Construction of Moiré-like Structure to Efficiently Enhance the H2 Photogeneration
Abstract
:1. Introduction
2. Results and Discussion
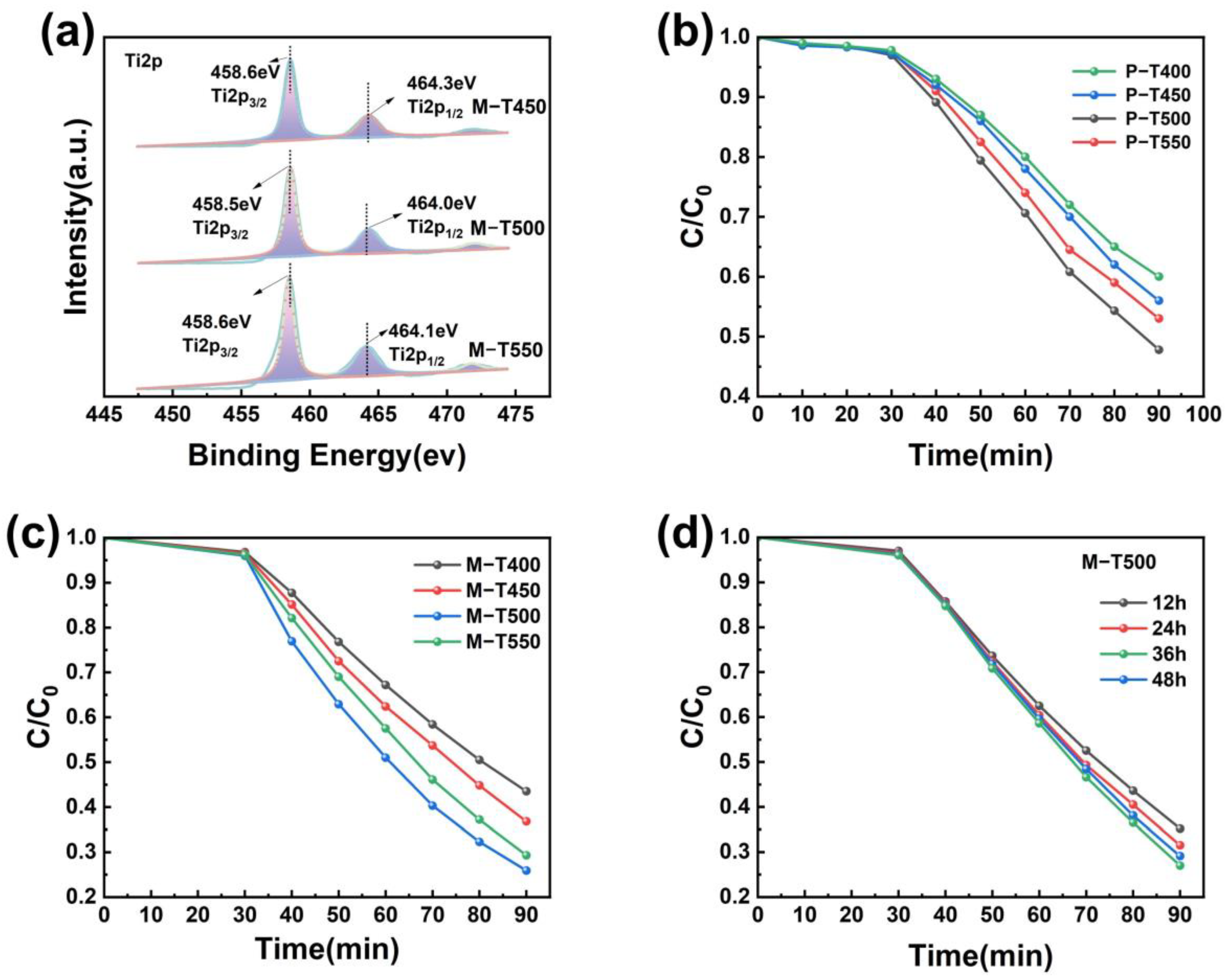
2.1. Physical Characterizations of M-TiO2
2.2. Analysis of the Photovoltaic Properties of M-TiO2
2.3. Analysis of Catalytic Experiments
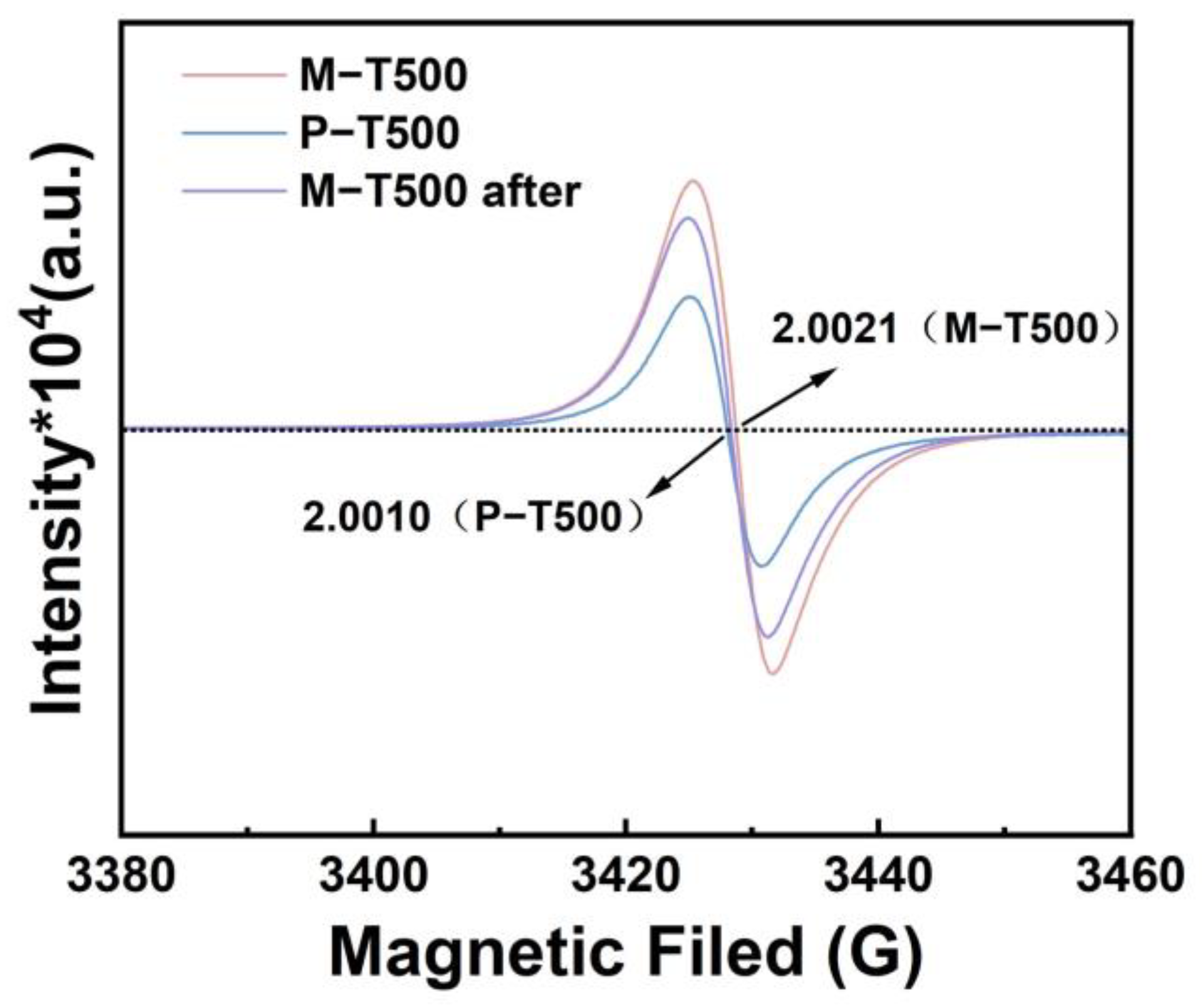
2.4. Photocatalytic Mechanism of M-TiO2
3. Experimental Section
3.1. Materials
3.2. Preparation of Moiré-like PDMS Film
3.3. Preparation of Moiré-like TiO2 by Sol-Gel Method
3.4. Characterizations
3.5. Catalysis Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| PDMS | Polydimethylsiloxane |
| DVD | Digital Versatile Disc |
| M-TiO2 | Moiré-like TiO2 |
| P-TiO2 | TiO2 without Moiré-like structure |
| M-T500 | Moiré-like TiO2 at the sintering temperature of 500 °C |
| M-T400 | Moiré-like TiO2 at the sintering temperature of 400 °C |
| M-T450 | Moiré-like TiO2 at the sintering temperature of 450 °C |
| M-T550 | Moiré-like TiO2 at the sintering temperature of 550 °C |
| P-T500 | TiO2 without Moiré-like structure at the sintering temperature of 500 °C |
| M-T500 before | M-T500 before catalytic reaction |
| M-T500 after | M-T500 after catalytic reaction |
References
- Liu, X.; Su, Y.; Li, Y.; Ma, Q.; Luo, J. Accompanying Bi Clusters as Charge Mediators Effectively Enhance Synergetic Redox of Bi2Sn2O7/ZnIn2S4 S-Scheme Heterostructure Composites. Small 2025, 21, 2410721. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Y.; Li, Y.; Yang, X.; Ma, Q.; Luo, J. MOF-on-MOF-derived CuO@In2O3 s-scheme heterojunction with core–shell structure for efficient photocatalytic CO2 reduction. Chem. Eng. J. 2024, 485, 149855. [Google Scholar] [CrossRef]
- Tayyab, M.; Kulsoom, U.E.; Liu, Y.; Mansoor, S.; Khan, M.; Akmal, Z.; Mushtaq, A.; Arif, M.; Shamriaz, U.; Zhou, L.; et al. Visible light-driven photocatalytic H2 evolution and dye degradation by electrostatic self-assembly of CdS nanowires on Nb2C MXene. Int. J. Hydrogen Energ. 2024, 51, 1400–1413. [Google Scholar] [CrossRef]
- Tayyab, M.; Mansoor, S.; Akmal, Z.; Khan, M.; Zhou, L.; Lei, J.; Zhang, J. A binary dumbbell visible light driven photocatalyst for simultaneous hydrogen production with the selective oxidation of benzyl alcohol to benzaldehyde. J. Colloid. Interface Sci. 2024, 665, 911–921. [Google Scholar] [CrossRef]
