Recent Advances in the Engineering of Cytochrome P450 Enzymes
Abstract
1. Introduction
2. Engineering Approaches for Cytochrome P450 Enzymes
2.1. Rational Design
2.2. Semi-Rational Design
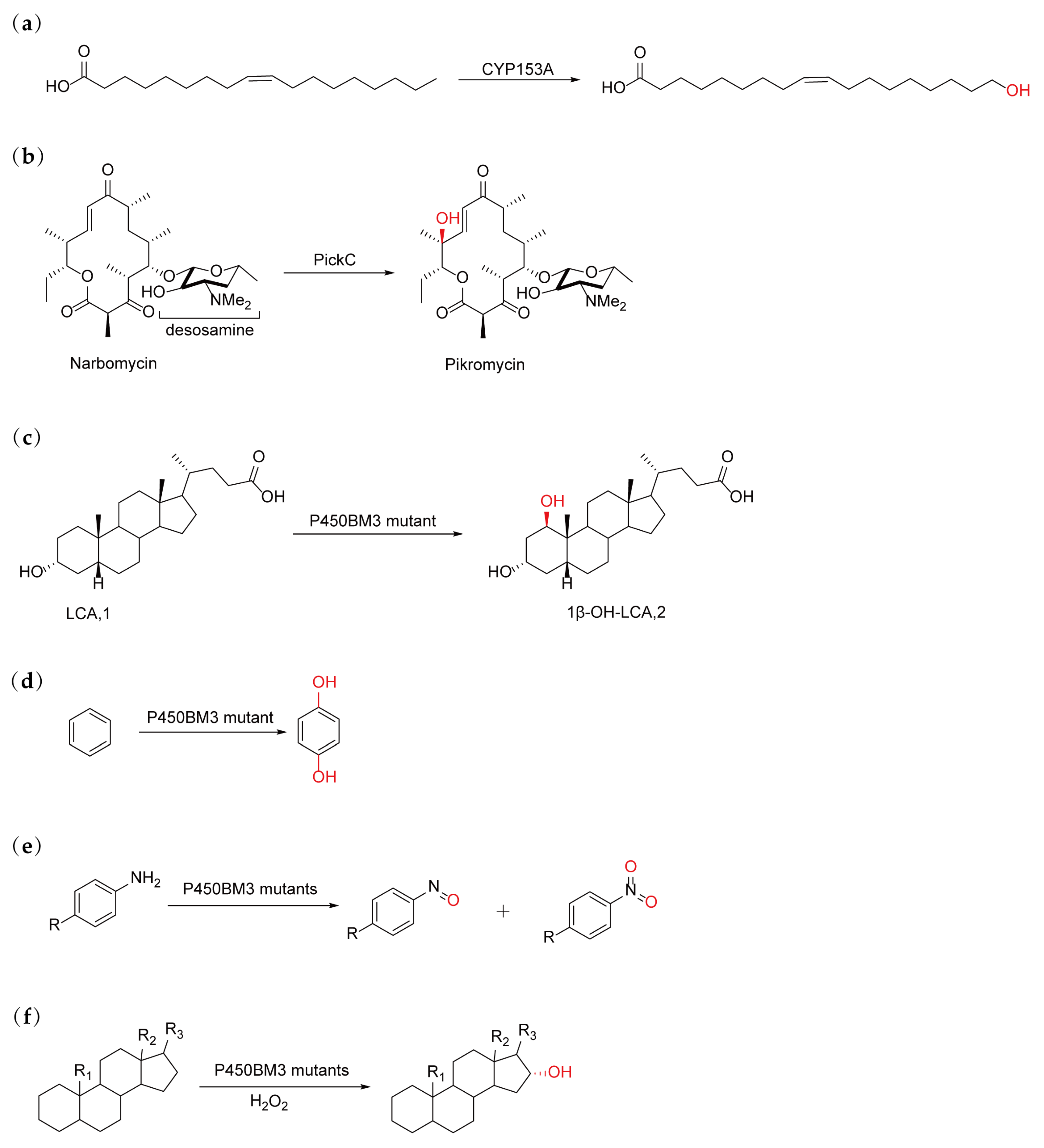
2.3. Directed Evolution
| P450 | Origin | Mutant | Substrate | Function | Application | Reference |
|---|---|---|---|---|---|---|
| P450BM3 | Priestia megaterium | E267A/T268V | 2,4,6-Triisopropylbenzenesulfonyl azide | C−H amination | Biocatalysis | [17] |
| GcoA | Amycolatopsis sp. ATCC 39116 | T296S | Guaiacol | Oxidative demethylation | Biocatalysis | [18] |
| CYP154C2. | Streptomyces avermitilis | L188F/M191F | Testosterone | 2α-hydroxylation | Biocatalysis | [19] |
| CYP DoxA | Streptomyces peucetius subsp. caesius | P88Y | 13-Deoxydaunorubicin | Hydroxylation at the C-14 position of daunorubicin | Pharmaceutical synthesis | [20] |
| CYP105AS1 | Amycolatopsis orientalis | Q127R/A265N | Compactin | Hydroxylation of compactin | Pharmaceutical synthesis | [21] |
| CYP102A1 | Priestia megaterium | L75I/A264G/A328V | Omeprazole | Hydroxylation | Pharmaceutical synthesis | [22] |
| P450 CYP153A/Pdx/Pdr | Pseudomonas putida | G307A/S120R/P165N/S453N | Oleic acid | Hydroxylation | Pharmaceutical synthesis | [25] |
| PikCH238pAcF | Streptomyces venezuelae | H238pAcF | Narbomycin | Hydroxylation | Biocatalysis | [27] |
| P450BM3 (CYP102A1) | Bacillus megaterium | G87A/W72T/A74L/ L181M | Lithocholic acid | Hydroxylation | Biocatalysis | [28] |
| P450BM3 | Priestia megaterium | A82F/A328F | Benzene | Bioconversion of benzene to hydroquinone | Chemical production | [29] |
| P450BM3 (CYP102A1) | Priestia megaterium NBRC 15308 = ATCC 14581 | F87L/V78S/A184V A82T/I263L | p-Substituted anilines | N-oxidation | Biocatalysis | [33] |
| CYP154C5 | Nocardia farcinica IFM 10152 | F92A/R114A/E282A/T248D | Steroids | Hydroxylation | Biocatalysis and pharmaceutical synthesis | [34] |
| P450LA1 | Labrenzia aggregata | T121A/N201K/N209S/Y385H/E418G/A103L/M118L/R120H/V123I/I326V/V327M/H385V/M391L | Styrene | Anti-Markovnikov oxidation | Chemical production | [38] |
| P411 (P450BM3) | Bacillus megaterium | A82L, A78V F263L, E267D | Phenethyl | Amination | Biocatalysis | [39] |
| P411-CIS | Bacillus megaterium | CIS/HF | 1-Methylindole | Carbene transfer to heterocycles and cyclic alkenes | Pharmaceutical synthesis | [40] |
| P411 | Bacillus megaterium | T327V/E70T/L177M/R226T/Y330V/L401P | N-phenylpyrrolidine | C(sp3)−H fluoroalkylation | Pharmaceutical synthesis | [41] |
| P411- FA-E3 | Bacillus megaterium | FA-E3 | N-phenyl morpholine | Cyanomethylation | Chemical production | [42] |
