Occurrence, Ecotoxicity, and Photocatalytic Remediation of Antiretroviral Drugs in Global Surface Water Matrices
Abstract
1. Introduction
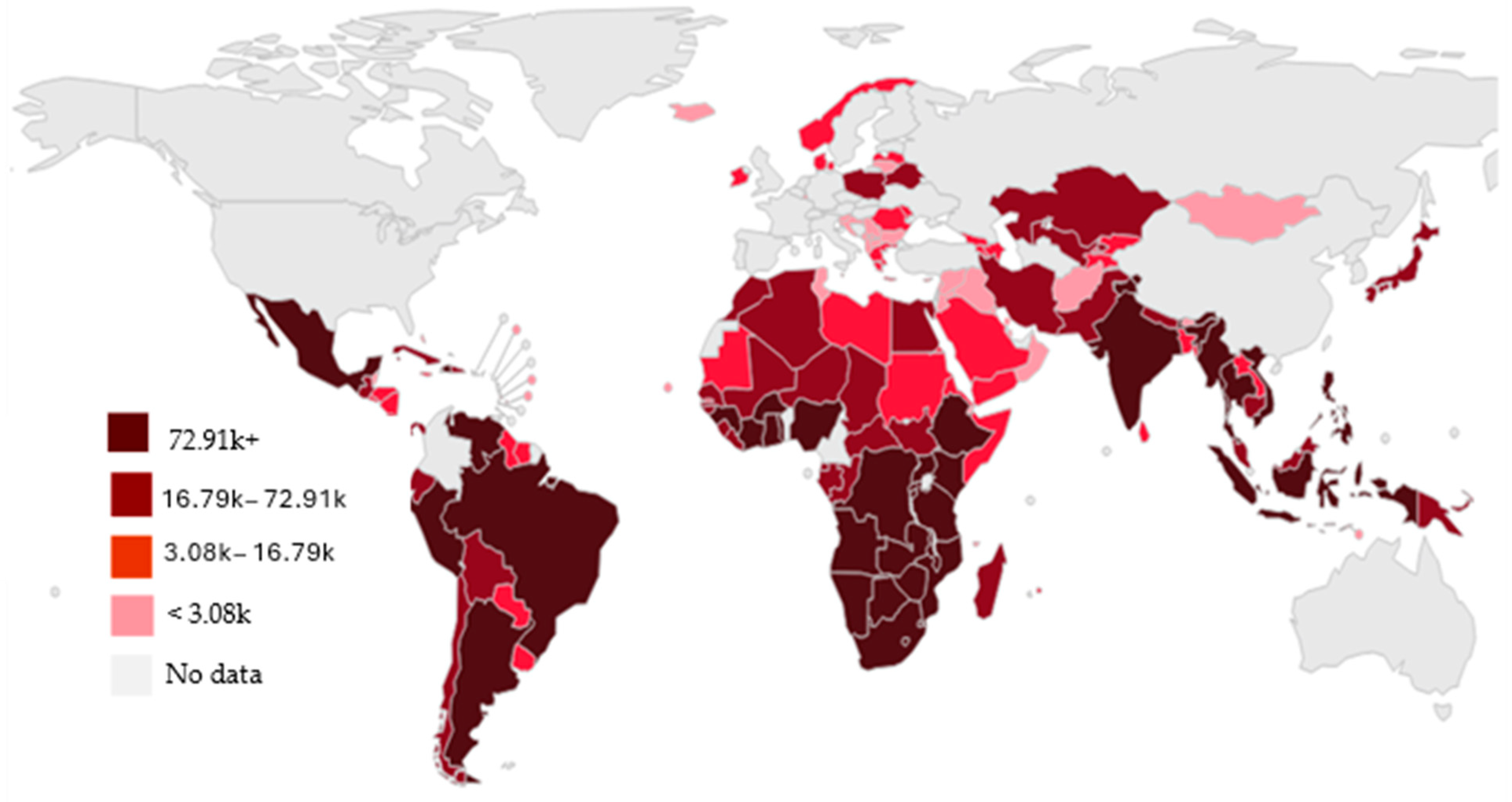
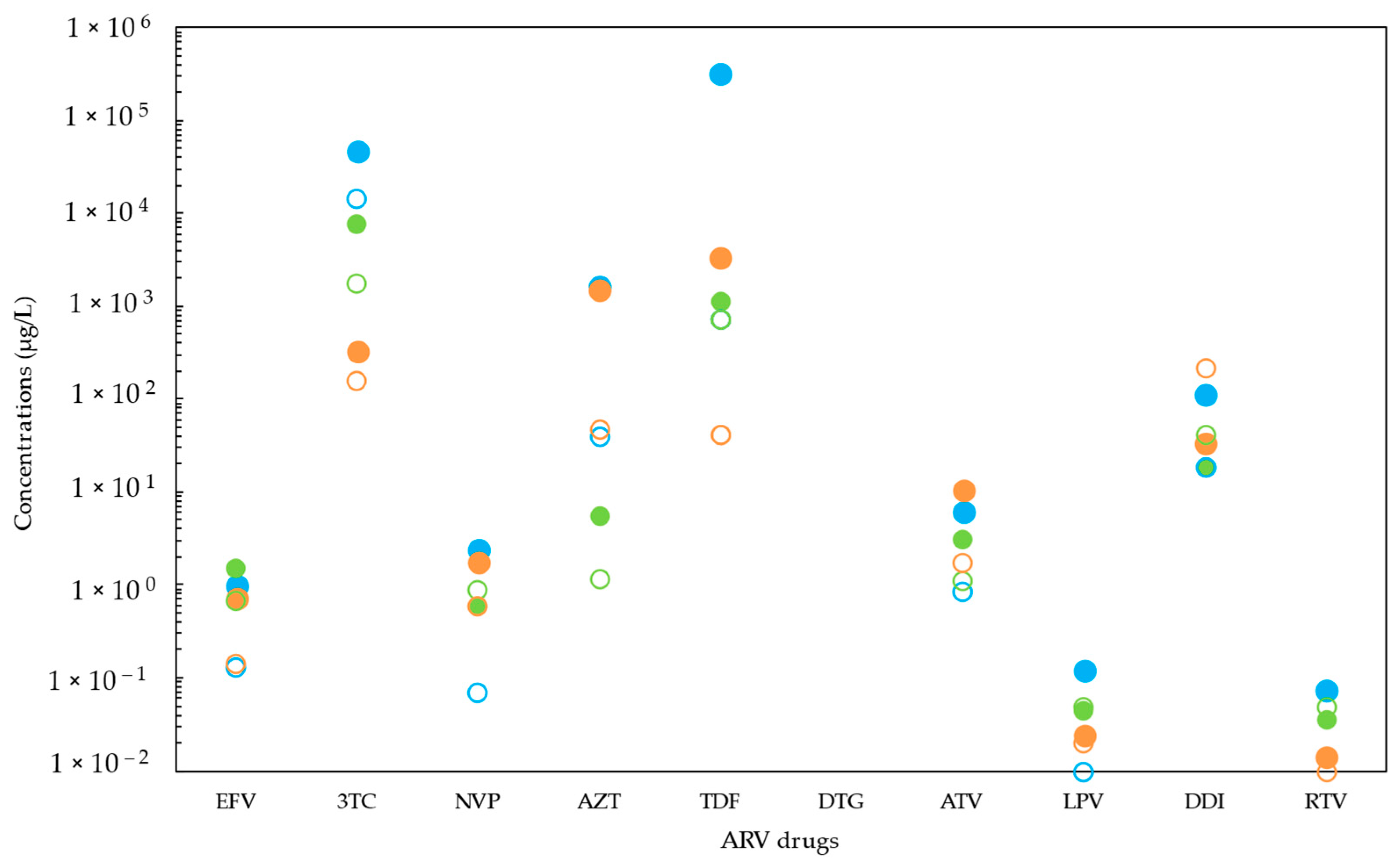
2. Occurrence of ARVs in Sub-Saharan Africa
3. Risks Associated with Antiretroviral Drugs
3.1. Ecotoxicity of Antiretroviral Drugs
3.2. Ecotoxicological Risk Assessment
3.3. Environmental Risk Characterization
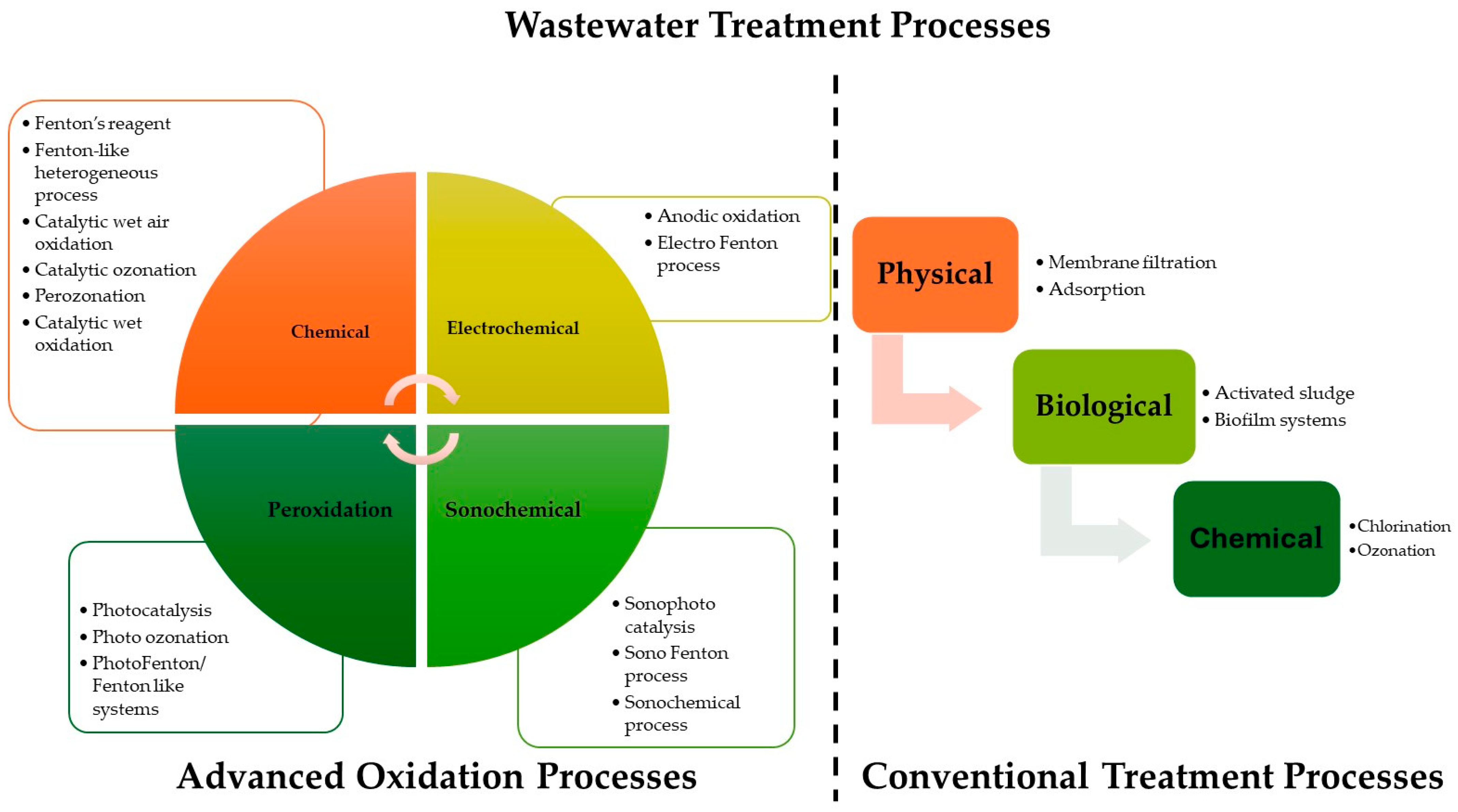
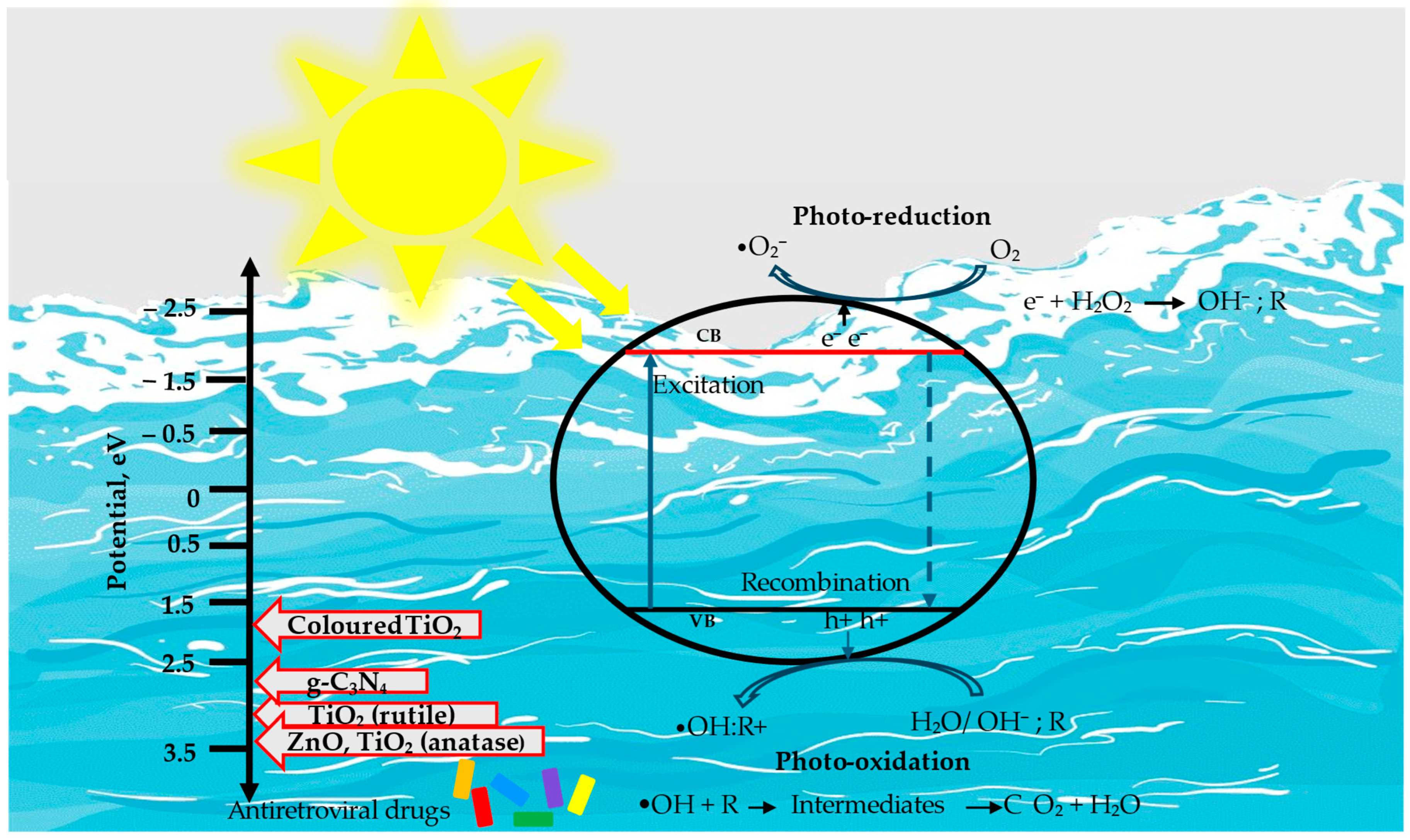
4. Mechanisms of Photocatalytic Degradation
4.1. Basic Principles of Photocatalytic Degradation Processes
4.2. Factors Influencing the Photocatalytic Degradation of Organic Pollutants
4.3. Various Photocatalysts Used in the Degradation of Pharmaceuticals
4.3.1. Titanium Dioxide
4.3.2. Black Titanium
4.3.3. Zinc Oxide
4.3.4. Graphitic Carbon Nitride-Based Photocatalysts for the Removal of Pharmaceutical Contaminants
4.3.5. Composite Photocatalysts for Application in Remediation of Pharmaceuticals
5. Challenges and Limitations
6. Future Research Directions
7. Conclusions and Recommendations
7.1. Conclusions
7.2. Recommendations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kümmerer, K. Pharmaceuticals in the Environment. Annu. Rev. Environ. Resour. 2010, 35, 57–75. [Google Scholar] [CrossRef]
- Kümmerer, K. The Presence of Pharmaceuticals in the Environment Due to Human Use—Present Knowledge and Future Challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.-A.; aus der Beek, T.; Bergmann, A.; Carius, A.; Grüttner, G.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A.; Rose, J.; et al. Pharmaceuticals in the Environment—The Global Perspective; German Environment Agency: Berlin, Germany, 2014. [Google Scholar]
- Health Care Without Harm a Multi-Stakeholder Approach to Pharmaceuticals in the Environment: Working Together Towards Effective Solutions. Available online: https://europe.noharm.org/resources/multi-stakeholder-approach-pharmaceuticals-environment-working-together-towards-effective (accessed on 17 April 2024).