- Tayyab, M.; Xie, Y.; Tan, X.; Usman, M.; Tang, M.-C.; Chen, S.S. A ternary dumbbell MoS2 tipped Zn0.1Cd0.9S nanorods visible light driven photocatalyst for simultaneous hydrogen production with organics degradation in wastewater. Chem. Eng. J. 2025, 505, 159064. [Google Scholar] [CrossRef]
- Kaya, S.; Saka, C.; Caglar, A.; Kaya, M.; Kivrak, H. Enhanced Photocatalytic Hydrogen Production on Cd-, Te-, Se-, and S-Doped Titanium Dioxide Catalysts. J. Electron. Mater. 2023, 52, 8227–8236. [Google Scholar] [CrossRef]
- Deng, X.; Liu, D.; Yuan, M.; Li, Y.; Yang, H.; Wang, C.; Wang, R.; Yang, X. Synergistic Effect of Boron Doping and Porous Structures on Titanium Dioxide for Efficient Photocatalytic Nitrate Reduction to Nitrogen in Pure Water. Inorg. Chem. 2025, 64, 2294–2302. [Google Scholar] [CrossRef]
- Li, F.; Huang, Y.; Peng, H.; Cao, Y.; Niu, Y. Preparation and Photocatalytic Water Splitting Hydrogen Production of Titanium Dioxide Nanosheets. Int. J. Photoenergy 2020, 2020, 3617312. [Google Scholar] [CrossRef]
- Mansoor, S.; Tayyab, M.; Khan, M.; Akmal, Z.; Zhou, L.; Lei, J.; Anpo, M.; Zhang, J. Recent advancements in Se- and Te-enriched cocatalysts for boosting photocatalytic splitting of water to produce hydrogen. Res. Chem. Intermediat 2023, 49, 3723–3745. [Google Scholar] [CrossRef]
- Thabet, S.M.; Abdelhamid, H.N.; Ibrahim, S.A.; El-Bery, H.M. Boosting photocatalytic water splitting of TiO2 using metal (Ru, Co, or Ni) co-catalysts for hydrogen generation. Sci. Rep. 2024, 14, 10115. [Google Scholar] [CrossRef]
- Liu, Y.; Tayyab, M.; Pei, W.; Zhou, L.; Lei, J.; Wang, L.; Liu, Y.; Zhang, J. The Precision Defect Engineering with Nonmetallic Element Refilling Strategy in g-C3N4 for Enhanced Photocatalytic Hydrogen Production. Small 2023, 19, e2208117. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Meng, X.; Liao, L.; Yuan, R.; Zhang, H.; Zhao, G.; Tang, K. Bottom-up construction of defective TiO2 microspheres with hierarchical architectures and controlled crystallites for visible-light photocatalysis. J. Alloys Compd. 2024, 1008, 176618. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, M.; Li, Y.; Sun, B.; Yang, X.; Su, Y.; Luo, J. Te2−modulated heterogeneous remodeling of S atoms in ultrathin ZnIn2S4 nanosheets containing S vacancies synergistically enhances CO2 photoreduction. J. Colloid. Interface Sci. 2024, 679, 772–784. [Google Scholar] [CrossRef] [PubMed]
