticides and fertilizers [5]. These substances, when released into air, water and soil, pose serious risks to human health and natural ecosystems. For example, the excessive use of antibiotics has resulted in the spread of resistant germs, posing serious problems for health systems worldwide [6,7]. In addition, indoor air quality (IAQ) has become a major problem, especially with the acceleration of the global warming consequences on our lifestyle and the emergence of new microbial epidemics such as COVID-19 [8,9,10]. In this context, bacterial air contamination has emerged as a significant concern, further intensified by rising urban density and climate change factors that create favorable conditions for bacterial proliferation [11].
Bacteria in the air can be responsible for a wide variety of illnesses, including upper and lower respiratory infections, skin infections, allergies, gastrointestinal illnesses and other health problems [12,13,14]. Moreover, certain bacteria can release toxins that exacerbate symptoms such as asthma or chronic obstructive pulmonary disease (COPD), leading to increased health risks and complications [15]. In addition, airborne microorganisms in closed environments (domestic habitations, schools, hospitals, etc.) often released through air ducts constitute the main cause of surface, space and equipment contamination and, therefore, human and animal infection and/or reinfection in these settings [16]. Relying on traditional strategies, mainly antibiotics, to tackle harmful microorganisms has led to natural pressure, causing the appearance of new resistant strains in the environment [7,17]. In addition to antibiotics, conventional methods for controlling airborne bacterial contamination include chemical disinfection, thermal sterilization, irradiation and physical filtration. However, these methods come with notable limitations, such as variable effectiveness, high operational costs and concerns over long-term environmental sustainability [18].
Over time, several studies have demonstrated the remarkable potential of nanomaterials in environmental remediation, particularly for photocatalytic degradation of organic pollutants. For example, TiO2-based composites, combined with metal oxides such as CuO, La2O3, and Bi2O3, have shown enhanced optical and photocatalytic properties with improved efficiency under visible light irradiation. Furthermore, the incorporation of porous supports like zeolites and hydroxyapatite enhances adsorption–photodegradation synergy, facilitating the complete removal of contaminants and highlighting the engineered nanomaterials’ versatility in addressing environmental challenges, reinforcing the relevance of developing innovative photocatalysts for air and water purification applications [19,20,21,22,23].
Recently, the use of nanotechnology to manage IAQ has led to innovative solutions to mitigate the harmful effects of pollution in aerial ecosystems [9,24,25]. Thus, integrating bioactive nanocomposites in air filters to capture, inactivate and/or kill bacteria present in ambient air is being considered [26,27]. Over the last decade, there has been growing interest in studying the antimicrobial properties of nanoparticles such as MgFe2O4 [28], TiO2, MgO, TiO2/MgO [29] and NiO2 [30], mainly due to the ineffectiveness of certain antibiotics against resistant bacteria. In this context, MgFe2O4 magnetic nanoparticles, among others, have emerged as a promising candidate due to their unique properties and potential mechanisms of action against microorganisms. In the same context, El-Khawaga et al. investigated the use of MgFe2O4 nanoparticles showing significant efficacy in eliminating Gram-positive and Gram-negative bacterial strains [28]. Sajid et al. showed that the highest inhibition rates (%I) are 85.4% for TiO2/MgO, 82.8% for TiO2 and 65.14% for MgO at a concentration of 50 mg/2 mL and a contact time of 2 h [29].
MFe2O4 nanocomposites are materials made of transition metal ferrite (MFe2O4) nanoparticles [31], where M generally represents a transition metal such as iron (Fe), cobalt (Co), zinc (Zn), copper (Cu), nickel (Ni), manganese (Mn), etc. These nanoparticles are generally integrated into a host matrix, which can be a polymer, a metal oxide, a ceramic or another material [32,33]. MFe2O4 nanocomposites are widely used as photocatalysts to degrade organic pollutants present in water, such as dyes, pesticides, pharmaceuticals, heavy metals, etc. Their ability to adsorb and degrade pollutants makes them effective for water and wastewater treatment [34,35]. Additionally, MFe2O4 nanocomposites can be used to reduce volatile organic compounds (VOCs) in the air by acting as photocatalysts to oxidize them to harmless products, such as carbon dioxide and water. This approach is particularly useful for purifying indoor and outdoor air [36]. Under sunlight or artificial light sources, ferrite nanoparticles can generate electron–hole pairs, which can initiate oxidation and degradation reactions of pollutants [37]. Their nanometric size and large specific surface area enhance their efficiency in removing air contaminants, allowing for more effective interaction and capture of pollutants [38,39]. It is important to note that air is a dynamic and living environment. Microorganisms present in air, known as bioaerosols, encompass a great diversity and richness, including bacteria, fungi, viruses, algae, and protozoa [40]. Bacteria, for example, represent a major component of bioaerosols. Common genera include Bacillus, Staphylococcus, Micrococcus, and Pseudomonas. Some of these bacteria can be pathogenic to humans, animals, or plants, while others play beneficial roles in the biogeochemical cycles [41,42,43].
In this context, nanoparticles with antibacterial activity are emerging as an innovative and promising solution to combat the spread of harmful microorganisms. Due to their unique physicochemical properties, they offer new perspectives for combating bacterial infections and the spread of harmful microorganisms in the environment [44]. In fact, nanoparticles’ antibacterial mechanisms are not well defined, and some of them include damage to the cell wall, plasma membrane, cytosolic proteins, enzymes, and the production of reactive oxygen species (ROS) [45].
Metal nanoparticles are among the most studied for their antibacterial properties. Some metals have been known for their antimicrobial capabilities and nanoparticles made from these metals maximize this effectiveness, thanks to their large specific surface area, allowing increased interaction with bacterial constituents [46,47,48]. For example, MgFe2O4 nanoparticles exhibit important antibacterial activity, particularly under light exposure, due to their ability to generate reactive oxygen species (ROS), which cause oxidative damage to bacterial membranes, proteins, and DNA. Additionally, these nanoparticles can disrupt bacterial antioxidant defenses, release metal ions (Mg2+ and Fe2+/Fe3+) that interfere with vital enzymes, and compromise membrane integrity. Their effectiveness is influenced by factors such as size, shape, and concentration, making them promising candidates for antimicrobial applications, especially in light-activated environments [49,50,51,52,53,54,55].
In general, nanoparticles have demonstrated a great capacity to interfere with the cellular components of bacteria, leading to their inactivation or total degradation [56]. Their large specific surface area promotes an effective adsorption of bacteria, allowing their antibacterial properties to eliminate the captured microorganisms. Such properties can be harnessed to manufacture air filters capable of capturing and neutralizing harmful microorganisms, making them ideal for environments where strict microbiological air quality control is essential [38]. Herein, magnesium ferrite (MgFe2O4) was used to fabricate a new air filtration system capable of eliminating airborne bacteria from the atmosphere.
2. Results
2.1. Material Characterization
2.1.1. X-Ray Diffraction
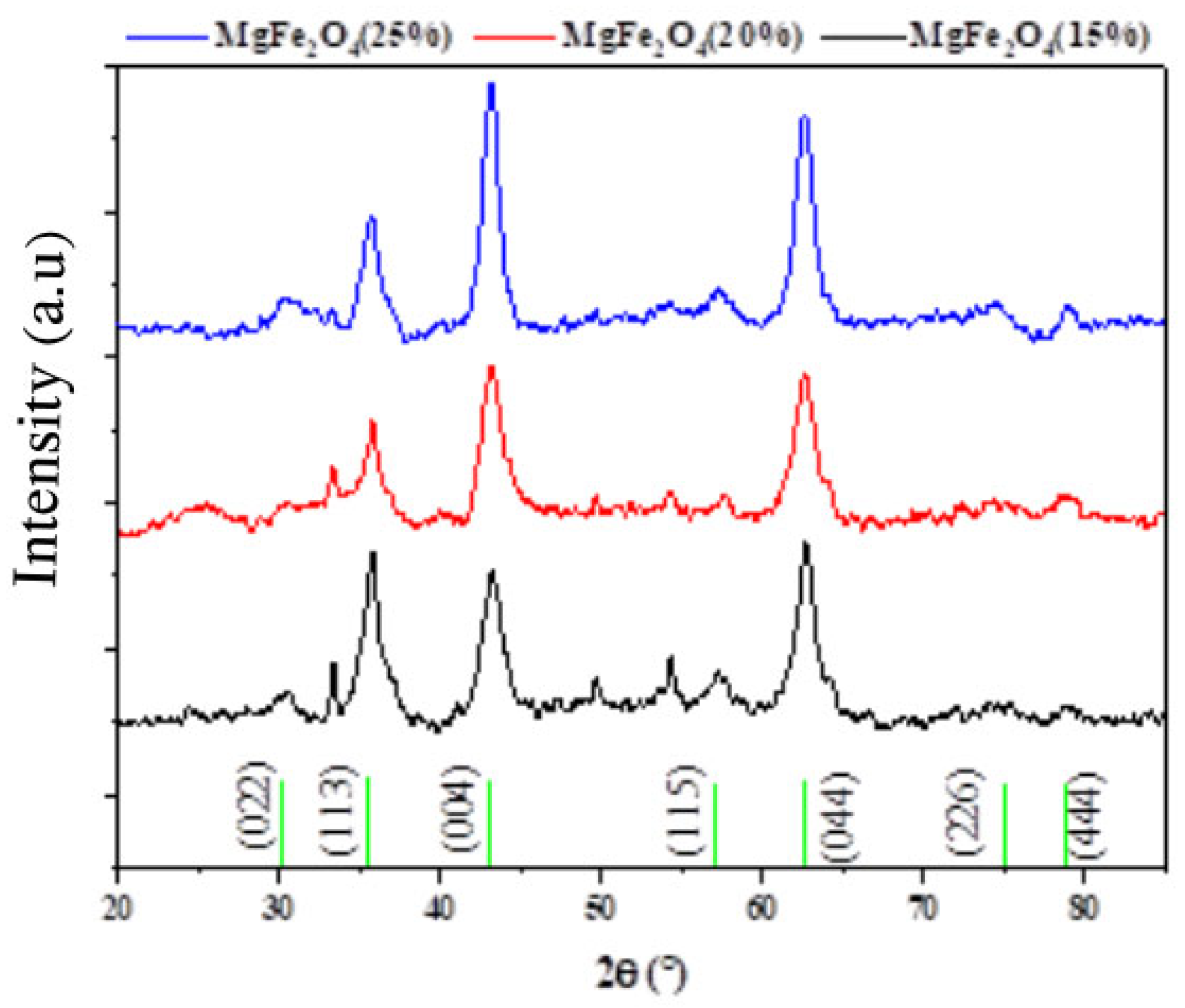
In order to identify the crystalline phase and the average crystallite size of the synthesized samples of MgFe2O4 (15%, 20% and 25%), XRD measurements were carried out as shown in Figure 1. Structural phase identification showed the presence of the Fe2O3 hematite phase (JCPDS No. 01-073-0603) characterized by peaks at 2θ = 35.5°, 43.2, 47.7°, and 57.3° attributed to the hkl, 110, 202, 024, and 122 planes, respectively. In addition, other peaks confirmed the formation of the cubic MgFe2O4 phase (JCPDS No. 01-073-1960) as shown in Figure 1. An overlap between the peaks of the two phases was also observed. A prior work by [57] found that increasing the amount of magnesium increased the lattice spacing due to a bigger incorporation of the ion (Mg2+), which in turn reduced the diffraction angles, and also discovered that magnesium ferrite had better diffraction angles and less magnesium than MgFe2O4.
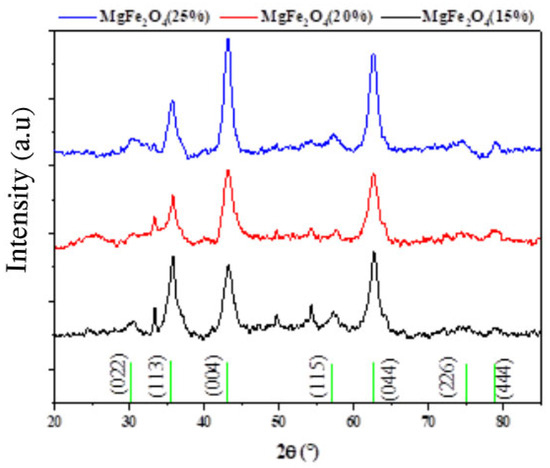
Figure 1.
XRD diagram of MgFe2O4 nanoparticles with different Mg ratios (15%, 20%, and 25%).
2.1.2. Fourier Transform Infrared Spectroscopy (FTIR)
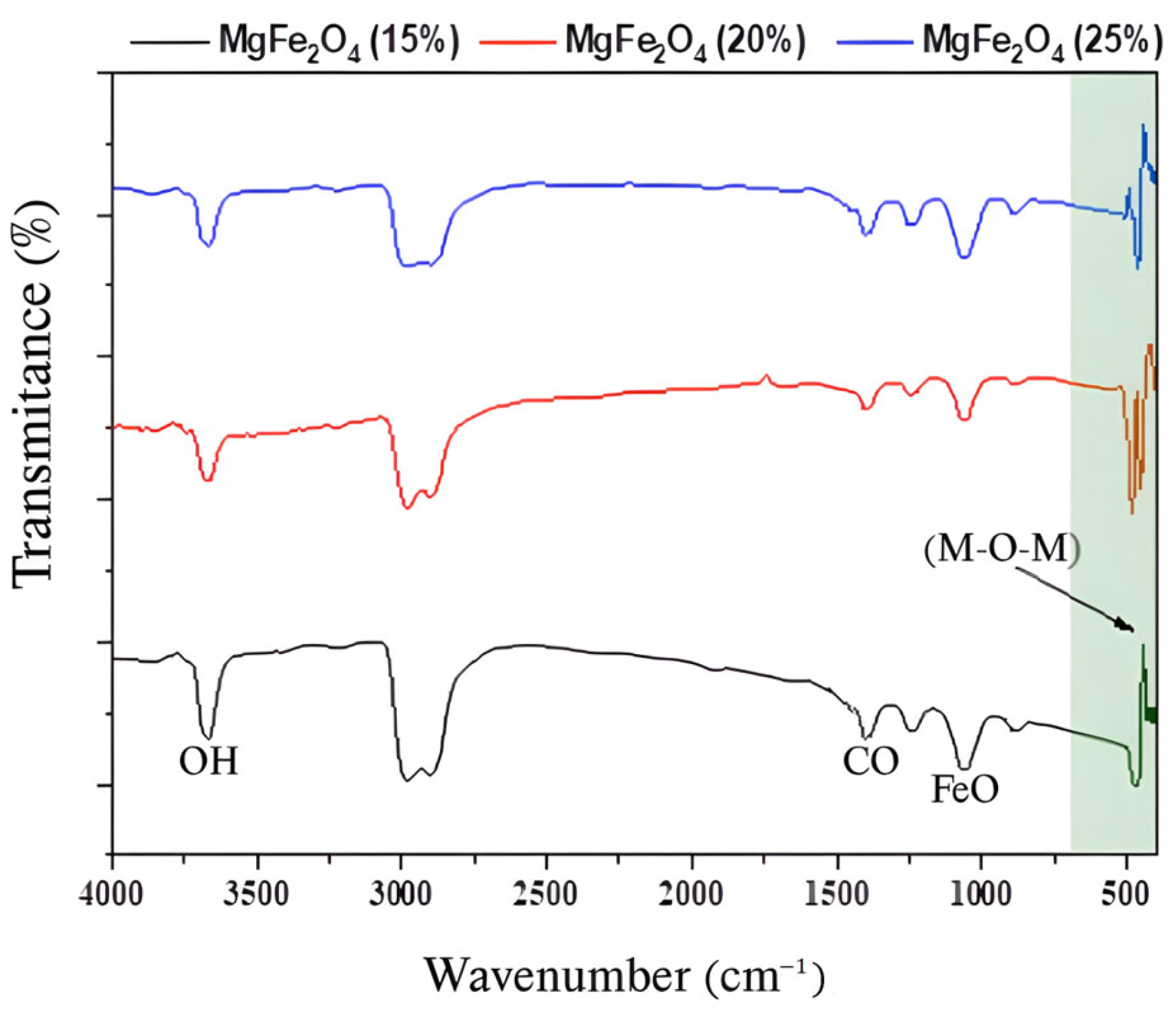
Using synthesized nanoparticles, the powder and both supported and non-supported tissue were analyzed using infrared spectroscopy by measuring the transmittance (%) as a function of frequency (cm−1) at room temperature. Fourier transformation equipment of the JASCO FT/IR-4200® type was used in the spectral range of 400–4000 cm−1. The FTIR spectra of MgFe2O4 powders with varying percentages in Mg ranging from 15% to 25% are displayed in Figure 2. The metal–oxygen–metal (M-O-M) links (where M = Fe, Mg) vibrated, resulting in the observed 420 and 510 cm⁻1 absorption bands. This confirms that the metal oxide was created. Furthermore, Fe-O stretching modes were responsible for the peaks at 1072 and 1253 cm−1. Moreover, the C-H vibrational modes were responsible for the peaks observed at 1442 and 1250 cm−1 [58]. Generally, the carbon–hydrogen vibrational bond is a result of the absorption of 2900–3000 cm−1, and the recorded peak at 3625 cm−1 is attributed to the (O-H), isolated OH in Mg(OH)2 [59].