| P450BM3 | Bacillus megaterium | G252E | Siloxane | Cleavage of silicon–carbon (Si–C) bonds | Biocatalysis | [43] |
3. Challenges and Future Directions for the Engineering of Cytochrome P450 Enzymes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CYPs | Cytochrome P450 enzymes |
References
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiotics 2021, 11, 94–114. [Google Scholar] [CrossRef]
- Annaval, T.; Teijaro, C.N.; Adhikari, A.; Yan, X.H.; Chen, J.J.; Crnovcic, I.; Yang, D.; Shen, B. Cytochrome P450 Hydroxylase TnmL Catalyzing Sequential Hydroxylation with an Additional Proofreading Activity in Tiancimycin Biosynthesis. ACS Chem. Biol. 2021, 16, 1172–1178. [Google Scholar] [CrossRef]
- Zheng, Z.; Clardy, J.; Liu, H.W. Biosynthesis of the Unusual Epoxy Isonitrile-Containing Antibiotics Aerocyanidin and Amycomicin. J. Am. Chem. Soc. 2024, 146, 21061–21068. [Google Scholar] [CrossRef]
- Matthews, S.; Belcher, J.D.; Tee, K.L.; Girvan, H.M.; McLean, K.J.; Rigby, S.E.J.; Levy, C.W.; Leys, D.; Parker, D.A.; Blankley, R.T.; et al. Catalytic Determinants of Alkene Production by the Cytochrome P450 Peroxygenase OleT (JE). J. Biol. Chem. 2017, 292, 5128–5143. [Google Scholar] [CrossRef]
- Lia, H.; Lib, W.; Songb, K.; Liuc, Y.; Zhaob, G.; Dua, Y.L. Nitric oxide synthase-guided genome mining identifies a cytochrome P450 enzyme for olefin nitration in bacterial specialized metabolism. Synth. Syst. Biotechnol. 2024, 9, 127–133. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Tateishi, Y.; McCarty, K.D. C-C bond cleavage reactions catalyzed by cytochrome P450 enzymes. Med. Chem. Res. 2023, 32, 1263–1277. [Google Scholar] [CrossRef]
- Hasemann, C.A.; Kurumbail, R.G.; Boddupalli, S.S.; Peterson, J.A.; Deisenhofer, J. Structure and function of cytochromes P450: A comparative analysis of three crystal structures. Structure 1995, 3, 41–62. [Google Scholar] [CrossRef]
- Guengerich, F.P. Mechanisms of Cytochrome P450-Catalyzed Oxidations. ACS Catal. 2018, 8, 10964–10976. [Google Scholar] [CrossRef]
- Fang, Y.P.; Tai, Z.; Hu, K.; Luo, L.F.; Yang, S.W.; Liu, M.M.; Xie, X. Comprehensive Review on Plant Cytochrome P450 Evolution: Copy Number, Diversity, and Motif Analysis from Chlorophyta to Dicotyledoneae. Genome Biol. Evol. 2024, 11, 11. [Google Scholar] [CrossRef]
- Singh, A.; Panwar, R.; Mittal, P.; Hassan, M.I.; Singh, I.K. Plant cytochrome P450s: Role in stress tolerance and potential applications for human welfare. Int. J. Biol. Macromol. 2021, 184, 874–886. [Google Scholar] [CrossRef]