- AlIssa, Y.; Hamidi, S.M.; Shahnazi, A.; Nabid, M.R. One dimensional efficient photocatalyst based on plasmonic grating. Opt. Quant. Electron. 2021, 53, 355. [Google Scholar] [CrossRef]
- Aravind, I.; Wang, Y.; Cai, Z.; Shen, L.; Zhao, B.F.; Yang, S.S.; Wang, Y.; Dawlaty, J.M.; Gibson, G.N.; Guignon, E.; et al. Hot Electron Plasmon-Resonant Grating Structures for Enhanced Photochemistry: A Theoretical Study. Crystals 2021, 11, 118. [Google Scholar] [CrossRef]
- Masuzawa, S.; Okazaki, S.; Maru, Y.; Mizutani, T. Catalyst-type-an optical fiber sensor for hydrogen leakage based on fiber Bragg gratings. Sens. Actuators B Chem. 2015, 217, 151–157. [Google Scholar] [CrossRef]
- Iqbal, T.; Ali, M.; Ahsan, S.; Afsheen, S.; Farrooq, M.; El-marghany, A.; Warad, I. Geometrical Optimization of TiO2-Noble Metal Grating for Enhanced Photocatalytic Activity and SPR Biosensor Application. Plasmonics 2024, 19, 2635–2652. [Google Scholar] [CrossRef]
- Iloglu, O.; Yurtsever, H.A. The effects of annealing temperature on the thickness, morphology, band gap energy, and photocatalytic performance of ZIF-8-derived ZnO/TiO2 thin films. J. Mater. Sci.-Mater. Electron. 2024, 35, 1211. [Google Scholar] [CrossRef]
- Sadia, S.I.; Shishir, M.K.H.; Ahmed, S.; Aidid, A.R.; Islam, M.M.; Rana, M.M.; Al-Reza, S.M.; Alam, M.A. Crystallographic biography on nanocrystalline phase of polymorphs titanium dioxide (TiO2): A perspective static review. S. Afr. J. Chem. Eng. 2024, 50, 51–64. [Google Scholar] [CrossRef]
- Shen, J.; Olfert, J.; Abbasi-Atibeh, E.; Semagina, N. The effect of catalyst particle size and temperature on CNT growth on supported Fe catalysts during methane pyrolysis. Catal. Today 2025, 453, 115275. [Google Scholar] [CrossRef]
- Yang, H.; Tao, Q. Preparation and characterization of doped titanium dioxide thin films. Optoelectron. Adv. Mat. 2008, 2, 806–810. [Google Scholar]
- Zou, W.W.; Zhang, J.L.; Chen, F.; Anpo, M.; He, D.N. A simple approach for preparing a visible-light TiO2 photocatalyst. Res. Chem. Intermediat 2009, 35, 717–726. [Google Scholar] [CrossRef]