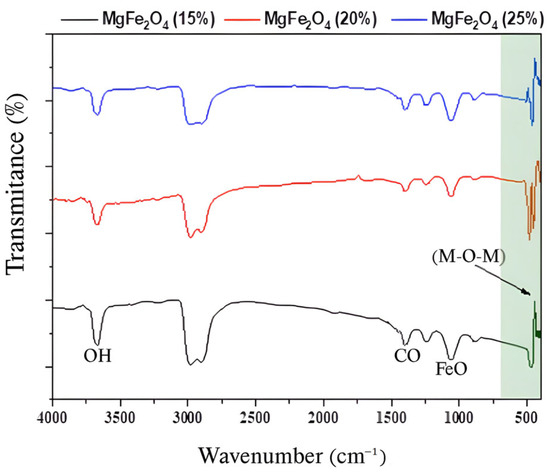
Figure 2.
FTIR spectra of MgFe2O4 powders with varying Mg percentages (15%, 20%, and 25%).
2.1.3. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
Scanning electron microscope and transmission electron microscope analyses of the prepared MgFe2O4 were carried out to assess their surface morphology at the micron and submicron levels. It was observed that the particles varied in size. SEM and TEM images show aggregated particles, probably due to the change in [Mg(NO3)2] concentration.
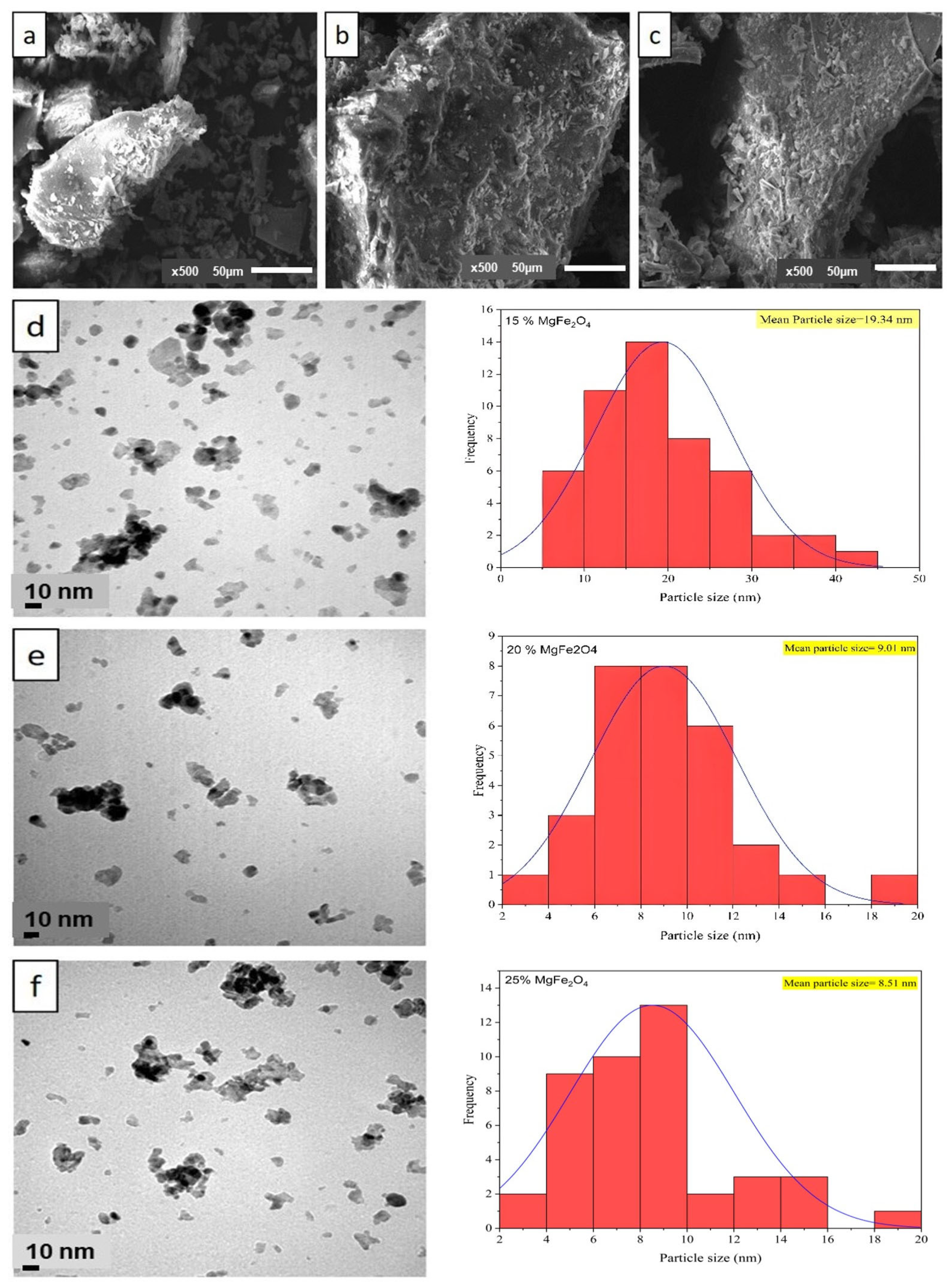
The SEM micrograph of the MgFe2O4 samples (Figure 3a–c) showed an irregular structure of MgFe2O4 (15%, 20% and 25%). The SEM images showed that the particle size of the calcined material became more significant with increasing [Mg(NO3)2] concentration. As synthesized, MgFe2O4 shows an agglomeration of particles resembling flattened flakes on a generally smooth surface (Figure 3a–c). Transmission electron microscopy (TEM) was used to obtain further information on the morphology and concentration distribution of [Mg(NO3)2] on the surface of MgFe2O4. The TEM images in Figure 3d–f show spherical particles and the presence of agglomerates resulting from intra-particle interactions due to van der Waals attractive forces.
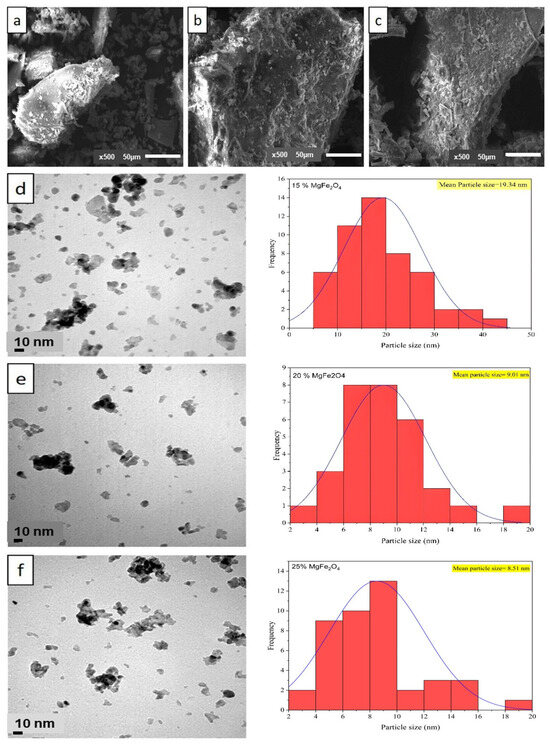
Figure 3.
SEM image (magnitude 5000×) of (a) 15% MgFe2O4; (b) 20% MgFe2O4; (c) 25% MgFe2O4; and TEM image and particle size distribution of (d) 15% MgFe2O4; (e) 20% MgFe2O4; (f) 25% MgFe2O4 at the magnification of 10 nm.
These images also show a spherical morphology of the particles, with a lower proportion of nanoparticle agglomerates (15%) in MgFe2O4. After change in [Mg(NO3)2] concentration, the dispersion of the particles decreased, as mentioned previously in SEM, due to the agglomeration of MgFe2O4 particles. This process provides a basis for the nucleation of MgFe2O4 particles, therefore weakening their tendency to aggregate. The annealed samples demonstrated a notable decrease in nanoparticle size for the calcined MgFe2O4. The size of primary (non-agglomerated) nanoparticles was obtained from TEM images using imageJ software (v.1.53), with an average size of 19.34 ± 0.1 nm, 9.01 ± 0.1 nm, and 8.51 ± 0.1 nm for MgFe2O4 (15%), MgFe2O4 (20%), and MgFe2O4 (25%), respectively. Moreover, distinct size categories of MgFe2O4 particles (aggregated particles) were between 44.08 nm and 65.5 nm. This finding is consistent with prior studies suggesting that the increase in concentration enhanced the aggregated particles [60,61]. A size distribution histogram (Figure 3d–f) revealed a moderate size distribution. In contrast, SEM analysis showed the formation of larger aggregates, with sizes up to 109, 261, and 400 μm, probably due to agglomeration of the nanoparticles during the coprecipitation process (drying, calcination). High-resolution TEM images (Figure 3d–f) confirmed the crystallinity of individual nanoparticles, while SEM images (Figure 3a–c) illustrated the overall morphology and aggregation behavior.
2.1.4. BET Analysis
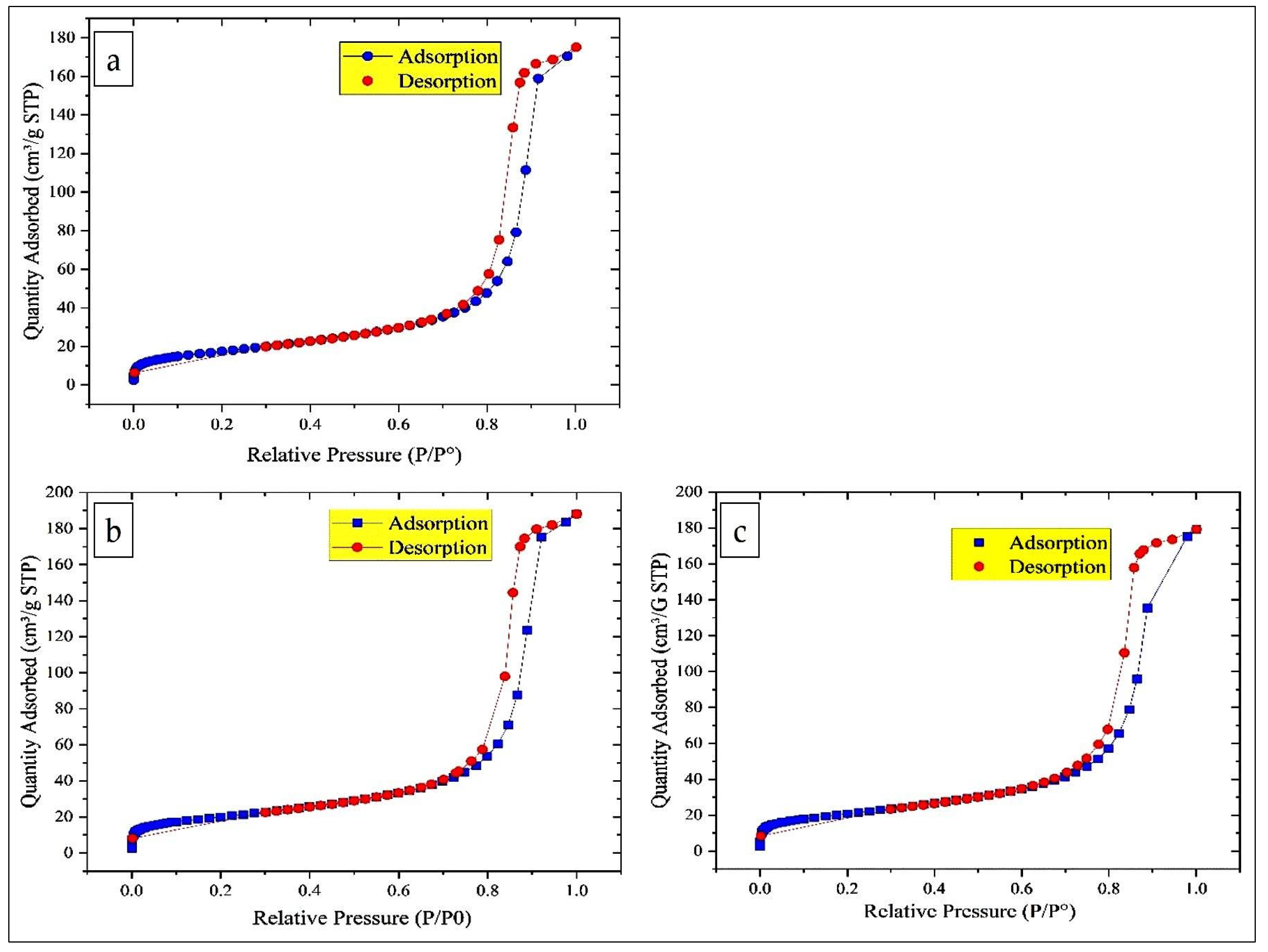
The nitrogen adsorption–desorption isotherms for all the catalysts are of type IV according to the IUPAC classification, indicating the presence of mesoporous materials. Moreover, the H1 type hysteresis observed is typical of cylindrical pores formed by the agglomeration of spherical particles of similar size arranged in a uniform packing pattern (Figure 4). The increase in specific surface area of MgFe2O4 (15% MgFe2O4-Sarea = 62.74 ± 0.1235 m2/g; 20% MgFe2O4-Sarea = 71.17 ± 0.1118 m2/g; 25% MgFe2O4-Sarea = 73.92 ± 0.1129 m2/g) with increasing Mg content can be attributed to several factors. As Mg ions may replace Fe in the Fe2O3 lattice, the crystal structure is perturbed, leading to the formation of surface defects and vacancies that expose more surface area. This substitution can also promote the formation of smaller particles or nanostructures, increasing the surface area to volume ratio. In addition, the incorporation of Mg can increase the porosity of the material, creating more mesopores that contribute to the overall surface area.
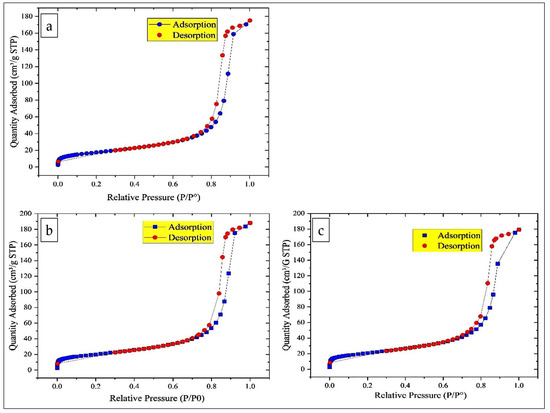
Figure 4.