- Date, M.; Jaspal, D. Pharmaceutical Wastewater Remediation: A Review of Treatment Techniques. Ind. Eng. Chem. Res. 2023, 62, 20492–20505. [Google Scholar] [CrossRef]
- Sanderson, H.; Johnson, D.J.; Reitsma, T.; Brain, R.A.; Wilson, C.J.; Solomon, K.R. Ranking and Prioritization of Environmental Risks of Pharmaceuticals in Surface Waters. Regul. Toxicol. Pharmacol. 2004, 39, 158–183. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xie, Y.; Li, J. Toxic Effects and Molecular Mechanisms of Sulfamethoxazole on Scenedesmus obliquus. Ecotoxicol. Environ. Saf. 2022, 232, 113258. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Thakker, A.; Gupta, K.K. Vestibular Schwannoma: What We Know and Where We Are Heading. Head Neck Pathol. 2020, 14, 1058–1066. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Liu, A.; Anadón, A.; Rodríguez, J.-L.; Martínez-Larrañaga, M.-R.; Yuan, Z.; Martínez, M.-A. Paracetamol: Overdose-Induced Oxidative Stress Toxicity, Metabolism, and Protective Effects of Various Compounds in Vivo and in Vitro. Drug Metab. Rev. 2017, 49, 395–437. [Google Scholar] [CrossRef]
- Russo, D.; Siciliano, A.; Guida, M.; Andreozzi, R.; Reis, N.M.; Li Puma, G.; Marotta, R. Removal of Antiretroviral Drugs Stavudine and Zidovudine in Water under UV254 and UV254/H2O2 Processes: Quantum Yields, Kinetics and Ecotoxicology Assessment. J. Hazard. Mater. 2018, 349, 195–204. [Google Scholar] [CrossRef]
- Akhtar, Z.R.; Tariq, K.; Mavian, C.; Ali, A.; Ullah, F.; Zang, L.-S.; Ali, F.; Nazir, T.; Ali, S. Trophic Transfer and Toxicity of Heavy Metals from Dengue Mosquito Aedes Aegypti to Predator Dragonfly Tramea Cophysa. Ecotoxicology 2021, 30, 1108–1115. [Google Scholar] [CrossRef]
- Hashem, M.S.; Qi, X. Treated Wastewater Irrigation—A Review. Water 2021, 13, 1527. [Google Scholar] [CrossRef]
- Tesfamariam, E.H.; Ogbazghi, Z.M.; Annandale, J.G.; Gebrehiwot, Y. Cost–Benefit Analysis of Municipal Sludge as a Low-Grade Nutrient Source: A Case Study from South Africa. Sustainability 2020, 12, 9950. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A. Feedstock and Process Influence on Biodiesel Produced from Waste Sewage Sludge. J. Environ. Manag. 2018, 216, 176–182. [Google Scholar] [CrossRef]
- Ahmed, Y.; Zhong, J.; Yuan, Z.; Guo, J. Roles of Reactive Oxygen Species in Antibiotic Resistant Bacteria Inactivation and Micropollutant Degradation in Fenton and Photo-Fenton Processes. J. Hazard. Mater. 2022, 430, 128408. [Google Scholar] [CrossRef]
- Madkhali, N.; Prasad, C.; Malkappa, K.; Choi, H.Y.; Govinda, V.; Bahadur, I.; Abumousa, R.A. Recent Update on Photocatalytic Degradation of Pollutants in Waste Water Using TiO2-Based Heterostructured Materials. Results Eng. 2023, 17, 100920. [Google Scholar] [CrossRef]
- Lazarotto, J.S.; de Lima Brombilla, V.; Silvestri, S.; Foletto, E.L. Conversion of Spent Coffee Grounds to Biochar as Promising TiO2 Support for Effective Degradation of Diclofenac in Water. Appl. Organomet. Chem. 2020, 34, e6001. [Google Scholar] [CrossRef]
- Peñas-Garzón, M.; Abdelraheem, W.H.M.; Belver, C.; Rodriguez, J.J.; Bedia, J.; Dionysiou, D.D. TiO2-Carbon Microspheres as Photocatalysts for Effective Remediation of Pharmaceuticals under Simulated Solar Light. Sep. Purif. Technol. 2021, 275, 119169. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, W.; Peng, T.; Liang, J.; Li, P.; Pan, D.; Fan, Q.; Wu, W. Visible Light Driven Ti3+ Self-Doped TiO2 for Adsorption-Photocatalysis of Aqueous U(VI). Environ. Pollut. 2020, 262, 114373. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Huang, X.; Dong, Y.; Huang, F. Conductive Black Titania Nanomaterials for Efficient Photocatalytic Degradation of Organic Pollutants. Catal. Lett. 2020, 150, 1346–1354. [Google Scholar] [CrossRef]
- Nasr, M.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Recent Progress on Titanium Dioxide Nanomaterials for Photocatalytic Applications. ChemSusChem 2018, 11, 3023–3047. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, T.; Huang, F. The Black and White Issue of Nanotitania. In Black TiO2 Nanomaterials for Energy Applications; World Scientific (Europe): London, UK, 2016; pp. 77–117. ISBN 978-1-78634-165-5. [Google Scholar]
- Morgan, K.K. HIV Medications: Antiretroviral Therapy (ART)—Types, Brand Names, and How They Work. Available online: https://www.webmd.com/hiv-aids/aids-hiv-medication (accessed on 15 January 2025).
- Ncube, S.; Madikizela, L.M.; Chimuka, L.; Nindi, M.M. Environmental Fate and Ecotoxicological Effects of Antiretrovirals: A Current Global Status and Future Perspectives. Water Res. 2018, 145, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Ngumba, E.; Gachanja, A.; Tuhkanen, T. Occurrence of Selected Antibiotics and Antiretroviral Drugs in Nairobi River Basin, Kenya. Sci. Total Environ. 2016, 539, 206–213. [Google Scholar] [CrossRef]
- Castellino, S.; Moss, L.; Wagner, D.; Borland, J.; Song, I.; Chen, S.; Lou, Y.; Min, S.S.; Goljer, I.; Culp, A.; et al. Metabolism, Excretion, and Mass Balance of the HIV-1 Integrase Inhibitor Dolutegravir in Humans. Antimicrob. Agents Chemother. 2013, 57, 3536–3546. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, P.; Vyas, R.K.; Pandit, P.; Dalai, A.K. Occurrence and Removal of Antiviral Drugs in Environment: A Review. Water. Air Soil Pollut. 2013, 224, 1410. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Ncube, S.; Chimuka, L. Analysis, Occurrence and Removal of Pharmaceuticals in African Water Resources: A Current Status. J. Environ. Manag. 2020, 253, 109741. [Google Scholar] [CrossRef] [PubMed]
- Ofrydopoulou, A.; Nannou, C.; Evgenidou, E.; Lambropoulou, D. Sample Preparation Optimization by Central Composite Design for Multi Class Determination of 172 Emerging Contaminants in Wastewaters and Tap Water Using Liquid Chromatography High-Resolution Mass Spectrometry. J. Chromatogr. A 2021, 1652, 462369. [Google Scholar] [CrossRef]
- K’oreje, K.O.; Vergeynst, L.; Ombaka, D.; De Wispelaere, P.; Okoth, M.; Van Langenhove, H.; Demeestere, K. Occurrence Patterns of Pharmaceutical Residues in Wastewater, Surface Water and Groundwater of Nairobi and Kisumu City, Kenya. Chemosphere 2016, 149, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Madikizela, L.M.; Tavengwa, N.T.; Chimuka, L. Status of Pharmaceuticals in African Water Bodies: Occurrence, Removal and Analytical Methods. J. Environ. Manag. 2017, 193, 211–220. [Google Scholar] [CrossRef]
- Reddy, K.; Renuka, N.; Kumari, S.; Bux, F. Algae-Mediated Processes for the Treatment of Antiretroviral Drugs in Wastewater: Prospects and Challenges. Chemosphere 2021, 280, 130674. [Google Scholar] [CrossRef]
- Kebede, T.G.; Seroto, M.B.; Chokwe, R.C.; Dube, S.; Nindi, M.M. Adsorption of Antiretroviral (ARVs) and Related Drugs from Environmental Wastewaters Using Nanofibers. J. Environ. Chem. Eng. 2020, 8, 104049. [Google Scholar] [CrossRef]
- Nannou, C.; Ofrydopoulou, A.; Evgenidou, E.; Heath, D.; Heath, E.; Lambropoulou, D. Antiviral Drugs in Aquatic Environment and Wastewater Treatment Plants: A Review on Occurrence, Fate, Removal and Ecotoxicity. Sci. Total Environ. 2020, 699, 134322. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. Multiresidue Methods for the Analysis of Pharmaceuticals, Personal Care Products and Illicit Drugs in Surface Water and Wastewater by Solid-Phase Extraction and Ultra Performance Liquid Chromatography-Electrospray Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2008, 391, 1293–1308. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Vogt, T.; Gerber, E.; Vogt, B.; Bouwman, H.; Pieters, R. HIV-Antiretrovirals in River Water from Gauteng, South Africa: Mixed Messages of Wastewater Inflows as Source. Sci. Total Environ. 2022, 806, 150346. [Google Scholar] [CrossRef]
- Ngwenya, P.; Musee, N. Modelling Ecological Risks of Antiretroviral Drugs in the Environment. Environ. Chem. Ecotoxicol. 2023, 5, 145–154. [Google Scholar] [CrossRef]