- Greule, A.; Stok, J.E.; Voss, J.J.D.; Cryle, M.J. Unrivalled diversity: The many roles and reactions of bacterial cytochromes P450 in secondary metabolism. Nat. Prod. Rep. 2018, 35, 757–791. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Z.; Ma, J.S.; Li, M.; Zhang, Y.T.; Jiang, B.X.; Zhao, X.L.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef] [PubMed]
- Omura, T. Contribution of cytochrome P450 to the diversification of eukaryotic organisms. Biotechnol. Appl. Biochem. 2013, 60, 4–8. [Google Scholar] [CrossRef]
- Kamel, A.; Harriman, S. Inhibition of cytochrome P450 enzymes and biochemical aspects of mechanism-based inactivation (MBI). Drug Discov. Today Technol. 2013, 10, e177–e189. [Google Scholar] [CrossRef] [PubMed]
- Brindha, J.; Balamurali, M.M.; Chanda, K. Evolutionary approaches in protein engineering towards biomaterial construction. RSC Adv. 2019, 9, 34720–34734. [Google Scholar]
- Korendovych, I.V. Rational and Semirational Protein Design. Methods Mol. Biol. 2018, 1685, 15–23. [Google Scholar]
- Steck, V.; Kolev, J.N.; Ren, X.; Fasan, R. Mechanism-Guided Design and Discovery of Efficient Cytochrome P450-Derived C-H Amination Biocatalysts. J. Am. Chem. Soc. 2020, 142, 10343–10357. [Google Scholar] [CrossRef]
- Ellis, E.S.; Hinchen, D.J.; Bleem, A.; Bu, L.; Mallinson, S.J.B.; Allen, M.D.; Streit, B.R.; Machovina, M.M.; Doolin, Q.V.; Michener, W.E.; et al. Engineering a Cytochrome P450 for Demethylation of Lignin-Derived Aromatic Aldehydes. JACS Au 2021, 1, 252–261. [Google Scholar] [CrossRef]
- Gao, Q.L.; Ma, B.B.; Wang, Q.W.; Zhang, H.; Fushinobu, S.; Yang, J.; Lin, S.S.; Sun, K.K.; Han, B.N.; Xu, L.H. Improved 2alpha-Hydroxylation Efficiency of Steroids by CYP154C2 Using Structure-Guided Rational Design. Appl. Environ. Microbiol. 2023, 89, e0218622. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, L.X.; Chen, W.; Zhong, J.J.; Qian, C.; Zhou, W.W. Rational Design of Daunorubicin C-14 Hydroxylase Based on the Understanding of Its Substrate-Binding Mechanism. Int. J. Mol. Sci. 2023, 24, 8337. [Google Scholar] [CrossRef]
- Ashworth, M.A.; Bombino, E.; Wijma, H.J.; Janssen, D.B.; McLean, K.J.; Munro, A.W. Computation-Aided Engineering of Cytochrome P450 for the Production of Pravastatin. ACS Catal. 2022, 12, 13259–13270. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Sun, Y.; Osawa, Y.; Chen, Y.E.; Zhang, H. Computational redesign of cytochrome P450 CYP102A1 for highly stereoselective omeprazole hydroxylation by UniDesign. J. Biol. Chem. 2023, 299, 105050. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tao, C.M.; Shen, X.L.; Sun, X.X.; Wang, J.; Yuan, Q.P. Unlocking the potential of enzyme engineering via rational computational design strategies. Biotechnol. Adv. 2024, 73, 108376. [Google Scholar] [CrossRef] [PubMed]