Nitrogen adsorption/desorption isotherm and distribution of nanoparticles: (a) 15% MgFe2O4; (b) 20% MgFe2O4; (c) 25% MgFe2O4.
2.1.5. X-Ray Photoelectron Spectroscopy (XPS) Analysis
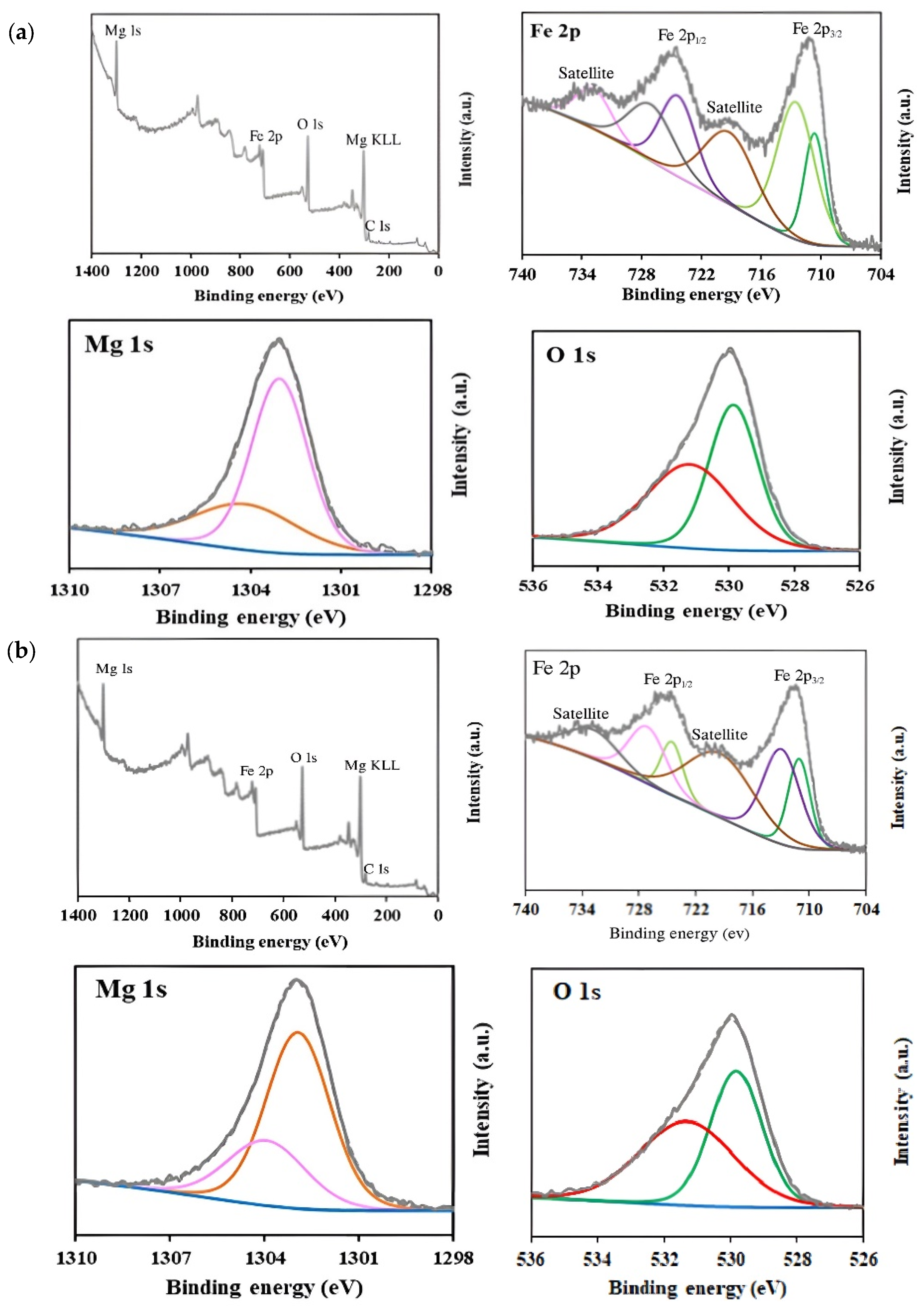
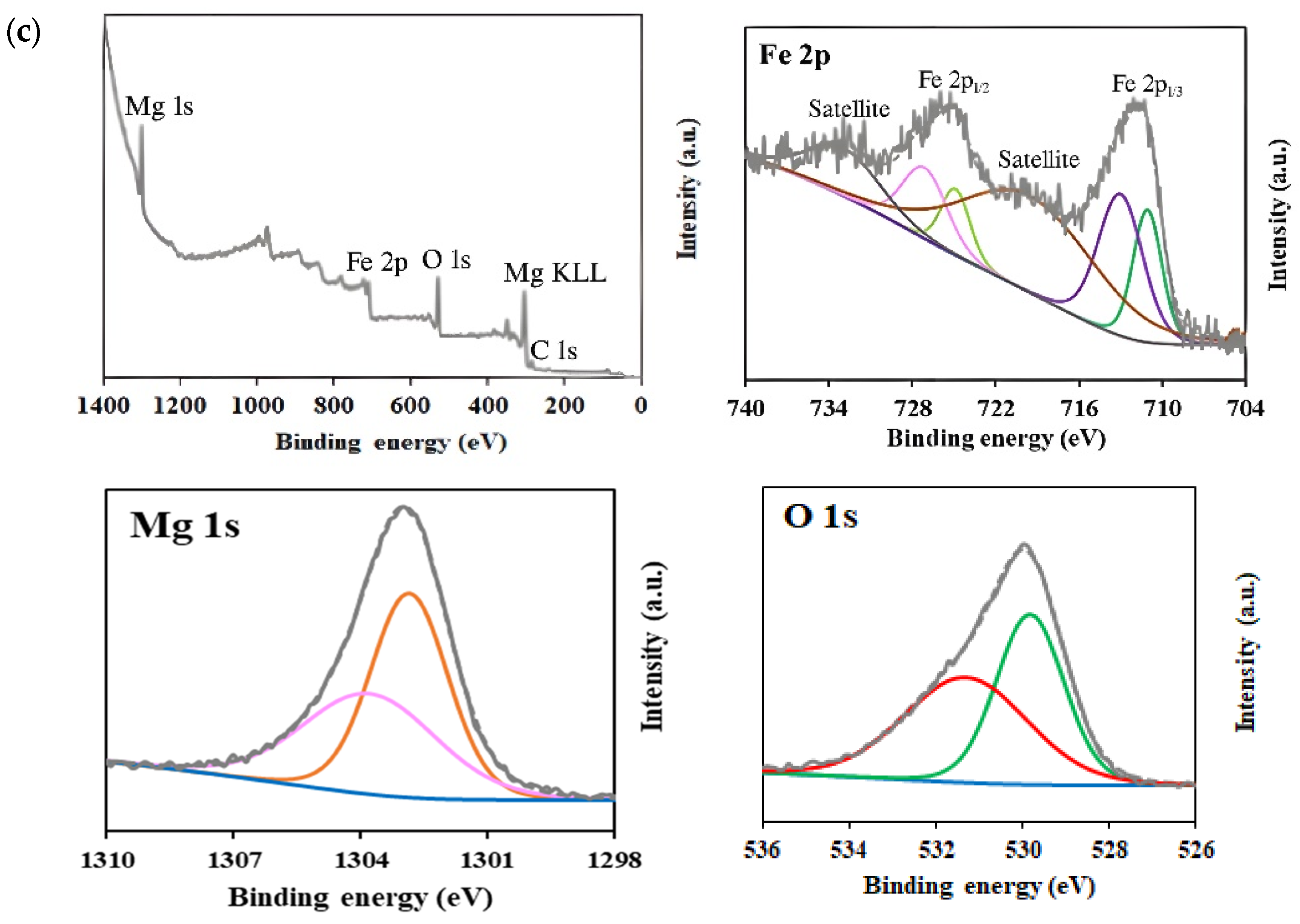
XPS analysis was carried out to determine the surface chemical composition and the oxidation states of elements in MgFe2O4 samples (Figure 5). Survey spectra confirmed the presence of Mg, Fe, and O elements in all samples. High-resolution spectra of Mg 1 s exhibited two peaks centered at ~1303.9 eV and ~1302.9 eV, which are characteristic of Mg2+ in both tetrahedral and octahedral sublattices [62,63]. With the increase in Mg content during synthesis, a slight increase in the relative intensity of the peak at ~1302.9 eV was observed, from 67.6% in the 15% MgFe2O4 sample to 71.2% in the 25% MgFe2O4 sample, suggesting a higher occupancy of octahedral sites.
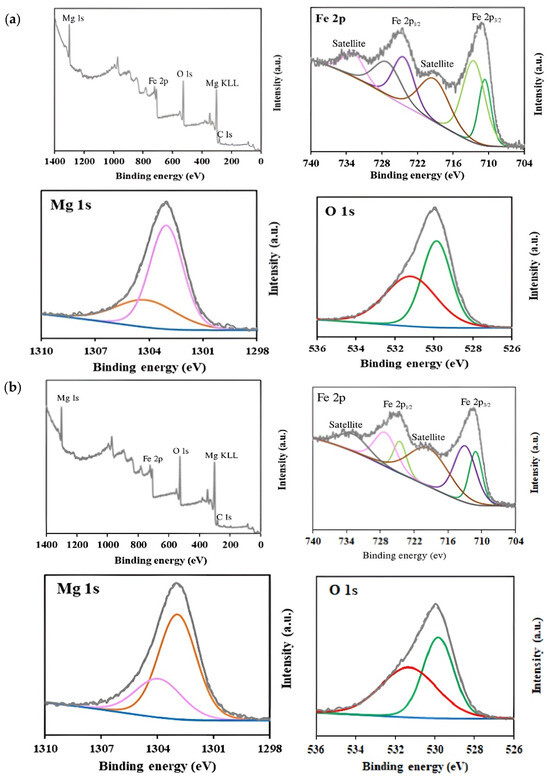
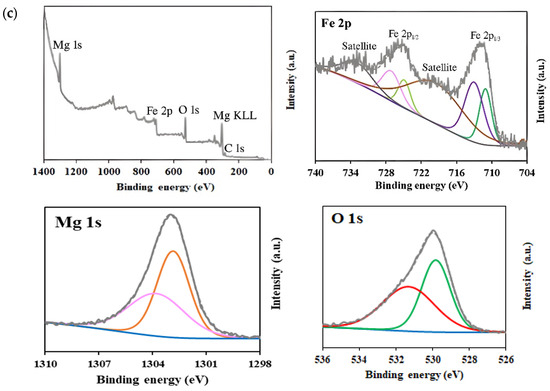
Figure 5.
XPS survey and high-resolution spectra corresponding to Mg 1s, O 1s, and Fe 2p regions for (a) 15% MgFe2O4; (b) 20% MgFe2O4; (c) 25% MgFe2O4.
In the Fe 2p high-resolution spectra, two main peaks were observed at 711.0 and 724.8 eV, corresponding to Fe 2p3/2 and Fe 2p1/2, respectively. The deconvolution of Fe 2p3/2 revealed two peaks at ~711 eV and ~713 eV, indicating the presence of Fe2+ and Fe3+ oxidation states, respectively [64,65]. Similarly, Fe 2p1/2 showed peaks at ~724.5 and ~727 eV, corresponding to Fe2+ and Fe3+, respectively [65]. Additionally, satellite peaks attributed to shake-up processes were detected at 719.29 eV and 732.82 eV [66]. The analysis of the Fe2+/Fe3+ ratios revealed a progressive increase in Fe3+ content with increasing Mg(NO3)2 precursors: from 59.7% (Fe3+) in the 15% sample to 64.6% in the 25% sample. This trend suggests that Mg incorporation promotes a higher oxidation state of iron, possibly due to charge compensation effects within the spinel structure. For the O 1s spectra, the peak centered at 530 eV was deconvoluted into two components, reflecting different chemical environments. The peak at ~529.9 eV was assigned to lattice oxygen (metal–oxygen bonds), while the peak at ~531.1 eV was attributed to surface-adsorbed oxygen species and oxygen vacancies [67,68,69]. The relative contribution of surface oxygen increased with Mg content (from 50.1% in the 15% MgFe2O4 sample to 53.3% in the 25% MgFe2O4 sample), possibly due to increased defect formation or surface reactivity.
2.2. Photocatalytic Degradation of Crystal Violet (CV)
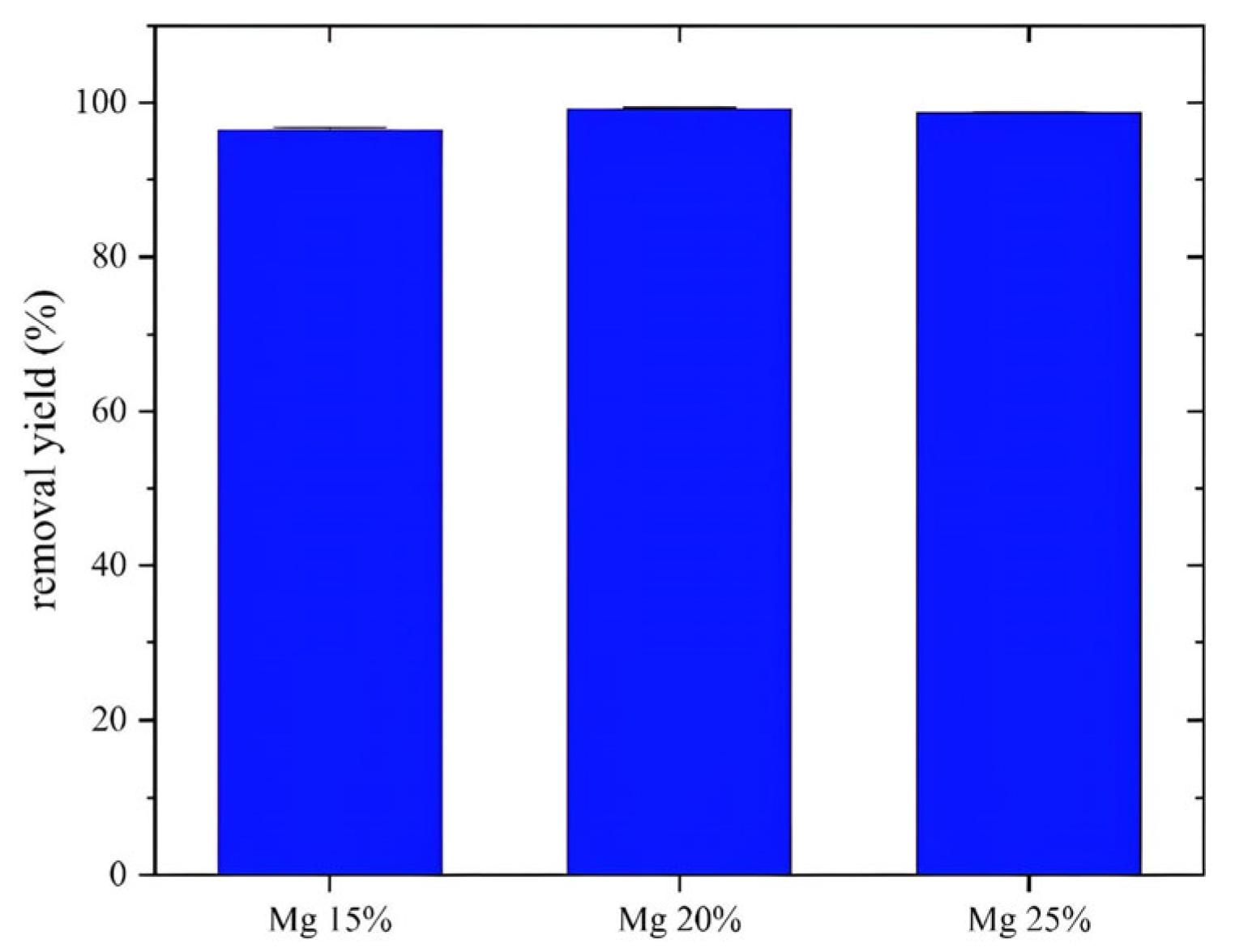
The efficiency of crystal violet (CV) degradation was assessed spectrophotometrically by tracking absorbance at its maximum wavelength, λmax = 591 nm. Figure 6 shows the degradation yield after 360 min of visible light irradiation, revealing almost complete elimination of CV, with an efficiency close to 100% for an initial concentration of 20 mg/L. This significant performance clearly shows that the photocatalytic activity of the synthesized MgFe2O4 nanoparticles under visible light is very high.