- Keller, V.D.J.; Williams, R.J.; Lofthouse, C.; Johnson, A.C. Worldwide Estimation of River Concentrations of Any Chemical Originating from Sewage-Treatment Plants Using Dilution Factors. Environ. Toxicol. Chem. 2014, 33, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Mtolo, S.P.; Mahlambi, P.N.; Madikizela, L.M. Synthesis and Application of a Molecularly Imprinted Polymer in Selective Solid-Phase Extraction of Efavirenz from Water. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2019, 79, 356–365. [Google Scholar] [CrossRef]
- K’oreje, K.O.; Okoth, M.; Van Langenhove, H.; Demeestere, K. Occurrence and Treatment of Contaminants of Emerging Concern in the African Aquatic Environment: Literature Review and a Look Ahead. J. Environ. Manag. 2020, 254, 109752. [Google Scholar] [CrossRef]
- Rimayi, C.; Odusanya, D.; Weiss, J.M.; De Boer, J.; Chimuka, L. Contaminants of Emerging Concern in the Hartbeespoort Dam Catchment and the uMngeni River Estuary 2016 Pollution Incident, South Africa. Sci. Total Environ. 2018, 627, 1008–1017. [Google Scholar] [CrossRef]
- Wooding, M.; Rohwer, E.R.; Naudé, Y. Determination of Endocrine Disrupting Chemicals and Antiretroviral Compounds in Surface Water: A Disposable Sorptive Sampler with Comprehensive Gas Chromatography—Time-of-Flight Mass Spectrometry and Large Volume Injection with Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2017, 1496, 122–132. [Google Scholar] [CrossRef]
- Gani, K.M.; Hlongwa, N.; Abunama, T.; Kumari, S.; Bux, F. Emerging Contaminants in South African Water Environment- a Critical Review of Their Occurrence, Sources and Ecotoxicological Risks. Chemosphere 2021, 269, 128737. [Google Scholar] [CrossRef]
- Mutua, G.K.; Kinyari, P.; Githuku, C.; Kironchi, G.; Kang’ethe, E.; Prain, G.; Njenga, M.; Karanja, N.N. Assessment of Environmental and Public Health Hazards in Wastewater Used for Urban Agriculture in Nairobi, Kenya. Trop. Subtrop. Agroecosystems 2010, 12, 85–97. [Google Scholar]
- Roura, M.; Watson-Jones, D.; Kahawita, T.M.; Ferguson, L.; Ross, D.A. Provider-Initiated Testing and Counselling Programmes in Sub-Saharan Africa: A Systematic Review of Their Operational Implementation. AIDS 2013, 27, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Semiyaga, S.; Okure, M.A.E.; Niwagaba, C.B.; Katukiza, A.Y.; Kansiime, F. Decentralized Options for Faecal Sludge Management in Urban Slum Areas of Sub-Saharan Africa: A Review of Technologies, Practices and End-Uses. Resour. Conserv. Recycl. 2015, 104, 109–119. [Google Scholar] [CrossRef]
- Hawkins, T. Understanding and Managing the Adverse Effects of Antiretroviral Therapy. Antiviral Res. 2010, 85, 201–209. [Google Scholar] [CrossRef]
- Olabode, G.; Somerset, V. Advances in Chromatographic Determination of Selected Anti-Retrovirals in Wastewater. In Nano and Bio-Based Technologies for Wastewater Treatment; Fosso-Kankeu, E., Ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 105–127. ISBN 978-1-119-57709-6. [Google Scholar]
- Almeida, L.C.; Mattos, A.C.; Dinamarco, C.P.G.; Figueiredo, N.G.; Bila, D.M. Chronic Toxicity and Environmental Risk Assessment of Antivirals in Ceriodaphnia Dubia and Raphidocelis Subcapitata. Water Sci. Technol. 2021, 84, 1623–1634. [Google Scholar] [CrossRef]
- Robson, L.; Barnhoorn, I.E.J.; Wagenaar, G.M. The Potential Effects of Efavirenz on Oreochromis mossambicus after Acute Exposure. Environ. Toxicol. Pharmacol. 2017, 56, 225–232. [Google Scholar] [CrossRef]
- Blas-García, A.; Apostolova, N.; Ballesteros, D.; Monleón, D.; Morales, J.M.; Rocha, M.; Victor, V.M.; Esplugues, J.V. Inhibition of Mitochondrial Function by Efavirenz Increases Lipid Content in Hepatic Cells. Hepatology 2010, 52, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Pitso, K.M. Mozambique Tilapia Oreochromis mossambicus; University of Johannesburg: Johannesburg, South Africa, 2020. [Google Scholar]
- Omotola, E.O.; Genthe, B.; Ndlela, L.; Olatunji, O.S. Environmental Risk Characterization of an Antiretroviral (ARV) Lamivudine in Ecosystems. Int. J. Environ. Res. Public Health 2021, 18, 8358. [Google Scholar] [CrossRef]
- Minguez, L.; Pedelucq, J.; Farcy, E.; Ballandonne, C.; Budzinski, H.; Halm-Lemeille, M.-P. Toxicities of 48 Pharmaceuticals and Their Freshwater and Marine Environmental Assessment in Northwestern France. Environ. Sci. Pollut. Res. 2016, 23, 4992–5001. [Google Scholar] [CrossRef]
- Gosset, A.; Wiest, L.; Fildier, A.; Libert, C.; Giroud, B.; Hammada, M.; Hervé, M.; Sibeud, E.; Vulliet, E.; Polomé, P.; et al. Ecotoxicological Risk Assessment of Contaminants of Emerging Concern Identified by “Suspect Screening” from Urban Wastewater Treatment Plant Effluents at a Territorial Scale. Sci. Total Environ. 2021, 778, 146275. [Google Scholar] [CrossRef]
- European Chemical Bureau. Technical Guidance Document on Risk Assessment In-LBNC20418ENC.Pdf; European Commission Joint Research Centre: Brussels, Belgium, 2003. [Google Scholar]
- Musee, N.; Ondiaka, M.; Chimphango, A.; Aldrich, C. Modelling the Fate, Behaviour and Toxicity of Engineered Nanomaterials in Aquatic Systems; Water Research Commission: Pretoria, South Africa, 2015. [Google Scholar]
- Hernando, M.D.; Mezcua, M.; Fernández-Alba, A.R.; Barceló, D. Environmental Risk Assessment of Pharmaceutical Residues in Wastewater Effluents, Surface Waters and Sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in Wastewater Treatment Plants in Greece: Occurrence, Removal and Environmental Risk Assessment. Sci. Total Environ. 2014, 466–467, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Molnar, E.; Maasz, G.; Pirger, Z. Environmental Risk Assessment of Pharmaceuticals at a Seasonal Holiday Destination in the Largest Freshwater Shallow Lake in Central Europe. Environ. Sci. Pollut. Res. 2021, 28, 59233–59243. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal Occurrence, Removal, Mass Loading and Environmental Risk Assessment of 55 Pharmaceuticals and Personal Care Products in a Municipal Wastewater Treatment Plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, T.; Guo, C.; Lv, J.; Hua, Z.; Hou, S.; Zhang, Y.; Meng, W.; Xu, J. Drugs of Abuse and Their Metabolites in the Urban Rivers of Beijing, China: Occurrence, Distribution, and Potential Environmental Risk. Sci. Total Environ. 2017, 579, 305–313. [Google Scholar] [CrossRef]
- Muriuki, C.; Kairigo, P.; Home, P.; Ngumba, E.; Raude, J.; Gachanja, A.; Tuhkanen, T. Mass Loading, Distribution, and Removal of Antibiotics and Antiretroviral Drugs in Selected Wastewater Treatment Plants in Kenya. Sci. Total Environ. 2020, 743, 140655. [Google Scholar] [CrossRef]
- Cid, R.S.; Roveri, V.; Vidal, D.G.; Dinis, M.A.P.; Cortez, F.S.; Salgueiro, F.R.; Toma, W.; Cesar, A.; Guimarães, L.L. Toxicity of Antiretrovirals on the Sea Urchin Echinometra Lucunter and Its Predicted Environmental Concentration in Seawater from Santos Bay (Brazilian Coastal Zone). Resources 2021, 10, 114. [Google Scholar] [CrossRef]
- Guo, J.; Boxall, A.; Selby, K. Do Pharmaceuticals Pose a Threat to Primary Producers? Crit. Rev. Environ. Sci. Technol. 2015, 45, 2565–2610. [Google Scholar] [CrossRef]
- Cayman Chemicals Safety Data Sheet. Available online: https://cdn.caymanchem.com/cdn/msds/14412m.pdf (accessed on 16 January 2025).
- Vogt, B.; Bouwman, H.; Bezuidenhout, C.; Horn, S.; Bothma, L.; Gerber, E.; van Aswegen, D.; Blom, K.; Fouché, D.; Potgieter, J. Quantification, Fate and Hazard Assessment of HIV-ARVs in Water Resources; Water Research Commission: Pretoria, South Africa, 2020. [Google Scholar]
- Kumar, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Kumar Thakur, V.; Nguyen, V.-H.; Singh, P. C-, N-Vacancy Defect Engineered Polymeric Carbon Nitride towards Photocatalysis: Viewpoints and Challenges. J. Mater. Chem. A 2021, 9, 111–153. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Kamali, M.; Zhang, X.; Feijoo, S.; Al-Salem, S.M.; Dewil, R.; Appels, L. Biochar in Hydroxyl Radical-Based Electrochemical Advanced Oxidation Processes (eAOPs)—Mechanisms and Prospects. Chem. Eng. J. 2023, 467, 143291. [Google Scholar] [CrossRef]
- Bermúdez, L.A.; Pascual, J.M.; Martínez, M.d.M.M.; Poyatos Capilla, J.M. Effectiveness of Advanced Oxidation Processes in Wastewater Treatment: State of the Art. Water 2021, 13, 2094. [Google Scholar] [CrossRef]