- Chica, R.A.; Doucet, N.; Pelletier, J.N. Semi-rational approaches to engineering enzyme activity: Combining the benefits of directed evolution and rational design. Curr. Opin. Biotechnol. 2005, 16, 378–384. [Google Scholar] [CrossRef]
- Duan, Y.; Ba, L.; Gao, J.W.; Gao, X.X.; Zhu, D.M.; René, M.D.J.; Mink, D.; Kaluzna, I.; Lin, Z.L. Semi-rational engineering of cytochrome CYP153A from Marinobacter aquaeolei for improved omega-hydroxylation activity towards oleic acid. Appl. Microbiol. Biotechnol. 2016, 100, 8779–8788. [Google Scholar] [CrossRef]
- Liu, C.C.; Schultz, P.G. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 2010, 79, 413–444. [Google Scholar] [CrossRef]
- Pan, Y.J.; Li, G.B.; Liu, R.X.; Guo, J.W.; Liu, Y.J.; Liu, M.Y.; Zhang, X.W.; Chi, L.P.; Xu, K.W.; Wu, R.; et al. Unnatural activities and mechanistic insights of cytochrome P450 PikC gained from site-specific mutagenesis by non-canonical amino acids. Nat. Commun. 2023, 14, 12508. [Google Scholar] [CrossRef]
- Li, H.Y.; Dai, W.; Qin, S.S.; Li, S.; Yu, Y.D.; Zhang, L. Regio-and stereo-selective 1beta-hydroxylation of lithocholic acid by cytochrome P450 BM3 mutants. Biotechnol. Bioeng. 2023, 120, 2230–2241. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Wang, B.J.; Wang, F.; Yu, X.J.; Ma, L.X.; Li, A.; Reetz, M.T. Chemo-and Regioselective Dihydroxylation of Benzene to Hydroquinone Enabled by Engineered Cytochrome P450 Monooxygenase. Angew. Chem. Int. Ed. 2019, 58, 764–768. [Google Scholar] [CrossRef]
- Roiban, G.D.; Agudo, R.; Reetz, M.T. Cytochrome P450 catalyzed oxidative hydroxylation of achiral organic compounds with simultaneous creation of two chirality centers in a single C-H activation step. Angew. Chem. Int. Ed. 2014, 53, 8659–8663. [Google Scholar] [CrossRef]
- Agudo, R.; Roiban, G.D.; Reetz, M.T. Achieving Regio-and Enantioselectivity of P450-Catalyzed Oxidative CH Activation of Small Functionalized Molecules by Structure-Guided Directed Evolution. Chembiochem 2012, 13, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.D.A.; Dhoke, G.V.; Davari, M.D.; Ruff, A.J.; Schwaneberg, U. Directed Evolution of P450 BM3 towards Functionalization of Aromatic O-Heterocycles. Int. J. Mol. Sci. 2019, 20, 3353. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, F.Q.; Jiang, Y.P.; Qin, X.Q.; Xian, M.; Feng, Y.G.; Cong, Z.Q. Diverse N-Oxidation of Primary Aromatic Amines Controlled by Engineered P450 Peroxizyme Variants Facilitated by Dual-Functional Small Molecule. Adv. Sci. 2025, 12, e2412100. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Qin, M.; Wang, Q.; Jiang, Y.P.; Cong, Z.Q. Semirationally Engineering an Efficient P450 Peroxygenase for Regio-and Enantioselective Hydroxylation of Steroids. ACS Catal. 2025, 15, 2977–2986. [Google Scholar] [CrossRef]