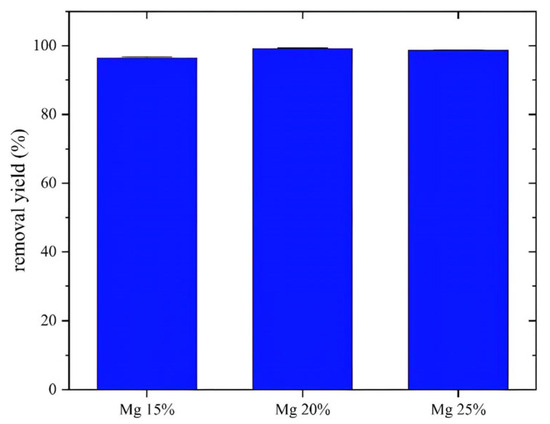
Figure 6.
Effect of Mg proportions on crystal violet removal yield after 2 h contact time, T = 25 °C, m = 0.08 g, v = 200 mL.
As shown in the histogram (Figure 5), the degradation rates for MgFe2O4 materials at 15%, 20%, and 25% were 97.6%, 99.4%, and 98.9%, respectively. The 20% MgFe2O4 nanoparticles showed the best performance and were selected for further work, including the study of antibacterial activity and the preparation of air filters. The photocatalytic activity of MgFe2O4 is based on the generation of electron–hole pairs upon light exposure, followed by the formation of reactive oxygen species (O₂* and *OH) that are responsible for degrading organic pollutants. Increasing the Mg content reduces the band gap, leading to improved absorption of visible light [70]. In addition, the incorporation of Mg generates defect states that act as traps for charge carriers, reducing the hole–electron recombination time and improving charge separation efficiency. The higher specific surface area observed for Mg-rich samples further enhances photocatalytic activity by providing more active sites for pollutant adsorption and ROS generation.
2.3. Antibacterial Activity Against Escherichia coli ATCC 8739
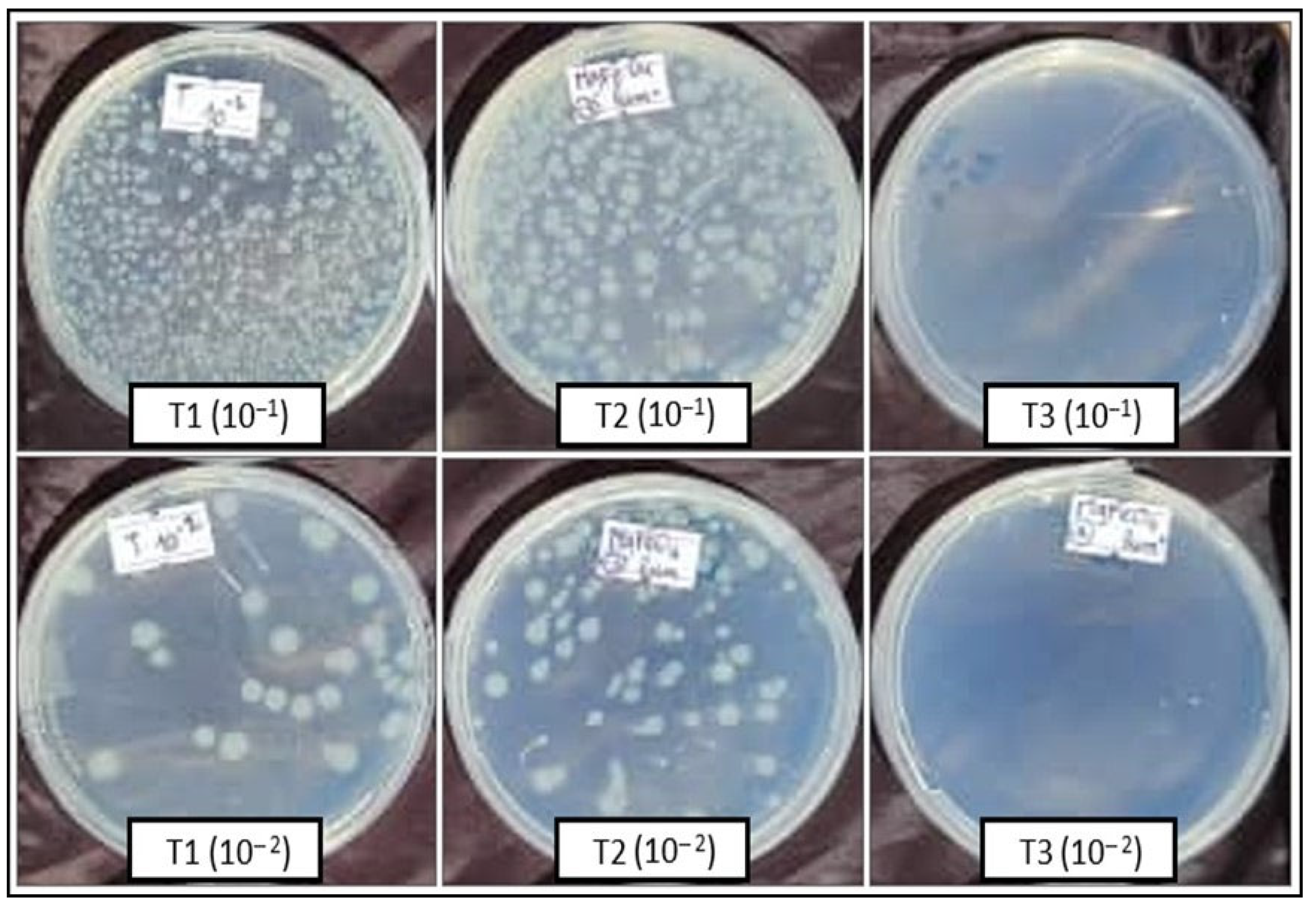
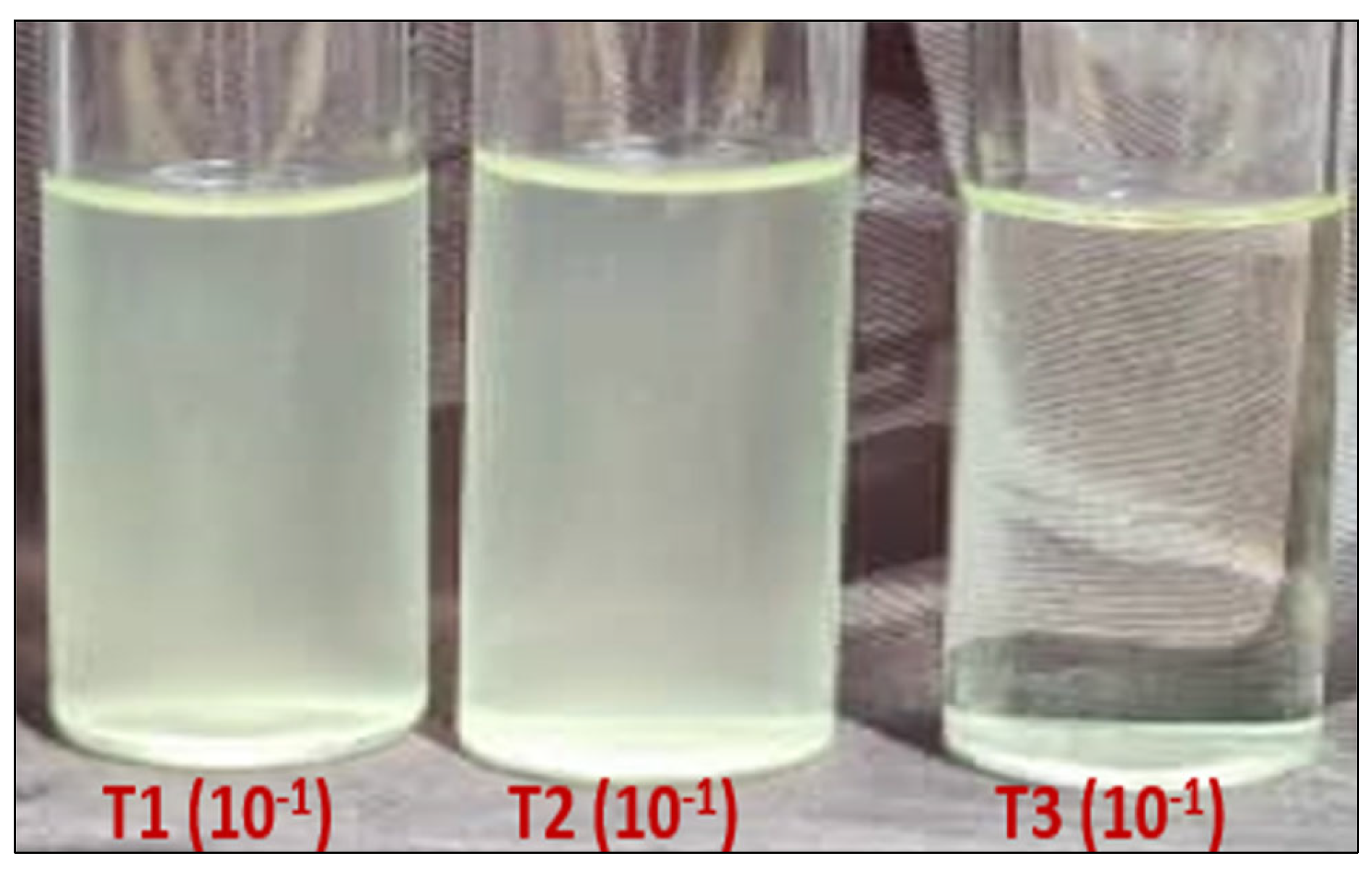
Escherichia coli ATCC 8739 was tested for its sensitivity to MgFe2O4 (20%) in the presence and absence of visible light. The obtained results clearly showed that light significantly promotes the activity of the tested material. Indeed, concerning the 10−1 bacterial dilution, the number of colonies obtained after a bacteria–MgFe2O4 contact time of 1 h, in absence of exposure to light, surpassed 300 colonies (uncountable plates), results comparable to those of the untreated control. However, the T3 treatment (1 h bacteria–MgFe2O4 contact in the presence of light) resulted in only one colony obtained in the three replicates (Table 1, Figure 7).

Table 1.
ATCC 8739 numbering (CFU) from the 10−1 and 10−2 dilutions after incubation.
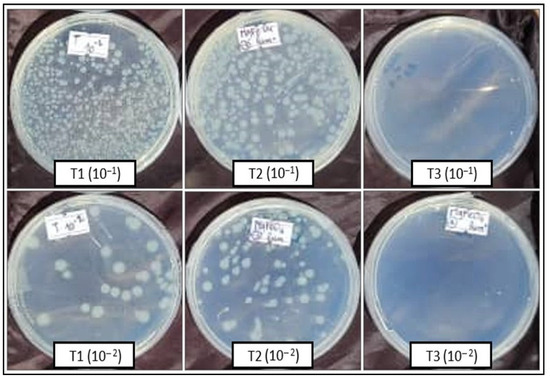
Figure 7.
ATCC 8739 numbering (CFU) from the 10−1 and 10−2 dilutions after incubation. T1: spreading after 1 h ATCC 8739-physiological water contact; T2: spreading after 1 h ATCC 8739-MgFe2O4 contact in absence of light; T3: spreading after 1h ATCC 8739-MgFe2O4 contact under light exposure.
The results obtained with the 10−2 bacterial dilution confirm those of the previous one. Thus, 64 CFU were obtained with the untreated control (1 h bacteria–physiological water contact), 112 CFU with the T2 treatment (1 h bacteria–MgFe2O4 contact in absence of light), and 0 CFU were obtained with the T3 treatment (1 h bacteria–MgFe2O4 contact in the presence of light) (Table 1, Figure 7).
Similar results were obtained by spreading the three treatments on the liquid LB medium. Table 2 shows the average absorbance values obtained by spectrophotometer (600 nm) after incubation for 24 h. Thus, significant differences, translated by a visible difference in the media turbidity from one treatment to another, were observed (Figure 8). Overall, no bacterial viability was obtained from the T3, indicating that light excitation of the MgFe2O4 particles leads to complete elimination of viable bacteria after one hour contact in a liquid solution (Table 2).

Table 2.
ATCC 8739 absorbance (600 nm) after 24 h incubation from each treatment.
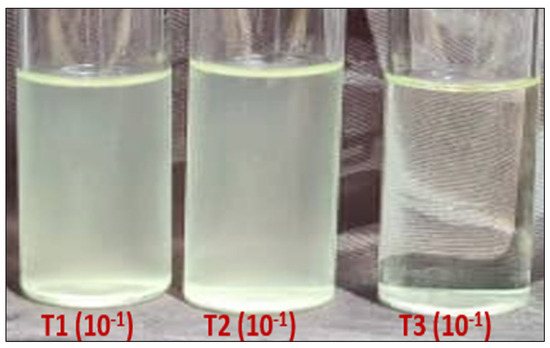
Figure 8.
Bacterial load (turbidity) after 24 h incubation of the 3 treatments on a liquid LB medium. T1: spreading after 1 h ATCC 8739-physiological water contact; T2: spreading after 1 h ATCC 8739-MgFe2O4 contact in absence of light; T3: spreading after 1 h ATCC 8739-MgFe2O4 contact under light exposure.