- Cai, Q.Q.; Lee, B.C.Y.; Ong, S.L.; Hu, J.Y. Fluidized-Bed Fenton Technologies for Recalcitrant Industrial Wastewater Treatment–Recent Advances, Challenges and Perspective. Water Res. 2021, 190, 116692. [Google Scholar] [CrossRef] [PubMed]
- Lebron, Y.A.R.; Moreira, V.R.; Maia, A.; Couto, C.F.; Moravia, W.G.; Amaral, M.C.S. Integrated Photo-Fenton and Membrane-Based Techniques for Textile Effluent Reclamation. Sep. Purif. Technol. 2021, 272, 118932. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent Advances in Ozone-Based Advanced Oxidation Processes for Treatment of Wastewater- A Review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Reggiane de Carvalho Costa, L.; Guerra Pacheco Nunes, K.; Amaral Féris, L. Ultrasound as an Advanced Oxidative Process: A Review on Treating Pharmaceutical Compounds. Chem. Eng. Technol. 2021, 44, 1744–1758. [Google Scholar] [CrossRef]
- Oluwole, A.O.; Omotola, E.O.; Olatunji, O.S. Pharmaceuticals and Personal Care Products in Water and Wastewater: A Review of Treatment Processes and Use of Photocatalyst Immobilized on Functionalized Carbon in AOP Degradation. BMC Chem. 2020, 14, 62. [Google Scholar] [CrossRef]
- Friedmann, D. A General Overview of Heterogeneous Photocatalysis as a Remediation Technology for Wastewaters Containing Pharmaceutical Compounds. Water 2022, 14, 3588. [Google Scholar] [CrossRef]
- Nabgan, W.; Saeed, M.; Jalil, A.A.; Nabgan, B.; Gambo, Y.; Ali, M.W.; Ikram, M.; Fauzi, A.A.; Owgi, A.H.K.; Hussain, I.; et al. A State of the Art Review on Electrochemical Technique for the Remediation of Pharmaceuticals Containing Wastewater. Environ. Res. 2022, 210, 112975. [Google Scholar] [CrossRef]
- Finn, M.; Giampietro, G.; Mazyck, D.; Rodriguez, R. Activated Carbon for Pharmaceutical Removal at Point-of-Entry. Processes 2021, 9, 1091. [Google Scholar] [CrossRef]
- Vinayagam, V.; Murugan, S.; Kumaresan, R.; Narayanan, M.; Sillanpää, M.; Viet N Vo, D.; Kushwaha, O.S.; Jenis, P.; Potdar, P.; Gadiya, S. Sustainable Adsorbents for the Removal of Pharmaceuticals from Wastewater: A Review. Chemosphere 2022, 300, 134597. [Google Scholar] [CrossRef]
- Abujazar, M.S.S.; Karaağaç, S.U.; Abu Amr, S.S.; Alazaiza, M.Y.D.; Bashir, M.J.K. Recent Advancement in the Application of Hybrid Coagulants in Coagulation-Flocculation of Wastewater: A Review. J. Clean. Prod. 2022, 345, 131133. [Google Scholar] [CrossRef]
- Ahmad, A.; Tariq, S.; Zaman, J.U.; Martin Perales, A.I.; Mubashir, M.; Luque, R. Recent Trends and Challenges with the Synthesis of Membranes: Industrial Opportunities towards Environmental Remediation. Chemosphere 2022, 306, 135634. [Google Scholar] [CrossRef]
- Su, Z.; Wu, X.; Kuo, D.-H.; Yang, B.; Wu, B.; Chen, L.; Zhang, P.; Lin, J.; Lu, D.; Chen, X. Synergistic Vacancy Defects and Bandgap Engineering in an Ag/S Co-Doped Bi 2 O 3 -Based Sulfur Oxide Catalyst for Efficient Hydrogen Evolution. J. Mater. Chem. A 2024, 12, 10494–10506. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Advances in Heterogeneous Photocatalytic Degradation of Phenols and Dyes in Wastewater: A Review. Water. Air Soil Pollut. 2011, 215, 3–29. [Google Scholar] [CrossRef]
- Valencia, S.H.; Marín, J.M.; Restrepo, G.M. Evolution of Natural Organic Matter by Size Exclusion Chromatography during Photocatalytic Degradation by Solvothermal-Synthesized Titanium Dioxide. J. Hazard. Mater. 2012, 213–214, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Al-Rasheed, R.A. Water Treatment by Heterogeneous Photocatalysis an Overview. In Proceedings of the 4th SWCC Acquired Experience Symposium, Jeddah, Saudi Arabia, 7 May 2005. [Google Scholar]
- Mohamadpour, F.; Amani, A. Mohammad Photocatalytic Systems: Reactions, Mechanism, and Applications. RSC Adv. 2024, 14, 20609–20645. [Google Scholar] [CrossRef]
- Umar, M.; Aziz, H.A. Photocatalytic Degradation of Organic Pollutants in Water. In Organic Pollutants—Monitoring, Risk and Treatment; IntechOpen: London, UK, 2013; ISBN 978-953-51-0948-8. [Google Scholar]
- Singh, S.; Garg, A. Characterisation and Utilization of Steel Industry Waste Sludge as Heterogeneous Catalyst for the Abatement of Chlorinated Organics by Advanced Oxidation Processes. Chemosphere 2020, 242, 125158. [Google Scholar] [CrossRef] [PubMed]
- Gozdecka, A.; Wiącek, A.E. Effect of UV Radiation and Chitosan Coating on the Adsorption-Photocatalytic Activity of TiO2 Particles. Mater. Sci. Eng. C 2018, 93, 582–594. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Holmberg, K. Solubilization of Hydrophobic Dyes in Surfactant Solutions. Materials 2013, 6, 580–608. [Google Scholar] [CrossRef]
- Marin, R.-C.; Behl, T.; Negrut, N.; Bungau, S. Management of Antiretroviral Therapy with Boosted Protease Inhibitors—Darunavir/Ritonavir or Darunavir/Cobicistat. Biomedicines 2021, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Pascariu, P.; Cojocaru, C.; Samoila, P.; Airinei, A.; Olaru, N.; Rotaru, A.; Romanitan, C.; Tudoran, L.B.; Suchea, M. Cu/TiO2 Composite Nanofibers with Improved Photocatalytic Performance under UV and UV–Visible Light Irradiation. Surf. Interfaces 2022, 28, 101644. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, J.; Deng, Y.; Wang, M.; Li, D.; Xia, C. Electric Double Layer-Mediated Polarization Field for Optimizing Photogenerated Carrier Dynamics and Thermodynamics. Nat. Commun. 2023, 14, 3592. [Google Scholar] [CrossRef]
- Dugandžić, A.M.; Tomašević, A.V.; Radišić, M.M.; Šekuljica, N.Ž.; Mijin, D.Ž.; Petrović, S.D. Effect of Inorganic Ions, Photosensitisers and Scavengers on the Photocatalytic Degradation of Nicosulfuron. J. Photochem. Photobiol. Chem. 2017, 336, 146–155. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, G. A Review on the Factors Affecting the Photocatalytic Degradation of Hazardous Materials. Mater. Sci. Eng. Int. J. 2017, 1, 106–114. [Google Scholar] [CrossRef]
- Armaković, S.J.; Armaković, S.; Finčur, N.L.; Šibul, F.; Vione, D.; Šetrajčić, J.P.; Abramović, B.F. Influence of Electron Acceptors on the Kinetics of Metoprolol Photocatalytic Degradation in TiO2 Suspension. A Combined Experimental and Theoretical Study. RSC Adv. 2015, 5, 54589–54604. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Paumo, H.K.; Dalhatou, S.; Katata-Seru, L.M.; Kamdem, B.P.; Tijani, J.O.; Vishwanathan, V.; Kane, A.; Bahadur, I. TiO2 Assisted Photocatalysts for Degradation of Emerging Organic Pollutants in Water and Wastewater. J. Mol. Liq. 2021, 331, 115458. [Google Scholar] [CrossRef]
- Mahlambi, M.M.; Ngila, C.J.; Mamba, B.B. Recent Developments in Environmental Photocatalytic Degradation of Organic Pollutants: The Case of Titanium Dioxide Nanoparticles—A Review. J. Nanomater. 2015, 2015, 790173. [Google Scholar] [CrossRef]
- Slusarski-Santana, V.; Fiorentin-Ferrari, L.D.; Fiorese, M.L. Antimicrobial Activities of Photocatalysts for Water Disinfection. In Nanophotocatalysis and Environmental Applications: Detoxification and Disinfection; Inamuddin Asiri, A.M., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 217–243. ISBN 978-3-030-12619-3. [Google Scholar]
- Kapilashrami, M.; Zhang, Y.; Liu, Y.-S.; Hagfeldt, A.; Guo, J. Probing the Optical Property and Electronic Structure of TiO2 Nanomaterials for Renewable Energy Applications. Chem. Rev. 2014, 114, 9662–9707. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent Progress in Metal-Doped TiO2, Non-Metal Doped/Codoped TiO2 and TiO2 Nanostructured Hybrids for Enhanced Photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Aljaafari, A. Effect of Metal and Non-Metal Doping on the Photocatalytic Performance of Titanium Dioxide (TiO2): A Review. Curr. Nanosci. 2022, 18, 499–519. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; Thomas, N.; Louis, J.; Mathew, D.T.; Ganguly, P.; John, H.; Pillai, S.C. Graphene Coupled TiO2 Photocatalysts for Environmental Applications: A Review. Chemosphere 2021, 271, 129506. [Google Scholar] [CrossRef]