- Farinas, E.T.; Bulter, T.; Arnold, F.H. Directed enzyme evolution. Curr. Opin. Biotech. 2001, 12, 545–551. [Google Scholar] [CrossRef]
- Arnold, F.H. Directed Evolution: Bringing New Chemistry to Life. Angew. Chem. Int. Ed. 2018, 57, 4143–4148. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the third wave of biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef]
- Hammer, S.C.; Kubik, G.; Watkins, E.; Huang, S.; Minges, H.; Arnold, F.H. Anti-Markovnikov alkene oxidation by metal-oxo-mediated enzyme catalysis. Science 2017, 358, 215–218. [Google Scholar] [CrossRef]
- Prier, C.K.; Zhang, R.J.K.; Buller, A.R.; Brinkmann-Chen, S.; Arnold, F.H. Enantioselective, intermolecular benzylic C-H amination catalysed by an engineered iron-haem enzyme. Nat. Chem. 2017, 9, 629–634. [Google Scholar] [CrossRef]
- Brandenberg, O.F.; Chen, K.; Arnold, F.H. Directed Evolution of a Cytochrome P450 Carbene Transferase for Selective Functionalization of Cyclic Compounds. J. Am. Chem. Soc. 2019, 141, 8989–8995. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Zhang, R.K.; Arnold, F.H. Enantiodivergent alpha-Amino C-H Fluoroalkylation Catalyzed by Engineered Cytochrome P450s. J. Am. Chem. Soc. 2019, 141, 9798–9802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Maggiolo, A.O.; Alfonzo, E.; Mao, R.; Porter, N.J.; Abney, N.; Arnold, F.H. Chemodivergent C(sp3)-H and C(sp2)-H cyanomethylation using engineered carbene transferases. Nat. Catal. 2023, 6, 152–160. [Google Scholar] [CrossRef]
- Sarai, N.S.; Fulton, T.J.; Meara, L.R.O.; Johnston, K.E.; Brinkmann-Chen, S.; Maar, R.R.; Tecklenburg, R.E.; Roberts, J.M.; Reddel, J.C.T.; Arnold, F.H. Directed evolution of enzymatic silicon-carbon bond cleavage in siloxanes. Science 2024, 383, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Polic, V.; Auclair, K. Controlling substrate specificity and product regio-and stereo-selectivities of P450 enzymes without mutagenesis. Bioorganic Med. Chem. 2014, 22, 5547–5554. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, P.; Li, S. Functional expression and regulation of eukaryotic cytochrome P450 enzymes in surrogate microbial cell factories. Eng. Microbiol. 2022, 2, 100011. [Google Scholar] [CrossRef]
- Pang, C.; Zhang, G.; Liu, S.; Zhou, J.W.; Li, J.H.; Du, G.C. Engineering sigma factors and chaperones for enhanced heterologous lipoxygenase production in Escherichia coli. Biotechnol. Biofuels Bioprod. 2022, 15, 105. [Google Scholar] [CrossRef]