2.4. Antibacterial Activity of Air Filters in the Air Circulation System
2.4.1. LB Solid Medium
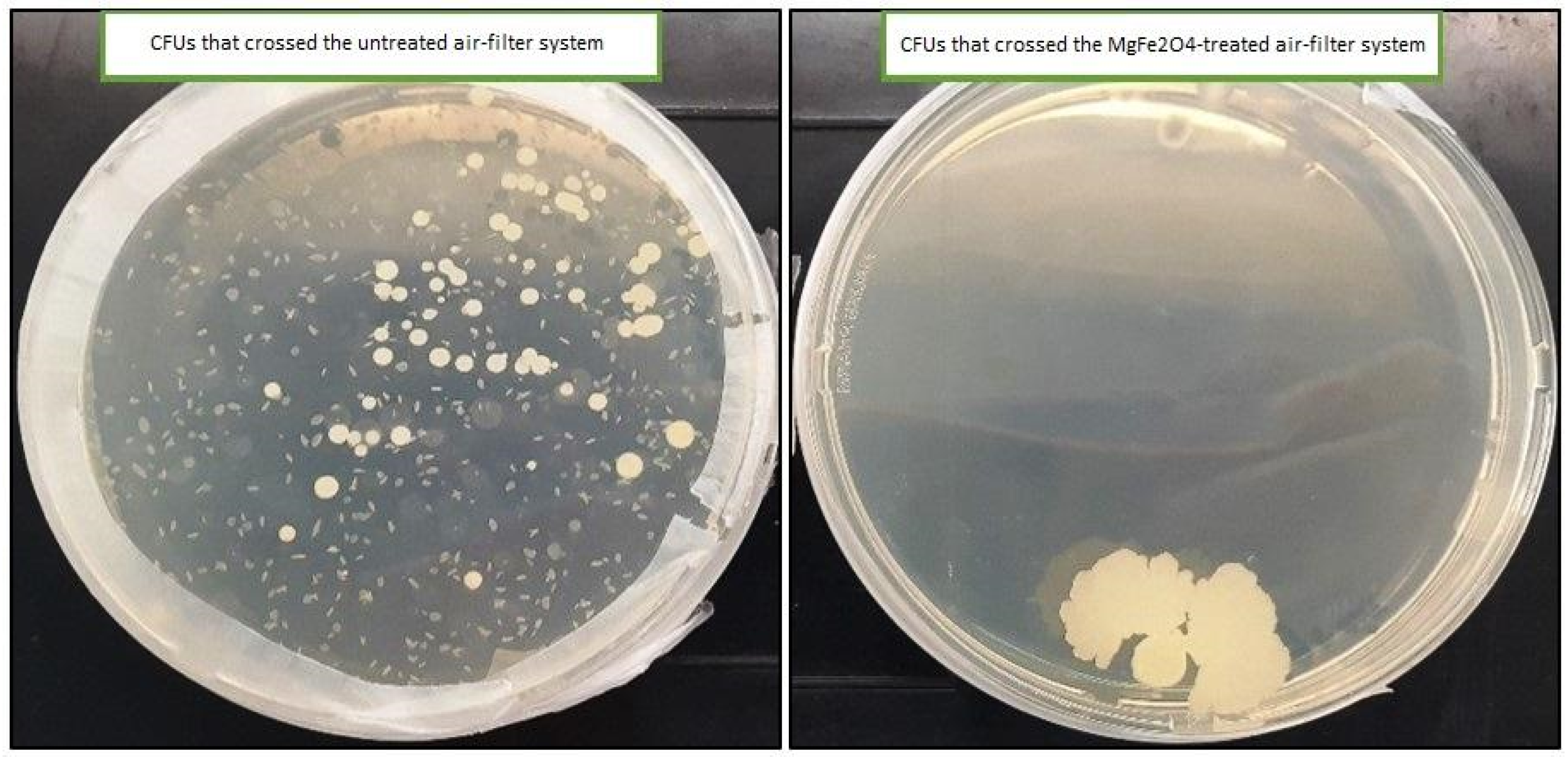
As shown in Figure 9, only one colony was obtained on the LB solid medium that was deposited in the inoculation chamber that receives air from the treated air filters (impregnated with 20% MgFe2O4). However, the number of colonies obtained on the solid LB medium in the inoculation chamber that received air from the untreated air filters was uncountable (˃300 CFU).
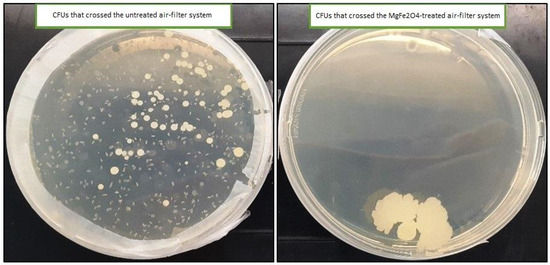
Figure 9.
UFCs obtained on the solid LB medium from both untreated and MgFe2O4 air filtration chambers.
2.4.2. LB Liquid Medium
- a.
- Direct measurement of the optical density of the LB liquid media
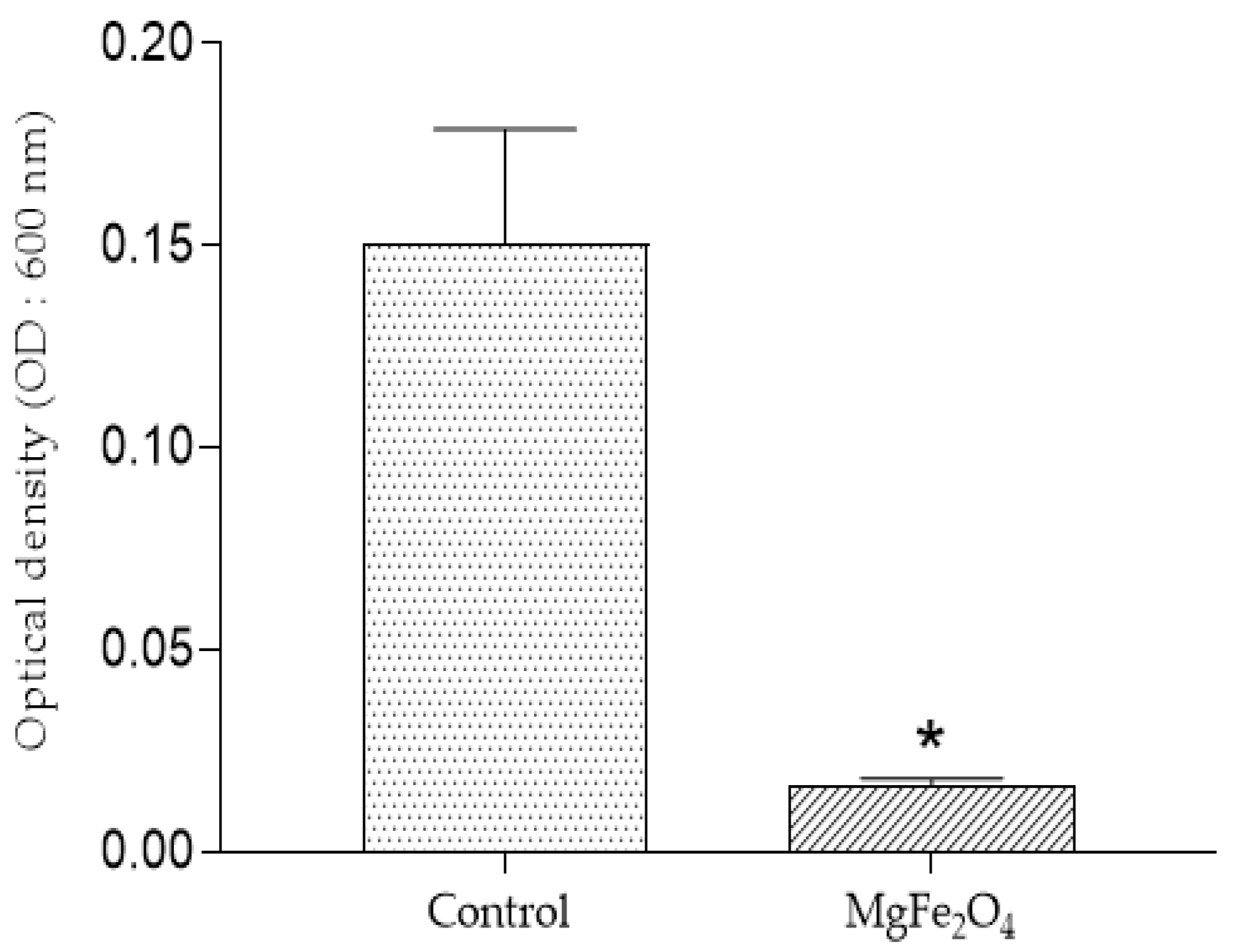
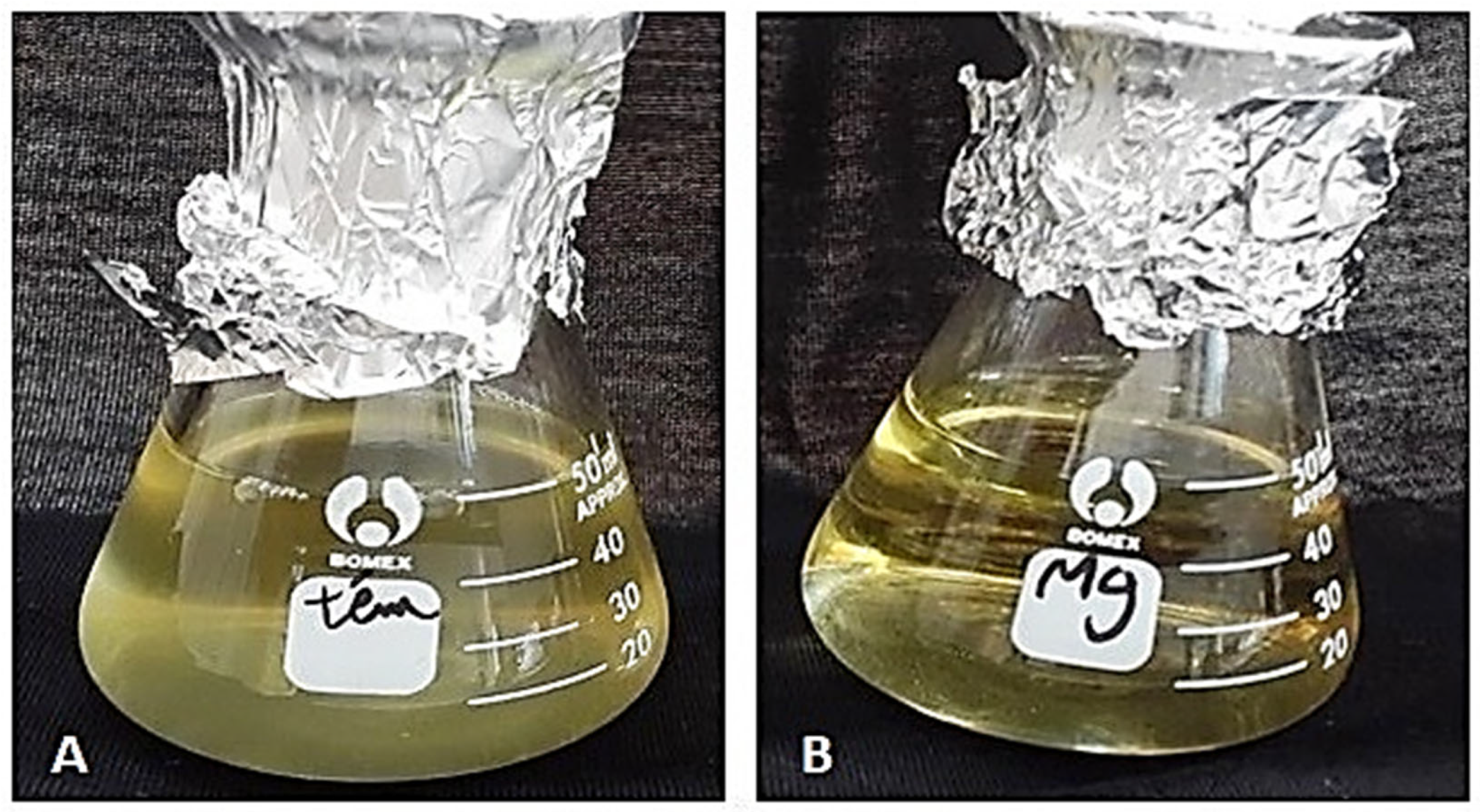
The direct reading of optical density (OD) of the Erlenmeyer deposited in both untreated and MgFe2O4 treated air filtration chambers then incubated for 48 h confirmed the results obtained on the solid LB medium. Indeed, a highly significant difference was observed, translated by naked eye-visible difference in the turbidites of the media belonging to the two treatments (control filters: DO = 0.15; MgFe2O4-treated filters: DO = 0.0163) (Figure 10 and Figure 11).
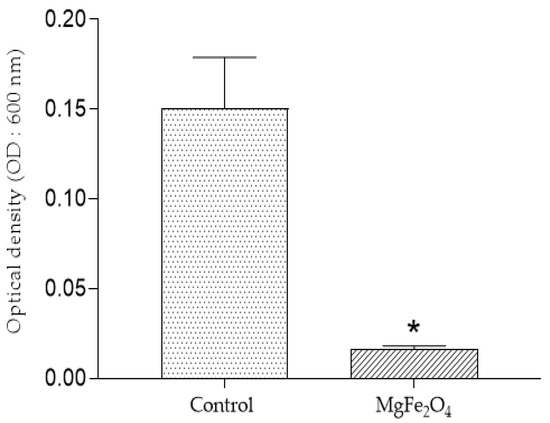
Figure 10.
Optical density (OD: 600 nm) after incubation of the LB liquid media after incubation: control (inoculation chamber receiving air from the untreated air filtration chamber); MgFe2O4 (inoculation chamber receiving air from the MgFe2O4-treated air filtration chamber). Statistical analysis carried out using GraphPad Prism V 9.3.1 software through the unpaired t-test for comparison between two means. *: significant difference (p ≤ 0.05).

Figure 11.
Visible turbidity of the liquid LB media after 48 h incubation. (A): Erlenmeyer that received air from the untreated air filtration chamber. (B): Erlenmeyer that receives air from the MgFe2O4-treated air filtration chamber.
- b.
- Colony-forming Units (UFCs) numbering from the liquid LB media
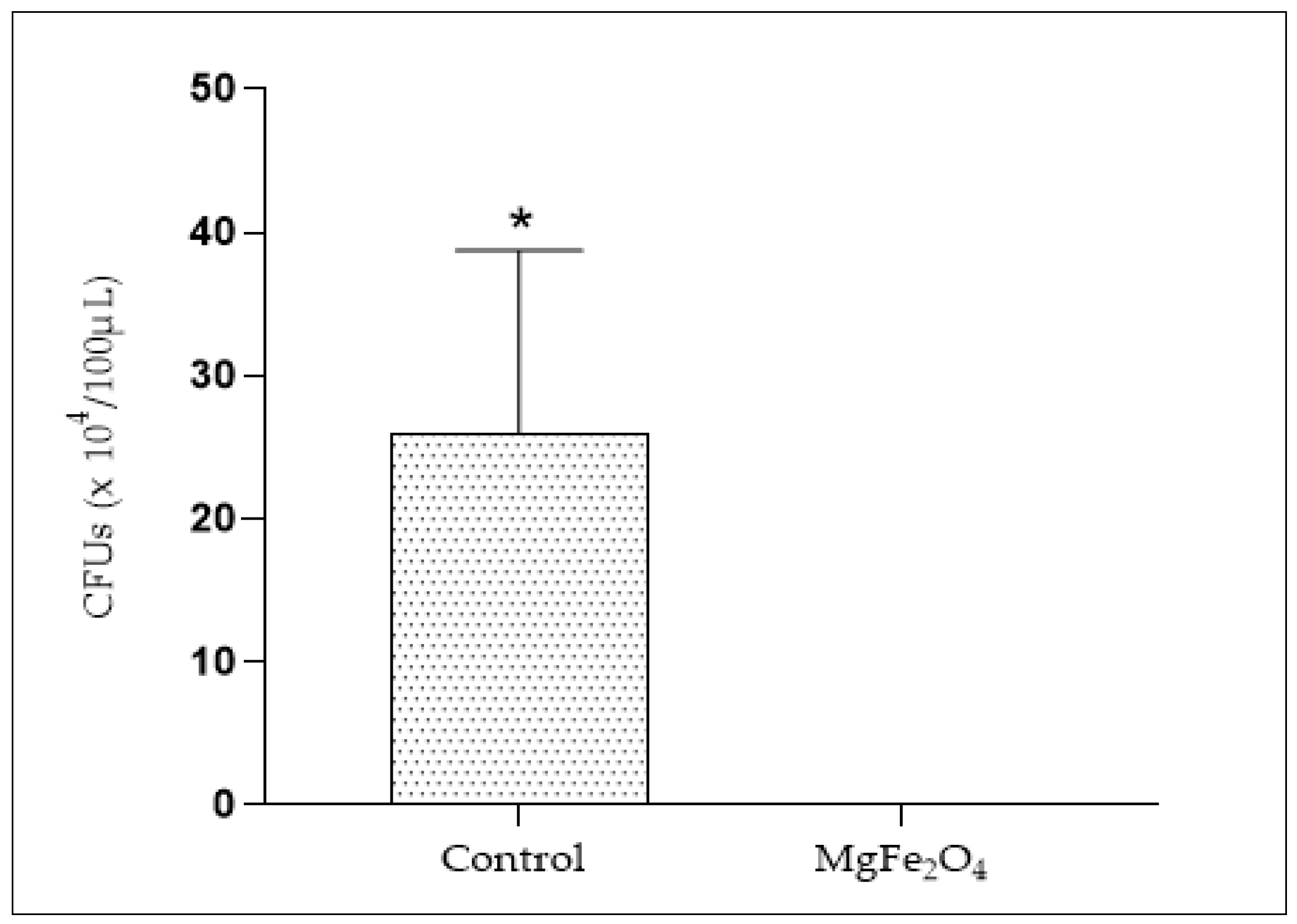
A total of 26 × 104 CFUs were obtained from the Erlenmeyer exposed to air coming from the untreated air filtration chamber. However, no viable bacteria were obtained from the MgFe2O4-treated air filtration chamber (Figure 12), revealing a complete inhibition of bacterial viability through the use of air filters impregnated with MgFe2O4 and exposed to light.