- Chauke, N.M.; Mohlala, R.L.; Ngqoloda, S.; Raphulu, M.C. Harnessing Visible Light: Enhancing TiO2 Photocatalysis with Photosensitizers for Sustainable and Efficient Environmental Solutions. Front. Chem. Eng. 2024, 6, 1356021. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.-C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, N.; Nowrouzi, M.; Madadi Avargani, V.; Sayadi, M.H.; Zendehboudi, S. Design and Optimization of TiO2-Based Photocatalysts for Efficient Removal of Pharmaceutical Pollutants in Water: Recent Developments and Challenges. J. Water Process Eng. 2024, 57, 104597. [Google Scholar] [CrossRef]
- Wu, S.; Hu, H.; Lin, Y.; Zhang, J.; Hu, Y.H. Visible Light Photocatalytic Degradation of Tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Yang, H.; Li, G.; An, T.; Gao, Y.; Fu, J. Photocatalytic Degradation Kinetics and Mechanism of Environmental Pharmaceuticals in Aqueous Suspension of TiO2: A Case of Sulfa Drugs. Catal. Today 2010, 153, 200–207. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants Using TiO2-Based Photocatalysts: A Review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Zou, L.; Zhu, Y.; Hu, Z.; Cao, X.; Cen, W. Remarkably Improved Photocatalytic Hydrogen Evolution Performance of Crystalline TiO2 Nanobelts Hydrogenated at Atmospheric Pressure with the Assistance of Hydrogen Spillover. Catal. Sci. Technol. 2022, 12, 5575–5585. [Google Scholar] [CrossRef]
- Qiang, C.; Li, N.; Zuo, S.; Guo, Z.; Zhan, W.; Li, Z.; Ma, J. Microwave-Assisted Synthesis of RuTe2/Black TiO2 Photocatalyst for Enhanced Diclofenac Degradation: Performance, Mechanistic Investigation and Intermediates Analysis. Sep. Purif. Technol. 2022, 283, 120214. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Zhang, S.; Zhong, Q. A Study on the NH 3 -SCR Performance and Reaction Mechanism of a Cost-Effective and Environment-Friendly Black TiO2 Catalyst. Phys. Chem. Chem. Phys. 2018, 20, 22744–22752. [Google Scholar] [CrossRef] [PubMed]
- Andronic, L.; Ghica, D.; Stefan, M.; Mihalcea, C.G.; Vlaicu, A.-M.; Karazhanov, S. Visible-Light-Active Black TiO2 Nanoparticles with Efficient Photocatalytic Performance for Degradation of Pharmaceuticals. Nanomaterials 2022, 12, 2563. [Google Scholar] [CrossRef] [PubMed]
- Gad-Allah, T.A.; Ali, M.E.M.; Badawy, M.I. Photocatalytic Oxidation of Ciprofloxacin under Simulated Sunlight. J. Hazard. Mater. 2011, 186, 751–755. [Google Scholar] [CrossRef]
- Padmapriya, G.; Manikandan, A.; Krishnasamy, V.; Jaganathan, S.K.; Antony, S.A. Spinel NixZn1−xFe2O4 (0.0 ≤ x ≤ 1.0) Nano-Photocatalysts: Synthesis, Characterization and Photocatalytic Degradation of Methylene Blue Dye. J. Mol. Struct. 2016, 1119, 39–47. [Google Scholar] [CrossRef]
- El Bouraie, M.M.; Ibrahim, S.S. Comparative Study Between Metronidazole Residues Disposal by Using Adsorption and Photodegradation Processes onto MgO Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2021, 31, 344–364. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wang, Y.; Wang, H.; Wu, H.; Gao, D. Efficient Photocatalytic Degradation of Sulfonamides in Wastewater Using G-C3N4 Heterostructures: A Critical Review. Environ. Technol. Innov. 2024, 36, 103854. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef]
- Tokode, O.; Prabhu, R.; Lawton, L.A.; Robertson, P.K.J. The Effect of pH on the Photonic Efficiency of the Destruction of Methyl Orange under Controlled Periodic Illumination with UV-LED Sources. Chem. Eng. J. 2014, 246, 337–342. [Google Scholar] [CrossRef]
- Sarafraz, M.; Sadeghi, M.; Yazdanbakhsh, A.; Amini, M.M.; Sadani, M.; Eslami, A. Enhanced Photocatalytic Degradation of Ciprofloxacin by Black Ti3+/N-TiO2 under Visible LED Light Irradiation: Kinetic, Energy Consumption, Degradation Pathway, and Toxicity Assessment. Process Saf. Environ. Prot. 2020, 137, 261–272. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Oba, S.N.; Aniagor, C.O.; Adeniyi, A.G.; Ighalo, J.O. Adsorption of Ciprofloxacin from Water: A Comprehensive Review. J. Ind. Eng. Chem. 2021, 93, 57–77. [Google Scholar] [CrossRef]
- Abdullah, F.H.; Bakar, N.H.H.A.; Bakar, M.A. Current Advancements on the Fabrication, Modification, and Industrial Application of Zinc Oxide as Photocatalyst in the Removal of Organic and Inorganic Contaminants in Aquatic Systems. J. Hazard. Mater. 2022, 424, 127416. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Cheng, M.; Lai, C.; Li, L.; Yang, X.; Du, L.; Zhang, G.; Wang, G.; Yang, L. Recent Progress in the Applications of Non-Metal Modified Graphitic Carbon Nitride in Photocatalysis. Coord. Chem. Rev. 2023, 474, 214846. [Google Scholar] [CrossRef]
- Ringu, T.; Ghosh, S.; Das, A.; Pramanik, N. Zinc Oxide Nanoparticles: An Excellent Biomaterial for Bioengineering Applications. Emergent Mater. 2022, 5, 1629–1648. [Google Scholar] [CrossRef]
- Sun, C.; Xu, Q.; Xie, Y.; Ling, Y.; Hou, Y. Designed Synthesis of Anatase–TiO2 (B) Biphase Nanowire/ZnO Nanoparticle Heterojunction for Enhanced Photocatalysis. J. Mater. Chem. A 2018, 6, 8289–8298. [Google Scholar] [CrossRef]
- Ali, S.; Muhammad Ismail, P.; Khan, M.; Dang, A.; Ali, S.; Zada, A.; Raziq, F.; Khan, I.; Shakeel Khan, M.; Ateeq, M.; et al. Charge Transfer in TiO2-Based Photocatalysis: Fundamental Mechanisms to Material Strategies. Nanoscale 2024, 16, 4352–4377. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Yu, J. Chapter 8—Modification of ZnO-Based Photocatalysts for Enhanced Photocatalytic Activity. In Interface Science and Technology; Yu, J., Jaroniec, M., Jiang, C., Eds.; Surface Science of Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 265–284. [Google Scholar]
- He, Y.; Sutton, N.B.; Rijnaarts, H.H.H.; Langenhoff, A.A.M. Degradation of Pharmaceuticals in Wastewater Using Immobilized TiO2 Photocatalysis under Simulated Solar Irradiation. Appl. Catal. B Environ. 2016, 182, 132–141. [Google Scholar] [CrossRef]
- Raliya, R.; Avery, C.; Chakrabarti, S.; Biswas, P. Photocatalytic Degradation of Methyl Orange Dye by Pristine Titanium Dioxide, Zinc Oxide, and Graphene Oxide Nanostructures and Their Composites under Visible Light Irradiation. Appl. Nanosci. 2017, 7, 253–259. [Google Scholar] [CrossRef]
- Han, J.; Liu, Y.; Singhal, N.; Wang, L.; Gao, W. Comparative Photocatalytic Degradation of Estrone in Water by ZnO and TiO2 under Artificial UVA and Solar Irradiation. Chem. Eng. J. 2012, 213, 150–162. [Google Scholar] [CrossRef]
- Ravbar, M.; Kunčič, A.; Matoh, L.; Možina, S.S.; Šala, M.; Šuligoj, A. Controlled Growth of ZnO Nanoparticles Using Ethanolic Root Extract of Japanese Knotweed: Photocatalytic and Antimicrobial Properties. RSC Adv. 2022, 12, 31235–31245. [Google Scholar] [CrossRef]
- Zhang, Z.; Zada, A.; Cui, N.; Liu, N.; Liu, M.; Yang, Y.; Jiang, D.; Jiang, J.; Liu, S. Synthesis of Ag Loaded ZnO/BiOCl with High Photocatalytic Performance for the Removal of Antibiotic Pollutants. Crystals 2021, 11, 981. [Google Scholar] [CrossRef]
- Al-Ahmed, A. Photocatalytic Properties of Graphitic Carbon Nitrides (g-C3N4) for Sustainable Green Hydrogen Production: Recent Advancement. Fuel 2022, 316, 123381. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, Y.; Fan, J.; Xue, Y.; Chang, H.; Masubuchi, Y.; Yin, S. Synthesis of Graphitic Carbon Nitride from Different Precursors by Fractional Thermal Polymerization Method and Their Visible Light Induced Photocatalytic Activities. J. Alloys Compd. 2018, 735, 1297–1305. [Google Scholar] [CrossRef]
- Attri, P.; Garg, P.; Sharma, P.; Singh, R.; Chauhan, M.; Lim, D.-K.; Kumar, S.; Chaudhary, G.R. Precursor-Dependent Fabrication of Exfoliated Graphitic Carbon Nitride (gCN) for Enhanced Photocatalytic and Antimicrobial Activity under Visible Light Irradiation. J. Clean. Prod. 2023, 422, 138538. [Google Scholar] [CrossRef]
- Nejad, M.S.; Vakily, Z.; Mostafavi, A.; Sheibani, H. Fabrication of Covalently Linked Ruthenium Complex onto Carbon Nitride Nanotubes for the Photocatalytic Degradation of Tetracycline Antibiotic. preprint, 2022. [Google Scholar] [CrossRef]
- John, A.; Rajan, M.S.; Thomas, J. Carbon Nitride-Based Photocatalysts for the Mitigation of Water Pollution Engendered by Pharmaceutical Compounds. Environ. Sci. Pollut. Res. 2021, 28, 24992–25013. [Google Scholar] [CrossRef]