- Wichai, T.; Sooksai, S.; Noitang, S.; Vangnai, A.S.; Kotchaplai, P. Pylb-based overexpression of cytochrome P450 in Bacillus subtilis 168. Enzym. Microb. Technol. 2025, 185, 110587. [Google Scholar] [CrossRef]
- Chun, Y.J.; Shimada, T.; Sanchez-Ponce, R.; Martin, M.V.; Lei, L.; Zhao, B.; Kelly, S.L.; Waterman, M.R.; Lamb, D.C.; Guengerichet, F.P. Electron transport pathway for a Streptomyces cytochrome P450: Cytochrome P450 105D5-catalyzed fatty acid hydroxylation in Streptomyces coelicolor A3(2). Biol. Chem. 2007, 282, 17486. [Google Scholar] [CrossRef]
- Hou, Q.; Rooman, M.; Pucci, F. Enzyme Stability-Activity Trade-Off: New Insights from Protein Stability Weaknesses and Evolutionary Conservation. J. Chem. Theory Comput. 2023, 19, 3664–3671. [Google Scholar] [CrossRef]
- Miller, S.R. An appraisal of the enzyme stability-activity trade-off. Evolution 2017, 71, 1876–1887. [Google Scholar] [CrossRef]
- Behrendorff, J.B.; Gillam, E.M. Prospects for Applying Synthetic Biology to Toxicology: Future Opportunities and Current Limitations for the Repurposing of Cytochrome P450 Systems. Chem. Res. Toxicol. 2017, 30, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Krishna, S.H. Developments and trends in enzyme catalysis in nonconventional media. Biotechnol. Adv. 2002, 20, 239–267. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayanan, G.; Haapala, M.; Kiiski, I.; Sikanen, T. Digital microfluidic immobilized cytochrome P450 reactors with integrated inkjet-printed microheaters for droplet-based drug metabolism research. Anal. Bioanal. Chem. 2018, 410, 6677–6687. [Google Scholar] [CrossRef]
- Reinen, J.; Ferman, S.; Vottero, E.; Vermeulen, N.P.; Commandeur, J.N. Application of a fluorescence-based continuous-flow bioassay to screen for diversity of cytochrome P450 BM3 mutant libraries. J. Biomol. Screen. 2011, 16, 239–250. [Google Scholar] [CrossRef]
- Vanella, R.; Kovacevic, G.; Doffini, V.; Fernandez, D.S.J.; Nash, M.A. High-throughput screening, next generation sequencing and machine learning: Advanced methods in enzyme engineering. Chem. Commun. 2022, 58, 2455–2467. [Google Scholar] [CrossRef]
- Liu, Y.H.; Li, Z.Y.; Cao, C.Q.; Zhang, X.H.; Meng, S.Q.; Davari, M.D.; Xu, H.J.; Ji, Y.; Schwaneberg, U.; Liu, L. Engineering of Substrate Tunnel of P450 CYP116B3 though Machine Learning. Catalysts 2023, 13, 1228. [Google Scholar] [CrossRef]
- Hu, X.M.; Hou, Y.Y.; Teng, X.R.; Liu, Y.; Li, Y.; Li, W.; Li, Y.; Ai, C.Z. Prediction of cytochrome P450-mediated bioactivation using machine learning models and in vitro validation. Arch. Toxicol. 2024, 98, 1457–1467. [Google Scholar] [CrossRef]