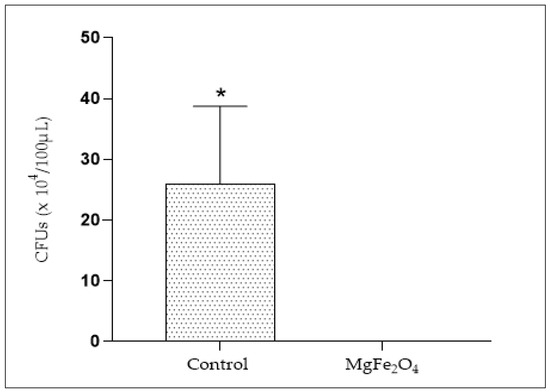
Figure 12.
Viable bacteria numbering (CFUs) from Erlenmeyer deposited in both the untreated air filtration chamber (Control) and the MgFe2O4-treated air filtration chamber (MgFe2O4). Statistical analysis carried out using GraphPad Prism V 9.3.1 software through the unpaired t-test for comparison between two means. *: significant difference (p ≤ 0.05).
In this work, magnesium ferrite (MgFe2O4) nanoparticles showed strong antibacterial activity. This activity must be expanded to ensure its use in the fight against pathogenic microorganisms, particularly those resistant to conventional antimicrobials. Magnesium ferrite nanoparticles are a class of nanomaterials that have attracted increasing interest due to their potential applications in various fields, including medical and environmental applications [71,72]. However, their antibacterial activity is a growing research topic [28].
The bacterial strain, Escherichia coli ATCC 8739 (American Type Culture Collection) was used to evaluate MgFe2O4 antibacterial activity. The entire strain’s genome is already sequenced. It is widely used as a quality control strain in antimicrobial formulation testing, culture media testing, efficacy testing and bioresistance testing [73]. Like all other bacteria, this species is affected by its environment. Its cytoplasmic membrane is permeable to water, but rarely to other metabolites. Nanoparticles, especially metallic ones, can interact directly with the cell membrane of bacteria, causing structural disturbances that lead to increased permeability or complete destruction of the membrane [49], which could explain the complete inhibition of E. coli ATCC 8739 by the synthesized magnesium ferrite (MgFe2O4) nanoparticles.
Our results clearly show that MgFe2O4 nanoparticles have significant antibacterial activity against E. coli ATCC 8739 strains when exposed to light, but they are less effective in the absence of light. This could be due to the ability of light-excited MgFe2O4 to activate reactive oxygen species (ROS) generation. In fact, ROS are known to cause oxidative damage to bacterial cell membranes, proteins, and DNA, leading to bacterial death [50]. Some nanoparticles can also induce indirect oxidative stress by disrupting the natural antioxidant mechanisms of microorganisms by interfering with the antioxidant enzyme systems of microbial cells, such as superoxide dismutase and catalase, thus increasing their vulnerability to reactive oxygen species [51,52].
Nanoparticles can also disrupt the structure and function of bacterial cell lipid membranes. This disruption can lead to altered membrane permeability, ion leakage, and membrane disorganization, compromising cell stability and survival. For example, silver nanoparticles have been observed to induce significant changes in the lipid composition of bacterial membranes, leading to disruptions in membrane function and increased cytotoxicity [53].
When cells are exposed to metal nanoparticles, they can also partially dissolve, releasing metal ions (such as Mg+ in the case of magnesium nanoparticles). These ions can interfere with bacterial metabolic processes and bind to enzymes and proteins, inhibiting their vital functions [54]. Nanoparticles can also penetrate bacterial cells and interfere with various metabolic processes such as DNA and protein synthesis or inhibit enzymes critical for cell survival [55].
The fine mechanisms of action of nanoparticles are poorly explored. They vary depending on their composition, size, and shape. Small nanoparticles tend to have a large specific surface area, improving their interaction with bacterial membranes. Similarly, higher concentrations of nanoparticles generally increase the antibacterial effect [74].
To our knowledge, the use of magnesium ferrite nanoparticles for microbiological air filtration is carried out for the first time in this study. Very little work has been conducted on the antibacterial activity of magnesium-based nanoparticles. Indeed, the maximum activity, observed in the presence of visible light, is probably due to the photocatalytic effects of our material. Some metallic nanoparticles, such as those based on titanium dioxide (TiO2) or zinc (ZnO), have already demonstrated photocatalytic properties that activate the generation of ROS upon exposure to light [75].
Lagashetty et al. [76] conducted a study on the antibacterial activity of (Ag-MgFe2O4) nanocomposites against various bacteria. The results showed that silver-doped magnesium ferrites (Ag-MgFe2O4) exhibit moderate activity against bacteria. The addition of silver is known for its antimicrobial properties, which can improve the effectiveness of magnesium ferrites. However, the results of this work clearly show that MgFe2O4 nanoparticles possess strong antibacterial activity once excited by visible light, which can significantly reduce the viability of airborne bacteria upon simple contact.
Ehi-Eromosele et al. [77] reported the synthesis and antibacterial activity of magnesium ferrite (MgFe2O4) and cobalt-doped magnesium ferrite (Co0.8Mg0.2Fe2O4) nanoparticles. The synthesized nanoparticles were tested for their antibacterial activity against several bacterial strains (Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, and Serratia marcescens). Their results showed that the tested nanoparticles, especially cobalt-doped magnesium ferrite (Co0.8Mg0.2Fe2O4), had good antibacterial properties against these bacteria.
In our study, the constructed device demonstrates the high microbial load of the filtered air volume (control filter: viable bacteria count ˃ 300 CFU); it also demonstrates the effectiveness of the studied nanoparticles in the elimination of bacteria from the same sample of tested air (1 CFU). Recently, the application of nanoparticles in air filtration systems seems to have attracted great interest due to their potential to improve air quality by eliminating microorganisms and harmful particles.
Mekapothula et al. [78] conducted a study exploring the use of copper nanoparticles coated on polymeric fibers for air filtration, particularly in healthcare environments. Copper oxide nanoparticles were synthesized and coated on polyethylene and polypropylene fibers. These nanoparticle-coated materials showed high efficiency in reducing various pathogens, including SARS-CoV-2 and bacterial strains such as Pseudomonas aeruginosa and Escherichia coli. The authors considered that this coating not only provides antiviral and antibacterial properties but also prevents biofouling, making it a promising solution for air conditioning systems and medical masks.
In another approach, adopted by Bonfim et al. [79] and discussed by De Riccardis [80], the use of electrospun nanofibrous membranes for air filtration seems a promising alternative. These membranes are created by electrospinning polymers into fibers with diameters in the nanometer range, resulting in a large specific surface area. According to the authors, this structure is very effective in trapping fine particles and microorganisms. The flexibility of electrospinning allows the use of various polymers and the incorporation of inorganic nanoparticles to improve antimicrobial properties.
Similarly, Bortolassi et al. [52] developed membranes based on polyacrylonitrile (PAN) nanofibers incorporating titanium dioxide (TiO₂), zinc oxide (ZnO), and silver (Ag) nanoparticles. These membranes showed high filtration efficiency, with near-total efficiency (≈100%) for sodium chloride (NaCl) particles of 9–300 nm diameter. The silver-containing nanofibers also demonstrated significant antibacterial activity against Escherichia coli bacteria.
Regarding the dispersion of nanoparticles on filtration media, Vilar et al. [81] used well-dispersed silver nanoparticles on cellulose filter paper. These filters showed substantial bacterial reduction (up to 99%) under gravity filtration. The combination of polydopamine (PDA) and polyethyleneimine (PEI) allowed homogeneous distribution of silver nanoparticles, increasing their efficacy against Staphylococcus aureus and Escherichia coli.
It should be noted that nanoparticles may pose potential risks to human health due to their nanometric size and potential for interaction with biological systems. Studies have shown that some nanoparticles such as ZnFe2O4 and CoFe2O4, can be toxic to human cells, affecting their viability and function [82]. Similarly, nanocomposites that integrate nanoparticles in a polymer or ceramic matrix may pose risks to human health. Studies have shown that nanocomposites containing titanium dioxide (TiO2) or zinc oxide (ZnO) nanoparticles can have toxic effects on human cells and living organisms [83]. These potential risks arise from exposure to the nanoparticles themselves as well as the chemicals used in their manufacture. A thorough assessment of the safety and risks associated with these materials to ensure their safety in various applications seems necessary [84]. In our study, MgFe2O4 nanoparticles were used to impregnate air filters that are not in intimate contact with humans, reducing the risk of exposure to such materials and their negative impact on human health.
This work opens a wide field of perspectives to demonstrate the effect of nanoparticles on the antibacterial effect of molecules used in therapy. By exploring the synergy between nanoparticles and antibiotics, developing targeted nanoparticles, assessing their safety, innovating in their design, and addressing chronic infections, research can pave the way for more effective and specific antibacterial therapies, addressing the challenges posed by bacterial resistance and difficult-to-treat infections.
3. Materials and Methods
3.1. Chemicals
Magnesium (II) nitrate Mg(NO3)2, crystal violet C25H30ClN3, iron (III) chloride FeCl3, sodium hydroxide NaOH, and silver nitrate (AgNO3) were all acquired from Sigma Aldrich. All reagents were used without further purification. Locally sourced deionized (DI) water was used for experimental procedures.
3.2. Biological Material
The bacterial strain, Escherichia coli ATCC 8739 (American Type Culture Collection), was used to evaluate MgFe2O4 antibacterial activity. It is widely used as a quality control strain in antimicrobial formulation testing, culture media testing, efficacy testing, and bioresistance testing.
3.3. MgFe2O4 Synthesis
The MgFe2O4 nanoparticles were synthesized using the coprecipitation method. Three MgFe2O4 spinel solutions were prepared using 4.59, 6.12, and 7.65 g of magnesium nitrate [Mg(NO3)2], corresponding to 15, 20, and 25% Mg, respectively. Thus, each of the three Mg(NO3)2 concentrations was dissolved in 30 mL deionized water, and then, 2.9 g iron chloride (FeCl3) was added to each mixture. The solutions were kept under stirring for 1 h 30 min at 50 °C to ensure complete dissolution of the powders. A solution of NaOH was then added dropwise to the mixture to adjust the pH to 12, resulting in the formation of a precipitate.
The obtained solutions were sonicated for 20 min, kept under stirring for 30 min, then subjected to a washing process that involved alternating rinses with ethanol and deionized water. Ethanol helped to break down any organic residues and promote effective removal of contaminants, while deionized water removed water-soluble impurities. A few drops of silver nitrate (AgNO3) solution were added to a small sample of the wash solution to ensure the absence of chloride ions (no white precipitate of silver chloride (AgCl) are formed), indicating that chloride ions were successfully removed.
The samples were dried in the oven for 10 h at 80 °C to remove the adsorbed organic products and ensure complete dryness. At the end, the powders were collected, grounded, and placed in crucibles for calcination at 500 °C for 2 h.
3.4. MgFe2O4 Characterization
X-ray diffraction (XRD) was carried out to determine the structural characteristics, including crystallite size, phase identification, and crystallinity of the synthesized MgFe2O4 spine. XRD measurements of powdered MgFe2O4 spinel were performed using a model Empyrean Panalytical diffractometer at 40 kV using Cu K radiation (λ = 1.54178 Å), with scans lasting 15 min over a range of angles from 20° to 80°. The functional groups were identified using a Nicolet 8700® Fourier transform infrared spectrometer (Tokyo, Japan), at wavelengths of 400–4000 cm−1. Surface morphology of the prepared samples was observed using SEM (JEOL ISM 6610) (Tokyo, Japan) operating at an accelerating voltage of 20 kV to examine the synthesized material and to elucidate the morphology. A transmission electron microscope (JEOL 2000 EX II) operating at an accelerating voltage of 120 kV and equipped with ultra-high-resolution images was used to determine particle size of the nanoparticles. The Brunauer–Emmett–Teller (BET) surface areas of the synthesized samples were recorded by nitrogen (N2) physisorption at −196 °C using a Micromeritics (Norcross, GA, USA) ASAP-2020. The XPS analysis of MgFe2O4 (15%), MgFe2O4 (20%) and MgFe2O4 (25%) was conducted using a SPECS (Berlin, Germany) spectrometer equipped with a Phoibos 100 MCD analyzer and a monochromatized X-ray Al Kα (1486.6 eV). High-resolution spectra of Mg, O, and Fe elements were taken with 30 eV of energy pass and 0.1 eV of energy step.
3.5. Photocatalytic Activity of MgFe2O4
The photocatalytic activity of the synthesized MgFe2O4 nanoparticles was evaluated using crystal violet dye as a model pollutant. In the experiment, 0.08 g of MgFe2O4 nanoparticles with different compositions (15%, 20% and 25%) was dispersed in 50 mL of crystal violet dye solution. The mixture was stirred continuously for 30 min in the absence of irradiated light to achieve adsorption equilibrium and uniform dispersion of the MgFe2O4 nanoparticles. The solution was then exposed to light irradiation, using three xenon lamps BEETRO® (75 W, wavelength = 400 nm). The degradation of the crystal violet dye was monitored over a period of 360 min. After exposure, a 20 mL aliquot of the solution was centrifuged at 8000 rpm for 5 min to separate the MgFe2O4 nanoparticles. The supernatant was then analyzed using UV–visible spectroscopy to measure absorbance and determine the rate of dye degradation [1,2,3].
3.6. Results of the Antibacterial Activity Against Escherichia coli
The antibacterial activity of magnesium ferrite (MgFe2O4) at a 20% concentration was assessed against the reference strain Escherichia coli ATCC 8739. Pure colonies from a fresh culture of E. coli were suspended in sterile physiological water, with the bacterial suspension adjusted to match a McFarland 0.5 standard, resulting in an initial bacterial concentration of approximately 108 CFU/mL (CFU: colony-forming units). Two serial dilutions (10−1 and 10−2) were prepared by diluting 1 mL of the stock suspension into 9 mL of sterile physiological water.
Each dilution (1 mL) was then incubated with 3 mL of sterile physiological water containing/or not MgFe2O4 (20%) for 1 hour. Three different treatments were conducted: T1 (control) involved bacterial suspension in sterile physiological water (9 g/L NaCl) exposed to light during the contact time (Philips® (Eindhoven, The Netherlands) Lamp: 50 w, 400 nm); T2 included bacterial suspension with 2 mg of MgFe2O4 (20%) in the dark; and T3 included bacterial suspension with 2 mg MgFe2O4 (20%) exposed to light during the contact time (Philips® Lamp: 50 w, 400 nm).