- She, S.; Wang, Y.; Chen, R.; Yi, F.; Sun, C.; Hu, J.; Li, Z.; Lu, G.; Zhu, M. Ultrathin S-Doped Graphitic Carbon Nitride Nanosheets for Enhanced Sulpiride Degradation via Visible-Light-Assisted Peroxydisulfate Activation: Performance and Mechanism. Chemosphere 2021, 266, 128929. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Al-Sehemi, A.G.; El-Nasser, K.S.; Taha, T.A.; Al-Ghamdi, A.A.; Syed, J.A.S.; Amin, M.A.; Ali, T.; Bashir, T.; Palamanit, A.; et al. Graphitic Carbon Nitride (g–C3N4)–Based Semiconductor as a Beneficial Candidate in Photocatalysis Diversity. Int. J. Hydrogen Energy 2022, 47, 5142–5191. [Google Scholar] [CrossRef]
- Pazoki, M.; Parsa, M.; Farhadpour, R. Removal of the Hormones Dexamethasone (DXM) by Ag Doped on TiO2 Photocatalysis. J. Environ. Chem. Eng. 2016, 4, 4426–4434. [Google Scholar] [CrossRef]
- Evgenidou, Ε.; Vasilopoulou, K.; Ioannidou, E.; Koronaiou, L.A.; Nannou, C.; Trikkaliotis, D.G.; Bikiaris, D.; Kyzas, G.Z.; Lambropoulou, D. Photocatalytic Degradation of the Antiviral Drug Abacavir Using Titania-Graphene Oxide Nanocomposites in Landfill Leachate. J. Photochem. Photobiol. Chem. 2023, 439, 114628. [Google Scholar] [CrossRef]
- Chen, J.; Luo, H.; Shi, H.; Li, G.; An, T. Anatase TiO2 Nanoparticles–Carbon Nanotubes Composite: Optimization Synthesis and the Relationship of Photocatalytic Degradation Activity of Acyclovir in Water. Appl. Catal. Gen. 2014, 485, 188–195. [Google Scholar] [CrossRef]
- Ayodhya, D. Ag-SPR and Semiconductor Interface Effect on a Ternary CuO@Ag@Bi2S3 Z-Scheme Catalyst for Enhanced Removal of HIV Drugs and (Photo)Catalytic Activity. New J. Chem. 2022, 46, 15838–15850. [Google Scholar] [CrossRef]
- Bhembe, Y.A.; Lukhele, L.P.; Hlekelele, L.; Ray, S.S.; Sharma, A.; Vo, D.-V.N.; Dlamini, L.N. Photocatalytic Degradation of Nevirapine with a Heterostructure of Few-Layer Black Phosphorus Coupled with Niobium (V) Oxide Nanoflowers (FL-BP@Nb2O5). Chemosphere 2020, 261, 128159. [Google Scholar] [CrossRef]
- Tabana, L.; Booysens, D.-R.; Tichapondwa, S. Photocatalytic Degradation of Efavirenz and Nevirapine Using Visible Light-Activated Ag-AgBr-LDH Nanocomposite Catalyst. J. Photochem. Photobiol. Chem. 2023, 444, 114997. [Google Scholar] [CrossRef]
- An, T.; An, J.; Yang, H.; Li, G.; Feng, H.; Nie, X. Photocatalytic Degradation Kinetics and Mechanism of Antivirus Drug-Lamivudine in TiO2 Dispersion. J. Hazard. Mater. 2011, 197, 229–236. [Google Scholar] [CrossRef]
- Katal, R.; Davood Abadi Farahani, M.H.; Jiangyong, H. Degradation of Acetaminophen in a Photocatalytic (Batch and Continuous System) and Photoelectrocatalytic Process by Application of Faceted-TiO2. Sep. Purif. Technol. 2020, 230, 115859. [Google Scholar] [CrossRef]
- Feng, X.; Wang, P.; Hou, J.; Qian, J.; Wang, C.; Ao, Y. Oxygen Vacancies and Phosphorus Codoped Black Titania Coated Carbon Nanotube Composite Photocatalyst with Efficient Photocatalytic Performance for the Degradation of Acetaminophen under Visible Light Irradiation. Chem. Eng. J. 2018, 352, 947–956. [Google Scholar] [CrossRef]
- Li, G.; Nie, X.; Gao, Y.; An, T. Can Environmental Pharmaceuticals Be Photocatalytically Degraded and Completely Mineralized in Water Using G-C3N4/TiO2 under Visible Light Irradiation?—Implications of Persistent Toxic Intermediates. Appl. Catal. B Environ. 2016, 180, 726–732. [Google Scholar] [CrossRef]
- Wu, M.; Lv, H.; Wang, T.; Ao, Z.; Sun, H.; Wang, C.; An, T.; Wang, S. Ag2MoO4 Nanoparticles Encapsulated in G-C3N4 for Sunlight Photodegradation of Pollutants. Catal. Today 2018, 315, 205–212. [Google Scholar] [CrossRef]
- Hu, X.; Fan, J.; Zhang, K.; Yu, N.; Wang, J. Pharmaceuticals Removal by Novel Nanoscale Photocatalyst Bi4VO8Cl: Influencing Factors, Kinetics, and Mechanism. Ind. Eng. Chem. Res. 2014, 53, 14623–14632. [Google Scholar] [CrossRef]
- Benjedim, S. Preparation of Activated Carbons by Chemical Activation of Argan Seed Shell Waste. Application for the Removal of Paracetamol and Amoxicillin from Aqueous Solutions; Universidad de Granada: Granada, Spain, 2022. [Google Scholar]
- Kar, P.; Aggarwal, D.; Shukla, K.; Gupta, R.K. Defect State Modulation of TiO2 Nanostructures for Photocatalytic Abatement of Emerging Pharmaceutical Pollutant in Wastewater Effluent. Adv. Energy Sustain. Res. 2022, 3, 2100162. [Google Scholar] [CrossRef]
- Jin, D.; Lv, Y.; He, D.; Zhang, D.; Liu, Y.; Zhang, T.; Cheng, F.; Zhang, Y.; Sun, J.; Qu, J. Photocatalytic Degradation of COVID-19 Related Drug Arbidol Hydrochloride by Ti3C2 MXene/Supramolecular g-C3N4 Schottky Junction Photocatalyst. Chemosphere 2022, 308, 136461. [Google Scholar] [CrossRef] [PubMed]
- Bhamare, V.S.; Kulkarni, R.M. Photocatalytic degradation of pharmaceutical drug zidovudine by undoped and 5% barium doped zinc oxide nanoparticles during water treatment: Synthesis and characterisation. Int. J. Appl. Pharm. 2019, 11, 227. [Google Scholar] [CrossRef]
- Masunga, N.; Mamba, B.B.; Kefeni, K.K. Magnetically Separable Samarium Doped Copper Ferrite-Graphitic Carbon Nitride Nanocomposite for Photodegradation of Dyes and Pharmaceuticals under Visible Light Irradiation. J. Water Process Eng. 2022, 48, 102898. [Google Scholar] [CrossRef]
- Fang, Y.; Li, Y.; Zhou, F.; Gu, P.; Liu, J.; Chen, D.; Li, N.; Xu, Q.; Lu, J. An Efficient Photocatalyst Based on Black TiO2 Nanoparticles and Porous Carbon with High Surface Area: Degradation of Antibiotics and Organic Pollutants in Water. ChemPlusChem 2019, 84, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Chen, X.; Ma, J.; Gu, C.; Xian, Q.; Gong, T.; Sun, C. Carbon Nitride-Modified Defective TiO2–x@Carbon Spheres for Photocatalytic H2 Evolution and Pollutants Removal: Synergistic Effect and Mechanism Insight. J. Phys. Chem. C 2018, 122, 20444–20458. [Google Scholar] [CrossRef]
- He, J.; Ye, J.; Zhang, Y.; Kong, L.; Zhou, X.; Ma, Y.; Yang, Y. Synergistic RGO/Black TiO2/2D-ZIF-8 Ternary Heterogeneous Composite with Highly Efficient Photocatalytic Activity. ChemistrySelect 2020, 5, 3746–3755. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Czech, B.; Wasilewska, A.; Boguszewska-Czubara, A.; Yubuta, K.; Wagata, H.; Daminova, S.S.; Kadirova, Z.C.; Vargas, R. Detoxifying SARS-CoV-2 Antiviral Drugs from Model and Real Wastewaters by Industrial Waste-Derived Multiphase Photocatalysts. J. Hazard. Mater. 2022, 429, 128300. [Google Scholar] [CrossRef]
- Yuan, X.; Sun, M.; Yao, Y.; Lin, X.; Shi, J. N/Ti3+-Codoped Triphasic TiO2/g-C3N4 Heterojunctions as Visible-Light Photocatalysts for the Degradation of Organic Contaminants. New J. Chem. 2019, 43, 2665–2675. [Google Scholar] [CrossRef]
- Wang, W.-L.; Wu, Q.-Y.; Wang, Z.-M.; Hu, H.-Y.; Negishi, N.; Torimura, M. Photocatalytic Degradation of the Antiviral Drug Tamiflu by UV-A/TiO2: Kinetics and Mechanisms. Chemosphere 2015, 131, 41–47. [Google Scholar] [CrossRef]
- Singh, J.; Palsaniya, S.; Soni, R.K. Mesoporous Dark Brown TiO2 Spheres for Pollutant Removal and Energy Storage Applications. Appl. Surf. Sci. 2020, 527, 146796. [Google Scholar] [CrossRef]
- Cao, S.; Du, M.; Li, Y.; Ye, X.; Wang, Y.; Ye, J. Nanosized Carbonate-Doped TiO2–x Mesocrystals for Visible-Light-Driven Photocatalytic Removal of Water Pollutants. ACS Appl. Nano Mater. 2020, 3, 4197–4208. [Google Scholar] [CrossRef]
- Wu, S.; Li, X.; Tian, Y.; Lin, Y.; Hu, Y.H. Excellent Photocatalytic Degradation of Tetracycline over Black Anatase-TiO2 under Visible Light. Chem. Eng. J. 2021, 406, 126747. [Google Scholar] [CrossRef]
- Alalm, M.G.; Djellabi, R.; Meroni, D.; Pirola, C.; Bianchi, C.L.; Boffito, D.C. Toward Scaling-Up Photocatalytic Process for Multiphase Environmental Applications. Catalysts 2021, 11, 562. [Google Scholar] [CrossRef]
- Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and Prospects for Wastewater Treatment by UV and Visible-Light-Active Heterogeneous Photocatalysis: A Critical Review. In Heterogeneous Photocatalysis: Recent Advances; Muñoz-Batista, M.J., Navarrete Muñoz, A., Luque, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 225–264. ISBN 978-3-030-49492-6. [Google Scholar]