- Doong, R.A.; Chang, S.M.; Hung, Y.C.; Kao, I.L. Preparation of highly ordered titanium dioxide porous films: Characterization and photocatalytic activity. Sep. Purif. Technol. 2007, 58, 192–199. [Google Scholar] [CrossRef]
- Paul, K.K.; Sreekanth, N.; Biroju, R.K.; Narayanan, T.N.; Giri, P.K. Solar light driven photoelectrocatalytic hydrogen evolution and dye degradation by metal-free few-layer MoS nanoflower/TiO(B) nanobelts heterostructure. Sol. Energ. Mat. Sol. C 2018, 185, 364–374. [Google Scholar] [CrossRef]
- Balamurugan, M.; Silambarasan, M.; Saravanan, S.; Soga, T. Synthesis of anatase and rutile mixed phase titanium dioxide nanoparticles using simple solution combustion method. Phys. B Condens. Matter 2022, 638, 413843. [Google Scholar] [CrossRef]
- Brella, M.; Taabouche, A.; Gharbi, B.; Gheriani, R.; Bouachiba, Y.; Bouabellou, A.; Serrar, H.; Touil, S.; Laggoune, K.; Boudissa, M. Comparison of Thin Films of Titanium Dioxide Deposited by Sputtering and Sol-Gel Methods for Waveguiding Applications. Semiconductors 2022, 56, 234–239. [Google Scholar] [CrossRef]
- Makula, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Li, Y.D.; Jiang, Y.Q.; Ruan, Z.H.; Lin, K.F.; Yu, Z.B.; Zheng, Z.F.; Xu, X.Z.; Yuan, Y. Simulation-guided synthesis of graphitic carbon nitride beads with 3D interconnected and continuous meso/macropore channels for enhanced light absorption and photocatalytic performance. J. Mater. Chem. A 2017, 5, 21300–21312. [Google Scholar] [CrossRef]
- Guizard, C.; Princivalle, A. Preparation and characterization of catalyst thin films. Catal. Today 2009, 146, 367–377. [Google Scholar] [CrossRef]
- Li, Y.D.; Ruan, Z.H.; He, Y.Z.; Li, J.Z.; Li, K.Q.; Jiang, Y.Q.; Xu, X.Z.; Yuan, Y.; Lin, K.F. In situfabrication of hierarchically porous g-CN and understanding on its enhanced photocatalytic activity based on energy absorption. Appl. Catal. B Environ. 2018, 236, 64–75. [Google Scholar] [CrossRef]
- Li, Y.D.; Ruan, Z.H.; He, Y.Z.; Li, J.Z.; Li, K.Q.; Yang, Y.L.; Xia, D.B.; Lin, K.F.; Yuan, Y. Enhanced photocatalytic H2 evolution and phenol degradation over sulfur doped meso/macroporous g-C3N4 spheres with continuous channels. Int. J. Hydrogen Energ. 2019, 44, 707–719. [Google Scholar] [CrossRef]