Following a 60 min contact time, bacterial samples from each treatment were inoculated onto two types of media to determine bacterial viability. The first inoculation was on Luria–Bertani (LB) agar plates (tryptone 10 g/L, yeast extract 5 g/L, NaCl 5 g/L, agar 20 g/L, pH 7), where 100 µL of each treated suspension was spread and incubated for 24 h at 37 °C. Bacterial viability was determined by CFU counts at the end of the incubation period. The second inoculation was carried by transferring 50 µL of treated suspension into test tubes containing 9 mL of LB broth (without agar). The tubes were incubated under the same conditions, and bacterial charge was estimated via optical density measurements at 600 nm.
3.7. Air Pollution Control
3.7.1. Air Filter Fabrication
The air filters were fabricated using Vlieseline. Vlieseline is a non-woven textile made from synthetic fibers, typically polyester or polypropylene, bonded through thermal fusion or chemical binding. This type of fabric is lightweight, breathable, durable, and relatively inexpensive, making it a popular material for a wide range of applications [85].
Vlieseline fabric’s impregnation with MgFe2O4 (20%) nanoparticles was performed in a series of structured steps to ensure proper adhesion and preservation of nanoparticles functionality. First, the fabric was prepared through a cleaning process that involved washing to remove any impurities, grease, or residual finishing agents that could hinder nanoparticle binding. Following cleaning, the fabric surface was activated by abrasion with a hard brush, creating a rougher texture to enhance nanoparticles’ adhesion.
Once prepared, the fabric underwent nanoparticle deposition. Three pieces of the fabric were immersed in a beaker containing pure water and placed on a magnetic stirrer. Gradually, 0.5 g of MgFe2O4 (20%) nanoparticles was added to the beaker, with continuous stirring for 10 h, allowing sufficient contact time for nanoparticles’ adhesion onto the fabric surface. The impregnated fabrics were placed in an oven and dried at 80 °C overnight, ensuring that the nanoparticles were firmly attached to the fabric and ready for use.
3.7.2. Construction of the Air Circulation System
The setup consisted of a closed system with four chambers connected by transparent tubing, and ending in an air pump. The air pump drew air from the outside (the non-sterile lab atmosphere) at a flow rate of 600 mL/min and directed it evenly into two small filtration chambers. Each filtration chamber measured 12.5 cm × 5.8 cm × 5 cm, and contained a filtration system made from Vlieseline layers impregnated (or not) with MgFe2O4 (20%). The filtration system was composed of 7 parallelly overlapping layers (5 cm2 each). The layers were spaced approximately 1.5 cm apart. They were either impregnated with MgFe2O4 (treated filtration chamber) or left untreated as a control (untreated filtration chamber).
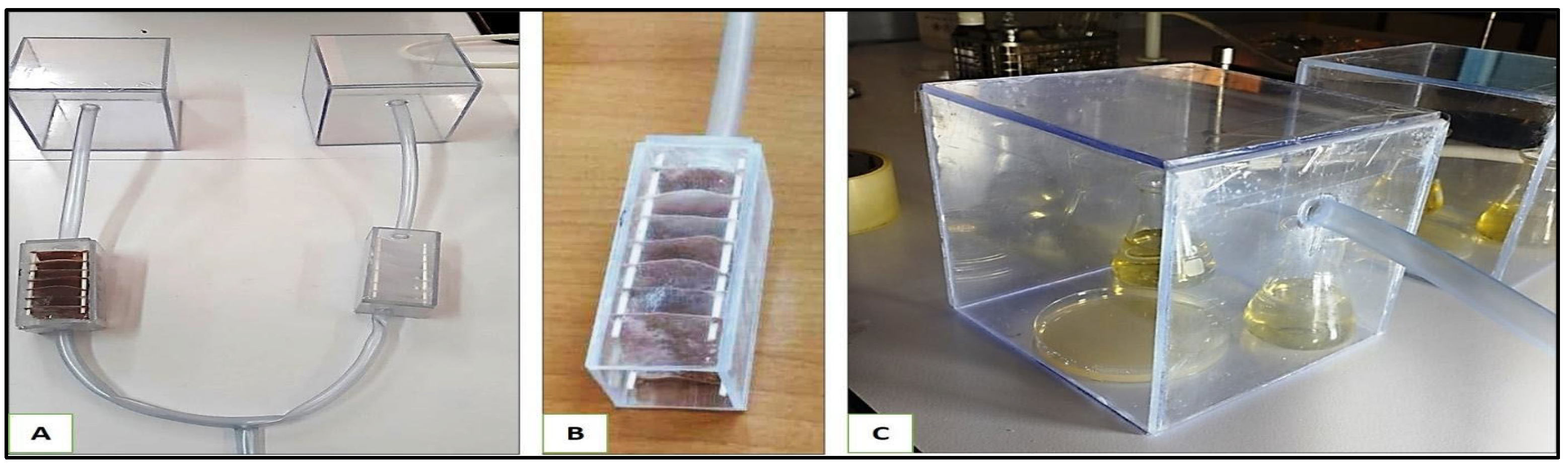
The air from each filtration chamber was then conducted to its corresponding inoculation chamber (Figure 13). Each inoculation chamber (15.5 cm × 15 cm × 15 cm) contained both liquid and solid LB media in open Petri dishes and open Erlenmeyer, respectively. Bacteria that pass through the filtration systems should settle onto the open culture media inside the inoculation chambers (Figure 6).
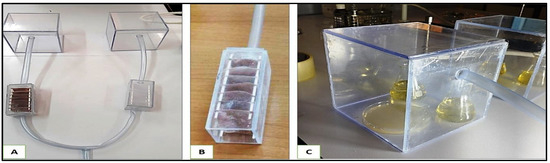
Figure 13.
The air filtration system. (A): Entire setup without the pump. (B): filtration chamber, (C): inoculation chamber.
The entire system was sterilized before use, and the filtration chambers were exposed to light (Philips® Lamp 50 w, 400 nm) during air aspiration to activate the MgFe2O4. After 60 min of air circulation (36 L of air), the culture media were aseptically recovered and incubated at 25 °C for 48 h. Microbial growth was measured by direct CFU count on the solid LB medium, and estimated by measuring absorbance at λ = 600 nm of the liquid LB medium. Finally, a CFU count of the liquid medium was realized according to [86].
3.8. Statistical Analysis
The air filtering activity results were compared using GraphPad Prism V 9.3.1 software, using the unpaired t-test for comparison between two means.
4. Conclusions
This work explored the antibacterial activity of magnesium ferrite (MgFe2O4) nanoparticles, aiming to develop innovative air filters capable of inhibiting bacterial propagation in the air, thus improving its quality and health safety in various closed environments. MgFe2O4 nanoparticles were successfully synthesized using the coprecipitation method. They were evaluated for their antibacterial activity against the reference strain Escherichia coli ATCC 8739, then integrated into Vlieseline matrices using an impregnation–deposition method. The fabrics were used as bacterial air filters through a lab setup for air circulation. The results showed significant reduction in the viable bacterial load that crosses the enlightened MgFe2O4-treated filters compared to the untreated ones, confirming the effectiveness of MgFe2O4 antibacterial activity in the presence of visible light. Consequently, the MgFe2O4-based air filters have significant potential to improve indoor air quality by providing additional protection against bacterial pathogens. The air filters made with MgFe2O4 show a good stability over time (several uses), due to its chemical, thermal, and mechanical robustness, as well as its long-lasting antibacterial and catalytic activity. Another property of MgFe2O4 that makes it easy to recycle is its magnetic property.
Advances in the synthesis and incorporation of MgFe2O4 nanoparticles in conventional air filtration systems would open the way to potential commercialization of these filters in various sectors, including healthcare facilities, industrial environments, and public spaces. It is also important to evaluate MgFe2O4 antimicrobial activity against a broader range of microorganisms, including viruses and fungi. It would also be relevant to study the long-term effect of MgFe2O4 nanoparticles on human health and the microbial ecosystem to assess any potential side effects.
Author Contributions
A.R., C.B.M., M.B. and L.M.: investigation, original draft writing, formal analysis, methodology; A.A., L.M. and N.M.: project administration, validation, supervision; R.B., S.O.T. and L.M.: writing, methodology; M.R., L.M. and A.R.: supervision, validation, reviewing, editing; L.M., M.N.A., A.A. and P.O.: reviewing, editing; M.B, C.B.M. and M.N.A.: data curation, methodology; A.R. and M.N.A.: software, data curation; P.O., M.R., R.B. and M.N.A.: funding, resources; L.M., N.M., A.R. and S.O.T.: conceptualization, writing, validation, project administration, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Researchers Supporting Project number (PNURSP2025R481), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Acknowledgments
The authors are grateful to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R481), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors are grateful to the Algerian direction of research and technology (DGRSDT). MN and PO thank the support from the Employment, Industry, and Tourism Office of the Principality of Asturias (Spain) for the project SEK-25-GRU-GIC-24-010.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lascoumes, P. Les instruments d’action publique, traceurs de changement: L’exemple des transformations de la politique française de lutte contre la pollution atmosphérique (1961–2006). Polit. Soc. 2007, 26, 73–89. [Google Scholar] [CrossRef]
- Voronina, Y.; Lopushynskyi, I.; Grechanyk, B.; Vahonova, O.; Kondur, A.; Akimov, O. Economic and environmental component in the field of sustainable development management. Calitatea 2024, 25, 7–14. [Google Scholar]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Chaitanya, M.V.N.L.; Arora, S.; Pal, R.S.; Ali, H.S.; El Haj, B.M.; Logesh, R. Assessment of environmental pollutants for their toxicological effects on human and animal health. In Organic Micropollutants in Aquatic and Terrestrial Environments; Springer: Cham, Switzerland, 2024; pp. 67–85. [Google Scholar]
- Sharma, G.; Verma, A.; Wang, T.; Naushad, M.; Kumar, A.; Dhiman, P. Remediation of antibiotics using coordination polymers. Coord. Chem. Rev. 2024, 519, 216120. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014; Available online: https://apps.who.int/iris/handle/10665/112642 (accessed on 22 February 2025).
- Halawa, E.M.; Fadel, M.; Al-Rabia, M.W.; Behairy, A.; Nouh, N.A.; Abdo, M.; Abdeen, A. Antibiotic action and resistance: Updated review of mechanisms, spread, influencing factors, and alternative approaches for combating resistance. Front. Pharmacol. 2024, 14, 1305294. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Calheiros, C.S.C.; Villanueva, F.; Alonso-Cuevilla, N.P.; Gabriel, M.F.; Silva, G.V. Indoor air quality: A review of cleaning technologies. Environments 2022, 9, 118. [Google Scholar] [CrossRef]
- Nguyen, M.; Burciaga, B.; Ghosh, N. History of Implementation of Nanotechnology in Novel Air Purifiers with Special Reference to Reduction of Pollen, Mold Spores, and PM2.5 Indices; West Texas A&M University: Canyon, TX, USA, 2024; Available online: https://wtamu-ir.tdl.org/items/0c9854cf-7203-4616-849f-6a9fcf546055 (accessed on 22 February 2025).
- Rodríguez, M.; Seseña, S.; Valiente, N.; Palop, L.; Rodríguez, A. Indoor air quality in elderly care centers: A multidisciplinary approach. Build. Environ. 2024, 262, 111832. [Google Scholar] [CrossRef]
- Donnet, M.; Mensi, M.; Lussi, A. La contamination bactérienne de l’air ambiant lors d’un traitement AIRFLOW®. Swiss Dent. J. SSO 2020, 130, 913–916. [Google Scholar]
- Morawska, L.; Johnson, G.R.; Ristovski, Z.D.; Hargreaves, M.; Mengersen, K.; Corbett, S.; Chao, C.Y.H.; Li, Y.; Katoshevski, D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009, 40, 256–269. [Google Scholar] [CrossRef]
- Gupta, J.K.; Lin, C.-H.; Chen, Q. Characterizing exhaled airflow from breathing and talking. Indoor Air 2010, 20, 31–39. [Google Scholar] [CrossRef]
- Matys, J.; Kensy, J.; Gedrange, T.; Zawiślak, I.; Grzech-Leśniak, K.; Dobrzyński, M. A molecular approach for detecting bacteria and fungi in healthcare environment aerosols: A systematic review. Int. J. Mol. Sci. 2024, 25, 4154. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Air Pollution and Child Health: Prescribing Clean Air: Summary; World Health Organization: Geneva, Switzerland, 2018; WHO/CED/PHE/18.01; Available online: https://iris.who.int/handle/10665/275545 (accessed on 22 February 2025).
- Baranovsky, S. Circulation et Persistance de Pathogènes Nosocomiaux Multirésistants et Hautement Résistants Émergents dans l’environnement Hospitalier: Complexité des Unités de Transmission. Ph.D. Thesis, University of Montpellier, Montpellier, France, 2020. Available online: https://theses.hal.science/tel-03118483 (accessed on 22 February 2025).
- Gattinger, D.; Schlenz, V.; Weil, T.; Sattler, B. From remote to urbanized: Dispersal of antibiotic-resistant bacteria under the aspect of anthropogenic influence. Sci. Total Environ. 2024, 924, 171532. [Google Scholar] [CrossRef] [PubMed]
- Jann, J. Développement d’une Technologie de Surface Bactéricide à Base d’aluminium. Ph.D. Thesis, University of Sherbrooke, Sherbrooke, QC, Canada, 2023. Available online: https://savoirs.usherbrooke.ca/bitstream/11143/20032/18/jann_jessica_PhD_2023.pdf (accessed on 22 February 2025).