- Davis, A.M.; Plowright, A.T.; Valeur, E. Directing evolution: The next revolution in drug discovery? Nat. Rev. Drug Discov. 2017, 16, 681–698. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, M. Navigating the landscape of enzyme design: From molecular simulations to machine learning. Chem. Soc. Rev. 2024, 53, 8202–8239. [Google Scholar] [CrossRef]
- Munro, A.W.; Girvan, H.M.; McLean, K.J. Cytochrome P450--redox partner fusion enzymes. Biochim. Biophys. Acta 2007, 1770, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Yan, J.Y.; Cao, S.; Bai, F.; Yang, Y.; Huang, S.H.; Yao, L.S.; Anzai, Y.; Kato, F.; et al. New Reactions and Products Resulting from Alternative Interactions between the P450 Enzyme and Redox Partners. J. Am. Chem. Soc. 2014, 136, 3640–3646. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Dong, H.D.; Zhao, X.M.; Wang, X.X.; Liu, Z.Q.; Zheng, Y.G. Functional Expression and Construction of a Self-Sufficient Cytochrome P450 Chimera for Efficient Steroidal C14α Hydroxylation in Escherichia coli. Biotechnol. Bioeng. 2025, 122, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Beyer, N.; Kulig, J.K.; Bartsch, A.; Hayes, M.A.; Janssen, D.B.; Fraaije, M.W. P450(BM3) fused to phosphite dehydrogenase allows phosphite-driven selective oxidations. Appl. Microbiol. Biotechnol. 2017, 101, 2319–2331. [Google Scholar] [CrossRef]
- Kokorin, A.; Parshin, P.D.; Bakkes, P.J.; Pometun, A.A.; Tishkov, V.I.; Urlacher, V.B. Genetic fusion of P450 BM3 and formate dehydrogenase towards self-sufficient biocatalysts with enhanced activity. Sci. Rep. 2021, 11, 21706. [Google Scholar] [CrossRef]
- Fujioka, K.; Casida, J.E. Glutathione S-transferase conjugation of organophosphorus pesticides yields S-phospho-, S-aryl-, and S-alkylglutathione derivatives. Chem. Res. Toxicol. 2007, 20, 1211–1217. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Chen, X. Recent Advances in the Engineering of Cytochrome P450 Enzymes. Catalysts 2025, 15, 374. https://doi.org/10.3390/catal15040374
Liu C, Chen X. Recent Advances in the Engineering of Cytochrome P450 Enzymes. Catalysts. 2025; 15(4):374. https://doi.org/10.3390/catal15040374
Chicago/Turabian StyleLiu, Chang, and Xi Chen. 2025. "Recent Advances in the Engineering of Cytochrome P450 Enzymes" Catalysts 15, no. 4: 374. https://doi.org/10.3390/catal15040374
APA StyleLiu, C., & Chen, X. (2025). Recent Advances in the Engineering of Cytochrome P450 Enzymes. Catalysts, 15(4), 374. https://doi.org/10.3390/catal15040374