- Yang, X.X.; Lu, F.H. Enhancement of photoelectrochemical performance for nitrogen-doped titanium dioxide-based thin films produced by a facile air-based sputtering deposition. Ceram. Int. 2024, 50, 7817–7826. [Google Scholar] [CrossRef]
- Bermudez, S.; Castaneda, L.; Salazar, L.; Sanchez-Saenz, C.; Carmona, D. Photoelectrochemical Methods for Flat Band Potential Estimation: Case Studies of ZnO Nanorods and TiO2 Compact Films. J. Electrochem. Soc. 2022, 169, 096513. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Yang, R.J.; Mei, L.; Fan, Y.Y.; Zhang, Q.Y.; Zhu, R.S.; Amal, R.; Yin, Z.Y.; Zeng, Z.Y. ZnIn2S4-Based Photocatalysts for Energy and Environmental Applications. Small Methods 2021, 5, 2100887. [Google Scholar] [CrossRef]
- Shishi, S.; Xibao, L.; Yingtang, Z.; Lu, H.; Yu, X.; Fang, D.; Juntong, H.; Zhi, C.; Zhijun, F.; Jilin, X.; et al. Novel BiOBr/Bi2S3 high-low junction prepared by molten salt method for boosting photocatalytic degradation and H2O2 production. J. Mater. Sci. Technol. 2023, 155, 148–159. [Google Scholar]
- Li, X.; Han, T.; Zhou, Y.; Xie, Y.; Luo, Y.; Huang, J.; Chen, Z.; Deng, F. Photoelectrocatalytic hydrogen evolution and synchronous degradation of organic pollutants by pg-C3N4/β-FeOOH S-scheme heterojunction. Sci. China Technol. Sci. 2024, 67, 1238–1252. [Google Scholar] [CrossRef]
- Zan, Z.; Li, X.; Gao, X.; Huang, J.; Luo, Y.; Han, L. 0D/2D Carbon Nitride Quantum Dots (CNQDs)/BiOBr S-Scheme Heterojunction for Robust Photocatalytic Degradation and H2O2 Production. Acta Phys.-Chim. Sin. 2023, 39, 2209016. [Google Scholar]
- Zhao, S.; Shen, S.S.; Han, L.; Tian, B.C.; Li, N.; Chen, W.; Li, X.B. Ferroelectric perovskite PbTiO3 for advanced photocatalysis. Rare Met. 2024, 43, 4038–4055. [Google Scholar] [CrossRef]
- Mechiakh, R.; Gheriani, R.; Chtourou, R. Preparation of Nanocrystalline Titanium Dioxide (TiO2) Thin Films by the Sol-gel dip Coating Method. J. Nano Res. 2011, 16, 105–111. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Zhu, J.; Wang, Q.; Xie, M.W.; Meng, K.; Mao, L.B.; Yang, L.; Pan, T.S.; Gao, M.; Yao, G.; et al. Flexible Antibacterial Respiratory Monitoring Sensor Based on Controllable Au-Modified Surface of Highly {001} Preferred Anatase Titanium Dioxide Thin Film. ACS Biomater. Sci. Eng. 2024, 10, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Xu, Y.; Chen, S.Q.; Zhou, Y.F.; Zhu, J.Y.; Chen, G. (3-Aminopropyl)trimethoxysilane-Functionalized Titanium Dioxide Thin-Film Nanocomposite Membrane: Enhanced Rejection Performance for Unary and Binary High-Salt Wastewater. ACS Appl. Polym. Mater. 2023, 5, 10137–10147. [Google Scholar] [CrossRef]
- Sari, Y.; Gareso, P.L.; Armynah, B.; Tahir, D. A review of TiO2 photocatalyst for organic degradation and sustainable hydrogen energy production. Int. J. Hydrogen Energ. 2024, 55, 984–996. [Google Scholar] [CrossRef]
- Khatibnezhad, H.; Sani, M.A.F. Preparation and characterization of nanostructured TiO2 thin film codoped with nitrogen and vanadium on glass surface by sol-gel dip-coating method. Res. Chem. Intermediat 2015, 41, 7349–7361. [Google Scholar] [CrossRef]






| Material | Transmittance | Absorptivity |
|---|---|---|
| P-T500 | 61.5 | 38.5 |
| M-T500 | 32.4 | 67.6 |
| Material | Specific Surface Area (m2/g) | Average Pore Diameter (nm) |
|---|---|---|
| P-T500 | 42.2 | 6.1 |
| M-T500 | 60.4 | 8.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Yuan, M.; Hao, Y.; Wang, Y.; Jiao, H.; Li, Y.; Zhou, D.; Liu, X.; Yang, H.; Wang, C. Construction of Moiré-like Structure to Efficiently Enhance the H2 Photogeneration. Catalysts 2025, 15, 398. https://doi.org/10.3390/catal15040398
Yang G, Yuan M, Hao Y, Wang Y, Jiao H, Li Y, Zhou D, Liu X, Yang H, Wang C. Construction of Moiré-like Structure to Efficiently Enhance the H2 Photogeneration. Catalysts. 2025; 15(4):398. https://doi.org/10.3390/catal15040398
Chicago/Turabian StyleYang, Guanglu, Meng Yuan, Yuexi Hao, Yizhe Wang, Haochen Jiao, Yudong Li, Desheng Zhou, Xing Liu, Haiyue Yang, and Chengyu Wang. 2025. "Construction of Moiré-like Structure to Efficiently Enhance the H2 Photogeneration" Catalysts 15, no. 4: 398. https://doi.org/10.3390/catal15040398
APA StyleYang, G., Yuan, M., Hao, Y., Wang, Y., Jiao, H., Li, Y., Zhou, D., Liu, X., Yang, H., & Wang, C. (2025). Construction of Moiré-like Structure to Efficiently Enhance the H2 Photogeneration. Catalysts, 15(4), 398. https://doi.org/10.3390/catal15040398