- Boudraa, R.; Talantikite-Touati, D.; Souici, A.; Djermoune, A.; Saidani, A.; Fendi, K.; Amrane, A.; Bollinger, J.C.; Tran, H.N.; Hadadi, A.; et al. Optical and photocatalytic properties of TiO2–Bi2O3–CuO supported on natural zeolite for removing Safranin-O dye from water and wastewater. J. Photochem. Photobiol. A Chem. 2023, 443, 114845. [Google Scholar] [CrossRef]
- Cheikh, S.; Imessaoudene, A.; Bollinger, J.-C.; Hadadi, A.; Manseri, A.; Bouzaza, A.; Assadi, A.; Amrane, A.; Zamouche, M.; El Jery, A.; et al. Complete Elimination of the Ciprofloxacin Antibiotic from Water by the Combination of Adsorption–Photocatalysis Process Using Natural Hydroxyapatite and TiO2. Catalysts 2023, 13, 336. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Mechraoui, O.; Aberkane, B.; Benabbas, A.; Manseri, A.; Moussaoui, Y.; Bollinger, J.C.; Amrane, A.; Zoukel, A.; Mouni, L. Synthesis of a TiO2/zeolite composite: Evaluation of adsorption-photodegradation synergy for the removal of Malachite Green. Nano-Struct. Nano-Objects 2024, 38, 101191. [Google Scholar] [CrossRef]
- Boudraa, R.; Talantikite-Touati, D.; Souici, A.; Djermoune, A.; Saidani, A.; Fendi, K.; Amrane, A.; Bollinger, J.C.; Tran, H.N.; Mouni, L. Breaking new grounds: Solid-state synthesis of TiO2–La2O3–CuO nanocomposites for degrading brilliant green dye under visible light. J. Clean. Prod. 2024, 481, 144126. [Google Scholar] [CrossRef]
- Boudraa, R.; Talantikite-Touati, D.; Djermoune, A.; Souici, A.; Kebir, M.; Ait Merzeg, F.; Amrane, A.; Bollinger, J.C.; Mouni, L. Comprehensive characterization and unprecedented photocatalytic efficacy of TiO2-CuO-La2O3 and TiO2-CuO-Bi2O3 nanocomposites: A novel approach to environmental remediation. Mater. Sci. Eng. B 2025, 312, 117863. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F. Impacts of agricultural pesticides on terrestrial ecosystems. In Ecological Impacts of Toxic Chemicals; Sánchez-Bayo, F., van den Brink, P.J., Mann, R.M., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2011; pp. 63–87. [Google Scholar] [CrossRef]
- Arain, M.F. Nanotools for air remediation: An introduction. In Nanotechnology to Monitor, Remedy, and Prevent Pollution; Arain, M.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 125–140. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Fatolahi, L. Evaluation of photocatalysis inactivation in indoor air purification of pathogenic microbes by using the different nanomaterials based on TiO2 nanomaterials. J. Environ. Sci. Health A 2024, 59, 213–222. [Google Scholar] [CrossRef]
- El-Khawaga, A.M.; Ayman, M.; Hafez, O.; Shalaby, R.E. Photocatalytic, Antimicrobial and Antibiofilm Activities of MgFe2O4 Magnetic Nanoparticles. Sci. Rep. 2024, 14, 12877. [Google Scholar] [CrossRef]
- Sajid, S.; Kodape, S.M.; Kurhade, P. A study on bacterial inactivation using green synthesized photocatalytically active TiO2, MgO, TiO2/MgO nanoparticles. J. Indian Chem. Soc. 2024, 101, 101425. [Google Scholar] [CrossRef]
- Beena, V.; Parvathiraja, C.; Paulkumar, K.; Gupta, J.K.; Almutairi, T.M.; Mouni, L. Bio-engineered NiO nanoparticles for photocatalytic and antibacterial treatments. J. Dispers. Sci. Technol. 2025, 1–13. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Manohar, A.; Suvarna, T.; Vattikuti, S.V.P.; Manivasagan, P.; Jang, E.S.; Sudhani, H.P.; Kim, K.H. Structural, morphological, magnetic, electrochemical and biocompatible properties of ZnFe2O4/MgFe2O4/NiFe2O4/CeO2 nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2024, 705, 135667. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Z.; Dong, H.; Guan, X.; Ren, Q.; Lv, X.; Jin, X. Simple combination of oxidants with zero-valent iron (ZVI) achieved very rapid and highly efficient removal of heavy metals from water. Water Res. 2016, 88, 671–680. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.S.H.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S. Removal of Indoor Volatile Organic Compounds via Photocatalytic Oxidation: A Short Review and Prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef]
- Laouici, R. Élaboration des Matériaux Semi-Conducteurs et Étude de Leurs Applications Environnementales. Ph.D. Thesis, University of Jijel, Jijel, Algeria, 2023. [Google Scholar]
- Simon-Deckers, A. Biological Effects of Manufactured Nanoparticles: Influence of Their Characteristics. Ph.D. Thesis, AgroParisTech, Paris, France, 2008. [Google Scholar]
- Salih, S.J.; Mahmood, W.M. Review on magnetic spinel ferrite (MFe2O4) nanoparticles: From synthesis to application. Heliyon 2023, 9, e16601. [Google Scholar] [CrossRef]
- Nie, C.; Qiu, Y.; Pei, T.; Qin, Y. Specific Sources Exert Influence on the Community Structures of Bioaerosols. Aerobiology 2024, 2, 72–84. [Google Scholar] [CrossRef]
- Hart, D. Pathogenic Bacteria in the Air of Operating Rooms: Their Widespread Distribution and the Methods of Control. Arch. Surg. 1938, 37, 521–530. [Google Scholar] [CrossRef]
- Stryjakowska-Sekulska, M.; Piotraszewska-Pajak, A.; Szyszka, A.; Nowicki, M.; Filipiak, M. Microbiological Quality of Indoor Air in University Rooms. Pol. J. Environ. Stud. 2007, 16, 623. [Google Scholar]
- Brągoszewska, E.; Biedroń, I. Indoor Air Quality and Potential Health Risk Impacts of Exposure to Antibiotic-Resistant Bacteria in Office Rooms in Southern Poland. Int. J. Environ. Res. Public Health 2018, 15, 2604. [Google Scholar] [CrossRef] [PubMed]
- Jonidi Jafari, A.; Moslemzadeh, M. The Effect of TiO₂ Nanoparticles on Bacterial Growth: The Effect of Particle Size and Their Structure—A Systematic Review. Int. J. Environ. Health Res. 2024, 34, 697–707. [Google Scholar] [CrossRef]
- Salas-Orozco, M.F.; Lorenzo-Leal, A.C.; de Alba Montero, I.; Marín, N.P.; Santana, M.A.C.; Bach, H. Mechanism of Escape from the Antibacterial Activity of Metal-Based Nanoparticles in Clinically Relevant Bacteria: A Systematic Review. Nanomedicine 2024, 55, 102715. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial Activity of Silver Nanoparticles: A Surface Science Insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Dutta, S.; Erchinger, J.E.; Strieth-Kalthoff, F.; Kleinmans, R.; Glorius, F. Energy Transfer Photocatalysis: Exciting Modes of Reactivity. Chem. Soc. Rev. 2024, 53, 1068–1089. [Google Scholar] [CrossRef]
- Tessema, B.; Gonfa, G.; Hailegiorgis, S.M.; Workneh, G.A.; Tadesse, T.G. Synthesis and Evaluation of the Anti-Bacterial Effect of Modified Silica Gel Supported Silver Nanoparticles on E. coli and S. aureus. Results Chem. 2024, 7, 101471. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-Spectrum Bioactivities of Silver Nanoparticles: The Emerging Trends and Future Prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef]
- Ivask, A.; Juganson, K.; Bondarenko, O.; Mortimer, M.; Aruoja, V.; Kasemets, K.; Blinova, I.; Heinlaan, M.; Slaveykova, V.; Kahru, A. Mechanisms of Toxic Action of Ag, ZnO and CuO Nanoparticles to Selected Ecotoxicological Test Organisms and Mammalian Cells In Vitro: A Comparative Review. Nanotoxicology 2014, 8 (Suppl. S1), 57–71. [Google Scholar] [CrossRef] [PubMed]
- Bortolassi, A.C.C.; Nagarajan, S.; de Araújo Lima, B.; Guerra, V.G.; Aguiar, M.L.; Huon, V.; Soussan, L.; Cornu, D.; Miele, P.; Bechelany, M. Efficient Nanoparticles Removal and Bactericidal Action of Electrospun Nanofibers Membranes for Air Filtration. Mater. Sci. Eng. C 2019, 102, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Fayez, K.A.; El-Deeb, B.A.; Mostafa, N.Y. Toxicity of Biosynthetic Silver Nanoparticles on the Growth, Cell Ultrastructure and Physiological Activities of Barley Plant. Acta Physiol. Plant. 2017, 39, 155. [Google Scholar] [CrossRef]
- Xiu, Z.M.; Zhang, Q.B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Da Costa, D. Développement D’une Thérapie Combinatoire à Base D’agents Anti-Biofilms et de Particules D’acide Poly-Lactique Pour la Délivrance D’antibiotiques Contre des Biofilms Bactériens. Ph.D. Thesis, University of Lyon, Lyon, France, 2020. Available online: https://theses.hal.science/tel-03612040 (accessed on 22 February 2025).
- Jomini, S. Effets des Nanoparticules de Dioxyde de Titane Sur Les Bactéries: De la Cellule à la Communauté. Ph.D. Thesis, University of Lorraine, Nancy, France, 2014. Available online: https://hal.univ-lorraine.fr/tel-01750816 (accessed on 22 February 2025).
- Uddin, M.J.; Jeong, Y.K. Application of Magnesium Ferrite Nanomaterials for Adsorptive Removal of Arsenic from Water: Effects of Mg and Fe Ratio. Chemosphere 2022, 307, 135817. [Google Scholar] [CrossRef]
- Manohar, A.; Vijayakanth, V.; Reddy Pallavolu, M.; Hyeon Kim, K. Effects of Ni-Substitution on Structural, Magnetic Hyperthermia, Photocatalytic, and Cytotoxicity Study of MgFe2O4 Nanoparticles. J. Alloys Compd. 2021, 868, 160515. [Google Scholar] [CrossRef]
- Ilhan, S.; Izotova, S.G.; Komlev, A.A. Synthesis and Characterization of MgFe2O4 Nanoparticles Prepared by Hydrothermal Decomposition of Co-Precipitated Magnesium and Iron Hydroxides. Ceram. Int. 2015, 41, 577–585. [Google Scholar] [CrossRef]
- Phenrat, T.; Saleh, N.; Sirk, K.; Tilton, R.D.; Lowry, G.V. Aggregation and Sedimentation of Aqueous Nanoscale Zerovalent Iron Dispersions. Environ. Sci. Technol. 2007, 41, 284–290. [Google Scholar] [CrossRef]
- de Hoyos-Sifuentes, D.H.; Reséndiz-Hernández, P.J.; Díaz-Guillén, J.A.; Ochoa-Palacios, R.M.; Altamirano-Guerrero, G. Synthesis and Characterization of MgFe2O4 Nanoparticles and PEG-Coated MgFe2O4 Nanocomposite. J. Mater. Res. Technol. 2022, 18, 3130–3142. [Google Scholar] [CrossRef]
- Uma, S.; Vignesh, D.; Shobana, M.K. Synthesis of heterostructured nanocomposite MgFe2O4/MoO3 for H2S sensing: Experimental and theoretical approach by DFT. Sens. Actuators B Chem. 2025, 425, 136950. [Google Scholar] [CrossRef]
- Padhan, A.M.; Nayak, S.; Sahu, M.; Jagličić, Z.; Koželj, P.; Kim, H.J. Cationic redistribution induced magnetic properties of Zn²⁺ substituted MgFe₂O₄ spinel ferrite. Phys. B Condens. Matter 2023, 668, 415245. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Zhao, Q.; Qu, Z.; Yuan, D.; Liu, S.; Hu, X.; Chen, G. Synthesis, characterization and adsorptive performance of MgFe2O4 nanospheres for SO2 removal. J. Hazard. Mater. 2010, 184, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Taha, T.A.; Fayed, M.G.; Mohamed, S.G. Enhanced electrochemical performance of MgFe2O4/SrTiO3 and MgFe2O4/SiO2 nanocomposite structures. J. Alloys Compd. 2022, 925, 166660. [Google Scholar] [CrossRef]
- Kaur Ubhi, M.; Kaur, M.; Singh, D.; Javed, M.; Oliveira, A.C.; Kumar Garg, V.; Sharma, V.K. Hierarchical Nanoflowers of MgFe2O4, Bentonite and B-,P- Co-Doped Graphene Oxide as Adsorbent and Photocatalyst: Optimization of Parameters by Box–Behnken Methodology. Int. J. Mol. Sci. 2022, 23, 9678. [Google Scholar] [CrossRef]
- Heiba, Z.K.; Sanad, M.M.S.; Mohamed, M.B. Influence of Mg-deficiency on the functional properties of magnesium ferrite anode material. Solid State Ion. 2019, 341, 115042. [Google Scholar] [CrossRef]
- Yan, Z.; Gao, J.; Li, Y.; Zhang, M.; Guo, M. Hydrothermal synthesis and structure evolution of metal-doped magnesium ferrite from saprolite laterite. RSC Adv. 2015, 5, 92778–92787. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, Y.J.; Kim, J.H.; Jang, J.W.; Choi, S.H.; Lee, J.S. Defective ZnFe2O4 nanorods with oxygen vacancy for photoelectrochemical water splitting. Nanoscale 2015, 7, 19144–19151. [Google Scholar] [CrossRef]
- Soukeur, A.; Kaci, M.; Omeiri, S.; Bellal, B.; Amara, M.; Trari, M. Photocatalytic degradation of bromothymol blue over MgFe2O4 under sunlight exposure. Opt. Mater. 2023, 142, 114108. [Google Scholar] [CrossRef]
- Rajadurai, L.; Dash, C.S.; Revathi, S.; Dhiwahar, A.T.; Sundararajan, M.; Ravisankar, P.; Alagarasan, J.K.; Mohandoss, S.; Sambasivam, R. Photocatalytic Degradation of Tetracycline Hydrochloride Using Pure and Copper-Doped Magnesium Ferrite Nanoparticles: Efficiency, Kinetics, and Mechanism. Inorg. Chem. Commun. 2024, 162, 112197. [Google Scholar] [CrossRef]
- Salama, R.S.; Gouda, M.S.; Aboud, M.F.A.; Alshorifi, F.T.; El-Hallag, A.A.; Badawi, A.K. Synthesis and Characterization of Magnesium Ferrite-Activated Carbon Composites Derived from Orange Peels for Enhanced Supercapacitor Performance. Sci. Rep. 2024, 14, 8223. [Google Scholar] [CrossRef]
- Dadi, R. Synthèse de Nanoparticules D’oxydes Métalliques et Leur Activité Antibactérienne. Ph.D. Thesis, Université Paris-Nord-Paris XIII, Villetaneuse, France, 2019. Available online: https://theses.hal.science/tel-03119316 (accessed on 22 February 2025).
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Kim, D.; Lee, H.; Kim, Y.; Lee, Y.; Kim, M.; Kim, H.; Lee, H. Biodegradable Nanofiber/Metal–Organic Framework/Cotton Air Filtration Membranes Enabling Simultaneous Removal of Toxic Gases and Particulate Matter. Polymers 2023, 15, 3965. [Google Scholar] [CrossRef] [PubMed]
- Lagashetty, A.; Pattar, A.; Ganiger, S.K. Synthesis, Characterization and Antibacterial Study of Ag-Doped Magnesium Ferrite Nanocomposite. Heliyon 2019, 5, e01760. [Google Scholar] [CrossRef]
- Ehi-Eromosele, C.O.; Olugbuyiro, J.A.O.; Taiwo, O.S.; Bamgboye, O.A.; Ango, C.E. Synthesis and Evaluation of the Antimicrobial Potentials of Cobalt-Doped and Magnesium Ferrite Spinel Nanoparticles. Bull. Chem. Soc. Ethiop. 2018, 32, 451–458. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Airborne Bioaerosols and Their Impact on Human Health. J. Environ. Sci. 2018, 67, 23–35. [Google Scholar] [CrossRef]
- Bonfim, D.P.; Cruz, F.G.; Guerra, V.G.; Aguiar, M.L. Development of Filter Media by Electrospinning for Air Filtration of Nanoparticles from PET Bottles. Membranes 2021, 11, 293. [Google Scholar] [CrossRef]
- De Riccardis, M.F. Electrospun Nanofibrous Membranes for Air Filtration: A Critical Review. Compounds 2023, 3, 390–410. [Google Scholar] [CrossRef]
- Vilar, M.R.; Ferraria, A.M.; do Rego, A.M.B.; Boufi, S. Cellulose: De Nouvelles Perspectives pour un Matériau Millénaire. L’Actualité Chim. 2015, 393–394, 102. [Google Scholar]
- Zaich, Y.; Lourghi, A.; Rouibah, K.E.; Akika, F.Z. Adsorption et Photo-Dégradation sous Irradiation Solaire de Quelques Polluants Organiques Contenus dans les Effluents Liquides. Ph.D. Thesis, Université de Jijel, Jijel, Algeria, 2022. [Google Scholar]
- Rossano, M. Utilisation des Nanoparticules de Dioxyde de Titane dans les Émulsions Cosmétiques: Impact sur la Santé Humaine et l’Environnement. Ph.D. Thesis, Université Le Havre, Le Havre, France, 2014. Available online: https://theses.fr/2014LEHA0003 (accessed on 22 February 2025).
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Zhao, Y. Safety of Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef]
- Mao, N.; Russell, S.J.; Pourdeyhimi, B. Characterisation, Testing, and Modelling of Nonwoven Fabrics. In Handbook of Nonwovens; Woodhead Publishing: Sawston, UK, 2022; pp. 509–626. [Google Scholar] [CrossRef]
- Brugger, S.D.; Baumberger, C.; Jost, M.; Jenni, W.; Brugger, U.; Mühlemann, K. Automated Counting of Bacterial Colony Forming Units on Agar Plates. PLoS ONE 2012, 7, e33695. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).