- Constantino, D.S.M.; Dias, M.M.; Silva, A.M.T.; Faria, J.L.; Silva, C.G. Intensification Strategies for Improving the Performance of Photocatalytic Processes: A Review. J. Clean. Prod. 2022, 340, 130800. [Google Scholar] [CrossRef]
- Beil, S.B.; Bonnet, S.; Casadevall, C.; Detz, R.J.; Eisenreich, F.; Glover, S.D.; Kerzig, C.; Næsborg, L.; Pullen, S.; Storch, G.; et al. Challenges and Future Perspectives in Photocatalysis: Conclusions from an Interdisciplinary Workshop. JACS Au 2024, 4, 2746–2766. [Google Scholar] [CrossRef]
- Sibhatu, A.; Kassegn, G.; Imteyaz, S.; Suresh, S.; Tran, N.; Hessel, V. Synthesis and Process Parametric Effects on the Photocatalyst Efficiency of CuO Nanostructures for Decontamination of Toxic Heavy Metal Ions. Chem. Eng. Process.—Process Intensif. 2022, 173, 108814. [Google Scholar] [CrossRef]
- Motamedi, M.; Yerushalmi, L.; Haghighat, F.; Chen, Z. Recent Developments in Photocatalysis of Industrial Effluents ։ A Review and Example of Phenolic Compounds Degradation. Chemosphere 2022, 296, 133688. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Akram, S.; khalid, S.; Lal, B.; Hassan, S.U.; Ashraf, R.; Kezembayeva, G.; Mushtaq, M.; Chinibayeva, N.; Hosseini-Bandegharaei, A. Advanced Photocatalysis as a Viable and Sustainable Wastewater Treatment Process: A Comprehensive Review. Environ. Res. 2024, 253, 118947. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Singh, P. Photocatalytic Water Decontamination Using Graphene and ZnO Coupled Photocatalysts: A Review. Mater. Sci. Energy Technol. 2019, 2, 509–525. [Google Scholar] [CrossRef]
- Kranz, C.; Wächtler, M. Characterizing Photocatalysts for Water Splitting: From Atoms to Bulk and from Slow to Ultrafast Processes. Chem. Soc. Rev. 2021, 50, 1407–1437. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; Liu, H.; Chen, J.; Ma, S.; Wen, M.; Kong, J.; An, T. Photocatalytic Degradation Mechanism of Gaseous Styrene over Au/TiO2@CNTs: Relevance of Superficial State with Deactivation Mechanism. Appl. Catal. B Environ. 2020, 272, 118969. [Google Scholar] [CrossRef]
- Bagheri, S.; TermehYousefi, A.; Do, T.-O. Photocatalytic Pathway toward Degradation of Environmental Pharmaceutical Pollutants: Structure, Kinetics and Mechanism Approach. Catal. Sci. Technol. 2017, 7, 4548–4569. [Google Scholar] [CrossRef]
- Otor, H.O.; Steiner, J.B.; García-Sancho, C.; Alba-Rubio, A.C. Encapsulation Methods for Control of Catalyst Deactivation: A Review. ACS Catal. 2020, 10, 7630–7656. [Google Scholar] [CrossRef]
- Zhang, Z.; He, D.; Zhao, S.; Qu, J. Recent Developments in Semiconductor-Based Photocatalytic Degradation of Antiviral Drug Pollutants. Toxics 2023, 11, 692. [Google Scholar] [CrossRef] [PubMed]

| Drug Class | Drug Name | Formulation (mg/tablet) | Dosage (tablet. d−1) | ||
|---|---|---|---|---|---|
| Generic | Acronym | Brand | |||
| NRTIs | Abacavir | ABC | Ziagen | 300 | 2 |
| Emtricitabine | FTC | Emtriva | 200 | 1 | |
| Didanosine | DDI | Videx EC | 250 | 1 | |
| Lamivudine | 3TC | Epivir | 300 | 1 | |
| Tenofovir disoproxil Fumarate | TDF | Viread | 300 | 1 | |
| Stavudine | d4T | Zerit | 60 | 1 | |
| Zalcitabine | ddC | Hivid | 0.750 | 3 | |
| Zidovudine | AZT, ZDV | Retrovir | 300 | 1 | |
| NNRTIs | Doravirine | DOR | Pifeltro | 100 | 1 |
| Efavirenz | EFV | Sustiva | 600 | 1 | |
| Etravirine | ETR | Intelence | 200 | 2 | |
| Nevirapine | NVP | Viramune | 200 | 2 | |
| Rilpivirine | RPV | Edurant | 25 | 1 | |
| PIs | Atazanavir | ATV | Reyataz | 300 | 1 |
| Darunavir | DRV | Prezista | 800 | 1 | |
| Fosamprenavir | FPV | Lexiva | 700 | - | |
| Ritonavir | RTV | Norvir | 100 | - | |
| Saquinavir | SQV | Invirase | 200 | - | |
| Tipranavir | TPV | Aptivus | 250 | - | |
| Indinavir | IDV | Crixivan | 400 | - | |
| Nelfinavir | NFV | Viracept | 1250 | 2 | |
| Lopinavir | LPV | Kaletra | 200 | 1 | |
| Amprenavir | APV | Agenerase | - | - | |
| INIs | Cabotegravir | CAB | Vocabria | 30 | 1 |
| Dolutegravir | DTG | Tivicay | 50 | 1 | |
| Raltegravir | RAL | Isentress | 400 | 2 | |
| E&FIs | Enfuvirtide | T-20 | Fuzeon | 90 | 2 |
| Maraviroc | MVC | Selzentry | 300 | 2 | |
| PEs | Cobicistat | COBI, c | Tybost | 150 | 1 |
| Pharmaceutical | Photocatalyst | Catalyst Dosage (g/L) | Irradiation | Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|
| 3TC | P25 | 1 | 365 UV | >95 | [147] |
| ABC | GO-TiO2 | 0.1 | 500 W m−2 | 99 | [142] |
| ACE | Black TiO2 | 3 | UV | 99 | [148] |
| ACE | OVPTCN | 0.5 | Visible: 300 W Xe lamp | 96 | [149] |
| ACV | TNPs-MWCNTs | 0.02 | 125 W Hg lamp | 98.6 | [143] |
| ACV | g-CN/TiO2 | 0.03 | 300 W Xe lamp | 100 | [150] |
| ACV | Ag2MoO4/g-C3N4 | 0.25 | 300 W Xe lamp | 100 | [151] |
| ACV | Bi4VO8Cl | 1 | 300 W Xe lamp | 100 | [152] |
| ACV | TNPs-MWCNTs | 0.4 | 365 | 98 | [143] |
| ACV | g-CN/TiO2 | 0.3 | >420 | 100 | [150] |
| ACV | Ag2MoO4/g-C3N4 | 0.25 | >420 | 100 | [151] |
| ACV | BiVO8Cl | 0.05 | 200–700 | 100 | [152] |
| AMX | Black TiO2 | 0.6 | F18W/T8, UVA | 100 | [116] |
| AMX | AC/TixOy | 0.01 | 100 W/m2 LED lamp | 92 | [153] |
| Antipyrine | Defective-TiO2 | 0.01 | Visible: 36 W lamp | 100 | [154] |
| Arbidol Hydrochloride | Ti3C2MXene/g-C3N4 | 0.1 | >420 | 99 | [155] |
| AZT | 5%BZONPs | 0.1 | 8 W UV lamp | 100 | [156] |
| AZT | CuSm0.06Fe1.94O4@g-C3N4 | 0.12 | Visible | 71.5 | [157] |
| AZT | CuO@Ag@Bi2S3 | 0.02 | 200 UV-A | 87.4 | [144] |
| AZT | CuSm0.06Fe1.94O4@g-C3N4 | 1.2 | >420 | 72 | [157] |
| AZT | CuO@Ag@Bi2S3 | 0.02 | 200 UV-A | 87 | [144] |
| Carbamazepine | BTN@PCs | 0.6 | Visible: Xe lamp | 63 | [158] |
| CIP | CSs/TiO2-x@gC3N4 | 1 | Visible: 1000 W Xe lamp | 90 | [159] |
| CIP | BTN@PCs | 0.6 | Visible: Xe lamp | 84 | [123] |
| DCF | RuTe2/B-TiO2 | 0.3 | 250W Xe lamp | 95.2 | [158] |
| Doxycycline Hydrochloride | RGO/BTiO2/2D-ZIF-8 | 0.1 | Visible: 300 W Xe lamp | 76 | [114] |
| EFV | Ag-AgBr-LDH | 2 | 84 | [160] | |
| IBP | B-N-TiO2 | 0.4 | Visible: 5 W LED lamp | 96 | [146] |
| LPV | Ammonium molybdate (WU and WW photocatalysts) | 0.4 | 500–550 | 95 | [123] |
| LVFX | NTTC | 0.3 | Visible: Xe lamp | ∼60 | [161] |
| Norfloxacin | BTN@PCs | 0.6 | Visible: Xe lamp | 45 | [162] |
| NVP | FL-BP@Nb2O5 | 0.1 | >420 | 68 | [158] |
| NVP | Ag-AgBr-LDH | 2 | 100 | [145] | |
| Oseltamivir | P25 | 0.5 | 365 UV | 96 | [146] |
| Oxytetracycline | BTN@PCs | 0.6 | Visible: Xe lamp | 94 | [163] |
| Oxytetracycline Hydrochloride | Dark brown TiO2 spheres | Natural sunlight | 80 | [158] | |
| PAR | AC/TixOy | 0.01 | 100 W/m2 LED lamp | 100 | [164] |
| RBV | Bi4VO8Cl | 0.05 | 200–780 | 100 | [153] |
| RTV | (WU and WW photocatalysts) | 0.4 | 500-550 | 95 | [152] |
| STV | CuO@Ag@Bi2S3 | 0.02 | 200 UV-A | 92.1 | [161] |
| Sulfisoxale | C-doped TiO2-x | 0.4 | Visible: CEL–HXF300 Xe lamp | ~70 | [144] |
| Sulfisoxale | BTN@PCs | 0.6 | Visible: Xe lamp | 75 | [165] |
| TC | Black anatase-TiO2 | 0.2 | Visible: 1000 W Xe lamp | 66.2 | [158] |
| TC | γ-Fe2O3/b-TiO2 | 0.3 | Solar: 300 W Xe lamp | 99.3 | [166] |
| TC | BTN@PCs | 0.6 | Visible: Xe lamp | 90 | [167] |
| TC | RGO@BT | 0.2 | Solar | 94 | [149] |
| TC | Ag/La-TiO2−x | 0.5 | 300 W Xe | 98 | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngwenya, P.; Tabana, L.S.; Tichapondwa, S.M.; Chirwa, E.M.N. Occurrence, Ecotoxicity, and Photocatalytic Remediation of Antiretroviral Drugs in Global Surface Water Matrices. Catalysts 2025, 15, 381. https://doi.org/10.3390/catal15040381
Ngwenya P, Tabana LS, Tichapondwa SM, Chirwa EMN. Occurrence, Ecotoxicity, and Photocatalytic Remediation of Antiretroviral Drugs in Global Surface Water Matrices. Catalysts. 2025; 15(4):381. https://doi.org/10.3390/catal15040381
Chicago/Turabian StyleNgwenya, Phephile, Lehlogonolo S. Tabana, Shepherd M. Tichapondwa, and Evans M. N. Chirwa. 2025. "Occurrence, Ecotoxicity, and Photocatalytic Remediation of Antiretroviral Drugs in Global Surface Water Matrices" Catalysts 15, no. 4: 381. https://doi.org/10.3390/catal15040381
APA StyleNgwenya, P., Tabana, L. S., Tichapondwa, S. M., & Chirwa, E. M. N. (2025). Occurrence, Ecotoxicity, and Photocatalytic Remediation of Antiretroviral Drugs in Global Surface Water Matrices. Catalysts, 15(4), 381. https://doi.org/10.3390/catal15040381










